Abstract
The Ixodes scapularis embryo-derived cell line ISE6 is the most widely utilized tick-derived cell line due to its susceptibility to a wide variety of tick- and non-tick-vectored pathogens. Little is known about its tissue origin or biological background. Protein expression of ISE6 cells was compared with that of another Ixodes scapularis-derived cell line, IDE12, and dissected tick synganglia. Results demonstrated the presence of a neuronal marker protein, type 3 β-tubulin, in all three samples, as well as other shared and unique neuronal and immune response-associated proteins. Of neuronal proteins shared between the two cell lines, ISE6 expressed several in significantly greater quantities than IDE12. Stimulation of ISE6 cells by in vivo exposure to the hemocoel environment in unfed larval and molting nymphal ticks, but not unfed nymphal ticks, resulted in the development of neuron-like morphologic characteristics in the implanted cells.
Keywords: Ixodes scapularis, cell culture, ISE6, IDE12, proteome, synganglion, neuron
Introduction
The blacklegged tick, Ixodes scapularis, is the most medically significant tick of North America due to the disease agents it can transmit by feeding on its vertebrate hosts. This species is known to vector a variety of bacterial, viral, and protozoan pathogens to humans including several species of intracellular bacteria in the order Rickettsiales. Because species in the genera Rickettsia, Anaplasma, and Ehrlichia are obligate intracellular bacteria, they require cell culture systems to be propagated and genetically manipulated in the laboratory environment. Tick cells allow for the propagation of microorganisms that do not grow in mammalian cell culture, and for the study of tick-borne pathogens or symbionts in a tick environment (Simser et al. 2001). Tick cell cultures can also be used to study tick physiological and immune responses (Simser et al. 2004), and potentially can be an antigen source for the development of anti-tick vaccines (Bell-Sakyi et al. 2007).
More than 50 tick cell lines have been generated as tools to investigate tick- and tick-borne pathogen biology, of which 21 were generated in our lab, and these have been used for the cultivation and maintenance of numerous obligate intracellular pathogens. Ixodes scapularis-derived cell lines have proved the most useful for the isolation and cultivation of intracellular tick-borne microbes (Bell-Sakyi et al. 2007). Cell line ISE6 (I. scapularis embryonic 6) is a highly permissive and extensively used tick cell line. ISE6 cells have been successfully used to isolate into culture many tick-associated bacteria including members of the genera Rickettsia (Simser et al. 2002; Pornwiroon et al. 2006; Baldridge et al. 2010), Anaplasma (Munderloh et al. 1996a; Munderloh et al. 1996b; Woldehiwet et al. 2002; Munderloh et al. 2003; Tate et al. 2013),Ehrlichia (Munderloh et al. 2009), Borrelia (Varela et al. 2004), and Neoehrlichia (Munderloh et al. 2007). Ixodes scapularis-derived cells have also been used to study vector-pathogen interactions, such as the expression of variant proteins of several Anaplasmataceae members in response to the tick environment (Nelson et al. 2008; Kuriakose et al. 2011; Chávez et al. 2012), and differential expression ofRickettsia prowazekii genes during growth in mammalian and arthropod cells (Tucker et al. 2011). Arboviruses and eukaryotes such as Babesia have also been propagated and studied in I. scapularis cells (Lawrie et al. 2004; Garcia et al. 2005; Ribeiro et al. 2009). Furthermore, ISE6 cells have become an important model for molecular study using RNAi of tick genes important for decreasing pathogen transmission and understanding tick biology, and are amenable to genetic manipulation (Kurtti et al. 2008; Naranjo et al. 2013). Despite the wide use of I. scapularis cell lines for propagating tick-borne pathogens and studying tick biology, little is known about their tissue origin and protein composition.
Proteomic analyses have been used extensively to characterize human-derived cell lines (Negoro et al. 2011; Beckmann et al. 2011), and identify specific markers in cancer cells (Antwi et al. 2009). Proteomic profiling of rabbit embryonic cells helped identify proteins specific to each of three embryo-derived cell lines (Intawicha et al. 2013). To learn more about the tissue origin and protein complement of I. scapularis cell lines, we used proteomic and immunochemical methods, as well as microscopy. We included two different I. scapularis embryo-derived cell lines, ISE6 (resembling neurons) and IDE12 (resembling hemocytes), to determine if their contrasting appearance and behavior (Mattila et al. 2007) was reflected in their protein profiles. In addition, we selected the tick synganglion for comparison to understand the degree to which embryonic I. scapularis cell lines may be taken to represent the cells of a specific tick organ that they resemble.
Materials and Methods
Cell culture
ISE6 cells, in vitro passage 115, (Kurtti et al. 1996) and IDE12 cells, in vitro passage 24, (Munderloh et al. 1994) were maintained at 32°C in supplemented L15C300 medium (Oliver et al. 2014) in sealed 25 cm2 flasks. To distinguish cells injected into I. scapularis ticks from native tissue, ISE6 cells were transformed to express mCherry-Lifeact protein, which fluorescently labels filamentous actin without affecting its normal function (Kurtti et al. 2008; Riedl et al. 2008). mCherry-Lifeact-expressing ISE6 cells were maintained in the same manner as untransformed ISE6. The rhesus monkey endothelial cell line RF/6A (CRL-1780, American Tissue Type Culture Association, Manassas, VA, USA) was cultured in Glutamax RPMI medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum and an additional 2 mM L-glutamine. Neither of the tick cell lines used, nor the mammalian cell line, have been cloned.
Tick maintenance and dissections
Engorged I. scapularis females were purchased from the Oklahoma State University Tick Rearing Facility as they were beginning to lay eggs. Ticks of all stages were kept in sealed humidors over a saturated solution of potassium sulfate to maintain a constant relative humidity of 97% at room temperature (Rockland 1960). Larval and nymphal ticks were fed on male Angora hamsters (Mesocricetus auratus).
For analysis by mass spectrometry, synganglia were dissected from unfed adult I. scapularis. Tissues were kept in cold phosphate buffered saline (PBS) and maintained on ice for 2-3 hours until protein purification. Synganglia were lysed in radio-immunoprecipitation assay (RIPA) buffer (Cold Spring Harbor Protocols 2006) and protein concentration was measured using a BCA protein assay kit (Pierce, Rockford, IL, USA) with a spectrophotometer (Eppendorf, Hamburg, Germany) to determine the amount needed for the proteomic analysis. This revealed that ~1μg of protein could be extracted from a single tick synganglion and, subsequently, synganglia from two male and two female ticks were pooled for each of the three mass spectrometry replicates.
Protein preparation and Mass Spectrometry
Mass spectrometry and protein purifications were performed at the University of Minnesota Center for Mass Spectrometry and Proteomics according to the following protocol.
Protein extraction
Cultured cell proteins were extracted in 200 μl of protein extraction buffer [7 M urea, 2 M thiourea, 0.4 M triethylammonium bicarbonate pH 8.5, 20% acetonitrile and 4 mM tris(2-carboxyethyl)phosphine], whereas the proteins from the synganglia were extracted in 20 μl of the same buffer. Samples were bath sonicated in ice water for 3 min, placed in pressure cycling technology tubes (Pressure Biosciences, Inc., South Easton, MA, USA), and processed in the Barocycler NEP2320 (Pressure Biosciences, Inc.) at 35 kpsi for 30 sec and 0 kpsi for 15 sec for 40 cycles at 37 °C. After running the samples in the barocycler, methyl methanethiosulfonate (MMTS, 200 mM) was added to the tubes to a final concentration of 8 mM MMTS, tubes were inverted several times, and incubated for 15 min at room temperature. Samples were transferred to new 1.5 mL microfuge Protein LoBind tubes (Eppendorf, Hamburg, Germany), and two aliquots of each sample were taken for protein concentration determination by Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The samples were stored at −80 °C until the protein concentrations were determined and the samples were digested.
In-solution proteolytic digestion
Twenty μg each of ISE6 and IDE12 cell protein and 5 μg of synganglion protein were transferred to new 1.5 mL microfuge tubes. Samples were diluted four-fold with ultra-pure water, and trypsin (Promega, Madison, WI, USA) was added at a 1:35 ratio of trypsin to total protein. Samples were incubated for 16 hr at 37 °C after which they were frozen at −80 °C for 0.5 hrs and dried in a vacuum centrifuge. Ten μg of cells and 5 μg of synganglia were desalted as previously described (Rappsilber et al. 2003).
Peptide liquid chromatography fractionation & mass spectrometry
Samples were resuspended in load solvent (98:2:0.01, water:acetonitrile:formic acid) and 1-1.5 μg aliquots were analyzed on a Velos Orbitrap mass spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) as described previously (Lin-Moshier et al. 2013), with the exception that the liquid chromatography gradient and MS acquisition times were 120 minutes.
Database searching and protein identification
MS/MS samples were analyzed using Sequest (Thermo Fisher Scientific, San Jose, CA, USA; version 27, rev. 12). Thermo Fisher mass spectrometer .RAW files were converted to mzxml data format with msconvert (http://proteowizard.sourceforge.net/tools.shtml), and the online TINT tool (https://github.com/jmchilton/tint/) converted mzxml files to .DTA for Sequest. Sequest was set up to search the NCBI Reference sequence Ixodes scapularis (taxon 6945; February 18, 2014) protein FASTA database to which the cRAP contaminants database (http://www.thegpm.org/cRAP/index.html) was appended (41058 forward plus reversed entries) assuming the digestion enzyme trypsin. Sequest was searched with a fragment ion mass tolerance of 0.80 Da and a precursor ion tolerance of 100 ppm. Cysteine MMTS was specified in Sequest as a fixed modification and oxidation of methionine was specified as a variable modification.
Criteria for protein identification
Scaffold (version 3.6.0, Proteome Software Inc., Portland, OR, USA) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm (Keller et al. 2002). Protein identifications were accepted if they could be established at greater than 90.0% probability and contained at least 3 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al. 2003). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Normalized spectral counting was used to compare the relative abundance of certain proteins between the cell lines examined, but not the synganglia.
Protein functions were evaluated by inputting Scaffold-derived identities into Blast2GO (version 2.8.0) (Conesa et al. 2005) to annotate gene ontology (GO) numbers. Protein sequences were blasted with blastp using the non-redundant database at NCBI (http://www.ncbi.nlm.nih.gov), then mapped and annotated with Blast2GO in November, 2014. Motifs identified by the InterProScan 5 tool at EMBL-EBI (http://www.ebi.ac.uk) were integrated into the GO number annotation. Proteins with known functions were examined using KEGG pathways (www.genome.jp/kegg/pathway.html). Additionally, several proteins known to have neuronal associations but not annotated as such by Blast2GO were manually annotated as noted in Supplemental Table S1.
Western Blot
Protein from wild-type ISE6, IDE12, and RF/6A cells was extracted with 2x sample buffer, loaded onto 4 – 15% polyacrylamide Mini-Protean TGX gels (Bio-Rad Laboratories, Hercules, CA, USA), and separated by electrophoresis as previously described (Chávez et al. 2012). Gels were blotted onto Immobilon-P membranes (Millipore, Bedford, MA, USA) at 4°C overnight at 30V, and incubated with rabbit polyclonal β-3 tubulin antibody or a mouse monoclonal β-3 tubulin antibody (Sigma-Aldrich, St. Louis, MO, USA) to test for β-3 tubulin, a neuronal cell marker (Lee et al. 1990; Kim et al. 2004), in ISE6, IDE12, and RF/6A cells (Leiss et al. 1988). Anti-rabbit goat horseradish peroxidase (HRP)-conjugated or anti-mouse donkey HRP-conjugated secondary antibodies were used (Jackson ImmunoResearch, West Grove, PA, USA). Metal-enhanced DAB substrate (Thermo Fisher Scientific, Waltham, MA, USA) was used to visualize bands.
Injections
Unfed larval and nymphal I. scapularis were injected with mCherry-Lifeact-expressing ISE6 cells to observe changes stimulated in these cells by innate tick factors. For injection, 7.5 × 106 cells were concentrated into 300 μl of L15C300 medium by centrifugation at 500xg for 5 min at 4°C.
Tick injections were performed with pulled-glass capillary needles affixed with 2-part epoxy to 27 gauge steel needles. Ticks were immobilized dorsum down on double-sided adhesive tape on the stage of a dissecting microscope. Needles were guided into place using a manipulator/micromanipulator (model MN-153; Narishige, Tokyo, Japan), and nymphs were injected through the cuticle on the dorsal side of a forecoxa into the hemocoel with 0.1 μl cell suspension. Larvae were injected with <0.05 μl of cell suspension into the ventral side posterior to the basis capitulum and anterior to the gut.
In vivo ISE6 observation
Ixodes scapularis larvae injected with ISE6 cells were incubated for two weeks prior to microscopic observation. Live larvae were slide-mounted, dorsum down, in PBS for microscopic through-cuticle observation.
Nymphs injected with mCherry-Lifeact-expressing ISE6 cells were incubated for 6 weeks and dissected without an intervening feeding. Ticks that were injected as nymphs but dissected as adults were fed upon hamsters 1 to 2 days after injection and were dissected 6 weeks later, after molting into adults.
Nymphs, as well as adult ticks injected with mCherry-Lifeact-expressing ISE6 cells as nymphs, were dissected to examine changes in the injected cells. Prior to dissection, ticks were adhered ventral side down to double-sided adhesive tape and legs were secured with strips of tape. While moistened with a drop of PBS, the dorsum of the tick was separated with a 26-gauge needle, using the bevel of the needle to cut around the outside margins of the carapace until it could be lifted free. The backless tick was immersed in more PBS and a very light sprinkling of 50 μm glass beads was added around the tick to help support a coverslip placed over the tick. The coverslips were sealed with drops of Valap (equal parts Vaseline, lanolin, and paraffin wax).
Microscopy and Imaging
Fluorescent microscopy was performed using an Olympus BX61 disk-scanning unit confocal microscope (Olympus America, Center Valley, PA, USA). Confocal cell images were acquired with a Photometrics Quantem:512SC EMCCD camera (Photometrics, Tucson, AZ, USA). An Olympus UPlanSApo 60x (N.A. 1.35) objective and an X-cite Exacte fluorescent light source (Lumen Dynamics, Mississauga, Ontario, Canada) were used to visualize all confocal samples. A Nikon Diaphot inverted microscope (Chiyoda, Tokyo, Japan) with Nikon DXM1200 color camera was used to obtain in vitro phase contrast and fluorescent images. Confocal image capture software Metamorph (Molecular Devices, Sunnyvale, CA, USA) and inverted microscope image capture software Nikon ACT-1 were used. ImageJ (US National Institutes of Health) was used for still image z-projections, cropping, and adjustment of brightness/contrast. Dark field subtraction was performed on fluorescent color camera images using Photoshop (Adobe Systems, Mountain View, CA, USA) to remove hot pixels.
Results
Mass spectrometry & Bioinformatics
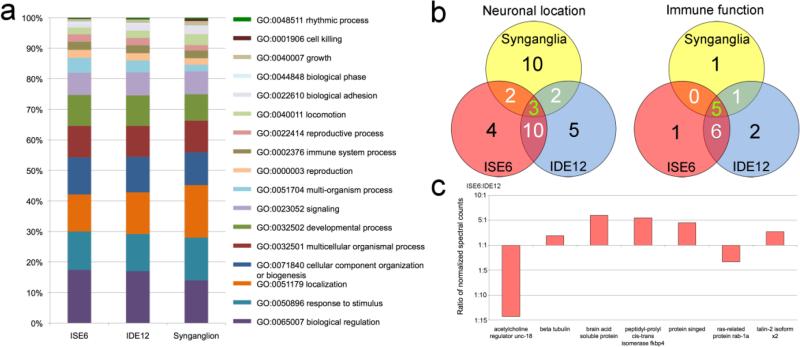
A global proteome analysis of ISE6 cells, IDE12 cells, and synganglia was performed to determine the similarity between the protein profiles of all samples and to identify proteins that could provide a clue to the possible tissue identity of each cell line. Only proteins with ≥3 identifiable peptides were considered in the analysis. The number of proteins identified in each sample set differed greatly, with 200 proteins from the synganglia, 322 proteins from the ISE6 cell sample, and 355 proteins from the IDE12 cell sample (Online resource 1). The proteomic profile from each sample set was defined by protein function using the bioinformatics tools described above. All three tissues produced substantially similar level 2 biological process GO term profiles with ~55% of proteins falling into the broad categories of metabolic process (GO:0008152), cellular process (GO:0009987), and single-organism process (GO:0044699). Among the remaining level 2 categories (Figure 1), the synganglia presented the more divergent profile, with somewhat more localization (GO:0051179) and fewer biological regulation (GO:0065007) and biological phase (GO:0044848) proteins. 7.7% of synganglia proteins were not assigned GO terms (in comparison to 4.1% of ISE6 and 4.5% of IDE12), indicating a greater proportion of unknown, hypothetical, or highly tick-specific proteins not yet assigned GO terms. Level 2 GO profiles of the two cell lines were very similar to one another with few GO term proportions diverging more than 10%. The most divergent GO term proportions between the cell lines were in the sparsely represented terms biological adhesion (GO:0022610; 68% higher in IDE12) and rhythmic process (GO:0048511; 84% higher in IDE12). Complete GO annotations of proteins are included in the supplemental material for I. scapularis (Online resource 1), ISE6 cells (Online resource 2), and IDE12 cells (Online resource 3).
Figure 1.
Characterization of unique and shared proteins with specialized function in I. scapularis synganglia, ISE6, and IDE12 cells. a) Representation of the proportion of level 2 GO terms associated with the proteins identified in the 3 cell and tissue samples. The three most common level 2 GO terms, metabolic process (GO:0008152), cellular process (GO:0009987), and single-organism process (GO:0044699), which represent 50-55% of annotated terms have been excluded to allow greater resolution amongst the rarer terms. b) Number of unique and shared neuronal and immune proteins by sample set. The green number in the center of the Venn diagram represents the number of proteins common to all sample sets. White numbers in overlapping regions represent proteins shared by two sample sets, and the black numbers represents unique proteins. Details are in Supplemental Table S1. c) Relative abundance of significantly different neuronal proteins shared between the ISE6 and IDE12 cells. The relative abundance was determined by the normalized count of the spectra used to identify each of the proteins.
Annotation of proteins with cellular component GO terms provided an indication of the tissue or organelle types associated with the proteins in each sample. Of particular interest to this study were proteins strongly associated with neuronal tissues. Cellular and synganglia proteins were deemed neuronal if labeled with either of two GO terms: “synapse” (GO:0045202, level 2) or “neuron part” (GO:0097458, level 4). Several more proteins known to have neuronal association were also annotated manually. Proteins annotated with any neuronal labels comprised 9.5% (19/200) of synganglia proteins, 6.5% of ISE6 proteins (21/322), and 6.2% of IDE12 proteins (22/355).
Mass spectrometry identified numerous shared proteins in all of the sample sets. Both cell lines expressed a relatively high percentage of proteins involved in neuronal functions, with 5 of these proteins shared between all sample types, including synganglia (Figure 1b). The identities of the neuronal proteins discovered in each tissue type are listed in Supplemental Table S1. Beta tubulin was the second most abundant protein in the ISE6 and IDE12 cells, and showed high homology with β-3 tubulin from chicken (data not shown). It was one of the proteins with the greatest coverage (70 - 72%) and the largest number of spectra (323 - 314) in all three sample sets. Beta-3 tubulin is a commonly used marker for neurons and is conserved within vertebrate (Sullivan and Cleveland 1986) and insect (Leiss et al. 1988; Siebert et al. 2008) lineages, though the amino acid make-up of this tubulin isoform diverges somewhat between vertebrates and insects (Rudolph et al. 1987). Spectral counting indicated that beta tubulin was significantly more abundant in ISE6 cells (109.1 counts) than in IDE12 cells (53.95 counts; p-value <0.0001), suggesting that more cells in the ISE6 population expressed the protein or that the protein was more highly expressed in ISE6, overall (Figure 1c). Spectral counting also indicated several other shared neuronal proteins with significantly different rates of expression between the cell types (Figure 1c). Both cell lines also produced other unique neuronal proteins that were not present in the synganglia (Figure 1b; Supplemental Table S1).
Proteins related to immune response were also deemed of interest and proteins labeled with the “immune system process” term (GO:0002376; level 2) were compared between the three samples (Figure 1b). Overall, fewer immune system-associated proteins were annotated than neuronal tissue-associated proteins in all samples, representing 3.5% (7/200) of synganglia proteins, 3.7% of ISE6 proteins (12/322), and 3.9% of IDE12 proteins (14/355). Most were found in both cell lines, with a few exceptions (Supplemental Table S1). Spectral counting indicated different quantities detected between the cell lines for some proteins, but the most substantial quantity differences in quantity were for two proteins that were also labeled with neuronal GO terms. These proteins (beta tubulin and protein singed; Figure 1c) were significantly more abundant in the ISE6 cell line.
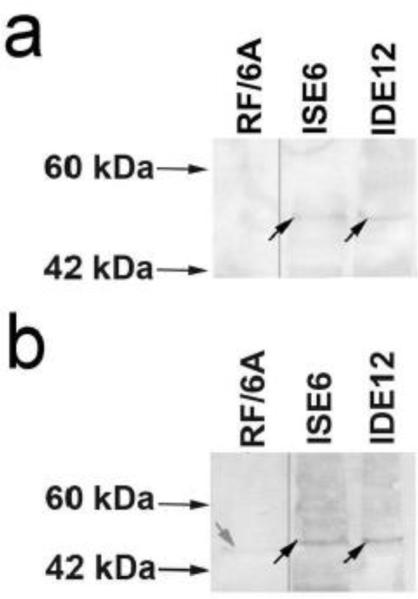
Western blot for β-3 tubulin
To further investigate the expression of neuronal markers in both cell lines, we performed Western blots using monoclonal and polyclonal antibodies against β-3 tubulin, high levels of which had been detected by proteomic analysis. Both types of antibodies identified a band at ~50 kDa (Figure 2), which agrees with the molecular weight (MW) predicted for β-3 tubulin by mass spectrometry . The monoclonal antibodies clearly showed a single band in both ISE6 and IDE12 cells (Figure 2a). This band was not detected in primate-derived RF/6A microvascular endothelial cells. A band at the same MW was also identified with the polyclonal antibodies (Figure 2b). A light band of slightly lower MW was detected in the RF/6A protein extract, possibly the result of cross-reactivity with other β-tubulins common to non-neuronal cell types, especially given the polyclonal nature of the antibodies and the presence of conserved epitopes within all β-tubulin proteins. The presence of faint but distinct bands of the correct MW in both samples confirmed the proteomics results regarding the expression of neuronal markers in ISE6 and IDE12 cell lines.
Figure 2.
Western blots, using a) a monoclonal antibody and b) a polyclonal antibody to detect the 3β-tubulin protein in ISE6 and IDE12 cells, were used to confirm the expression of the neuronal marker in both cell lines. Bands at around 50 kDa, consistent with the predicted molecular weight of 3β-tubulin, were detected by both antibodies in both tick cell protein samples (black arrows). The endothelial cell line RF/6A was used as negative control. A lighter band around 45 kDa was detected in the negative control with the polyclonal antibody (grey arrow). The lower molecular weight and intensity of this band suggest it could be a result of cross-reactivity with other β-tubulins, as conserved peptides are shared between the different subunits. RF/6A lane images adjacent to ISE6 and IDE12 lanes originate from the same blots with which they are pictured.
In vivo observation of ISE6 cells
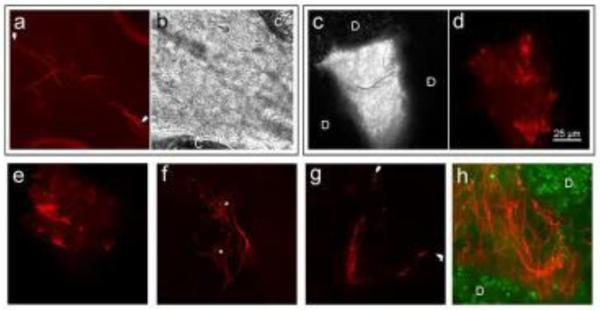
Using confocal microscopy, mCherry-Lifeact-expressing ISE6 cells injected into I. scapularis larvae were visible through the cuticle of the live tick where they adhered to the interior ventral epithelium (Figure 3a & 3b). Cells appeared very different morphologically from ISE6 cells grown in vitro (Figure 4), demonstrating elongation of axon-like cell filopodia that reached lengths in excess of 100 μm in comparison to the 10-50 μm length of in vitro-cultured ISE6 filopodia. The axon-like region of the cell displayed numerous branching filopodia, and more filopodia overall than ISE6 cells observed in vitro. Of 5 larvae examined microscopically, fluorescent cells were clearly discernable through the cuticle in two larvae.
Figure 3.
Red fluorescent mCherry-Lifeact-expressing ISE6 cells in I. scapularis. a and b) A single injected cell adhered to the interior ventral cuticle of a larval tick between coxae (labeled C). Branching filopodium (between arrows) extends >100 μm from the cell body which is off the right side of the field of view. a) fluorescent image and b) corresponding bright field image. c-e) Cells injected into nymphs that did not feed and molt are morphologically similar to ISE6 cells in culture (Figure 4c) and formed much smaller tumors. The fluorescent tumor pictured in d) corresponds to the grey monochrome bright field image in c) showing the location of the tumor between gut diverticula (labeled D). f) Two cells with neuron-like morphology, nuclei labeled with asterisks. g) A single bipolar cell from a molted tick ~125 μm long with terminal ends marked by white arrowheads. Filamentous actin is absent in some portions of the very fine cell processes, but is present at the tips. h) Large tumor of elongated cells from a molted tick between gut diverticula (green; labeled D). The scale bar in 3d is applies to all panels.
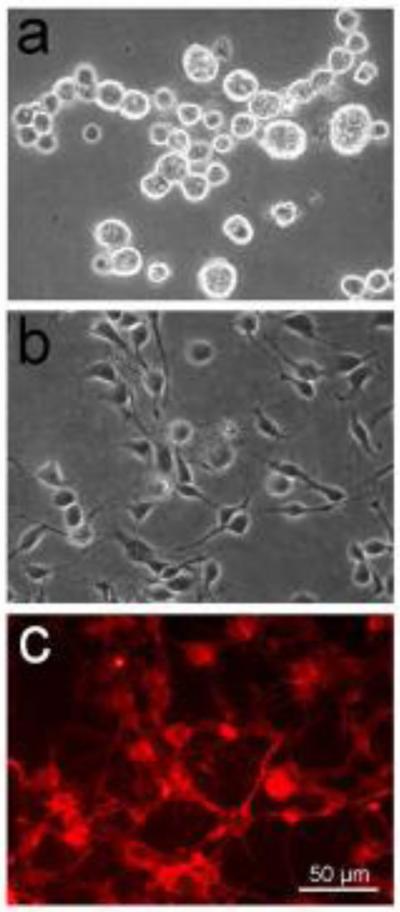
Figure 4.
IDE12 and mCherry-Lifeact-expressing ISE6 cells growing at low density in vitro to demonstrate cell morphology. a) IDE12 cells with typical spherical shape. b & c) bright field and red fluorescent views of mCherry-Lifeact ISE6 cells. The scale bar in 4c applies to all panels.
mCherry-Lifeact-expressing ISE6 cells injected into unfed nymphs developed into tumors as the cells divided within the hemocoel (Figure 3c – 3e). Cell appearance was comparable to cultured cells, with filopodia reaching a typical length of about 20-25 μm in ticks or 50 μm in newly subcultured cells in vitro (Figure 4b). ISE6 tumors were small, typically ~75 μm in diameter, and located adjacent to the injection site (Figure 3e), or between gut diverticula (Figure 3c & 3d). Cells not associated with tumors were absent. In unfed nymphs, ISE6 cells were only seen associated with tumors and did not develop abnormally or differentiate into more neuron-like forms. Six unfed nymphs were dissected and fluorescent tumors were found in all instances.
The final group of nymphs was injected with mCherry-Lifeact-expressing ISE6 cells, fed upon hamsters, and allowed to molt into adult ticks. Substantial growth and morphological change of cells occurred in this group. ISE6 cells were distributed as scattered single cells or small groups (Figure 3f & 3g), and as large tumors mainly situated near the nymphal injection site or between gut diverticula (Figure 3h). Cells were enlarged and cell shape was greatly altered with elongate axon-like and dendrite-like structures present (Figure 3f) and a far greater number of filopodia overall in comparison to ISE6 cells in vitro or injected into unmolted unfed nymphs. Though cell body diameter remained comparable at ~10-15 μm, cell length was greatly increased in the molted adults with some cells extending to >100 μm in length (Figure 3g). Adult ISE6 tumors were larger than those formed in unfed nymphs, extending to >200 μm across. Elongate axon-like filopodia were visible, sometimes appearing bundled with the axon-like structures of nearby cell bodies (Figure 3h). Six adult ticks (3 females and 3 males) were dissected and examined with morphologically neuron-like fluorescent cells and tumors found in all individuals.
Discussion
Tick-derived cell lines have become important tools for the study and propagation of tick-vectored pathogens, as well as for the study of biological processes in different tick species. ISE6 and other I. scapularis-derived cell lines are the most extensively used for gene and protein expression studies in ticks (Bell-Sakyi et al. 2007). Unlike BME26, a Rhipicephalus microplus-derived cell line, which has been characterized on a molecular basis (Esteves et al. 2008), only cytogenetic analyses and karyotyping have been done to understand the origin of I. scapularis cell lines (Chen et al. 1994; Meyer et al. 2010), and their specific tissue origin remains unknown.
Tick cell lines often comprise a mixture of cell types; their appearance under phase contrast microscopy (Chen et al. 1994) and their behavioral phenotype differs greatly. Two cell lines derived from I. scapularis embryos, ISE6 and IDE12, behaved differently after inoculation with heat inactivated B. burgdorferi, with 80% of IDE12 phagocytosing bacteria compared to less than 1% of ISE6 (Mattila et al. 2007). This suggested possible differentiation of embryonic tissues into distinct cell types. We decided to compare IDE12 and ISE6 because of their dissimilar appearance (Figure 4) and phagocytic (Mattila et al. 2007; Kurtti and Keyhani 2008) and humoral immune responses to bacterial pathogens vectored by I. scapularis.
The proteomic profiles of the cell lines generated using mass-spectrometry and identification based on synaptic or neuron cellular component GO term annotations indicated that both ISE6 and IDE12 cells expressed neuronal proteins (Supplemental Table S1). The beta tubulin family was identified by mass spectrometry, and immunoblotting further specified the presence of the neural cytoskeletal protein β-3 tubulin in both cell lines (Figures 1 & 2). β-3 tubulin is a diagnostic marker of neuronal cells (Ludueña 1998) and is rarely present in other cell types (Jouhilahti et al. 2008), providing an indication that both tick embryo-derived cell lines may be derived from, or are potential precursors to, neuronal tissue. In addition, several other neuron-associated proteins were identified in both cell line samples, including proteins involved in axon development, formation of mechanosensory receptors, and neurotransmitters (Supplemental Table S1). ISE6 cells presented a higher percentage of neuron-related proteins for their total sample size, with 6.5% of the proteins participating in neuronal functions versus 6.2% in IDE12. Additionally, β-3 tubulin and some other neuron-associated proteins were much more abundant in ISE6 than in IDE12 cells (Figure 1c). The neuronal protein most abundant in IDE12 relative to ISE6 cells, acetylcholine unc regulator, is, interestingly, associated with mechanosensory and motor neurons rather than the neurons of the central nervous system.
Synganglia possessed more neuronal proteins unique to that tissue (10 unique proteins) than ISE6 (4), and IDE12 (5). Though I. scapularis transcriptome analysis predicted the presence of a number of neuropeptides (Egekwu et al. 2014), these proteins were not directly detected, with the exception of crustacean hyperglycemic hormone (pericardial organ-type) precursor. It is likely that other neuropeptides were expressed but fell below the detection limit of our proteomic analysis. Ideally, these peptides should be sequenced de novo and their existence and function verified experimentally, since their small size complicates their annotation in big genomes (Ons et al. 2009).
The observed phagocytic behavior of IDE12 cells and their susceptibility to infection by a lower range of intracellular organisms relative to ISE6 (Mattila et al. 2007, and our unpublished observations) led us to also examine proteins annotated as related to immune response (GO:0002376, immune system process, a level 2 term). Few differences in the identity or abundance of these proteins were notable between ISE6 and IDE12 cells, however (Figure 1b, Supplemental Table S1). One protein that was present in IDE12 but absent in ISE6 was Ixoderin, a lectin originally identified in I. ricinus based on its high homology with the hemocyte and salivary gland-produced lectin Dorin M, of the soft tick Ornithodoros moubata (Rego et al. 2005). Lectins are involved in the identification of self-/non-self tissues and can bind the carbohydrate cell walls of invading microorganisms, so Ixoderin may well function as a defense response protein in IDE12 cells.
The proteomic characteristics observed in both cell lines proved remarkably similar considering the substantial variations between the lines in appearance and behavior. That both the level 2 GO term proportions and the majority of neuronal proteins were shared indicated substantial similarity between the cell lines relative to the more divergent synganglion tissue. This similarity may be based on the origin of these cells in embryonic tissue; both ISE6 and IDE12 are relatively undifferentiated and may retain attributes of multipotent embryonic cells, including the ability to propagate indefinitely in culture. The retention of some developmental potential in ISE6 could also explain its partial differentiation into neuron-like cells when exposed to certain physiological conditions within molting or larval ticks. In contrast, synganglion tissue is a highly differentiated and specialized tissue that has effectively reached its endpoint of development, particularly in the adult ticks from which it was acquired. Differences in developmental trajectory or the extent of differentiation may explain the observed differences in protein profiles.
ISE6 cells typically demonstrate an elongate phenotype in culture, presenting numerous filopodia in comparison to IDE12 cells, which are spherical and mostly lack protrusions (Figure 4). Further differentiation of ISE6 into elongate neuron-like cells with structures reminiscent of dendrites occurred after injection into unfed larvae, or nymphs that were subsequently allowed to engorge, and later molted. Injection of ISE6 cells into nymphal ticks that were incubated for the same duration but left unfed did not result in differentiation into neuron-like structures. In the latter ticks, ISE6 cells were apparent only as small clusters of undifferentiated cells within the adult hemocoel. This suggested that the presence of a molting-associated hormone in the hemolymph was necessary for ISE6 cells to progress to the phenotype that resembled neurons. In larval ticks, it may be that hemolymph factors are retained from the egg stage, when cells are rapidly differentiating, resulting in the similar phenotype observed for injected ISE6 cells. Future research will aim at developing an in vitro method of stimulating neuron-like ISE6 formation by chemical methods such as exposure to ecdysteroids and other hormones that regulate molting (Kurtti and Munderloh 1983; Jenson et al. 2012).
Stable Drosophila melanogaster cell lines transfected to express neurotransmitter receptors have found important application in the study of pharmaceuticals and development of insecticides. However, endogenously-expressed receptors may interfere with correct interpretation of results, but are frequently not detected or characterized (Towers and Sattelle 2002). Given the potential for neuronal differentiation of the ISE6 cell line and the abundance of neuronal proteins, including the insecticide target acetylcolinesterase, this cell line could be used for the study of neuropeptides and other neuronal factors, ultimately aiding in the development of more specific acaricides.
The neuron-like phenotype of ISE6 cells may also explain the utility of this cell line for the propagation of arthropod-borne viruses (Bell-Sakyi et al. 2007; Offerdahl et al. 2012; Bell-Sakyi et al. 2012). Numerous mosquito-borne arboviruses are known for neurotropism and induction of encephalitis in vertebrates. Though few tissue tropism studies in mosquitoes have been published, there is evidence that some arboviruses likewise thrive in arthropod neuronal tissue, for example dengue virus (Salazar et al. 2007). Similarly, in ticks, Thogoto virus is maintained transstadially by infection of the synganglion (Booth et al. 1989). The presence of numerous neuron-associated proteins in ISE6 may help explain why it is a popular host cell line for the study of neurotropic viruses or other intracellular pathogens with a propensity to infect nervous tissue, such as the Ehrlichia muris-like agent (Lynn et al. 2015).
Continuous arthropod cell lines are largely undifferentiated, and probably represent a cell type found in developing embryos or imaginal tissues found in other immature life-stages, such as caterpillars, that are capable of continuous cell division without the need for transformation, mutagenesis, or use of cells derived from tumors. The morphological similarities of ISE6 cells to neurons and the expression of neural proteins do not prove that this cell line is derived from neural embryonic tissue. Nonetheless, expression of neuron-associated proteins and peptides in tick cell lines provides valuable information to researchers seeking suitable in vitro systems to investigate the responses of neuronal genes to cellular or chemical stimuli, or the biological relevance of corresponding gene products. The I. scapularis cell lines ISE6 and IDE12 and, to a lesser extent the tick synganglion, demonstrated substantial overlap in protein profile, but differed in the amount of peptides produced. All three sample sets contained the protein β-3 tubulin, well characterized as a neuronal marker, although the protein was substantially more abundant in ISE6 than in IDE12 cells. ISE6 cells exposed to the internal milieu of larval or molting nymphal I. scapularis, developed neuron-like morphological characteristics not seen in cells injected into nymphal ticks that did not molt or cells grown in cell culture. This potential for ISE6 to develop neuron-like characteristics and proteins may make it an attractive research tool for tick neural physiology and toxicology and the development of improved acaricides. Moreover, studies investigating the relationship of tick-borne pathogens targeting the tick synganglion may benefit from the use of this cell line.
Supplementary Material
Acknowledgements
This research was funded by grants from the US National Institutes of Health, numbers R01AI042792 and R01AI049424 to Ulrike G. Munderloh. Mass spectrometry was performed at The Center for Mass Spectrometry and Proteomics at the University of Minnesota.
Footnotes
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Minnesota. Hamsters were maintained in accordance with an approved University of Minnesota IACUC protocol.
Bibliography
- Antwi K, Hanavan PD, Myers CE, Ruiz YW, Thompson EJ, Lake DF. Proteomic identification of an MHC-binding peptidome from pancreas and breast cancer cell lines. Mol Immunol. 2009;46:2931–7. doi: 10.1016/j.molimm.2009.06.021. doi: 10.1016/j.molimm.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Baldridge GD, Burkhardt NY, Labruna MB, Pacheco RC, Paddock CD, Williamson PC, Billingsley PM, Felsheim RF, Kurtti TJ, Munderloh UG. Wide dispersal and possible multiple origins of low-copy-number plasmids in rickettsia species associated with blood-feeding arthropods. Appl Environ Microbiol. 2010;76:1718–31. doi: 10.1128/AEM.02988-09. doi: 10.1128/AEM.02988-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann T, Thüte T, Heinrich C, Büntemeyer H, Noll T. Proteomic and metabolomic characterization of CHO DP-12 cell lines with different high passage histories. BMC Proc 5 Suppl. 2011;8:P92. doi: 10.1186/1753-6561-5-S8-P92. doi: 10.1186/1753-6561-5-S8-P92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L, Kohl A, Bente DA, Fazakerley JK. Tick cell lines for study of Crimean-Congo hemorrhagic fever virus and other arboviruses. Vector Borne Zoonotic Dis. 2012;12:769–81. doi: 10.1089/vbz.2011.0766. doi: 10.1089/vbz.2011.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L, Zweygarth E, Blouin EF, Gould EA, Jongejan F. Tick cell lines: tools for tick and tick-borne disease research. Trends Parasitol. 2007;23:450–7. doi: 10.1016/j.pt.2007.07.009. doi: 10.1016/j.pt.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Booth TF, Davies CR, Jones LD, Staunton D, Nuttall PA. Anatomical basis of Thogoto virus infection in BHK cell culture and in the ixodid tick vector, Rhipicephalus appendiculatus. J Gen Virol. 1989;70(Pt 5):1093–104. doi: 10.1099/0022-1317-70-5-1093. [DOI] [PubMed] [Google Scholar]
- Chávez ASO, Felsheim RF, Kurtti TJ, Ku P-S, Brayton KA, Munderloh UG. Expression patterns of Anaplasma marginale Msp2 variants change in response to growth in cattle, and tick cells versus mammalian cells. PLoS One. 2012;7:e36012. doi: 10.1371/journal.pone.0036012. doi: 10.1371/journal.pone.0036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Munderloh UG, Kurtti TJ. Cytogenetic characteristics of cell lines from Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 1994;31:425–34. doi: 10.1093/jmedent/31.3.425. [DOI] [PubMed] [Google Scholar]
- Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–6. doi: 10.1093/bioinformatics/bti610. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Egekwu N, Sonenshine DE, Bissinger BW, Roe RM. Transcriptome of the female synganglion of the black-legged tick Ixodes scapularis (Acari: Ixodidae) with comparison between Illumina and 454 systems. PLoS One. 2014;9:e102667. doi: 10.1371/journal.pone.0102667. doi: 10.1371/journal.pone.0102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves E, Lara FA, Lorenzini DM, Costa GHN, Fukuzawa AH, Pressinotti LN, Silva JRMC, Ferro JA, Kurtti TJ, Munderloh UG, Daffre S. Cellular and molecular characterization of an embryonic cell line (BME26) from the tick Rhipicephalus (Boophilus) microplus. Insect Biochem Mol Biol. 2008;38:568–80. doi: 10.1016/j.ibmb.2008.01.006. doi: 10.1016/j.ibmb.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia S, Billecocq A, Crance J-M, Munderloh U, Garin D, Bouloy M. Nairovirus RNA sequences expressed by a Semliki Forest virus replicon induce RNA interference in tick cells. J Virol. 2005;79:8942–7. doi: 10.1128/JVI.79.14.8942-8947.2005. doi: 10.1128/JVI.79.14.8942-8947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intawicha P, Wang S-H, Hsieh Y-C, Lo N-W, Lee K-H, Huang S-Y, Ju J-C. Proteomic profiling of rabbit embryonic stem cells derived from parthenotes and fertilized embryos. PLoS One. 2013;8:e67772. doi: 10.1371/journal.pone.0067772. doi: 10.1371/journal.pone.0067772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson LJ, Paulson SL, Bloomquist JR. Induction and inhibition of an apparent neuronal phenotype in Spodoptera frugiperda insect cells (Sf21) by chemical agents. Invert Neurosci. 2012;12:119–27. doi: 10.1007/s10158-012-0138-5. doi: 10.1007/s10158-012-0138-5. [DOI] [PubMed] [Google Scholar]
- Jouhilahti E-M, Peltonen S, Peltonen J. Class III beta-tubulin is a component of the mitotic spindle in multiple cell types. J Histochem Cytochem. 2008;56:1113–9. doi: 10.1369/jhc.2008.952002. doi: 10.1369/jhc.2008.952002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Kim JS, Chang M-Y, Yu IT, Kim JH, Lee S-H, Lee Y-S, Son H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem. 2004;89:324–36. doi: 10.1046/j.1471-4159.2004.02329.x. doi: 10.1046/j.1471-4159.2004.02329.x. [DOI] [PubMed] [Google Scholar]
- Kuriakose JA, Miyashiro S, Luo T, Zhu B, McBride JW. Ehrlichia chaffeensis transcriptome in mammalian and arthropod hosts reveals differential gene expression and post transcriptional regulation. PLoS One. 2011;6:e24136. doi: 10.1371/journal.pone.0024136. doi: 10.1371/journal.pone.0024136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti TJ, Keyhani NO. Intracellular infection of tick cell lines by the entomopathogenic fungus Metarhizium anisopliae. Microbiology. 2008;154:1700–9. doi: 10.1099/mic.0.2008/016667-0. doi: 10.1099/mic.0.2008/016667-0. [DOI] [PubMed] [Google Scholar]
- Kurtti TJ, Mattila JT, Herron MJ, Felsheim RF, Baldridge GD, Burkhardt NY, Blazar BR, Hackett PB, Meyer JM, Munderloh UG. Transgene expression and silencing in a tick cell line: A model system for functional tick genomics. Insect Biochem Mol Biol. 2008;38:963–968. doi: 10.1016/j.ibmb.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti TJ, Munderloh UG. The effects of 20-hydroxyecdysone and juvenile hormone III on tick cells. J Parasitol. 1983;69:1072–8. [PubMed] [Google Scholar]
- Kurtti TJ, Munderloh UG, Andreadis TG, Magnarelli LA, Mather TN. Tick cell culture isolation of an intracellular prokaryote from the tick Ixodes scapularis. J Invertebr Pathol. 1996;67:318–21. doi: 10.1006/jipa.1996.0050. doi: 10.1006/jipa.1996.0050. [DOI] [PubMed] [Google Scholar]
- Lawrie CH, Uzcátegui NY, Armesto M, Bell-Sakyi L, Gould EA. Susceptibility of mosquito and tick cell lines to infection with various flaviviruses. Med Vet Entomol. 2004;18:268–74. doi: 10.1111/j.0269-283X.2004.00505.x. doi: 10.1111/j.0269-283X.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–32. doi: 10.1002/cm.970170207. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Leiss D, Hinz U, Gasch A, Mertz R, Renkawitz-Pohl R. Beta 3 tubulin expression characterizes the differentiating mesodermal germ layer during Drosophila embryogenesis. Development. 1988;104:525–31. doi: 10.1242/dev.104.4.525. [DOI] [PubMed] [Google Scholar]
- Lin-Moshier Y, Sebastian PJ, Higgins L, Sampson ND, Hewitt JE, Marchant JS. Re-evaluation of the role of calcium homeostasis endoplasmic reticulum protein (CHERP) in cellular calcium signaling. J Biol Chem. 2013;288:355–67. doi: 10.1074/jbc.M112.405761. doi: 10.1074/jbc.M112.405761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludueña RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–75. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- Lynn GE, Oliver JD, Nelson CM, Felsheim RF, Kurtti TJ, Munderloh UG. Tissue Distribution of the Ehrlichia muris-Like Agent in a Tick Vector. PLoS One. 2015;10:e0122007. doi: 10.1371/journal.pone.0122007. doi: 10.1371/journal.pone.0122007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila JT, Munderloh UG, Kurtti TJ. Phagocytosis of the Lyme disease spirochete, Borrelia burgdorferi, by cells from the ticks, Ixodes scapularis and Dermacentor andersoni, infected with an endosymbiont, Rickettsia peacockii. J Insect Sci. 2007;7:58. doi: 10.1673/031.007.5801. doi: 10.1673/031.007.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Kurtti TJ, Van Zee JP, Hill CA. Genome organization of major tandem repeats in the hard tick, Ixodes scapularis. Chromosome Res. 2010;18:357–70. doi: 10.1007/s10577-010-9120-4. doi: 10.1007/s10577-010-9120-4. [DOI] [PubMed] [Google Scholar]
- Munderloh UG, Blouin EF, Kocan KM, Ge NL, Edwards WL, Kurtti TJ. Establishment of the tick (Acari:Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales:Anaplasmataceae) in tick cell culture. J Med Entomol. 1996a;33:656–64. doi: 10.1093/jmedent/33.4.656. [DOI] [PubMed] [Google Scholar]
- Munderloh UG, Liu Y, Wang M, Chen C, Kurtti TJ. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J Parasitol. 1994;80:533–43. [PubMed] [Google Scholar]
- Munderloh UG, Madigan JE, Dumler JS, Goodman JL, Hayes SF, Barlough JE, Nelson CM, Kurtti TJ. Isolation of the equine granulocytic ehrlichiosis agent, Ehrlichia equi, in tick cell culture. J Clin Microbiol. 1996b;34:664–70. doi: 10.1128/jcm.34.3.664-670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munderloh UG, Silverman DJ, MacNamara KC, Ahlstrand GG, Chatterjee M, Winslow GM. Ixodes ovatus Ehrlichia exhibits unique ultrastructural characteristics in mammalian endothelial and tick-derived cells. Ann N Y Acad Sci. 2009;1166:112–9. doi: 10.1111/j.1749-6632.2009.04520.x. doi: 10.1111/j.1749-6632.2009.04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munderloh UG, Tate CM, Lynch MJ, Howerth EW, Kurtti TJ, Davidson WR. Isolation of an Anaplasma sp. organism from white-tailed deer by tick cell culture. J Clin Microbiol. 2003;41:4328–35. doi: 10.1128/JCM.41.9.4328-4335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munderloh UG, Yabsley MJ, Murphy SM, Luttrell MP, Howerth EW. Isolation and establishment of the raccoon Ehrlichia-like agent in tick cell culture. Vector Borne Zoonotic Dis. 2007;7:418–25. doi: 10.1089/vbz.2007.0640. doi: 10.1089/vbz.2007.0640. [DOI] [PubMed] [Google Scholar]
- Naranjo V, Ayllón N, Pérez de la Lastra JM, Galindo RC, Kocan KM, Blouin EF, Mitra R, Alberdi P, Villar M, de la Fuente J. Reciprocal regulation of NF-kB (Relish) and Subolesin in the tick vector, Ixodes scapularis. PLoS One. 2013;8:e65915. doi: 10.1371/journal.pone.0065915. doi: 10.1371/journal.pone.0065915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro E, Yamauchi T, Urasaki Y, Nishi R, Hori H, Ueda T. Characterization of cytarabine-resistant leukemic cell lines established from five different blood cell lineages using gene expression and proteomic analyses. Int J Oncol. 2011;38:911–9. doi: 10.3892/ijo.2011.933. doi: 10.3892/ijo.2011.933. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Herron MJ, Felsheim RF, Schloeder BR, Grindle SM, Chavez AO, Kurtti TJ, Munderloh UG. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics. 2008;9:364. doi: 10.1186/1471-2164-9-364. doi: 10.1186/1471-2164-9-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Offerdahl DK, Dorward DW, Hansen BT, Bloom ME. A three-dimensional comparison of tick-borne flavivirus infection in mammalian and tick cell lines. PLoS One. 2012;7:e47912. doi: 10.1371/journal.pone.0047912. doi: 10.1371/journal.pone.0047912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD, Burkhardt NY, Felsheim RF, Kurtti TJ, Munderloh UG. Motility Characteristics Are Altered for Rickettsia bellii Transformed To Overexpress a Heterologous rickA Gene. Appl Environ Microbiol. 2014;80:1170–6. doi: 10.1128/AEM.03352-13. doi: 10.1128/AEM.03352-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ons S, Richter F, Urlaub H, Pomar RR. The neuropeptidome of Rhodnius prolixus brain. Proteomics. 2009;9:788–92. doi: 10.1002/pmic.200800499. doi: 10.1002/pmic.200800499. [DOI] [PubMed] [Google Scholar]
- Pornwiroon W, Pourciau SS, Foil LD, Macaluso KR. Rickettsia felis from cat fleas: isolation and culture in a tick-derived cell line. Appl Environ Microbiol. 2006;72:5589–95. doi: 10.1128/AEM.00532-06. doi: 10.1128/AEM.00532-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protocols CSH. RIPA buffer (05-01). Cold Spring Harb Protoc. 20062006 pdb.rec10617–pdb.rec10617. doi: 10.1101/pdb.rec10617. [Google Scholar]
- Rappsilber J, Ishihama Y, Mann M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem. 2003;75:663–70. doi: 10.1021/ac026117i. [DOI] [PubMed] [Google Scholar]
- Rego ROM, Hajdusek O, Kovár V, Kopácek P, Grubhoffer L, Hypsa V. Molecular cloning and comparative analysis of fibrinogen-related proteins from the soft tick Ornithodoros moubata and the hard tick Ixodes ricinus. Insect Biochem Mol Biol. 2005;35:991–1004. doi: 10.1016/j.ibmb.2005.04.001. doi: 10.1016/j.ibmb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ribeiro MFB, Bastos C V, Vasconcelos MMC, Passos LMF. Babesia bigemina: in vitro multiplication of sporokinetes in Ixodes scapularis (IDE8) cells. Exp Parasitol. 2009;122:192–5. doi: 10.1016/j.exppara.2009.03.011. doi: 10.1016/j.exppara.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–7. doi: 10.1038/nmeth.1220. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland LB. Saturated Salt Solutions for Static Control of Relative Humidity between 5° and 40° C. Anal Chem. 1960;32:1375–1376. doi: 10.1021/ac60166a055. [Google Scholar]
- Rudolph JE, Kimble M, Hoyle HD, Subler MA, Raff EC. Three Drosophila beta-tubulin sequences: a developmentally regulated isoform (beta 3), the testis-specific isoform (beta 2), and an assembly-defective mutation of the testis-specific isoform (B2t8) reveal both an ancient divergence in metazoan isotypes an. Mol Cell Biol. 1987;7:2231–42. doi: 10.1128/mcb.7.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert KS, Lorenzen MD, Brown SJ, Park Y, Beeman RW. Tubulin superfamily genes in Tribolium castaneum and the use of a Tubulin promoter to drive transgene expression. Insect Biochem Mol Biol. 2008;38:749–55. doi: 10.1016/j.ibmb.2008.04.007. doi: 10.1016/j.ibmb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Simser JA, Macaluso KR, Mulenga A, Azad AF. Immune-responsive lysozymes from hemocytes of the American dog tick, Dermacentor variabilis and an embryonic cell line of the Rocky Mountain wood tick, D. andersoni. Insect Biochem Mol Biol. 2004;34:1235–46. doi: 10.1016/j.ibmb.2004.07.003. doi: 10.1016/j.ibmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Simser JA, Palmer AT, Fingerle V, Wilske B, Kurtti TJ, Munderloh UG. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl Environ Microbiol. 2002;68:4559–66. doi: 10.1128/AEM.68.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simser JA, Palmer AT, Munderloh UG, Kurtti TJ. Isolation of a spotted fever group Rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Appl Environ Microbiol. 2001;67:546–52. doi: 10.1128/AEM.67.2.546-552.2001. doi: 10.1128/AEM.67.2.546-552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KF, Cleveland DW. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc Natl Acad Sci U S A. 1986;83:4327–31. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate CM, Howerth EW, Mead DG, Dugan VG, Luttrell MP, Sahora AI, Munderloh UG, Davidson WR, Yabsley MJ. Anaplasma odocoilei sp. nov. (family Anaplasmataceae) from white-tailed deer (Odocoileus virginianus). Ticks Tick Borne Dis. 2013;4:110–9. doi: 10.1016/j.ttbdis.2012.09.005. doi: 10.1016/j.ttbdis.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers PR, Sattelle DB. A Drosophila melanogaster cell line (S2) facilitates post-genome functional analysis of receptors and ion channels. Bioessays. 2002;24:1066–73. doi: 10.1002/bies.10178. doi: 10.1002/bies.10178. [DOI] [PubMed] [Google Scholar]
- Tucker AM, Driskell LO, Pannell LK, Wood DO. Differential proteomic analysis of Rickettsia prowazekii propagated in diverse host backgrounds. Appl Environ Microbiol. 2011;77:4712–8. doi: 10.1128/AEM.05140-11. doi: 10.1128/AEM.05140-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela AS, Luttrell MP, Howerth EW, Moore VA, Davidson WR, Stallknecht DE, Little SE. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163–9. doi: 10.1128/JCM.42.3.1163-1169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldehiwet Z, Horrocks BK, Scaife H, Ross G, Munderloh UG, Bown K, Edwards SW, Hart CA. Cultivation of an ovine strain of Ehrlichia phagocytophila in tick cell cultures. J Comp Pathol. 2002;127:142–9. doi: 10.1053/jcpa.2002.0574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.