Abstract
In recent years, percutaneous radiofrequency ablation (RFA) has been developed as a new tool in the treatment of non-small-cell lung cancer (NSCLC) in non-surgical patients. There is growing evidence that RFA-mediated necrosis can modulate host immune responses. Here we analysed serum inflammatory factors as well as immunosuppressive cells in the peripheral blood to discover possible prognostic indicators. Peripheral blood and serum samples were collected before RFA and within 3 months after the treatment in a total of 12 patients. Inflammatory cytokines and growth factors were measured in serum by the Bio-Plex assay. Myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) were evaluated in the peripheral blood via flow cytometry. In patients developing local or lymphogenic tumour relapse (n = 4), we found an early significant increase in the concentration of tumour necrosis factor (TNF)-α as well as chemokine (C-C motif) ligand (CCL)-2 and CCL-4 compared to patients without relapse (n = 4) and healthy donors (n = 5). These changes were associated with an elevated activity of circulating MDSC indicated by an increased nitric oxide (NO) production in these cells. Elevated serum levels of TNF-α, CCL-2 and CCL-4 associated with an increased NO production in circulating MDSCs might be an early indicator of the incomplete RFA and subsequently a potential tumour relapse in NSCLC.
Keywords: chronic inflammatory factors, myeloid-derived suppressor cells, non-small-cell lung cancer, radiofrequency ablation
Introduction
In recent years, percutaneous radiofrequency ablation (RFA) has shown promising oncological results in the treatment of non-small-cell lung cancer in non-surgical patients 1,2. Tumour tissue is destroyed by a high-frequency alternating current with ionic agitation and frictional heating. Large amounts of tumour debris are released as a consequence of cell membrane alteration, protein denaturation and heat-induced necrosis 3,4. It has been reported that RFA-mediated necrosis can modulate the host immune responses and there is growing evidence that RFA may induce anti-tumour immune reactions 5. In a rabbit tumour model, RFA therapy stimulated the local inflammatory response with dense infiltration and activation of tumour-specific T cells 6. This finding was confirmed in a mouse model, together with the induction of dendritic cell (DC) maturation upon RFA treatment 7,8. Several studies of RFA effects on immune cells were also conducted in cancer patients. It was reported that RFA could induce acute inflammation in lung cancer patients 9,10. Moreover, upon RFA treatment of liver metastases, systemic inflammatory effects, such as fever and an increase in neutrophil and monocyte numbers, was found 11. In addition, these patients demonstrated an elevated tumour-specific cytolytic activity of CD8 T cells 12. In patients with hepatocellular carcinoma (HCC), RFA treatment induced an increase in the frequency of monocytes 11, and tumour-specific activated T cells and natural killer (NK) cells were observed 13,14. This effect depended in part on the DC maturation driven by cellular debris released after RFA 15. Conversely, the role of numerous released proinflammatory mediators such as interleukin (IL)-6, C-reactive protein (CRP), tumour necrosis factor (TNF)-α and secretory phospholipase A2 has been discussed controversially 16–20. In lung cancer patients, a moderate and self-limiting systemic inflammatory response indicated by the increase in amounts of neutrophils and monocytes, as well as plasma levels of multiple proinflammatory factors, was detected soon after RFA. In addition, the frequency of peripheral regulatory T cells (Tregs) was reduced, associated with an improvement in anti-tumour T cell reactivity 21.
The rate of local tumour recurrence following RFA of primary lung neoplasms was reported to range up to 40% 1,22,23. Recurrences resulted from the residual vital tumour tissue enclosing vessels or located in the marginal zone of the ablated tumours 4. The immunological profile of patients with incomplete ablation developing early tumour recurrence compared to completely ablated patients has not been studied so far.
The objective of this study was to investigate the impact of RFA on the profile of inflammatory factors in the serum and immunosuppressive cells in the peripheral blood of patients with complete or incomplete ablation. We found that patients developing local or lymphogenic tumour relapse displayed an early elevation of TNF-α, chemokine (C-C motif) ligand (CCL)-2 and CCL-4 concentrations in serum associated with an increased activity of myeloid-derived suppressor cells (MDSCs) in the peripheral blood [indicated by enhanced nitric oxide (NO) production] compared to patients without relapse and healthy donors. These data suggest that the above-mentioned factors may be applied as early biomarkers of potential tumour relapse in patients with non-operable non-small-cell lung cancer (NSCLC) upon RFA treatment.
Material and methods
Patients
Twelve patients (nine male, three female; median age 72 years) with histologically proven stage I NSCLC were enrolled into this study. In all patients, positron emission tomography–computed tomography (PET-CT) has shown a significant fludeoxyglucose (FDG) uptake in the pulmonary nodule, whereas mediastinal and hilar lymph nodes lacked detectable FDG uptake. No cerebral metastases were observed on contrast-enhanced cerebral magnetic resonance imaging (MRI) or CT. Inoperability was determined by a thoracic surgeon in all patients due to the limited pulmonary function or other co-morbidities (Table1). After discussion by the interdisciplinary tumour board, percutaneous RFA was performed. All patients underwent general anaesthesia and endobronchial double-lumen intubation. RFA probes were placed into the pulmonary tumours under CT guidance and bipolar RFA (Celon®LABPower; bipolar Celon®ProSurge RFA probe; Olympus Surgical Technologies Europe, Hamburg, Germany) was performed. All patients underwent clinical and radiological follow-up at 1, 2 and 3 months subsequent to the RFA treatment, and then every 3 months.
Table 1.
Clinical data of the patients included in this study
| Patient gender, age (years) | Co-morbidity | Tumour diameter (mm) | Histology | Follow-up |
|---|---|---|---|---|
| F, 77 | CHD, COD, HYP, CRF, PVD | 15 | Adeno Ca | CA |
| M, 78 | HA, COD, PNP | 15 | Squamous cell Ca | CA |
| F, 67 | COD, DM, HYP | 20 | Adeno Ca | CA |
| M, 65 | HYP, COD, | 12 | Adeno Ca | CA |
| M, 64 | HYP, DM, PVD | 34 | Adeno Ca | ICA |
| M, 74 | COD, DM, PVD, CHD | 20 | Adeno Ca | ICA |
| M, 72 | CHD, DCM | 35 | Adeno Ca | ICA |
| M, 83 | COD, HYP | 25 | Squamous cell Ca | ICA |
CHD = coronary heart disease; COD = chronic obstructive pulmonary disease; HYP = hypertension; DM = diabetes mellitus II; PVD = peripheral vascular disease; HA = heart arrhythmia; CRF = chronic renal failure; PNP = polyneuropathis; DCM = dilative cardiomyopathy; CA = complete ablation; ICA = incomplete ablation.
The Ethics Committee of the University of Heidelberg approved this study. Each patient was informed comprehensively; written consent was given prior to participation. The principles of the Declaration of Helsinki were followed.
Preparation of peripheral blood samples
Peripheral blood was taken from each patient 1 day before the RFA and 3 days subsequent to the RFA procedure and again 1, 2 and 3 months thereafter. Peripheral blood mononuclear cells (PBMCs) were isolated by the gradient centrifugation according to the manufacturer's protocol (Biocoll; Biochrom, Berlin, Germany), washed twice in cold phosphate-buffered saline (PBS) without Ca2+ and Mg2+ and adjusted to 107 cells supplemented with heat-inactivated fetal calf serum (FCS) (Biochrom) and 10% dimethylsulphoxide (DMSO). Serum samples were prepared at the same time-points and stored together with PBMC aliquots at −80 °C until analysis.
Flow cytometry analysis
The following fluorescent-labelled monoclonal antibodies (mAbs) were used for surface staining: anti-CD3-allophycocyanin (APC)-cyanin 7 (Cy7), CD4-phycoerythrin (PE)-Cy7, CD25-PE, human leucocyte antigen D-related (HLA-DR)-APC-Cy7, CD14-peridinin chlorophyll (PerCP) and CD11b-APC (all from BD Biosciences, San Jose, CA,USA). For the intracellular stainings, mAbs for transforming growth factor (TGF)-β-PerCP, interleukin (IL)-10-fluorescein isothiocyanate (FITC) (both from R&D Systems, Abingdon, UK) and forkhead box protein 3 (FoxP3)-APC (eBioscience, San Diego, CA, USA) were used, as well as the fixation and permeabilization buffer from the FoxP3 staining set (eBioscience). Staining with 4,5-diaminofluorescein diacetat (DAF-2DA; Gentaur, Kampenhout, Belgium) was performed for intracellular NO measurement, as recommended by the manufacturer. Acquisition was performed by six-colour flow cytometry using a fluorescence activated cell sorter (FACS)Canto II with FACSDiva software (both from BD Biosciences) with dead cell exclusion based on scatter profile or 7-aminoactinomycin D (7-AAD) (Biolegend, San Diego, CA, USA). FlowJo software (TreeStar, Ashland, OR, USA) was used to analyse at least 100 000 events. Data were expressed as dot-plots.
Bio-plex assay
Concentrations of 28 various chronic inflammatory factors in serum of treated patients and healthy donors of matched age and gender were measured by the multiplex technology (Millipore, Darmstadt, Germany), according to the manufacturer's protocol.
Statistical analysis
All data are shown as means ± standard error (s.e.) for the indicated time-points. Results were assessed with a Student's t-test and Mann–Whitney U-test. Statistical analyses were performed using GraphPad Prism software (San Diego, CA, USA). A value of P < 0·05 was considered statistically significant.
Results
Patient characteristics and clinical responses
All patients underwent CT-guided RFA without intraprocedural complications. The median duration of RFA was 62 min. In the post-treatment chest radiograph we observed a mild pneumothorax in three patients (33%); it was self-limiting, therefore no patient required chest drainage. Five patients (62·5%) developed pleural effusion that was reabsorbed within 30 days; no thoracentesis was required. One patient developed a haemothorax after the RFA, which was evacuated by videoassisted thoracoscopy (VATS). All patients were discharged 3–7 days upon the RFA procedure. Four patients had to be excluded from the analysis; two patients for technical reasons (repeated coagulation of blood samples during processing), one due to a post-interventional viral infection and one due to post-interventional complications (haemothorax). Finally, the samples from eight patients were included in this study. In a median follow-up period of 12 months, four patients (50%) developed local tumour recurrence in the ablated area, which was verified by contrast-enhanced CT and PET-CT (Table1).
Profile of inflammatory factors in patients' serum upon the RFA
Inflammatory cytokines, chemokines and growth factors were measured in patients' serum before and after the RFA using a bio-plex assay (Fig. 1). We found that the concentration of vascular endothelial growth factor (VEGF) was elevated significantly in serum of patients before therapy (baseline) compared to healthy donors (Fig. 1a). Some other mediators, such as TGF-β1, granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-6, TNF-α and IFN-γ, displayed a strong tendency for elevation in serum before the RFA compared to healthy donors (Fig. 1b–f). Interestingly, we detected a significant decrease in TGF-β1 and GM-CSF levels shortly after the treatment (at day 3) compared to baseline, followed by their increase at day 30 (Fig. 1b,c). While the TGF-β1 concentration remained at the same high level during the whole monitoring time (until day 90; Fig. 1b), the GM-CSF level was found to be elevated markedly at day 30 compared to healthy donors, and then decreased again to baseline level (Fig. 1c). Concentrations of the other studied factors were elevated at days 30 and 60 compared to the baseline and healthy donors followed by a decrease to baseline levels at day 90 (Fig. 1a,d–h).
Fig 1.
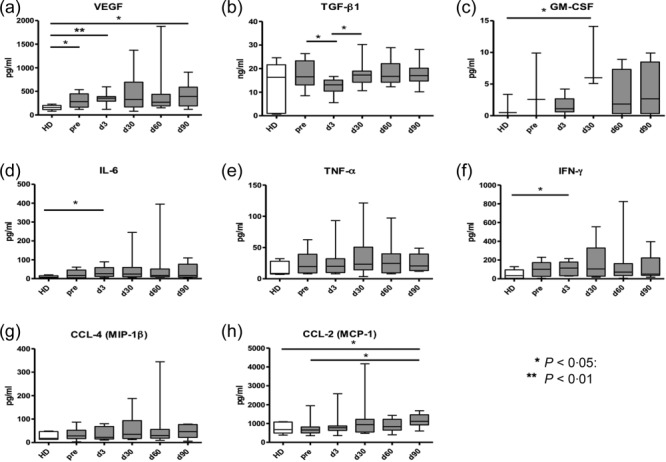
Effect of radiofrequency ablation (RFA) on various chronic inflammatory factors in serum of lung cancer patients. Patients with histologically proven non-small-cell lung cancer (NSCLC) were treated with percutaneous RFA performed under general anaesthesia and endobronchial double-lumen intubation. Levels of vascular endothelial growth factor (VEGF) (a), transforming growth factor (TGF)-β1, (b), granulocyte–macrophage colony-stimulating factor (GM-CSF) (c), interleukin (IL)-6 (d), tumour necrosis factor (TNF)-α (e), interferon (IFN)-γ (f), chemokine (C-C motif) ligand (CCL)-4 (g) and CCL-2 (h) were measured in serum of patients before the treatment (baseline) and at days 3, 30, 60 and 90 after the RFA as well as in healthy donors (HD) of matched age and gender by the bio-plex assay. Cumulative data are expressed as pg/mg protein [mean ± standard error of the mean (s.e.m.)]. *P < 0·05; **P < 0·01, differences between indicated groups.
As shown in Table1, four patients developed local or lymphogenic tumour relapse, due to an incomplete ablation of the tumour tissue. Based on this observation, we analysed the profile of serum inflammatory factors in patients with complete (CA) and incomplete ablation (ICA) (Fig. 2). Patients with ICA showed an early and stable significant increase in serum TNF-α concentrations associated with a markedly enhanced production of CCL-2 and CCL-4 compared to healthy donors or patients with CA (Fig. 2a–c). At each time-point, the serum TNF-α concentration in patients with ICA showed a tendency for elevation compared to patients with CA (Fig. 2a). Moreover, two of four patients with ICA responded to the treatment with an elevation of IL-6 concentrations (data not shown). In contrast, in patients with CA, no significant increase of TNF-α, CCL-4 (Fig. 2a,c) and IL-6 (data not shown) was observed compared to patients' baseline levels and healthy donors. In addition, serum CCL-2 in CA patients remained similar to that in healthy donors and was elevated significantly only at day 90 after the RFA (Fig. 2b). Interestingly, the pattern of changes in TGF-β1 and VEGF in serum of patients with CA and ICA was found to be similar. Thus, levels of TGF-β1 decreased in both patient groups at day 5 after therapy compared to baseline values, followed by recovery to the before-treatment levels (Fig. 2d). Serum concentration of VEGF in both groups of patients remained constantly higher than in healthy donors (Fig. 2e). We detected no differences in amounts of other mediators such as IL-1β, IL-10, IFN-γ and IL-12p70 compared to those before treatment (data not shown).
Fig 2.
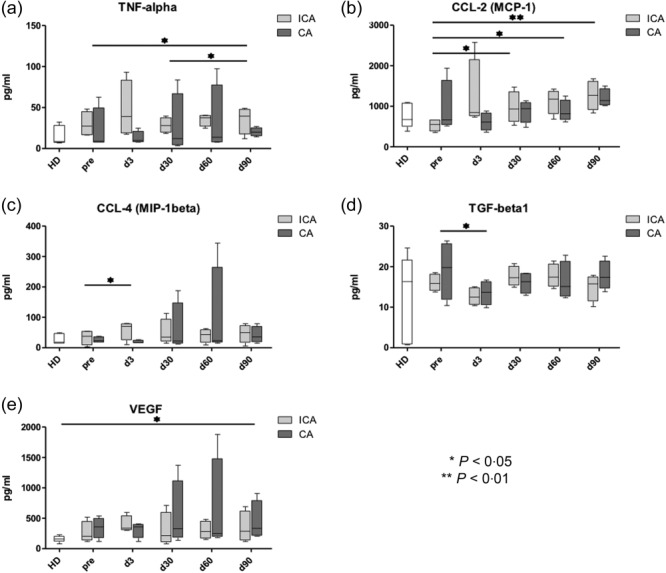
Chronic inflammatory mediators in serum of patients with complete ablation (CA) and incomplete ablation (ICA) upon the radiofrequency ablation (RFA). Concentrations of tumour necrosis factor (TNF)-α (a), chemokine (C-C motif) ligand (CCL)-2 (b), CCL-4 (c), transforming growth factor (TGF)-β1 (d) and vascular endothelial growth factor (VEGF) (e) were determined in serum of patients with CA and ICA before therapy and at days 3, 30 and 60 upon the radiofrequency ablation (RFA) as well as in healthy donors (HD) by the bio-plex assay. Results are presented as pg/mg protein [mean ± standard error of the mean (s.e.m.)]. *P < 0·05; **P < 0·01.
In summary, we observed an early elevation of TNF-α, CCL-2 and CCL-4 levels in the serum of patients developing tumour relapse upon the RFA treatment. In an additional analysis, however, we could not find a correlation between these parameters and the frequency of MDSC.
Characterization of immunosuppressive cells in the peripheral blood of treated patients
Next we addressed the question of whether or not the RFA could influence the two most important immunosuppressive cell populations, MDSCs and Tregs. MDSCs were found highly enriched in tumour lesions and lymphoid organs, both in mouse tumour models and in cancer patients 5–8. According to the current view, human MDSCs consist of granulocytic (grMDSCs) and monocytic (mMDSCs) subsets defined as HLA-DR–CD14–CD15+CD11b+ and HLA-DR–CD14+CD11b+ cells, respectively 24,25. As the grMDSC subset was hardly detectable in the PBMCs of studied patients (data not shown), using flow cytometry we analysed only the subpopulation of mMDSCs (referred to hereinafter as the MDSCs). A typical gating strategy pattern is shown in Fig. 3a. Three of eight patients from both groups had strongly increased frequencies of MDSCs before treatment compared to their counterparts in healthy donors (Fig. 3b,c). We found that two of the CA patients contained MDSCs during the whole monitoring period at levels detected in the peripheral blood of healthy donors (Fig. 3b). Almost all MDSCs from both patients and donors produced NO detected by intracellular staining (Fig. 3d). However, levels of NO production in MDSCs from patients with CA and ICA were considerably different. CA patients showed no significant changes in the NO concentration upon therapy compared to healthy donors (Fig. 3e). In contrast, in ICA patients, NO production remained at relatively high levels, showing a tendency for elevation compared to CA patients at each observation time-point (Fig. 3e). Analysing CD4+CD25+FoxP3+ Tregs in the peripheral blood, we found that their numbers in patients with CA failed to differ significantly from those in patients with ICA or healthy donors (Fig. 4b). In addition, we could not detect any changes in both TGF-β1 and IL-10 intracellular production in Tregs in responding patients compared to non-responders or healthy donors (data not shown).
Fig 3.
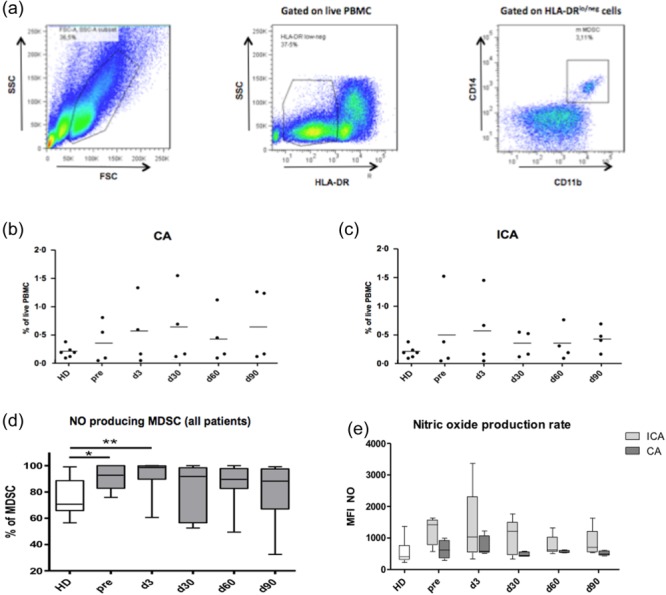
Analysis of circulating myeloid-derived suppressor cells (MDSCs) in lung cancer patients after the radiofrequency ablation (RFA). MDSCs from the peripheral blood of patients subjected to the RFA were analysed by flow cytometry. (a) Monocytic CD14+CD11b+ MDSCs were gated on live human leucocyte antigen D-related (HLA-DR) low/negative peripheral blood mononuclear cells (PBMCs). Representative dot-plots with the gating strategy are shown. Amounts of MDSCs in patients with complete ablation (CA) (b) and incomplete ablation (ICA) (c) are shown as the percentage within live PBMCs at different time-points before and after the therapy in comparison to those in healthy donors (HD). (d) Frequencies of nitric oxide (NO)-producing MDSCs in patients before and after ablation are expressed as the percentage within total MDSCs [mean ± standard error of the mean (s.e.m.)]. Levels of NO production in MDSCs from patients with CA and ICA (e) are presented as mean fluorescent intensity (MFI; mean ± s.e.m.). *P < 0·05; **P < 0·01.
Fig 4.
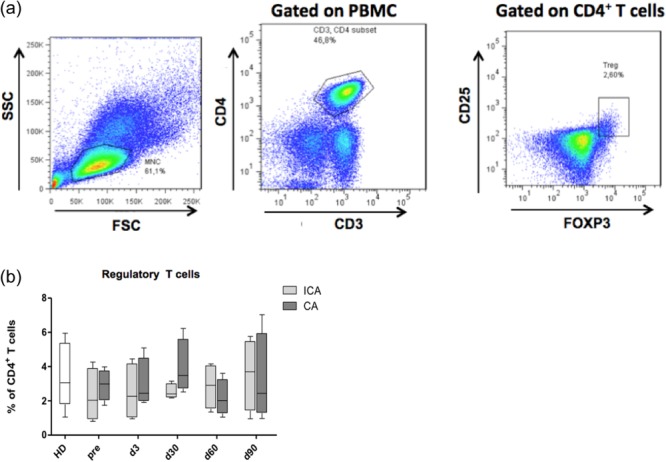
Effect of the radiofrequency ablation (RFA) on circulating regulatory T cells (Tregs) in patients with non-small-cell lung cancer (NSCLC). CD4+CD25+forkhead box protein 3 (FoxP3)+ cells. Tregs from the peripheral blood of patients treated with the RFA were tested by flow cytometry. (a) Representative dot plots with the gating strategy are demonstrated. Numbers of Tregs in patients with CA and ICA (b) are presented as the percentage among CD4+ T cells [mean ± standard error of the mean (s.e.m.)].
In summary, in patients demonstrating tumour relapse, an early enrichment was found in TNF-β, CCL-2 and CCL-4 serum levels. This was associated with an enhanced NO production in MDSCs from the peripheral blood, suggesting that these factors can be applied as early biomarkers of the ICA.
Discussion
Chronic inflammatory conditions have been reported to play an important role in the progression of lung cancer 26–28. The role of different inflammatory cytokines, chemokines and growth factor as predictive markers of lung cancer prognosis and treatment efficacy is under discussion. Although some authors report that TGF-β1 and VEGF could serve as potential prognostic markers in lung cancer treatment 29,30, in this study we failed to demonstrate the changes of these factors in patients with CA compared to patients with ICA, and therefore their prognostic value. Our data on TGF-β1 were in line with reports showing its inability to predict the response to RFA of liver metastases 18,19. In contrast, we demonstrated that serum levels of TNF-α, CCL-2 and CCL-4 were increased in patients with ICA immediately upon therapy compared to these values in patients with CA or healthy donors, thereby suggesting that these inflammatory factors might be used as biomarkers for RFA efficiency. Interestingly, increased serum levels of TNF-α and IL-1β were also observed in HCC patients upon RFA treatment 31. Although the levels of other chronic inflammatory mediators measured in this study showed a strong variation at different time-points upon RFA, they were not dependent upon the clinical response to the treatment.
While the phenotypical markers of MDSCs in mouse tumour models are well determined, the markers for human MDSCs are not well established, and differ among various cancers 25,32–34. MDSCs are immature cells of myeloid origin displaying immunosuppressive activities mediated by numerous mechanisms 24,33,35. These cells consist of granulocytic and monocytic subsets and were found to be recruited and expanded under chronic inflammatory conditions typical for the tumour microenvironment 24,25,32–35. The role of MDSCs in lung cancer progression and their changes in the response to various therapies have not yet been studied. Here we report, for the first time to our knowledge, that monocytic but not granulocytic MDSC numbers are elevated in the peripheral blood of NSCLC patients independent of their response to RFA. Upon curative RFA of patients with HCC, the frequency of MDSCs showed various changes and was correlated inversely with recurrence-free survival and the positive prognosis of these patients 36.
Because NO has been reported to be one of the major mediators of the MDSC immunosuppressive function 24,25,33–35, we examined its production. A higher level of NO production was found in MDSCs after treatment in patients with ICA, but not in the CA group, indicating that intracellular NO levels in circulating MDSCs could be used as another potential biomarker for RFA efficiency. It has been reported that the chemokine CCL-2 could interact with MDSC to support progression and metastasis in prostate cancer patients 37, which is in agreement with our observations demonstrating a close association between the CCL-2 elevation in serum and the stimulation of NO production by MDSCs in the peripheral blood. Moreover, TNF-α and CCL-4 have been demonstrated to stimulate the migration, expansion and activation of MDSCs in cancer patients and tumour-bearing mice [24,25,32,34,35].
We have shown previously that another immunosuppressive cell population, of Tregs, was recruited into the tumour lesions in patients with lung adenocarcinoma 38. In contrast to another report, we failed to observe any differences in numbers of Tregs in the patients' peripheral blood upon RFA in CA as well as ICA. 21. We suggest that the clinical response to RFA in lung cancer patients seems to be more dependent upon the immunosuppressive activity of MDSCs and chronic inflammatory factors stimulating their activation.
In conclusion, we found that the levels of chronic inflammatory factors in lung cancer patients were significantly higher than in healthy donors. This was associated with an enhancement of NO production in circulating MDSCs. Moreover, if the treatment of lung cancer patients resulted in tumour relapse, the intracellular concentration of NO in MDSCs as well as the level of serum TNF-α, CCL-2 and CCL-4 remained significantly increased compared to the group with CA. We suggest that measurement of the above-mentioned values in lung cancer patients subsequent to RFA may potentially be useful to predict the clinical efficacy of the ablation.
Disclosure
All authors declare that there are no conflicts of interest.
References
- Hiraki T, Gobara H, Mimura H, Matsui Y, Toyooka S, Kanazawa S. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142:24–30. doi: 10.1016/j.jtcvs.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the rapture study) Lancet Oncol. 2008;9:621–8. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- Clasen S, Krober SM, Kosan B, et al. Pathomorphologic evaluation of pulmonary radiofrequency ablation: proof of cell death is characterized by DNA fragmentation and apoptotic bodies. Cancer. 2008;113:3121–9. doi: 10.1002/cncr.23882. [DOI] [PubMed] [Google Scholar]
- Schneider T, Reuss D, Warth A, et al. The efficacy of bipolar and multipolar radiofrequency ablation of lung neoplasms – results of an ablate and resect study. Eur J Cardiothorac Surg. 2009;39:968–73. doi: 10.1016/j.ejcts.2010.08.055. [DOI] [PubMed] [Google Scholar]
- Nierkens S, den Brok MH, Ruers TJ, Adema GJ. Radiofrequency ablation in cancer therapy: tuning in to in situ tumor vaccines. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. pp. :39–59. [Google Scholar]
- Wissniowski TT, Hansler J, Neureiter D, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the vx2 hepatoma in rabbits. Cancer Res. 2003;63:6496–500. [PubMed] [Google Scholar]
- den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–9. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- den Brok MH, Sutmuller RP, Nierkens S, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Williams J, Ding I, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol. 2002;12:26–33. doi: 10.1053/srao.2002.31360. [DOI] [PubMed] [Google Scholar]
- Nomura M, Yamakado K, Nomoto Y, et al. Complications after lung radiofrequency ablation: risk factors for lung inflammation. Br J Radiol. 2008;81:244–9. doi: 10.1259/bjr/84269673. [DOI] [PubMed] [Google Scholar]
- Rughetti A, Rahimi H, Rossi P, et al. Modulation of blood circulating immune cells by radiofrequency tumor ablation. J Exp Clin Cancer Res. 2003;22:247–50. [PubMed] [Google Scholar]
- Hansler J, Wissniowski TT, Schuppan D, et al. Activation and dramatically increased cytolytic activity of tumor specific t lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol. 2006;12:3716–21. doi: 10.3748/wjg.v12.i23.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448–57. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- Zerbini A, Pilli M, Penna A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific t-cell responses. Cancer Res. 2006;66:1139–46. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- Zerbini A, Pilli M, Fagnoni F, et al. Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J Immunother. 2008;31:271–282. doi: 10.1097/CJI.0b013e318160ff1c. [DOI] [PubMed] [Google Scholar]
- Schell SR, Wessels FJ, Abouhamze A, Moldawer LL, Copeland EM, III . Pro- and antiinflammatory cytokine production after radiofrequency ablation of unresectable hepatic tumors. J Am Coll Surg. 2002;195:774–81. doi: 10.1016/s1072-7515(02)01333-9. [DOI] [PubMed] [Google Scholar]
- Kallio R, Sequeiros R, Surcel HM, Ohtonen P, Kiviniemi H, Syrjala H. Early cytokine responses after percutaneous magnetic resonance imaging guided laser thermoablation of malignant liver tumors. Cytokine. 2006;34:278–83. doi: 10.1016/j.cyto.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Evrard S, Menetrier-Caux C, Biota C, et al. Cytokines pattern after surgical radiofrequency ablation of liver colorectal metastases. Gastroenterol Clin Biol. 2007;31:141–5. doi: 10.1016/s0399-8320(07)89344-4. [DOI] [PubMed] [Google Scholar]
- Jansen MC, van Wanrooy S, van Hillegersberg R, et al. Assessment of systemic inflammatory response (SIR) in patients undergoing radiofrequency ablation or partial liver resection for liver tumors. Eur J Surg Oncol. 2008;34:662–7. doi: 10.1016/j.ejso.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Hoshida Y, Kanai F, et al. Proteomic analysis of sera from hepatocellular carcinoma patients after radiofrequency ablation treatment. Proteomics. 2005;5:4287–95. doi: 10.1002/pmic.200401287. [DOI] [PubMed] [Google Scholar]
- Fietta AM, Morosini M, Passadore I, et al. Systemic inflammatory response and downmodulation of peripheral CD25+FoxP3+ T-regulatory cells in patients undergoing radiofrequency thermal ablation for lung cancer. Hum Immunol. 2009;70:477–86. doi: 10.1016/j.humimm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:160–6. doi: 10.1016/j.jtcvs.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg. 2007;134:857–64. doi: 10.1016/j.jtcvs.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–53. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Cho WC, Kwan CK, Yau S, So PP, Poon PC, Au JS. The role of inflammation in the pathogenesis of lung cancer. Expert Opin Ther Targets. 2011;15:1127–37. doi: 10.1517/14728222.2011.599801. [DOI] [PubMed] [Google Scholar]
- Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9:375–383. doi: 10.1016/j.coph.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLOS ONE. 2011;6:e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RJ, Tin AW, Brown NJ, Jitlal M, Lee SM, Woll PJ. Analysis of circulating angiogenic biomarkers from patients in two Phase III trials in lung cancer of chemotherapy alone or chemotherapy and thalidomide. Br J Cancer. 2012;106:1153–9. doi: 10.1038/bjc.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ji W, Zhang L, et al. Changes of circulating transforming growth factor-beta1 level during radiation therapy are correlated with the prognosis of locally advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:521–5. doi: 10.1097/JTO.0b013e3181cbf761. [DOI] [PubMed] [Google Scholar]
- Ali MY, Grimm CF, Ritter M, et al. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol. 2005;43:817–22. doi: 10.1016/j.jhep.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61:255–63. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35:107–15. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arihara F, Mizukoshi E, Kitahara M, et al. Increase in CD14+HLA-DR–/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421–30. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–8. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Kimpfler S, Warth A, et al. Foxp3(+) regulatory Tcells and natural killer cells distinctly infiltrate primary tumors and draining lymph nodes in pulmonary adenocarcinoma. J Thorac Oncol. 2011;6:432–8. doi: 10.1097/JTO.0b013e31820b80ca. [DOI] [PubMed] [Google Scholar]


