Abstract
Programmed cell death-1 (PD-1) is a co-stimulatory molecule that inhibits T cell proliferation. We aimed to clarify PD-1 expression in CD4+ T cells and the association between PD-1 expression and the 7785C/T polymorphism of PDCD1, with a focus on the two subtypes of type 1 diabetes, type 1A diabetes (T1AD) and fulminant type 1 diabetes (FT1D), in the Japanese population. We examined 22 patients with T1AD, 15 with FT1D, 19 with type 2 diabetes (T2D) and 29 healthy control (HC) subjects. Fluorescence-activated cell sorting (FACS) and real-time PCR were utilized to analyse PD-1 expression quantitatively. Genotyping of 7785C/T in PDCD1 was performed using the TaqMan method in a total of 63 subjects (21 with T1AD, 15 with FT1D and 27 HC). FACS revealed a significant reduction in PD-1 expression in CD4+ T cells in patients with T1AD (mean: 4·2 vs. 6·0% in FT1D, P = 0·0450; vs. 5·8% in T2D, P = 0·0098; vs. 6·0% in HC, P = 0·0018). PD-1 mRNA expression in CD4+ T cells was also significantly lower in patients with T1AD than in the HC subjects. Of the 63 subjects, PD-1 expression was significantly lower in individuals with the 7785C/C genotype than in those with the C/T and T/T genotypes (mean: 4·1 vs. 5·9%, P = 0·0016). Our results indicate that lower PD-1 expression in CD4+ T-cells might contribute to the development of T1AD through T cell activation.
Keywords: fulminant type 1 diabetes, PD-1, type 1A diabetes
Introduction
Type 1 diabetes results from absolute insulin deficiency. According to the classifications of the American Diabetes Association, the World Health Organization and the Japanese Diabetes Society, type 1 diabetes is divided into two subcategories: autoimmune type 1 (type 1A) diabetes and idiopathic type 1 (type 1B) diabetes 1–3. There are several lines of evidence indicating that T cell-dependent autoimmunity plays a critical role in destructing insulin-producing pancreatic beta cells in T1AD 4,5. For example, T lymphocyte infiltration has been observed in pancreatic tissue upon autopsy of patients with T1AD 6,7. Additionally, islet reactive T cells have been detected in the peripheral blood of patients with T1AD 8,9.
FT1D is a subtype of type 1 diabetes characterized by markedly rapid disease progression within an average of several days and almost complete insulin deficiency resulting from the destruction of pancreatic beta cells 10,11. This variant accounts for approximately 20% of acute onset type 1 diabetes cases in Japan 10. Macrophages and T cells are detected as the main components of the infiltrates in the islets as well as in exocrine pancreas, but islet-related autoantibodies are usually negative 12,13.
Programmed cell death-1 (PD-1) is a member of the B7-CD28 family and is one of the core co-stimulatory molecules, like cytotoxic T lymphocyte antigen-4 (CTLA-4), delivering critical inhibitory signals regulating the T cell response and maintaining peripheral tolerance. PD-1 expression can be induced not only in T cells but also in other immunocytes, whereas CTLA-4 expression is restricted only to T cells, suggesting that PD-1 plays a broader role in immune suppression 14,15. Moreover, it has been suggested that PD-1 inhibits T cell activation more potently than CTLA-4 in analyses of the expression levels of T cell transcription by CD3/CD28/PD-1- or CD3/CD28/CTLA-4-coated beads stimulation 16. Therefore, inhibiting the PD-1 pathway would result in excessive T cell proliferation, failure of tolerance and autoimmune activation.
The role of PD-1 in human autoimmune diseases, such as T1AD, has been studied using animal models. PD-1 deficiency was shown to accelerate the onset and frequency of T1AD in non-obese diabetic (NOD) mice 17. Destructive insulitis was observed in these mice, characterized by increased infiltration of CD4+ and CD8+ T lymphocytes into the islets compared with wild-type NOD mice. PD-1 deficiency in antigen-specific CD4+ T cells in NOD mice resulted in increased T-cell numbers in the spleen, pancreatic lymph nodes and pancreas, and induced T1AD 18. However, little has been reported on the role of the PD-1 pathway in patients with type 1 diabetes.
The contribution of human leucocyte antigen (HLA) genes, particularly class II DR and DQ genes, to susceptibility to T1AD is well known 19. Regarding non-HLA genes, variable-number tandem repeats in the insulin gene, CTLA-4 and PTPN22, are also well known. Recently, several reports have demonstrated that polymorphisms in the PD-1 gene, PDCD1, are associated with T1AD 20–27. We previously identified four sequence variants in PDCD1 (834D/I, 7625C/T, 7785C/T and 8185[TGC]n) and clarified the contribution of PDCD1 to genetic susceptibility to T1AD in the Japanese population 22. Of these variants, the 7785C/T (PD-1·5: rs2227981) polymorphism was shown repeatedly to exhibit a protective effect against the development of T1AD 21,23,24,26,27.
Based on these findings, the present study was performed to clarify PD-1 expression in CD4+ T cells in patients with T1AD, FT1D and T2D as well as healthy controls (HC), with special reference to 7785C/T polymorphism in PDCD1.
Methods
Study population and design
We studied 22 patients with T1AD, 15 with FT1D, 19 with T2D and 29 HC (Table1). Patients with GAD and/or IA-2 antibodies and insulin dependency were diagnosed with type 1A diabetes 1. Fulminant type 1 diabetes was diagnosed according to the established criteria 28. There was a significant difference in age between each of the four groups and in HbA1c between subjects with T1AD, FT1D and T2D. All patients with type 1A and fulminant type 1 diabetes were controlled appropriately with multiple daily insulin injections and did not exhibit acidosis at the beginning of the study. HbA1c values (%) were expressed as the National Glycohemoglobin Standardization Program (NGSP) value 29.
Table 1.
Clinical characteristics
| Type 1A diabetes (T1AD) | Fulminant type 1 diabetes (FT1D) | Type 2 diabetes (T2D) | Healthy control subjects (HC) | P-value | |
|---|---|---|---|---|---|
| n | 22 | 15 | 19 | 29 | |
| Gender (male/female) | 5/17 | 8/7 | 12/7 | 12/17 | n.s.* |
| Age (years) | 42 (18–77) | 55 (27–72) | 62 (44–71) | 43 (25–63) | 0·0003** |
| Disease duration (years) | 7·8 (1·5–33·0) | 5·7 (0·4–9·3) | 8·0 (0·1–32·0) | n.d. | n.s.** |
| HbA1c (%) | 7·9 (5·8–9·7) | 7·4 (6·6–9·5) | 9·4 (6·4–13·9) | n.d. | 0·0459** |
| Serum C-peptide (ng/ml) | 0·01 (<0·01–2·18) | 0·04 (<0·01–0·06) | n.d. | n.d. | n.s.** |
| GAD/IA-2 antibody positive (%) | 100/18·2 (n = 6) | 6·67/0 | 0/n.d. | n.d./n.d. |
Median (range).
P-values were calculated by Fisher's exact probability test. Bonferroni's correction of multiple comparison was made to the level of significance (P < 0·05/6).
P-values were calculated by Kruskal–Wallis test. GAD = glutamic acid decarboxylase; HbA1c = glycated haemoglobin; IA-2 = insulinoma-associated antigen-2; n.d. = not determined; n.s. = not significant.
This study was approved by the ethics committee of Osaka Medical College. All patients and HC provided written informed consent.
Fluorescence activated cell sorter (FACS) analysis
Peripheral blood mononuclear cells (PBMCs) were obtained from fresh whole blood via density gradient centrifugation (Lymphoprep; Axis-Shield PoC AS, Oslo, Norway), then washed twice in phosphate-buffered saline (PBS) via density gradient centrifugation and subjected immediately to cellular staining without blood cell preservation.
A total of 1 × 106 PBMCs per tube were aliquoted and incubated with 20 μl of each test antibody or the respective isotype control. The cells were stained with the following antibodies: phycoerythrin (PE) anti-CD279 (PD-1) (clone MIH4) and peridinin chlorophyll protein (PerCP) anti-CD4 (clone SK3) (BD Bioscience, San Jose, CA, CA). The following isotype control antibodies were used: PE mouse immunoglobulin (Ig)G1, κ (clone MOPC-31) and PerCP mouse IgG1, κ (clone MOPC-31) (BD Biosciences). After surface staining for 30 min at 4 °C in the dark, the cells were washed twice with PBS. The stained cells were subsequently analysed by FACS (BD FACSAria™). Each sample had gates set separately according to isotype controls for PD-1 in our study. In all samples, gates were set so that negative controls stained uniformly <0·5% as false positive cells (Fig. 1c). A sample from a single healthy subject was used as a control every time to confirm the accuracy. At least 10,000 events were acquired for each sample. The frequency of CD4+PD-1+ T cells was calculated as a percentage of the total CD4+ T cell population.
Fig 1.
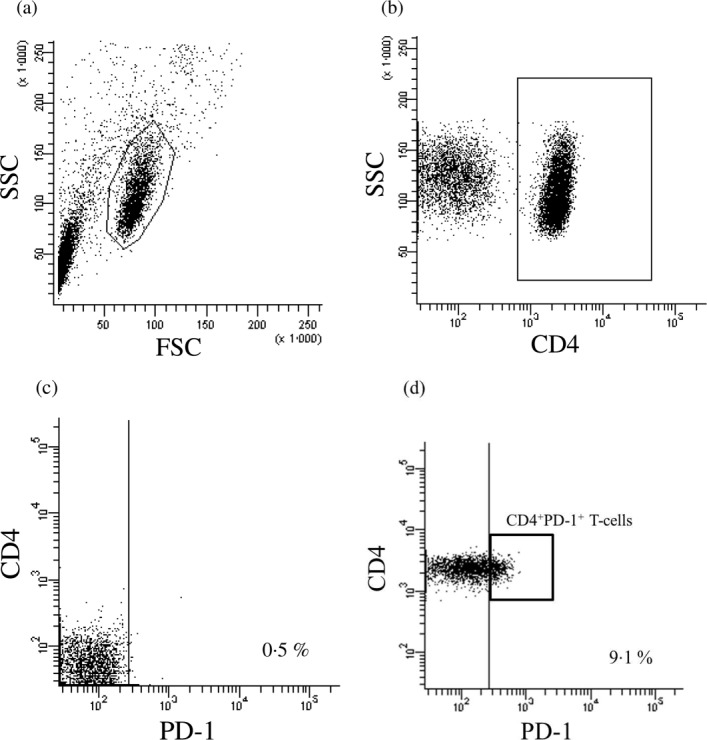
Fluorescence activated cell sorter (FACS) analysis of programmed cell death-1 (PD-1) in peripheral CD4+ T cells. Representative plots showing one healthy control sample gated on lymphocytes (a) showing CD4 (b) and staining for PD-1 (d) as well as the isotype control (c).
RNA isolation and quantitative real-time PCR
PBMCs were separated using lymphocyte separation medium (Lymphoprep) and washed twice in PBS via density gradient centrifugation. CD4+ T cells were isolated from PBMCs using anti-CD4 monoclonal antibody magnetic beads (BD IMag™ Human CD4 T Lymphocyte Enrichment Set DM; BD Biosciences). Using FACS, the purity of the magnetic beads-isolated CD4+ T cells was greater than 97·6%. Total RNA was extracted from CD4+ T cells using the RNeasy MiniKit (QIAGEN, Tokyo, Japan), and first-strand cDNA was synthesized using the SuperScript VILO cDNA Synthesis Kit (Life Technologies Corporation, Carlsbad, CA, USA). We quantified PD-1 mRNA expression via the TaqMan real-time PCR method (StepOnePlus™; Applied Biosystems, Foster City, CA, USA). Each specimen was analysed in duplicate. We used TaqMan® gene expression assays (Assay ID no. Hs01550088_m1 and Assay ID no. Hs99999901_s1; Applied Biosystems) to confirm the quantification of PDCD1 and 18S expression. The gene expression level was reported as the relative ratio of PDCD1 expression to that of the internal control (18S gene).
Genotyping for the 7785C/T polymorphism
We extracted DNA from PBMCs from the patients and the healthy controls using the Blood and Cell Culture DNA Midi Kit (QIAGEN). Genotyping for the 7785C/T polymorphism was performed in 63 subjects (21 patients with T1AD, 15 with FT1D and 27 HC) using the TaqMan method with the StepOnePlus Real-Time PCR System (Applied Biosystems) and TaqMan® SNP Genotyping Assays.
Statistical analysis
Fisher's exact probability test was performed for gender comparison. Bonferroni's correction of multiple comparison was made to the level of significance (P < 0·05/6). Kruskal–Wallis tests were performed for multiple comparisons in age, disease duration, HbA1c and serum C-peptide.
Differences in PD-1 protein and mRNA expression in peripheral CD4+ T cells were assessed using the two-tailed unpaired Student's t-test and the Mann–Whitney U-test, respectively. The significance of the differences in the distribution of alleles and genotypes between the patients with T1AD or FT1D and HC was determined using Fisher's exact probability test. P-values of less than 0·05 were considered significant.
Results
PD-1 expression in CD4+ T cells
Figure 1a–d shows a representative FACS result on the frequency of CD4+PD-1+ T cells in HC. Figure 2 shows that there was a significant reduction in the frequency of CD4+PD-1+ T cells in patients with T1AD (mean: 4·2%) compared with patients with FT1D (mean: 6·0%, P = 0·045), patients with T2D (mean 5·8%, P = 0·0098) and the HC (mean: 6·0%, P = 0·0018).
Fig 2.
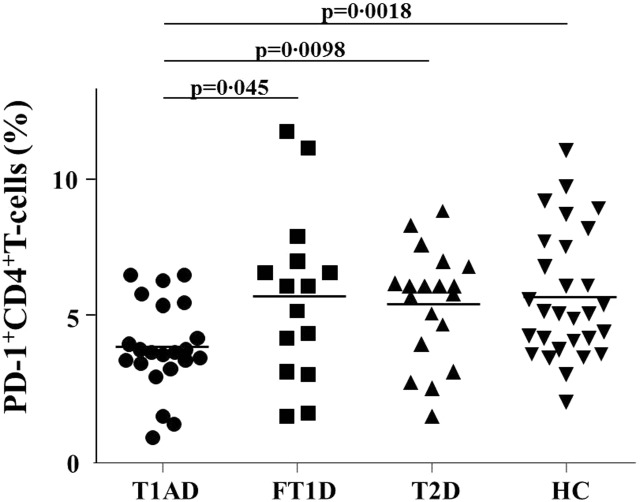
Programmed cell death-1 (PD-1) expression in peripheral CD4+ T cells as determined by fluorescence activated cell sorter (FACS). The data represent PD-1 expression in CD4+ T cells from patients with type 1A diabetes (T1AD, circles), fulminant type 1 diabetes (FT1D, squares) or type 2 diabetes (T2D, upward-facing triangles) or healthy control subjects (HC, downward-facing triangles). The bars represent the mean.
There were no significant correlations between the frequency of PD-1 expression and various clinical characteristics, including age, disease duration, HbA1c levels and serum C-peptide levels (data not shown). In patients with T1AD, there was also no significant correlation between the frequency of PD-1 expression and their duration of diabetes (data not shown).
PD-1 mRNA expression in CD4+ T cells
There was a significant reduction in PD-1 mRNA levels in patients with T1AD (median: 0·584) compared to HC (median: 1·877, P = 0·0023), but not to those with FT1D (median: 0·6096, n.s.) (Fig. 3).
Fig 3.
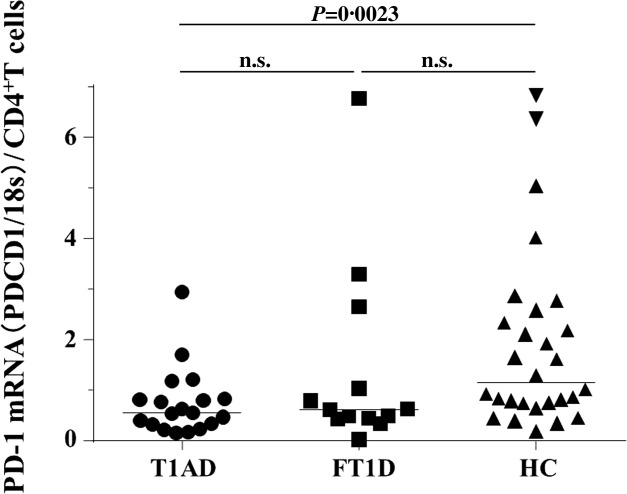
Programmed cell death-1 (PD-1) mRNA expression in peripheral CD4+ T cells. PD-1 mRNA expression was quantified in CD4+ T cells from patients with type 1A diabetes (T1AD, circles) or fulminant type 1 diabetes (FT1D, squares) or healthy control subjects (HC, downward-facing triangles). The bars represent the median.
Frequency of the 7785C/T genotype and allele
We then analysed the association between each genotype and PD-1 expression in CD4+ T cells. Of the 63 subjects whose 7785C/T genotype in PDCD1 was examined, the frequency of CD4+PD-1+ T cells was significantly lower in subjects with the C/C genotype than in those with the C/T and T/T genotypes (mean: 4·1 versus 5·9%, P = 0·0016) (Fig. 4).
Fig 4.
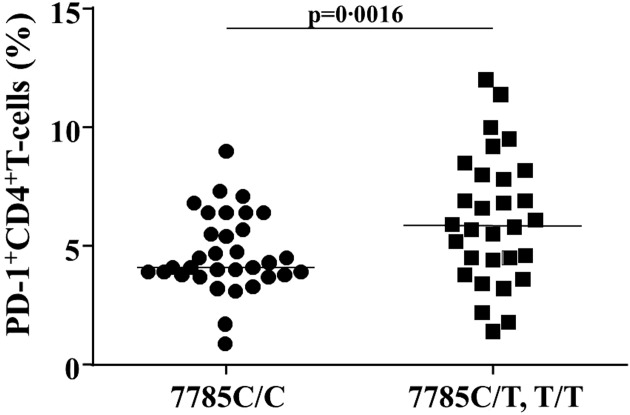
The association between the 7785 C/T polymorphism and programmed cell death-1 (PD-1) expression in CD4+T cells (%) among total subjects. PD-1 expression in CD4+ T cells was determined in subjects with the 7785 C/C genotype (circles) and C/T and T/T genotypes (squares). The bars represent the mean.
Of the 63 subjects, the 7785C/C, C/T and T/T genotypes were present in 33 subjects (52·4%), 21 subjects (33·0%) and nine subjects (14·3%), respectively. Of the 21 subjects with T1AD, these genotypes were present in 13 (61·9%), 6 (28·6%) and 2 subjects (9·5%), respectively. Of the 15 subjects with FT1D, they were present in 7 (46·7%), 6 (40·0%) and 2 subjects (13·3%), respectively. Of the 27 HC, they were present in 13 (48·2%), 9 (33·3%) and 5 subjects (18·5%), respectively. No significant differences in the allele and genotype frequencies of the 7785C/T polymorphism were observed among the patients with T1AD, FT1D and HC (Supporting information, Table S1).
Discussion
The present study revealed that the frequency of CD4+PD-1+ T cells was significantly lower in patients with T1AD compared to patients with FT1D, T2D and HC. The frequency of CD4+PD-1+ T cells was significantly lower in patients with the C/C genotype than in those with the C/T and T/T genotypes.
Low PD-1 expression in patients with T1AD might increase T cell proliferation and activation, leading to the destruction of beta cells, as shown in mouse models 17,18, providing a possible common mechanism underlying human autoimmune diseases. For example, PD-1 expression on CD4+ T cells in peripheral blood was significantly lower in patients with rheumatoid arthritis and systemic lupus erythematosus 30,31. In multiple sclerosis patients, increased expression of PD-1 was associated with disease remission 32. Various autoimmune diseases occurred in PD-1 knockout mice (PDCD1–/– mice) as a result of their genetic background. C57BL/PDCD1–/– mice, BALB/c PDCD1–/– mice and NOD-PDCD1–/– mice develop lupus-like glomerulonephritis and arthritis, dilated cardiomyopathy and T1AD, respectively33,34,17.
The 7785C/T polymorphism is located in exon 5, and the phenotype of this synonymous (Ala268Ala) polymorphism would be associated with alteration in the level of PDCD1 expression via linkage disequilibrium with other PDCD1 polymorphisms that may lead to an alteration in gene transcription 35,36. Although the 7785C/T polymorphism could play some role in the development of T1AD 21,23,24,26,27, it is unclear whether the allelic variation of 7785C/T contributes to the function of PD-1 in T1AD. In this study, when all 63 subjects were taken into account, the frequency of CD4+PD-1+ T cells was decreased significantly in subjects carrying the 7785C/C genotype, but not so when only patients with T1AD were analysed (data not shown). Further studies are warranted to clarify the association between decreased PD-1 expression in patients with T1AD and PDCD1. Gene–gene interactions, such as those between PDCD1 and class II HLA genes, or interactions between 7785C/T and the other PD-1 polymorphisms (834D/I, 7625C/T and 8185[TGC]n) should be evaluated by stratifying the patients included in our study.
PD-1 and CTLA-4, both of which act as inhibitory molecules in T cell proliferation, might be associated with the differences in the aetiology between T1AD and FT1D. In the present study, low expression of PD-1 was shown to be associated with T1AD. Previously, we reported that CTLA-4 expression was significantly lower in patients with FT1D, but not with T1AD 37. The role of PD-1 and CTLA-4 in the pathogenesis of type 1 diabetes needs further evaluation.
This study has a limitation. Disease duration was different among patients with T1AD in our study. We did not perform longitudinal analysis; however, there was no significant correlation between the duration of diabetes and the frequency of PD-1 expression in patients with T1AD.
In conclusion, PD-1 expression in peripheral CD4+ T cells was decreased in Japanese patients with T1AD compared with patients with FT1D, T2D and HC. Lower PD-1 expression in CD4+ T cells might contribute to the development of T1AD.
Acknowledgments
We gratefully acknowledge support from Mr. Teruo Ueno for his excellent technical assistance. This study was supported by a Grant-in-Aid from the Japanese Society for the Promotion of Science (KAKENHI 24591345).
Disclosure
The authors declare that there are no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1. The frequency of 7785C/T genotype and allele in patients with type 1 diabetes and healthy control subjects.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl. 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–28. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–8. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- Roep BO. The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia. 2003;46:305–21. doi: 10.1007/s00125-003-1089-5. [DOI] [PubMed] [Google Scholar]
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–33. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313:353–60. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Hawkes CJ, Schloot NC, Marks J, et al. T-cell lines reactive to an immunodominant epitope of the tyrosine phosphatase-like autoantigen IA-2 in type 1 diabetes. Diabetes. 2000;49:356–66. doi: 10.2337/diabetes.49.3.356. [DOI] [PubMed] [Google Scholar]
- Nagata M, Kotani R, Moriyama H, Yokono K, Roep BO, Peakman M. Detection of autoreactive T cells in type 1 diabetes using coded autoantigens and an immunoglobulin-free cytokine ELISPOT assay: report from the fourth immunology of diabetes society T cell workshop. Ann NY Acad Sci. 2004;1037:10–5. doi: 10.1196/annals.1337.002. [DOI] [PubMed] [Google Scholar]
- Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM study group. N Engl J Med. 2000;342:301–7. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26:2345–52. doi: 10.2337/diacare.26.8.2345. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kobayashi T, Momotsu T. A novel subtype of type 1 diabetes mellitus. N Engl J Med. 2000;342:1835–7. [PubMed] [Google Scholar]
- Shibasaki S, Imagawa A, Tauriainen S, et al. Expression of Toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J. 2010;57:211–9. doi: 10.1507/endocrj.k09e-291. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–82. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1–/– mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823–8. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes. 2013;62:2859–69. doi: 10.2337/db12-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–67. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Nielsen C, Hansen D, Husby S, Jacobsen BB, Lillevang ST. Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens. 2003;62:492–7. doi: 10.1046/j.1399-0039.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Ni R, Ihara K, Miyako K, et al. PD-1 gene haplotype is associated with the development of type 1 diabetes mellitus in Japanese children. Hum Genet. 2007;121:223–32. doi: 10.1007/s00439-006-0309-8. [DOI] [PubMed] [Google Scholar]
- Hiromine Y, Ikegami H, Fujisawa T, et al. Trinucleotide repeats of programmed cell death-1 gene are associated with susceptibility to type 1 diabetes mellitus. Metabolism. 2007;56:905–9. doi: 10.1016/j.metabol.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Momin S, Flores S, Angel B B, Codner D E, Carrasco P E, Perez-Bravo F. Interactions between programmed death 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) gene polymorphisms in type 1 diabetes. Diabetes Res Clin Pract. 2009;83:289–94. doi: 10.1016/j.diabres.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Asad S, Nikamo P, Tö rn C, et al. No evidence of association of the PDCD1 gene with Type 1 diabetes. Diabet Med. 2007;24:1473–7. doi: 10.1111/j.1464-5491.2007.02297.x. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Smyth DJ, Bailey R, et al. The candidate genes TAF5L, TCF7, PDCD1, IL6 and ICAM1 cannot be excluded from having effects in type 1 diabetes. BMC Med Genet. 2007;8:71. doi: 10.1186/1471-2350-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores S, Beems M, Oyarzún A, Carrasco E, Pérez F. Programmed cell death 1 (PDCD1) gene polymorphisms and type 1 diabetes in Chilean children. Rev Med Chil. 2010;138:543–50. [PubMed] [Google Scholar]
- Lee YH, Bae SC, Kim JH, Song GG. Meta-analysis of genetic polymorphisms in programmed cell death 1: Associations with rheumatoid arthritis, ankylosing spondylitis, and type 1 diabetes susceptibility. Z Rheumatol. 2014 doi: 10.1007/s00393-014-1415-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab. 2007;3:36–45. doi: 10.1038/ncpendmet0351. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to national glycohemoglobin standardization program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatachi S, Iwai Y, Kawano S, et al. CD4+ PD-1+ T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol. 2003;30:1410–9. [PubMed] [Google Scholar]
- Kristjansdottir H, Steinsson K, Gunnarsson I, Gröndal G, Erlendsson K, Alarcón-Riquelme ME. Lower expression levels of the programmed death 1 receptor on CD4+CD25+ T cells and correlation with the PD-1.3A genotype in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:1702–11. doi: 10.1002/art.27417. [DOI] [PubMed] [Google Scholar]
- Trabattoni D, Saresella M, Pacei M, et al. Costimulatory pathways in multiple sclerosis: distinctive expression of PD-1 and PD-L1 in patients with different patterns of disease. J Immunol. 2009;183:4984–93. doi: 10.4049/jimmunol.0901038. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- Prokunina L, Castillejo-López C, Oberg F, et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32:666–9. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- Lin SC, Yen JH, Tsai JJ, et al. Association of a programmed death 1 gene polymorphism with the development of rheumatoid arthritis, but not systemic lupus erythematosus. Arthritis Rheum. 2004;50:770–5. doi: 10.1002/art.20040. [DOI] [PubMed] [Google Scholar]
- Haseda F, Imagawa A, Murase-Mishiba Y, et al. Low CTLA-4 expression in CD4+ helper T-cells in patients with fulminant type 1 diabetes. Immunol Lett. 2011;139:80–6. doi: 10.1016/j.imlet.2011.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The frequency of 7785C/T genotype and allele in patients with type 1 diabetes and healthy control subjects.


