Abstract
MicroRNAs, activated by the enzyme Dicer1, control post-transcriptional gene expression. Dicer1 has important roles in the epithelium during nephrogenesis, but its function in stromal cells during kidney development is unknown. To study this we inactivated Dicer1 in renal stromal cells. This resulted in hypoplastic kidneys, abnormal differentiation of the nephron tubule and vasculature, and perinatal mortality. In mutant kidneys, genes involved in stromal cell migration and activation were suppressed as were those involved in epithelial and endothelial differentiation and maturation. Consistently, polarity of the proximal tubule was incorrect, distal tubule differentiation was diminished, and elongation of Henle’s loop attenuated resulting in lack of inner medulla and papilla in stroma-specific Dicer1 mutants. Glomerular maturation and capillary loop formation were abnormal while peritubular capillaries, with enhanced branching and increased diameter, formed later. In Dicer1-null renal stromal cells, expression of factors associated with migration, proliferation and morphogenic functions including α-smooth muscle actin, integrin-α8, -β1, and the WNT pathway transcriptional regulator LEF1 were reduced. Dicer1 mutation in stroma led to loss of expression of distinct microRNAs. Of these, miR-214, -199a-5p and -199a-3p regulate stromal cell functions ex vivo, including WNT pathway activation, migration and proliferation. Thus, Dicer1 activity in the renal stromal compartment regulates critical stromal cell functions that, in turn, regulate differentiation of the nephron and vasculature during nephrogenesis.
Keywords: DICER1, FOXD1, microRNA, stroma, podocytes, tubule differentiation
INTRODUCTION
Increasing evidence suggests that the renal stroma plays critical, instructive roles through tissue-interactions during kidney development.1 Recent cell ablation studies of the renal stroma have provided evidence that it plays a role in regulating nephron progenitor cells and vasculature.2,3 Inactivation of the transcription factor FOXD1 in the cortical stroma results in expansion of nephron progenitor cells and a severe deficit in differentiation.1,4,5 Inactivation of β-catenin in stromal tissues of the developing kidney leads to loss of elongation of the loop of Henle of the differentiating nephron tubule,6 and recent studies have also identified Notch signaling in the renal stroma as a regulator of vascular patterning.7,8
MicroRNAs (miRNAs) are a family of more than 2000 small non-coding RNAs that function as post-transcriptional regulators and are increasingly recognized as important regulators of gene expression.9,10 miRNAs are synthesized in the nucleus, processed by the RNase III enzyme Drosha, exported to the cytoplasm and cleaved for subsequent activation by the RNase III known as Dicer1.11,12 Therefore, Dicer1 inactivation results in complete inactivation of miRNA function. Activated miRNAs are loaded into a complex including the Argonaute protein, which enables the miRNA to bind by sequence complementarity to mRNA.9,13 A single miRNA can bind to 50–100 functionally related mRNA. This binding leads to gene silencing by miRNA mediated degradation, and translational suppression by disruption of the ribosomal complex.9,12,13 Therefore miRNA activity may regulate sets of genes for specific biological processes during development, metabolism, and homeostasis. Recent studies have identified important roles for post transcriptional regulators including miRNAs in podocytes,14,15 juxtaglomerular (JG) cells,16 nephron epithelium and collecting duct system of the developing kidney17,18 and in epithelial and stromal cells during adult kidney diseases.10,19,20 However, the importance of miRNAs in stromal cells has not been explored during kidney development.
Renal stromal cells derive from the cortical stroma overlying the cap mesenchyme.6,21 This layer of mesenchymal cells in the zone of nephrogenesis expresses the transcription factor FOXD1. These Foxd1+ progenitor cells give rise to all the stroma of the developing kidney. Renal stromal cells become vascular smooth muscle cells (VSMCs), glomerular mesangial cells, pericytes and fibroblasts of the mature kidney.21 As described above, mice lacking Foxd1 show severe defects in kidney organogenesis including markedly reduced kidney volume, longitudinal fusion, ventral rotation, smaller collecting system and a marked decrease in the number of nephrons. The defects are so severe that it is difficult to understand, from studying these mutants, the functional role of Foxd1+ mesenchymal progenitors and the stroma they give rise to in nephrogenesis.1,4
We therefore tested the hypothesis that deletion of the miRNA activating enzyme Dicer1 in Foxd1+ stromal progenitors may define the importance of post-transcriptional regulation by miRNAs in the stromal tissues during kidney organogenesis. Dicer1 inactivation in the renal stroma resulted in hypoplastic kidneys with abnormal differentiation of the nephron tubule and vasculature. Three miRNAs -214, -199a-5p and -199a-3p were enriched in the renal stroma and regulate stromal cell functions ex vivo. Taken together, these observations suggest that Dicer1 activity in the renal stromal compartment regulates differentiation of nephron and vascular compartments of the developing kidney.
RESULTS
Dicer1 inactivation in the Foxd1+ cortical stroma results in multiple defects of nephrogenesis
Foxd1+ nephrogenic progenitors are located in the cortical stroma surrounding the cap mesenchyme in the nephrogenic zone (Supplementary Figure S1A). These progenitors give rise to all of the stromal cells of the developing kidney, including mesangium and vascular smooth muscle (Supplementary Figure S1B).21,22 Many of these stromal cells are attached to forming capillaries whereas others are closely associated with the developing tubule (Supplementary Figure S1B).
To inactivate the miRNA processing RNase III gene Dicer1 in the stromal tissues during kidney development, we crossed the Foxd1-eGFPCre (Foxd1 GC) allele with the Dicer1 flox allele (Figure 1A). In the Dicer1 flox allele, the exon 23 of the Dicer1 gene is flanked by two loxP sites.23 This exon encodes most of the second RNase III domain, and therefore removal of the exon results in a null allele.23 Offspring with the genotype Foxd1+/GC; Dicer1fl/fl were born at below the expected Mendelian ratio (expected 12.5%, actual 9.8% [n=22/225]), and survived for a maximum of 2 days after birth (Figure 1B). Dicer1 is highly expressed in kidney during development (www.genepaint.org) and Cre activity sufficient to cause widespread recombination under the Foxd1 regulatory sites was confirmed from E10.5 onward (Supplementary Figure S1B).21 Inactivation of the DICER1 enzyme only in stromal compartment of the kidney was confirmed by immunostaining using an antibody that recognizes an epitope present on full-length protein (Figure 1C and F).24 Kidneys of these mutant mice were smaller and bladders uniformly empty (Figure 1, D and G, Supplementary Table 1), suggesting that the vascular supply and nephrons are sufficiently disrupted that there is no effective production of urine. Although kidney failure is evident from these findings, the uniform death of mice within two days of birth, the observation of cyanosis (Supplementary Figure S2A), and the histological abnormalities in the lung at birth including reduction in branching, and septation resulting in fewer alveolae with smaller diameter (Supplementary Figure S2B), suggests respiratory failure may also contribute to the high mortality in the early post natal period. Consistent with such observations, we previously identified that the perivascular stromal lineage in the lung derives from Foxd1+ lung progenitor cells.25
Figure 1. Dicer1 inactivation in renal stromal progenitors results in neonatal lethality and profound disruption of nephrogenesis.

(A) Gene map showing the site of recombination of genomic DNA in cells that activated Foxd1 (B) Kaplan-Meier plots showing survival after birth of mutant mice. (C) Immunodetection of DICER1 (arrowheads, interstitial positively stained cell) indicates absence in renal stromal cells of P0 mutant mice. Bar, 25 μm. (D) Wholemount urological tract from mutant vs control mice showing typical features including small kidney size, and empty small bladder. (E) Low power images of PAS stained kidneys showing characteristic abnormalities, including absence of papilla, shortened medulla and cystic changes. (F) Higher power PAS stained images of medulla and cortex showing typical changes including loss of papilla, shortened medulla and abnormalities of tubules including reduced brush border, dilatation of tubules, loss of polarization of epithelial cells (arrowheads), and glomerular abnormalities including cystic change and cuboidal glomerular morphology. Many podocytes retain an epithelial morphology. (G) The total number of glomeruli per unit area of the kidney was reduced and (H) the width of the nephrogenic zone is reduced. Bar, 25 μm. **P < 0.01, n = 3/group.
Coronal section of Dicer1 mutant kidneys revealed absence of the inner medulla and a shortened outer medulla (Figure 1E and H, Supplementary Figure S1D). Higher magnification revealed that glomeruli were present close to the innermost portion of kidneys and proximal tubules could be detected in this area, suggesting attenuation or absence of the outer medulla as well as the inner medulla. Nephron tubules in the cortex were abnormal showing reduced polarization identified by nuclei not restricted to the basolateral position, lack of columnar dimensions, a reduction in formation of brush border and, in some instances, tubular distension/dilatation and occasionally frank cyst formation (Figure 1I–N, Supplementary Figure S1D). Glomeruli were also abnormal. Many glomeruli were cystic and some showed dilated vessels. In addition podocytes retained a cuboidal epithelial morphology (Figure 1K and N) which is normally lost as podocyte differentiation proceeds. Finally, the number of glomeruli in mutant kidneys was reduced (Figure 1O), suggesting that branching morphogenesis or nephrogenesis within the nephrogenic zone was reduced. Consistent with a reduction in nephrogenesis, the nephrogenic zone was reduced in thickness (Figure 1P).
Global Transcriptional analysis of mutant kidneys identifies impaired development of stromal cells, epithelial maturation and vasculogenesis
To explore the global effects of the Dicer1-null renal stromal cells on nephrogenesis we compared the global transcriptome of Foxd1+/GC; Dicer1fl/fl kidneys at E15.5 with Foxd1+/GC; Dicer1fl/+ kidneys at the same timepoint (Figure 2). This comparison identified 372 differentially regulated genes with the majority of genes, downregulated. One of the most significantly affected pathways was the pathway defined by hepatic stellate cell activation for which all 16 genes of the annotated pathway were downregulated (Figure 2A). Genes which regulate microvascular adhesion and endothelial junction formation were the next most downregulated (Agranulocyte adhesion and diapedesis), and epithelial tight junction signaling and vesicle transport were prominently downregulated (tight junction signaling) (Figure 2A). In addition integrin-like kinase (ILK) signaling was downregulated. A more detailed look at prominently affected gene ontologies identified 149 genes that regulated functions critical to stromal cell biology (Figure 2B). These included stromal cell activation and migration with many downregulated genes. In addition to impaired stromal cell biology inferred from these findings, there were also many genes affected that are involved in epithelial differentiation and vascular development (Figure 2B). Furthermore many genes involved in TGFβ, WNT, AKT and IGFBP1 signaling were under-expressed in mutant kidneys compared with wild type.
Figure 2. Global transcriptional analysis of kidneys from Foxd1+/GC; Dicer1fl/fl and control mice provides unbiased insight to the effect of Dicer1 mutation in stromal cells on nephrogenesis.
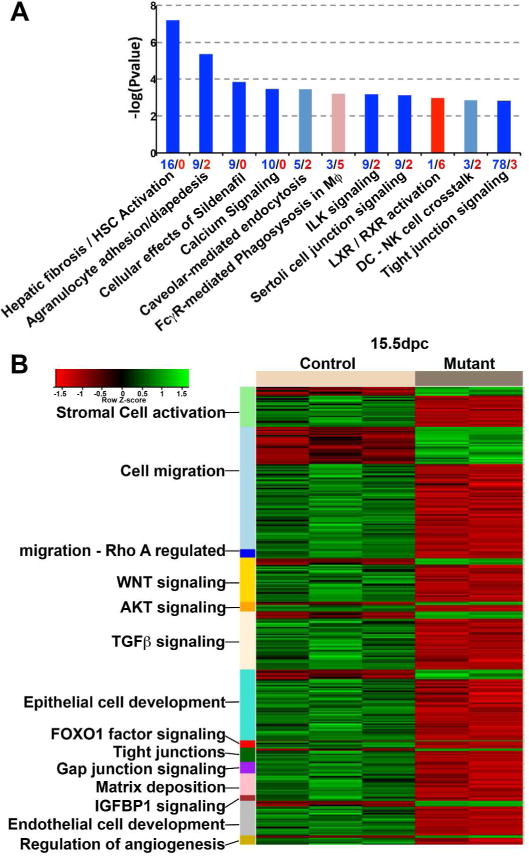
(A) Pathways analysis of Foxd1+/GC; Dicer1fl/fl kidneys compared to control at E15.5 showing the most significantly regulated canonical pathways where red indicates mainly upregulated genes and blue, downregulated genes. Height of the bar reflects the statistical enrichment (−log10 P-value) for the pathways, while the numbers on the x-axis reflect the total number of regulated genes in each pathway (red = up, blue = down). (B) Heatmap showing subsets of the significantly differentially expressed genes comparing control and Foxd1+/GC; Dicer1fl/fl kidneys that belong to specific ontologies.
Stromal cells are impaired in mice lacking Dicer1 in the renal stroma
As predicted by the transcriptional analysis the Dicer1-null renal stromal cells showed reduction in the expression of many typical genes that denote migration. PDGFRβ, a marker of stromal cells, is a receptor involved in migration and vasculogenesis, and was decreased in mutant kidneys, particularly in the innermost zone (Figure 3A and B). The contractile protein α-smooth muscle actin (αSMA) which is strongly expressed in the medullary interstitium and weakly in the cortical interstitium, was almost absent in Foxd1+/GC; Dicer1fl/fl kidneys, while expression surrounding the arteriolar vasculature was intact (Figure 3A and C). Tenascin C, normally expressed predominantly in the nephrogenic and cortical interstitium, was reduced in the mutant kidney (Figure 3D, Supplementary Figure S3A). Integrin-α8 and -β1 receptors, implicated in adhesion, migration and angiogenesis and upstream of ILK signaling,26,27 are highly expressed in renal stromal cells in both medulla and cortex, with integrin-α8 most highly expressed also in the cap mesenchyme. Expression of integrin-α8 and integrin β1 was delayed in development of Foxd1+/GC; Dicer1fl/fl kidneys, such that both receptor subunits were reduced at embryonic day 15.5 (E15.5), E18.5 and postnatal day 0 (P0) (Figure 3E and 3F, Supplementary Figure S3B and S3C). In addition to impaired expression of receptors and proteins involved in activation, stromal cells showed impaired proliferative capacity from E15.5 onward as determined by the cell cycle marker Ki67 (Figure 3G–I).
Figure 3. Dicer1 inactivation in renal stromal progenitors impairs their activity, patterning and expression of integrins.
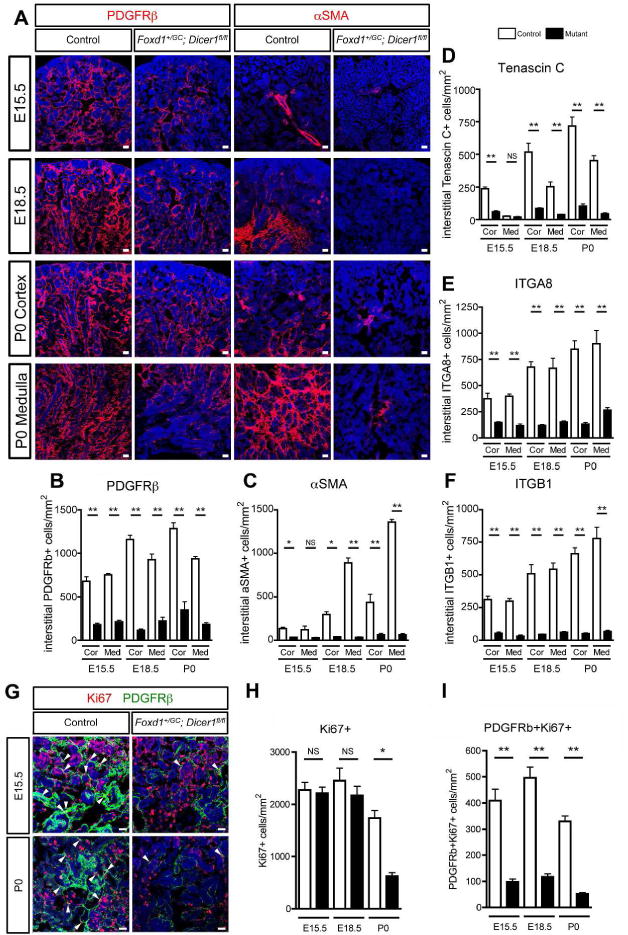
(A) Images of PDGFRβ and αSMA positive stromal cells of the developing kidney. Note that mutant stromal cells showed strikingly reduction in the expression of these genes at P0. (B–C) Quantification of number of PDGFRβ+ (B) and αSMA+ (C), Tenascin C+ (D), integrin α8+ (E) and integrin β1+ (F) stromal cells per unit area of kidney. (G) Images of Ki67− and PDGFRβ-expressing cells of the developing kidney. Note that mutant stromal cells show reduction in the expression of Ki67 at E15.5 and P0. Arrowheads indicate interstitial positively stained cells. (H–I) Quantification of number of Ki67+ (H) and Ki67+PDGFRβ+ (I) cells per unit area of kidney. Bar, 25 μm. *P < 0.05, **P < 0.01, n = 3/group.
Abnormal differentiation of ureteric bud and nephron differentiation in mice lacking Dicer1 in the renal stroma
Transcriptional analysis indicated epithelial cell differentiation was perturbed when stromal cells lacked Dicer1 (Figure 2). To explore this further, differentiation of epithelial tissues was examined using tissue-specific makers for the proximal tubule (lotus lectin [Lotus Tetragonolobus Lectin (LTL)]), thick ascending limb of the loop of Henle (Uromodulin, [UMOD] also known as Tamm–Horsfall protein), and collecting duct ([Dolichos bifloris agglutinin (DBA)]) at E15.5, E18.5 and P0. At E15.5, the number of epithelial cells expressing all the above markers was significantly decreased (Figure 4), suggesting that differentiation of the ureteric and nephron epithelium was disturbed. At P0, the number of tubules in the medulla (defined by inner area without glomeruli) expressing the collecting duct marker DBA of mutant mice was significantly reduced (Figure 4A and B), suggesting the distribution of collecting ducts was also disturbed in mutant kidneys. By contrast even in P0 kidneys very few cortical tubules expressed UMOD (distal tubules) and in the medulla many fewer tubules (loop of Henle) expressed UMOD, suggesting differentiation of the distal tubule is markedly impaired and that loop of Henle formation is also significantly impaired (Figure 4A and C). The UMOD+ loop of Henle did not show extended structures in mutant kidneys at P0, suggesting lack of elongation of the loop of Henle. Finally the number of cortical tubules expressing LTL was reduced, consistent with impaired differentiation of the proximal tubules (Figure 4A and D). The aberrant expression of LTL in the medulla in mutant kidneys at P0 was observed due to lack of elongation of the loop of Henle. At higher magnification (insets in Figure 4A), LTL expression, which is normally found predominantly at the brush border in controls, was expressed evenly throughout the tubules of mutant kidneys, consistent with impaired differentiation of the proximal tubules. These findings indicate nephron differentiation was delayed or halted in premature states while ureteric differentiation was retarded.
Figure 4. Dicer1 inactivation in renal stromal progenitors results in impaired nephron elongation, segmentation and polarization.
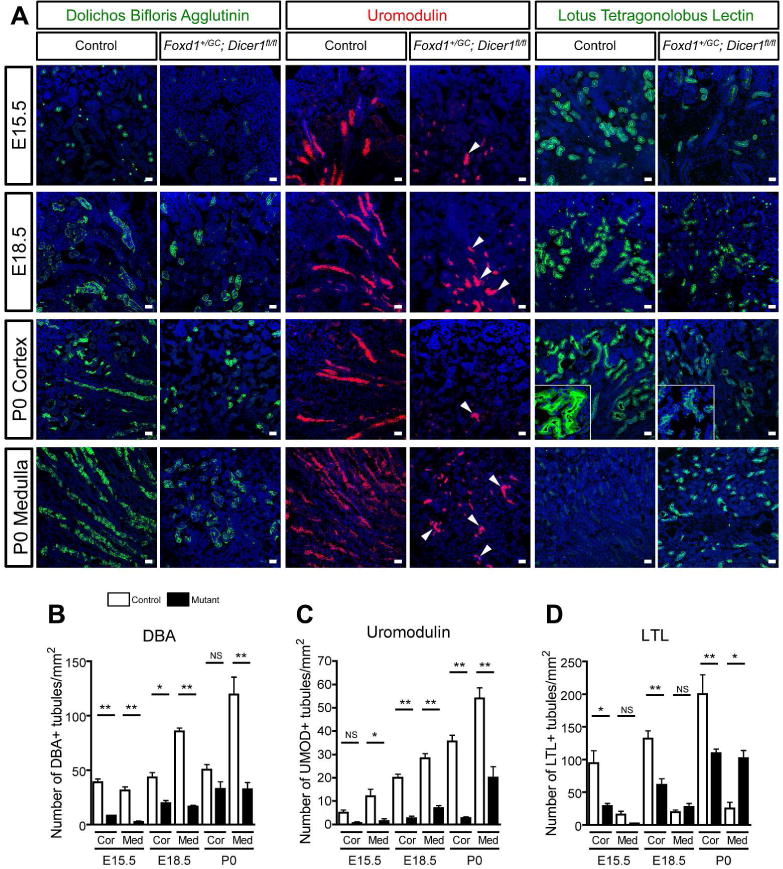
(A) Images showing markers of proximal tubule (LTL), collecting duct (DBA) and loop of Henle / Distal tubule (Uromodulin) differentiation in E15.5, E18.5 and P0 kidneys. Note the presence of proximal tubules in the medulla of mutant mice, and the loss of polarization of proximal tubules (inset). Note that expression of all markers is delayed in mutant embryos and that Uromodulin is almost absent in the cortex of mutant P0 kidneys. In the medulla, Uromodulin labels very short U-shaped turns in the tubules (arrowheads) in mutants whereas in controls the tubules are long and linear. (B–D) Quantification of positive stained tubules per unit area of kidney. Bar, 25 μm. *P < 0.05, **P < 0.01, n = 3/group.
Differentiation of podocytes is impaired when Dicer1 was inactivated by Foxd1-Cre in the mouse
Although podocytes derive from the proximal end of the developing nephron epithelia and therefore arise from Six2+ nephron epithelial progenitors in the cap mesenchyme, podocyte precursors in developing glomeruli activate the transcription factor FOXD1 late in development from E15.5 onward (Supplementary Figure S1A–B).28 Foxd1 is necessary for cortical stromal progenitor cells to differentiate into stromal cells of the interstitium including mural cells (VSMCs and pericytes)21 as exemplified by the severe developmental defects, including the persistence of cuboidal epithelial morphology seen when Foxd1 was mutated (Figure 5A).4 It is likely therefore that late expression of Foxd1 in maturing podocytes is important in the transition of embryonic epithelial podocyte precursors into mature podocytes which exhibit some pericyte-like functions and characteristics.29,30 The failure of formation of mature podocytes was apparent in Foxd1−/− kidneys (Figure 5A). It was striking therefore that Dicer1 was deleted in podocytes in neonates (Supplementary Figure S1C) and that differentiating podocyte precursors in Foxd1+/GC; Dicer1fl/fl kidneys did not adopt certain mature podocyte characteristics (Figure 1N, Figure 5F). Podocytes from mutant mice were larger, more cuboidal, and present in smaller numbers (Figure 5B–G). The expression intensity of the transcriptional regulator Wilms tumor 1 (WT1), normally expressed by all mature podocytes, was reduced in E18.5 and P0 glomeruli (Figure 5E). Although mutant podocytes closely associated with capillary loops and formed foot processes, glomerular endothelial cell fenestration was reduced (Figure 5F). Overall these findings confirm a role for podocyte miRNA in maturation, and implicate FOXD1 as an important transcription factor in podocyte maturation.
Figure 5. Dicer1 inactivation in renal FOXD1 lineage results in abnormal podocyte differentiation.

(A) Images of Foxd1−/− glomeruli. The failure of podocytes to mature from epithelial progenitors is readily apparent in Foxd1−/− kidneys by the persistence of cuboidal epithelial morphology. (B) Images showing expression of the mesenchymal transcriptional regulator WT1 in podocytes and the nephrogenic zone during nephrogenesis. Note that in mutant kidneys the expression of WT1 in glomerular cells is markedly reduced at E18.5 and P0 (inset). (C–D) Quantification of positive stained cells of WT1 in the nephrogenic zone (C) and glomeruli (D). (E) Quantification of glomerular fluorescence intensity of WT1. (F) Representative EM images showing lack of mesangium in mutant glomeruli. Note in mutant mice the podocytes show bigger and there is wrinkling and collapse of the capillary loop structure and extensive foot process effacement (arrowheads) (F) Quantification of podocytes diameter. Bar, 25 μm (A,B), 2 μm (E).
Microvascular patterning is abnormal in mice lacking Dicer1 in the renal stroma
Transcriptional analysis indicated vasculogenesis was perturbed in mutant kidneys. To ascertain the role of stromal cells in capillary and arteriolar development, kidneys were labeled with a capillary marker, CD31 (Figure 6A). At E15.5 the proportion of endothelial progenitors that remained unattached to other endothelial cells and therefore not forming a capillary structure was increased in mutant kidneys (Figure 6A–B). By P0, peritubular capillaries had formed but the patterning of capillaries was abnormal. They exhibited increased branching and increased capillary diameter. These changes were more prominent in the cortex (Figure 6A, C–E), implicating roles for stromal cell miRNAs in vasculogenesis. Pericytes of the peritubular capillaries had abnormal vacuolation (Figure 6E). Mesangial cells lacked Dicer1 in Foxd1+/GC; Dicer1fl/fl kidneys (Supplementary Figure S1C) and the mesangium of a number of glomeruli was also abnormal (Figure 5F, 6F and G). All glomeruli showed reduced mesangial cellularity and eighteen percent of glomeruli lacked a mesangium and showed a single lumen capillary surrounded by immature podocytes. Together with the changes to podocytes, these defects in the mesangium probably resulted in a failure of glomerular maturation in the Dicer1 mutant kidneys (Figure 5F, 6H and I). Arteriolar covering by VSMCs was also evaluated. Although there were fewer arterioles identified in mutant kidneys, the covering by the αSMA+ vascular smooth muscle was also modestly diminished (Figure 6J–L).
Figure 6. Dicer1 inactivation in renal stromal progenitors results in abnormal vascular patterning.

(A) Images of CD31+ endothelium of the developing kidney. Note that at E15.5 many endothelial cells of the PTCs are not connected to one another in mutant kidneys (arrowheads) whereas in controls they already form a connected vasculature. In P0 kidney cortex, mutant capillaries are now connected but show very wide capillaries with enhanced branching (arrow). These differences were less apparent in the medulla. (B–D) Quantification of number of unconnected vessels (B), number of branching points per unit area (C) and diameter of CD31+ vessels (D). (E) Representative EM images of peritubular capillaries showing abnormal dilatation, and pericytes containing abnormal vacuoles in mutant kidneys (arrowheads). L, capillary lumen; Pc, pericytes; EC, endothelial cells; Fb, fibroblasts. (F) PAS stained sections showing absent or attenuated mesangium (arrow) in some mutant glomeruli at P0. (G) Quantification of number of glomeruli lacking mesangium. Glomeruli which lacked a mesangium showed a single lumen capillary surrounded by immature podocytes. (N.D., not detected). (H–I) Quantification of glomerular maturity index at P0 (3 > 2 > 1). Mature glomeruli were decreased both in outer (H) and inner cortex (I) in mutant kidneys. (J–K) Images of VSMCs (J; PAS stain, K; αSMA+ expression) in control and mutant kidneys showing a reduction in number of layers of smooth muscle. (L) Quantification of thickness of αSMA+ VSMCs. Bar, 25 μm (A, F, J, K). 2 μm (E). *P < 0.05, **P < 0.01, n = 3–5/group.
Maintenance of the nephrogenic zone is impaired in mice lacking Dicer1 in the renal stroma
Because mutant kidneys had a thinner nephrogenic zone (Figure 1) with fewer glomeruli than control counterparts, we evaluated the maintenance of Six2+/Cited1+ nephron epithelial progenitors in the cap mesenchyme of the nephrogenic zone. We found that the number of SIX2+ cap mesenchyme cells was reduced in the Dicer1 mutant kidneys at E15.5, E18.5 and P0 (Figure 7A and B). In addition, a subset population of CITED1+ cells in the cap mesenchyme was reduced in cell number in the mutant kidney at all stages (Figure 7A and C). The number of FOXD1+ cortical stromal cells in the nephrogenic zone was also decreased (Supplementary Figure S1A).
Figure 7. Dicer1 inactivation in renal stromal progenitors reduces the maintenance of epithelial progenitors.

(A) Images of SIX2 and CITED1 positive cap mesenchyme cells of the developing kidney. Note that mutant stromal cells showed markedly reduction in the expression of these genes both at E15.5 and P0. (B–C) Quantification of number of SIX2+ (B) and CITED1+ (C) cap mesenchyme cells in the nephrogenic zone. Bar, 25 μm. *P < 0.05, **P < 0.01, n = 3/group.
Dicer1 inactivation in stromal cells results in reduced WNT signaling and downstream target genes involved in nephron elongation
Previous reports indicate that Wnt/β-catenin signaling in stromal cells plays important roles in nephron elongation through crosstalk between the collecting duct and stroma and also the loop of Henle of the nephron, where ineffective WNT signaling to stromal cells results in failure of elongation of the loop of Henle with lack of the inner medulla and papilla.6 WNT pathway activation is deficient in mutant kidneys (Figure 2B). In addition, Dicer1 inactivation in the stromal cells also results in insufficient elongation of the loop of Henle, and absence of the inner medulla and papilla. The expression of Wnt4 and 11 ligands, implicated in signaling from stromal cells to the nephron tubule, was significantly reduced in Foxd1+/GC; Dicer1fl/fl kidneys at P0 (Figure 8A). In contrast Wnt7b (expressed by the developing nephron) was unaffected (Figure 8A).6,31 Consistent with perturbed WNT signaling, the expression of WNT/β-catenin target genes including Axin2, Lef1 and Wnt1 inducible signaling pathway 1 (Wisp1) were reduced in Foxd1+/GC; Dicer1fl/fl kidneys (Figure 8A). Strikingly, nuclear LEF1, a WNT responsive nuclear factor, was almost absent from the stromal cells of the Dicer1 mutant kidneys. β-catenin accumulation in stromal cells in the medulla of Foxd1+/GC; Dicer1fl/fl kidneys was also decreased both in the cytosol and nuclei (Figure 8B–C, E–F). p57Kip2 encodes a cyclin dependent kinase inhibitor, which regulates progression at the G1 checkpoint in the cell-cycle. It has been reported to be a stromal cell target gene of Wnt7b signaling from the developing nephron and to play roles in renal medulla development.6,32 p57Kip2 expression was restricted to a subset of stromal cells in the normal renal medulla (Figure 8D and G), but Foxd1+/GC; Dicer1fl/fl kidneys showed a marked reduction in p57Kip2 protein expression at both E15.5 and P0 (Figure 8D and G). Collectively, these findings are consistent with the loss of miRNA activity in the renal stromal cells leading to abnormal regulation of canonical WNT/β-catenin signaling in Foxd1+/GC; Dicer1fl/fl kidneys.
Figure 8. Dicer1 mutation disrupts WNT signaling in renal stromal cells.

(A) qPCR data showing changes in the expression of Wnt4, Wnt 11 and regulators of the canonical Wnt signaling, Axin2, Lef1 and Wisp1 in whole kidney. (B–D) Confocal images of LEF1 (B), β-catenin (C) and p57Kip2 (D) in developing renal medulla. Arrowheads indicate interstitial positively stained cells. Note that mutant stromal cells showed marked reduction in the expression of LEF1 and β-catenin accumulation as well as reduction in p57Kip2 at both E15.5 and P0. (E–G) Quantification of number of LEF1+ (E), nuclear β-catenin+ (F) and p57Kip2+ (G) per mm2 of kidney. Bar, 25 μm. **P < 0.01, n = 3–5/group.
Dicer1 mutation in stromal cells inhibits expression of stromal cell miRNA involved in migration, proliferation and cell-cell signaling
To identify the effect of Dicer1 deficiency in stromal cells on miRNA expression, the miRNAome was interrogated by miRCURY LNA™ miRNA Array of E15 and P0 mutant and wild type kidneys (Figure 9A). Forty-six miRNA were expressed at significantly lower levels in mutant kidneys, and this result was unaffected by stage of development. To identify which of these candidate miRNAs were expressed by stromal cells and might therefore be functionally important, PDGFRβ+ renal stromal cells were purified from wild type embryos and examined for miRNA expression levels using miRNA Array (Supplementary Figure S4A). Compared with the PDGFRβ− renal cells, PDGFRβ+ renal stromal cells were enriched in a restricted number of miRNAs. Several miRNA clusters were enriched in stromal cells consistently throughout the developmental stages evaluated and were subsequently downregulated in post-natal kidneys. When the two datasets were crossed-referenced, a restricted number of miRNA both downregulated in mutant kidneys and enriched in stromal cells was identified. These included miR-214, miR-199a-3p, miR-199a-5p, miR199b-5p miR-143-3p and miR32-5p (Figure 9A, Supplementary Figure S4B). Predicted target genes of these miRNAs include Wnt signaling pathway regulators, vascular patterning regulators including Notch, cell cycle regulators, and regulators of the migratory machinery (Supplementary Figure S4C).
Figure 9. Dicer1 mutation results in loss of stromal cell microRNA that serve to promote human renal stromal cell migration, proliferation and expression of factors.
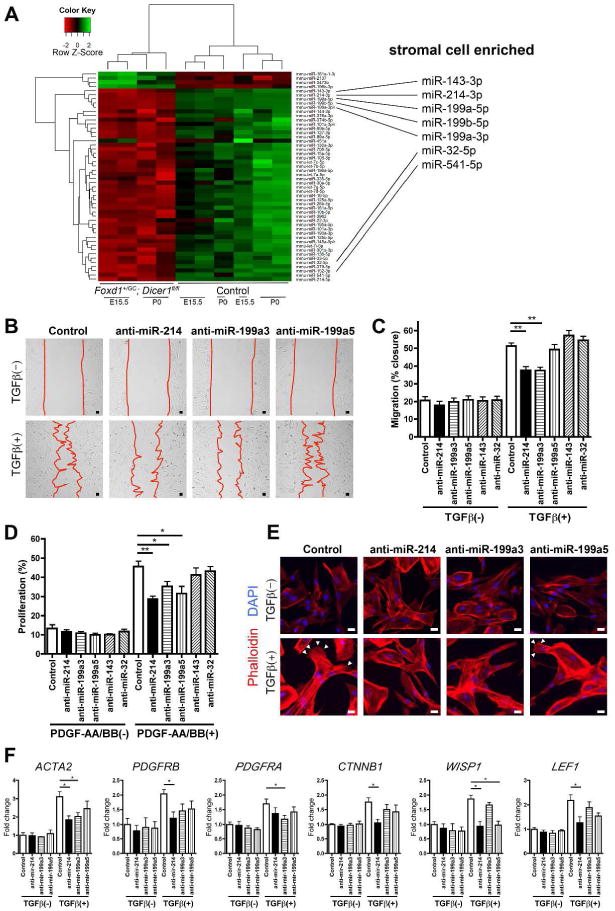
(A) Heatmap showing hierarchical cluster analysis of significantly differentially regulated microRNA between control, Foxd1+/GC; Dicer1fl/fl, E15.5 and P0 kidneys. Mutant kidneys cluster together and show 46 miRNA with reduced expression compared with 4 which were increased. When these miRNA were interrogated against miRNA that are enriched in stromal cells 7 miRNA were identified (right panel). (B) Photomicrographs showing effect of anti-miR-214 and -199a-3p on migration. (C) Graph showing the effect of different anti-miRNAs on migration when stimulated by TGFβ after 24h. (D) Graph showing the effect of different anti-miRNAs on proliferation when stimulated by a combination of PDGF-AA and -BB after 16h. (E) Images of phalloidin-Cy3 stained stromal cells showing the formation of lamellipodia and filopodia in response to TGFβ at 24h (arrowheads), a response markedly diminished following silencing of miR-214 or miR-199-3p (F) Graphs showing the effect of anti-miR treatment on transcript levels. Bar, 25 μm. *P < 0.05, **P < 0.01. n = 3–5/group
Silencing of Dicer1-regulated miRNAs in human renal stromal cells impairs cell migration, proliferation and WNT pathway responses
Candidate miRNAs were evaluated for their effect on human fetal renal stromal cells, using specific anti-miRNA oligonucleotides which bind by sequence complementarity and silence specific miRNA activity.19 Silencing of miR-214 or miR-199a-3p inhibited human stromal cells in a migration assay (Figure 9B–C). Silencing of miR-214, miR-199a-3p or miR-199a-5p reduced the capacity of stromal cells to proliferate in response to PDGFs (Figure 9D). Close examination of the morphology of human renal stromal cells indicated that upon silencing of miR-214 or miR-199a-3p, TGFβ did not induce the formation of organized lamellipodia and filopodia, the cytoskeletal projections necessary for migration (Figure 9E). The effect of silencing these miRNAs on expression of transcripts for WNT pathway components, WNT response genes, PDGF receptors, integrins and contractile proteins was evaluated. Similar to mouse stromal cells, human stromal cells were enriched in WNT4 and WNT11 and transcripts indicative of active WNT pathway activity (Supplementary Figure S5). Silencing of miR-214 markedly attenuated the upregulation of ACTA2, PDGFRB, and WNT pathway genes, CTNNB1, WISP1 and LEF1 in response to TGFβ stimulation, whereas silencing of miR-199a-3p attenuated PDGFRA upregulation, and silencing of miR-199a-5p prevented upregulation of the WNT/βcatenin responsive gene WISP1 (Figure 9F). Silencing of these miRNAs had no effect on WNT4 or WNT11 expression and had no effect on expression of integrin-β1 or -α8 (not shown).
DISCUSSION
These studies highlight a critical role for post-transcriptional modification of gene expression by miRNA in renal stromal cell function during nephrogenesis. Dicer1 deficiency leads to loss of a restricted number of individual stromal cell miRNA that when silenced disrupt important stromal functions, including migration, and proliferation and cell-signaling. Such stromal cell miRNA include the co-regulated miR-214/199 cluster. The studies also uncover many unappreciated facets of stromal cell biology in nephrogenesis, and provide further insight into the critical role for stromal cells in maintaining nephron progenitors, regulating both nephron differentiation as well as microvascular patterning. Many of these critical functions of stromal cells are disrupted by the loss of miRNA activity, culminating in perinatal lethality. One of these functions is regulation of the differentiation and maturation of the nephron tube. Without Dicer1, stromal cells do not support nephron elongation or normal segmentation. Finally, the expression of Foxd1 and consequent activation of miRNA late in nephrogenesis by epithelial podocyte precursor cells plays important roles in their differentiation from cuboid epithelial cells into highly branched and functioning vascular wall cells.
Progenitor cells lying in the cortical stroma overlying cap mesenchyme, activate Foxd1 from E10.5 onward until P4. We have previously shown that Foxd1+ progenitors silence Foxd1 expression as they migrate to the renal interstitium and differentiate into stromal cells that surround developing nephrons and microvasculature.21 Foxd1 progenitor-derived stromal cells seen during nephrogenesis, develop into VSMCs, pericytes, perivascular fibroblasts and mesangial cells as the kidney matures.21,33,34 Therefore from the time that progenitor cells activate Foxd1 throughout the nephrogenic period, both progenitors and their descendent cells lack Dicer1 expression.
Inactivation of Foxd1 by targeted deletion has been reported to impact nephrogenesis severely.1,2,4 However the defects in kidneys from mice lacking Foxd1 are so severe that characterization of Foxd1 progenitor-derived stromal functions has not been feasible. Deletion of the transcriptional regulator, β-catenin in renal stroma was reported to disrupt loop of Henle elongation as a result of WNT/β-catenin bidirectional crosstalk with epithelial cells,6 providing evidence that renal stromal cells play important roles in the nephrogenic process. Expression of Wnt ligands 7b, 4 and 11 were proposed to contribute to that bidirectional signaling.6,31 These studies presented here show similar attenuated elongation of the loop of Henle. We show that Dicer1 inactivation results in loss of WNT signaling activity and accompanied by deregulation of WNT ligands including 4, 11 with reduced LEF1 and WNT/β-catenin signaling in medullary stroma, suggesting that miRNA regulate WNT pathway signaling in renal stroma. Dicer1 inactivation in stromal cells, however, has more widespread effects on nephron tube differentiation, including markedly attenuated distal tubule differentiation as well as impaired polarization of the proximal tubule. Therefore Dicer1 inactivation has broader effects on stromal cell functions than simply disrupting WNT/β-catenin signaling. Additional pathways may include TGFβ and FGF signaling,35 integrin signaling, signaling by the FOXO1 transcription factor, as well as disruption of endocytic and phagocytic signaling pathways.
Dicer1 inactivation in the renin-secreting arteriolar VSMCs including JG cells has been reported to cause striped fibrosis in adult kidneys.16 This is presumably a consequence of systemic hypotension due to lack of renin secretion and also nephron hypoperfusion as a result of afferent arteriolar abnormalities. Unlike these reports, we did not see evidence of fibrosis in mutant kidneys, although our observations have been limited to neonatal kidneys. Although Foxd1 progenitor-derived cells become VSMCs including JG cells, the inactivation in Foxd1 lineage stroma resulted in deletion of Dicer1 in the interstitial stoma as well as VSMCs and as a consequence resulted in a much more profound phenotype than that restricted to arteriolar VSMCs.
Dicer1 inactivation in Foxd1+ stroma has profound effects on gene expression and patterning within the stromal compartment itself, suggesting that stromal cells lacking Dicer1 lack a broad range of functions including normal migration, activation and angiogenic function, and that miRNA are critical in stromal cell organization and differentiation. Stromal cells in other organs including the lung where epithelium is derived from endoderm,36,37 have been shown to play broad roles in regulating epithelial differentiation, therefore it is noteworthy that although renal epithelium is derived largely by a mesenchyme to epithelial transition process, it nevertheless requires crosstalk with surrounding stromal cells for correct differentiation and segmentation.
Our analysis identified approximately 25 miRNA enriched in the renal stroma during development, 10 of which were enriched at both E15.5 and E18.5 but down regulated after birth. Seven of these were significantly under-represented in Dicer1 mutant embryonic kidneys implicating these miRNA in stromal cell functions. We evaluated 5 of these in functional and transcriptional assays of purified human fetal renal stromal cells. Strikingly miR-214, -199a-3p and -199a-5p all contributed to migration, proliferation and WNT pathway activation. These miRNA cluster on the opposite strand of the Dynamin-3 gene and have been reported to play important roles in skin and muscle development, as well as regeneration after injury.38–40 Further understanding of these miRNA and others that are temporally regulated in renal stroma are warranted, since our findings implicate these miRNA as positive regulators of critical stromal cell functions.
Recent studies have indicated that many Foxd1 progenitor-derived cells become pericytes of the renal peritubular capillary plexus,21,41 but their functions in creation of that plexus have not been explored. The findings reported here indicate that stromal cells, many of which are attached to forming capillaries do contribute to microvascular patterning as has been shown in other tissue beds.42,43 Mesangial cells derive from Foxd1+ progenitors, and in almost 20% of glomeruli there was a failure of the mesangium to form altogether suggesting misdirection of mesangial cells. In such glomeruli, no capillary loops formed, suggesting mesangial cells regulate capillary loop formation. Importantly, despite the failure of loop formation, endothelium had developed in apposition to immature podocytes forming a single, wide capillary. In these and other glomeruli at P0, there was an additional striking feature. Podocytes appeared uniformly immature. This was characterized by the persistence of cuboidal epithelial phenotype of podocytes and the lack of integration with the capillaries. Podocytes arise from the S-shaped body at the blind end of the developing nephron,31 and derive from Six2+/Cited1+ progenitors or cap mesenchyme similar to the other cells of the non-collecting duct nephron,31,44 Somewhat confusingly, Foxd1 is expressed late during embryogenesis in these podocyte progenitors once the glomerular capillaries form. Since Foxd1 is critical for stromal cell differentiation, which become mural cells of the vasculature, it seems that activation of Foxd1 is a critical step in transition of podocyte epithelial progenitors into mature podocytes which share many characteristics with mural cells. It is striking, therefore, that as Dicer1 and miRNA activity is silenced in podocyte progenitors only after E18.5, this silencing of miRNA is sufficient to disrupt normal podocyte differentiation. These observations suggest podocyte miRNA are critical in differentiation steps and that the differentiation of podocytes requires activation of a stromal cell program. An alternate explanation for the podocyte phenotype is that the mesangium is necessary for podocyte maturation. Future experiments in which Foxd1 is deleted only in podocytes is would resolve the role of Foxd1 in podocyte differentiation.
Another observation in these studies is that the number of nephrons that formed was significantly reduced. Whereas Foxd1 mutant kidneys have fewer than 25% of the normal glomerular complement,1,4 Dicer1 inactivation in Foxd1+ progenitors resulted in about a 50% reduction in glomeruli. Dicer1 mutant kidneys had two defects that may explain this reduction. Firstly the nephrogenic zone is reduced in cellularity and this is largely due to reductions in Six2+/Cited1+ progenitors. Secondly the differentiation and branching of the ureteric bud is reduced. A reduction in branching of the ureteric bud will result in fewer glomeruli and this observation suggests Foxd1+ cortical stroma may regulate ureteric bud branching. Since the pool of Six2+ progenitors that form epithelial cells is also reduced it indicates that Foxd1+ cortical stromal progenitors regulate the maintenance of the Six2+ epithelial progenitors, and is consistent with recent observations when cortical stroma was ablated.2 Collectively these studies identify novel functions for both cortical stromal progenitors and the stoma itself in nephron differentiation.
Compared with deletion of Dicer1 in Six2+ progenitors of the kidney,17,18 the phenotype of Dicer1 inactivation in Foxd1+ progenitors shows broader effects on organ development. This may be because of the dual role of stromal cells in vascular and epithelial development, but it also suggests that miRNA in stroma may play greater roles in regulation of critical genes. Although beyond the scope of these current studies, identification of additional functionally significant miRNA in renal stromal cells and podocytes during differentiation will lead to greater understanding of miRNA and cell functions in nephrogenesis.
We conclude that Dicer1 activity in Foxd1+ progenitors of the cortical stroma and their progeny is critical to nephron and vascular development in the kidney.
MATERIALS AND METHODS
Animals
The conditional Dicer1 allele (Dicer1c) was previously described23 and purchased from Jackson Labs. The Dicer1− allele was generated from the Dicer1c allele with the Foxd1GFP-Cre (Foxd1GC). The Foxd1+/GC mouse was generated using standard techniques as described.21 Age-matched littermates with Foxd1+/GC; Dicer1fl/+ were used as controls. Foxd1+/GC mice were also bred with Rs26tdTomato allele45 (B6.Cg-Gt(ROSA)26Sor<tm14(CAG-tdTomato)Hze>/J) to report offspring of Foxd1 progenitors in the developing kidney. Genotyping was performed with the following primer pairs: (i) Dicer1 forward: CCTGACAGTGACGGTCCAAAG, Dicer1 reverse: CATGACTCTTCAACTCAAACT (ii) Foxd1-GC common: TCTGGTCCAAGAATCCGAAG, Foxd1-GC wild-type forward: CTCCTCCGTGTCCTCGTC, Foxd1-GC mutant forward: GGGAGGATTGGGAAGACAAT. Stromal cells were purified from CD-1 mouse kidneys. All studies were carried out under approved IACUC protocols held at University of Washington and Harvard Medical School.
Experimental Mouse kidney collection
Mouse embryos were collected from pregnant females at E15.5 and E18.5 using standard methods, or neonates euthanized immediately at birth (P0). Kidneys and the urological system were dissected for imaging or transferred to fixatives for analysis. In some experiments pups remained with mothers for up to 72h and were censored at each 24h after birth, and then euthanized at 72h.
Statistical analysis
Results are presented as mean ± SEM (standard error of the mean). Statistical analyses were carried out using GraphPad Prizm (GraphPad Software). To analyze the difference between 2 groups, 2-tailed Student’s t test was used. When more than 2 groups were present, one-way analysis of variance (ANOVA) followed by Turkey’s honestly significant difference (HSD) post hoc test was used. A P value less than 0.05 was considered significant.
Supplementary Material
Figure S1. Expression and lineage tracing of Foxd1 in nephrogenic interstitium, cortical stroma and podocytes. (A) Images of FOXD1 positive cells of the developing kidney. FOXD1+ cortical stroma progenitors, detected by anti-GFP antibodies in Foxd1+/GC kidneys, can be seen in the nephrogenic zone overlying cap mesenchyme and are unaffected in Foxd1+/GC; Dicer1fl/fl kidneys. Foxd1 expression persists in stroma of the nephrogenic zone surrounding the ureteric bud and renal vesicle but is not detected in differentiated stroma of renal cortex and medulla which derives from the Foxd1+ progenitors. In Foxd1+/GC; Dicer1fl/fl kidneys the expression of Foxd1 around ureteric bud and renal vesicles is markedly attenuated. Although podocyte progenitors also express Foxd1 from at E15.5 (insets), Foxd1 expression in podocytes is also attenuated in Foxd1+/GC; Dicer1fl/fl kidneys. (B) Images of Foxd1+/GC; R26tdTomato/+ kidneys at P0. tdTomato reflects Foxd1 expression. tdTomato+ cells are seen in stromal PDGFRβ+ cells but not in LTL+ proximal tubules, CD31+ vasculature and CD1 1b+ leukocytes. Note that tdTomato+ cells are also seen in many of WT1+ podocytes. (C) Images showing representative glomeruli indicating loss of full-length DICER1 expression in mesangial cells and also podocytes of the mutant glomerulus. (D) Images of PAS stained sections showing the medulla of control P0 kidney and Foxd1+GC; Dicer1fl/fl kidney. Note the presences of cysts and shortened loop of Henle (arrows) Bar, 25 μm
Figure S2. Lung development is disrupted by Dicer1 inactivation in Foxd1+ progenitor-derived stroma in the developing lung. (A) Images of newborn pups showing cyanosis in mutants. (B–C) Photomicrographs from E18.5 and P0 (B) and quantification at P0 (C) of H&E stained lung sections showing a reduction in branching, and septation (arrows) resulting in fewer alveolae with smaller diameter (2-way arrows). Bar, 25 μm. **P < 0.01, n = 3/group.
Figure S3. Dicer1 inactivation in renal stromal progenitors impaired their activity, patterning and expression of integrins (A–C) Images of Tenascin C (A), Integrin α8 (B) and β1 (C) positive stromal cells of the developing kidney. In mutant kidneys the expression of integrin α8 is reduced in the cap mesenchyme and medullary stromal cells at E15.5 whereas its expression is reduced modestly in the stromal compartment at P0. Integrin β1 expression is more profoundly reduced in the stromal cells in both cortex and medulla in mutant kidneys. Bar, 25 μm.
Figure S4. Renal stromal cells exhibit temporally regulated enrichment of miRNA during development compared to the epithelium and endothelium (A) Schema showing the purification of PDGFRβ+ and PDGFRβ− cell fractions from whole kidney at E15.5, E18.5 and P0 by magnetic immunoaffinity separation (B) Heat Map showing unsupervised hierarchical clustering for stromal miRNA (PDGFRβ+) vs. non-stromal (PDGFRβ−) miRNA at E15.5, E18.5 and P0. The clustering was performed on all samples, and on the 50 most highly expressed miRNAs with highest standard deviation. Green indicates higher values and Red indicates lower values. Enlarged is a cluster of miRNA that are enriched in PDGFRβ+ stroma at E15.5 and E18.5 but not at P0 (C) Abbreviated list of candidate target genes for each of these miRNA based on the Targetscan search algorithm (www.targetscan.org) for seed sequences complimentary to the miRNA in the 3′ untranslated regions and translated regions of all mRNAs.
Figure S5. Human fetal stromal cells express transcripts for WNT ligands and WNT response genes. Graph of Q-PCR transcript levels normalized to GAPDH
Acknowledgments
We wish to thank Lynn and Mike Garvey Microscopy Suite, Akio Kobayashi, Kelly Hudkins and Kimberly Muczynski (University of Washington), Bill Stallcup (Burnham Institute, San Diego, CA). These studies were supported by the Institute for Stem Cell and Regenerative Medicine, the University of Washington School of Medicine, NIH grants DK93493, DK94768, DK87389, AHA grant 12040023 to J.S.D, and by Biogen Idec. N.N. is supported by the Nephrology Division of Asahikawa Medical University.
Footnotes
DISCLOSURES
J.S.D. is employed by Biogen Idec, is on the scientific advisory board for Promedior Inc. and Regulus Therapeutics, has stock or stock options with Promedior Inc., Biogen Idec and Muregen LLC, is the co-founder of Muregen LLC, and has recently consulted for Pharmaceuticals: Abbvie, Takeda, Bristol-Myers Squibb, GlaxoSmithKline, Biogen Idec, and Boehringer Ingelheim.
References
- 1.Levinson RS, Levinson RS, Batourina E, et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 2.Das A, Tanigawa S, Karner CM, et al. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol. 2013;15:1035–1044. doi: 10.1038/ncb2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sims-Lucas S, Schaefer C, Bushnell D, et al. Endothelial Progenitors Exist within the Kidney and Lung Mesenchyme. PLoS ONE. 2013;8:e65993. doi: 10.1371/journal.pone.0065993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatini V, Hatini V, Huh SO, et al. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 5.Fetting JL, Fetting JL, Guay JA, et al. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development. 2014;141:17–27. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Yu J, Carroll TJ, et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle SC, Liu Z, Kopan R. Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development. 2014;141:346–354. doi: 10.1242/dev.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin EE, Sequeira-Lopez MLS, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol. 2014;306:F249–58. doi: 10.1152/ajprenal.00313.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yates LA, Norbury CJ, Gilbert RJC. The long and short of microRNA. Cell. 2013;153:516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Gomez IG, Grafals M, Portilla D, et al. MicroRNAs as potential therapeutic targets in kidney disease. J Formos Med Assoc. 2013;112:237–243. doi: 10.1016/j.jfma.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutvágner G, Hutvágner G, McLachlan J, et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga R, Han BW, Hung J-H, et al. Dicer partner proteins tune the length of mature miRNAs in flies and mammals. Cell. 2012;151:533–546. doi: 10.1016/j.cell.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo I, Heo I, Ha M, et al. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Cell. 2012;151:521–532. doi: 10.1016/j.cell.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Ho J, Ho J, Ng KH, et al. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol. 2008;19:2069–2075. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey SJ, Harvey SJ, Jarad G, et al. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sequeira-Lopez MLS, Sequeira-Lopez MLS, Weatherford ET, et al. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagalakshmi VK, Nagalakshmi VK, Ren Q, et al. Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney Int. 2011;79:317–330. doi: 10.1038/ki.2010.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho J, Ho J, Pandey P, et al. The pro-apoptotic protein Bim is a microRNA target in kidney progenitors. J Am Soc Nephrol. 2011;22:1053–1063. doi: 10.1681/ASN.2010080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chau BNB, Chau BNB, Xin CC, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4:121ra18–121ra18. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrasekaran K, Chandrasekaran K, Karolina DS, et al. Role of microRNAs in kidney homeostasis and disease. Kidney Int. 2012;81:617–627. doi: 10.1038/ki.2011.448. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys BD, Lin S-L, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harfe BD, Harfe BD, McManus MT, et al. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grelier G, Grelier G, Voirin N, et al. Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype. Br J Cancer. 2009;101:673–683. doi: 10.1038/sj.bjc.6605193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung C, Hung C, Linn G, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew S, Mathew S, Chen X, et al. Integrins in renal development. Pediatr Nephrol. 2012;27:891–900. doi: 10.1007/s00467-011-1890-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Zhang X, Mernaugh G, et al. beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development. 2009;136:3357–3366. doi: 10.1242/dev.036269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunskill EW, Brunskill EW, Georgas K, et al. Defining the molecular character of the developing and adult kidney podocyte. PLoS ONE. 2011;6:e24640. doi: 10.1371/journal.pone.0024640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin J, Jin J, Sison K, et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell. 2012;151:384–399. doi: 10.1016/j.cell.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Campanholle G, Ligresti G, Gharib SA, et al. Cellular Mechanisms of Tissue Fibrosis. 3. Novel mechanisms of kidney fibrosis. American journal of physiology Cell physiology. 2013;304:C591–603. doi: 10.1152/ajpcell.00414.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little MH, McMahon AP. Mammalian kidney development: principles, progress and projections. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P, Zhang P, Liégeois NJ, et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- 33.Rojas A, Chang F-C, Lin S-L, et al. The role played by perivascular cells in kidney interstitial injury. Clin Nephrol. 2012;77:400–408. doi: 10.5414/CN107371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffield JS, Humphreys BD. Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney Int. 2011;79:494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- 35.Plisov SY, Yoshino K, Dove LF, et al. TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development. 2001;128:1045–1057. doi: 10.1242/dev.128.7.1045. [DOI] [PubMed] [Google Scholar]
- 36.Zorn AM, Zorn AM, Wells JM, et al. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin Y, Yin Y, Wang F, et al. Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development. 2011;138:3169–3177. doi: 10.1242/dev.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aurora AB, Aurora AB, Mahmoud AI, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2• overload and cell death. J Clin Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed M, Ahmed M, Emelianov V, et al. microRNA-214 controls skin and hair follicle development via modulating the activity of Wnt, Edar and Bmp signalling pathways. J Invest Derm. 2012;132:S104–S110. [Google Scholar]
- 40.Shatseva T, Shatseva T, Lee DY, et al. MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. Journal of Cell Science. 2011;124:2826–2836. doi: 10.1242/jcs.077529. [DOI] [PubMed] [Google Scholar]
- 41.Dirocco DP, Kobayashi A, Taketo MM, et al. Wnt4/β-Catenin Signaling in Medullary Kidney Myofibroblasts. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20:205–214. doi: 10.1634/stemcells.20-3-205. [DOI] [PubMed] [Google Scholar]
- 43.Jeansson M, Jeansson M, Gawlik A, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi A, Kobayashi A, Valerius MT, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madisen L, Madisen L, Zwingman TA, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression and lineage tracing of Foxd1 in nephrogenic interstitium, cortical stroma and podocytes. (A) Images of FOXD1 positive cells of the developing kidney. FOXD1+ cortical stroma progenitors, detected by anti-GFP antibodies in Foxd1+/GC kidneys, can be seen in the nephrogenic zone overlying cap mesenchyme and are unaffected in Foxd1+/GC; Dicer1fl/fl kidneys. Foxd1 expression persists in stroma of the nephrogenic zone surrounding the ureteric bud and renal vesicle but is not detected in differentiated stroma of renal cortex and medulla which derives from the Foxd1+ progenitors. In Foxd1+/GC; Dicer1fl/fl kidneys the expression of Foxd1 around ureteric bud and renal vesicles is markedly attenuated. Although podocyte progenitors also express Foxd1 from at E15.5 (insets), Foxd1 expression in podocytes is also attenuated in Foxd1+/GC; Dicer1fl/fl kidneys. (B) Images of Foxd1+/GC; R26tdTomato/+ kidneys at P0. tdTomato reflects Foxd1 expression. tdTomato+ cells are seen in stromal PDGFRβ+ cells but not in LTL+ proximal tubules, CD31+ vasculature and CD1 1b+ leukocytes. Note that tdTomato+ cells are also seen in many of WT1+ podocytes. (C) Images showing representative glomeruli indicating loss of full-length DICER1 expression in mesangial cells and also podocytes of the mutant glomerulus. (D) Images of PAS stained sections showing the medulla of control P0 kidney and Foxd1+GC; Dicer1fl/fl kidney. Note the presences of cysts and shortened loop of Henle (arrows) Bar, 25 μm
Figure S2. Lung development is disrupted by Dicer1 inactivation in Foxd1+ progenitor-derived stroma in the developing lung. (A) Images of newborn pups showing cyanosis in mutants. (B–C) Photomicrographs from E18.5 and P0 (B) and quantification at P0 (C) of H&E stained lung sections showing a reduction in branching, and septation (arrows) resulting in fewer alveolae with smaller diameter (2-way arrows). Bar, 25 μm. **P < 0.01, n = 3/group.
Figure S3. Dicer1 inactivation in renal stromal progenitors impaired their activity, patterning and expression of integrins (A–C) Images of Tenascin C (A), Integrin α8 (B) and β1 (C) positive stromal cells of the developing kidney. In mutant kidneys the expression of integrin α8 is reduced in the cap mesenchyme and medullary stromal cells at E15.5 whereas its expression is reduced modestly in the stromal compartment at P0. Integrin β1 expression is more profoundly reduced in the stromal cells in both cortex and medulla in mutant kidneys. Bar, 25 μm.
Figure S4. Renal stromal cells exhibit temporally regulated enrichment of miRNA during development compared to the epithelium and endothelium (A) Schema showing the purification of PDGFRβ+ and PDGFRβ− cell fractions from whole kidney at E15.5, E18.5 and P0 by magnetic immunoaffinity separation (B) Heat Map showing unsupervised hierarchical clustering for stromal miRNA (PDGFRβ+) vs. non-stromal (PDGFRβ−) miRNA at E15.5, E18.5 and P0. The clustering was performed on all samples, and on the 50 most highly expressed miRNAs with highest standard deviation. Green indicates higher values and Red indicates lower values. Enlarged is a cluster of miRNA that are enriched in PDGFRβ+ stroma at E15.5 and E18.5 but not at P0 (C) Abbreviated list of candidate target genes for each of these miRNA based on the Targetscan search algorithm (www.targetscan.org) for seed sequences complimentary to the miRNA in the 3′ untranslated regions and translated regions of all mRNAs.
Figure S5. Human fetal stromal cells express transcripts for WNT ligands and WNT response genes. Graph of Q-PCR transcript levels normalized to GAPDH


