Abstract
Objectives
A common complaint of cancer patients is the experience of cognitive difficulty during and after chemotherapy. We hypothesized that cognitive impairment may result from dysfunction in large-scale brain networks, particularly those involved in attentional control.
Methods
Using a case-control design, this study includes women with a history of invasive ductal or lobular, triple-negative breast cancer who completed standard adjuvant chemotherapy within two years of study entry. Women who reported cognitive impairment by the Global Rating of Cognition question were considered to be cases (n= 15). Women who reported no cognitive impairment were considered to be controls (n= 13). All enrolled participants were eligible for MRI investigation and underwent resting state-functional connectivity MRI.
Results
Women who self-reported cognitive impairment were found to have disrupted resting-state functional connectivity, as measured by MRI, when compared to women who did not self-report cognitive impairment. These findings suggest that some women may be more sensitive to the standard treatments for breast cancer and that this increased sensitivity may result in functional connectivity alterations in the brain networks supporting attention and executive function.
Conclusions
Neuroimaging analyses confirmed self-reported cognitive deficits in women with breast cancer treated with chemotherapy.
Keywords: cognitive disorders, diagnostic imaging, neuroimaging, breast neoplasms, chemotherapy, complications
Introduction
Chemotherapy-associated cognitive impairment (CACI), or “chemobrain,” is a phenomenon in which a subset of cancer survivors suffers cognitive dysfunction after chemotherapy. A recently published report from the International Cognition and Cancer Task Force(ICCTF)[1] concluded that “neuropsychological studies have shown cognitive dysfunction in 13–70% of patients receiving chemotherapy”. This cognitive impairment manifests in a variety of ways, most notably as a memory impairment, a decreased capability to perform executive functions including working memory, and deficits in psychomotor and processing speed.[2] For many patients, cognitive impairment proves to be debilitating as it negatively impacts quality of life and hinders occupational goals.[1] Nelson and Suls[2] recently published a review of the literature on the relationship between chemotherapy and cognitive impairment and observed a similarly wide range of estimates for the incidence of chemotherapy-associated cognitive impairment as cited by the ICCTF. They concluded that this wide estimate for CACI from various studies could be attributed to a variety of reasons, including methodological differences in their assessment of cognitive function, and suggested that new research approaches were needed to study chemotherapy-associated cognitive changes.
Implementing advanced neuroimaging techniques to investigate CACI constitutes a promising complement to self-reports and neuropsychological assessments, both of which are not capable of revealing the neural mechanisms underlying CACI. Resting-state functional-connectivity magnetic resonance imaging (rs-fcMRI) is used to infer functional relatedness between various regions of the brain.[3] Since cognition relies on synergistic activities of large neural populations organized by function, a network-based analytical approach is useful for understanding the underlying neurobiological mechanisms of cognitive deficits.[4] Resting-state fcMRI has previously been used to define network disruptions resulting in the cognitive deficits associated with various pathologic conditions, including, but not limited to, Alzheimer’s disease[5], stroke[6], and depression.[7] Bruno, Hosseini, and Kesler[8] used rs-fcMRI and graph theoretical analysis to examine the functional connectivity in breast cancer survivors treated with chemotherapy relative to healthy women. Compared to healthy controls, the breast cancer group displayed altered global brain network organization characterized by significantly decreased global clustering as well as disrupted network characteristics in frontal, striatal, and temporal regions. These authors suggest that this pattern of altered network organization is likely to result in reduced efficiency of information transfer. This same group[9]using rs-fcMRI found that disrupted default mode network (DMN) connectivity among breast cancer patients who received chemotherapy may explain long-term cognitive difficulties. Studies using structural MRI have revealed decreased gray matter volume in frontal, temporal, and cerebellar regions in breast cancer patients following a course of adjuvant chemotherapy.[10] Other studies based on resting-state functional connectivity MRI suggested alterations in the functional network architecture of the brains of women with breast cancer who received chemotherapy leading to decreased network efficiency and implicated brain systems important for executive function.[8] In their review of the literature, O'Farrell, MacKenzie, and Collins[10] cited several studies investigating the effects of CACI, and found increased activation in the prefrontal cortex and cerebellum. The authors conclude that these findings might represent a compensatory mechanism following a decrease in cognitive ability and pretreatment baseline assessments are necessary to reveal changes in brain integrity resulting from the neurotoxic effects of chemotherapy. In contrast to these studies, the present study investigates within a population of women whose breast cancer has been treated with chemotherapy, using self-report to delineate subgroups who do or do not describe cognitive impairment.
Based on the contention that systemic chemotherapy causes functional disruptions in several neural systems, most notably the networks responsible for attention and executive control[11;12], our focus in the current manuscript is on the fronto-parietal attention network[13;14], composed of the precuneus, bilateral inferior parietal and dorsolateral prefrontal cortex; and the cingulo-opercular control network, composed of bilateral medial frontal, mid cingulate, frontal operculum, and right supramarginal gyrus. The fronto-parietal network is associated with moment to moment top-down task control[13] and flexibly supporting goal-oriented processes.[15] The cingulo-opercular network is associated with stable maintenance of overall task configuration. These functions are consistent with the broad range of deficits subjectively reported by many patients after chemotherapy.[11;12;16] A novel aspect of this study is the use of self-report rather than results from neurocognitive testing to define subgroups of patients with and without complaints of CACI.
Materials and Methods
Design and Setting
This was a case-control study of female breast cancer survivors who received chemotherapy as part of their cancer treatment. The impaired cohort (i.e., cases) was defined as women who affirmed cognitive impairment and the nonimpaired cohort (i.e., controls) were women who did not affirm cognitive impairment. Washington University’s Human Research Protection Office approval was obtained prior to recruitment.
Participants
Recruited participants were between the ages of 35 and 70 years, had been diagnosed with invasive ductal or lobular breast cancer Stages I, II, or III (American Joint Committee on Cancer (AJCC) Staging Manual, 7th edition, 2010) within the previous two years, and finished chemotherapy treatment at least 30 days prior to participation. Participants could be pre-or-postmenopausal with early stage breast cancer, who received standard adjuvant chemotherapy including either anthracyclin and/or taxane. Exclusion criteria included (1) evidence of other active cancers within the past year, (2) receipt of skull-base radiation treatment within the past year, or (3) history of brain trauma or disease.
Participants completed the following assessment forms: (1) medical history and health information, (2) Cognitive Failures Questionnaire (CFQ)[17], and (3) Global Rating of Cognition (GRC). The Cognitive Failures Questionnaire is a validated self-report questionnaire that contains 25 items and measures failures in perception, memory, and motor function. The GRC is a single-item, self-report question that uses a Likert-type scale to rate the impact of cognitive impairments on daily life. Based upon responses to the Global Rating of Cognition question, subjects were assigned to the Impaired or Non-Impaired groups. There were 15 subjects who endorsed a GRC response of Extremely affected, Strongly affected, or Moderately affected by their impairment and were classified as Impaired. There were 13 subjects who endorsed a GRC response of Slightly affected or Not affected and were classified as Non-Impaired.
Neuroimaging Data Collection
Scans were performed on a Siemens 3T Tim Trio MRI scanner at Washington University. Resting-state functional-connectivity MRI (rs-fcMRI) and anatomical images were collected during the same imaging session. An asymmetric spin-echo echo-planar pulse sequence (EPI) (TR=2200ms, TE=27ms, flip angle=90°, 4 × 4 × 4 mm voxels) captured images of blood oxygenation level-dependent (BOLD) contrast responses.[18;19] EPI images of the whole brain involved volume acquisitions across 36 odd-even, contiguously interleaved, bicommissurally aligned axial slices. A T1-weighted structural magnetization prepared rapid gradient echo (MP-RAGE) image was acquired across 176 sagittal slices (TR=2400ms; TE=3.09ms; flip angle=8°; inversion time [TI]=1000ms; 1 × 1 × 1 mm voxels). Additionally, a T2-weighted structural image obtained across 36 axial slices (TR=6150ms, TE=86ms, flip angle=120°, 1 × 1 × 4 mm voxels) was in-register with the EPI and aided alignment between axial EPI and sagittal MP-RAGE image slices.[20] Three 164-frame (6 minute) EPI runs recorded spontaneous brain activity while participants were awake, performed no task, and remained with their eyes closed in a darkened room. Three runs, six minutes each, were collected so that 1) there would be enough data remaining after removing frames with motion, 2) we had available a sufficient representation of the lowest frequencies of spontaneous BOLD signal fluctuations for resting state functional connectivity MRI analysis, and 3) so as to avoid a single 18-minute run, which would be demanding on patients asked to hold still and not fall asleep. This strategy of concatenating BOLD volumes for resting state fMRI is common and has been adopted in multiple studies [21–24] as well as the Human Connectome Project.[23]
Image Preprocessing
EPI image preprocessing started with compensation for systematic slice-dependent differences from interleaved odd-even slice acquisition and alignment of the time for each slice to the beginning of each volume acquisition using sinc interpolation. Next, corrections for intensity differences within runs utilized a whole brain mean signal intensity normalized to mode 1000. These time- and intensity-adjusted slices were realigned within and across runs using rigid body correction for inter-frame head motion.[25;26;26;27] The across-run-realigned slices were resampled to 3mm3 voxels and registered to an atlas template by computing 12 parameter affine transforms between an average from the first frames of each EPI run and the atlas template using the individual’s T2 and MP-RAGE images as intermediaries.[20] This atlas template was created using MP-RAGE structural images from 12 normal middle-age individuals (mean 48 yrs, SD +10.7) and registered to the Talairach atlas space[28;29] based on spatial normalization methods.[30]
Additional resting state preprocessing steps were applied in MATLAB (2007a, The Mathworks, Natick, MA) to reduce noise from sources unlikely to reflect neural activity.[31] These steps include demeaning and detrending each BOLD run, temporal filtering with a bandpass filter to remove frequencies > 0.009Hz and <0.08Hz, and spatial smoothing with a 6 mm full width at half-maximum Gaussian kernel. BOLD signal modifications per voxel removed, through linear regression, 24 motion-related and 6 tissue-related sources of nuisance variance. The motion regressors were the six previously computed linear corrections for head movement, their squares, and the same for the immediately preceding timepoint, as derived by Volterra expansion.[32] The tissue-related regressors were a global whole-brain signal averaged over all voxels in the brain, signals in the ventricles and white matter, and their associated temporal derivatives.[32] A standard mask was used for whole-brain, ventricles, and white matter in each subject.[25;27;33;34]
We applied a volume censoring method[31], which removed frames of data with either > .4mm of frame by frame displacement (FD) or > 4 of the quality control parameter dvars (DV), which measures the rate of change in signal intensity across the entire brain at each frame.[35] No additional frames before or after the censored frames were removed, and interpolation was not performed.[36] Spatial smoothing and temporal filtering as well as nuisance variable regression were repeated on the original preprocessed data, leaving out the censored frames. Since these parameters were more lenient than recommended by Power, et al.[31], we plotted correlation between region pairs versus the distance between regions to check for the distance dependent artifact often caused by even sub-millimeter head motion.[31;37] Our resting state fMRI pre-processing and processing approaches are based on methods we and others have developed over the past 5–7 years.[4;31;38–40]
Resting State Analysis of Correlations among Control Regions
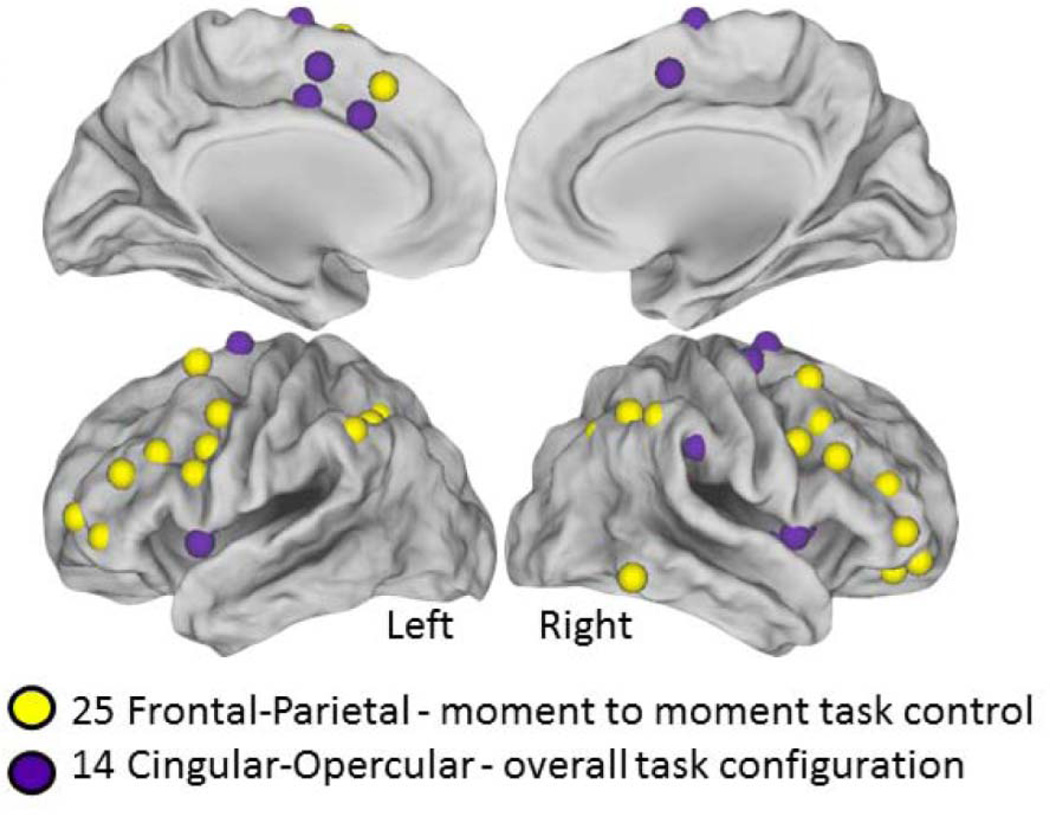
A total of 25 fronto-parietal and 14 cingulo-opercular cognitive control regions of Dosenbach, et al.[13] were created by placing a 10mm diameter sphere around the reported coordinates (Figure 1). A time-series of BOLD signal intensity was calculated in each of the 39 regions for each subject, and within subject Pearson’s correlation coefficients (R) were calculated between each pair of regions and then the Fisher R-Z transformation was performed.[41] For example, a 39 × 39 correlation matrix was generated for each subject with each cell containing the Fisher Z transformed Pearson’s R for each pair-wise correlation. Student’s t-tests (two-tailed, independent samples t-tests assuming equal variance were calculated in MATLAB between the matrices for impaired and non-impaired subjects for each region pair. Bonferroni’s correction was used to correct for multiple comparisons. A p value smaller than 6.75×10−5 was considered significant. Simple linear regression was used to explore relationship between the behavioral scores (GRC/CFQ) and the Fisher z standardized R correlation values (one value per subject) in the reported connection.
Figure 1.
39 Task control regions tested for functional connection differences between impaired and non-impaired patients. The 25 Fronto-Parietal regions and 14 Cingulo-Opercular regions used. Note, the spheres are shown larger than the actual 10mm diameter size.
Results
Participants
Study population consisted of 28 females, with a median age of 53 (range 36–69) years. Of the 28 participants, 20 (71%) were post-menopausal at the time of data acquisition. There was no significant difference between cases and controls in distribution of menopausal status, tumor stage, type of chemotherapy treatment received, or presence of other comorbid ailments. The percentage of women receiving hormonal therapy in the control groups (n=8) 67% was significantly greater than the percentage of women receiving hormonal therapy in the cases (n=4) 27%. Additional participant characteristics are shown in Table 1. The two groups, impaired and non-impaired, differed in self-reported cognitive impairment measured by the GRC (used to define the groups) as well as on the CFQ.
Table 1.
Description of Study Population
| Demographics | Total (n=28) |
Impaired (n=15) |
Non-Impaired (n=13) |
p-value* |
|---|---|---|---|---|
| Age, Median (Min-Max) | 53 (36–69) | 54 (36–69) | 52 (40–67) | 0.555 |
| Race | ||||
| White | 18 (64%) | 9 (60%) | 9 (69%) | 0.596 |
| Black | 8 (29%) | 4 (27%) | 4 (31%) | |
| Asian | 2 (7%) | 2 (13%) | 0 | |
| Employment | ||||
| Full-time | 14 (50%) | 6 (40%) | 8 (62%) | 0.464 |
| Part-time | 6 (21%) | 4 (26%) | 2 (15%) | |
| Unemployed | 3 (11%) | 1 (7%) | 2 (15%) | |
| Retired | 3 (11%) | 3 (20%) | 0 | |
| Other | 2 (7%) | 1 (7%) | 1 (8%) | |
| Education | ||||
| High school/GED equivalent | 8 (29%) | 6 (40%) | 2 (15%) | 0.202 |
| Associate Degree or some college | 7 (25%) | 4 (27%) | 3 (24%) | |
| Bachelor’s Degree | 5 (18%) | 1 (6%) | 4 (31%) | |
| Master’s Degree | 6 (21%) | 4 (27%) | 2 (15%) | |
| PhD, MD3, JD, or other higher degree | 2 (7%) | 0 | 2 (15%) | |
| CFQ, Median (Min-Max) | 47 (12–81) | 57 (46–81) | 28 (12–56) | <0.001 |
| Global Rating of Cognition | ||||
| Not at all | 6 (21%) | 0 | 6 (46%) | <0.001 |
| Slightly | 7 (25%) | 0 | 7 (54%) | |
| Moderately | 5 (18%) | 5 (33%) | 0 | |
| Strongly | 7 (25%) | 7 (47%) | 0 | |
| Extremely | 3 (11%) | 3 (20%) | 0 | |
Mann-Whitney test was used for continuous variables and Fisher’s exact test was used for categorical variables
Resting State Functional Connectivity Results
All 28 participants had sufficient usable fMRI data after motion censoring; the smallest number of usable frames was 138; the largest 476. There was no difference between impaired and non-impaired groups in number of frames kept after motion censoring (impaired mean 396 (STD 89); non-impaired 390 (104); t-test p=.87), RMS movement numbers on each run (impaired mean .203mm (STD .05); non-impaired .197 (.06); t-test p=.80), or the quality control measure DV (impaired mean 1.44 (STD .25); non-impaired 1.55 (STD .24); t-test p=.26). All usable frames were included for each subject.
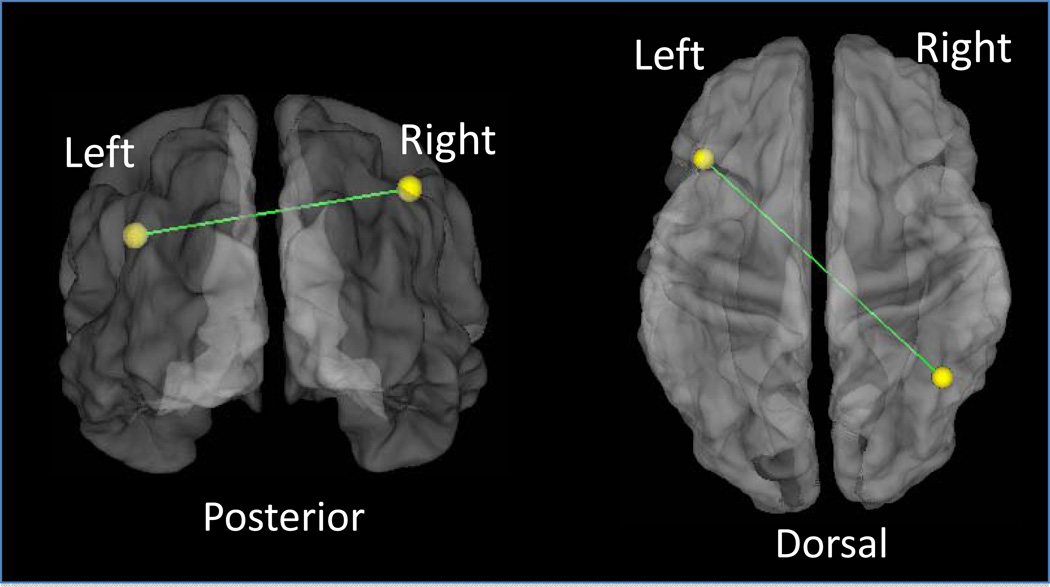
A significant difference (p=1.4 × 10−5) in connection strength between impaired (average R=0.118) and non-impaired (average R=0.346) subjects was identified between two regions of the fronto-parietal system; impaired subjects showed weaker functional connectivity. The functional connection between the left fronto-parietal region (Talairach coordinates x=−41, y=20, and z=31) and a right parietal region (x=41, y=−55, and z=45) is shown in Figure 2.
Figure 2.
A functional connection in the fronto-parietal system shows reliable differences between impaired and non-impaired patients. Student’s t-tests between impaired and non-impaired patients results in a single functional connection between frontal-parietal regions with a correlation strength difference, non-impaired more correlated than impaired, that meets the stringent Bonferroni correction for multiple comparisons. The left frontal (−41, 20, 31) to right parietal (41, −55, 45) connection, shown on a translucent brain in 2 views (left panel posterior view; right panel dorsal view) has mean standardized R of 0.346 for non-impaired and 0.118 for impaired (p = 0.00001).
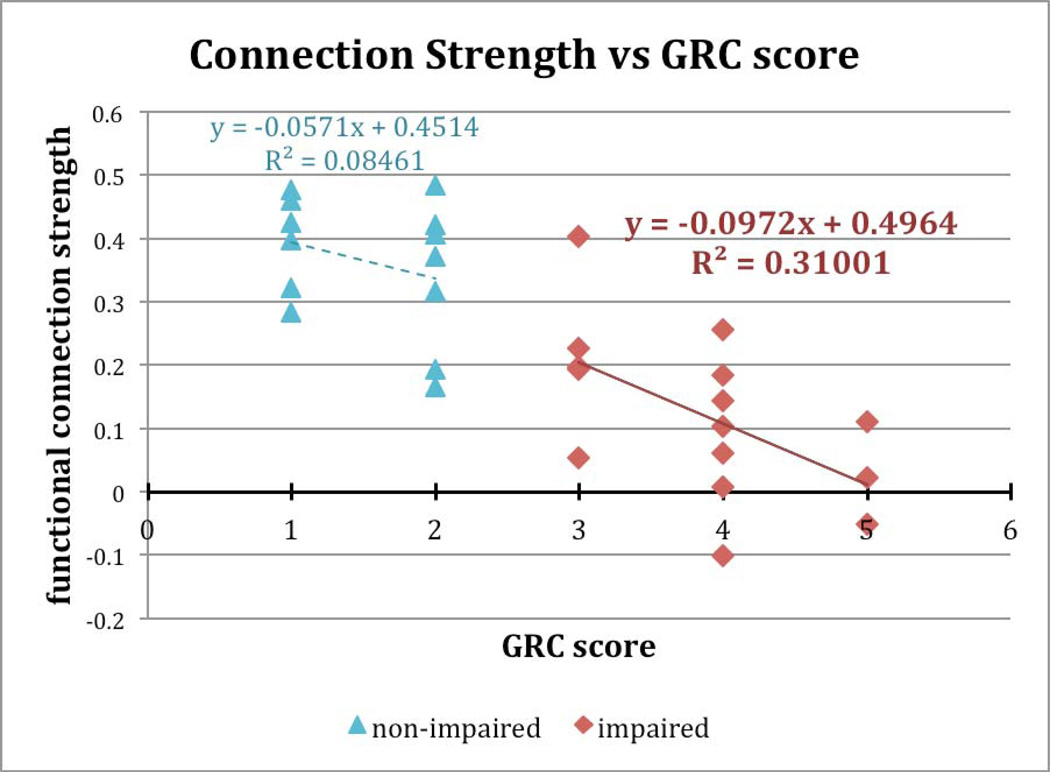
Further evidence that this functional connection may be atypical in the setting of chemotherapy-associated cognitive impairment is the relationship between an individual’s functional connection strength and her scores on both of the behavioral measures (CFQ and GRC) (Figure 3).
Figure 3.
Correlation values (standardized R) for the functional connection shown in Figure 2 plotted against the self-reported measure of severity of cognitive impairment, Global Rating of Cognition (GRC). Functional connection strength shows a robust correlation with cognitive impairment for the impaired patients, but not for the non-impaired patients.
The subject groups were defined by the GRC. Thus, we assessed whether correlation strength for this particular functional connection related to GRC scores only within groups. Simple linear regression was performed between the behavioral scores and the Fisher Z standardized R values for the reported connection, one value per subject. Within the impaired group, the correlation strength had a negative relationship with the GRC: with severely affected individuals (highest GRC scores) having the lowest functional connection strength and 31% of the variance explained (p = .031). By contrast, within the non-impaired group, there was a non-significant relationship between connection strength and GRC score (p = .34).
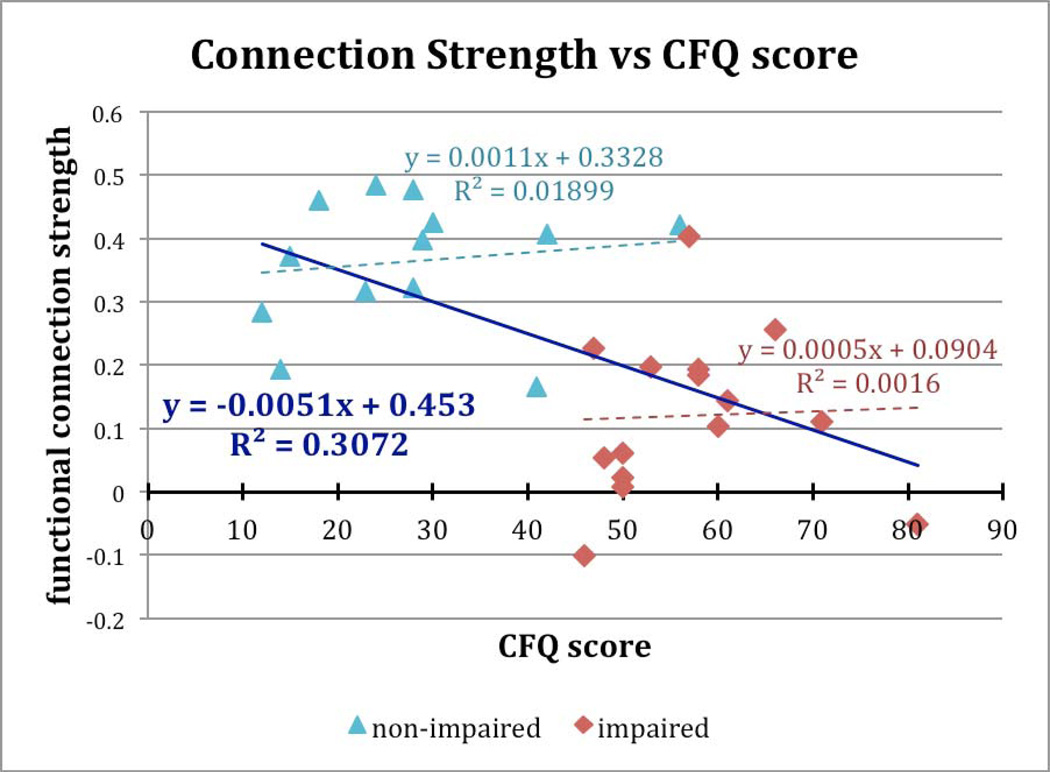
We also tested the relationship between the CFQ score and correlation strength across both groups (Figure 4). The negative correlation explains 31% of the variance (p = .0022). By contrast, no such relationship was determined within either the impaired (p= .89) or non-impaired (p = .65) group.
Figure 4.
Correlation values for the functional connection shown in Figure 2 plotted against the Cognitive Failures Questionnaire (CFQ) score. Higher values indicate greater perceived cognitive impairment. There is a robust negative correlation across all individuals with a higher functional connection strength associated with lower perceived impairment. However, no significant relationship was detected within either group alone.
To assess the impact of subject motion, we examined the present data based on both the 39 × 39 correlation matrix, as well as a larger matrix generated using a set of 264 regions[31] that includes those 39 regions and has a broader coverage over the cerebrum. Importantly, no distance-dependent artifact was found for either region set, indicating that the choices made for preprocessing adequately removed the potential contaminating effect of motion artifact from these data.
Discussion
The findings from this case-control, cross-sectional study of cognitive impairment in female breast cancer patients demonstrate that women who self-reported cognitive impairment were found to have disrupted functional connectivity within brain networks implicated in cognitive control. In addition, the identified disrupted functional connection indexed the extent of cognitive impairment within the group reporting impairment. These findings suggest that the standard therapeutic levels of chemotherapy for some breast cancer patients may result in altered functional connectivity in the brain networks supporting attention and executive function. This effect, in turn, may contribute to the self-reported cognitive difficulties after receiving chemotherapy among a subset of breast cancer patients.
We hypothesized that the executive dysfunction described by women with chemotherapy-induced cognitive impairment would localize to brain systems critical for executive or “top-down” control. Specifically, building upon our “dual networks architecture for top-down control”[13], where a distinct fronto-parietal system oversees rapid, adaptive online control and a separate cingulo-opercular system oversees stable, resilient task set maintenance, we hypothesized that there would be disrupted resting-state functional connectivity within these now well-defined systems. The primary result from the functional connectivity data, that a single functional connection between two regions within the fronto-parietal system shows a robust difference in strength between chemotherapy-treated breast cancer survivors who do or do not describe CACI, is partially consistent with this hypothesis. In addition, the observation that the strength of this functional connectivity seems to provide an index of the perceived severity of impairment with the group experiencing impairment, lends plausibility to the relationship.
The majority of published studies that have investigated CACI have only used batteries of standard neurocognitive tests. The International Cognition and Cancer Task Force (ICCTF)[1] defines impairment as scoring 1.5 standard deviations below average on one or more standard neurocognitive assessments. Unfortunately, this definition reflects a population level pre-post function difference rather than an individual difference. In addition, the use of neuropsychological tests is problematic because these tests are subject to practice effects and do not fully describe the extent of cognitive impairment.[16;42] Patients may report difficulty in performing mental tasks while simultaneously scoring within a normal range of cognitive function. Self-reported measures of cognition are more sensitive in detecting subtle cognitive changes that may be functionally relevant to the patient.[2]
Previous neuroimaging research demonstrated abnormalities in brain structure after chemotherapy among breast cancer patients.[43–45] For example, Deprez et al[46;47] used diffusion tensor imaging (DTI) to study the cerebral white matter (WM) integrity in women with breast cancer who received chemotherapy. Compared to controls, the breast cancer patients showed decreased fractional anisotropy (FA) in frontal and temporal WM tracts and increased mean diffusivity (MD) in frontal WM. Reduced FA is typically interpreted to reflect reduced white matter integrity. Analysis of the study’s results showed a significant correlation between FA and performance on standard neuropsychological tests. In a subsequent nested case control study, there were significant decreases of FA in breast cancer patients after exposure to chemotherapy. In addition, performance changes in attention and verbal memory correlated with mean regional FA changes. The authors concluded that concurrent longitudinal changes in WM integrity and cognition were observed after chemotherapy treatment.
The fronto-parietal system is thought to be important for the initiation and rapid adjustment of control during the carrying out of attention-demanding tasks.[13] Future work should test further the hypothesis that this system is differentially affected in patients with CACI. For example, it would be helpful to relate strength of functional connectivity to psychometric measures of executive control. In addition, task-based fMRI, using tasks designed to address rapid, adaptive online control, could be helpful in investigating the relationship discerned based on resting state functional connectivity data.
Bruno, Kesler, and Hosseini[8] in 2012demonstrated that the functional network architecture of the brains of women with breast cancer who received chemotherapy differed on standard network metrics in comparison to women who did not receive chemotherapy. The findings based on resting-state functional connectivity MRI and graph theory based approaches suggested alterations leading to decreased network efficiency and implicated brain systems important for executive function. Subsequent work, in collaboration with Kesler et al.[9] demonstrated that using machine learning and a multivariate pattern classification approach on resting state functional connectivity MRI data of the Default Mode Network, the authors were able to classify single individuals as belonging to either a healthy control group, or either a chemotherapy-treated or non-chemotherapy treated breast cancer survivor group. In follow-up work, implementing the same machine learning, pattern classification approach but this time using task-based fMRI from an attention demanding task for functional connectivity, these authors[48]demonstrated a comparably robust capacity to classify the same set of individuals accurately as belonging either to healthy controls, chemotherapy or non-chemotherapy treated patients with breast cancer. Successful classification appeared to place greatest weight on brain regions in frontal and parietal cortex. Findings of McDonald et al.[11] showed decreased frontal gray matter density after chemotherapy, which was accompanied by self-reported difficulties in executive functioning. Kesler et al[12] found significantly reduced activation in the left middle dorsolateral prefrontal cortex and premotor cortex in breast cancer survivors compared with healthy controls. Breast cancer survivors who received chemotherapy demonstrated significantly reduced left caudal lateral prefrontal cortex activation and increased perseverative errors and reduced processing speed. And finally, Kesler et al.[9] concluded that disrupted DMN connectivity may help explain long-term cognitive difficulties following chemotherapy in breast cancer patients. Taken together, these observations implicate alterations in the overall functional network architecture in the brains of chemotherapy-treated breast cancer patients and fit the notion that chemotherapy effects on cognition are unlikely to be restricted to a specific region or set of regions. Thus, we do not believe that a single functional connection is sufficient as an explanation for the pathobiology associated with chemotherapy-associated cognitive changes. Along those lines, multivariate pattern classification of the form implemented by Hosseinni, Kesler and colleagues[48] has the substantial likelihood of providing additional capacity to demonstrated CACI effects beyond that available from standard univariate analyses. While these studies were designed to address the question of how chemotherapy in the setting of breast cancer influences the brain’s functional network architecture, our study is designed to address the question of which brain systems are altered differentially in patients who, in the setting of chemotherapy, report cognitive deficits. A larger sample size than the current study may make it possible to discern additional group differences.
Limitations
The present study has several limitations. First, the numbers of patients with and without self-reported cognitive impairment is relatively small. Low power produces an increased risk of both Type 1 and Type II errors. While stringent multiple comparison correction methods were implemented to reduce the likelihood of type II errors, there remains a concern for both spurious false positive and false negative findings. Thus, the results reported in the present study must be considered those of a pilot investigation. Second, the percentage of women receiving hormonal therapy was significantly different between the two groups. We believe this finding is spurious and does not reflect a true biological effect or diminish the findings of disrupted functional connectivity among women who received chemotherapy. To explore the role of hormonal therapy in breast cancer women with CACI a larger study should be conducted. Third, as the women are received a large number of chemotherapy agents, we were unable to explore whether specific agents are more likely to cause CACI and changes in functional connectivity.
Clinical Implications
The clinical relevance of the results from this pilot investigation is noteworthy. Understanding the brain systems implicated in chemotherapy-associated cognitive impairment has a substantial likelihood of shaping rational interventions, both pharmacotherapeutic and cognitive/behavioral. In addition, having neuroimaging biomarkers for CACI, in combination with increasingly sophisticated analysis strategies, such as multivariate pattern analysis, increases the possibility that we will be able to predict which patient with breast cancer is most likely to suffer from CACI. That sort of predictive power could lead to alterations in chemotherapeutic regimens, or implementation of preemptive interventions, or other approaches to mitigate cognitive burdens.
Acknowledgments
This research was supported, in part, by a grant from the Foundation for Barnes-Jewish Hospital- Cancer Frontier Fund (7540-55), NIH CTSA Grant (UL1 TR000448), and the Intellectual and Developmental Disabilities Research Center at Washington University (NIH/NICHD P30 HD062171).
This research used REDCap for data collection and storage and this part was supported by Clinical and Translational Science Award (CTSA) Grant [UL1 TR000448] and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842).
We acknowledge the assistance of Caroline Bumb, MHS in the identification and recruitment of study subjects, Timothy Wolf, OTD, MSCI, OTR/L for intellectual input regarding measuring and reporting results of cognitive impairment in the study protocol and manuscript, and Jonathan Peelle, PhD for his contribution to the interpretation of the data and critical review of the manuscript.
Footnotes
Presented at the 2013 San Antonio Breast Cancer Symposium, San Antonio Texas, December 2013.
The studies presented in this work were conducted using the scanning and special services in the MIR Center for Clinical Imaging Research located at the Washington University Medical Center.
References
- 1.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WL, Suls J. New Approaches to Understand Cognitive Changes Associated With Chemotherapy for Non-Central Nervous System Tumors. [available online March 21, 2013];J Pain Symptom Manage. 2013 doi: 10.1016/j.jpainsymman.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 4.Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79(4):798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbetta M, Tansy AP, Stanley CM, Astafiev SV, Snyder AZ, Shulman GL. A functional MRI study of preparatory signals for spatial location and objects. Neuropsychologia. 2005;43(14):2041–2056. doi: 10.1016/j.neuropsychologia.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48(3):329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A. 2013;110(28):11600–11605. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Farrell E, MacKenzie J, Collins B. Clearing the air: a review of our current understanding of "chemo fog". Curr Oncol Rep. 2013;15(3):260–269. doi: 10.1007/s11912-013-0307-7. [DOI] [PubMed] [Google Scholar]
- 11.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30(Suppl):S117–S125. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesler SR, Kent JS, O'Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68(11):1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Tannock IF, Ahles TA, Ganz PA, van D. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. Journal of Clinical Oncology. 2004;22(11):2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 17.Broadbent DE, Cooper PF, Fitzgerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 18.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 21.Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM. Functional connectivity of the amygdala in early-childhood-onset depression. J Am Acad Child Adolesc Psychiatry. 2011;50(10):1027–1041. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry. 2012;53(9):964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MP, Kelly M, Laumann T, Miller KL, Moeller S, Petersen S, Power J, Salimi-Khorshidi G, Snyder AZ, Vu AT, Woolrich MW, Xu J, Yacoub E, Ugurbil K, Van E, Glasser MF. Resting-state fMRI in the Human Connectome Project. Neuroimage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sylvester CM, Barch DM, Corbetta M, Power JD, Schlaggar BL, Luby JL. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1326–1336. doi: 10.1016/j.jaac.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talairach J, Tousignant P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- 29.Talairach J, Tournoux P, Missir O. Referentially oriented cerebral MRI anatomy: An atlas of sterotaxic anatomical correlations for gray and white matter. New York: G. Thieme Verlag; 1993. [Google Scholar]
- 30.Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality-independent approach to spatial normalization of tomographic images of the human brain. Human Brain Mapping. 1995;3(3):209–223. [Google Scholar]
- 31.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 33.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 34.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31(4):1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 35.Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power JD, Petersen SE. Control-related systems in the human brain. Curr Opin Neurobiol. 2013;23(2):223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, Gennatas ED, Jackson C, Prabhakaran K, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2014.10.044. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinately large population. Biometrika. 2014;10(4):507–521. [Google Scholar]
- 42.Rubens FD, Boodhwani M, Nathan H. Interpreting studies of cognitive function following cardiac surgery: a guide for surgical teams. Perfusion. 2007;22(3):185–192. doi: 10.1177/0267659107080943. [DOI] [PubMed] [Google Scholar]
- 43.Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 44.Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103(3):303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 45.De Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33(12):2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den SJ, Smeets A, Christiaens MR, Leemans A, Van HW, Vandenberghe J, Vandenbulcke M, Sunaert S. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32(3):480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van HW, Verhoeven JS, Christiaens MR, Vandenberghe J, Vandenbulcke M, Sunaert S. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30(3):274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 48.Hosseini SM, Kesler SR. Multivariate Pattern Analysis of fMRI in Breast Cancer Survivors and Healthy Women. J Int Neuropsychol Soc. 2013:1–11. doi: 10.1017/S1355617713001173. [DOI] [PMC free article] [PubMed] [Google Scholar]