Abstract
Dendritic cells (DCs) play an important role in the induction of the primary immune response to infection. DCs may express the tryptophan-catabolizing enzyme indolamine2,3-dioxygenase (IDO), which is an inducer of immune tolerance. Because there is evidence that chronic hepatitis C virus (HCV) infection leads to functional impairment of certain DC populations, we analysed IDO expression in DCs and monocytes from chronically infected and recovered HCV patients. The IDO1 and -2 expression was increased significantly in the monocytes of chronic HCV patients but, interestingly, not in those from recovered patients. The myeloid DCs from chronically infected HCV patients also showed enhanced IDO1 expression, while no change in either IDO1 or -2 was found for plasmacytoid DCs. Up-regulation of IDO1 gene expression was confirmed by the presence of enhanced kynurenine/tryptophan ratios in the plasma from chronic HCV patients. Increased IDO1 and -2 expression was also observed in monocytes from healthy donors infected with an adapted mutant of the HCV JFH-1 strain ex vivo, confirming a direct effect of HCV infection. These changes in IDO expression could be prevented by treatment with the IDO inhibitor 1-methyl tryptophan (1-mT). Furthermore, maturation of monocyte-derived DCs from chronically infected HCV patients, as well as well as monocyte-derived DCs infected ex vivo with HCV, was impaired, but this was reversed by 1-mT treatment. This suggests that IDO inhibitors may be used to treat chronic HCV patients in vivo, in conjunction with current therapies, or to activate DCs from patients ex vivo, such that they can be administered back as a DC-based therapeutic vaccine.
Keywords: dendritic cell, hepatitis C virus, monocyte, IDO
Introduction
Hepatitis C virus (HCV) infection is a serious health problem, with approximately 150 million chronically infected individuals worldwide, which is likely to increase during the coming years. Although HCV infection can occur in two forms, acute or chronic, up to 80% of all patients become chronically infected, which may be followed by hepatic cirrhosis, liver failure or hepatocellular carcinoma 1 years after the initial acquisition of the infection. In addition, co-infections with human immunodeficiency virus (HIV), hepatitis B virus (HBV) or extended alcohol consumption are associated with more severe HCV-induced liver disease 2. Whereas in the late 1970s and early 1980s the spread was due primarily to contaminated blood transfusions, today most cases are acquired through the sharing of infected needles while injecting drugs or, to a much lesser extent, via prenatal and sexual transmission 3. Recently, the nucleotide polymerase inhibitor sofosbuvir (Sovaldi™) was approved for the treatment of chronic HCV in combination with other anti-viral drugs. Sofosbuvir inhibits the HCV NS5B RNA polymerase, which plays an important role in the replication of the virus. Fewer side effects and an up to 90% success rate makes it the most promising treatment at present 4. However, there is still no licensed vaccine available. Developing vaccine strategies to generate effective immune responses to HCV as well as for potential treatment of HCV infections to improve the life of infected people is of great importance. The absence of an effective T cell response in HCV-infected individuals is thought to play a role in the establishment of a chronic infection 5, which underlines the importance of the adaptive immune response for the clearance of HCV infection.
Dendritic cells (DC) play a key role in initiating a fast and effective anti-viral T cell response and are derived from haematopoietic bone marrow progenitor cells. Immature DCs (iDC), which have low T cell activation potential but high endocytic capacity, are localized specifically in the skin and the inner lining of the nose, lungs, stomach and intestines. Once in contact with potential harmful pathogens they mature and migrate to the lymph nodes. Maturation of DCs is critical for the uptake and presentation of antigens to naive T cells. Mature DCs are defined by morphological changes, the up-regulation of major histocompatibility complex (MHC) and co-stimulatory molecules, their ability to produce cytokines influencing T cell activation and their high ability for antigen presentation 6.
Humans have two major subsets of DCs: DC1, also called myeloid DCs (mDCs) and DC2, or plasmacytoid DCs (pDCs), which can be distinguished by the expression of their surface molecules. Myeloid DCs are characterized as CD1c+CD11c+CD14– human leucocyte antigen D-related (HLA-DR+) cells. Once activated and mature, they produce T helper 1 (Th1) or Th-2 promoting cytokines to prime naive T cells. pDCs are CD123+ô CD11c–CD14–HLA-DR+ cells and secrete interferon (IFN)-α 7. Both types are necessary to link the innate and adaptive immune response.
Indoleamine 2,3-dioxygenase 1 (IDO1) is the rate-limiting enzyme for the tryptophan (Trp) catabolism through the kynurenine pathway (Kyn), and catalyzes the degradation of L-tryptophan to N-formylkynurenine 8. Its expression in DCs and monocytes is influenced by different soluble [IFN-α and -β, prostaglandin E2 (PGE2), tumour necrosis factor (TNF)-α] and membrane-bound [cytotoxic T lymphocyte antigen (CTLA)-4/CD28, CD200] molecules. Moreover, T cell activation and proliferation are modulated by the induction of IDO1 in antigen-presenting DCs 9. IDO1-expressing DCs and macrophages inhibit antigen-specific allergenic T cell activation in both human and murine cells. It is known that IDO1 is produced mainly by pDCs 10, but is also expressed by mDCs.
Recently, several studies have suggested that the expression of IDO1 is an important mediator of immune tolerance and is changed in cancer and HIV patients 9,10. There are different theories as to what triggers the increase in IDO1 expression in these chronically infected patients or if IDO1 actively suppresses immune responses to HIV antigens 8. Recent studies on liver samples of chronic HCV patients showed an up-regulation of IDO1 11,12. Higashitani 12 also showed that the degree of liver inflammation and fibrosis is correlated with enhanced IDO1 activity in DCs, whereas its level was down-regulated in cultured macrophages from chronic HCV patients 13. The recently discovered IDO2 gene is also expressed by human DCs, but seems to be mainly inactive despite its similarity to IDO1 14. 1-Methyltryptophan (1-mT) is used widely as a competitive IDO inhibitor. It exists in two stereoisomers, the levo (L-) and dextro (D-) isoforms. Recent studies have shown that L-1-mT is more effective at inhibiting IDO1 and its effect on the T cell response, whereas the IDO2 gene seems to be affected by D-1-mT 15.
In this study, we demonstrated that the IDO1 and -2 expression levels in monocytes and DCs and Kyn/Trp ratios in plasma are enhanced in chronic HCV patients in comparison to healthy individuals, while the IDO1 and -2 levels are normal in recovered patients. Similarly, IDO1 and -2 expression is increased in monocytes infected with HCV ex vivo, and maturation of monocyte-derived DCs from chronic HCV patients is impaired. The changes in IDO expression could be reversed by 1-mT treatment, which may have implications for HCV treatment and DC vaccine development.
Materials and methods
Subjects
A total number of nine chronically infected (seven male, two female; age 43 ± 15 years) and five spontaneously recovered (four male, one female; age 37 ± 20 years) HCV patients were studied. Patients were referred from the out-patient clinic at the Department of Medicine of the Royal University Hospital, Saskatoon and fulfilled the following inclusion criteria: (a) active [HCV-RNA detected by polymerase chain reaction (PCR)] or former HCV infection and (b) absence of hepatitis B and/or HIV infection. All patients signed written and informed consent to participate in the study. Furthermore, 10 healthy volunteers (five male, five female; age 30.5 ± 2) were evaluated. All age values and ranges are listed as median values with interquartile range. Table1 shows the characteristics of the patients and healthy controls. The study was performed in accordance with the guidelines of the Biomedical Research Ethics Board (University of Saskatchewan).
Table 1.
Characteristics of hepatitis C patients and controls.
| Patient | Age (years) | Sex | ALT | Viral load log (eq/ml) | Genotype | IL-8 conc. (pg/ml) | TNF-α conc. (pg/ml) |
|---|---|---|---|---|---|---|---|
| HCV PCR+ | 33 | M | 73 | 6·238 | 1b | 26·049 | 27·092 |
| 51 | M | 177 | 6·138 | 3a | 67·342 | 14·589 | |
| 71 | F | 89 | 6·031 | 1a | 53·957 | 11·572 | |
| 40 | M | 33 | 6·146 | 1b | 48·871 | 17·176 | |
| 23 | M | 88 | 5·954 | 1 | 22·647 | 26·488 | |
| 53 | M | 51 | 5·942 | 1b | 42·103 | 12·488 | |
| 25 | M | 64 | 6·169 | 2 | 67·970 | 28·069 | |
| 43 | M | 105 | 6·860 | 4 | 71·757 | 25·757 | |
| 58 | F | 116 | 7·011 | 1 | 69·985 | 27·005 | |
| HCV PCR– | 30 | M | 21 | n.a. | 13·779 | 5·093 | |
| 37 | M | 10 | n.a. | 6·696 | 4·251 | ||
| 48 | M | 9 | n.a. | 14·804 | 4·554 | ||
| 35 | M | 30 | n.a. | 16·921 | 8·418 | ||
| 70 | F | 30 | n.a. | 9·304 | 5·102 | ||
| Controls | 24 | F | 0·492 | 0·744 | |||
| 34 | M | 0·518 | 0·891 | ||||
| 33 | M | 0·865 | 0·697 | ||||
| 30 | F | 0·429 | 0·560 | ||||
| 29 | F | 0·605 | 1·028 | ||||
| 29 | M | 0·979 | 0·693 | ||||
| 33 | M | 0·903 | 0·688 | ||||
| 29 | M | 0·936 | 1·551 | ||||
| 31 | F | 0·479 | 0·643 | ||||
| 31 | F | 0·645 | 0·753 |
ALT = alanine aminotransferase; IL = interleukin; n.a. = not applicable; PCR = polymerase chain reaction; TNF = tumour necrosis factor; M = male; F = female.
Cell isolation and generation of mature DCs
Myeloid DCs, pDCs and CD14+ monocytes were isolated from 100 ml blood of HCV patients and healthy volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient separation using Ficoll-Plaque Premium (GE Healthcare Life Science, Baie d'Urfe, QE, Canada). Plasma was frozen at −80°C until further use. Myeloid DCs, pDCs and monocytes were subsequently isolated using specific antibodies conjugated to microbeads (Miltenyi Biotech, Auburn, CA, USA), according to the manufacturer's instructions. Briefly, CD19+ B cells and CD1c mDCs were labelled with CD19/CD1c- (BDCA-1) biotin for depletion of the B cell fraction (negative selection using an LD column), followed by positive selection with anti-biotin microbeads on an LS column to obtain CD1c (BDCA-1) mDCs. The flow-through was incubated with a human CD304 (BDCA-4/neuropilin-1) antibody for the isolation of pDCs. In a last step, CD14+ monocytes were isolated on an LS-column after incubation with a human CD14-specific antibody. Immature DCs (iDCs) and mature DCs (matDCs) were generated as described previously 16. Briefly, CD14+ monocytes were resuspended in complete RPMI medium (CRPMI) (RPMI-1640; Invitrogen Canada Inc., Burlington, ON, Canada), containing 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 µM non-essential amino acids, 1 mM sodium pyruvate, 50 mM 2-mercaptoethanol (ME), 10 mM HEPES and 50 mg/ml gentamycin (Gibco Life Technologies, Burlington, ON, Canada). The cells were distributed into six-well plates after adding recombinant human granulocyte–macrophage colony-stimulating factor (rhGM-CSF) (100 ng/ml, PeproTech Inc., Rocky Hill, NJ, USA) and interleukin (IL)-4 (200 ng/ml, PeproTech Inc.), collected on day 3 in RNA lysis buffer (Zymo Research, Irvine, CA, USA) and stored at −80°C until use. To mature these cells (matDC) the medium was replaced on day 3, and 10 ng/ml recombinant human (rh)IL-1β, 10 ng/ml rhIL-6, 20 ng/ml rhTNF-α (PeproTech Inc.) and 500 ng/ml PGE2 (Sigma-Aldrich Canada Ltd, Oakville, ON, Canada) were added 48 h before the cells were collected in RNA lysis buffer.
Analysis of mRNA expression of co-stimulatory molecules and IDO
To compare the mRNA levels of different co-stimulatory molecules by quantitative reverse transcription–PCR (qRT–PCR), total RNA was extracted from all cell types (mDCs, pDCs, CD14+ monocytes, iDCs, matDC) of HCV patients and healthy controls using ZR RNA MicroPrep (Zymo Research). The isolated RNA was treated with TurboDNase (Ambion Life Technologies, Burlington, ON, Canada), and its integrity and concentration were determined using spectrophotometry. Reverse transcription was performed using a QuantiTect reverse transcription kit (Qiagen, Mississauga, ON, Canada) with 20 ng total RNA in accordance with the manufacturer's protocol. The cDNA product was assessed by qRT–PCR using 96-well plates (Bio-Rad, Mississauga, ON, Canada) and actin was used as internal control to normalize the input. All assays were performed in triplicate with 0·5 µl cDNA using platinum SYBR green qPCR SuperMix-UDG (Invitrogen) in a final volume of 20 µl. The primers to determine the mRNA levels of IDO1, IDO2, CD40, CD80 and CD83 (Table2) were used at a concentration of 10 pmol per reaction and fluorescence was measured over 40 cycles. Reverse transcription was performed with the following cycles in a C1000 Thermal Cycler (CFX96 Real Time System; Bio-Rad): 2 min at 55°C, 2 min at 95°C, followed by 40 cycles of 30 s at 95°C and 30 s at 56·3°C (annealing temperature). The PCR products were analysed further by a melt curve program from 55°C to 95 °C with 5 s each, ending with 1 min at 95°C.
Table 2.
Primer sequences for qRT–PCR.
| β-actin, forward | AGCGAGCATCCCCCAAAGTT |
| β-actin, reverse | GGGCACGAAGGCTCATCATT |
| IDO1, forward | GGCACACGCTATGGAAAACT |
| IDO1, reverse | CGGACATCTCCATGACCTTT |
| IDO2, forward | TGCTTCATGCCTTTGATGAG |
| IDO2, reverse | GAAGGCCTTATGGGAAGGAG |
| CD40, forward | CTCATGCTCGCCCGGCTTTGG |
| CD40, reverse | CGATCCTGGGGACCACAGACAACA |
| CD80, forward | GTGGCAACGCTGTCCTGTGGT |
| CD80, reverse | GTGCCCTCGTCAGATGGGCG |
| CD83, forward | TCACTTGTAAGTTTGCACGGCTACA |
| CD83, reverse | CAGACAGGCACACCCCTGAGC |
IDO = indolamine 2,3-dioxygenase; qRT–PCR = quantitative reverse transcription–polymerase chain reaction.
Measurement of tryptophan and kynuridine concentration
One ml plasma from each patient and healthy volunteer was thawed on ice and inactivated at 56°C for 40 min to eliminate HCV infectivity 17. Detection of Trp and Kyn was performed by liquid chromatography-mass spectrometry (LC-MS) with the assistance of Yong Huang, Department of Bioengineering and Therapeutic Science, UCSF School of Pharmacy, San Francisco, CA, USA.
Evaluation of proinflammatory cytokines and chemokines and IDO
Serum cytokines and chemokines were quantified using the electrochemiluminescence (ECL) detection-based Meso Scale Discovery (MSD) multiplex platform and Sector Imager 2400 (MSD, Gaithersburg, MD, USA), according to the manufacturer's instructions. The human proinflammatory-10 ultrasensitive kit from MSD, which measures IFN-γ, IL-10, IL-12 p70, IL-13, IL-1α, IL-2 IL-4, IL-5, IL-8 and TNF-α, was used. The MSD Discovery Workbench software was used to convert relative luminescent units into protein concentrations using interpolation from several log calibrator curves.
Production and titration of infectious HCV strain JFH-1
A plasmid containing the cell culture-adapted HCV cDNA, JFH-1T (pJFH-1T), modified with coding mutations in E2, p7 and NS2 18, was kindly provided by Dr Rodney Russell (University of Newfoundland). pJFH-1T was linearized with Xba1, and the end was blunted using Mung Bean DNA nuclease (New England Biolabs, Pickering, ON, Canada). pJFH-1 was treated with Xba1 twice, followed by a single digestion with mung bean DNA nuclease (New England Biolabs). The linearized DNA was used for in-vitro transcription with the MEGAscript T7 kit (Ambion), according to the manufacturer's instructions. Infection of the human hepatoma cell line Huh7·5 was carried out using electroporation. The trypsinized cells were washed twice and resuspended in phosphate-buffered saline (PBS) pH 7·2 (Gibco-Life Technologies) to a final concentration of 1.5 × 107 cells/ml. Four hundred µl of the cell suspension were mixed with 10 µg of mRNA, transferred to a 4-mm sterile disposable cuvette (VWR, Edmonton, AB, Canada) and electroporated in the presence of J6/JFH-1 RNA in a Gene Pulser™ (Bio-Rad) with a voltage of 270 V and a capacitance of 950 µF. Subsequently, the cells were immediately resuspended in Dulbecco's minimal essential medium (DMEM; Invitrogen) supplemented with 10% heat-inactivated FBS, 1% non-essential amino acids and 0.1% gentamycin (Gibco-Life Technologies), and incubated at 37 °C and 5% CO2. After 3 days the supernatant was collected and concentrated using Amicon-15-Plus columns (Fisher Scientific, Edmonton, AB, Canada) at 2000 g for 20 min at 4°C. Infectious supernatants were divided into aliquots and stored at −80°C for further experiments. The titre was measured by using focus-forming assays. Briefly, Huh7·5 cells were infected with virus dilutions for 5 days, and infected foci were visualized by staining with mouse anti-HCV NS5a IgG (Meridian-Life Science, Saco, ME, USA) and goat anti-mouse Alexa Fluor 488 (Invitrogen).
Infection of CD14+ monocytes
HCV infection of monocytes was carried out using MACSductin (Miltenyi Biotech), according to the manufacturer's instructions. Monocytes were isolated on an LS-column after incubation with a specific human CD14-specific antibody which was conjugated to microbeads, following the manufacturer's instructions (Miltenyi Biotech). Subsequently, 2·5 × 106 freshly isolated monocytes were washed twice in PBS, pH 7·2 (Gibco-Life Technologies) and resuspended in 100 µl magnetic affinity cell sorter (MACS) buffer [PBS pH 7·4, 10% bovine serum albumin (BSA) and 250 mM ethylenediamine tetraacetic acid (EDTA)] (Gibco-Life Technologies). Subsequently, the target population was labelled magnetically with CD14+ MACS microbeads for the second time, and incubated at 4°C for 15 min. After completion of the cell labelling, the cells were washed in serum-free RPMI medium and resuspended in 1 ml. For infection with a multiplicity of infection (MOI) of 0·1, 7·5 µl MACSductin reagent was mixed with 2·5 × 105 virus particles and incubated for 20 min at room temperature (RT). Target cells and the virus-MACSductin complex were mixed and applied onto an already washed LS column. After one more washing step the virus–cell complexes were eluted with 3 ml CRPMI medium incubated at 37°C and 5% CO2. Forty-eight h later 0·2 mM L-1-mT (Sigma-Aldrich) was added to one part of the cells to determine the effects of the IDO1 inhibitor, whereas the rest of the monocytes remained untreated. Gene expression was measured 5 days later by qRT–PCR as described above.
Analysis of HCV infection and replication in monocytes
The HCV- and mock-infected CD14+ monocytes with or without 1-mT treatment were washed twice to remove any remaining virus. Isolation of RNA and generation of cDNA was performed as described above. Gene-specific primers for HCV plus and HCV minus strand RNA as well as NS5A were designed using NCBI (www.ncbi.nlm.nih.gov/), as follows. NS5a, forward: GGCTGCACAGGTACGCTCCG; NS5a, reverse: TCCTGCCGCCACAGGAGGTT; HCV-positive strand, forward: CTCGCAAGCACCCTATCAGGCAGT; HCV-positive strand, reverse: GCAGAAAGCGTCTAGCCATGGCGT 19; the same primers were used in reverse order for detection of negative-strand RNA. RT–PCR was performed with Invitrogen one-step RT–PCR (Invitrogen), following the manufacturer's instructions with some modifications. Briefly, 21·5 µl diethylpyrocarbonate (DEPC)-treated water (Gibco-Life Technology), mixed with 25 µl reaction mix buffer (containing 0·4 mM of each dNTP and 2.4 mM MgSO4) and 1 µl RT/platinum Taq mix were mixed with 0·5 µl RNA. This solution was incubated for 3 min at 50°C for cDNA synthesis. Subsequently, the primer mix (10 µM each of forward and reverse primers) was added. Samples were heated first to 94°C for 2 min, followed by 35 cycles of 94°C/45 s, 59·2°C (NS5A) or 60°C (positive and negative strand)/30 s and 72°C/1 min. After a last amplification step of 10 min at 72°C, the samples were cooled down to 4°C before application on a 1% agarose gel to identify the PCR products. Gene expression of NS5a was measured by qRT–PCR, as described above.
Western blotting and immunohistochemistry
HCV-infected cells were collected in 100 µl sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer on days 2 or 5 after infection and boiled for 5 min at 97°C. Twenty µl of each sample was separated by SDS-PAGE on a 7·5% gel. After transfer to a nitrocellulose membrane, the membrane was incubated with 1 : 1000 diluted mouse anti-NS5a antibody (1 : 1000; Meridian-Life Science), followed by a 1 : 15 000-diluted Alexa488-labelled anti-mouse immunoglobulin (Ig)G (LI-COR Inc., Lincoln, NB, USA). Western blots were analysed using an Odyssey-Licor scanner (LI-COR Inc.). To evaluate expression of NS5A by fluorescence, monocytes from healthy individuals and Huh7·5 cells were infected with J6/JFH-1 at an MOI of 0·1, as described above. Two and 5 days after infection the cells were collected, plated onto an eight-chamber slide (Millipore, Billerica, MA, USA) and fixed with an acetone–methanol solution. Subsequently, they were incubated for 1 h with 1 : 200 diluted mouse anti-NS5a antibody (Meridian-Life Science), followed by 1 h incubation with 1 : 100 diluted Alexa488-labelled anti-mouse IgG (Invitrogen). Images were analysed using a Zeiss axiovert 200 M inverted microscrope.
Flow cytometry
HCV-infected and uninfected monocytes were fixed and permeabilized to stain for NS5A and IDO1 using a BD Cytofix/Cytosperm Fixation/Permeabililzation Kit (BD Bioscience, Mississauga, ON, Canada), according to the manufacturer's instructions. After incubation in 100 µl Cytofix/Cytosperm solution for 30 min on ice, the cells were washed twice in ×1 Perm/Wash solution, and then incubated with an anti-NS5A antibody (Meridian Life Science, Memphis, TN, USA) for 30 min on ice. After two washing steps, allophycocyanin (APC)-conjugated anti-mouse IgG (BioLegend, San Diego, CA, USA) was applied for another 30 min. For detection of IDO1, cells were also incubated with anti-IDO1 antibody (Sigma-Aldrich, St Louis, MO, USA) followed by goat anti-rabbit IgG-fluorescein isothiocyanate (FITC) (Southern Biotech, Birmingham, AL, USA). Finally, cells were resuspended in 200 µl PBS, pH 7·2 (Gibco-Life Technologies) and acquired by flow cytometry (BD Biosciences). Data were analysed using Kaluza software (version 1·2).
Statistical analysis
The difference between chronic and recovered HCV patients as well as healthy controls was assessed using one-way analysis of variance (anova). Significant differences were analysed further using the Kruskal–Wallis test and Dunn's multiple comparison test. Differences were considered significant if P < 0·05.
Results
Chronic and recovered HCV patients have lower numbers of PBMCs and elevated serum proinflammatory cytokine levels
In order to determine the effect of HCV infection on the numbers of circulating mDCs, pDCs and CD14+ monocytes in chronic (HCV PCR+) and recovered (HCV PCR–) HCV patients, in comparison with healthy controls (Ctrl), we enumerated the purified cells using a counting chamber. The numbers of PBMCs were decreased significantly in chronically infected HCV patients compared with control subjects (P < 0·01), and even recovered patients did not reach the cell numbers of healthy individuals (P < 0·05) (Fig. 1a). In addition, the mean number of pDCs was significantly lower in HCV PCR+ patients compared to control subjects (P < 0·05) (Fig. 1c). No significant reduction was seen in the numbers of mDCs and CD14+ monocytes, although a trend towards a decrease was found for both HCV PCR– and PCR+ patients (Fig. 1b,c). This was not due to differences in median age, as there were no statistical differences between the three groups.
Fig 1.
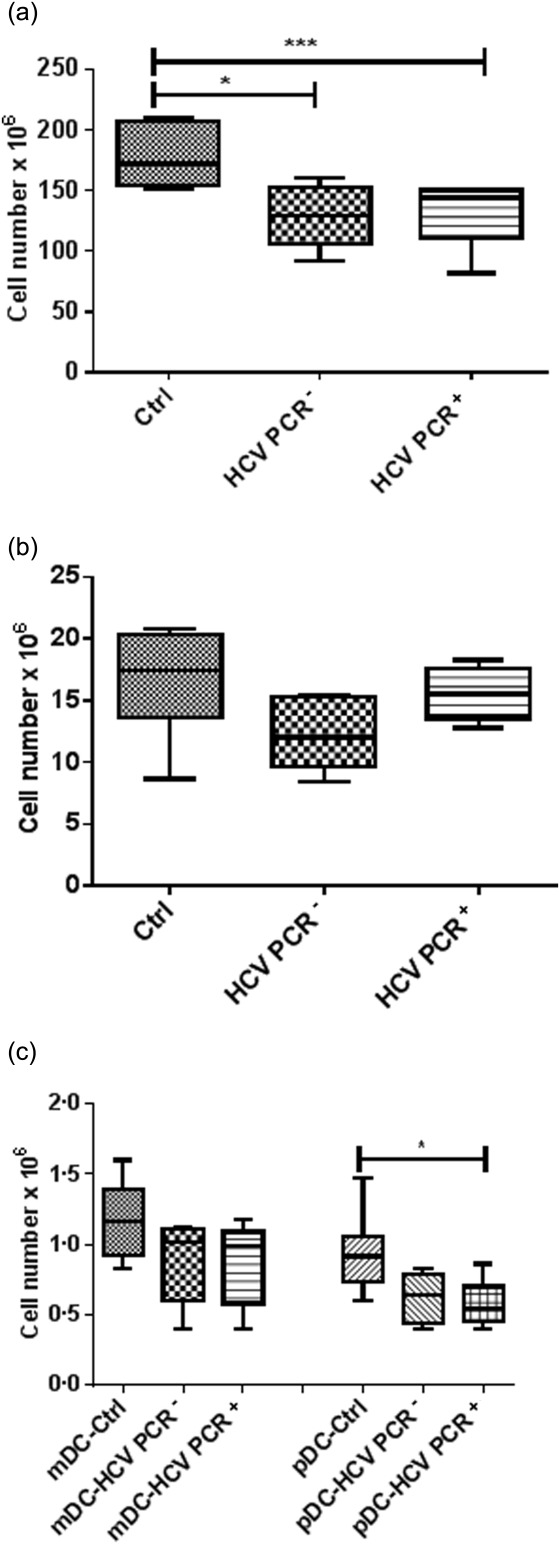
Cell numbers in peripheral blood mononuclear cells (PBMCs) (a), monocytes (b), mature dendritic cells (mDCs) and plasmacytoid DCs (pDCs) (c) in healthy controls (Ctrl), recovered hepatitis C virus (HCV) [HCV polymerase chain reaction (PCR)–] and chronic HCV (HCV PCR+) patients. Results are shown as median value and interquartile range. Significant difference is shown as **P < 0·05; *P < 0·01.
To determine if there is a link between the presence of inflammatory cytokines and an ongoing or a former HCV infection, we analysed the plasma of HCV patients, as well as controls, for their occurrence. The IL-8 and TNF-α concentrations of patients and controls are shown in Table1. Chronic HCV PCR+ patients had higher levels of IL-8 and TNF-α than recovered HCV PCR– patients (P < 0·001), and both groups had higher levels than healthy volunteers (P < 0·0001 and P < 0·001, respectively). The ALT levels were higher in the HCV PCR+ patients than in the HCV PCR– patients (P < 0·01).
Effect of HCV infection on the expression of IDO1 and -2 in DCs
As there is evidence that DCs in chronically infected HCV patients are phenotypically altered and functionally impaired, these DCs may express IDO and be tolerogenic. To evaluate this, IDO1 and -2 expression was analysed in DCs from chronic and recovered HCV patients as well as from healthy volunteers. Myeloid DCs, pDCs and monocytes were isolated, and IDO1 and IDO2 mRNA was quantified using qRT–PCR. The IDO1 mRNA levels were significantly higher in mDCs and monocytes from HCV PCR+ patients than in those from HCV PCR– patients and healthy volunteers (P < 0·0001 and P < 0·001, respectively; Fig. 2a,b). However, no differences were observed in pDCs (Fig. 2c). IDO1 is known to mediate the degradation of Trp to Kyn in the kynuridine pathway, so the serum Kyn level depends strongly on the IDO1 activity. To investigate further the effect of HCV infection, the Kyn/Trp levels in the plasma were examined as a measure of the biological activity of IDO1. As shown in Fig. 3, the serum Kyn/Trp ratio was significantly higher in chronic HCV PCR+ patients than in healthy subjects (P < 0·01) and recovered HCV PCR– patients (P < 0·05).
Fig 2.
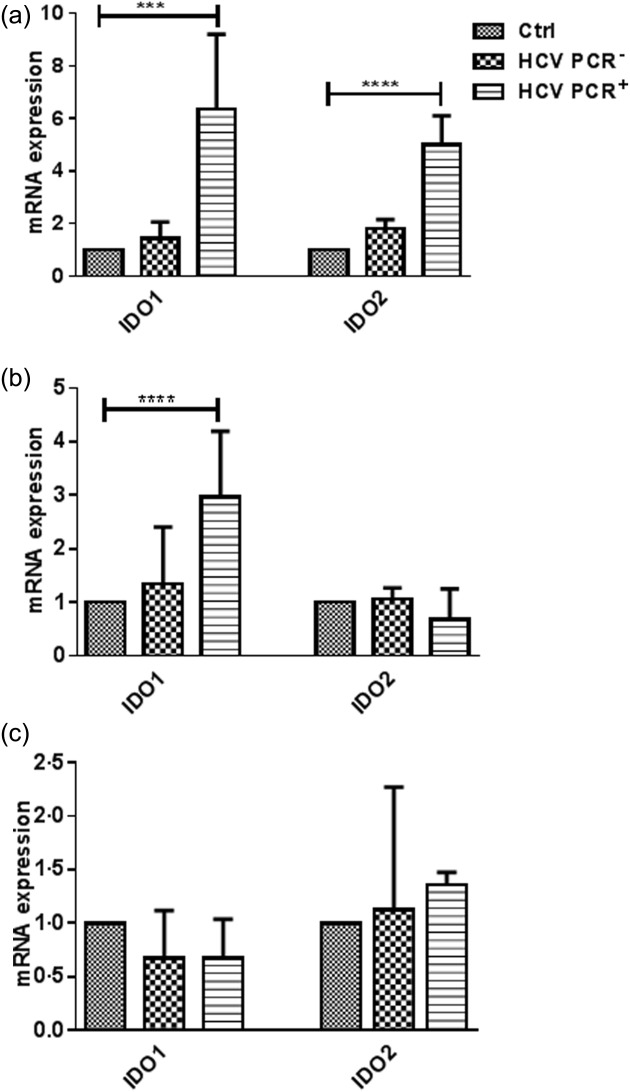
Comparison of the indolamine2,3-dioxygenase 1 (IDO1) and -2 expression between healthy volunteers (Ctrl), recovered hepatitis C virus (HCV) [HCV polymerase chain reaction (PCR)–] and chronic (HCV PCR+) HCV patients in monocytes (a), myeloid dendritic cells (mDCs) (b) and plasmacytoid DCs (pDCs) (c). The change in gene expression is calculated in comparison to the values of the Ctrl monocytes. Results are shown as median value and interquartile range. Significant difference is shown as ***P < 0·001, ****P < 0·0001.
Fig 3.
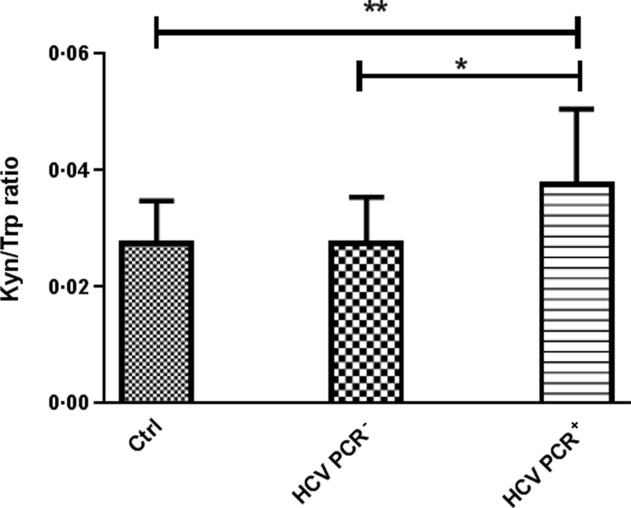
The kynurenine/tryptophan (Kyn/Tryp) ratio in the serum of healthy subjects (Ctrl), recovered hepatitis C virus (HCV) [HCV polymerase chain reaction (PCR)–] and chronic (HCV PCR+) HCV patients. Results are shown as median values and interquartile range. Significant difference is shown as *P < 0·05; **P < 0·01.
Maturation of DCs is impaired during chronic HCV infection
If monocytes and DCs from chronically infected HCV patients display markers that are indicative of a tolerogenic phenotype, which is reflected in enhanced Kyn levels in the serum, these cells might be difficult to stimulate into inducing an appropriate immune response in vivo. In order to determine whether or not monocytes from chronically infected HCV patients can be differentiated into DCs and matured monocytes from Ctrl, HCV PCR– and PCR+ subjects were treated with IL-4 and GM-CSF for 3 days, and then matured for 2 days with IL-6, IL-1β, TNF-α and PGE2. DCs derived from healthy or HCV PCR– individuals displayed the characteristics of fully mature DCs, like a veiled morphology, by microscopy (data not shown). Furthermore, these cells expressed increased levels of CD80, CD83 and CD40, well-known cell-surface markers for matDCs (Fig. 4). However, these morphological features were not observed in matDCs of chronically infected HCV patients. They remained immature and showed the morphology of iDCs. This correlates with the qRT–PCR results, as they showed almost no up-regulation of any maturation marker (Fig. 4). Therefore, iDCs of HCV PCR+ patients could not be stimulated to their mature state using a maturation cocktail of TNF-α, IL-6, IL-1β and PGE2 ex vivo.
Fig 4.
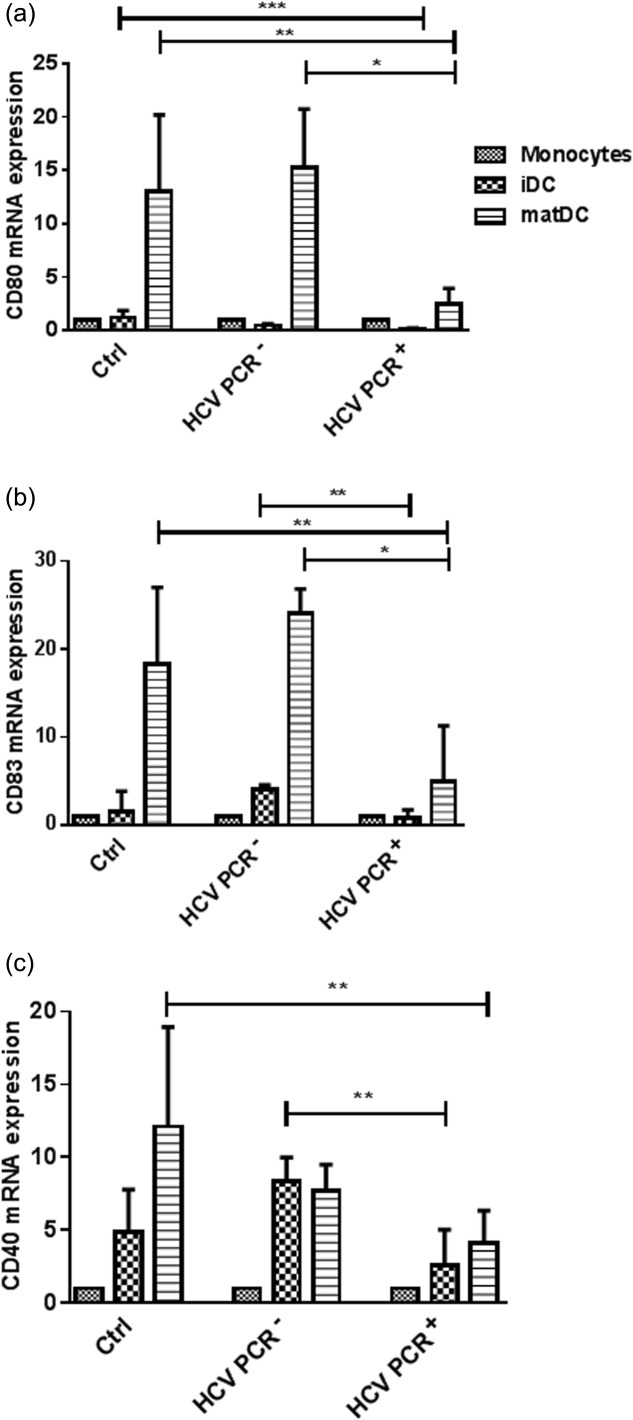
Maturation of dendritic cells (DCs) from healthy volunteers (Ctrl), recovered hepatitis C virus (HCV) [HCV polymerase chain reaction (PCR)–] and chronic (HCV PCR+) HCV patients. Monocytes were differentiated into immature DCs (iDCs) with granulocyte–macrophage colony-stimulating growth factor (GM-CSF) and interleukin (IL)-4, and matured with a cocktail of tumour necrosis factor (TNF)-α, IL-6, IL-1β and prostaglandin E2 (PGE2). The change in gene expression of CD80 (a), CD83 (b) and CD40 (c) is calculated in comparison to the values of the Ctrl monocytes. Results are shown as median values and interquartile range. Significant difference is shown as *P < 0·05; **P < 0·01; ***P < 0·001.
A comparison between monocytes, iDCs and matDCs revealed that the IDO1 expression was higher in monoytes from HCV PCR+ patients (P < 0·001), as well as in matDCs from both HCV PCR+ (P < 0·01) and PCR– (P < 0·05) patients, in comparison to those from healthy subjects (Fig. 5a). The up-regulation of IDO1 in matDCs seemed to be similar in all three groups, because HCV PCR– and HCV PCR+ patients already began with a higher IDO1 level in monocytes. Therefore, this indicates that chronic HCV patients show the highest IDO1 expression in an absolute manner when compared to healthy controls. The IDO2 expression revealed a similar pattern in monocytes, with a significant difference in expression between Ctrl and HCV PCR+ patients (P < 0·0001; Fig. 5b), but no difference between the IDO2 level in matDCs; no IDO2 mRNA was detected in iDCs (data not shown).
Fig 5.
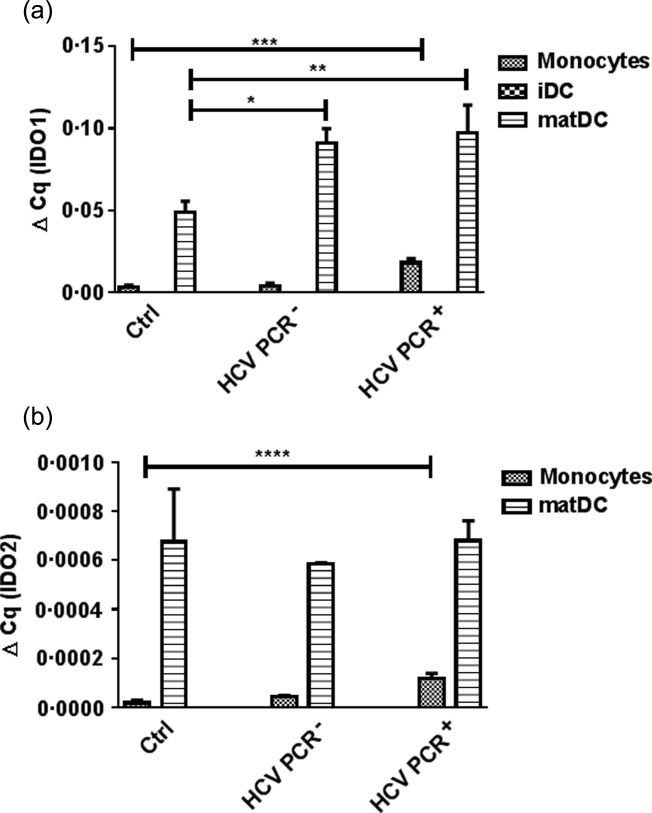
CD14+ monocytes, isolated from healthy individuals (Ctrl), recovered hepatitis C virus (HCV) [HCV polymerase chain reaction (PCR)–] and chronic (HCV PCR+) HCV patients were differentiated with granulocyte–macrophage colony-stimulating growth factor (GM-CSF) and interleukin (IL)-4 for 3 days and matured with a cocktail of tumour necrosis factor (TNF)-α, IL-6, IL-1β and prostaglandin E2 (PGE2). The gene expression of indolamine2,3-dioxygenase 1 (IDO1) (a) and -2 (b) was measured by quantitative reverse transcription–polymerase chain reaction (qRT–PCR), and analysed against β-actin. Results are shown as median values and interquartile range. Significant difference is shown as *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
IDO1 expression is inhibited by addition of L-1-mT
As the expression of IDO1 was increased most significantly in monocytes from HCV PCR+ patients, we studied its expression in monocytes infected ex vivo with HCV. Peripheral blood CD14+ monocytes were infected with an adapted mutant of HCV JFH-1, JFH-1T at an MOI of 0·1. Infection was confirmed using RT–PCR and Western blot analysis. Two or 5 days after infection and extensive washing, RT–PCR revealed the presence of both NS5A and positive-strand RNA, but no negative-strand RNA (Fig. 6b,c) in all samples. This indicates that HCV RNA is actually on or in the cells, but there is no detectable HCV replication. Negative-strand RNA was detected in Huh7·5 cells infected with JFH-1T (MOI 0·1), which confirms the validity of the assay (Fig. 6a), although this does not exclude the possibility that very low copy numbers might have been present. A Western blot probed with NS5A-specific antibodies showed that the HCV RNA entered the monocytes and expresses NS proteins (Fig. 6d), and this was confirmed further by detection of NS5A in monocytes and Huh7·5 cells by immunofluorescence (Fig. 6e).
Fig 6.
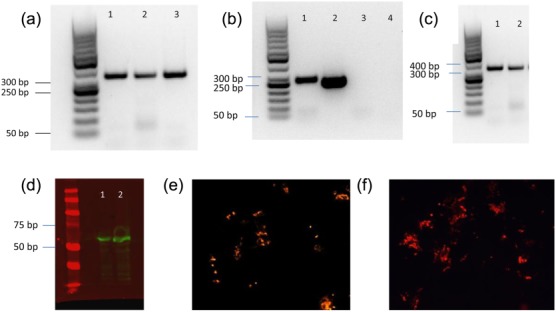
Verification of hepatitis C virus (HCV) infection of monocytes. (a) HCV infection of Huh7·5 cells with JFH-1T [multiplicity of infection (MOI) 0·1]. Cells were analysed by reverse transcription–polymerase chain reaction (RT–PCR). Negative-strand HCV RNA 2 days after infection (lane 1), 3 days after infection (lane 2) and 5 days after infection (lane 3). (b) HCV infection of monocytes 2 and 5 days after inoculation with JFH-1T (MOI 0·1). Positive-strand HCV RNA was analysed by RT–PCR 2 days after infection (lane 1) and 5 days after infection (lane 2) and negative-strand HCV RNA 2 days after infection (lane 3) and 5 days after infection (lane 4) in mature dendritic cells (DCs). (c) HCV NS5a RNA identified by RT–PCR in monocytes. (d) Western blot of NS5a expression in JFH-1T-infected monocytes after 2 (lane 1) and 5 (lane 2) days. (e) HCV-NS5a detected in JFH-1T-infected monocytes using fluorescent microscopy (×20). (f) HCV-NS5a in JFH-1T-infected Huh7·5 cells as positive control (×20).
As was found for chronic HCV patients, monocytes infected with HCV significantly up-regulated the expression of IDO1 (P < 0·0001) and IDO2 (P < 0·001); however, IDO1 and -2 expression was reduced by treatment of the HCV-infected monocytes with 1-mT (Fig. 7). IDO1 protein production and reduction by 1-mT in in-vitro HCV-infected monocytes were analysed by flow cytometry. The gating strategy is shown in Fig. 8a,b. The number of HCV-infected monocytes – as indicated by NS5A protein expression (Fig. 8c) – that express IDO1 was increased significantly (P < 0·01), whereas the addition of 1-mT reversed this effect (P < 0·01) (Fig. 8d).Treatment with 1-mT did not affect NS5A expression significantly.
Fig 7.
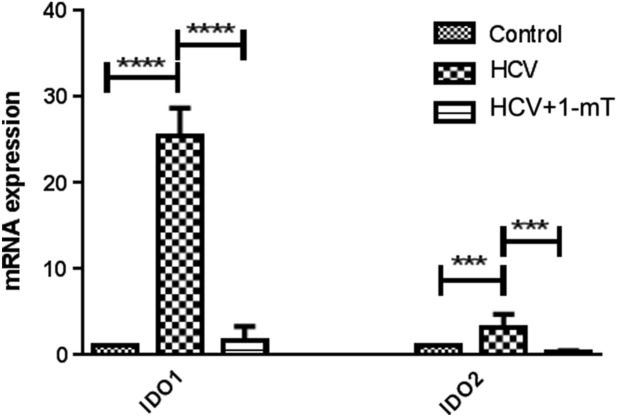
Monocytes, isolated from healthy individuals, were transduced with hepatitis C virus (HCV) JFH-1T for 48 h, and then treated with levo-1-methyl tryptophan (L-1-mT) or left untreated for 5 days. (A). Gene expression of indolamine2,3-dioxygenase 1 (IDO1) and -2 was measured by quantitative reverse transcription–polymerase chain reaction (qRT–PCR). mRNA expression is compared to uninfected monocytes. Significant difference is shown as ***P < 0·001; ****P < 0·0001.
Fig 8.
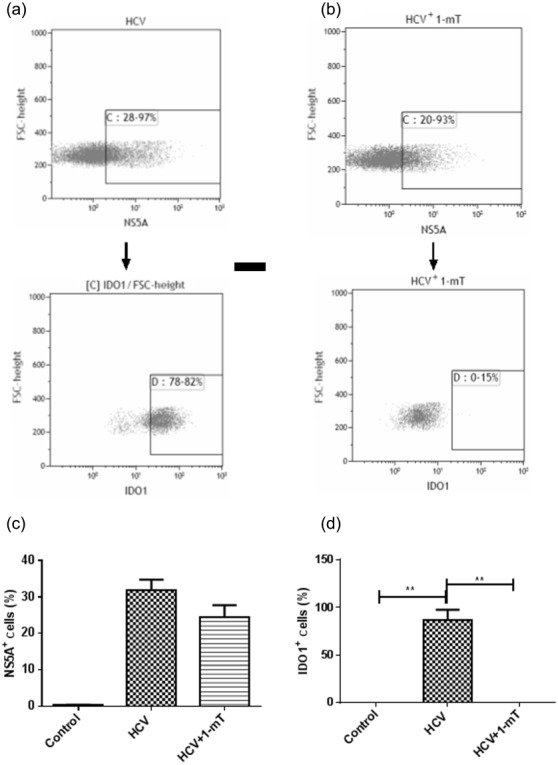
Monocytes, isolated from healthy individuals, were transduced with JFH-1T for 48 h, and then treated with levo-1-methyl tryptophan (L-1-mT) or left untreated for 5 days. Sequential gating strategy for analysis of NS5A+ and then indolamine2,3-dioxygenase 1 (IDO-1)+ cells in (a) HCV JFH-1T-infected and (b) JFH-1T-infected L-1-mT-treated monocytes. The percentage of NS5A (c) and IDO-1 (d)-positive cells in control monocytes, JFH-1T-infected monocytes and JFH-1T-infected L-1-mT-treated monocytes was enumerated by flow cytometry. Results are shown as median values and interquartile range. Significant difference is shown as **P <0·01.
Impaired maturation of HCV-infected monocyte-derived iDCs is reversed by 1-L-mT treatment
In addition to the elevated IDO1 expression in monocytes, the maturation of monocyte-derived iDCs in HCV+ patients was inhibited significantly, which leads to the question of whether or not maturation is possible when using the IDO inhibitor 1-mT. To investigate this, monocytes were infected with HCV in the absence or presence of 1-mT, and the maturation was examined by analysing the markers CD40, CD80 and CD83. Fig. 9 shows that the maturation of monocyte-derived iDCs after HCV infection was clearly inhibited, as the expression of all three markers was decreased strongly in comparison to uninfected cells. Addition of 1-mT reversed this effect. Taken together, these data suggest that L-1-mT not only has a strong modulatory effect on the expression of IDO1 and IDO2, but also leads to a restored maturation of HCV-infected monocyte-derived iDCs.
Fig 9.
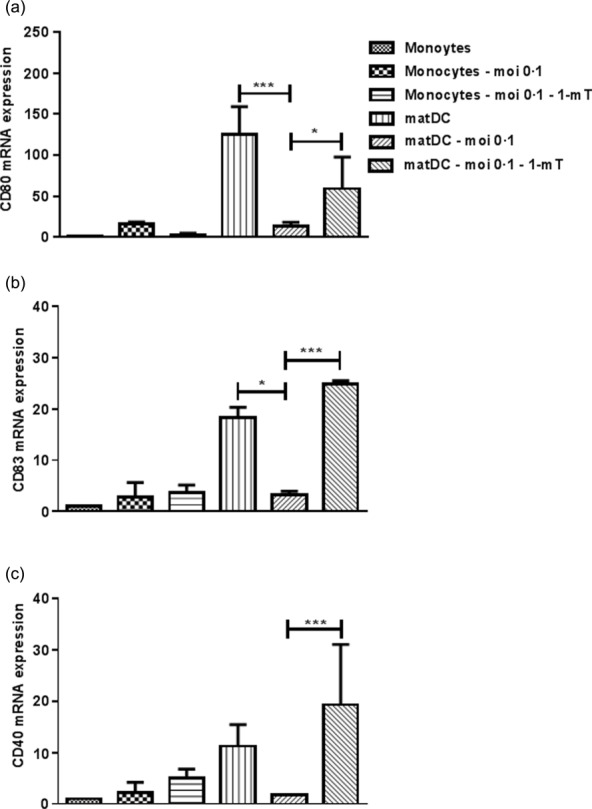
Monocytes, isolated from healthy individuals, were transduced with JFH-1T for 48 h, and then treated with granulocyte–macrophage colony-stimulating growth factor (GM-CSF) and interleukin (IL)-4, in the absence or presence of levo-1-methyl tryptophan (L-1-mT). Three days later a maturation cocktail of tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β and prostaglandin E2 (PGE2) was added. Gene expression of CD80 (a), CD83 (b) and CD40 (c) was measured by quantitative reverse transcription–polymerase chain reaction (qRT–PCR). mRNA expression is compared to uninfected monocytes. Results are shown as median value and interquartile range. Significant difference is shown as *P < 0·05; ***P < 0·001.
Discussion
In this study we demonstrated that (i) the IDO1 mRNA level was enhanced in mDCs and monocytes of chronically infected HCV patients in comparison to recovered HCV patients and healthy individuals, and IDO2 mRNA expression was higher in monocytes from PCR+ HCV patients; (ii) a significantly increased serum Kyn/Trp ratio in the serum of the HCV PCR+ patients was in accordance with the elevated IDO1 expression; (iii) infection with JFH-1T, which was modified with coding mutations in E2, p7 and NS2 18 ex vivo, also resulted in up-regulation of IDO1 and IDO2 mRNA expression, and this effect could be reversed by blocking IDO1 with L-1-mT; and (iv) maturation of monocyte-derived DCs from chronically infected HCV patients, as well as monocyte-derived DCs infected ex vivo with HCV, was impaired, and this was also reversed by 1-mT treatment. This information extends our understanding of the effects of HCV on monocytes and DC subsets and may provide a target for HCV therapy.
PBMCs constitute a critical part of the immune system and contain lymphocytes including natural killer (NK), T and B cells, monocytes, DCs and macrophages. We found that the number of PBMCs is reduced significantly in chronic and spontaneously recovered HCV patients; thus, a decline in PBMC numbers upon HCV infection appear to be irreversible. Among these, the monocyte and mDC numbers appeared to be reduced, but were not affected significantly by chronic HCV infection, whereas pDC numbers were lower in HCV PCR+ patients. It is generally known that both DC subsets may be reduced in HCV infection 20–23 and are thought to correlate with the severity of the disease, while mDCs and pDC numbers are increased significantly in the liver of chronic infected patients 24. Furthermore, Shiina and Rehermann 25 reported that cell culture-produced HCV inhibited IFN-expression through Toll-like receptor (TLR)-9, indicating a functional impairment. However, in other studies, pDCs from chronic HCV patients seemed phenotypically and functionally not different from those of healthy subjects in vivo 25–27, although they occurred in lower numbers 28. Both mDCs and pDCs also showed a decrease in their cell-surface molecules and co-stimulatory cytokine production as well as their allostimulatory activity 23, which leads to an incapacity to prime T cells and to a much lower anti-viral immune response to HCV.
The IDO1 mRNA levels were enhanced in mDCs and monocytes of chronically infected HCV patients in comparison to recovered HCV patients and healthy individuals. The expression of IDO1 is also changed in HIV patients 9,10, and even ex vivo HIV-infected macrophages produce high IDO levels 8. Furthermore, a recent study revealed that the degree of liver inflammation and fibrosis in HCV patients is correlated with an enhanced IDO1 activity in DCs 12. Conversely, the IDO1 level was found to be down-regulated in cultured macrophages from chronic HCV patients 13. An association between inflammation and IDO activity in plasma has been reported repeatedly, but the responsible cell types have not yet been identified. It appears that HCV efficiently enables IDO over-expression in an inflammatory milieu, as recovered HCV patients have the same IDO expression pattern as healthy individuals.
The decline of IDO1 and absence of IDO2 expression in iDCs in all three groups is related to the fact that IDO expression depends upon the maturation state of the cells. The expression of IDO is associated with different cell surface markers, i.e. CD80, CD86 or PDL-1/2, which are expressed in iDCs only to a low extent. Conversely, it is also possible that tolerogenic DCs were triggered to increase CD80/CD86 expression, which leads to reverse signalling and mediation of an IDO-dependent environment 29. The increased serum Kyn/Trp ratio in the plasma of the HCV PCR+ patients was in accordance with the elevated IDO1 expression. This might or might not be significant biologically. However, cancer patients show enhanced IDO activity and therefore an increased Tryp degradation which seems to be involved in the disease progression 30,31. Furthermore, the level of increase in Kyn/Trp ratio may depend upon the cell type. One possibility is that the elevated IDO1 expression is a strategy of HCV to prevent or weaken the innate defence mechanism of DCs and, as a result, to suppress the immune response to HCV antigens; thus, the use of 1-mT as selective IDO1 inhibitor might be promising as a treatment of the infection. Our data provide evidence that L-1-mT inhibits the IDO1 activity effectively in HCV-infected monocytes ex vivo, and simultaneously supports former studies 32,33. This may suggest a potential strategy to optimize the functional properties of DCs by treatment with immunomodulatory compounds or L-1-mT. It would also be feasible to use the IDO inhibitor in vivo. Different studies have already shown that mice receiving 1-mT during tumour growth developed tumours more slowly than the control group 34,35. This may add some time to the life of cancer patients. However, the question remains of whether or not the toxicity of the IDO inhibitor is acceptable in HCV patients and the benefits outweigh the safety issues, or if other treatments are more acceptable at this stage.
Because monocytes were infected with HCV, as proved by the expression of NS5A, we could show that these cells express more IDO1 than uninfected monocytes. After the addition of 1-mT the expression of IDO1 was down-regulated. The mechanism as to how 1-mT affects the IDO1 mRNA expression is still unclear, and needs to be investigated further. However, others have reported effects of 1-mT that are independent of IDO1, suggesting that it has another function apart from targeting IDO activity 34.
Infection of monocytes with JFH-1T ex vivo resulted in up-regulation of IDO1, similar to the observations in chronic HCV patients. PBMCs have been studied in terms of whether HCV uses them simply as a reservoir or if the virus can actually infect and replicate in PBMCs. Many studies provide evidence of HCV in macrophages, B and T cells 36–39. HCV infection and replication in PBMCs might contribute to the development of resistance and impaired clearance of the virus. However, there is still no consensus on whether HCV can infect, replicate or express its proteins in human DCs. In a recent study on the effects of HCV on mDCs and pDCs, the observed positive strand RNA was interpreted as cell-associated virus or virus adherent to the surface that was resistant to washing 40. Furthermore, JFH-1 and JFH-1T clones were shown to be unable to establish a productive infection in human primary T cells, PBMCs and T cell lines 41. Because, in our study, the monocytes were infected in vitro with an initial MOI of 0·1 progressing to ∼30% of the monocytes expressing NS5A by flow cytometry, it is possible that the virus entered and replicated, but at levels too low to detect. This is most probably the case, as in studies published by Pham et al. 41, nested PCR was used to detect negative-strand PCR products. The other possibility is that there could be many non-infectious virions in the pool that did not contribute to the titre but entered cells and translated viral proteins. Non-infectious virions can possibly enter cells and express proteins, but cannot produce a plaque (foci) on Huh7·5 cells. Claudin-1, also known as CD81, is an entry factor for HCV and expressed by many blood cell types, especially B cells, which may capture HCV from the circulation 42. While HCV may not replicate in DCs, viral RNA might enter the DCs and be transcribed, but perhaps not complete the infectious cycle. We found no evidence of virus replication in HCV-infected monocytes, as no minus-strand HCV RNA was detected; however, we detected NS5a and positive-strand RNA, indicating that HCV entered the cells, and protein was expressed de novo. Opinions diverge and depend upon the definition of infection: if infection requires replication there would be no evidence for infection. However, virus replication may not be necessary for the effects of HCV on monocytes, which might be associated with factors other than replication. For instance, for herpes simplex virus-1 binding of the entry glycoproteins gB, gC and gH/gL to DCs was sufficient for the induction of DC maturation and the associated release of cytokines to effect maturation of DCs, but an interaction between the entry glycoproteins was necessary 43. Similarly, entry of the HCV genome, and de novo protein production appeared to be sufficient to up-regulate IDO1 and -2 expression in monocytes.
Monocyte-derived iDCs from chronic HCV patients did not respond to maturation stimuli and remained in their immature state ex vivo. Our results are in accordance with several reports indicating a deficient maturation during HCV infection 44,45; interestingly, monocyte-derived iDCs from recovered patients showed a similar level of maturation as in healthy subjects. It has been suggested that the defective DC function of chronic HCV patients could be alleviated by increasing the number of DCs or the quantity of maturation stimuli 46. Thus, it is important to elucidate the reason for the lack of DC maturation, as this could lead to impaired T cell function and therefore viral persistence. Inducing and studying functional maturation ex vivo would be a starting point. We proved that the addition of 1-mT leads to a restored maturation of HCV-infected monocytes. Unfortunately, until now there have been no detailed studies on how 1-mT influences cell maturation. However, Agaugué 34 stated that the effect of 1-mT on the functional orientation of DCs depends upon the type of maturation signal, but that its effects on maturation are not correlated with the inhibition of the IDO activity. Overall, as 1-mT can interfere with other pathways and molecules, it seems to be a promising reagent to change the immune response in vivo. The next step would be to analyse how 1-mT intervenes in DC maturation, and especially if maturation of monocyte-derived DCs from chronic HCV patients can also be restored by this inhibitor.
Over-expression of IDO1 has indeed far-reaching consequences: if the essential amino acid Trp, necessary to synthesize proteins and induce proliferation and cell survival, is not present or in a lower amount, T cell activation is inhibited 47. Conversely, the enzyme activation of IDO1 is strictly controlled, as sustained IDO expression would probably have a toxic effect on the immune system. Normally, IDO is induced mainly in response to proinflammatory cytokines or activated T cells 11; furthermore, IFN-γ production or CD80/CD86 ligation are also potent inducers of up-regulation of IDO, although the pathways are still not fully defined 8. It is noteworthy that IDO expression is probably dependent upon the maturation status of the cells. PGE2 is often used in maturation cocktails, mainly together with CD40L, LPS, poly (I:C) or a combination of IL-6, IL-1β and TNF-α. Mature DCs can then be loaded with antigens and activate antigen-specific T cells. PGE2 is important for the maturation of monocytes, in particular migration after stimulation, but is also responsible for the induction of IDO. This would support our findings where, after using a maturation cocktail containing PGE2, the IDO1 mRNA activity was increased in healthy subjects as well as in HCV patients. The same effect was also found for IDO2, but to a much lower extent. Unfortunately, the effects of PGE2 on mature DCs ex vivo are highly controversial. Braun and colleagues 48 stated that no active IDO enzyme activity could be measured after maturation with PGE2. TNF or TLR receptors seemed to be necessary to reactivate IDO. Also, PGE2-matured DCs were not differentiated fully, and no proinflammatory cytokines were produced 49. A recent study by Kraus et al. 50, however, proved that PGE2 is not necessary for the maturation of DCs, but enhances the ability of the cells to stimulate CD4+ and CD8+ T cells. In addition, while the IDO mRNA was up-regulated by PGE2, it was not enough to initiate IDO protein expression; further maturation signals were necessary. The expression of IDO probably requires both PGE2 and maturation signals 51. However, all these experiments were performed ex vivo, so it is still unclear how matDCs behave in vivo. PGE2 is naturally occurring and increased in HCV-expressing cells 52, which indicates that in HCV-infected patients PGE2 could be responsible for an increase of IDO1 in matDCs. Regardless, it is important to specify the inclusion of PGE2 to mature DCs, at least until the complex actions of PGE2 are analysed further and its effect on the IDO activity is clear.
In summary, while IDO1 and -2 expression was increased in the mDCs and monocytes of chronically infected HCV patients, recovered patients showed similar levels as healthy volunteers. In addition, increased IDO1 and -2 expression was found in monocytes from healthy donors infected with JFH-1-HCV ex vivo, and this could be reversed by treatment with 1-MT. These data suggest that IDO inhibitors may be used to treat chronic HCV patients in vivo, in conjunction with current therapies, or to activate DCs from patients ex vivo, such that they can be readministered as an effective DC-based therapeutic vaccine.
Acknowledgments
We would like to thank all of our blood donors as well as Drs. S. Sanche and S. Skinner for their help in patient recruiting. We would also like to thank Dr. Rodney Russell for providing the cell-adapted JFH-1T cDNA clone. None of the authors have competing interests to declare. This study was supported by the Canadian Institutes of Health Research and the Saskatchewan Health Research Foundation. SS was supported by a fellowship from SHRF. This is VIDO manuscript number 705.
Author contributions
S. v. D. L.-v. d. H. and A. L. designed the study; A. L. established the methods for isolation of monocytes, mDCs, pDCs and the in-vitro generation of iDCs and matDCs; S. S. and R. G. performed the experiments; J. A. W. contributed to the in-vitro HCV infections, S. S. and S. v. D. L.-v. d. H. wrote the paper; A. L., R. G. and J. A. W. critically read the paper.
References
- Bialek SR, Terrault NA. The changing epidemiology and natural history of hepatitis C virus infection. Clin Liver Dis. 2006;10:697–715. doi: 10.1016/j.cld.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- McHutchison JG. Understanding hepatitis C. Am J Manag Care. 2004;10:S21–9. [PubMed] [Google Scholar]
- The Medical Letter I. 2014. pp. 5–6. The Medical Letter for Drugs and Therapeutics,
- Liu B, Woltman AM, Janssen HL, Boonstra A. Modulation of dendritic cell function by persistent viruses. J Leukoc Biol. 2009;85:205–14. doi: 10.1189/jlb.0408241. [DOI] [PubMed] [Google Scholar]
- Landi A, Babiuk LA, van Drunen Littel-van den Hurk S. Dendritic cells matured by a prostaglandin E2-containing cocktail can produce high levels of IL-12p70 and are more mature and Th1-biased than dendritic cells treated with TNF-alpha or LPS. Immunobiology. 2011;216:649–62. doi: 10.1016/j.imbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Wieder E. Dendritic cells: a basic review. International Society for Cellular Therapy; 2003. [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra6. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrea E, Riezu-Boj JI, Gil-Guerrero L, et al. Upregulation of indoleamine 2,3-dioxygenase in hepatitis C virus infection. J Virol. 2007;81:3662–6. doi: 10.1128/JVI.02248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashitani K, Kanto T, Kuroda S, et al. Association of enhanced activity of indoleamine 2,3-dioxygenase in dendritic cells with the induction of regulatory T cells in chronic hepatitis C infection. J Gastroenterol. 2013;48:660–70. doi: 10.1007/s00535-012-0667-z. [DOI] [PubMed] [Google Scholar]
- Cozzi A, Zignego AL, Carpendo R, et al. Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J Viral Hepat. 2006;13:402–8. doi: 10.1111/j.1365-2893.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- Lob S, Konigsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153–7. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Liao J, Villella J, et al. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol Immunother. 2012;61:2013–20. doi: 10.1007/s00262-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi A, Babiuk LA, Littel-van den Hurk SV. High transfection efficiency, gene expression, and viability of monocyte-derived human dendritic cells after nonviral gene transfer. J Leukoc Biol. 2007;82:849–60. doi: 10.1189/jlb.0906561. [DOI] [PubMed] [Google Scholar]
- Song H, Li J, Shi S, Yan L, Zhuang H, Li K. Thermal stability and inactivation of hepatitis C virus grown in cell culture. Virol J. 2010;7:40. doi: 10.1186/1743-422X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RS, Meunier JC, Takikawa S, et al. Advantages of a single-cycle production assay to study cell culture-adaptive mutations of hepatitis C virus. Proc Natl Acad Sci USA. 2008;105:4370–5. doi: 10.1073/pnas.0800422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castet V, Fournier C, Soulier A, et al. Alpha interferon inhibits hepatitis C virus replication in primary human hepatocytes infected in vitro. J Virol. 2002;76:8189–99. doi: 10.1128/JVI.76.16.8189-8199.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicinnati VR, Kang J, Sotiropoulos GC, et al. Altered chemotactic response of myeloid and plasmacytoid dendritic cells from patients with chronic hepatitis C: role of alpha interferon. J Gen Virol. 2008;89:1243–53. doi: 10.1099/vir.0.83517-0. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Asabe S, Wieland S, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431–6. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EJ, O'Farrelly C. The affect of chronic hepatitis C infection on dendritic cell function: a summary of the experimental evidence. J Viral Hepat. 2011;18:601–7. doi: 10.1111/j.1365-2893.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- Losikoff PT, Self AA, Gregory SH. Dendritic cells, regulatory T cells and the pathogenesis of chronic hepatitis C. Virulence. 2012;3:610–20. doi: 10.4161/viru.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitani H, Shimizu Y, Murata H, Higuchi K, Watanabe A. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronic liver diseases. J Hepatol. 2002;36:734–41. doi: 10.1016/s0168-8278(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–95. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]
- Decalf J, Fernandes S, Longman R, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007;204:2423–37. doi: 10.1084/jem.20070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H, Laird ME, Saleh R, et al. Circulating plasmacytoid dendritic cells in acutely infected patients with hepatitis C virus genotype 4 are normal in number and phenotype. J Infect Dis. 2010;202:1671–5. doi: 10.1086/656777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Decalf J, Pol S. Plasmacytoid dendritic cells move down on the list of suspects: in search of the immune pathogenesis of chronic hepatitis C. J Hepatol. 2008;49:1069–78. doi: 10.1016/j.jhep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Von Bubnoff D, Scheler M, Wilms H, Fimmers R, Bieber T. Identification of IDO-positive and IDO-negative human dendritic cells after activation by various proinflammatory stimuli. J Immunol. 2011;186:6701–9. doi: 10.4049/jimmunol.1003151. [DOI] [PubMed] [Google Scholar]
- de Jong RA, Nijman HW, Boezen HM, et al. Serum tryptophan and kynurenine concentrations as parameters for indoleamine 2,3-dioxygenase activity in patients with endometrial, ovarian, and vulvar cancer. Int J Gynecol Cancer. 2011;21:1320–7. doi: 10.1097/IGC.0b013e31822017fb. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Suda T, Furuhashi K, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2010;67:361–5. doi: 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–4. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Tone S, Kanouchi H, Miyawaki C, Ono S, Minatogawa Y. Transcriptional regulation of indoleamine 2,3-dioxygenase (IDO) by tryptophan and its analogue: down-regulation of the indoleamine 2,3-dioxygenase (IDO) transcription by tryptophan and its analogue. Cytotechnology. 2007;54:107–13. doi: 10.1007/s10616-007-9081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agaugue S, Perrin-Cocon L, Coutant F, Andre P, Lotteau V. 1-Methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J Immunol. 2006;177:2061–71. doi: 10.4049/jimmunol.177.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T, Ino K, Kajiyama H, et al. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol. 2009;115:185–92. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Revie D, Salahuddin SZ. Human cell types important for hepatitis C virus replication in vivo and in vitro: old assertions and current evidence. Virol J. 2011;8:346. doi: 10.1186/1743-422X-8-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Thimme R. T cell responses in hepatitis C: the good, the bad and the unconventional. Gut. 2012;61:1226–34. doi: 10.1136/gutjnl-2011-300620. [DOI] [PubMed] [Google Scholar]
- Revie D, Salahuddin SZ. Role of macrophages and monocytes in hepatitis C virus infections. World J Gastroenterol. 2014;20:2777–84. doi: 10.3748/wjg.v20.i11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi M, Ito T, Uchikoshi M, et al. Infection of B cells with hepatitis C virus for the development of lymphoproliferative disorders in patients with chronic hepatitis C. J Med Virol. 2009;81:619–27. doi: 10.1002/jmv.21388. [DOI] [PubMed] [Google Scholar]
- Liang H, Russell RS, Yonkers NL, et al. Differential effects of hepatitis C virus JFH1 on human myeloid and plasmacytoid dendritic cells. J Virol. 2009;83:5693–707. doi: 10.1128/JVI.02671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TN, MacParland SA, Mulrooney PM, Cooksley H, Naoumov NV, Michalak TI. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J Virol. 2004;78:5867–74. doi: 10.1128/JVI.78.11.5867-5874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marukian S, Jones CT, Andrus L, et al. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology. 2008;48:1843–50. doi: 10.1002/hep.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske A, Pollara G, Krummenacher C, Katz DR, Chain BM. Glycoprotein-dependent and TLR2-independent innate immune recognition of herpes simplex virus-1 by dendritic cells. J Immunol. 2008;180:7525–36. doi: 10.4049/jimmunol.180.11.7525. [DOI] [PubMed] [Google Scholar]
- Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–6. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- Saito K, Ait-Goughoulte M, Truscott SM, et al. Hepatitis C virus inhibits cell surface expression of HLA-DR, prevents dendritic cell maturation, and induces interleukin-10 production. J Virol. 2008;82:3320–8. doi: 10.1128/JVI.02547-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AJ,SemperAE, Libri NA, Rosenberg WM. Monocyte-derived dendritic cell function in chronoc hepatitis C is impaired at physiological numbers of dendritic cells. Clin Exp Immunol. 2007;148:494–500. doi: 10.1111/j.1365-2249.2007.03367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–81. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzinger M, Jurgens B, Hainz U, et al. Ambivalent effects of dendritic cells displaying prostaglandin E2-induced indoleamine 2,3-dioxygenase. Eur J Immunol. 2012;42:1117–28. doi: 10.1002/eji.201141765. [DOI] [PubMed] [Google Scholar]
- Krause P, Singer E, Darley PI, Klebensberger J, Groettrup M, Legler DF. Prostaglandin E2 is a key factor for monocyte-derived dendritic cell maturation: enhanced T cell stimulatory capacity despite IDO. J Leukoc Biol. 2007;82:1106–14. doi: 10.1189/jlb.0905519. [DOI] [PubMed] [Google Scholar]
- von Bergwelt-Baildon MS, Popov A, Saric T, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–37. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- Waris G, Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: role of prostaglandin E2 in RNA replication. J Virol. 2005;79:9725–34. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


