Abstract
Social inequalities in birth weight are an important population health concern as low birth weight is one mechanism through which inequalities are reproduced across generations. Yet we don’t understand what causes adverse birth outcomes. This study draws together theoretic and empiric findings from disparate disciplines—sociology, economics, public health, and behavior genetics—to develop a new integrative intra- and inter-generational model of preconception processes influencing birth weight. This model is empirically tested using structural equation modeling and population-level data containing linked mother-daughter pairs from the National Longitudinal Survey of Youth (NLSY79) and the Children of the NLSY79 (N=1,580 mother-daughter pairs). Results reveal that birth weight is shaped by preconception factors dating back to women’s early life experiences as well as conditions dating back three generations, via integrative intra- and inter-generational processes. These processes reveal specific mechanisms through which social inequality can transmit from mothers to children via birth weight.
Social inequalities in birth weight are well-documented. Rates of low birth weight (less than 2500 grams at birth) and small-for-gestational age (membership in the lowest decile of birth weight at each gestational age) are consistently higher among infants of poor and unmarried women, as well as among infants of non-Hispanic black and some Hispanic women (Blumenshine, Egerter, Barclay, Cubbin, and Braveman 2010; Goldenberg and Culhane 2007). For example, all else equal, infants born to married women weigh 76–80 grams more at birth than their counterparts born to unmarried women (Buckles and Price 2013; Kane Forthcoming). Similarly, in 2012, 7% of births to white women were low birth weight whereas 13% of births to black women—nearly twice as many—were low birth weight (Martin, Hamilton, Osterman, Curtin, and Mathews 2013). Although these disparities are, to some extent, inter-related, marital status, race-ethnicity, and socioeconomic status appear to independently affect birth outcome (Sullivan, Raley, Hummer, and Schiefelbein 2012).
Being born low birth weight has long-term implications for children—including higher risk of physical, cognitive, and psychosocial disadvantages (Paneth 1995). As a result, birth weight has been identified as one mechanism through which inequalities can be transmitted from parents to children (Case, Lubotsky, and Paxson 2002; Currie 2009; Currie and Moretti 2007). It is therefore unsurprising that improving perinatal health is a highly prioritized population health concern (U.S. Department of Health and Human Services 2014), as such efforts could have dramatic implications not only for the health and social well-being of future generations but also for population-level patterns of social inequality.
However, despite significant efforts advanced in multiple disciplines across the health and social sciences, we still don’t fully understand the etiological factors contributing to low birth weight or small-for-gestational age. This may be due, at least in part, to the fact that most studies on this topic have examined exposure to risk factors only during pregnancy. Yet prenatal behaviors and conditions that are linked with birth outcomes are also socially patterned (Blumenshine et al. 2010), suggesting they are likely rooted in processes pre-dating pregnancy. Accordingly, recent work has expanded the period of exposure to also include preconception risk factors, but many of these studies focus on risks and resources present within the twelve months leading up to conception, to the exclusion of earlier life events and experiences (Johnson, Posner, Biermann, Cordero, Atrash, Parker, Boulet, and Curtis 2006; van Dyck 2010).
This study adds to the literature by implementing a longer-term intra-generational approach that examines risk factors and resources presenting within individuals across childhood, adolescence, and young adulthood. Such an approach is theoretically consonant with a life course perspective of health (Braveman and Barclay 2009; Halfon and Hochstein 2002; Kuh, Ben-Shlomo, Lynch, Hallqvist, and Power 2003; Richardson, Hussey, and Strutz 2012) and is empirically supported by a large body of work on social inequalities in health documenting long-term effects of early life course experiences, such as exposure to persistent poverty in childhood, on numerous indicators of adult health (Poulton, Caspi, Milne, Thomson, Taylor, Sears, and Moffitt 2002). This approach will contribute new knowledge to the preconception health literature by detailing intricate intra-generational pathways through which early life exposures shape birth weight.
Social scientists and behavior geneticists have also studied this topic but tend to approach it from a different angle, by linking birth weight across parents and children. This inter-generational approach demonstrates striking and persistent similarities in low birth weight among mother-daughter pairs (Conley and Bennett 2000; Conley and Bennett 2001; Currie and Moretti 2007), and shows that fetal and maternal genetic processes explain a portion (less than half) of the intergenerational similarity (Lunde, Melve, Gjessing, Skjærven, and Irgens 2007; Magnus 1984; Magnus, Berg, Bjerkedal, and Nance 1984; Magnus, Gjessing, Skrondal, and Skjaerven 2001; Magnus, Bakketeig, and Skjaerven 1993; Vlietinck, Derom, Neale, Maes, Van Loon, Derom, and Thiery 1989). However, no studies in this area have also explored social factors, such as intergenerational similarities in maternal educational attainment prior to birth, that may further describe these intergenerational processes. This study implements this more comprehensive approach, and, in doing so, expands our understanding of intergenerational processes that ultimately affect birth outcomes.
In sum, promising new directions to more fully understand the etiological factors contributing to low birth weight and small-for-gestational age include implementing both longer-term intra-generational processes and more comprehensive inter-generational processes, but empirical work in both of these areas remains nascent. This study takes a novel approach by expanding, and ultimately combining, each of these approaches to contribute a broader preconception model of factors influencing birth weight.
In the sections that follow, I first introduce and develop a conceptual model integrating intra- and inter-generational preconception processes that may influence birth outcomes, by drawing together theory and empirical findings from disparate disciplines. Next, I empirically test this model using a structural equation modeling approach that simultaneously estimates the numerous pathways proposed, with data containing linked mother-daughter pairs from the National Longitudinal Survey of Youth (NLSY79) and the Children of the NLSY79. I conclude by describing the ways in which this integrative approach offers new insights into specific mechanisms through which inequality can be reproduced across generations via birth weight.
BACKGROUND
Seminal work in social stratification indicates that both intra- and inter-generational processes are involved in the development of social inequality and in the reproduction of social inequality across generations (Blau and Duncan 1967; Featherman and Hauser 1978). The original Blau and Duncan model proposed that father’s education and occupation (‘social origins’) are linked with child’s occupation (‘social destination’) via child’s education. Thus, social destination is accounted for by both inter-generational processes (social origins) and intra-generational processes (child’s education). Consistent with their approach, this study develops a model of integrative intra- and inter-generational preconception processes influencing birth weight, which have been identified as mechanisms through which social inequality can be reproduced across generations (Case, Lubotsky, and Paxson 2002; Currie 2009; Currie and Moretti 2007).
To develop such a model, several elements need to be explicitly connected, each of which stem from a different literature. The full model is presented in Figure 1; each component will be systematically introduced in the sections that follow. I first develop the intra-generational component—or, within-person processes through which earlier-life social disadvantages contribute to later-observed birth outcomes—by drawing on literature in public health and social inequalities in health. Next I develop the inter-generational components—or, processes driving social and biologic similarities observed across parents and children—by drawing on sociology, economics, and behavior genetics.
Figure 1.
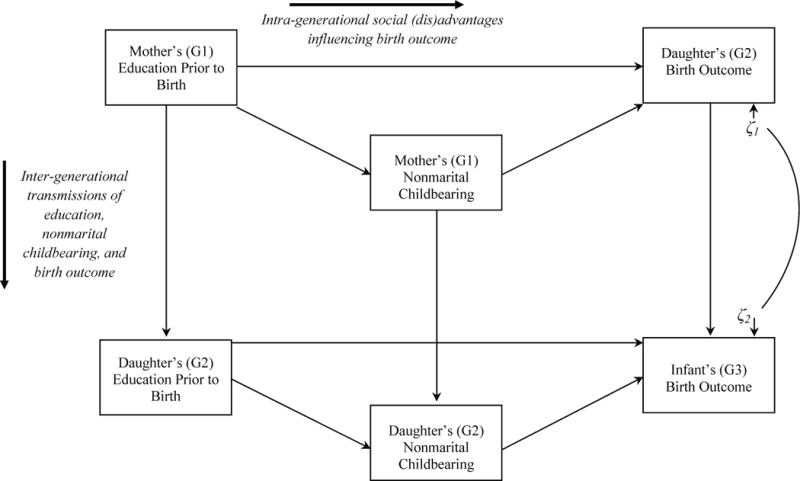
Inter- and Intra-generational Model of Birth Outcome, Human Capital, and Nonmarital Childbearing
Intra-Generational Mechanisms Linking Preconception Experiences to Birth Weight
Poor health can stem from a variety of sources, such as biologic/genetic pathways, social relationships, and the physical environment, but a key determinant of adult health is socioeconomic status (Warnecke, Oh, Breen, Gehlert, Paskett, Tucker, Lurie, Rebbeck, Goodwin, and Flack 2008), and, in particular, low socioeconomic status (SES) in childhood and adolescence (Chen, Martin, and Matthews 2006; Poulton et al. 2002).
To understand the mechanisms by which preconception socioeconomic disadvantage may influence birth weight, I turn to the broader literature on social inequalities in health. SES can determine access to health-related resources (Link and Phelan 1995). Education is one dimension of SES, and is a primary driver of health disparities; the mechanism being that lower levels of human or financial capital lead to differential access or utilization of healthcare (Ross and Wu 1996), and are associated with lower levels of social support (Thoits 1995) and greater exposure to cumulative stress and poor physical conditions in the environment (Adler, Bush, and Pantell 2012; Adler and Ostrove 1999; McEwen 1998). Education also increases individual agency, self-efficacy, and problem-solving capacity, each of which foster good health—a notion known as ‘education as learned effectiveness’ (Mirowsky and Ross 2003). Applied to the case of birth weight, a woman’s preconception level of education may stratify her employment opportunities, access to high-quality healthcare services or health-promoting activities, and level of social support in instrumental relationships (spouse, family members); in turn, these can be sources of stress that ultimately affect birth weight (Collins, Dunkel-Schetter, Lobel, and Scrimshaw 1993; Dole, Savitz, Hertz-Picciotto, Siega-Riz, McMahon, and Buekens 2003; Dunkel-Schetter, Gurung, Lobel, and Wadhwa 2001; Lobel, Dunkel-Schetter, and Scrimshaw 1992; Schetter 2009).
Another potential mechanism linking preconception education to birth weight is nonmarital childbearing. Lower levels of preconception human capital are associated with a higher likelihood of nonmarital childbearing (Carlson and England 2011; McLanahan 2004). In turn, being unmarried at birth is consistently associated with LBW (Albrecht, Miller, and Clarke 1994; Bennett 1992; Buckles and Price 2013; Shah, Balkhair, Ohlsson, Beyene, Scott, and Frick 2011). The latter association may reflect a number of circumstances. Unmarried women are more likely to live in materially deprived neighborhoods, report higher levels of prenatal anxiety and prenatal smoking, and report lower levels of prenatal social support and financial resources—each of which can affect birth outcomes (Kane Forthcoming; O’Campo, Xue, Wang, and Caughy 1997; Pagel, Smilkstein, Regen, and Montano 1990; Wadhwa, Sandman, Porto, Dunkel-Schetter, and Garite 1993). Although racial disparities in birth weight are not a focus of this study, it should be noted that a growing literature cites discrimination—a factor associated with many of these same circumstances—as a likely mechanism explaining stark racial disparities observed (Braveman 2011; Giscombé and Lobel 2005; Rosenthal and Lobel 2011).
In sum, intra-generational social disadvantage may influence birth weight through many different pathways. This study focuses on two pathways which are depicted in Figure 1 with horizontal arrows: a direct effect of preconception education on birth weight (through the implicit pathways described above), and an indirect effect of preconception education via nonmarital childbearing.
Inter-Generational Mechanisms Influencing Birth Weight
Inter-generational (parent-child) transmissions of birth weight, education, and nonmarital childbearing have been examined in sociology, economics, and behavior genetics. Social science studies using two different large-scale datasets (Panel Study of Income Dynamics and California birth certificate records) estimate that the odds of having a LBW infant are between 1.5 (Currie and Moretti 2007) and 2.0 (Conley and Bennett 2000) greater for mothers who were born LBW compared with mothers who were not born LBW. Thus, the intergenerational transmission of LBW is well-established. This association is represented in Figure 1 with a vertical line linking the birth outcomes of mothers and daughters.
Behavior geneticists have identified genetic influences on parent-child similarities in birth weight. Genetic factors account for somewhere between a quarter (Lunde et al. 2007; Magnus, Gjessing, Skrondal, and Skjaerven 2001) and 39 percent of the variance in birth weight (Vlietinck et al. 1989) and 14 percent of the variance in gestational age (Lunde et al. 2007). Although these effects are likely to be polygenic, one gene that may be involved in the intergenerational association of growth restriction is the gene for insulin-like growth factor I (Vaessen, Janssen, Heutink, Hofman, Lamberts, Oostra, Pols, and van Duijn 2002; Woods, Camacho-Hübner, Savage, and Clark 1996).
Unfortunately, as genetic factors are often unobserved in population-level survey data, it can be difficult to incorporate these associations into an empirical model. However, a statistical correction can be applied by correlating the residual of mother’s birth weight with that of daughter’s birth weight. This approach, proposed by Heckman (1979), is taken here (see Figure 1). Substantively, correlating residuals indicates that an unobserved (e.g., genetic) factor affecting one variable is associated with an unobserved (e.g., genetic) factor affecting another variable. Recall that within a regression approach, residuals of the dependent variable capture factors that remain unexplained by the model, genetic or otherwise. Therefore, this correlation parameter logically includes genetic and social similarities between mothers and children that affect birth weight yet remain unobserved in the model. This fact will be kept in mind in the interpretation of this parameter. Importantly however, explicitly estimating this correlation affords the opportunity to statistically control for the influence of shared genetic factors on birth weight (among other unobserved shared social traits), while also allowing for the estimation of other intra- and inter-generational pathways net of this genetic similarity—two features that are important in order to bridge the behavioral genetics literature in this area with that of public health and social science.
Theory and empirical evidence from family sociology and demography documents mother-daughter similarities in nonmarital childbearing and educational attainment around the time of a birth (Bumpass and McLanahan 1989; Furstenberg Jr, Levine, and Brooks-Gunn 1990; Kahn and Anderson 1992; Manlove 1997). Several mechanisms may account for these associations. One perspective highlights the role of socialization or role modeling. The family unit is an important social institution that shapes children’s values and beliefs, and socialization is the primary mechanism through which values are transmitted from parents to children (Bengtson 1975). Through socialization, children can adopt similar attitudes, values, and preferences related to childbearing and/or educational attainment as their parents; thus, socialization can account for intergenerational similarities in fertility timing and schooling (Anderton, Tsuya, Bean, and Mineau 1987; Axinn and Thornton 1993; Barber 2001a; Barber 2001b; Kahn and Anderson 1992; Manlove 1997; Thornton 1991; Thornton and Camburn 1987). Socialization can take on two forms: parents can establish priorities for their children or lead by example (Mustillo, Wilson, and Lynch 2004); either approach is an effective means to transmit beliefs and values from parents to children. In the case of human capital for example, parents may establish specific educational aspirations for their children and provide resources and guidance to help their children achieve these goals, or, they can lead by example—meaning, children may seek to achieve the same level of human capital as their parents achieved.
Another perspective emphasizes the role of family instability in intergenerational transmissions of childbearing behavior (Barber 2001b). Growing up in a single parent home is associated with greater odds of nonmarital childbearing among daughters (Amato and Kane 2011; Aquilino 1996). Similarly, marital instability and changes in family structure can spur early home-leaving, childbearing, and union formation (Amato, Landale, Havasevich-Brooks, Booth, Eggebeen, Schoen, and McHale 2008; Amato and Keith 1991; Aquilino 1991; Goldscheider and Goldscheider 1998; Wu 1996). Thus, intergenerational transmissions of nonmarital childbearing may reflect instability in women’s childhood environment.
In sum, these intergenerational transmissions are depicted in Figure 1 with vertical lines connecting mother’s and daughter’s preconception education, and mother’s and daughter’s nonmarital childbearing. This completes the description of the intra- and inter-generational mechanisms depicted in Figure 1.
Contributions of these Integrative Processes to Population-Level Social Inequality
Although several distinct pathways have been discussed, it is critical to acknowledge that maternal education, nonmarital childbearing, and birth outcomes are intricately related in a broader fashion that reflects and likely perpetuates population-level trends of social inequality. Nonmarital childbearing has risen rapidly in the U.S. over the past several decades, from 18% of all births in 1980 to 41% in 2012 (Martin et al. 2013; Martin, Hamilton, Ventura, Osterman, Kirmeyer, Mathews, and Wilson 2011), and has become increasingly selective of socioeconomically disadvantaged women as their more advantaged counterparts capitalize on new opportunities to secure higher levels of educational attainment and delay births until after marriage (Carlson and England 2011; McLanahan and Percheski 2008). For example, in 1990, nearly a quarter (23%) of marital childbearers in one U.S. state (North Carolina) had completed a Bachelor’s degree, compared with only 17% of nonmarital childbearers. This 6% difference in college education by marital status grew to a 15% difference by 2012 (42% versus 27%, respectively) (author’s own calculations based on birth certificate record data). Unfortunately, given the substantial economic and parenting resources associated with marital (versus nonmarital) childbearing, this suggests that children born outside of marital unions are becoming increasingly disadvantaged over time, effectively creating two increasingly polarized subpopulations of children with very different social and economic prospects (McLanahan 2004). These trends are consequential given that consistently observed risk factors of low birth weight include low levels of maternal human capital and nonmarital childbearing (previously described). Therefore, this study seeks to understand more about intra- and inter-generational preconception processes contributing to birth weight with the broader goal of shedding new light on population-level trends in social inequality.
DATA AND METHODS
Sample
This study links data from the National Longitudinal Survey of Youth 1979 cohort (NLSY79), a longitudinal survey of over 12,000 male and female participants collected annually from 1979 to 1994 and biennially from 1996 to the present, with data from the Children of the NLSY79 (CNLSY79), a longitudinal survey of the children of the NLSY79 cohort, collected biennially since 1994. The analytic sample includes CNLSY79 daughters (5,624) who ever had a child by 2010 (N = 1,580). Each daughter was matched with her mother from the NLSY79 file, creating a sample of 1,580 mother-daughter pairs. In the case of multiparous mothers, one daughter per mother was randomly selected. Hereafter, NLSY79 mothers will be referred to as “G1” (Generation 1), CNLSY79 daughters as “G2” (Generation 2), and the infants of CNLSY79 daughters as “G3” (Generation 3).
Measures
Birth Outcomes
This study examines two weight-related birth outcomes. Similar to past research (Morenoff 2003), growth restriction is indicated by a continuous variable, birth weight (range = 227–5,613 grams), and equations predicting birth weight control for preterm birth status (1 = preterm birth). (Here, preterm birth approximates gestational length as gestational length was only measured for G2 and not G3.) Low birth weight is indicated by a binary variable, where a value of 1 indicates less than 2500 grams at birth.
Explanatory Variables
Maternal education is indicated by the number of years of schooling completed prior to birth (G1 range = 1–16, G2 range = 0–20). Nonmarital childbearing for G1 and G2 is indicated as 1 = unmarried in the year prior to birth, and 0 = married in the year prior to birth.
Control Variables
Following past research (Chomitz, Cheung, and Lieberman 1995; Goldenberg, Culhane, Iams, and Romero 2008; Reichman 2005), I control for three potential confounders of birth weight: race-ethnicity, infant sex (1 = male), and infant birth order. In addition, based on past work (Bumpass and McLanahan 1989), race-ethnicity, G1 family structure in adolescence (1 = non-intact, or, not living with both biological parents), and grandmother’s (G0) education (range = 0 – 20) are included as controls of maternal education prior to birth and nonmarital childbearing.
Statistical Analyses
Structural equation models (SEMs) are used to test the proposed model and are ideal in this study for three key reasons: SEMs simultaneously estimate multiple equation systems; SEMs facilitate the estimation of total, direct, and indirect effects of numerous pathways (Bollen 1989); and, in SEMs, the residuals of endogenous (interval) variables can be correlated, which is the approach taken for the interval variable in this study, growth restriction.
SEMs of growth restriction and LBW were estimated (separately) in Mplus 7 using maximum likelihood estimation; the latter SEM was specified using a generalized linear (logit) model. Endogenous variables in both SEMs include birth outcome, preconception maternal education, and nonmarital childbearing; all control variables (previously described) are exogenous. Pathways through which family-of-origin SES, preconception education, and nonmarital childbearing operate directly and indirectly on birth outcome are assessed by examining total, direct, and indirect effects on birth outcome. Three goodness-of-fit statistics are provided for each model: the Confirmatory Fit Index (CFI) and Tucker-Lewis Index (TLI), both of which must be above .90 to accept the model and above .95 to deem the model as a good fit, as well as the Root Means Square Error of Approximation (RMSEA), for which scores of less than 0.05 indicate adequate model fit. The TLI performs particularly well for large sample sizes while adjusting for model complexity; the RMSEA adjusts for error in the population, making it ideal for use with large population-level samples (Bollen and Long 1993). All multivariate analyses are unweighted in accordance with methodological advice to refrain from applying survey weights when the variables used to calculate survey weights are a function of the independent variables used in a regression model (Winship and Radbill 1994), as is the case here. G1 descriptive statistics are weighted using NLSY79 survey weights; G2 descriptive statistics are weighted using CNLSY79 survey weights.
RESULTS
Table 1 presents descriptive statistics for study variables. Among G2, the average birth weight is 3,231 grams and the proportion of LBW is 8 percent. The proportion of LBW is similar to that reported at the national-level, 7 percent, for births in 1980, the average year of first birth for G1 (Ventura 1982). Levels of education and nonmarital childbearing are also comparable. G1 report an average of 11 years of schooling prior to first birth; the median level of education at birth among all-parity U.S. mothers in 1980 is 12.6 years (Ventura 1982)—a similar level given the latter is measured at, and not prior to, birth. Comparisons of nonmarital childbearing with national-level data require race-specific figures: nearly a quarter (24%) of G1 white mothers and eighty-one percent of G1 black mothers had a nonmarital birth (results not shown); the corollary at the national-level in 1980 was 18% (white) and 83% (black).
Table 1.
Weighted Descriptive Statistics
| Mean or Percent | Standard Deviation | |
|---|---|---|
| Birth Outcomes | ||
| Daughter’s (G2) birth weight (range = 227 – 5,613 grams) | 3,230.8 | 523.6 |
| Infant’s (G3) birth weight (range = 312 – 5,245 grams) | 3,183.2 | 587.1 |
| Daughter’s (G2) low birth weight (1 = yes) | 7.9% | |
| Infant’s (G3) low birth weight (1 = yes) | 9.6% | |
| Explanatory Variables | ||
| Mother’s (G1) education prior to birth (range = 1 – 16 years) | 11.0 | 1.8 |
| Daughter’s (G2) education prior to birth (range = 0 – 20 years) | 11.2 | 2.2 |
| Mother’s (G1) nonmarital childbearing (1 = yes) | 40.0% | |
| Daughter’s (G2) nonmarital childbearing (1 = yes) | 87.3% | |
| Control Variables | ||
| Race/ethnicity (reference = non-Hispanic White/Other) | ||
| non-Hispanic Black | 28.2% | |
| Hispanic | 9.7% | |
| G3 sex (1 = male) | 52.3% | |
| G3 parity (range = 1 – 7) | 1.8 | 1.0 |
| Mother’s (G1) family structure in adolescence (1 = non-intact) | 36.8% | |
| Grandmother’s (G0) education (range = 0 – 20) | 10.3 | 2.8 |
| Daughter’s (G2) preterm birth (1 = yes) | 10.4% | |
| Infant’s (G3) preterm birth (1 = yes) | 11.4% |
Note: N = 1,580 mother (G1) — daughter (G2) pairs.
Source: National Longitudinal Survey of Youth 1979 cohort (NLSY79) and Children of the NLSY79.
Comparisons for G2 can be drawn against young women in the National Longitudinal Study of Adolescent Health (Add Health) who had a birth by the 2008–9 interview. Comparing basic demographics (authors own calculations; not shown), shows that, in 2010, the average age of G2 was 27 (range = 17 – 38); in 2008–9, Add Health mothers were, on average, 29 (range = 24 – 34). Age at first birth is also similar although slightly lower among G2 (20.2) versus Add Health (21.9). Comparing key study variables shows that average birth weight is similar (3,183 grams in G2 and 3,243 grams in Add Health), though the proportion LBW is somewhat higher (10% among G2; 8% among Add Health). (It is difficult to draw comparisons of nonmarital childbearing for measurement reasons: in CNLSY79, I measure marital status in the year prior to birth, while in Add Health, marital status is captured at the time of birth.) Overall, these comparisons suggest G2 are similar in many ways to a subsample of women in Add Health, a nationally-representative population-based sample, who have transitioned to motherhood.
I now empirically test the model proposed in this study. Figure 2 depicts model results for growth restriction. I begin by examining intergenerational associations. The path coefficient from G2 birth weight to G3 birth weight indicates that, for each additional gram of G2 birth weight, G3 birth weight is, on average, 0.13 grams heavier, net of controls, the correlation between residuals of G2 and G3 birth weight, and all other pathways in the model. This demonstrates a statistically significant intergenerational transmission of birth weight net of unobserved mother-daughter genetic (and/or social) similarities. Path coefficients indicating intergenerational transmissions of education and nonmarital childbearing are positive and statistically significant, as expected. Each additional year of schooling completed by G1 prior to birth is associated with nearly a quarter-year increase in G2’s schooling prior to birth (b = 0.22). Similarly, G1 nonmarital childbearing is associated with a 66% increase in the odds of G2 nonmarital childbearing (odds ratio = e0.51 = 1.66).
Figure 2.
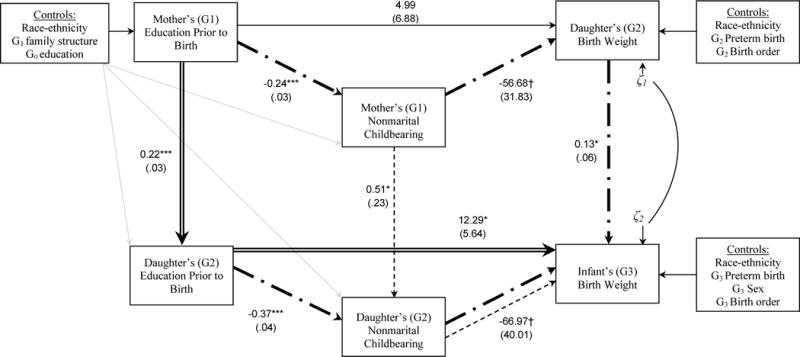
Structural Equation Model Results for the Integrative Inter- and Intra-generational Model of Growth Restriction, Education, and Nonmarital Childbearing
Notes: N = 1,580 mother (G1) — daughter (G2) pairs. Double, dash, and dash-dot lines indicate statistically significant indirect pathways (p < .10 level or better).
Source: National Longitudinal Survey of Youth 1979 cohort (NLSY79) and Children of the NLSY79.
Next I examine intra-generational pathways operating on growth restriction. G2 education affects G3 birth weight directly, such that each additional year of G2 schooling is associated with an increase in G3 birth weight of 12 grams. G2 education also affects G3 birth weight indirectly via nonmarital birth status. Each additional year of G2 schooling is associated with a 31% decrease in the odds of nonmarital childbearing (odds ratio = e−.37 = .69). In turn, G2 nonmarital childbearing is associated with a 67 gram decline in G3 birth weight. Among G1, the influence of education on birth weight is limited to an indirect effect via nonmarital childbearing: each additional year of G1 schooling is associated with a 21% decrease in the odds of nonmarital childbearing (OR = e−.24 = .79); in turn, G1 nonmarital childbearing is associated with a decrease of 57 grams in G2 birth weight (although the latter association is only marginally significant).
Comparing analogous path coefficients across generations produces interesting results. The direct effect of education on nonmarital childbearing appears to be stronger among G2 than G1, based on the magnitude of the path coefficients and the similarity in standard errors. Indeed, Wald tests of equality suggest we can reject the hypothesis that these path coefficients are equal across generations (test statistic = 6.79, df = 1, p = .01). On the other hand, Wald tests suggest we cannot reject the hypothesis that the direct effect of nonmarital childbearing on birth weight (test statistic = .02, df = 1, p = .89), nor the direct effect of education on birth weight (test statistic = .60, df = 1, p = .44), are equal across generations. Thus, these two path coefficients are statistically indistinguishable across generations.
I now examine integrative inter- and intra-generational pathways that depict a richer perspective of longer-term processes influencing birth weight. I examine two types of pathways that comprise the total effect of G1 education on G3 birth weight (total effect = 4.29 (SE = 1.68), p = .01). First, I consider whether pathways related to inter-generational transmissions of education and nonmarital childbearing have longer-term effects on birth outcomes of future generations. Indeed, a statistically significant indirect pathway, denoted in Figure 2 with a double line, shows that G1’s level of education prior to birth is positively associated with G2’s level of education prior to birth, and this in turn is associated with G3 birth weight (indirect effect = 2.67 (SE = 1.32), p < .05). In fact, this pathway can be traced back even further, to G0’s (grandmother’s) education, which is positively associated with G1’s education prior to birth (b = 0.24, p < .001; see Table 2, Panel A). Thus, maternal education is transmitted across (at least) three generations and ultimately affects growth restriction in the most recent generation.
Table 2.
Unstandardized Path Coefficients from the Structural Equation Model of Birth Weight (Panel A) and Low Birth Weight (Panel B)
| Panel A: Birth Weight | Panel B: Low Birth Weight | |
|---|---|---|
| Infant’s (G3) Birth Outcome ← | ||
| Race/ethnicity (reference = non-Hispanic White/Other) | ||
| non-Hispanic Black | −128.15*** (27.67) |
.53* (.21) |
| Hispanic | −52.55† (31.23) |
.25 (.24) |
| G3 Preterm birth | −993.69*** (52.77) |
– |
| G3 Sex (1 = male) | 137.09*** (23.45) |
−.18 (.17) |
| G3 Parity | 17.24 (13.79) |
−.14† .08 |
| Daughter’s (G2) Birth Outcome ← | ||
| Race/ethnicity (reference = non-Hispanic White/Other) | ||
| non-Hispanic Black | −197.01*** (30.89) |
.83*** (.25) |
| Hispanic | −18.05 (36.05) |
.38 (.28) |
| G2 Preterm birth | −603.79*** (52.70) |
– |
| Mother’s (G1) Education Prior to Birth ← | ||
| Race/ethnicity (reference = non-Hispanic White/Other) | ||
| non-Hispanic Black | −.01 (.10) |
−.01 (.10) |
| Hispanic | −.25† (.14) |
−.25† (.14) |
| Mother’s (G1) family structure in adolescence (1 = non-intact) | −.36*** (.09) |
−.36*** (.09) |
| Grandmother’s (G0) Education | .24*** (.02) |
.24*** (.02) |
| Daughter’s (G2) Education Prior to Birth ← | ||
| Race/ethnicity (reference = non-Hispanic White/Other) | ||
| non-Hispanic Black | −.38** (.12) |
−.38** (.12) |
| Hispanic | −.32* (.15) |
−.32* (.15) |
| Mother’s (G1) Nonmarital Childbearing ← | ||
| Race/ethnicity (reference = non-Hispanic White/Other) | ||
| non-Hispanic Black | 2.49*** (.14) |
2.49*** (.14) |
| Hispanic | .21 (.16) |
.21 (.16) |
| Daughter’s (G2) Nonmarital Childbearing ← | ||
| Race/ethnicity (reference = non-Hispanic White/Other) | ||
| non-Hispanic Black | 1.61*** (.29) |
1.61*** (.29) |
| Hispanic | .25 (.21) |
.25 (.21) |
| Intergenerational correlation (G3:G2) of birth weight | 7,291.83 (18,154.98) |
– |
|
| ||
| CFI | .982 | .975 |
| TLI | .971 | .957 |
| RMSEA | .025 | .026 |
Notes: p <.001,
p <.01,
p <.05,
p <.10 (two-tailed). N = 1,580 mother—daughter pairs.
Another indirect pathway is denoted with a single dotted line, and shows that inter-generational transmissions of nonmarital childbearing shape birth outcomes of future generations (indirect effect = .14 (SE = .08), p < .10). G1 nonmarital childbearing (which is partially shaped by G1 education) is positively associated with G2 nonmarital childbearing, which in turn affects G3 birth weight. Together, these two indirect pathways provide evidence that growth restriction is affected by longer-term inter-generational transmissions of education and nonmarital childbearing. (The marginal statistical significance of this second indirect pathway reflects the fact that one path coefficient in the pathway is marginally significant: G2 nonmarital childbearing → G3 birth weight. But, this does not detract from the substantive importance of this path coefficient, nor of the entire pathway. For example, a 67 gram difference in birth weight between married and unmarried G2 mothers is equal to one-ninth (11%) of a standard deviation of birth weight (587), and nearly half of the raw difference in birth weight between single (3,140 grams) and married G2 mothers (3,304 grams) in this sample (67/(3304 – 3140) = .41). Similarly, a 57 gram difference in in birth weight between married and unmarried G2 mothers is equal to one-ninth (11%) of a standard deviation of birth weight (524), and more than one-third of the raw difference in birth weight between single (3,108 grams) and married G2 mothers (3,271 grams) in this sample (57/(3271 – 3108) = .35).)
The second type of pathway examined is the long-term effect of intra-generational social inequalities on birth weight. Indeed, evidence emerges along these lines. The indirect pathway denoted with a dash-dot line among G1 illustrates that G1 education is inversely related to the probability of nonmarital childbearing, which is associated with G2 birth weight; in turn, G2 birth weight is positively associated with G3 birth weight (indirect effect = .34 (SE = .20), p < .10). The parallel pathway among the G2 generation, previously described, operates similarly (indirect effect = .49 (SE = .29), p < .10). Thus, intra-generational social disadvantages appear to affect the growth restriction not only of the most proximate generation, but of future generations as well.
Model parameters not presented in Figure 2 are depicted in Table 2, Panel A, as are model fit indices. Note that the parameter indicating the inter-generational correlation of birth weight was not statistically significant. The model fit indices indicate good model fit: the CLI and TLI are above .95 (.98 and .97, respectively) and the RMSEA is below .05 (.03). The full model explains 35% of the variance in G3 birth weight and 15% of the variance in G2 birth weight; similarly, the residual variances for each are low (0.22 and 0.26, respectively). Thus, the proposed model fits the data well.
Next I present findings from the SEM of low birth weight. Similar to findings for growth restriction, results depicted in Figure 3 indicate significant intergenerational transmissions of LBW, maternal education, and nonmarital childbearing. G2 LBW is associated with a 70% increase in the odds of having a LBW infant (G3) (OR = e0.53 = 1.70). (The intergenerational transmissions of education and nonmarital childbearing are identical to those previously described, given that this portion of the SEM is identical between the models depicted in Figures 2 and 3.) Unlike growth restriction however, no evidence emerges to suggest that inter-generational transmissions of education and nonmarital childbearing have longer-term effects on LBW of future generations. The statistical significance of these pathways is precluded by the lack of a significant association of G2 education with G3 LBW and of G2 nonmarital childbearing with G3 LBW.
Figure 3.
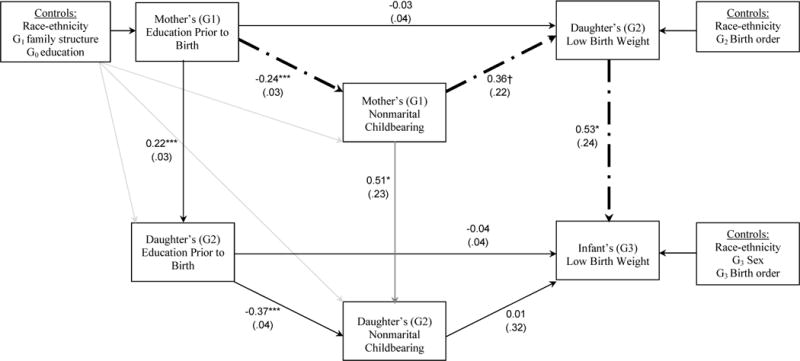
Structural Equation Model Results for the Integrative Inter- and Intra-generational Model of Low Birth Weight, Education, and Nonmarital Childbearing
Notes: N = 1,580 mother (G1) — daughter (G2) pairs. Dash-dot lines indicate a significant indirect pathway (p < .10 or better).
Source: National Longitudinal Survey of Youth 1979 cohort (NLSY79) and Children of the NLSY79.
Yet the pathway depicting long-term effects of intra-generational social inequalities on adverse birth outcome is statistically significant: G1 education prior to birth is inversely associated with G1 nonmarital childbearing (previously described); nonmarital childbearing is positively associated with G2 LBW, which in turn is positively associated with G3 LBW.
Model parameters not shown in Figure 3 are presented in Table 2, Panel B. This model fits the data very well: the CFI and TLI exceed 0.95 (0.98 and 0.96, respectively), and the RMSEA is below 0.05 (0.03). Note the logit specification of LBW does not permit the inclusion of an inter-generational covariance term for G2 LBW and G3 LBW. In supplementary analyses (not shown), I re-specified this SEM using a probit model which allows for the inclusion of this covariance term. Similar to the model for growth restriction however, this parameter was not statistically significant (b = .55, SE = .29, p = .15).
DISCUSSION
This study proposed and tested an integrative inter- and intra-generational model of preconception factors influencing birth weight. In doing so, this study bridged literature on the etiology of factors contributing to adverse birth outcome across disparate disciplines including public health, behavior genetics, economics, and sociology, and offered new insights into longer-term preconception processes that may underlie adverse birth outcome, not only within a single generation but across multiple generations. Study results offer three key contributions.
First, results documented longer-term, preconception influences of intra-generational social (dis)advantages on growth restriction. Maternal education prior to birth is associated with growth restriction both directly and indirectly via nonmarital childbearing. This evidence is consistent with studies identifying lower maternal education at birth and out-of-wedlock childbearing as risk factors for poor birth outcome (Paneth 1995; Shah et al. 2011), as well as studies showing that childhood socioeconomic disadvantage and early life chronic stressors have long-reaching effects on birth weight (Harville, Boynton-Jarrett, Power, and Hypponen 2010; Strutz, Hogan, Siega-Riz, Suchindran, Halpern, and Hussey 2014). But, this finding extends this literature by providing new knowledge as to the salient influence of preconception maternal education on birth weight, both directly, and indirectly via nonmarital childbearing, above and beyond all other pathways in the model. This is knowledge that can only be demonstrated when maternal education, along with early life SES, are measured prospectively, before women had any knowledge of a pregnancy, which is the case here.
Based on the finding that preconception maternal education has a salient effect on birth weight, both directly, and indirectly via nonmarital childbearing, I speculate these associations may reflect several underlying intra-generational processes. Lower levels of preconception education may restrict women’s future employment opportunities to positions within the low-wage labor market; these positions tend to be unstable or less-permanent and are often associated with inflexible time requirements, higher levels of on-the-job stress, a lack of health insurance, lower levels of personal autonomy, and fewer intrinsic rewards (Kalleberg 2011). Each of these factors can be sources of stress, which in turn can influence birth outcomes (Schetter 2009). Through the mechanism of learned effectiveness, lower levels of preconception education may also limit the development of individual agency, self-efficacy, and problem-solving capacity—each of which can limit participation in health-promoting activities (Mirowsky and Ross 2003). In turn, health-promoting activities, such as abstaining from prenatal smoking, can influence birth weight (Cnattingius 2004). The indirect effect of preconception education on birth weight via nonmarital childbearing may reflect any number of stressors that unmarried women disproportionately face, including poorer living conditions (materially deprived neighborhoods, higher crime rates) as well as lower levels of prenatal social support and financial resources—each of which can affect birth outcome (Landale and Oropesa 2001; O’Campo, Xue, Wang, and Caughy 1997).
These potential mechanisms provide clues as to how and where to intervene in order to improve birth outcomes. For example, if these findings are replicated in future research, programmatic and policy efforts that offer structural and institutional support to women in terms of achieving their desired level of education early on in the life course could indirectly improve birth outcomes. Indeed, early investments in human capital have the potential to influence a broad range of outcomes (Heckman 2000).
Supplementary analyses added another new finding: the association of maternal education with nonmarital childbearing was stronger among the more recent generation, while that of nonmarital childbearing and growth restriction had not weakened or strengthened across generations. Substantively, the stronger association between maternal education and nonmarital childbearing in the more recent generation is consistent with the notion that, over time, nonmarital childbearing has become increasingly selective of disadvantaged women as more advantaged women benefit from opportunities to secure more education and delay births until after marriage (Carlson and England 2011; McLanahan and Percheski 2008). In turn, this trend is effectively creating two increasingly polarized subpopulations of children with very different social and economic prospects (McLanahan 2004). The implication of this study’s findings suggests that these polarizing population trends also affect a critical marker of child health: growth restriction.
Second, analyses revealed that inter-generational transmissions of maternal education and nonmarital childbearing, potentially reflecting underlying mechanisms of parent-child socialization, role modeling, or family instability, appear to exert a long-term influence on birth outcomes of future generations. This was supported by two findings: the level of maternal education completed prior to birth was transmitted across (at least) three generations and was ultimately associated with growth restriction; and, nonmarital childbearing was transmitted across (at least) two generations and was ultimately associated with growth restriction. Intergenerational transmissions of education and nonmarital childbearing are well-documented in sociology and demography; that low levels of maternal education and nonmarital childbearing are associated with adverse birth outcome is well-known in public health. Yet, by integrating these pathways, this study shows, for the first time, that preconception factors (education, union status) dating back at least three generations can affect the birth weight of future generations. This supports the notion that the period of exposure examined in preconception health studies should be shifted farther back in the life course, beyond the twelve months leading to conception (Johnson et al. 2006; van Dyck 2010), to include women’s early life experiences. But, this finding goes beyond past research by showing that the period of exposure should extend back even further—to include factors dating back multiple generations. The logic underlying this proposed shift is consistent with the notion that risk factors cluster within some at-risk populations over time. It is possible that future studies applying this longer-term perspective may offer new insights into why stark and persistent racial disparities in birth weight are observed.
Third, findings suggest that the effects of intra-generational social inequalities on birth outcomes extend beyond that of one generation, to impact the birth outcomes of future generations as well. Maternal education is associated with nonmarital childbearing, which in turn is associated with both growth restriction and low birth weight; in turn, intergenerational transmissions of growth restriction and LBW, net of unobserved shared genetic (and/or social) factors, were documented here. This entire pathway bridges findings from behavior genetics, economics, sociology, and the social inequalities in health literature to offer a new, longer-term perspective of how birth weight outcomes may be shaped. The final piece of the pathway, significant intergenerational transmissions of birth weight and LBW, net of unobserved mother-daughter genetic or social similarities, also contributes a new finding to the literature. Intervention efforts that seek to eliminate or reduce social inequality may indirectly reduce stark disparities in birth weight among future generations.
The second and third key findings have important implications for understanding population-level patterns of social inequality. The integrative pathways emerging in this study reveal specific mechanisms through which social inequality can be transmitted from mothers to children via birth outcome, extending past work in this area (Case, Lubotsky, and Paxson 2002; Currie 2009; Currie and Moretti 2007). These findings also speak more broadly to the role of marriage in shaping birth outcomes. Consistent with past work, a significant association between nonmarital childbearing and adverse birth outcome was noted. But, study results show that this association is rooted in a more complex process spanning multiple generations. This has implications for how we conceptualize any health advantages marriage may incur for women and children, which is a broader topic of debate in the literature (Buckles and Price 2013; Kane Forthcoming; Umberson and Montez 2010; Waite 1995). At debate is whether health advantages observed among married women, such as lower rates of low birth weight or growth restriction, reflect confounding by unobserved selection factors, or if marriage in some way causes women to adopt heathier attitudes or behaviors that ultimately translate to better health. This study contributes to that debate by revealing more about the selection process that puts women at risk of being unmarried at birth than has been previously shown—specifically, by showing that this selection likely reflects social processes involving maternal education and union status dating back at least one generation.
Study limitations should be noted. Due to data limitations, I speculate on, but do not directly test, the role of some potential mechanisms underlying the associations observed in this study—for example, preconception and prenatal levels of stress and social support. Future studies should explicitly test these intermediate pathways. More broadly, future studies should further test the model proposed in this study and seek to replicate study findings using other population-level data sources. To that end, this study strove for simplicity in model development in an expressed effort to produce a framework that could be easily replicated and tested in future work using a variety of datasets. But, as G2 sample members have not yet completed their fertility, it is possible that the associations presented here are most relevant to patterns of education, nonmarital childbearing, and birth weight among earlier-timed or first births. Different associations may arise from utilizing a different population-level, multi-generation dataset where all sample members (mothers and children) have completed their fertility.
In conclusion, this study presents theoretic and empiric support for a new model of integrative inter- and intra-generational preconception pathways that shifts the focus of understanding the etiology of factors contributing to birth weight away from an examination of risk and protective factors contained within the prenatal period alone, or even within the twelve months prior to conception, and towards a framework including not only women’s earlier life course experiences, but also longer-term intergenerational effects. If replicated in future work, this shift could have important implications for planning and implementing population-health efforts seeking to improve birth outcomes and ameliorate stark social and racial inequalities in perinatal health in the U.S.
Footnotes
This research received support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant K99 HD075860), and benefited from NICHD support awarded to UNC’s Carolina Population Center (grants T32 HD007168 and R24 HD050924) and Penn State’s Population Research Institute (grants T32 HD007514 and R24 HD041025). Opinions reflect those of the authors and not necessarily those of the granting agencies. The author thanks Paul Amato, Alan Booth, Marianne Hillemeier, and Nancy Landale for their comments on an earlier draft of this manuscript.
References
- Adler Nancy, Bush Nicole R, Pantell Matthew S. Rigor, vigor, and the study of health disparities. Proceedings of the National Academy of Sciences. 2012;109:17154–17159. doi: 10.1073/pnas.1121399109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Annals of The New York Academy of Sciences. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Albrecht SL, Miller MK, Clarke LL. Assessing the importance of family structure in understanding birth outcomes. Journal of Marriage and the Family. 1994:987–003. [Google Scholar]
- Amato Paul, Kane Jennifer. Parents’ Marital Distress, Divorce, and Remarriage: Links With Daughters’ Early Family Formation Transitions. Journal of Family Issues. 2011;32:1073–1103. doi: 10.1177/0192513X11404363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato Paul, Landale Nancy S, Havasevich-Brooks Tara C, Booth Alan, Eggebeen David J, Schoen Robert, McHale Susan M. Precursors of Young Women’s Family Formation Pathways. Journal of Marriage and Family. 2008;70:1271–1286. doi: 10.1111/j.1741-3737.2008.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato Paul R, Keith Bruce. Parental divorce and the well-being of children: a meta-analysis. Psychological Bulletin. 1991;110:26. doi: 10.1037/0033-2909.110.1.26. [DOI] [PubMed] [Google Scholar]
- Anderton Douglas L, Tsuya Noriko O, Bean Lee L, Mineau Geraldine P. Intergenerational transmission of relative fertility and life course patterns. Demography. 1987;24:467–480. [PubMed] [Google Scholar]
- Aquilino William S. Family structure and home-leaving: A further specification of the relationship. Journal of Marriage and the Family. 1991:999–1010. [Google Scholar]
- Aquilino William S. The life course of children born to unmarried mothers: Childhood living arrangements and young adult outcomes. Journal of Marriage and the Family. 1996:293–310. [Google Scholar]
- Axinn William G, Thornton Arland. Mothers, children, and cohabitation: The intergenerational effects of attitudes and behavior. American Sociological Review. 1993:233–246. [Google Scholar]
- Barber Jennifer S. Ideational influences on the transition to parenthood: Attitudes toward childbearing and competing alternatives. Social Psychology Quarterly. 2001a:101–127. [Google Scholar]
- Barber Jennifer S. The intergenerational transmission of age at first birth among married and unmarried men and women. Social Science Research. 2001b;30:219–247. [Google Scholar]
- Bengtson Vern L. Generation and Family Effects in Value Socialization. American Sociological Review. 1975;40:358–371. [Google Scholar]
- Bennett T. Marital status and infant health outcomes. Social Science & Medicine. 1992;35:1179–1187. doi: 10.1016/0277-9536(92)90230-n. [DOI] [PubMed] [Google Scholar]
- Blau Peter M, Duncan Otis Dudley. The American occupational structure 1967 [Google Scholar]
- Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic Disparities in Adverse Birth Outcomes:: A Systematic Review. American Journal of Preventive Medicine. 2010;39:263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. 1989;8 [Google Scholar]
- Bollen Kenneth A, Long J Scott. Testing structural equation models. Vol. 154. Sage; 1993. [Google Scholar]
- Braveman Paula A. Black-White Disparities in Birth Outcomes: Is Racism-Related Stress a Missing Piece of the Puzzle? Handbook of African American Health. 2011:155–163. [Google Scholar]
- Braveman Paula, Colleen Barclay. Health disparities beginning in childhood: a life-course perspective. Pediatrics. 2009;124:S163–S175. doi: 10.1542/peds.2009-1100D. [DOI] [PubMed] [Google Scholar]
- Buckles Kasey S, Price Joseph. Selection and the Marriage Premium for Infant Health. Demography. 2013:1–25. doi: 10.1007/s13524-013-0211-7. [DOI] [PubMed] [Google Scholar]
- Bumpass Larry, McLanahan Sara. Unmarried motherhood: Recent trends, composition, and black-white differences. Demography. 1989;26:279–286. [PubMed] [Google Scholar]
- Carlson M, England P. Social Class and Changing Families in an Unequal America. Stanford University Press; 2011. [Google Scholar]
- Case A, Lubotsky D, Paxson C. Economic Status and Health in Childhood: The Origins of the Gradient. American Economic Review. 2002:1308–1334. doi: 10.1257/000282802762024520. [DOI] [PubMed] [Google Scholar]
- Chen E, Martin AD, Matthews KA. Socioeconomic status and health: Do gradients differ within childhood and adolescence? Social Science & Medicine. 2006;62:2161–2170. doi: 10.1016/j.socscimed.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Chomitz VR, Cheung LWY, Lieberman E. The role of lifestyle in preventing low birth weight. The Future of Children. 1995:121–138. [PubMed] [Google Scholar]
- Cnattingius Sven. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Collins Nancy L, Dunkel-Schetter Christine, Lobel Marci, Scrimshaw Susan C. Social support in pregnancy: psychosocial correlates of birth outcomes and postpartum depression. Journal of Personality and Social Psychology. 1993;65:1243. doi: 10.1037//0022-3514.65.6.1243. [DOI] [PubMed] [Google Scholar]
- Conley D, Bennett NG. Is biology destiny? Birth weight and life chances. American Sociological Review. 2000:458–467. [Google Scholar]
- Conley D, Bennett NG. Birth weight and income: interactions across generations. Journal of Health and Social Behavior. 2001:450–465. [PubMed] [Google Scholar]
- Currie Janet. Healthy, Wealthy, and Wise: Socioeconomic Status, Poor Health in Childhood, and Human Capital Development. Journal of Economic Literature. 2009:87–122. [Google Scholar]
- Currie Janet, Moretti Enrico. Biology as Destiny? Short-and Long-Run Determinants of Intergenerational Transmission of Birth Weight. Journal of Labor Economics. 2007;25 [Google Scholar]
- Dole Nancy, Savitz David A, Hertz-Picciotto Irva, Siega-Riz Anna Maria, McMahon Michael J, Buekens Pierre. Maternal stress and preterm birth. American Journal of Epidemiology. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- Dunkel-Schetter C, Gurung RAR, Lobel M, Wadhwa PD. Stress processes in pregnancy and birth: Psychological, biological, and sociocultural influences. Handbook of health psychology. 2001:495–518. [Google Scholar]
- Featherman David L, Hauser Robert Mason. Opportunity and Change. Academic Press; New York: 1978. [Google Scholar]
- Furstenberg Frank F, Jr, Levine Judith A, Brooks-Gunn Jeanne. The children of teenage mothers: patterns of early childbearing in two generations. Family Planning Perspectives. 1990:54–61. [PubMed] [Google Scholar]
- Giscombé Cheryl L, Lobel Marci. Explaining disproportionately high rates of adverse birth outcomes among African Americans: the impact of stress, racism, and related factors in pregnancy. Psychological Bulletin. 2005;131:662. doi: 10.1037/0033-2909.131.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg Robert L, Culhane Jennifer F. Low birth weight in the United States. The American Journal of Clinical Nutrition. 2007;85:584S–590S. doi: 10.1093/ajcn/85.2.584S. [DOI] [PubMed] [Google Scholar]
- Goldscheider Frances K, Goldscheider Calvin. The effects of childhood family structure on leaving and returning home. Journal of Marriage and the Family. 1998:745–756. [Google Scholar]
- Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. Milbank Quarterly. 2002;80:433–479. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harville Emily W, Boynton-Jarrett Renée, Power Chris, Hypponen Elina. Childhood hardship, maternal smoking, and birth outcomes: A prospective cohort study. Archives of Pediatrics & Adolescent Medicine. 2010;164:533. doi: 10.1001/archpediatrics.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman James J. Sample selection bias as a specification error. Econometrica: Journal of the Econometric Society. 1979:153–161. [Google Scholar]
- Heckman James J. Policies to foster human capital. Research in Economics. 2000;54:3–56. [Google Scholar]
- Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, Boulet S, Curtis MG. Recommendations to improve preconception health and health care–United States. Morbidity and Mortality Weekly Report. 2006;55 [PubMed] [Google Scholar]
- Kahn Joan R, Anderson Kay E. Intergenerational patterns of teenage fertility. Demography. 1992;29:39–57. [PubMed] [Google Scholar]
- Kalleberg Arne L. Good jobs, bad jobs: The rise of polarized and precarious employment systems in the United States, 1970s–2000s. Russell Sage Foundation; 2011. [Google Scholar]
- Kane Jennifer B. Marriage Advantages in Perinatal Health: Evidence of Marriage Selection or Marriage Protection? Journal of Marriage and Family. doi: 10.1111/jomf.12257. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. Journal of Epidemiology and Community Health. 2003;57:778. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landale Nancy S, Oropesa RS. Migration, social support and perinatal health: an origin-destination analysis of Puerto Rican women. Journal of Health and Social Behavior. 2001;42:166–183. [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995:80–94. [PubMed] [Google Scholar]
- Lobel Marci, Dunkel-Schetter Christine, Scrimshaw Susan C. Prenatal maternal stress and prematurity: a prospective study of socioeconomically disadvantaged women. Health Psychology. 1992;11:32. doi: 10.1037//0278-6133.11.1.32. [DOI] [PubMed] [Google Scholar]
- Lunde A, Melve KK, Gjessing HK, Skjærven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. American Journal of Epidemiology. 2007;165:734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- Magnus P. Causes of variation in birth weight: a study of offspring of twins. Clinical Genetics. 1984;25:15–24. doi: 10.1111/j.1399-0004.1984.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Magnus P, Berg K, Bjerkedal T, Nance WE. Parental determinants of birth weight. Clinical Genetics. 1984;26:397–405. doi: 10.1111/j.1399-0004.1984.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Magnus P, Gjessing HK, Skrondal A, Skjaerven Rj. Paternal contribution to birth weight. Journal of Epidemiology and Community Health. 2001;55:873–877. doi: 10.1136/jech.55.12.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Bakketeig LS, Skjaerven R. Correlations of birth weight and gestational age across generations. Annals of Human Biology. 1993;20:231–238. doi: 10.1080/03014469300002662. [DOI] [PubMed] [Google Scholar]
- Manlove Jennifer. Early motherhood in an intergenerational perspective: The experiences of a British cohort. Journal of Marriage and the Family. 1997:263–279. [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. National Vital Statistics Report. 2013;62 [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, Wilson EC. Births: final data for 2009. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;60:1. [PubMed] [Google Scholar]
- McEwen Bruce S. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McLanahan Sara. Diverging destinies: How children are faring under the second demographic transition. Demography. 2004;41:607–627. doi: 10.1353/dem.2004.0033. [DOI] [PubMed] [Google Scholar]
- McLanahan Sara, Percheski Christine. Family structure and the reproduction of inequalities. Annual Review of Sociology. 2008;34:257–276. [Google Scholar]
- Mirowsky John, Ross Catherine E. Education, social status, and health. Transaction Books; 2003. [Google Scholar]
- Morenoff JD. Neighborhood Mechanisms and the Spatial Dynamics of Birth Weight. American Journal of Sociology. 2003;108:976–1017. doi: 10.1086/374405. [DOI] [PubMed] [Google Scholar]
- Mustillo Sarah, Wilson John, Lynch Scott M. Legacy volunteering: A test of two theories of intergenerational transmission. Journal of Marriage and Family. 2004;66:530–541. [Google Scholar]
- O’Campo Patricia, Xue Xiaonan, Wang Mei-Cheng, Caughy M. Neighborhood risk factors for low birthweight in Baltimore: a multilevel analysis. American journal of public health. 1997;87:1113–1118. doi: 10.2105/ajph.87.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel Mark D, Smilkstein Gabriel, Regen Hari, Montano Dan. Psychosocial influences on new born outcomes: a controlled prospective study. Social Science & Medicine. 1990;30:597–604. doi: 10.1016/0277-9536(90)90158-o. [DOI] [PubMed] [Google Scholar]
- Paneth NS. The problem of low birth weight. The Future of Children. 1995;5:19–34. [PubMed] [Google Scholar]
- Poulton Richie, Caspi Avshalom, Milne Barry J, Thomson W Murray, Taylor Alan, Sears Malcolm R, Moffitt Terrie E. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman NE. Low birth weight and school readiness. The Future of Children. 2005;15:91–116. doi: 10.1353/foc.2005.0008. [DOI] [PubMed] [Google Scholar]
- Richardson Liana J, Hussey Jon M, Strutz KellyL. A Life Course Perspective in Maternal and Child Health. In: Kotch JB, editor. Maternal and Child Health: Programs, Problems, and Policy. Gaithersburg, MD: Aspen Publishers, Inc; 2012. pp. 65–85. [Google Scholar]
- Rosenthal Lisa, Marci Lobel. Explaining racial disparities in adverse birth outcomes: Unique sources of stress for Black American women. Social Science & Medicine. 2011;72:977–983. doi: 10.1016/j.socscimed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Ross Catherine E, Wu Chia-Ling. Education, age, and the cumulative advantage in health. Journal of Health and Social Behavior. 1996:104–120. [PubMed] [Google Scholar]
- Schetter Christine Dunkel. Stress processes in pregnancy and preterm birth. Current Directions in Psychological Science. 2009;18:205–209. [Google Scholar]
- Shah Prakesh S, Balkhair Taiba, Ohlsson Arne, Beyene Joseph, Scott Fran, Frick Corine. Intention to become pregnant and low birth weight and preterm birth: A systematic review. Maternal and Child Health Journal. 2011;15:205–216. doi: 10.1007/s10995-009-0546-2. [DOI] [PubMed] [Google Scholar]
- Strutz Kelly L, Hogan Vijaya K, Siega-Riz Anna Maria, Suchindran Chirayath M, Halpern Carolyn Tucker, Hussey Jon M. Preconception Stress, Birth Weight, and Birth Weight Disparities Among US Women. American Journal of Public Health. 2014;104:e125–e132. doi: 10.2105/AJPH.2014.301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan Kate, Raley R Kelly, Hummer Robert A, Schiefelbein Emily. The potential contribution of marital-cohabitation status to racial, ethnic, and nativity differentials in birth outcomes in Texas. Maternal and Child Health Journal. 2012;16:775–784. doi: 10.1007/s10995-011-0801-1. [DOI] [PubMed] [Google Scholar]
- Thoits Peggy A. Stress, coping, and social support processes: Where are we? What next? Journal of Health and Social Behavior. 1995:53–79. [PubMed] [Google Scholar]
- Thornton Arland. Influence of the marital history of parents on the marital and cohabitational experiences of children. American Journal of Sociology. 1991:868–894. [Google Scholar]
- Thornton Arland, Donald Camburn. The influence of the family on premarital sexual attitudes and behavior. Demography. 1987;24:323–340. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Healthy People 2020. Washington, DC: 2014. [Google Scholar]
- Umberson D, Montez JK. Social Relationships and Health A Flashpoint for Health Policy. Journal of Health and Social Behavior. 2010;51:S54–S66. doi: 10.1177/0022146510383501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen Norbert, Janssen Joop A, Heutink Peter, Hofman Albert, Lamberts Steven WJ, Oostra Ben A, Pols Huibert AP, van Duijn Cornelia M. Association between genetic variation in the gene for insulin-like growth factor-1 and low birthweight. The Lancet. 2002;359:1036–1037. doi: 10.1016/s0140-6736(02)08067-4. [DOI] [PubMed] [Google Scholar]
- van Dyck PC. Celebrating 75 Years of Title V (Maternal and Child Health) and Re-exploring Our Roots. Maternal and Child Health Journal. 2010;14:817–821. doi: 10.1007/s10995-010-0674-8. [DOI] [PubMed] [Google Scholar]
- Ventura Stephanie J. Trends in first births to older mothers 1970–79. Monthly Vital Statistics Report. 1982;31:1–15. [Google Scholar]
- Vlietinck R, Derom R, Neale MC, Maes H, Van Loon H, Derom C, Thiery M. Genetic and environmental variation in the birth weight of twins. Behavior Genetics. 1989;19:151–161. doi: 10.1007/BF01065890. [DOI] [PubMed] [Google Scholar]
- Wadhwa Pathik D, Sandman Curt A, Porto Manuel, Dunkel-Schetter Christine, Garite Thomas J. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. American Journal of Obstetrics and Gynecology. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- Waite LJ. Does marriage matter? Demography. 1995;32:483–507. [PubMed] [Google Scholar]
- Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, Lurie N, Rebbeck T, Goodwin J, Flack J. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. American Journal of Public Health. 2008;98:1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship Christopher, Larry Radbill. Sampling weights and regression analysis. Sociological Methods & Research. 1994;23:230–257. [Google Scholar]
- Woods Katie A, Camacho-Hübner Cecilia, Savage Martin O, Clark Adrian JL. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. New England Journal of Medicine. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- Wu LL. Effects of family instability, income, and income instability on the risk of a premarital birth. American Sociological Review. 1996:386–406. [Google Scholar]


