Abstract
Infants exposed to maternal HIV-1 provide an opportunity to assess correlates of HIV-1-specific interferon (IFN)-γ responses and may be informative in the development of HIV-1 vaccines. HIV-1-infected women with CD4 counts 200–500 cells/mm3 were randomized to short-course zidovudine/nevirapine (ZDV/NVP) or highly active anti-retroviral therapy (HAART) between 2003 and 2005. Maternal plasma and breastmilk HIV-1 RNA and DNA were quantified during the first 6–12 months postpartum. HIV-1 gag peptide-stimulated enzyme-linked immunospot (ELISPOT) assays were conducted in HIV-1-exposed, uninfected infants (EU), and correlates were determined using regression and generalized estimating equations. Among 47 EU infants, 21 (45%) had ≥1 positive ELISPOT result during follow-up. Infants had a median response magnitude of 177 HIV-1-specific spot-forming units (SFU)/106 peripheral blood mononuclear cells (PBMC) [interquartile range (IQR) = 117–287] directed against 2 (IQR = 1–3) gag peptide pools. The prevalence and magnitude of responses did not differ by maternal anti-retroviral (ARV) randomization arm. Maternal plasma HIV-1 RNA levels during pregnancy (P = 0·009) and breastmilk HIV-1 DNA levels at 1 month (P = 0·02) were associated with a higher magnitude of infant HIV-1-specific ELISPOT responses at 1 month postpartum. During follow-up, concurrent breastmilk HIV-1 RNA and DNA (cell-free virus and cell-associated virus, respectively) each were associated positively with magnitude of infant HIV-1-specific responses (P = 0·01). Our data demonstrate the importance of antigenic exposure on the induction of infant HIV-1-specific cellular immune responses in the absence of infection.
Keywords: HIV-1-EU, interferon gamma, paediatric immunity
Introduction
Globally, an estimated 370 000 children are newly infected with HIV-1 each year, the majority as a result of mother-to-child transmission 1. Infants born to HIV-1-infected mothers consume large volumes of breastmilk containing HIV-1, but despite this exposure ∼80% of these breastfeeding infants remain uninfected 2. It is possible that these infants escape infection due to natural resistance, either through genetics, innate immunity or acquired immunity, which protects them from acquiring HIV-1.
The discovery of HIV-1-specific cellular immune responses in individuals exposed to HIV-1 but who remain uninfected (EU) has been of particular interest, as adaptive immunity may protect against acquisition of infection. Among HIV-1-infected adults, HIV-1-specific cellular immune responses are associated with control of viral replication and viral clearance 3,4 and slower HIV-1 disease progression 5–11. In the pre-anti-retroviral era, waning of these responses correlated with disease progression 12–14. HIV-1-specific cellular immune responses have been reported in varied HIV-1 EU populations, including commercial sex workers 15–17, HIV-1-discordant couples 18–20 and infants born to HIV-1-infected women 21–23. CD4+ and CD8+ HIV-1-specific responses have been observed in EU infants, with prevalence ranging from 3 to 56% 24–27 and 0 to 47% 22,27–31, respectively, resulting in controversy around the detection of these responses and their potential protective role. However, vaccine development relies upon understanding the induction of immune responses, and so it remains important to identify the correlates of presence and magnitude of HIV-1-specific immune responses in EU individuals. Historic cohorts of infants of HIV-1-infected mothers who breastfeed offer a natural human challenge study, because they are exposed continuously to HIV-1 from their mothers. With both viral source and recipient identifiable, mother–infant cohorts provide a unique opportunity to investigate correlates of infant cellular immune responses.
We hypothesized that factors associated with exposure to increased levels of HIV-1 antigen would increase induction of HIV-1-specific immune responses. To test our hypothesis, we compared the prevalence, magnitude and breadth of infant HIV-1-specific T cell responses between breastfeeding HIV-1 EU infants born to women randomized to short-course zidovudine/nevirapine (ZDV/NVP) or highly active anti-retroviral therapy (HAART) [ZDV/lamivudine (3TC)/NVP], both shown to impact the levels of HIV-1 cell-free virus exposure in breastfeeding infants. Additionally, we examined maternal systemic and breastmilk HIV-1 viral levels as correlates of infant HIV-1-specific responses.
Materials and methods
Study population and sample collection
This study was a Phase II clinical trial conducted at the Mathare North City Council Clinic in Nairobi, Kenya between 2003 and 2005 and was approved by the Institutional Review Boards of the University of Washington and Kenyatta National Hospital (ClinicalTrials.gov number, NCT00167674). Methods for recruitment, randomization and follow-up for this trial, along with results of the primary study, have been described previously 32,33. Briefly, 60 HIV-1-positive pregnant women and their infants were followed for 1 year postpartum. Enrolled women had CD4 cell counts between 200 and 500 cells/mm3. At 34 weeks gestation, women were randomized to either ZDV/NVP or HAART. In the ZDV/NVP arm, women received ZDV from 34 weeks gestation until delivery and a single dose of NVP at labour, and infants were administered a single dose of NVP within 72 h of delivery, in accordance with Kenya national guidelines at the time. In the HAART arm, ZDV, 3TC and NVP were given to women at 34 weeks gestation until 6 months postpartum. Also as per the 2003–05 national guideline, all women were advised to stop breastfeeding 6 months after delivery, and women in the HAART arm were advised to discontinue taking HAART after breastfeeding cessation.
Maternal blood specimens were collected at 32 weeks gestation, within 2 days of delivery, then 2 weeks, 1 month and every 3 months after delivery for HIV-1 RNA levels. Breastmilk was obtained one to three times per week for the first month, then 3 and 6 months postpartum for breastmilk cell-free HIV-1 RNA and cell-associated HIV-1 DNA levels. Blood samples collected from infants at delivery and then at 1, 3, 6, 9 and 12 months of age were used to determine HIV-1 infection status and for enzyme-linked immunospot (ELISPOT) assays.
Laboratory methods
The processing of breastmilk specimens has been described elsewhere 34. Briefly, breastmilk samples were separated into supernatant and cells after discarding the lipid layer. Plasma and breastmilk HIV-1 RNA levels were determined using the Gen-Probe HIV-1 viral load assay (Gen-Probe Inc., San Diego, CA, USA), as described previously 34,35, with a lower limit of detection of 200 copies/ml and 100 copies/ml for plasma and breastmilk samples, respectively. Infant filter paper blood specimens were tested to determine HIV-1 status by HIV-1 DNA polymerase chain reaction (PCR) 36. HIV-1 DNA from breastmilk cells was extracted using the QIAmp DNA mini kit (Qiagen, Valencia, CA, USA) and quantified using real-time PCR as described previously 33,34. The lower limit of detection was one copy/reaction, and HIV-1 DNA levels were normalized to the number of cells tested (number of β-actin copies). CD4 counts were measured from blood samples using flow cytometry (FACScan; Becton Dickinson, San Jose, CA, USA).
Infant HIV-1 gag-specific T cell responses were assessed using an established interferon (IFN)-γ ELISPOT assay protocol on fresh peripheral blood mononuclear cells (PBMC). Briefly, 96-well nitrocellulose plates (Millipore, Billerica, MA, USA) were coated with 7·5 μg monoclonal antibody to IFN-γ (Mabtech, Stockholm, Sweden) for 2 h at 37 °C. Antibody was removed by washing the plates with RPMI-1640 and then blocked with R10 (RPMI-1640 containing 20 mM L-glutamine with 10% fetal calf serum) (all Sigma, St Louis, MO, USA) for 30 min at room temperature. Freshly isolated infant PBMC were then added in duplicate with 2 × 105 PBMC/well. Each infant PBMC sample was stimulated with R10 media alone as a negative control, 10 μg/ml phytohaemagglutinin (PHA) as a positive control or 20 μg/ml HIV-1 gag peptide pools. Seven peptide pools of overlapping 15-mers spanning HIV-1 p55 were derived from the clade A consensus sequence and were provided by the NIH AIDS Research and Reference Reagent program. Cells were stimulated overnight in a humidified incubator at 37 °C with 5% CO2 and were removed from the plates by washing with phosphate-buffered saline (PBS) containing 0·05% Tween-20. Biotinylated anti-IFN-γ antibody was applied for 3 h at room temperature, followed by washing, and then streptavidin alkaline phosphate (Mabtech) was added for 1·5 h at room temperature. After washing, alkaline phosphatase (Mabtech) was added for approximately 10 min or until spot-forming units (SFU) were visible in the PHA wells. The reaction was stopped by washing the plates under running water, and plates were dried overnight before being read on a CTL ImmunoSpot Core Analyzer (Cellular Technology Ltd, Shaker Heights, OH, USA).
Statistical methods
HIV-1-specific SFU was defined as the average number of spots in duplicate wells minus the background response (defined as the mean SFU in the negative control wells). ELISPOT responses were considered positive if experimental wells had ≥50 HIV-1-specific SFU/106 PBMC and more than twice the background response. Assays were excluded if PHA wells had <100 SFU/106 PBMC. Prevalence, breadth and magnitude of ELISPOT responses were evaluated by (1) including all valid assays or (2) excluding assays in which the background SFU >100/106 PBMC. Infants were defined as being positive responders if they had ≥1 peptide pool with a positive response. HIV-1 gag-specific immune responses were examined both as a dichotomous (using the predefined cut-offs above) and continuous (magnitude of responses) variable. Magnitude of responses was defined as the summed magnitude of HIV-1-specific SFU/106 PBMC across all peptide pools.
Viral loads below the limit of detection were recoded to the mid-point between zero and the limit of detection for that assay. Because a high percentage (55%) of breastmilk HIV-1 RNA assays were below the limit of detection, breastmilk HIV-1 RNA was modelled as a dichotomized covariate (detected/not detected). Infant HIV-1-specific IFN-γ responses were compared between the two randomization groups at each visit. ELISPOT prevalence was compared using Pearson's χ2 tests or Fisher's exact tests, and magnitude and breadth of responses were compared using Mann–Whitney U-tests. Linear regression was used to assess correlates of magnitude of ELISPOT HIV-1-specific responses (background subtracted) in all infants at specific time-points. Generalized estimating equation (GEE) models with a Poisson link and exchangeable correlation structure were used to examine associations between maternal viral load and infant ELISPOT responses over time. All regression models were adjusted for treatment arm and constructed with robust standard errors. Sensitivity analyses were performed in which samples with undetectable HIV-1 DNA levels and fewer than 10 000 cells tested were excluded from regression models. stata version 11.2 (College Station, TX, USA) was used for all analyses.
Results
Study population and characteristics
Of 60 mother–infant pairs, three infants acquired HIV-1 during follow-up and were excluded from the ELISPOT analyses; 47 (78%) infants had ELISPOT data at ≥1 visit. Among the selected mother–infant pairs, median age and CD4 cell count at 32 weeks gestation did not differ between trial arms (Table1). While plasma HIV-1 RNA levels were similar between the two groups at 32 weeks gestation, women randomized to ZDV/NVP had significantly higher plasma viral loads (∼2 log10 copies/ml higher) from delivery to 6 months postpartum compared to women randomized to HAART 33. Furthermore, more women in the ZDV/NVP arm had detectable breastmilk cell-free HIV-1 RNA levels at 1 month postpartum versus women in the HAART arm (82 versus 29%, P < 0·001). In contrast, breastmilk HIV-1 DNA levels did not differ by trial arm at any time-point. Follow-up time and number of valid assays did not differ between infants by randomization arm. Median breastfeeding duration was similar between infants in the ZDV/NVP arm [179 days, interquartile range (IQR) 91–184] and infants in the HAART arm (182 days, IQR 155–185).
Table 1.
Characteristics of HIV-1-infected mothers and their HIV-1-uninfected-infants with a valid enzyme-linked immunospot (ELISPOT) assay, by treatment arm
| ZDV/NVP (n = 23) | HAART (n = 24) | P-value* | |||
|---|---|---|---|---|---|
| n | Median (IQR) or n (%) | n | Median (IQR) or n (%) | ||
| Maternal characteristics | |||||
| Age (years) | 23 | 24 (20–30) | 24 | 26 (25–30) | 0·20 |
| CD4 cell count (cells/μl) at 32 weeks gestation | 23 | 333 (295–430) | 24 | 318 (264–421) | 0·57 |
| Plasma HIV-1 RNA level (log10 copies/ml) at | |||||
| 32 weeks gestation | 20 | 4·57 (4·21–5·31) | 23 | 4·81 (4·43–5·03) | 0·79 |
| Delivery | 13 | 4·05 (3·83–4·47) | 21 | 2·45 (2·00–2·75) | 0·0001 |
| Month 1 postpartum | 21 | 4·75 (4·09–5·45) | 24 | 2·45 (2·00–2·95) | <0·0001 |
| Month 3 postpartum | 21 | 4·88 (4·03–5·10) | 22 | 2·00 (2·00–2·54) | <0·0001 |
| Month 6 postpartum | 19 | 4·55 (3·80–5·02) | 21 | 2·00 (2·00–3·72) | 0·0004 |
| Month 9 postpartum | 13 | 4·66 (3·98–5·32) | 11 | 4·86 (4·02–5·28) | 0·84 |
| Month 12 postpartum | 8 | 5·27 (4·83–5·71) | 8 | 4·72 (4·18–5·24) | 0·14 |
| Breastmilk cell-free HIV-1 RNA detected at | |||||
| Delivery | 15 | 10 (67%) | 17 | 6 (35%) | 0·16 |
| Month 1 postpartum | 22 | 18 (82%) | 24 | 7 (29%) | <0·001 |
| Month 3 postpartum | 14 | 5 (36%) | 20 | 4 (20%) | 0·44 |
| Month 6 postpartum | 2 | 2 (100%) | 18 | 8 (44%) | 0·47 |
| Breastmilk HIV-1 DNA level (log10 copies/ml) at | |||||
| Delivery | 12 | 1·68 (1·37–2·15) | 12 | 1·76 (1·48–2·18) | 0·60 |
| Month 1 postpartum | 16 | 2·53 (1·98–2·78) | 17 | 2·27 (2·08–2·51) | 0·47 |
| Month 3 postpartum | 14 | 2·68 (2·23–3·06) | 13 | 2·25 (1·98–2·70) | 0·33 |
| Month 6 postpartum | 0 | – | 1 | 2·00 | – |
| Infant characteristics | |||||
| Follow-up time (days) | 23 | 364 (274–368) | 24 | 365 (277–368) | 0·69 |
| Number of assays† | 23 | 3 (2–5) | 24 | 4 (3–5) | 0·59 |
| Breastfeeding duration (days) | 23 | 179 (91–184) | 23 | 182 (155–185) | 0·36 |
Bold type indicates P ≤ 0·05.
By Mann–Whitney U-test.
Of a possible five time-points, reasons for not testing include missed visit or insufficient blood collection. IQR = interquartile range; n = number of individuals for whom data were available; ZDV/NVP = zidovudine/nevirapine; HAART = highly active anti-retroviral therapy.
Prevalence, durability, magnitude and breadth of HIV-1-specific IFN-γ responses and comparison by randomization arm
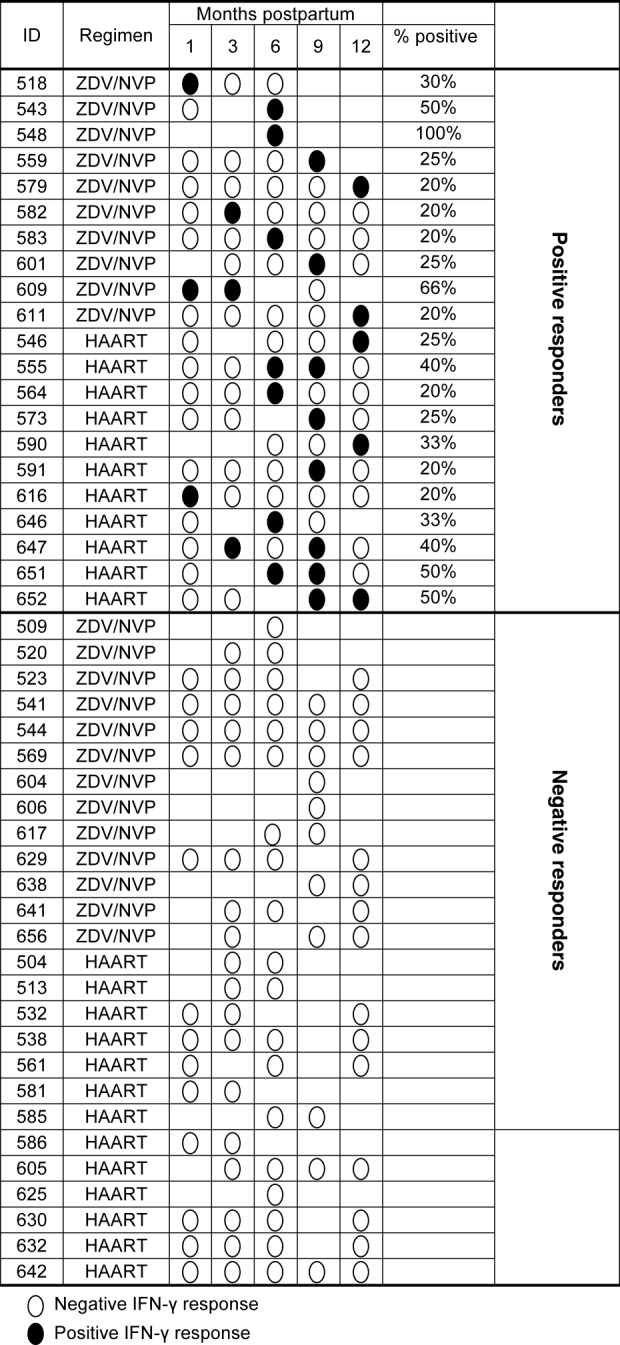
Ten (43%) infants in the ZDV/NVP arm and 11 (46%) infants in the HAART arm had positive HIV-1-specific IFN-γ responses at least once (Table2), and the prevalence of positive ELISPOTs did not differ between randomization arms at any visit (P > 0·05 for each visit). In the HAART arm, the prevalence of positive ELISPOTs was low early in life and increased thereafter, with the highest prevalence (43%) at 9 months of age. In contrast, prevalence of positive ELISPOTs among infants in the ZDV/NVP arm remained relatively constant throughout their first year of life.
Table 2.
Prevalence, magnitude and breadth of infant HIV-1-specific interferon (IFN)-γ responses over time, by treatment arm
| Prevalence* | Magnitude of all responses† | Magnitude of positive responses‡ | Breadth of positive responses§ | |||||
|---|---|---|---|---|---|---|---|---|
| ZDV/NVP | HAART | ZDV/NVP | HAART | ZDV/NVP | HAART | ZDV/NVP | HAART | |
| Month 1 | 2/13 (15%) | 1/18 (6%) | 65 (22–200) | 65 (22–105) | 1067 (547–1587) | 364 | 5·5 (4–7) | 4 |
| Month 3 | 2/16 (13%) | 1/17 (6%) | 42 (23–253) | 82 (52–120) | 598 (190–1006) | 302 | 2·5 (2–3) | 2 |
| Month 6 | 3/18 (17%) | 4/19 (21%) | 142 (47–360) | 217 (50–347) | 283 (196–724) | 165 (96–959) | 1 (1–3) | 1 (1–3·5) |
| Month 9 | 2/15 (13%) | 6/14 (43%) | 70 (27–192) | 182 (35–365) | 444 (110–778) | 211 (81–429) | 2·5 (2–3) | 2·5 (1–4) |
| Month 12 | 2/13 (15%) | 3/17 (18%) | 130 (32–165) | 70 (30–145) | 85 (61–109) | 218 (74–231) | 1 (1–1) | 3 (1–3) |
| Overall | 10/23 (43%) | 11/24 (46%) | 88 (45–187) | 96 (68–171) | 240 (110–776) | 231 (74–429) | 2 (1–3) | 2 (1–4) |
Proportion (%) of positive enzyme-linked immunospot (ELISPOT) results, defined as ≥ 1 peptide pool with experimental wells, ≥ 50 HIV-1-specific spot-forming units (SFU)/106 peripheral blood mononuclear cells (PBMC) and > ×2 background response.
Median interquartile range (IQR) summed HIV-1-specific responses among all infants tested, given in HIV-1-specifIc SFU/106 PBMC.
Median IQR summed HIV-1-specific responses among infants with positive ELISPOT results, given in HIV-1-specific SFU/106 PBMC.
Median (IQR) number of peptide pools recognized by infants with positive ELISPOT results. ZDV/NVP = zidovudine/nevirapine; HAART = highly active anti-retroviral therapy.
The median magnitude of all ELISPOT responses were similar between infants in the ZDV/NVP and HAART group overall (88 HIV-1-specific SFU/106 PBMC, IQR 45–187 versus 96 HIV-1-specific SFU/106 PBMC, IQR 68–171, respectively) and for every time-point (P > 0·05 for each visit). When restricted to positive responders at each visit or overall, the magnitudes of responses were not different by treatment arm (P > 0·05); however, statistical power for comparisons was limited. The median number of peptide pools recognized (breadth of response) also did not differ between randomization arms overall or at any single time-point (P > 0·05), and there were no specific pools recognized selectively in either arm (data not shown).
Of the 47 infants who had ELISPOT data during the study, 21 (45%) had at least one positive HIV-1-specific response (Fig. 1). Among the 21 positive responders, 13 had only one positive response, five infants (four HAART, one ZDV/NVP) had positive ELISPOT responses at two time-points, and three of these infants (two HAART, one ZDV/NVP) had repeated responses to identical gag pools (Fig. 2). The number of peptide pools that were recognized by infants with positive ELISPOT responses ranged from one to seven, with a median of two pools overall (Table2). Similar patterns of responses were observed when the analyses were restricted to assays with background responses ≤ 100 SFU/million PBMC. When assays with high backgrounds were removed, 17 (36%) had at least one positive response. The median magnitude of all responses were reduced to 63 HIV-1-specific SFU/106 PBMC, IQR 42–120 versus 72 HIV-1-specific SFU/106 PBMC, IQR 52–125, in the ZDV/NVP and HAART groups, respectively. The number of pepite pools recognized remained unchanged.
Fig 1.
Detection of negative and positive HIV-1-gag-specific interferon (IFN)-γ responses in HIV-1-exposed, uninfected (EU) infants during the first year postpartum. The detection of HIV-1-specific IFN-γ responses is shown for 47 EU infants born to mothers randomized to either short-course ZDV/NVP or HAART. Filled circles = detectable response; open circles = undetectable response; no circle = not tested.
Fig 2.
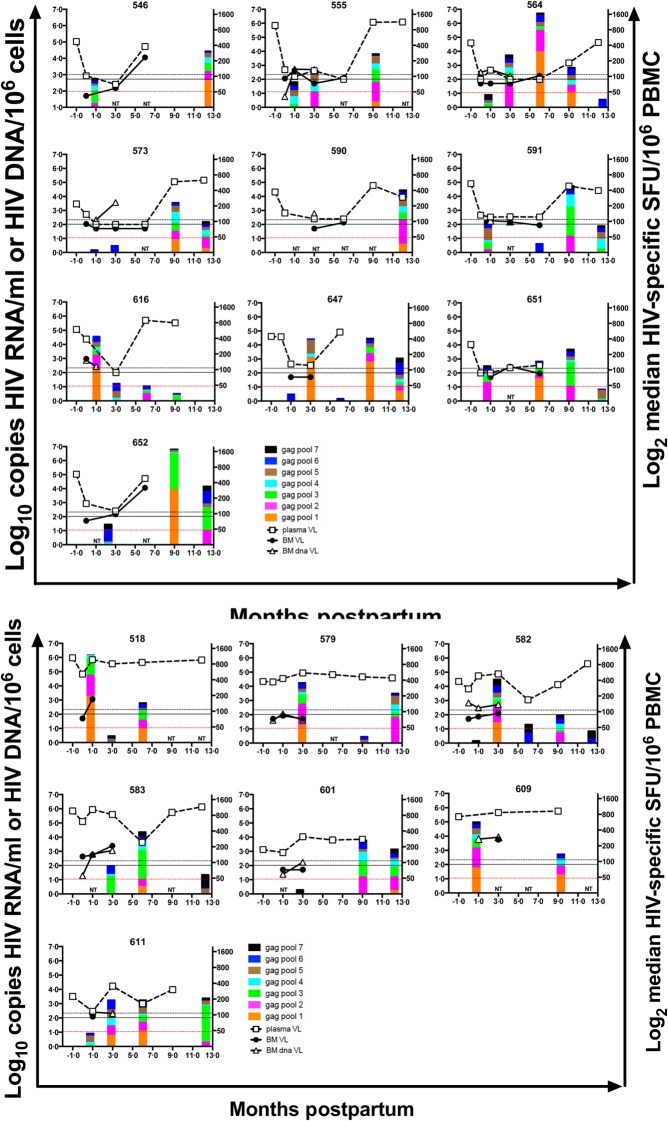
Infant HIV-1-specific peptide responses and maternal viral loads. Interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assays were conducted on freshly isolated peripheral blood mononuclear cells (PBMC) samples from HIV-1-exposed, uninfected infants using 2 × 105 PBMC per well with two wells per peptide pool. Data from assays with background spot-forming units (SFU) ≤ 100/106 PBMC are depicted. Pools with ≥ 50 HIV-1-specific SFU/106 PBMC and > ×2 the background response were defined as positive ELISPOT responses. Magnitude of HIV-1-specific peptide responses (stacked bar), plasma HIV-1 RNA (open squares, dashed line), breastmilk HIV-1 RNA (closed circles, solid line) and breastmilk HIV-1 DNA (open triangles, solid line) are shown for all infants with positive ELISPOT responses. Months −1 and 0 refer to 32 weeks gestation (initiation of antitetroviral regimen) and delivery, respectively. The mean days to delivery after the 32 weeks gestation visit was 39 (median 39 days, range 2–82 days). Data points marked NT indicate time-points when infants were not tested for ELISPOT responses. Black reference lines indicate the lower limits of detection for HIV-1 RNA/ml in plasma (200 copies/ml, dashed line) and breastmilk (100 copies/ml, solid line) and red reference lines indicate threshold for a positive HIV-1-specific IFN-γ response (50 HIV-1-specific SFU/106 PBMC).
Infant HIV-1-specific IFN-γ responses at 1 month of age are associated with maternal viral load
To evaluate the effect of antenatal exposure of infants to maternal virus on infant ELISPOT responses, we determined correlates of infant ELISPOT responses at 1 month postpartum utilizing data from all infants (both negative and positive ELISPOT results) and assessing all HIV-1-specific cellular responses (after subtraction of background) as a continuous variable. We utilized all infant data rather than the subset of positive responses to enhance potential analytical power because the biological threshold for a true positive response is unknown. Maternal HIV-1 viral levels and CD4 count were evaluated as correlates of infant responses at 1 month of age. For every log10 increase in maternal plasma viral load at 32 weeks gestation there was a significant association for a 0·44 log10 increase [95% confidence interval (CI) = −0·12–0·76, P = 0·009] in magnitude of infant IFN-γ responses (Table3). Thus, for every log10 increase in viral load during gestation, infants had ∼600/106 additional HIV-specific cells in circulation by 1 month of life. In contrast, for every log10 increase in breastmilk HIV-1 DNA month 1 postpartum, there was a 0·54 (95% CI = 0·11–0·97, P = 0·02) log10 increase in magnitude of infant IFN-γ responses (Table3A). Therefore, for every log10 increase in concurrent breastmilk viral load, infants have ∼3000/106 additional circulating HIV-specific cells. Similar results were found with sensitivity analyses excluding samples that had fewer than 10 000 cells and undetectable HIV-1 DNA (data not shown). When the analysis was restricted to assays with background ≤100 SFU/106 PBMC, the associations were similar: the contribution of plasma viral load during pregnancy was reduced to a trend (P = 0·08), while the contribution of concurrent breastmilk viral load remained a significant correlate for detection of infant HIV-1 specific IFN-γ responses 1 month after birth.
Table 3A.
Correlates of magnitude of infant HIV-1-specific interferon (IFN)-γ responses at month 1 postpartum
| n | aCoeff (95% CI) | P-value* | |
|---|---|---|---|
| Baseline (32 weeks gestation) | |||
| Plasma HIV-1 RNA level | 29 | 0·44 (0·12 to 0·76) | 0·009 |
| Maternal CD4 cell count (per 100 cells/μl) | 31 | −0·25 (−0·51 to 0·01) | 0·06 |
| Delivery | |||
| Plasma HIV-1 RNA level | 22 | 0·24 (−0·13 to 0·62) | 0·19 |
| Breastmilk cell-free HIV-1 RNA detected | 21 | 0·17 (−0·54 to 0·88) | 0·63 |
| Breastmilk HIV-1 DNA level | 18 | 0·12 (−0·36 to 0·59) | 0·61 |
| Month 1 postpartum | |||
| Plasma HIV-1 RNA level | 31 | 0·07 (−0·20 to 0·34) | 0·61 |
| Breastmilk cell-free HIV-1 RNA detected | 31 | 0·22 (−0·17 to 0·61) | 0·27 |
| Breastmilk HIV-1 DNA level | 25 | 0·54 (0·11 to 0·97) | 0·02 |
Bold type indicates P ≤ 0·05.
By linear regression models adjusting for treatment arm and using robust standard errors. Magnitude of responses was log-transformed; HIV-RNA and DNA levels were measured in log10 copies/ml. aCoeff = adjusted beta coefficient; CI = confidence interval; n = number of individuals for whom data were available.
Association between concurrent breastmilk HIV-1 levels and magnitude of HIV-1-specific IFN-γ responses
GEE models were developed to determine the relationship between HIV-1 exposure through different biological compartments and infant cellular immune responses longitudinally (Table4 and Fig. 2). The magnitude of infant ELISPOT responses was associated significantly with the concurrent detection of HIV-1 RNA in breastmilk (β = 0·84, 95% CI = 0·19–1·48, P = 0·01) and the concurrently measured level of breastmilk HIV-1 DNA (β = 0·84, 95% CI = 0·19–1·49, P = 0·01). Sensitivity analyses produced similar results when excluding samples with undetectable HIV-1 DNA in which fewer than 10 000 cells were tested (data not shown). There was a trend for a positive association between plasma viral load at 32 weeks gestation and magnitude of subsequent ELISPOT responses (β = 0·35, 95% CI = −0·03–0·72, P = 0·07).
Table 3b.
Correlates of magnitude of infant HIV-1-specific interferon (IFN)-γ responses over time
| n | aCoeff (95% CI) | P-value* | |
|---|---|---|---|
| Baseline (32 weeks gestation) | |||
| Plasma HIV-1 RNA levels | 43 | 0·35 (−0·03 to 0·72) | 0·07 |
| Delivery | |||
| Breastmilk cell-free HIV-1 RNA detected | 32 | −0·32 (−0·85 to 0·22) | 0·25 |
| Breastmilk HIV-1 DNA level | 24 | −0·12 (−0·45 to 0·20) | 0·46 |
| Time varying | |||
| Plasma HIV-1 RNA level | 45 | −0·03 (−0·37 to 0·31) | 0·87 |
| Breastmilk cell-free HIV-1 RNA detected | 40 | 0·84 (0·19 to 1·48) | 0·01 |
| Breastmilk HIV-1 DNA level | 27 | 0·84 (0·19 to 1·49) | 0·01 |
Bold type indicates P ≤ 0·05.
By generalized estimating equation models with a Poisson link and exchangeable correlation structure adjusting for treatment arm and using robust standard errors. Magnitude of responses was log-transformed; HIV-1 RNA and DNA levels measured in log10 copies/ml. aCoeff = adjusted beta coefficient; CI = confidence interval; n = number of individuals for whom data were available.
Discussion
In this study, prevalence and correlates of HIV-1-specific IFN-γ responses among breastfeeding HIV-1 EU infants born to mothers on anti-retroviral therapy were evaluated. We found that 45% of infants were able to generate cellular immune responses of substantial breadth and magnitude; however, most responses were transient. Our finding confirms previous studies that detected responses in HIV-1 EU infants 22,27,28,31, and is consistent with our previous study that observed 47% prevalence of at least one positive ELISPOT assay using human leucocyte antigen (HLA)-matched peptide stimulation in breastfeeding EU infants 29. We also found significant associations between maternal plasma and breastmilk HIV-1 viral levels and infant magnitude of HIV-1-specific ELISPOT responses, suggesting that antigen exposure modifies the induced infant HIV-1-specific immune responses.
In contrast to our study hypothesis, we did not observe that randomization to the ZDV/NVP arm was associated with higher infant HIV-1-specific immune responses. We may have been underpowered to detect a difference between the two arms; however, the absence of a difference by treatment is consistent with our finding that breastmilk cell-associated HIV-1 DNA predicted infant IFN-γ responses. We have demonstrated previously in this cohort that whereas breastmilk cell-free virus was decreased significantly in women on HAART, breastmilk cell-associated virus (as measured by HIV-1 DNA levels) remained similar to women in the ZDV/NVP arm 34, and the persistence of breastmilk HIV-1 DNA despite HAART has also been observed in a study from Botswana 37. Thus, although breastfeeding infants born to mothers on HAART had less exposure to maternal cell-free virus, there was persistent exposure to HIV-1 infected cells in breastmilk, and this may be a key determinant in generating infant immune responses.
We found a significant association between maternal pregnancy plasma HIV-1 RNA levels and magnitude of IFN-γ responses in EU infants at 1 month of age, suggesting that in-utero exposure influences infant immune responses in the absence of HIV-1 infection. Furthermore, ongoing HIV-1 exposure through breastmilk appears to induce responses as seen by the correlation between both breastmilk HIV-1 RNA and DNA and magnitude of infant IFN-γ responses during the postpartum period. The results from this study are consistent with other EU cohorts 29,38–40. Together these observations support the hypothesis that infant cellular immune responses are due to HIV-1 exposure and not randomly distributed false positives. However, it should be noted that not all studies have observed associations between increased transmitter virus exposure and EU cellular HIV-1 response. Some studies of HIV-1 discordant couples and mother–infant pairs have noted inverse associations with the partner's or mother's HIV-1 viral load 41,42. Consideration of the measures of transmitter virus (RNA, DNA), transmitter compartment (plasma, genital secretions or breastmilk), assay (ELISPOT or intracellular cytokine staining) and EU HIV-1-specific response score (positive/negative or magnitude) differ between studies, and may contribute to the differences in results. These predictors of cellular immune responses in EU individuals may reveal factors to consider in vaccine design in order to effectively induce immune responses.
We observed infant ELISPOT responses that were of relatively high magnitude and were comparable to levels noted after HIV-1 vaccines in trials among adults 43–45. Responses were detected in three infants at 1 month of age, suggesting that responses can be primed very early in life; however, these responses were not maintained and subsequently disappeared in all three infants. The characteristics of these infant responses are analogous to what may be expected among recipients of a prime-boost vaccine 46. The lack of persistent immune responses in infants suggests that initial in-utero priming of responses may not be sufficient for a sustained response, due perhaps to anti-retroviral treatment decreasing maternal HIV-1 viral load in the last trimester. With regard to the route of vaccine delivery, our data and others 19,38 have shown that oral exposure to HIV-1 induces systemic HIV-1-specific IFN-γ responses, lending support for discussing the potential role of mucosally administered HIV-1 vaccines. Recently, CD4+CCR5+ T cells have been noted to be prevalent in infant gut mucosa, yielding potential susceptibility to HIV-1 infection or vaccination 47.
This study benefited from the longitudinal assessment of HIV-1 EU infants to monitor durability of immune responses and to determine correlates over time, and to identify the infant's viral source and to collect detailed HIV-1 exposure data from the mothers. A limitation of this study was the relatively small number of mother–infant pairs. In the absence of a biological threshold or gold standard for HIV-1-specific SFU, cut-offs for positive assays are arbitrary and are based on laboratory-based comparisons to background wells or to control individuals. By using continuous HIV-1-specific SFU instead of dichotomous data, we were able to increase analytical power and precision to discern associations.
In summary, our findings suggest that HIV-1-specific IFN-γ responses in HIV-1 EU infants are associated with maternal levels of HIV-1 in plasma and breastmilk, and that the dose of infant exposure to maternal virus during and after pregnancy influences the induction of infant HIV-1-specific responses. Associations with breastmilk viral load suggest that these responses result from HIV-1 exposure at the oral and/or gut mucosal surfaces. Our results suggest that oral induction of immune responses is possible and related to dose of antigenic exposure; however, sustained responses are rare and the relevance of isolated cellular responses to protection is uncertain. It is likely that multi-pronged humoral and cellular responses induced by vaccines will be required.
Acknowledgments
The authors thank the research personnel, laboratory staff and data management teams in Nairobi, Kenya and Seattle, Washington; the Mathare North City Council Clinic for their participation and co-operation; and the Divisions of Obstetrics and Gynaecology and Paediatrics and Child Health at Kenyatta National Hospital for providing facilities for laboratory and data analysis. Most of all, we thank the mothers and children who participated in the study. This work was supported by grants to G. J.-S. from the Elizabeth Glaser Pediatric AIDS Foundation (no. 311-03) and grant K24HD054314 from the National Institute of Child Health and Diseases, National Institutes of Health (NICHD). A. Y. L. was supported by the University of Washington AIDS International Training and Research Program funded by the NIH Fogarty International Center (D43 TW00007). G. J.-S. was an Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) Scientist. Research support was also provided by the UW Center for AIDS Research (CFAR), an NIH program (P30 AI027757) which is funded by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI and NCCAM.
Disclosure
The authors declare no financial or commercial conflicts of interest.
References
- Joint United Nations Programme on HIV/AIDS (UNAIDS) 2010. Global report: UNAIDS report on the global AIDS epidemic 2010;. Available at: http://www.unaids.org/globalreport/Global_report.html. [PubMed]
- Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MBA. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–8. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- Hay CM, Ruhl DJ, Basgoz NO, et al. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J Virol. 1999;73:5509–19. doi: 10.1128/jvi.73.7.5509-5519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MR, van BaalenCA, Holwerda AM, et al. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–72. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- Musey LK, Krieger JN, Hughes JP, Schacker TW, Corey L, McElrath MJ. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J Infect Dis. 1999;180:278–84. doi: 10.1086/314868. [DOI] [PubMed] [Google Scholar]
- Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 2: genetic factors and implications for anti-retroviral therapeutics. Ann Intern Med. 2001;134:978–96. doi: 10.7326/0003-4819-134-10-200105150-00012. [DOI] [PubMed] [Google Scholar]
- Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 1: cellular and humoral immune responses. Ann Intern Med. 2001;134:761–76. doi: 10.7326/0003-4819-134-9_part_1-200105010-00013. [DOI] [PubMed] [Google Scholar]
- Wasik TJ, Wierzbicki A, Whiteman VE, Trinchieri G, Lischner HW, Kozbor D. Association between HIV-specific T helper responses and CTL activities in pediatric AIDS. Eur J Immunol. 2000;30:117–27. doi: 10.1002/1521-4141(200001)30:1<117::AID-IMMU117>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Kalams SA, Buchbinder SP, Rosenberg ES, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–20. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musey LK, Hughes J, Schacker T, Shea T, Corey L, McElrath MJ. Cytotoxic T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–74. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- Jennes W, Vuylsteke B, Borget MY, et al. HIV-specific T helper responses and frequency of exposure among HIV-exposed seronegative female sex workers in Abidjan, Cote d'Ivoire. J Infect Dis. 2004;189:602–10. doi: 10.1086/381454. [DOI] [PubMed] [Google Scholar]
- Rowland-Jones SL, Dong T, Fowke KR, et al. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Invest. 1998;102:1758–65. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowke KR, Kaul R, Rosenthal KL, et al. HIV-1-specific cellular immune responses among HIV-1-resistant sex workers. Immunol Cell Biol. 2000;78:586–95. doi: 10.1046/j.1440-1711.2000.00944.x. [DOI] [PubMed] [Google Scholar]
- Kebba A, Kaleebu P, Rowland S, et al. Distinct patterns of peripheral HIV-1-specific interferon- gamma responses in exposed HIV-1-seronegative individuals. J Infect Dis. 2004;189:1705–13. doi: 10.1086/383227. [DOI] [PubMed] [Google Scholar]
- Perez CL, Hasselrot K, Bratt G, Broliden K, Karlsson AC. Induction of systemic HIV-1-specific cellular immune responses by oral exposure in the uninfected partner of discordant couples. AIDS. 2010;24:969–74. doi: 10.1097/qad.0b013e328337aff8. [DOI] [PubMed] [Google Scholar]
- Schenal M, Lo Caputo S, Fasano F, et al. Distinct patterns of HIV-specific memory T lymphocytes in HIV-exposed uninfected individuals and in HIV-infected patients. AIDS. 2005;19:653–61. doi: 10.1097/01.aids.0000166088.85951.25. [DOI] [PubMed] [Google Scholar]
- Kuhn L, Coutsoudis A, Moodley D, et al. T-helper cell responses to HIV envelope peptides in cord blood: protection against intrapartum and breast-feeding transmission. AIDS. 2001;15:1–9. doi: 10.1097/00002030-200101050-00003. [DOI] [PubMed] [Google Scholar]
- De Maria A, Cirillo C, Moretta L. Occurrence of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T cell activity in apparently uninfected children born to HIV-1-infected mothers. J Infect Dis. 1994;170:1296–9. doi: 10.1093/infdis/170.5.1296. [DOI] [PubMed] [Google Scholar]
- Rowland-Jones S, Nixon D, Aldhous MC, et al. HIV-specific cytotoxic T cell activity in an HIV exposed but uninfected infant. Lancet. 1993;341:860–1. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- Borkowsky W, Krasinski K, Moore T, Papaevangelou V. Lymphocyte proliferative responses to HIV-1 envelope and core antigens by infected and uninfected adults and children. AIDS Res Hum Retroviruses. 1990;6:673–8. doi: 10.1089/aid.1990.6.673. [DOI] [PubMed] [Google Scholar]
- Clerici M, Sison AV, Berzofsky JA, et al. Cellular immune factors associated with mother-to-infant transmission of HIV. AIDS. 1993;7:1427–33. doi: 10.1097/00002030-199311000-00004. [DOI] [PubMed] [Google Scholar]
- Kuhn L, Meddows-Taylor S, Gray G, et al. Reduced HIV-stimulated T-helper cell reactivity in cord blood with short-course anti-retroviral treatment for prevention of maternal–infant transmission. Clin Exp Immunol. 2001;123:443–50. doi: 10.1046/j.1365-2249.2001.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik TJ, Bratosiewicz J, Wierzbicki A, et al. Protective role of beta-chemokines associated with HIV-specific Th responses against perinatal HIV transmission. J Immunol. 1999;162:4355–64. [PubMed] [Google Scholar]
- Aldhous MC, Watret KC, Mok JY, Bird AG, Froebel KS. Cytotoxic T lymphocyte activity and CD8 subpopulations in children at risk of HIV infection. Clin Exp Immunol. 1994;97:61–7. doi: 10.1111/j.1365-2249.1994.tb06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John-Stewart GC, Mbori-Ngacha D, Lohman-Payne B, et al. HIV-1-specific cytotoxic T lymphocytes and breast milk HIV-1 transmission. J Infect Dis. 2009;199:889–98. doi: 10.1086/597120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali DL, Sullivan JL. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J Immunol. 1995;154:433–43. [PubMed] [Google Scholar]
- McFarland EJ, Harding PA, Luckey D, Conway B, Young RK, Kuritzkes DR. High frequency of Gag- and envelope-specific cytotoxic T lymphocyte precursors in children with vertically acquired human immunodeficiency virus type 1 infection. J Infect Dis. 1994;170:766–74. doi: 10.1093/infdis/170.4.766. [DOI] [PubMed] [Google Scholar]
- Chung MH, Kiarie JN, Richardson BA, Lehman DA, Overbaugh J, John-Stewart GC. Breast milk HIV-1 suppression and decreased transmission: a randomized trial comparing HIVNET 012 nevirapine versus short-course zidovudine. AIDS. 2005;19:1415–22. doi: 10.1097/01.aids.0000181013.70008.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MH, Kiarie JN, Richardson BA, et al. Highly active anti-retroviral therapy versus zidovudine/nevirapine effects on early breast milk HIV type-1 RNA: a phase II randomized clinical trial. Antivir Ther. 2008;13:799–807. [PMC free article] [PubMed] [Google Scholar]
- Lehman DA, Chung MH, John-Stewart GC, et al. HIV-1 persists in breast milk cells despite anti-retroviral treatment to prevent mother-to-child transmission. AIDS. 2008;22:1475–85. doi: 10.1097/QAD.0b013e328302cc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–95. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–3. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RL, Ndung'u T, Lockman S, et al. Highly active anti-retroviral therapy started during pregnancy or postpartum suppresses HIV-1 RNA, but not DNA, in breast milk. J Infect Dis. 2005;192:713–9. doi: 10.1086/432489. [DOI] [PubMed] [Google Scholar]
- Farquhar C, Lohman-Payne B, Overbaugh J, et al. Breast milk HIV-1 RNA levels and female sex are associated with HIV-1-specific CD8+ T-cell responses in HIV-1-exposed, uninfected infants in Kenya. J Infect Dis. 2011;204:1806–10. doi: 10.1093/infdis/jir643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R, Rowland-Jones SL, Kimani J, et al. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol Lett. 2001;79:3–13. doi: 10.1016/s0165-2478(01)00260-7. [DOI] [PubMed] [Google Scholar]
- Willberg CB, McConnell JJ, Eriksson EM, et al. Immunity to HIV-1 is influenced by continued natural exposure to exogenous virus. PLOS Pathog. 2008;4:e1000185. doi: 10.1371/journal.ppat.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm DB, Meddows-Taylor S, Gray GE, Kuhn L, Tiemessen CT. Low maternal viral loads and reduced granulocyte-macrophage colony-stimulating factor levels characterize exposed, uninfected infants who develop protective human immunodeficiency virus type 1-specific responses. Clin Vaccine Immunol. 2007;14:348–54. doi: 10.1128/CVI.00464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebba A, Kaleebu P, Serwanga J, et al. HIV type 1 antigen-responsive CD4+ T-lymphocytes in exposed yet HIV Type 1 seronegative Ugandans. AIDS Res Hum Retroviruses. 2004;20:67–75. doi: 10.1089/088922204322749512. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept step study: a case–cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia F, Bernaldo de Quiros JC, Gomez CE, et al. Safety and immunogenicity of a modified pox vector-based HIV/AIDS vaccine candidate expressing Env, Gag, Pol and Nef proteins of HIV-1 subtype B (MVA-B) in healthy HIV-1-uninfected volunteers: a phase I clinical trial (RISVAC02) Vaccine. 2011;29:8309–16. doi: 10.1016/j.vaccine.2011.08.098. [DOI] [PubMed] [Google Scholar]
- Churchyard GJ, Morgan C, Adams E, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLOS ONE. 2011;6:e21225. doi: 10.1371/journal.pone.0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genesca M. Characterization of an effective CTL response against HIV and SIV infections. J Biomed Biotechnol. 2011;2011:103924. doi: 10.1155/2011/103924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunders MJ, van der Loos CM, Klarenbeek PL, et al. Memory CD4+CCR5+ T cells are abundantly present in the gut of newborn infants to facilitate mother-to child transmission of HIV-1. Blood. 2012;120:4383–90. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]