Abstract
Low birthweight is a risk factor for neonatal mortality and adverse metabolic health, both associated with inadequate prenatal adipose tissue development. Here we investigated the impact of maternal undernutrition on expression of genes regulating fetal perirenal adipose tissue (PAT) development and function at gestation days 89 and 130 (term=145d). Singleton fetuses were taken from adolescent ewes fed control (C) intake to maintain adiposity throughout pregnancy or undernourished (UN) to maintain conception weight but deplete maternal reserves (n=7/group). Fetal weight was independent of maternal intake at day 89 but by day 130 fetuses from UN dams were 17% lighter with lower PAT mass containing fewer unilocular adipocytes. Relative PAT expression of IGF1, IGF2, IGF2R and peroxisome-proliferator-activated receptor-gamma (PPARG) mRNA was lower in UN than in C, predominantly at day 89. Independent of maternal nutrition, PAT gene expression of PPARG, glycerol-3-phosphate dehydrogenase, hormone sensitive lipase, leptin, uncoupling protein-1 and prolactin receptor increased and IGF1, IGF2, IGF1R, IGF2R decreased between 89 and 130 days. Fatty acid synthase and lipoprotein lipase (LPL) mRNAs were not influenced by nutrition or stage of pregnancy. Females had greater LPL and leptin mRNA than males, and LPL, leptin and PPARG mRNAs were decreased by UN at day 89 in females only. PAT gene expression correlations with PAT mass were stronger at day 89 than day 130. These data suggest that key genes regulating adipose tissue development and function are active from mid-gestation when they are sensitive to maternal undernutrition. This leads to reduced fetal adiposity by late pregnancy.
Keywords: adipose tissue, gene expression, fetal, undernutrition, sheep
Introduction
Prenatal growth restriction leading to low birthweight remains a global health issue and in 2013 was estimated to impact more than 22 million babies (16%) born, with the highest incidence in Southeast Asia and Africa (http://data.unicef.org/nutrition/low-birthweight). Maternal malnutrition involving deficits in macro- and micro-nutrients remains the major cause of low birthweight in developing countries (http://www.countdown2015mnch.org/documents/2013Report/Countdown_2013-Update_withprofiles.pdf). Affected neonates have increased risk of neonatal mortality whereas surviving infants have greater risk of life-restricting complications involving stunted growth, poor immune function and low educational attainment (Raqib et al. 2007; Longo et al. 2013; Christian et al. 2014). Furthermore, low birthweight predicts metabolic syndrome and obesity in adulthood, and these consequences are exacerbated if the postnatal environment is nutrient-rich such as occurs in populations undergoing economic transition (Jain & Singhal 2012). Similarly, in agriculturally important mammals (ruminants, pigs) there is evidence that maternal undernutrition is the primary determinant of poor prenatal growth, with reductions in birthweight dependent on timing, duration and severity of the nutritional insult and age and/or parity of the dam (Luther et al. 2005; Wu et al. 2006). For these species low birthweight negatively impacts commercially important traits including neonatal survival and carcass fat content, with decreased financial returns for the producer (Greenwood et al. 2010; Nissen & Oksbjerg 2010).
In precocial mammals appropriate prenatal adipose tissue development is essential for adequate thermoregulation at birth to ensure immediate survival. In addition, adipose tissue is central to energy metabolism throughout the life-course (Klaus 2004; Galic et al. 2010), and derangements in its early development potentially impact body composition in later life. Accordingly, adipose tissue is considered to be a key target of developmental programming by maternal and/or fetal undernutrition (Sarr et al. 2012; Lukaszewski et al. 2013). In humans and sheep adipose tissue is present from mid-gestation onwards with most fat deposition occurring in the final third of pregnancy predominately in the perirenal region (Moragas & Torán 1983; Gemmell & Alexander 1978). At mid-gestation perirenal adipose tissue (PAT) is characterised by rapid multiplication of precursor cells or pre-adipocytes (Pope et al. 2014) and by late pregnancy this fat depot contains cells with the appearance of both white (unilocular) and brown (multilocular) adipocytes (Gemmell & Alexander 1978); these stages thereby reflect key windows in fetal fat development. In adults unilocular adipocytes are the major site of lipid storage and leptin secretion, and similarly in late gestation ovine fetuses plasma leptin correlates with unilocular fat mass when maternal nutrient intake is at or above maintenance requirements (Mühlhäusler et al. 2002). In contrast, multilocular adipocytes are predominantly associated with young animals; they are mitochondria-rich and play an essential role in neonatal thermogenesis via a unique uncoupling protein (UCP1) that burns fatty acids and glucose to release heat (Symonds 2013). Maternal undernutrition via its negative impact on fetal nutrient availability may alter relative proportions of white and brown fat in the fetus, with implications for subsequent survival and body composition, but this hypothesis has not been tested. Published studies have examined selected molecular markers of adipose tissue growth, differentiation and function in PAT of late gestation sheep fetuses whose mothers were undernourished during specific windows of gestation, but no clear consensus emerged regarding the impact of nutrition on fetal growth, PAT depot mass or gene expression (Symonds et al. 1998; Bispham et al. 2003; Budge et al. 2004; Lie et al. 2013).
Here we address this deficit by examining PAT gene expression at both mid and late pregnancy in a sheep model where maternal undernutrition throughout gestation reduced fetal weight, carcass fat content and PAT mass by late pregnancy (Luther et al. 2007). We examined genes involved in adipocyte proliferation and differentiation, namely IGF1, IGF2, IGF1R, IGF2R (Holly et al. 2006; Kleiman et al. 2013), and peroxisome-proliferator-activated receptor-gamma (PPARG) a transcriptional regulator playing a central role in adipocyte differentiation as well as co-ordinating genes involved in lipid deposition and metabolism (Semple et al. 2006). These include lipogenic genes such as lipoprotein lipase (LPL), which enhances fatty acid uptake into adipocytes, fatty acid synthase (FASN), which catalyses fatty acid synthesis, and glycerol-3-phosphate dehydrogenase (G3PDH), which is involved in glyceroneogenesis, and lipolytic genes such as hormone sensitive lipase (HSL), which is involved in hydrolysis of stored triglycerides to release non-esterified fatty acids (NEFA). We also measured gene expression for leptin, because previous studies suggest it reflects the proportion of white adipose tissue in the fetus (Yuen et al. 2003), and for UCP1 and prolactin receptor (PRLR) as markers of brown adipose tissue function (Pope et al. 2014). Accordingly, a secondary objective herein was to quantify the proportion of unilocular and multilocular cells in PAT of fetuses from undernourished versus optimally-nourished dams.
This study tested the hypothesis that molecular markers of fetal adipose tissue development are temporally sensitive to maternal undernutrition and are associated with the resulting late gestation lean fetal phenotype.
Materials and Methods
Animals and sample derivation
All procedures were licensed under the UK Animals (Scientific Procedures) Act 1986 and approved by local Ethical Review Committee. Day 4 embryos, recovered from adult ewes inseminated by a single sire, were transferred synchronously in singleton into adolescent recipients, as described previously (Wallace et al. 1997). Details of genotype, age, weight and adiposity of the animals, together with full details of experimental design and diet composition have been presented previously (Luther et al. 2007). Briefly, recipients of equivalent age, weight and adiposity were individually offered optimal control (C) or low quantity of the same complete diet (~0.7 × C intake) following embryo transfer. The C dietary level aimed to maintain normal maternal adiposity throughout gestation (promoting liveweight gain ~50g/day) and to provide 100% nutrient requirements of the adolescent sheep carrying a singleton fetus according to stage of pregnancy (AFRC 1993). The low dietary intake was calculated to maintain maternal liveweight at the initial value, thereby depleting maternal reserves throughout gestation as she attempts to meet the nutrient requirements of the developing conceptus: these dams were considered undernourished (UN). The level of feed offered was reviewed three-times weekly and adjusted as appropriate according to weekly bodyweight change data. Maternal body condition was subjectively assessed on a five-point scale fortnightly by the same highly experienced operator (1 = emaciated, 5 = obese; Russel et al. 1969). This score provides an external measure of adiposity, and is highly correlated with maternal carcass fat determined by chemical analysis (Wallace et al. 1999). Ultrasonography at 45d gestation revealed 28 viable pregnancies.
Immediately before necropsy maternal venous blood was sampled and the plasma used to confirm metabolic status: namely glucose, insulin and NEFA concentrations. Ewes were killed on either d89 or 130 gestation (n=7/group/stage; term =145 days) by i.v. sodium pentobarbitone (20ml Euthesate; 200mg pentobarbitone/ml; Willows Francis Veterinary, Crawley, UK) and exsanguination. The gravid uterus was weighed and opened, and fetal blood sampled by cardiac puncture immediately before administering intracardiac sodium pentobarbitone (3ml Euthesate); this plasma was analysed for glucose and insulin. The fetus was dried and weighed. PAT was weighed and samples either snap-frozen in isopentane chilled by liquid nitrogen and stored at −80°C until gene expression analysis (d89 and 130) or fixed in 10% neutral buffered formalin and embedded in paraffin for histological quantification of adipocytes (d130 only). Intact placentomes were dissected and weighed. The maternal carcass was weighed to confirm the effectiveness of nutritional treatments. The fat content of fetal carcasses was determined by chemical analysis (Wallace et al. 2006).
Plasma analyses
Maternal plasma NEFA was measured using an automated clinical analyzer with kits supplied by the manufacturer (Labmedics, Manchester, UK), with variation between duplicates <5%. Maternal and fetal insulin was measured by radioimmunoassay (MacRae et al. 1991; duplicate variation <10%) and glucose by dual-biochemistry analyser (YSI model 2700, Yellow Springs, OH, USA; duplicate variation <3%).
Fetal adipocyte histology
The method used to quantify the density of unilocular and multilocular cells in fetal PAT matched that of Mühlhäusler et al. (2002). Sections were cut (5μm), dried overnight, stained with haematoxylin and eosin and viewed at 200x magnification using a Leica microscope. Twelve separate complete fields of view per animal, ~1mm apart on a single section, were captured by digital camera and the images analysed using Image-Pro Plus (version 4.5.1, Media Cybernetics, Inc., Silver Spring, MD, USA). Standard point counting techniques (Weibel 1979) were employed: a standard grid was used to determine the adipose tissue component (i.e. unilocular or multilocular cell) falling below each of 35 grid points per image, thus totalling 420 points per animal. The volume density (Vd) of each cell type was calculated as Vd=N/T, where N is the number of points falling on unilocular or multilocular cells, and T is the total number of points counted. The total mass of the unilocular or multilocular component was calculated by multiplying the Vd of each component by the PAT mass. Relative unilocular (or multilocular) fat mass (g/kg fetus) was calculated by dividing by fetal weight.
Quantitative real-time reverse transcription-polymerase chain reaction analysis
Messenger RNA for genes involved in adipocyte proliferation (IGF1, IGF2, IGF1R, IGF2R) differentiation and function (PPARG, G3PDH, LPL, FAS, HSL, UCP1, PRLR, leptin) in fetal PAT were measured by quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) using probe and primer sets for sheep-specific sequences of these genes as previously described (Matsuzaki et al. 2006; Wallace et al. 2014). Briefly total RNA was extracted from 100mg frozen PAT using RNeasy Lipid Tissue Mini Kit (Qiagen, Crawley, West Sussex, UK). The quality and quantity of total RNA were determined via capillary electrophoresis using an Agilent 2100 Bioanalyzer (Agilent Technologies, Wilmington, DE, USA). Real-time RT-PCR reagents, probes, and primers were purchased from and used as recommended by Applied Biosystems (Warrington, UK). For each sample 54ng total RNA was subjected to reverse transcription (RT) in triplicate to generate first-strand cDNA using Taqman Reverse Transcription Reagents and Multiscribe Reverse Transcriptase. Polymerisation and amplification reactions for each RT sample were performed in duplicate in 20μl final volume using the Applied Biosystems 7500 Fast Real-Time PCR system. Quantification was performed using a relative standard curve method with serial dilutions of reference standard cDNA generated from RNA pooled from PAT of control and undernourished fetuses (3/stage/group). Individual mRNA levels of genes of interest were expressed relative to the sample’s own internal 18S RNA, determined using human 18S Pre-developed TaqMan Assay Reagents. Samples were randomised to ensure that each nutritional treatment and sex was represented in each of four 96-well plates. Also a quality control sample generated from the above RNA pool was run on each plate and used to calculate inter- and intra-assay coefficients of variation (cov). Intra-plate cov varied from 3.9 to 6.5 % (mean±sem, 5.3±0.23) while inter- plate cov varied from 0.76 to 10.76 % (7.1±0.80).
Statistical analysis
The power calculation carried out for the original study was based on the prediction that maternal undernutrition would impact fetal growth by the late gestation time-point. Accordingly seven animals per group were selected so that the experiment would have 80% power to detect (at 5% significance) a 20% change in fetal weight of 870g assuming animal variability of 515g. This was based on fetal weight data obtained from control-fed adolescent ewes of the same genotype, with identical paternal genetics and at the same stage of gestation. Statistical comparisons were made using Minitab (Minitab 16, Minitab Inc., State College, PA). ANOVA (general linear model, GLM) was used to determine effects of maternal nutrition and gestational stage, and their interaction, on maternal and fetal phenotype (Table 1) and on fetal PAT gene expression (Table 3). Post-hoc comparisons used Tukey’s method when one of the main effects or their interaction was significant. We had no control over the sex of the embryo/fetus and accordingly the study was not originally powered to examine gender effects; however, sex-specific effects were apparent upon initial examination of the data and so we additionally included sex as a factor in a second 3-factor ANOVA (nutrition*stage*sex, and all possible interactions) and present relevant findings separately (text and Figure 2). Paired Student’s t tests were used to determine differences in adipocyte cell type at d130 (Table 2). Pearson product-moment correlation was used to explore relationships between variables where indicated. Values are group mean ± sem throughout, statistical significance was taken as P<0.05 and a trend was indicated where P=0.06–0.1.
Table 1.
Maternal and fetal phenotype at necropsy on day 89 and 130 of gestation in relation to maternal nutrition (n=7/group)
| Stage of Gestation | Day 89 | Day 130 | P valueα | ||||
|---|---|---|---|---|---|---|---|
| Maternal Nutrition | Control | UN | Control | UN | Stage | Nutrition | Interaction |
| Maternal | |||||||
| Change in adiposity§ | 0±0a | −0.4±0.05b | 0±0a | −0.7±0.06c | 0.001 | 0.000 | 0.001 |
| Liveweight, Kg¥ | 46.4±0.67a | 42.3±1.12b | 49.6±0.58a | 41.3±0.93b | 0.236 | 0.000 | 0.024 |
| Carcass weight, Kg | 23.4±0.48a | 20.5±0.71b | 24.8±0.60a | 18.9±0.53b | 0.769 | 0.000 | 0.019 |
| Plasma glucose, μmol/l | 3.18±0.125a | 2.99±0.055ab | 3.29±0.098a | 2.75 ±0.062b | 0.484 | 0.000 | 0.057 |
| Plasma insulin, ng/ml | 0.69±0.058a | 0.53±0.031ab | 0.75±0.090a | 0.39±0.046b | 0.481 | 0.000 | 0.127 |
| Plasma NEFA, mmol/ml | 0.30±0.077a | 0.26±0.039a | 0.38±0.051ab | 0.76±0.187b | 0.012 | 0.129 | 0.063 |
| Fetal | |||||||
| Weight, g | 657±30a | 597±43a | 4274±84b | 3555±152c | 0.000 | 0.000 | 0.002 |
| Total placentome weight, g | 665±75a | 607±28ab | 480±28b | 469±39b | 0.002 | 0.595 | 0.751 |
| Brain: liver ratio | 0.314±0.017a | 0.359±0.020ab | 0.313±0.025a | 0.441±0.031b | 0.107 | 0.003 | 0.101 |
| Plasma glucose, μmol/l | 0.49±0.038a | 0.47±0.055a | 0.39±0.076ab | 0.22±0.030b | 0.004 | 0.076 | 0.150 |
| Plasma insulin, ng/ml | 0.44±0.022 | 0.44±0.019 | 0.47±0.083 | 0.29±0.038 | 0.215 | 0.076 | 0.086 |
| Perirenal fat weight, g | 2.1±0.29a | 1.45±0.33a | 27.4±1.11b | 19.5±1.01c | 0.000 | 0.000 | 0.000 |
| Perirenal fat, g/kg fetus | 3.3±0.52a | 2.3±0.34a | 6.4±0.23b | 5.5±0.33b | 0.000 | 0.019 | 0.890 |
Values are group mean ± sem.
Post-hoc comparisons (Tukey’s Method) used to further differentiate between four groups thus within rows values with a different superscript letter differ at P<0.05.
Change in external adiposity score from embryo transfer to necropsy
Liveweight minus weight of gravid uterus at necropsy. UN, undernourished; NEFA, non-esterified fatty acids
Table 3.
Fetal perirenal fat gene expression at necropsy on day 89 and 130 of gestation in relation to maternal nutrition (n=7/group)
| Stage of Gestation | Day 89 | Day 130 | P valueα | ||||
|---|---|---|---|---|---|---|---|
| Maternal Nutrition | Control | UN | Control | UN | Stage | Nutrition | Interaction |
| Male: female ratio | 3:4 | 2:5 | 3:4 | 3:4 | |||
| 18S | 0.023±0.001 | 0.024±0.001 | 0.024±0.001 | 0.024±0.001 | 0.551 | 0.214 | 0.150 |
| PPARG/18S | 20.6±1.89ab | 15.6±1.15a | 22.3±1.25b | 23.8±1.49b | 0.003 | 0.266 | 0.042 |
| G3PDH/18S | 20.9±2.21a | 18.8±1.47a | 41.9±2.95b | 50.8±3.29b | 0.000 | 0.213 | 0.051 |
| LPL/18S | 27.6±4.11 | 20.8±1.71 | 24.8±1.96 | 26.8±2.73 | 0.578 | 0.415 | 0.142 |
| FASN/18S | 22.6±3.95 | 21.2±2.47 | 27.4±3.57 | 20.9±2.00 | 0.973 | 0.433 | 0.423 |
| HSL/18S | 10.6±2.09a | 7.7±0.87a | 36.9±1.48b | 43.5±3.41b | 0.000 | 0.423 | 0.047 |
| Leptin/18S | 34.9±5.97ab | 23.8±1.88b | 47.9±5.08a | 40.3±3.96ab | 0.004 | 0.056 | 0.708 |
| UCP-1/18S | 0.53±0.07a | 0.38±0.08a | 37.3±3.05b | 33.9±2.94b | 0.000 | 0.431 | 0.469 |
| PRLR/18S | 0.9±0.12a | 0.9±0.15a | 25.6±3.26b | 26.1±2.84b | 0.000 | 0.905 | 0.901 |
| IGF1/18S | 30.5±1.48a | 23.1±1.83a | 10.9±3.30b | 5.9±0.32b | 0.000 | 0.006 | 0.563 |
| IGF2/18S | 35.3±2.36a | 27.0±2.49a | 9.0±2.76b | 7.1±0.41b | 0.000 | 0.031 | 0.163 |
| IGF1R/18S | 14.6±1.10a | 11.8±1.12ab | 8.9±0.50b | 8.4±0.64b | 0.000 | 0.082 | 0.204 |
| IGF2R/18S | 21.8±1.57a | 16.1±1.67b | 8.1±1.18c | 7.5±0.68c | 0.000 | 0.025 | 0.067 |
Values are group mean ± sem.
Post-hoc comparisons (Tukey’s Method) used to further differentiate between the four groups; thus within rows values with a different superscript letter differ at P<0.05. UN, undernourished.
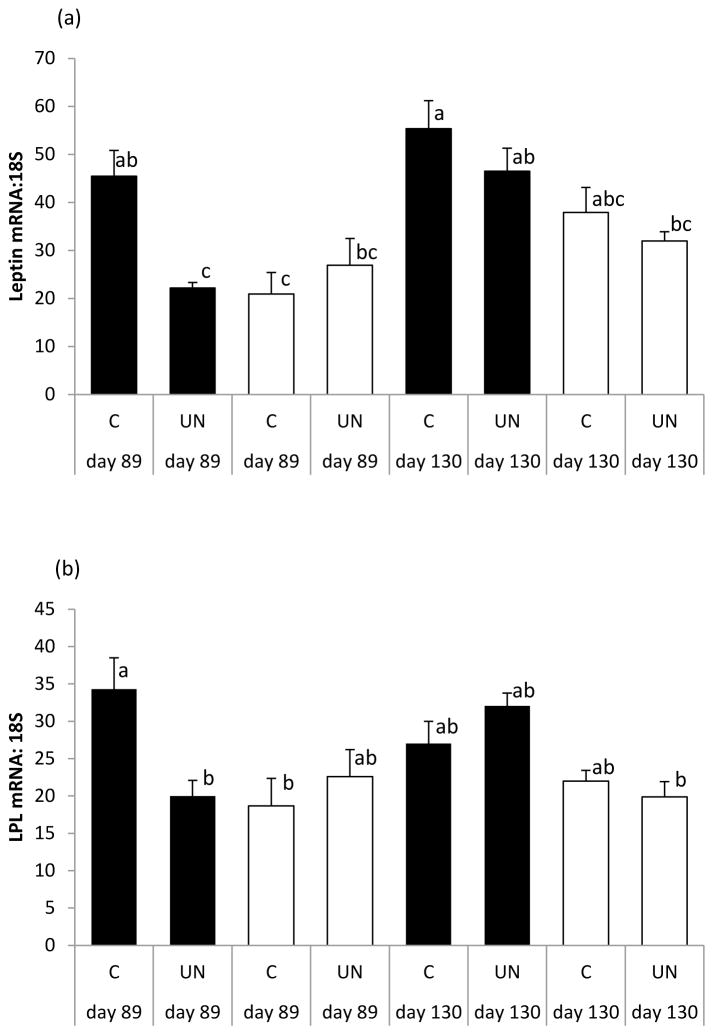
Figure 2.
Relative leptin (a) and LPL (b) mRNA expression in fetal perirenal fat of female (solid bar) and male (open bar) fetuses from control (C) and undernourished (UN) adolescent dams whose pregnancies were terminated on day 89 or day 130 of gestation. ANOVA was used to determine effects of sex, maternal nutrition, stage of gestation, and all possible interactions. Vertical bars are group mean ± sem and those with different letters differ P<0.05 using Tukey’s post-hoc method. Number of fetuses per group can be derived from Table 3. For leptin, there were effects of gender (P=0.001), stage of gestation (P=0.001), nutrition (P=0.029) and a gender x nutrition interaction (P=0.029). For LPL, there was an effect of gender (P=0.002), and a gender x nutrition x stage of gestation interaction (P=0.008).
Table 2.
Unilocular and multilocular adipose cell distribution in the fetal perirenal fat depot at day 130 of gestation in relation to maternal nutrition (n=7/group)
| Maternal Nutrition | Control | UN | P value, C vs UN |
|---|---|---|---|
| Unilocular fat | |||
| Volume density, % | 51.3±3.79 | 41.9±4.47* | 0.137 |
| Total mass, g | 14.0±1.07 | 8.2±1.01¥ | 0.002 |
| Relative mass, g/kg fetus | 3.3±0.26 | 2.4±0.37 | 0.078 |
| Multilocular fat | |||
| Volume density, % | 48.6±3.81 | 58.0±4.47* | 0.135 |
| Total mass, g | 13.4±1.37 | 11.3±0.96¥ | 0.231 |
| Relative mass, g/kg fetus | 3.1±0.29 | 3.1±0.18 | 0.954 |
UN, undernourished. Within a nutritional group values with the same symbol differ from each other, P<0.05.
Results
Maternal and fetal phenotype
By design maternal liveweight (42.9±0.41kg) and adiposity score (2.3±0.02) were equivalent between groups at embryo transfer and thereafter dams receiving a control (C) intake maintained their initial adiposity score until necropsy at gestational d89 or 130 (Table 1). In contrast, the external adiposity score of underfed (UN) dams gradually decreased, with the changes equivalent to average losses of 6.2 and 8.8% body fat at d89 and 130, respectively (Russel et al. 1969). Maternal liveweight and carcass mass had diverged by d89, with the difference between nutritional groups increased by d130. The relatively catabolic state of UN dams was mirrored by low plasma insulin and glucose, and by late pregnancy plasma NEFA was increased in UN versus C dams.
Fetal weight was independent of maternal dietary intake at d89 but by d130 fetuses from UN dams were 17% lighter than those in C dams (Table 1), although brain growth was preserved as indicated by higher fetal-weight specific brain weight (12.0±0.39 vs. 9.9±0.35g/kg, P<0.01) as well as greater brain: liver weight ratios (Table 1). Fetal plasma insulin and glucose were independent of maternal nutrition at d89 but there were trends towards lower insulin and glucose in the UN group at d130. Total placentome weight was not affected by maternal nutrition at either time-point (Table 1).
Fetal adiposity
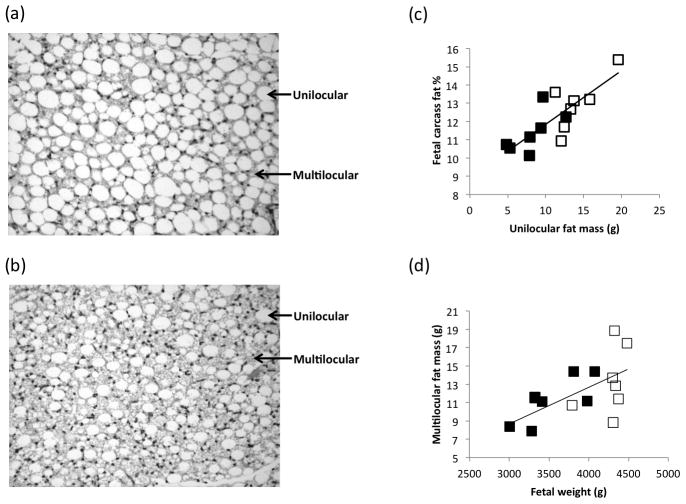
Absolute PAT mass increased >12-fold between gestational d89 and 130 and was lower in fetuses of UN dams at the late gestation time-point (Table 1). Moreover, PAT mass was positively related to fetal weight at both gestational ages (r=0.632, P=0.015 and r=0.738, P=0.003, n=14 at d89 and 130) and accordingly fetal weight-specific PAT mass was also negatively impacted by maternal undernutrition. At gestation d130, both absolute and fetal weight-specific unilocular fat cell mass was lower in fetuses from UN versus C dams (Table 2), but there were no significant effects of nutrition on multilocular cell mass. Representative images are presented in Figure 1. The relative proportion (volume density) of unilocular versus multilocular cells was equivalent within the C group but UN fetuses had more multilocular than unilocular cells (P<0.05). Irrespective of maternal nutrition, absolute and relative fetal unilocular fat mass was positively associated with carcass fat percentage (n=14; r=0.806, P=0.001 and r=0.763, P=0.001, Figure 1c). Multilocular fat cell mass was unrelated to fetal carcass fat percentage, but total multilocular fat mass was positively associated with fetal weight (r=0.605, P=0.022, n=14 Figure 1d), primarily due to the UN group (r=0.787, P=0.036, n=7).
Figure 1.
Haematoxylin and eosin-stained sections of perirenal fat from a representative fetus from a control and an undernourished pregnancy (both females) with unilocular and multilocular fat cells highlighted (a and b), and relationships between perirenal unilocular fat mass and percentage of fat in the carcass (c, r=0.806, P=0.001), and between fetal weight and multilocular fat mass (d, r=0.605, P=0.022) at day 130 of gestation in fetuses from control (□) and undernourished (■) adolescent dams.
Perirenal fat gene expression
The 2-factor ANOVA revealed an increase in PPARG mRNA expression in PAT as gestation progressed, while the stage x nutrition interaction reflected lower PPARG mRNA abundance in the UN group at gestational d89 but not at d130 (Table 3). G3PDH and HSL mRNA expression increased 2–3 fold between d89 and 130 irrespective of maternal nutrition, whereas LPL and FAS mRNA levels were independent of both gestational age and nutrition. UCP1 and PRLR mRNA were virtually undetectable at d89 and independent of maternal nutrition at d130. In contrast, gene expression for IGF1, IGF2 and their receptors was robustly greater at d89 than at d130, and these genes were impacted by maternal nutrition, largely due to lower expression in UN fetuses at d89. PAT leptin mRNA was influenced by stage of pregnancy (d130>89), with a trend for lower expression in UN fetuses.
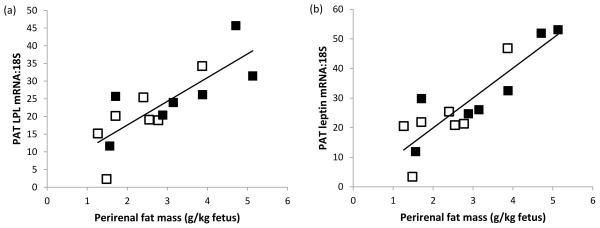
In view of the differential in fetal weight and PAT mass between the two stages of pregnancy and the dominant influence of gestational age on mRNA abundance of the majority of genes quantified, the relationships between fetal weight, PAT mass, metabolic status and adipose tissue gene expression were examined separately for the d89 and d130 groups (Table 4). At d89, the relative expression of genes involved in adipocyte differentiation and function, namely PPARG, G3PDH, LPL, FASN, HSL, leptin and UCP1, were positively related to both absolute and fetal weight-specific PAT mass, but unrelated to fetal weight (Table 4, Figure 3). Of the genes putatively involved in adipocyte proliferation, only IGF1 was weakly positively related to fetal weight-specific PAT mass. Also at d89, fetal plasma glucose was unrelated to abundance of any of the genes measured but fetal plasma insulin was modestly negatively associated with LPL, FASN, HSL and UCP1 mRNA expression. In contrast, at gestational d130 there were relatively weak negative relationships between fetal size, absolute PAT mass and gene expression for G3PDH and HSL (Table 4). Fetal weight-specific PAT mass was positively associated with UCP1 gene expression and was the only relationship detected at both stages of gestation. UCP1 gene expression and carcass fat percentage were positively associated at d130 (r=0.559, n=14, P=0.039). Fetal plasma glucose was positively related to leptin and all four IGF system genes at d130 and positive relationships were evident between fetal plasma insulin and both leptin and IGF1 gene expression.
Table 4.
Relationship between perirenal fat gene expression (relative to 18S) and fetal weight, fat mass and metabolic status at day 89 and 130 of gestation, irrespective of maternal nutrition and gender.
| PPARG | G3PDH | LPL | FASN | HSL | Leptin | UCP-1 | IGF1 | IGF2 | IGF1R | IGF2R | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 89 | |||||||||||
| Fetal weight, g | 0.137 | 0.139 | 0.161 | 0.333 | 0.274 | 0.122 | 0.203 | 0.257 | 0.189 | −0.132 | 0.066 |
| Perirenal fat mass, g | 0.619* | 0.636* | 0.707** | 0.807*** | 0.770*** | 0.759** | 0.630* | 0.505 | 0.389 | −0.060 | 0.325 |
| Perirenal fat, g/kg fetus | 0.729** | 0.717** | 0.795*** | 0.808*** | 0.805*** | 0.872*** | 0.702** | 0.558* | 0.442 | 0.049 | 0.420 |
| Plasma glucose | −0.207 | −0.282 | −0.233 | −0.177 | −0.166 | −0.215 | −0.397 | −0.105 | −0.075 | −0.323 | −0.234 |
| Plasma insulin | −0.497 | −0.413 | −0.584* | −0.538* | −0.696** | −0.489 | −0.697** | −0.377 | −0.436 | −0.205 | −0.400 |
| Day 130 | |||||||||||
| Fetal weight, g | −0.422 | −0.560* | −0.511 | 0.239 | −0.612* | 0.015 | 0.003 | 0.390 | 0.093 | −0.061 | −0.047 |
| Perirenal fat mass, g | −0.328 | −0.570* | −0.315 | 0.193 | −0.549* | 0.179 | 0.421 | 0.483 | 0.267 | 0.093 | 0.140 |
| Perirenal fat, g/kg fetus | −0.064 | −0.315 | 0.010 | 0.084 | −0.222 | 0.208 | 0.594* | 0.343 | 0.295 | 0.161 | 0.221 |
| Plasma glucose | 0.172 | 0.215 | 0.197 | 0.361 | −0.130 | 0.689** | 0.265 | 0.879*** | 0.796** | 0.631* | 0.741** |
| Plasma insulin | −0.059 | −0.137 | 0.129 | 0.016 | −0.241 | 0.716** | 0.192 | 0.597* | 0.477 | 0.449 | 0.475 |
Values are Pearson correlation coefficients with significant values shown in bold,
P<0.05,
P<0.01,
P<0.001.
Figure 3.
Relationships between body weight-specific perirenal adipose tissue (PAT) mass and perirenal fat (a) LPL mRNA (r=0.795, P<0.001) and (b) leptin mRNA (r=0.872, P<0.001) at day 89 of gestation in fetuses from control (□) and undernourished (■) adolescent dams.
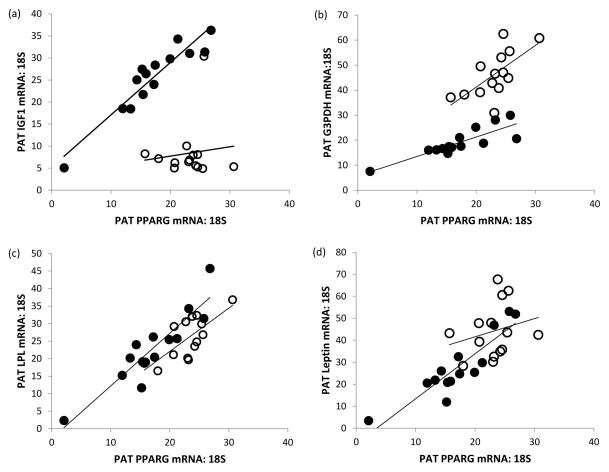
PRLR gene expression was unrelated to fetal weight, adiposity or metabolic status at both stages of gestation (not shown). Similarly PRLR mRNA abundance was not related to expression of any of the other genes measured in PAT. The relationships between the other 11 genes measured at gestational d89 and 130 are detailed in Table 5. At d89, robust positive relationships were evident between all possible comparisons (n=21) of PPARG, G3PDH, LPL, FASN, HSL, leptin and UCP1 mRNA expression. At d130, similar relationships were evident between PPARG, G3PDH, LPL and HSL but none of these genes was related to FASN, UCP1 or leptin expression and the latter genes were not correlated with each other. Similarly, IGF1 and IGF2 gene expression was positively related to the various genes involved in adipocyte differentiation and function at d89 (reaching significance in 13 of 14 comparisons, Table 5) but was unrelated to these genes at d130. Selected examples of these relationships are detailed in Figure 4.
Table 5.
Relationships between relative expression of genes in perirenal fat irrespective of nutrition status or gender at day 89 and day 130 of gestation
| Day 89 | G3PDH | LPL | FASN | HSL | Leptin | UCP1 | IGF1 | IGF2 | IGF1R | IGF2R |
|---|---|---|---|---|---|---|---|---|---|---|
| PPARG | 0.853*** | 0.910*** | 0.715** | 0.747** | 0.900*** | 0.768** | 0.940*** | 0.896*** | 0.593* | 0.882*** |
| G3PDH | 0.754** | 0.677** | 0.693** | 0.845*** | 0.715** | 0.710** | 0.738** | 0.429 | 0.670** | |
| LPL | 0.899*** | 0.891*** | 0.938*** | 0.754** | 0.805*** | 0.714** | 0.361 | 0.738** | ||
| FASN | 0.896*** | 0.833*** | 0.669** | 0.573* | 0.468 | −0.023 | 0.422 | |||
| HSL | 0.868*** | 0.837*** | 0.603* | 0.560* | 0.124 | 0.515 | ||||
| Leptin | 0.752** | 0.735** | 0.662** | 0.311 | 0.666** | |||||
| UCP1 | 0.663** | 0.626* | 0.333 | 0.599* | ||||||
| IGF1 | 0.951*** | 0.686** | 0.927*** | |||||||
| IGF2 | 0.776*** | 0.937** | ||||||||
| IGF1R | 0.874*** | |||||||||
| Day 130 | G3PDH | LPL | FASN | HSL | Leptin | UCP-1 | IGF1 | IGF2 | IGF1R | IGF2R |
|
| ||||||||||
| PPARGγ | 0.653* | 0.723** | 0.157 | 0.824*** | 0.242 | 0.171 | 0.118 | 0.345 | 0.548* | 0.427 |
| G3PDH | 0.645* | 0.189 | 0.713** | 0.347 | −0.113 | 0.192 | 0.356 | 0.252 | 0.321 | |
| LPL | −0.008 | 0.716** | 0.222 | 0.006 | 0.014 | 0.155 | 0.502 | 0.245 | ||
| FASN | 0.099 | 0.129 | −0.047 | 0.348 | 0.337 | 0.000 | 0.193 | |||
| HSL | 0.220 | −0.217 | −0.166 | 0.087 | 0.181 | 0.150 | ||||
| Leptin | 0.107 | 0.433 | 0.432 | 0.506 | 0.501 | |||||
| UCP1 | 0.390 | 0.446 | 0.419 | 0.423 | ||||||
| IGF1 | 0.934*** | 0.509 | 0.768*** | |||||||
| IGF2 | 0.598* | 0.896*** | ||||||||
| IGF1R | 0.816*** | |||||||||
Values are Pearson correlation coefficients with significant values shown in bold,
P<0.05,
P<0.01,
P<0.001.
Figure 4.
Relationships between relative PPARG gene expression and (a) IGF1, (b) G3PDH, (c) LPL and (d) leptin mRNA in fetal perirenal adipose tissue (PAT) at day 89 (●) and 130 (○) of gestation. At day 89 and 130, respectively: r=0.940, P<0.001 and r=0.118, not significant (NS) for (a); r=0.853, P<0.001 and r=0.653, P<0.05 for (b); r=0.910, P<0.001 and r=0.723, P<0.01 for (c); r=0.900, P<0.001 and r=0.242, NS for (d).
Adiposity and gene expression in relation to fetal sex
An effect of sex per se on absolute or fetal weight-specific PAT mass was not detected at either gestational d89 or 130 (P>0.3, data not shown). In late gestation, females had relatively more unilocular fat mass than males (3.2±0.34 versus 2.4±0.32g/kg) but this was not statistically significant (P=0.143). In relation to PAT gene expression, when sex was included as an additional factor in the ANOVA, females overall had greater leptin expression than males (P=0.001, Figure 2a) and the impact of maternal nutrition as well as gestational age was significant (P=0.029 and P=0.001, respectively). Sex also influenced PAT LPL gene expression (females>males, Figure 2b) and gene expression for both leptin and LPL was lower in female but not male UN versus C fetuses at gestational d89 only (Figure 2a, b). Although an effect of sex was not detected for any of the other genes measured, PPARG mRNA expression was lower in UN versus C female fetuses at d89 (nutrition x stage x gender interaction, P=0.011).
Discussion
This study reveals that a number of genes regulating prenatal adipose tissue development and function are expressed by mid-gestation when they are already sensitive to maternal undernutrition, and that their expression levels are markedly changed by late gestation. The early molecular sensitivity to poor nutrient supply may underlie the reduced fetal adiposity and altered proportions of unilocular and multilocular adipocytes evident by late pregnancy. In addition, the study indicates that some sex-specific differences in adipose tissue gene expression may emerge in utero.
Fetal growth and adiposity
Limiting maternal intake throughout gestation in young adolescent sheep results in gradual depletion of maternal reserves and slowing of fetal growth. By late gestation the fetuses are lighter and leaner than optimally-nourished controls reflected by lower carcass fat and reduced fetal PAT mass (Luther et al. 2007). Herein, we demonstrate that the PAT mass reduction is primarily due to lower numbers of unilocular adipocytes, suggesting that limiting maternal and hence fetal nutrient supply restricts the development of cells primarily involved in fat storage rather than the cells involved in neonatal thermogenesis. The positive relationship between PAT unilocular fat mass and carcass fat content implies a similar scenario is likely to operate throughout the fetus leading to a lean body phenotype. However, while the impact of maternal undernutrition on weight at birth is statistically significant in our model the effect is modest and previous studies using an equivalent prenatal nutritional insult have failed to detect persistent influences on postnatal growth or glucose metabolism up to 6 months of age (Wallace et al. 2010, 2012).
In this study a positive correlation between multilocular fat mass and fetal weight was observed within the undernourished group and furthermore those fetuses had more multilocular than unilocular adipocytes, which may be indicative of developmental delay. Nevertheless relative to the control group maternal undernutrition did not influence absolute or relative multilocular cell mass overall, suggesting that had these pregnancies progressed to term neonatal thermogenesis might not have been severely compromised. Indeed in two such studies no neonatal mortality occurred in offspring of undernourished dams following spontaneous delivery at term (Wallace 2011).
In our undernourished paradigm we restrict maternal intake throughout gestation rather than during specific “windows” as this reflects the most common scenario in human pregnancy. We also focus on adolescents because insufficient gestational weight gain (a proxy for maternal undernutrition) in this age group predicts low birthweight (Hediger et al. 1989; Scholl et al. 1991; Stevens-Simon & McAnarney 1992) and in developing countries where malnutrition predominates high pregnancy rates are commonplace in adolescents. As such there is a lack of directly comparable data in other precocial animal models.
Previous studies of adipose tissue development have largely involved adult ewes undernourished during discrete windows of gestation, with little consensus on the impact on fetal weight or PAT mass (Symonds et al. 1998; Bispham et al. 2003; Budge et al. 2004; Lie et al. 2013). None of these studies involving undernutrition quantified adipocyte cell populations, but conversely when fetal nutrient supply in normal late gestation pregnancies was increased by a 10-day intrafetal glucose infusion, the unilocular fat mass was enhanced and the size of the dominant lipid locule was positively correlated with fetal glucose concentrations (Mühlhäusler et al. 2005). The latter study and the data herein suggest that unilocular adipocytes within the PAT depot are sensitive to the prevailing nutrient supply in utero and when this supply is compromised multilocular cells predominate.
Perirenal fat gene expression: ontogeny and impact of undernutrition
The design herein allowed us to examine for the first time both the ontogeny of fetal PAT gene expression and the impact of maternal undernutrition. At mid-gestation when the adipose tissue first appears and begins to replicate, the abundance of all four IGF system genes was high, commensurate with the roles of IGF1 and IGF2 in adipocyte proliferation and differentiation (Holly et al. 2006; Kleiman et al. 2013). Moreover at this early stage of PAT development the relative gene expression of all four IGF system genes was already lower in undernourished fetuses, prior to any significant reduction in fetal body weight or PAT mass. These observations are consistent with attenuated IGF-mediated adipocyte proliferation and differentiation around mid-gestation most likely underlying the reduced mass and structural characteristics of PAT observed in the undernourished fetuses in late gestation. Although the consequences for postnatal body composition have not been established in this model, it is likely that the reduction in adipocyte number translates into increased susceptibility for cellular hypertrophy in later life (Heilbronn et al. 2004), particularly if the animal is exposed to a nutrient-rich diet postnatally.
IGF1 regulates transcription factors including PPARG (Scavo et al. 2004) and attenuated local IGF secretion within the developing PAT of undernourished fetuses may underlie the lower expression of PPARG at mid-gestation, with consequences for the progression of adipocyte differentiation and function. From the present data it is unlikely that deficits in glucose supply directly influence PPARG expression because fetal plasma glucose was not different between groups in mid-gestation and no correlation between fetal glucose and PPARG mRNA was observed. In contrast, when maternal nutrition was increased in late pregnancy (55% above maintenance), fetal plasma glucose and PAT PPARG mRNA were increased and positively correlated with each other, independent of changes in PAT mass or morphology (Mühlhäusler et al. 2007). IGF gene expression was not measured in the latter study but it is noteworthy that herein fetal glucose was positively related to IGF system mRNA in late pregnancy.
Herein, the gene expression of PPARG modestly increased with gestational age. A similar ontogenic increase in PPARG expression has recently been reported in normally-growing ovine fetuses sampled at either gestational d80 or 140 (Pope et al. 2014), commensurate with its role as a key regulator of adipose tissue-specific genes and with the increase in PAT mass as gestation proceeds. Accordingly a marked increase in relative gene expression between mid and late gestation was observed here for selected genes involved in lipid deposition, metabolism and signalling (G3PDH, HSL, leptin), whereas others involved in fatty acid synthesis and uptake into adipocytes (FASN, LPL) were surprisingly independent of gestational age.
For the genes specific to brown adipose tissue, namely PRLR and UCP1, abundance was extremely low in mid-pregnancy and high at late pregnancy in line with their role in promoting adequate thermogenesis after birth (Symonds et al. 1998; Pearce et al. 2005). Furthermore, the absence of an effect of maternal undernutrition on PRLR and UCP1 gene expression in the present late gestation fetuses matched the lack of effect on multilocular adipocyte mass. Conversely, the trend for attenuated PAT gene expression in UN fetuses for leptin, a white adipose tissue-specific gene (Yuen et al. 2003), was in line with the observed reduction in unilocular cell mass in late gestation.
Irrespective of the direction of ontogenic change, and with the exception of the aforementioned leptin expression, it is noteworthy that none of the other genes measured were impacted only in late gestation by maternal undernutrition, indicating that key molecular events underlying the lean fetal phenotype in this model originated earlier in gestation. In support we observed robust positive correlations between fetal weight-specific PAT mass and genes involved in adipocyte differentiation and function at gestational d89 (PPARG, G3PDH, LPL, FASN, HSL, leptin and UCP1), but by late gestation only a weak relationship between UCP1 and adiposity persisted. Moreover, expression of IGF1 and IGF2 and all the aforementioned genes were highly correlated with each other at the early stage of gestation, but fewer between-gene relationships were evident by late gestation. These findings may be attributable to differentiation of adipose tissue into the two main cell types by late gestation, and the relative proportion of white versus brown adipocytes being differentially impacted by maternal undernutrition by that stage.
Perirenal fat gene expression: impact of sex
We did not originally power the study to examine gender effects as we had no control over the sex of the embryo at the time of its transfer into the adolescent recipient. Irrespective of the low power when the gene expression data were examined it became clear that potentially interesting sex-specific effects were emerging. Accordingly when sex was included as a factor in the analysis, both leptin and LPL gene expression was higher in females than males overall. This agrees with reported higher LPL abundance in PAT and subcutaneous fat depots in female versus male lambs at 4 months postnatal age (Rattanatray et al. 2010). It also matches our data showing widespread sex differences in PAT gene expression, namely higher LPL, FASN and leptin, and lower IGF1, IGF2 and HSL in females, corresponding with their greater visceral and carcass fat deposition and larger adipocytes at 3 months of age (Wallace et al. 2014). Thus, the present study is the first in sheep to suggest that sex-specific differences in molecular markers of adipose tissue differentiation and/or function emerge in utero. We acknowledge that these potentially interesting gender differences in PAT gene expression require to be substantiated in a larger and more suitably powered study. Nevertheless greater expression of leptin in female fetal PAT aligns with the prenatal sexual dimorphism in leptin secretion and neonatal adiposity in humans (females> males). Relative to males, female gender is associated with greater leptin concentrations in amniotic samples at 16 weeks gestation and in cord blood at delivery (Cagnacci et al. 2006; Kayemba-Kay’s et al. 2008), and with greater percentage body fat as measured by air-displacement plethysmography neonatally (Hawkes et al. 2011). Furthermore, since only females were sensitive herein to the effects of maternal undernutrition with respect to PPARG, leptin and LPL gene expression at gestational d89, the implication is that sensitivity to prevailing nutrition occurs earlier in females than males and there is likely to be a temporal difference in adipose tissue development between sexes throughout the early life-course.
Previous studies investigating the impact of maternal nutrition on prenatal adipose gene expression in precocial animals have not reported fetal sex (Bispham et al. 2003; Budge et al. 2004; Mühlhäusler et al. 2007; Nguyen et al. 2010; Lie et al. 2013) but it is clearly an important consideration; imbalances in the male:female ratio may have confounded earlier results and may explain the lack of consensus in the sheep literature. Indeed, sexually-dimorphic differences in fetal growth and expression of genes involved in adipogenesis and brown adipose tissue development following in vitro adipocyte differentiation in culture are seen in fetal baboons following modest maternal undernutrition throughout gestation (Tchoukalova et al. 2014). Although we did not determine the long term impact of the mid-gestation sex-specific nutritional sensitivity of adipose tissue gene expression on postnatal adiposity, the present data align with findings from the Dutch famine of 1945. These show that maternal famine exposure during early (but not mid or late) pregnancy is associated with greater BMI and waist circumference at age 50 in women but not men (Ravelli et al. 1999) and may be due to altered DNA methylation of genes involved in adipose tissue metabolism (Tobi et al. 2009).
In conclusion, these data support the hypothesis that molecular markers of adipose tissue development in the fetus are temporally sensitive to maternal undernutrition in precocial mammals. Some key genes regulating adipose tissue development and function are active from mid-gestation when they are variously sensitive to maternal undernutrition leading to reduced fetal adiposity in late gestation. Furthermore, sex-specific differences in adipose tissue gene expression emerge in fetal life. Whether these prenatal events impact body composition in adult life remains unknown but it is clear that ensuring adequate maternal nutrition from the earliest stages of pregnancy is essential to optimise fetal growth and neonatal adipose development.
Acknowledgments
Funding
Funded by the Scottish Government’s Rural and Environment Science and Analytical Services Division (RESAS), including the Strategic Partnership for Animal Science Excellence (SPASE) and the U.S. National Institutes of Health (HD045784).
Footnotes
Declaration of interest
None of the authors had any financial or personal conflicts of interest.
References
- AFRC. An Advisory Manual Prepared by the AFRC Technical Committee on Responses to Nutrients. Wallingford: CAB International; 1993. Energy and Protein Requirements of Ruminants. [Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, Broughton Pipkin F, Stephenson T, Symonds ME. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- Budge H, Edwards LJ, McMillan IC, Bryce A, Warnes K, Pearce S, Stephenson T, Symonds ME. Nutritional manipulation of fetal adipose deposition and uncoupling protein 1 messenger RNA abundance in the sheep: differential effects of timing and duration. Biology of Reproduction. 2004;71:359–365. doi: 10.1095/biolreprod.103.018986. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Arangino S, Caretto S, Mazza V, Volpe A. Sexual dimorphism in the levels of amniotic fluid leptin in pregnancies at 16 weeks gestation: relation to fetal growth. European Journal of Obstetrics Gynecology and Reproductive Biology. 2006;124:53–57. doi: 10.1016/j.ejogrb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Christian P, Murray-Kolb LE, Tielsch JM, Katz J, LeClerq SC, Khatry SK. Associations between preterm birth, small-for-gestational age, and neonatal morbidity and cognitive function among school-age children in Nepal. BMC Pediatrics. 2014;27(14):58. doi: 10.1186/1471-2431-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Molecular and Cellular Endocrinology. 2010;25:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Gemmell RT, Alexander G. Ultrastructural development of adipose tissue in foetal sheep. Australian Journal of Biological Science. 1978;31:505–515. doi: 10.1071/bi9780505. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Thompson AN, Ford SP. Postnatal consequences of the maternal environment and growth during prenatal life for productivity of ruminants. In: Greenwood PL, Bell AW, Vercoe PE, Viljoen GJ, editors. Managing the Prenatal Environment to Enhance Livestock Productivity. Springer; 2010. pp. 3–36. [Google Scholar]
- Hawkes CP, Hourihane JO, Kenny LC, Irvine AD, Kiely M, Murray DM. Gender- and gestational age-specific body fat percentage at birth. Pediatrics. 2011;128:e645–651. doi: 10.1542/peds.2010-3856. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Scholl TO, Belsky DH, Ances IG, Salmon RW. Patterns of weight gain in adolescent pregnancy: effects on birth weight and preterm delivery. Obstetrics & Gynecology. 1989;74:6–12. [PubMed] [Google Scholar]
- Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type 11 diabetes mellitus. International Journal of Obesity. 2004;28(Suppl 4):S12–21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- Holly J, Sabin M, Perks C, Shield J. Adipogenesis and IGF-1. Metabolic Syndrome and Related Disorders. 2006;4:43–50. doi: 10.1089/met.2006.4.43. [DOI] [PubMed] [Google Scholar]
- Jain V, Singhal A. Catch up growth in low birth weight infants: striking a healthy balance. Reviews in Endocrine and Metabolic Disorders. 2012;13:141–147. doi: 10.1007/s11154-012-9216-6. [DOI] [PubMed] [Google Scholar]
- Kayemba-Kay’s S, Geary MP, Pringle J, Rodeck CH, Kingdom JC, Hindmarsh PC. Gender, smoking during pregnancy and gestational age influence cord leptin concentrations in newborn infants. European Journal of Endocrinology. 2008;159:217–224. doi: 10.1530/EJE-08-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus S. Adipose tissue as a regulator of energy balance. Current Drug Targets. 2004;5:241–250. doi: 10.2174/1389450043490523. [DOI] [PubMed] [Google Scholar]
- Kleiman A, Keats EC, Chan NG, Khan ZI. Elevated IGF2 prevents leptin induction and terminal adipocyte differentiation in hemangioma stem cells. Experimental and Molecular Pathology. 2013;94:126–136. doi: 10.1016/j.yexmp.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Lie S, Morrison JL, Williams-Wyss O, Ozanne SE, Zhang S, Walker SK, Kleeman DO, MacLaughlin SM, Roberts CT, McMillen IC. Impact of embryo number and periconception undernutrition on factors regulating adipogenesis, lipogenesis, and metabolism in adipose tissue in the sheep fetus. American Journal of Physiology Endocrinology and Metabolism. 2013;305:E931–941. doi: 10.1152/ajpendo.00180.2013. [DOI] [PubMed] [Google Scholar]
- Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR) Journal of Maternal, Fetal and Neonatal Medicine. 2013;26:222–225. doi: 10.3109/14767058.2012.715006. [DOI] [PubMed] [Google Scholar]
- Lukaszewski MA, Eberlé D, Vieau D, Breton C. Nutritional manipulation in the perinatal period program adipose tissue in offspring. American Journal of Physiology Endocrinology and Metabolism. 2013;305:E1195–1207. doi: 10.1152/ajpendo.00231.2013. [DOI] [PubMed] [Google Scholar]
- Luther JS, Redmer DA, Reynolds LP, Wallace JM. Nutritional paradigms of ovine fetal growth restriction: implications for human pregnancy. Human Fertility (Cambridge) 2005;8:179–187. doi: 10.1080/14647270500320121. [DOI] [PubMed] [Google Scholar]
- Luther J, Aitken R, Milne J, Matsuzaki M, Reynolds L, Redmer D, Wallace J. Maternal and fetal growth, body composition, endocrinology, and metabolic status in undernourished adolescent sheep. Biology of Reproduction. 2007;77:343–350. doi: 10.1095/biolreprod.107.061440. [DOI] [PubMed] [Google Scholar]
- MacRae JC, Bruce LA, Hovell FDB, Hart IC, Inkster J, Atkinson T. Influence of protein nutrition on the response of growing lambs to exogenous bovine growth hormone. Journal of Endocrinology. 1991;130:53–61. doi: 10.1677/joe.0.1300053. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Milne JS, Aitken RP, Wallace JM. Overnourishing pregnant adolescent ewes preserves perirenal fat deposition in their growth-restricted fetuses. Reproduction Fertility and Development. 2006;18:357–364. doi: 10.1071/rd05067. [DOI] [PubMed] [Google Scholar]
- Moragas A, Toran N. Prenatal development of brown adipose tissue in man. A morphometric and biomathematical study. Biology of the Neonate. 1983;43:80–85. doi: 10.1159/000241641. [DOI] [PubMed] [Google Scholar]
- Mühlhäusler BS, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Fetal leptin is a signal of fat mass independent of maternal nutrition in ewes fed at or above maintenance energy requirements. Biology of Reproduction. 2002;67:493–499. doi: 10.1095/biolreprod67.2.493. [DOI] [PubMed] [Google Scholar]
- Mühlhäusler BS, Adam CL, Marrocco EM, Findlay PA, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. Journal of Physiology. 2005;565(1):185–195. doi: 10.1113/jphysiol.2004.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlhäusler BS, Duffield JA, McMillan IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology. 2007;148:878–885. doi: 10.1210/en.2006-1115. [DOI] [PubMed] [Google Scholar]
- Nissen PM, Oksbjerg N. Quantification of prenatal effects on productivity in pigs. In: Greenwood PL, Bell AW, Vercoe PE, Viljoen GJ, editors. Managing the Prenatal Environment to Enhance Livestock Productivity. Springer; 2010. pp. 37–70. [Google Scholar]
- Nguyen LT, Mühlhäusler BS, Botting KJ, Morrison JL. Maternal undernutrition alters fat cell size distribution, but not lipogenic gene expression, in the visceral fat of the late gestation guinea pig fetus. Placenta. 2010;31:902–909. doi: 10.1016/j.placenta.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Pearce S, Budge H, Mostyn A, Genever E, Webb R, Ingleton P, Walker AM, Symonds ME, Stephenson T. Prolactin, the prolactin receptor and uncoupling protein abundance and function in adipose tissue during development in young sheep. Journal of Endocrinology. 2005;184:351–359. doi: 10.1677/joe.1.05732. [DOI] [PubMed] [Google Scholar]
- Pope M, Budge H, Symonds ME. The developmental transition of ovine adipose tissue through early life. Acta Physiologica. 2014;210:20–30. doi: 10.1111/apha.12053. [DOI] [PubMed] [Google Scholar]
- Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M, Moore SE, Fuchs G. Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. American Journal of Clinical Nutrition. 2007;85:845–852. doi: 10.1093/ajcn/85.3.845. [DOI] [PubMed] [Google Scholar]
- Rattanatray L, MacLaughlin SM, Kleeman DO, Walker SK, Mühlhäusler BS, McMillen IC. Impact of maternal periconceptional overnutrition on fat mass and expression of adipogenic and lipogenic genes in visceral and subcutaneous fat depots in the postnatal lamb. Endocrinology. 2010;151:5195–5205. doi: 10.1210/en.2010-0501. [DOI] [PubMed] [Google Scholar]
- Ravelli ACJ, van der Meulen JHP, Osmond C, Barker DJP, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. American Journal of Clinical Nutrition. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- Russel AJF, Doney JM, Gunn RG. Subjective assessment of body fat in live sheep. Journal of Agricultural Science Cambridge. 1969;72:451–454. [Google Scholar]
- Sarr O, Yang K, Regnault TRH. In utero programming of later adiposity: the role of fetal growth restriction. Journal of Pregnancy. 2012;12:Article ID 134758. doi: 10.1155/2012/134758. 10 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavo LM, Karas M, Murray M, Leroith D. Insulin-like growth factor -1 stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. Journal of Clinical Endocrinology and Metabolism. 2004;89:3543–3553. doi: 10.1210/jc.2003-031682. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Khoo CS, Healey MF, Rawson NL. Maternal weight gain, diet and infant birth weight: correlations during adolescent pregnancy. Journal of Clinical Epidemiology. 1991;44:423–428. doi: 10.1016/0895-4356(91)90081-j. [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. Journal of Clinical Investigation. 2006;116:581–590. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens-Simon C, McAnarney ER. Adolescent pregnancy. Gestational weight gain and maternal and infant outcomes. American Journal of Diseases of Children. 1992;146:1359–1364. doi: 10.1001/archpedi.1992.02160230117031. [DOI] [PubMed] [Google Scholar]
- Symonds ME. Brown adipose tissue growth and development. Scientifica. 2013:Article ID 305763. doi: 10.1155/2013/305763. 14 pages, http://dx.doi.org/10.1155/2013/305763. [DOI] [PMC free article] [PubMed]
- Symonds ME, Phillips ID, Anthony RV, Owens JA, McMillen IC. Prolactin receptor gene expression and foetal adipose tissue. Journal of Neuroendocrinology. 1998;10:885–890. doi: 10.1046/j.1365-2826.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- Tchoukalova YD, Krishnapuram R, White UA, Burk D, Fang X, Nijland MJ, Nathanielsz PW. Fetal baboon sex specific outcomes in adipocyte differentiation at 0.9 gestation in response to moderate maternal nutrient reduction. International Journal of Obesity. 2014;38:224–230. doi: 10.1038/ijo.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Human Molecular Genetics. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM. Adaptive maternal, placental and fetal responses to nutritional extremes in the pregnant adolescent: lessons from sheep. In: Mascie-Taylor CGN, Rosetta LL, editors. Reproduction and Adaptation. Cambridge University Press; 2011. pp. 112–127. [Google Scholar]
- Wallace JM, Da Silva P, Aitken RP, Cheyne MA. Maternal endocrine status in relation to pregnancy outcome in rapidly growing adolescent sheep. Journal of Endocrinology. 1997;155:359–368. doi: 10.1677/joe.0.1550359. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Cruickshank MA. Switching maternal dietary intake at the end of the first trimester has profound effects on placental development and foetal growth in adolescent ewes carrying singleton fetuses. Biology of Reproduction. 1999;61:101–110. doi: 10.1095/biolreprod61.1.101. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Matsuzaki M, Milne J, Aitken R. Late but not early gestational maternal growth hormone treatment increases fetal adiposity in overnourished adolescent sheep. Biology of Reproduction. 2006;75:231–239. doi: 10.1095/biolreprod.106.052605. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Milne JS, Aitken RP. Effect of weight and adiposity at conception and wide variations in gestational dietary intake on pregnancy outcome and early postnatal performance in young adolescent sheep. Biology of Reproduction. 2010;82:320–330. doi: 10.1095/biolreprod.109.080069. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Milne JS, Adam CA, Aitken RP. Adverse metabolic phenotype in low-birth-weight lambs and its modification by postnatal nutrition. British Journal of Nutrition. 2012;107:510–522. doi: 10.1017/S0007114511003175. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Milne JS, Aitken RP, Adam CL. Influence of birth weight and gender on lipid status and adipose tissue gene expression in lambs. Journal of Molecular Endocrinology. 2014;53:131–144. doi: 10.1530/JME-14-0123. [DOI] [PubMed] [Google Scholar]
- Wiebel ER. Stereological Methods. Vol. 1. London: Academic Press; 1979. Practical methods for biological morphometry. [Google Scholar]
- Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review: Intrauterine growth retardation: implications for the animal sciences. Journal of Animal Science. 2006;84:2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- Yuen SJ, Owens PC, Mühlhäusler BS, Roberts CT, Symonds ME, Keisler DH, McFarlane JR, Kauter KG, Evens Y, McMillan IC. Leptin alters the structural and functional characteristics of adipose tissue before birth. FASEB Journal. 2003;17:1102–04. doi: 10.1096/fj.02-0756fje. [DOI] [PubMed] [Google Scholar]