Abstract
The risk of developing systemic lupus erythematosus (SLE) is approximately nine times higher among women compared to men. However, very little is understood concerning the underlying mechanisms that contribute to this gender bias. Further, whether there is a link between immune response initiated in the gut mucosa, the progression of SLE and the associated gender bias has never been investigated. In this report, we show a potential link between the immune response of the gut mucosa and SLE and the gender bias of lupus for the first time, to our knowledge. Both plasma cell- and gut-imprinted- α4β7 T cell frequencies were significantly higher in the spleen and gut mucosa of female (SWR × NZB)F1 (SNF1) mice compared to that of their male counterparts. Importantly, female SNF1 mice not only showed profoundly higher CD45+ immune cell densities, but also carried large numbers of interleukin (IL)-17-, IL-22- and IL-9-producing cells in the lamina propria (LP) compared to their male counterparts. Intestinal mucosa of female SNF1 mice expressed higher levels of a large array of proinflammatory molecules, including type 1 interferons and Toll-like receptors 7 and 8 (TLR-7 and TLR-8), even before puberty. Our work, therefore, indicates that the gut immune system may play a role in the initiation and progression of disease in SLE and the associated gender bias.
Keywords: autoimmunity, gender bias, gut mucosa, inflammation, systemic lupus erythematosus
Introduction
The aetiology of systemic lupus erythematosus (SLE) is still unknown. SLE is an autoimmune disease characterized by the production of autoantibodies against nuclear material, and these immune complexes lead to glomerulonephritis and kidney failure1. It is generally believed that SLE is caused by a combination of genetic and environmental factors1,2. Variability in the genes that code for human leucocyte antigen (HLA), FcgRII and tumour necrosis factor (ligand) superfamily, member 4 (Tnfsf4) have been linked to SLE susceptibility1–3. In addition, viral infections including Epstein–Barr virus (EBV) and parvovirus in predisposed patients may also trigger the disease2,4. Diverse environmental stimuli such as cigarette smoking and silica dust have also been implicated in the onset of SLE1,2.
However, as has been observed in many other autoimmune diseases, including rheumatoid arthritis (RA) and multiple sclerosis (MS), there is a predisposition for SLE in women with a prevalence ratio close to 9 : 1 greater than men3. X-linked genes such as Foxp3, Tnf and Tlr7 have been correlated with this disproportionality5,6. Numerous studies have suggested that sex hormones, oestrogen in particular, can contribute to the onset and development of disease activities associated with SLE. Oestrogen is reported to have inductive effects on autoimmune-related immune responses such as production of antibody and proinflammatory cytokines3,6–8.
Recent studies using the non-obese diabetic (NOD) mouse model of type 1 diabetes (T1D) have reported a role for microbiota, independent of oestrogen-mediated effects, in determining the gender bias of autoimmune diseases9,10. While under a specific pathogen-free (SPF) environment, NOD mice showed a disease incidence ratio of approximately 4 : 1 in females greater than males; this difference does not exist under germ-free (GF) conditions9–11, suggesting that male NOD mice appear to be protected, at least in part, by the testosterone–gut microbiome interaction9,10. These studies indicate the strong involvement of immune responses initiated in the intestinal mucosa in determining this gender disparity. Even though lupus-susceptible GF mice develop disease12,13, whether the involvement of immune response that is initiated in the gut and the microbiota has a disease modulatory effect in SLE is not known. More recently, results from our laboratory show that change in the pH of drinking water using mouse models of T1D and SLE has a profound effect on the onset and severity of both diseases14 (Gaudreau et al., unpublished observation). This evidence suggests a potential role for mucosal immunity in shaping the systemic immune response observed in autoimmune diseases such as SLE.
Here we demonstrate, for the first time to our knowledge, the potential contribution of immune response initiated in the gut mucosa in determining the associated gender bias observed in SLE. Lupus-susceptible female (SWR × NZB)F1 (SNF1) mice carry profoundly higher numbers of plasma cells as well as gut-imprinted α4β7 T cells in the spleen and intestine. Further, higher numbers of proinflammatory cytokines interleukin (IL)-17-, IL-22- and IL-9-producing cells were found in the lamina propria (LP) of female mice. Importantly, the proinflammatory immune response in the gut mucosa of females appears to have initiated long before the onset of puberty, suggesting a lack of association between sex hormones and difference in the gut immune response in males and females. Overall, our study implies that proinflammatory responses that are initiated in the gut mucosa may have a contributory role in the initiation and progression of disease in SLE and the associated gender bias in lupus incidence.
Materials and methods
Mice
SWR and NZB mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and housed under SPF conditions at the animal facilities of the Medical University of South Carolina (MUSC). (SWR × NZB)F1 (SNF1) hybrids were bred at the SPF facility of MUSC. All animal experiments were performed according to ethical principles and guidelines approved by the institutional animal care committee.
Proteinuria
Urine samples were tested weekly for proteinuria. Protein level in the urine was determined by Bradford assay (BioRad, Hercules, CA, USA) against bovine serum albumin standards. Proteinuria was scored as follows; 0: 0–1 mg/ml, 1: 1–2 mg/ml, 2: 2–5 mg/ml, 3: 5–10 mg/ml and 4: ≥ 10 mg/ml. Mice that showed proteinuria >5 mg/ml were considered to have severe nephritis.
Enzyme-linked immunosorbent assay (ELISA)
Antibodies against nucleohistone and dsDNA in mouse sera were evaluated by ELISA. Briefly, 0·5 μg/well of nucleohistone (Sigma-Aldrich, St Louis, MO, USA) or 1 μg/well dsDNA from calf thymus (Sigma-Aldrich) was coated as antigen overnight onto ELISA plate wells in a carbonate buffer. Serial dilutions of the sera were made and immunoglobulin (Ig)G, IgG1, IgG2a or IgG3 were detected using horseradish peroxidase (HRP)-conjugated anti-mouse antibodies (Sigma-Aldrich, eBioscience, San Diego, CA, USA and Invitrogen, Carlsbad, CA, USA).
Flow cytometric analyses
Freshly isolated cells from spleen, mesenteric lymph node (MLN) and Peyer's patches (PP) were stained for surface markers using fluorochrome-labelled antibodies that are specific for mouse CD19, IgM, IgG and CD138 for B cells and CD4, CD44, CD62L, α4β7 and CCR9 for T cells (eBioscience and BD Biosciences, San Jose, CA, USA). Detection of immune cells was also performed using fluorochrome-labelled antibodies specific for mouse natural killer (NK)1.1, CD3ε, CD1d tetramer, CD11c, plasmacytoid dendritic cell antigen-1 (PDCA), T cell receptor (TCR)-β and TCR-γδ (NIH tetramer core; eBioscience and BD Biosciences). For detecting intracellular cytokines, cells were stimulated for 4 h at 37°C with phorbol myristate acetate (PMA) and ionomycin in the presence of brefeldin A/Golgiplug (BD Biosciences), fixed with paraformaldehyde, and permeabilized using 0·1% saponin. These cells were stained for interferon (IFN)-γ, IL-17, IL-4, IL-10 and IL-21. For some assays, cells were stimulated with PMA, ionomycin and bacterial lipopolysaccharide (LPS) (10 μg/ml) and B cells were stained for intracellular cytokines.
Immunofluorescence microscopy
Distal ileum pieces were snap-frozen in optimal cutting temperature (OCT) medium and 6-μm cryosections were made for immunofluorescence staining. Tissue sections were stained using Alexa Fluor 488-linked anti-mouse CD45, phycoerythrin (PE)-linked IL-17, IL-22 and IL-9 and 4′,6-diamidino-2-phenylindole (DAPI) and the images were acquired using either the BD CARVII or Olympus Fv10i inverted microscopes.
Quantitative polymerase chain reaction (PCR)
RNA was extracted from 2-cm pieces of the distal ileum using Isol-RNA Lysis Reagent (5′) according to the manufacturer's instructions. cDNA was prepared from RNA using Moloney murine leukaemia virus (MMLV) reverse transcriptase (Promega) and PCR was performed using SYBR-green master-mix (BioRad) and target-specific custom-made primer sets. A StepOne Plus (Applied Biosystems/ThermoFisher Scientific, Waltham, MA, USA) real-time PCR machine was used and relative expression of each factor was calculated by the 2-ΔCT cycle threshold method against β-actin control.
Statistical analysis
Proteinuria curves were analysed using the log-rank method and the proteinuria scores were analysed using Fisher's exact test. One- and two-tailed Student's t-tests were also employed to calculate P-values where indicated. A P-value ≤0·05 was considered statistically significant, as follows: *P ≤0·05, **P ≤ 0·01, ***P ≤ 0·001 for two-tailed and #P ≤0.05 for one-tailed tests. GraphPad Prism was used for calculating statistical significance for data from most experiments.
Results
Disease progression and antibodies against nuclear (nAgs) in SNF1 mice
SNF1 mice that develop lupus symptoms and proteinuria spontaneously and show gender bias in disease incidence similar to human SLE patients15,16 have been used widely for understanding the disease aetiology and for preclinical testing of therapeutics. To confirm this gender bias of disease progression and lupus in SNF1 mice housed in our facility, we have compared the progressive changes in the urinary protein levels and circulating anti-nucleohistone and anti-dsDNA antibodies in male and female mice. Consistent with earlier reports15–17, Fig. 1a shows that 80% of female SNF1 mice develop severe nephritis, as indicated by high proteinuria, within 32 weeks of age. In comparison, only 20% of male SNF1 mice at the same age showed severe nephritis, and only 50% of these developed severe nephritis by 40 weeks of age. Comparison of nephritis severity based on proteinuria levels, as reported previously15–17, showed that female SNF1 mice indeed develop significantly more nephritis than males at all time-points (Fig. 1b).
Fig 1.
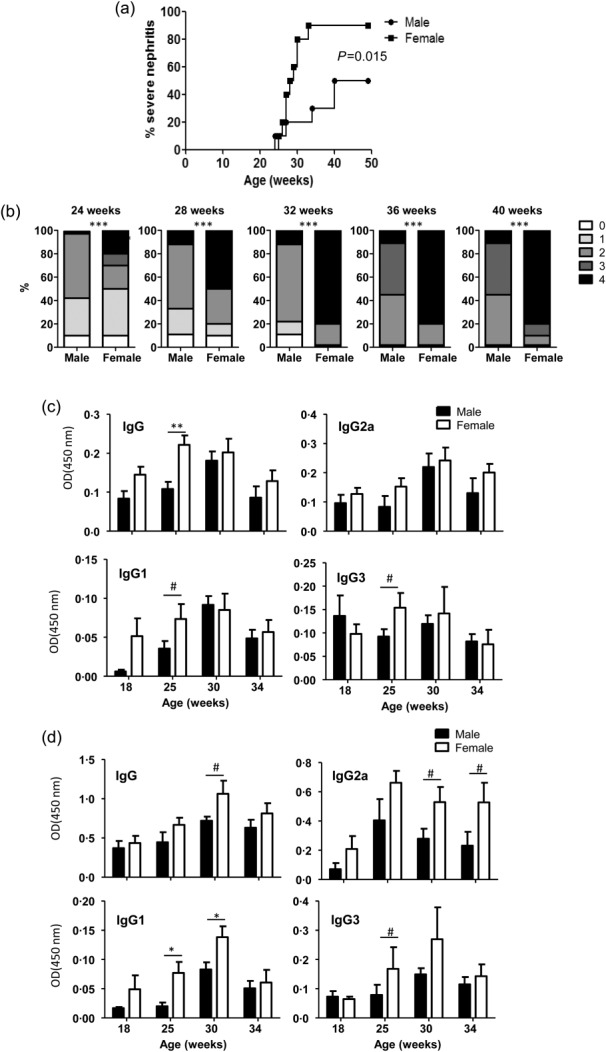
Gender difference in disease incidence and autoantibody levels in SNF1 mice. Male and female SWR × NZB F1 (SNF1) mice (10/group) were examined for proteinuria and autoantibodies. (a) Protein levels in urine samples were quantified by Bradford assay. Percentage of mice with severe nephritis as indicated by high proteinuria (≥5 mg/ml) is shown. (b) Severity of nephritis in male and female mice at different time-points is shown. Nephritis severity was scored based on urinary protein levels as follows; 0: 0–1 mg/ml, 1: 1–2 mg/ml, 2: 2–5 mg/ml, 3: 5–10 mg/ml and 4: ≥ 10 mg/ml. P-values were calculated using a two-tailed χ2 test. Serum levels of total immunoglobulin (Ig)G, IgG1, IgG2a and IgG3 antibodies specific against nucleohistone (c) or dsDNA (d) were assessed by enzyme-linked immunosorbent assay (ELISA) for indicated time-points. Each bar represents mean ± standard error of the mean of optical density values of samples from 10 mice/group.
Comparison of autoantibody levels in male and female SNF1 mice showed that, at 25 weeks of age, female SNF1 mice had significantly higher levels of overall serum IgG as well as the IgG3 and IgG1 isotypes against nucleohistone (nAg) (Fig. 1c). Moreover, female SNF1 mice also had significantly higher levels of circulating anti-dsDNA IgG, IgG2a, IgG1 and IgG3 antibodies than male mice, mainly at older ages (Fig. 1d). Overall, these results confirm the previously reported gender bias in lupus-like disease, in terms of nephritis and anti-nAg antibody levels, in SNF1 mice.
B cell phenotype in male and female SNF1 mice
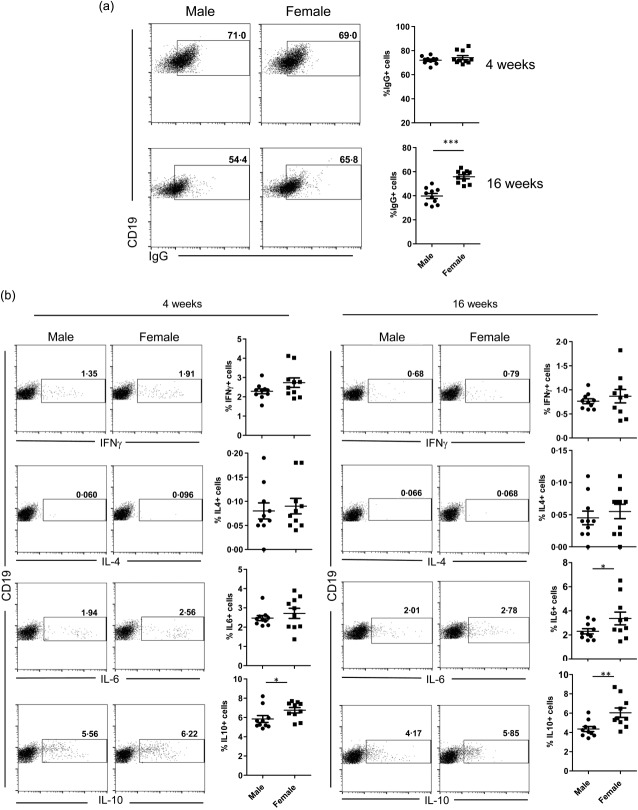
While oestrogens and testosterones are thought to have disease-promoting and -suppressing roles, respectively, in SLE18–20, the potential involvement of testosterone–gut microbiome interaction in protecting male NOD mice from T1D has been discussed9,10. Therefore, to assess if there is a link between the sex hormones and immune cell function towards their contribution to lupus incidence, splenic B and T cell properties of prepuberty (4-week-old) and adult (16-week-old) male and female SNF1 mice were studied. As the initiation and progression of autoimmunity are influenced by immunological events of early age, a prenephritic adult mouse 16 weeks of age, instead of a nephritic age, was used in this study. To realize the functionality of B cells, splenic cells were examined for surface IgG, intracellular cytokines IL-6, IL-10 and IL-4, as well as IFN-γ and the plasma cell frequencies. While the total number of IgG+ B cells was comparable in male and female mice at 4 weeks of age, 16-week-old adult female SNF1 mice harboured a significantly higher number of IgG+ B cells in the spleen compared to their male counterparts (Fig. 2a) which, as anticipated, corresponds to higher levels of IgG antibodies detected against nAg (Fig. 1c). Examination of the cytokines expressed revealed that B cells from female SNF1 mice showed considerably higher numbers of IL-10- and IL-6-positive B cells compared to their male counterparts, even at prepuberty age (Fig. 2b). These results show the potential contribution of IL-10 and IL-6 in rapid disease progression and higher lupus incidence in females. Examination of CD138+ plasma cell frequencies revealed that only 4-week-old, but not 16-week-old, females had higher numbers of IgMhi and IgMlo CD138+ cells compared to their age-matched male counterparts (Supporting information, Fig. S1). Phenotypical characterization of splenic T cells from male and female SNF1 mice aged 4 and 16 weeks revealed considerable differences in the memory-, regulatory- and cytokine-positive T cells only at prepuberty age, but not adult age (Supporting information, Fig. S2). These observations indicate that the T and B cell properties of lupus- susceptible male and female SNF1 mice may not be influenced by sex hormones.
Fig 2.
Difference in the phenotype of splenic B cells from male and female SWR × NZB F1 (SNF1) mice. (a) Spleen cells from male and female SNF1 mice aged 4 and 16 weeks were examined for surface CD19 and IgG by fluorescence activated cell sorter (FACS). Shown are representative FACS plots for male and female groups of mice and mean ± standard error of the mean (s.e.m.) values. (b) Intracellular cytokine expression in B cells was examined by FACS. Splenocytes were activated with PMA/ionomycin and LPS in the presence of brefeldin A and the cells were stained for surface CD19 and intracellular cytokines. Representative FACS plots and mean ± s.e.m. values are shown.
Adult female SNF1 mice harbour large numbers of gut mucosa-imprinted T cells
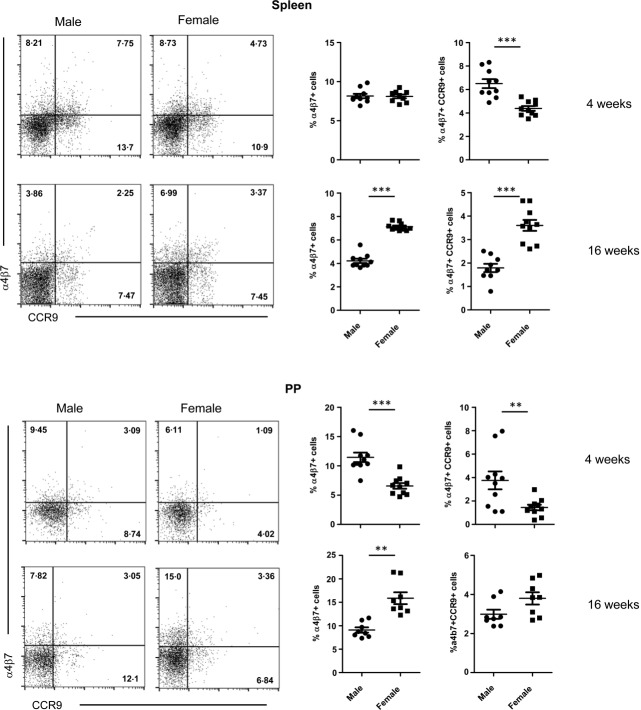
T cells that are activated in the intestinal mucosa express specific markers, such as α4β7 and CCR9. These markers are induced primarily by retinoic acid21,22, and also by the interaction with CD103+ dendritic cells (DCs)23,24. We therefore investigated the α4β7 integrin and CCR9 expression on CD4+ T cells that were isolated from the spleen and small intestinal PP. While spleen and PP of 4-week-old females showed lower numbers of α4β7+ and α4β7+CCR9+-positive cells compared to males, higher frequencies of these cells were detected in both spleen and PP of 16-week-old adult female mice (Fig. 3). These results suggest that these gut resident effector T cells are activated differently in male and female SNF1 mice before and after puberty and they may have a pathogenic role in lupus.
Fig 3.
Gut-associated T cells in male and female SWR × NZB F1 (SNF1) mice. Spleen and Peyer's patches of 4- and 16-week-old male and female SNF1 mice were examined for CD4, α4β7 and CCR9 expression by fluorescence activated cell sorter (FACS). Representative FACS plots for male and female (left panels) and mean ± standard error of the mean of percentage values of α4β7 single-positive or α4β7 and CCR9 double-positive cells (right panels) are shown.
Gut mucosa resident immune cells in male and female SNF1 mice are phenotypically different
Although the association between gut microbiome and disease incidence is less clear in lupus compared to other autoimmune diseases, a potential role for dietary factors is well recognized25–29, indicating a possible contribution of the immune responses initiated in the intestinal mucosa in lupus. Because the frequencies of mucosa-imprinted T cells are significantly different in male and female SNF1 mice, we examined if the characteristics of immune cells in the intestine of prepubertal and prenephritic adult-aged male and female SNF1 mice are different. Examination of plasma cells revealed that although there is no difference in the frequency of CD138+ cells in the PP of male and female SNF1 mice at prepuberty age, the females have significantly higher numbers of CD138+ B cells at prenephritic adult age (Fig. 4a). As in the case of spleen, adult female SNF1 mice showed a profoundly higher number of IgG+ B cells compared to their male counterparts (Fig. 4b). Importantly, 4-week-old females also showed significantly higher frequencies of IgG+ B cells in the PP compared to males. These observations suggest that B cells which are activated in the gut mucosa, beginning at prepuberty age, may indeed have an influence on the gender bias associated with lupus. Of note, gut mucosa-imprinted (IgA+) B cell frequencies were not significantly different in male and female SNF1 mice at prepuberty and adult ages (not shown).
Fig 4.
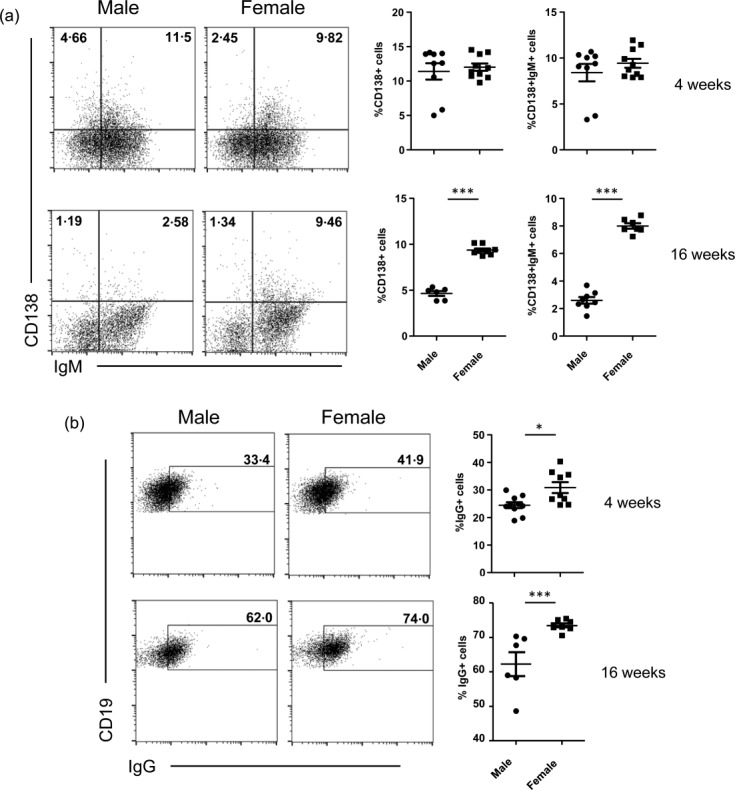
Female SWR × NZB F1 (SNF1) mice show higher numbers of plasma cells in the gut mucosa. Peyer's patches of 4- and 16-week-old male and female mice were stained for surface CD19, CD138, immunoglobulin (Ig)M and IgG and analysed by fluorescence activated cell sorter (FACS). (a) The frequencies of CD138 single-positive and CD138 and IgM double-positive plasma cells are shown. (b) CD19+ cells with surface expression of IgG are shown. Representative FACS plots (left panels) and mean ± standard error of the mean of percentage values (right panels) are shown.
The frequencies of splenic and intestinal plasmacytoid DCs (pDCs), TCR-γδ, NK T and NK cells were also determined in the male and female SNF1 mice. pDCs have been identified as key players in the pathology of SLE through the production of IFN-α, following the activation of Toll-like receptors 7 and 9 (TLR-7 and TLR-9)30–32. In addition, many studies have shown the oestrogen-mediated modulation of pDCs33,34. Only the frequencies of splenic pDCs, but not pDCs of PP, were significantly different in male and female SNF1 mice (Supporting information, Fig. S3). Interestingly, while the numbers of pDCs were found to be lower at 4 weeks of age in females, these cells were found in significantly higher numbers in females at 16 weeks of age compared to males. Of note, no profound difference was observed in the NK and NK T cell frequencies of 4- or 16-week-old male or female SNF1 mice. However, TCR-γδ Τ cells were relatively lower in PP of adult female SNF1 mice (not shown). Nevertheless, the functional significance of minor differences in the frequencies of these immune cell populations in male and female SNF1 mice is not known.
Gut mucosa of female SNF1 mice has high inflammatory factor levels
Differences in the frequencies of immune cells in the gut mucosa of male and female SNF1 mice prompted us to examine the expression profile of various pro- and anti-inflammatory factors. cDNA prepared from the distal ileum was subjected to a quantitative PCR analysis for an array of factors. As observed in Fig. 5, most proinflammatory factors, including IL-6, IL-9, IL-17, IL-22, IFN-α and IFN-β, are expressed at several-fold higher levels in the intestine of female SNF1 mice compared to their age-matched male counterparts. Albeit at relatively low expression levels in younger mice, both 4- and 16-week-old mice showed a similar trend in the expression of proinflammatory factors, suggesting that the lupus-associated proinflammatory response is initiated in the gut mucosa of female SNF1 mice, even before puberty and oestrogen production begins. Importantly, the expression level of IL-10 was also found to be higher in female SNF1 mice compared to males. While IL-10 has been shown to have a disease-promoting effect in lupus, it may also be expressed as part of an immune regulatory mechanism. Interestingly, the gut mucosa of female SNF1 mice also expresses higher levels of TLR-7 and TLR-8 mRNA, the two innate immune receptors that have been implicated in SLE. While the TLR-7 transcript levels were approximately twofold in females compared to males at prepuberty age, 16-week-old adult females expressed more than threefold TLR-7 mRNA compared to their male counterparts. Further, TLR-7 mRNA levels in the intestines of prepuberty and adult-aged females were three- and fivefold higher than in their respective-aged male counterparts. A similar trend in the expression levels of TLR-8 was observed in the gut mucosa of male and female SNF1 mice. These observations suggest that signalling through these X-linked TLRs may be responsible for higher proinflammatory cytokine expression, IFNs in particular, in the gut mucosa of female SNF1 mice.
Fig 5.
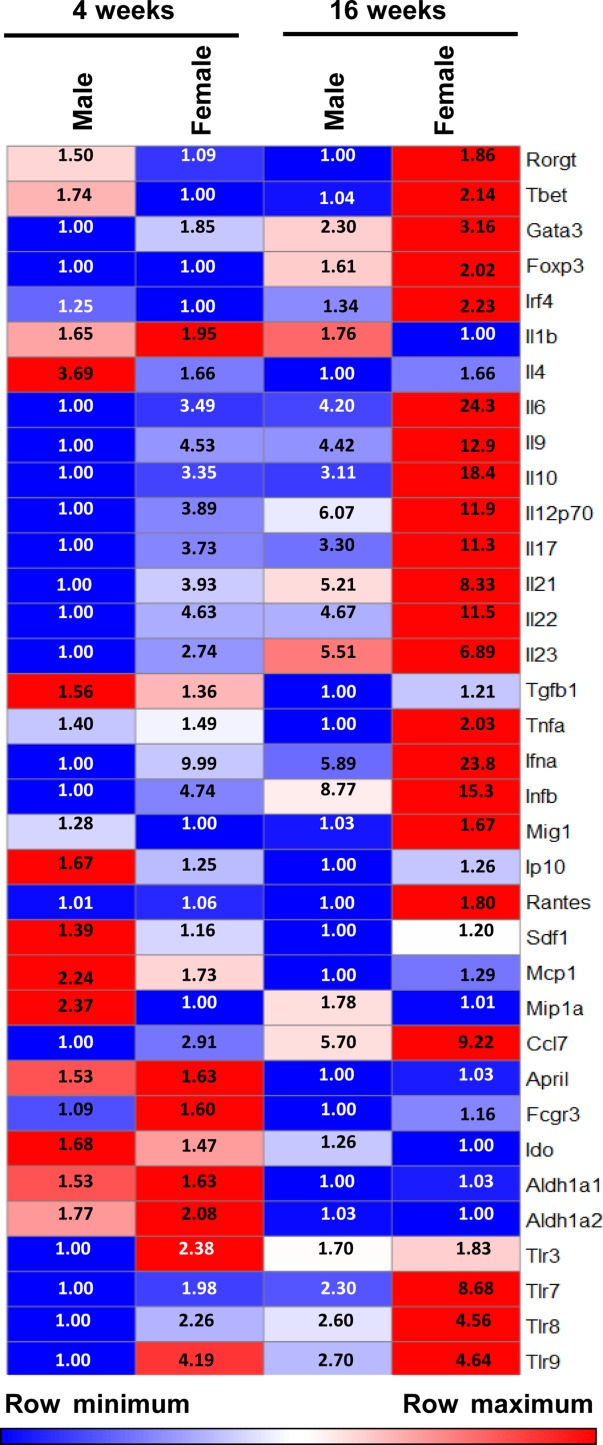
Female SWR × NZB F1 (SNF1) mouse intestine expresses a large number of proinflammatory factors. cDNA prepared from the distal ileum of 4- and 16-week-old male and female SNF1 mice was subjected to real-time quantitative polymerase chain reaction (PCR) to assess the expression levels of immune cell-associated factors including cytokines, transcription factors and chemokines. Expression levels of individual factors were calculated against the value of β-actin. Mean of these values (four to five mice/group) was used for generating the heat map. The lowest value of an individual factor among four groups of mice was considered as 1 (row minimum) for calculating fold expression values for each row.
Gender differences in proinflammatory factor expression is more prominent in the gut mucosa than in the systemic lymphoid organ
Considering the differences in the systemic B cell phenotype and gut immune responses of male and female SNF1 mice, we examined whether the T cell cytokine profiles are different in male and female SNF1 mice by fluorescence activated cell sorter (FACS). As observed in the Supporting information, Fig. S2, FACS analysis did not reveal a convincing trend in the expression of specific cytokine factors in different age groups of females compared to their male counterparts. Therefore, qPCR assay was performed to assess the overall expression pattern of various factors in spleens from prepuberty and adult-aged SNF1 mice. Supporting information, Fig. S4a, shows that while the majority of the proinflammatory factors expressed by younger age male and female SNF1 mice were comparable, adult SNF1 females expressed a considerable number of proinflammatory factors, Th17- and Th9-associated factors in particular, at relatively higher levels than those by their male counterparts. Nevertheless, the differences in the expression of these factors between adult male and female spleen cells were not as profound as that detected in the small intestine (Supporting information, Fig. S4b). Moreover, expression levels of X-linked TLRs, TLR-7 and TLR-8 in spleens were not considerably different between either males and females or different age groups of mice. Overall, these observations, in association with the results of Fig. 5, suggest that higher expressions of these proinflammatory factors are induced selectively in the gut mucosa of female mice.
Lamina propria (LP) of female SNF1 mice show large number of CD45+ immune cells
Because the expression levels of proinflammatory factors were profoundly different in male and female SNF1 mice, at adult ages in particular, distal ileum sections from 16-week-old mice were stained using anti-CD45 antibody to determine the immune cell density as a sign of their recruitment and/or proliferation. As observed in Fig. 6, CD45+ cell density was higher in the LP of female SNF1 mice compared to that of 16-week-old males. While the villi of distal ileum of female SNF1 mice appeared to be wider and tightly packed with CD45+ cells, male villi were found to be slender, with scattered CD45+ cells in a considerable proportion of the section. These observations indicate that higher levels of proinflammatory cytokines produced in the gut mucosa of female mice, even at prepuberty age, may be responsible for the recruitment and/or activation and proliferation of a large number of immune cells. This may cause a vicious cycle of inflammatory response in the female gut, which subsequently contributes to the systemic autoimmune process and gender bias observed in lupus.
Fig 6.
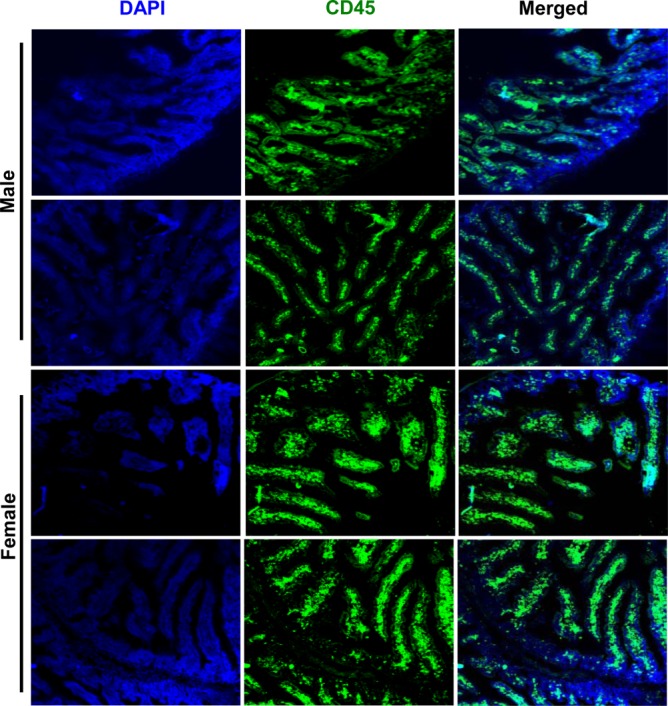
Lamina propria of female SWR × NZB F1 (SNF1) mice harbour a large number of immune cells. Six-μm cryosections of distal ileum from 16-week-old male and female SNF1 mice were stained using Alexa Fluor 488-linked anti-mouse CD45 and 4′,6-diamidino-2-phenylindole (DAPI) to visualize the immune cell density in lamina propria area. The images were acquired using the Olympus Fv10i inverted microscopes using a ×10 objective.
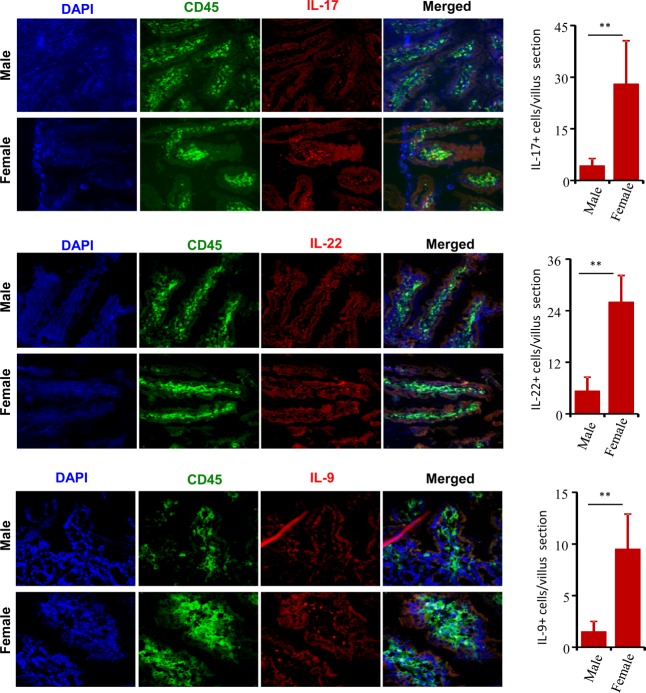
Immune cells in female SNF1 mice LP produce IL-17, IL-22 and IL-9
As shown in Fig. 5, the gut mucosa of female SNF1 mice, in particular 16-week-old adults, expresses a large array of proinflammatory cytokines. To confirm this observation, distal ileum sections were stained for IL-17, IL-9 and IL-22 expression. Figure 7 shows that the frequencies of IL-17-, IL-22- and IL-9-expressing CD45+ cells were profoundly higher in the LP of 16-week-old females compared to that of their male counterparts. While large numbers of IL-22- and IL-17-expressing cells were found within the LP of female SNF1 ileum, only a small number of scattered cells were found to express these cytokines in male SNF1 mice. These results reiterate the observations of Figs 5 and 6 and demonstrate clearly the strong proinflammatory immune response in the gut mucosa of female SNF1 mice. These observations also show that the proinflammatory response initiated in the gut mucosa at prepuberty may be responsible for the initiation and/or perpetuation of autoimmunity in lupus and determining its associated gender bias.
Fig 7.
Lamina propria of female SWR × NZB F1 (SNF1) mice harbour a large number of interleukin (IL)-17-, IL-22- and IL-9 producing cells. Six-μm cryosections of distal ileum from 16-week-old male and female SNF1 mice (three/group) were stained using Alexa Fluor 488-linked anti-mouse CD45, phycoerythrin (PE)-linked IL-17, IL-22 or IL-9 and 4',6-diamidino-2-phenylindole (DAPI). The images (left panels) were acquired using either the BD CARVII (for IL-17 and IL-22; using ×25 objective) or Olympus Fv10i (for IL-9: using ×60 objective) inverted microscopes. Cytokine-positive cells were counted in villi of comparable sizes and average numbers of cells (18–25 villi sections/group) are also shown (right panels).
Discussion
In this study, we demonstrate that the immune phenotype of gut mucosa is significantly different in lupus-prone male and female SNF1 mice and begins even before puberty. Our observations show that immune response initiated in the gut mucosa may be involved in the initiation and perpetuation of autoimmunity in lupus and determining the gender bias, which is prevalent in human SLE. The female SNF1 mouse intestine not only expresses a large array of proinflammatory factors, but also has a profoundly large number of immune cells compared to that of males. High expression of many proinflammatory cytokines in the female gut mucosa at as young as weaning (prepuberty) age indicates that they may have a role in gender bias, independent of sex hormones. This study represents the first report implying the role of gut immune system in the initiation and progression of disease process in SLE and the gender bias observed in this autoimmune disorder.
Gender bias is prevalent in many major autoimmune diseases, including SLE and rheumatoid arthritis (RA)35–37. The effect of sex hormones on autoimmunity, particularly in SLE, has been studied and oestrogen is linked to both the cause and pathology of the disease18,20,38. Conversely, testosterone has been found to be protective. For instance, not only does castration of males enhance the disease progression in animal models of SLE, but also treatment with testosterone reduced the disease incidence and severity19,20. Recent studies using NOD mice have linked the gut commensals and their metabolites to gender bias in autoimmune diseases9,10. Although sexual dimorphism has not been studied, similar to NOD mice, GF mouse models of SLE showed disease incidence in the absence of gut microbiota12,13. While the T1D incidence in female GF NOD mice was not profoundly different from that of their SPF counterparts9,10, higher levels of anti-nAg antibodies were detected in GF mice than in their SPF counterparts13, indicating that the contribution of gut microbiota in lupus or its gender bias may not be as prominent as in T1D. Nevertheless, gut mucosa, which is constantly exposed to microbes and dietary antigens and known to play a critical role in fighting pathogens and maintaining peripheral tolerance39,40, may influence the disease outcome in genetically predisposed subjects and preclinical models. It has been suggested that some of the gut bacteria and their DNA may serve as polyclonal B cell activators or as antigens cross-reacting with host DNA41,42. Our observation that the PP of female SNF1 mice carry higher numbers of IgG+ B cells compared to their male counterparts as early as 4 weeks of age indicates that the B cell response initiated in the gut mucosa, independently of sex hormones, may contribute to systemic autoimmunity in SLE and the gender bias in lupus incidence.
T cells that are activated in the gut mucosa, especially by CD103+ DCs, express specific integrins and chemokine receptors such as α4β7 and CCR9, and this imprinting enables these effector T cells to recirculate to the gut21,22. Involvement of α4β7 effector T cells in autoimmunity, inflammation and anti-viral immunity has been recognized and this integrin has been considered as one of the therapeutic targets for treating these inflammatory diseases43–45. Our observations that the frequency of α4β7+ CD4 T cells in the spleen and PP of female SNF1 mice is significantly higher than that of males at adult age suggest their potential contribution to gender bias in lupus. Intriguingly, α4β7/CCR9-positive T cell frequencies were significantly lower in female SNF1 mice at prepuberty age. However, whether oestrogen has a role in inducing α4β7 and/or CCR9 expression in T cells is not known.
While higher numbers of IgG+ B cells and mucosa-imprinted T cells in the systemic and intestinal lymphoid tissues of female SNF1 mice suggest that they may contribute to gender bias in lupus, the factors that are responsible for inducing these cells are not known. Expression profiling of male and female intestine demonstrates clearly that the intestine of female SNF1 mice produced higher levels of an array of proinflammatory cytokines and IL-10 compared to male SNF1 mice. Many of these factors, including IFN-α, IFN-β, IL-17, TLR-7 and TLR-8, have been implicated in the inflammation and pathogenesis associated with SLE46–51. The most intriguing of our novel observations is that the intestine of female SNF1 mice as young as 4 weeks of age expressed higher levels of these factors compared to males. This excludes the potential involvement of oestrogen as the cause for the inflammatory response in gut mucosa of these mice. Conversely, our observation also suggests a role for TLR-7 and TLR-8 in promoting higher expression of proinflammatory factors in the intestine of female SNF1 mice. In this regard, previous studies have shown that TLR-7 and TLR-8 stimulation promotes significantly higher IFN-α production in females than in males50–53. Interestingly, Tlr7 and Tlr8 genes are located on the X-chromosome and females potentially have two copies of these genes51. However, whether high levels of messenger RNA for TLR-7 and TLR-8 detected in the gut mucosa are due to this difference in the dosage of X-linked Tlr7 and Tlr8 in females is not clear. While this remains a possibility and requires further investigation, higher levels of these receptors in older mice compared to their prepubescent counterparts and their similar expression levels in the spleen suggest that the difference in TLR-7 and TLR-8 levels in the intestine of male and female mice may not be due to the dosage of these X-linked genes alone. A recent study using mouse models, although chromosome inactivation was not tested, has shown that dosage of X-linked Tlr8 plays a major role in the higher incidence of SLE in females51, another study using samples from lupus patients has demonstrated the lack of X-chromosome inactivation escape of the Tlr7 gene in females50. Our observation that a profound difference in TLR expression was observed in the gut mucosa, but not in the spleens of male and female SNF1 mice, suggests that the expression of these receptors and the associated cytokines are induced selectively in the gut mucosa of female SNF1 mice by unknown factors.
That the expression of proinflammatory cytokines is much higher in adult females compared to the prepubescent females suggests a progressive increase in the recruitment and/or activation of immune cells in the gut mucosa. It is possible that proinflammatory factors induced in the gut mucosa of females at early ages cause a vicious cycle of proinflammatory responses in the gut and contributes subsequently to rapid systemic autoimmune progression. It is also possible that the immune response initiated in the gut mucosa increases expression levels of TLR-7 and TLR-8, causing an amplified proinflammatory cytokine and chemokine response in older females. It is important to note that adult males and females express TLRs as well as proinflammatory cytokines at levels that are several-fold higher than the younger mice of their gender. This further implies that the immune response initiated in the gut mucosa may play a role not only in gender bias of SLE, but also in initiating and/or perpetuating the disease in both males and females. Although a similar trend in proinflammatory cytokines and chemokines was detected in the systemic lymphoid organ of older mice, the observation that the differences in the expression levels of these factors by female and male spleens were not as profound as that detected in the gut mucosa supports this notion. In addition, our results showing that spleen cells, unlike small intestine, from prepubescent males and females showed a comparable expression of various proinflammatory factors suggest that the proinflammatory immune response that contributes to lupus disease progression is initiated in the gut mucosa at young ages and spread out gradually to systemic lymphoid organs. Most importantly, as suggested by the cytokine profile of 4-week-old mice, the higher proinflammatory response in the gut mucosa of female SNF1 mice appears to be independent of sex hormone oestrogen.
Recent reports have suggested a dominant role for the Th17 pathway in the pathogenesis of rheumatoid diseases, including SLE48,54,55. While patients with SLE showed higher numbers of IL-17- and IL-22-producing T cells in the peripheral blood, studies using preclinical models demonstrated the pathogenic role of IL-17 in lupus48,55,56. IL-9 is known to promote high IgE production, lung eosinophilia and cause airway hyperresponsiveness57–60. In addition, cells that produce this cytokine have been implicated in skin inflammation61. Importantly, a recent study has shown a higher frequency of IL-9-producing T cells in the peripheral blood of SLE patients compared to controls62. Interestingly, while male and female SNF1 mice did not show a significant difference in T cells that express these cytokines in the spleen (not shown), we found that the frequencies of cells that expressed these cytokines were profoundly higher in the LP of female SNF1 mice. Further, the presence of higher number of CD45+ immune cells in the LP of female SNF1 mice indicates that these and other proinflammatory factors cause recruitment and/or activation of immune cells in the gut mucosa. The fact that CCL7 is expressed at higher levels in the intestine of female SNF1 mice indicates that this chemokine may have a role in recruiting monocytes to the inflammation site63.
Overall, our observations indicate that the proinflammatory immune response initiated in the gut mucosa may contribute to disease initiation and/or progression and the sexual dimorphism in SLE. However, what causes a higher proinflammatory response in the intestine of female SNF1 mice compared to males, even at prepubescent stage, is not known. A comprehensive set of future studies is needed to address many of the confounding questions associated with our novel observations. First, it appears that the degrees of involvement of genetic factors that are linked to lupus are different in male and female SNF1 mice. As mentioned above, it is not clear whether the X-linked Tlr7 gene contributes to the higher inflammatory response in females. Importantly, the percentage of plasmacytoid DCs that release type 1 IFNs upon TLR-7 engagement64,65 is comparable in the intestine of male and female SNF1 mice. However, whether these DCs from the female gut that express higher levels of TLR-7 and TLR-8, and/or this receptor on other activated antigen presenting cells (APCs) contribute to higher proinflammatory responses in female SNF1 mice, needs to be studied. Most importantly, whether Tlr7 and Tlr8 genes undergo X-inactivation escape, in gut mucosa in general and immune cell subpopulations in particular, causing higher expression levels of these receptors leading to an elevated proinflammatory cytokine response, needs to be addressed. Additional studies are also needed to understand how TLR-7/TLR-8 interaction with their putative ligands (ssRNA) in the gut mucosa of female SNF1 mice contributes to the accumulation of IL-17-, IL-22- and IL-9-expressing cells.
Another key question in this regard concerns the role of gut microbiota in a higher proinflammatory response in the gut mucosa of adult as well as prepubescent female SNF1 mice. Gut microbiota of prepubescent male and female mice is considered to be similar and mainly of maternal origin66,67. Studies using the T1D mouse model have shown that males and females show similar gut microbial composition before puberty and a testosterone-dependent selection of gut microbial communities occurs in males at adult ages9,10. Therefore, it remains to be determined whether gut microbiota have an influence on the gender-dependent differences in proinflammatory immune responses of SNF1 mice. Nevertheless, the possibility of the same gut microbial factors to interact with differentially expressed innate immune receptors such as TLR-7 and TLR-8, and produce different degrees of proinflammatory responses and cause the gender bias in SLE, cannot be ignored. Two recent reports68,69 and our unpublished study (Gaudreau et al.) showed a potential role of gut microbial communities in modulating the disease outcome in SLE. In spite of two recent reports on the role of gut microbiome in gender bias in a mouse model of T1D9,10, many aspects of the difference in gut microbial communities in males and females and the role of hormones in shaping the microbiome and immune responses are still unknown. Therefore, it is also possible that maternal microbiota is differentially acquired quantitatively and/or qualitatively by young males and females and these gut commensals cause the expression of TLR and proinflammatory cytokines at different levels that can contribute to the gender bias in lupus. Hence, the involvement of innate immune receptor–gut microbiota interaction in the initiation and progression of autoimmunity in SLE and associated sexual dimorphism needs to be investigated.
Acknowledgments
This work was supported by internal funds from MUSC, National Institutes of Health (NIH) grants R01AI073858 and Lupus Research Institute (LRI) award. M. C. G. performed the experiments and wrote the paper, B. M. J. performed the experiments and reviewed the paper, R. G. performed experiments and reviewed the paper, M. M. A. performed the experiments, and C. V. designed the study and wrote the paper. C. V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity and accuracy of the data and analysis.
Disclosure
Authors do not have any conflicts of interest to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Female SNF1 mice show higher number of plasma cells. Spleen cells of 4 and 16 weeks old male and mice were stained for surface CD19, CD138 and IgM, and analyzed by FACS. A) The frequencies of CD138 single positive and CD138 and IgM double positive plasma cells are shown. Representative FACS plots (left panels) and mean ±SEM of percentage values (right panels) are shown.
Fig. S2. Intracellular cytokine profiles of splenic and PP T cells. Spleen (A) and PP (B) of 4 and 16 week-old male and female SNF1 mice were stained for indicated intracellular cytokines as described in materials and methods and analyzed by FACS. Representative FACS plots for male and female (upper panels) and mean SEM of percentage values of cytokine positive cells (lower panels) are shown.
Fig. S3. Plasmacytoid dendritic cell (pDC) frequencies differ between male and female SNF1 mice. Spleen and PP of 4 and 16 weeks old SNF1 mice were stained using anti- mouse CD11c and PDCA-1 antibodies fo FACS analysis. Representative FACS plots for male and female and mean SEM values are shown.
Fig. S4. A) Spleen cells from adult female SNF1 mouse express large number of pro-inflammatory factors. cDNA prepared from freshly isolated spleen cells and subjected to real-time quantitative PCR as described for Fig. 5. Expression levels of individual factors were calculated against the value of b- actin. Mean values of cells from 3–4 mice tested individually were used for generating the heat map. The lowest value of an individual factor among 4 groups of mice was considered as 1 (row minimum) for calculating fold expression values for each row. Factors that were undetectable or produced extreme low values were excluded from this analysis. B) Comparison of expression profiles of various factors in small intestine and spleen. Relative expression ratios were calculated by dividing values of intestinal samples (presented in Fig. 5) with the respective values of spleen samples (Supporting Informtion, Fig. 4A) and shown. These intestine: spleen ratios indicate that majority of factors, Th17 and Th9 associated in particular, were expressed in the small intestine at profoundly higher levels than in the spleen of females even at prepubescent age.
References
- Li L, Mohan C. Genetic basis of murine lupus nephritis. Semin Nephrol. 2007;27:12–21. doi: 10.1016/j.semnephrol.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2010;10:3–7. doi: 10.1016/j.autrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clin Immunol. 2013;149:211–8. doi: 10.1016/j.clim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Draborg AH, Duus K, Houen G. Epstein–Barr virus and systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:370516. doi: 10.1155/2012/370516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore PD, Blankenhorn EP, Zachary JF, Teuscher C. Adult gonadal hormones selectively regulate sexually dimorphic quantitative traits observed in experimental allergic encephalomyelitis. Am J Pathol. 2004;164:167–75. doi: 10.1016/S0002-9440(10)63107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- Panchanathan R, Choubey D. Murine BAFF expression is up-regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity. Mol Immunol. 2013;53:15–23. doi: 10.1016/j.molimm.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med. 2013;207:1485–500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–12. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado MA, Kakkanaiah V, MacDonald GC, et al. The role of environmental antigens in the spontaneous development of autoimmunity in MRL-lpr mice. J Immunol. 1999;162:6322–30. [PubMed] [Google Scholar]
- Unni KK, Holley KE, McDuffie FC, Titus JL. Comparative study of NZB mice under germfree and conventional conditions. J Rheumatol. 1975;2:36–44. [PubMed] [Google Scholar]
- Sofi MH, Gudi R, Karumuthil-Melethil S, Perez N, Johnson BM, Vasu C. pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes. 2014;63:632–44. doi: 10.2337/db13-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalchin J, Nicklas JA, Eastcott JW, et al. Lupus prone (SWR × NZB)F1 mice produce potentially nephritogenic autoantibodies inherited from the normal SWR parent. J Immunol. 1985;134:885–94. [PubMed] [Google Scholar]
- Kalled SL, Cutler AH, Datta SK, Thomas DW. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritis: preservation of kidney function. J Immunol. 1998;160:2158–65. [PubMed] [Google Scholar]
- Stoll ML, Gavalchin J. Systemic lupus erythematosus-messages from experimental models. Rheumatology. 2000;39:18–27. doi: 10.1093/rheumatology/39.1.18. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM. Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive B cells. Curr Opin Rheumatol. 2006;18:456–61. doi: 10.1097/01.bor.0000240354.37927.dd. [DOI] [PubMed] [Google Scholar]
- Roubinian JR, Papoian R, Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977;59:1066–70. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melez KA, Reeves JP, Steinberg AD. Modification of murine lupus by sex hormones. Ann Immunol (Paris) 1978;129C:707–14. [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ohoka Y, Yokota A, Takeuchi H, Maeda N, Iwata M. Retinoic acid-induced CCR9 expression requires transient TCR stimulation and cooperativity between NFATc2 and the retinoic acid receptor/retinoid X receptor complex. J Immunol. 2011;186:733–44. doi: 10.4049/jimmunol.1000913. [DOI] [PubMed] [Google Scholar]
- Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CC, Lin BF. Dietary factors regulate cytokines in murine models of systemic lupus erythematosus. Autoimmun Rev. 2011;11:22–7. doi: 10.1016/j.autrev.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Muthukumar AR, Jolly CA, Zaman K, Fernandes G. Calorie restriction decreases proinflammatory cytokines and polymeric Ig receptor expression in the submandibular glands of autoimmune prone (NZB × NZW)F1 mice. J Clin Immunol. 2000;20:354–61. doi: 10.1023/a:1006620130114. [DOI] [PubMed] [Google Scholar]
- Pelajo CF, Lopez-Benitez JM, Miller LC. Vitamin D and autoimmune rheumatologic disorders. Autoimmun Rev. 2010;9:507–10. doi: 10.1016/j.autrev.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Terrier B, Derian N, Schoindre Y, et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Ther. 2012;14:R221. doi: 10.1186/ar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Meeker T, Gregorio J, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–9. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- Vollmer J, Tluk S, Schmitz C, et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–85. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillet C, Laffont S, Tremollieres F, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–64. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- Seillet C, Rouquie N, Foulon E, et al. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor alpha. J Immunol. 2013;190:5459–70. doi: 10.4049/jimmunol.1203312. [DOI] [PubMed] [Google Scholar]
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–44. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockshin MD Mary Kirkland Center for Lupus Research Consortium. Biology of the sex and age distribution of systemic lupus erythematosus. Arthritis Rheum. 2007;57:608–11. doi: 10.1002/art.22676. [DOI] [PubMed] [Google Scholar]
- Barragan-Martinez C, Amaya-Amaya J, Pineda-Tamayo R, et al. Gender differences in Latin-American patients with rheumatoid arthritis. Gend Med. 2012;9:490–510. doi: 10.1016/j.genm.2012.10.005. e5. [DOI] [PubMed] [Google Scholar]
- Young NA, Wu LC, Burd CJ, et al. Estrogen modulation of endosome-associated toll-like receptor 8: an IFNalpha-independent mechanism of sex-bias in systemic lupus erythematosus. Clin Immunol. 2014;151:66–77. doi: 10.1016/j.clim.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnet-Hughes A, Perez PF, Dore J, et al. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc Nutr Soc. 2010;69:407–15. doi: 10.1017/S0029665110001898. [DOI] [PubMed] [Google Scholar]
- Chen VL, Kasper DL. Interactions between the intestinal microbiota and innate lymphoid cells. Gut Microbes. 2014;5:129–40. doi: 10.4161/gmic.27289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperloo-Renkema HZ, Bootsma H, Mulder BI, Kallenberg CG, van der Waaij D. Host–microflora interaction in systemic lupus erythematosus (SLE): colonization resistance of the indigenous bacteria of the intestinal tract. Epidemiol Infect. 1994;112:367–73. doi: 10.1017/s0950268800057770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–43. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- Vaarala O. The role of the gut in beta-cell autoimmunity and type 1 diabetes: a hypothesis. Pediatr Diabetes. 2000;1:217–25. doi: 10.1046/j.1399543x.2000.010408.x. [DOI] [PubMed] [Google Scholar]
- Graham KL, Fleming FE, Halasz P, et al. Rotaviruses interact with alpha4beta7 and alpha4beta1 integrins by binding the same integrin domains as natural ligands. J Gen Virol. 2005;86:3397–408. doi: 10.1099/vir.0.81102-0. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- Elkon KB, Wiedeman A. Type I IFN system in the development and manifestations of SLE. Curr Opin Rheumatol. 2012;24:499–505. doi: 10.1097/BOR.0b013e3283562c3e. [DOI] [PubMed] [Google Scholar]
- Pisitkun P, Ha HL, Wang H, et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37:1104–15. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol. 2014;193:540–3. doi: 10.4049/jimmunol.1400931. [DOI] [PubMed] [Google Scholar]
- Jackson SW, Scharping NE, Kolhatkar NS, et al. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol. 2014;192:4525–32. doi: 10.4049/jimmunol.1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–96. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- Umiker BR, Andersson S, Fernandez L, et al. Dosage of X-linked Toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur J Immunol. 2014;44:1503–16. doi: 10.1002/eji.201344283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson KM, Christensen SR, Shupe J, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–8. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PY, Kumagai Y, Li Y, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zhu X, Zhao P, et al. Profile of Th17 cytokines (IL-17, TGF-beta, IL-6) and Th1 cytokine (IFN-gamma) in patients with immune thrombocytopenic purpura. Ann Hematol. 2008;87:899–904. doi: 10.1007/s00277-008-0535-3. [DOI] [PubMed] [Google Scholar]
- Chen DY, Chen YM, Wen MC, Hsieh TY, Hung WT, Lan JL. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis. Lupus. 2012;21:1385–96. doi: 10.1177/0961203312457718. [DOI] [PubMed] [Google Scholar]
- Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R97. doi: 10.1186/ar4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brule S, Heymans J, Havaux X, et al. Profibrotic effect of IL-9 overexpression in a model of airway remodeling. Am J Respir Cell Mol Biol. 2007;37:202–9. doi: 10.1165/rcmb.2006-0397OC. [DOI] [PubMed] [Google Scholar]
- Vink A, Warnier G, Brombacher F, Renauld JC. Interleukin 9-induced in vivo expansion of the B-1 lymphocyte population. J Exp Med. 1999;189:1413–23. doi: 10.1084/jem.189.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannin P, Delneste Y, Lecoanet-Henchoz S, Gretener D, Bonnefoy JY. Interleukin-7 (IL-7) enhances class switching to IgE and IgG4 in the presence of T cells via IL-9 and sCD23. Blood. 1998;91:1355–61. [PubMed] [Google Scholar]
- Petit-Frere C, Dugas B, Braquet P, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–51. [PMC free article] [PubMed] [Google Scholar]
- Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, Yu J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol. 2014;175:25–31. doi: 10.1111/cei.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H, Shi Y, Liu Z, et al. Increased interleukin9 and CD4+IL-9+ T cells in patients with systemic lupus erythematosus. Mol Med Rep. 2013;7:1031–7. doi: 10.3892/mmr.2013.1258. [DOI] [PubMed] [Google Scholar]
- Tsou CL, Peters W, Si Y, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–9. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–5. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls E, Shpiro N, Peggie M, et al. Essential role for IKKbeta in production of type 1 interferons by plasmacytoid dendritic cells. J Biol Chem. 2012;287:19216–28. doi: 10.1074/jbc.M112.345405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Lipuma L, Gobourne A, et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209:1445–56. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatric Res. 2014;76:2–10. doi: 10.1038/pr.2014.49. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liao X, Sparks JB, Luo XM. Dynamics of gut microbiota in autoimmune lupus. Appl Environ Microbiol. 2014;80:7551–60. doi: 10.1128/AEM.02676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevia A, Milani C, Lopez P, et al. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio. 2014;5:e01548–14. doi: 10.1128/mBio.01548-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Female SNF1 mice show higher number of plasma cells. Spleen cells of 4 and 16 weeks old male and mice were stained for surface CD19, CD138 and IgM, and analyzed by FACS. A) The frequencies of CD138 single positive and CD138 and IgM double positive plasma cells are shown. Representative FACS plots (left panels) and mean ±SEM of percentage values (right panels) are shown.
Fig. S2. Intracellular cytokine profiles of splenic and PP T cells. Spleen (A) and PP (B) of 4 and 16 week-old male and female SNF1 mice were stained for indicated intracellular cytokines as described in materials and methods and analyzed by FACS. Representative FACS plots for male and female (upper panels) and mean SEM of percentage values of cytokine positive cells (lower panels) are shown.
Fig. S3. Plasmacytoid dendritic cell (pDC) frequencies differ between male and female SNF1 mice. Spleen and PP of 4 and 16 weeks old SNF1 mice were stained using anti- mouse CD11c and PDCA-1 antibodies fo FACS analysis. Representative FACS plots for male and female and mean SEM values are shown.
Fig. S4. A) Spleen cells from adult female SNF1 mouse express large number of pro-inflammatory factors. cDNA prepared from freshly isolated spleen cells and subjected to real-time quantitative PCR as described for Fig. 5. Expression levels of individual factors were calculated against the value of b- actin. Mean values of cells from 3–4 mice tested individually were used for generating the heat map. The lowest value of an individual factor among 4 groups of mice was considered as 1 (row minimum) for calculating fold expression values for each row. Factors that were undetectable or produced extreme low values were excluded from this analysis. B) Comparison of expression profiles of various factors in small intestine and spleen. Relative expression ratios were calculated by dividing values of intestinal samples (presented in Fig. 5) with the respective values of spleen samples (Supporting Informtion, Fig. 4A) and shown. These intestine: spleen ratios indicate that majority of factors, Th17 and Th9 associated in particular, were expressed in the small intestine at profoundly higher levels than in the spleen of females even at prepubescent age.