Abstract
Klotho plays an important role in the pathogenesis of cardiovascular disease in chronic kidney disease (CKD). Klotho is highly expressed in the kidney and parathyroid glands, but its presence in the vasculature is debated. Renal Klotho is decreased in CKD, but the effect of uremia on Klotho in other tissues is not defined. The effect of vitamin D receptor activator therapy in CKD on expression of Klotho in various tissues is also in debate. In uremic rats (surgical 5/6th nephrectomy model), we compared 3-months of treatment with and without paricalcitol on Klotho immunostaining in the kidney, parathyroid glands and aorta. With uremia, Klotho was unchanged in the parathyroid, significantly decreased in the kidney (66%) and the intimal-medial area of the aorta (69%), and significantly increased in the adventitial area of the aorta (67%) compared with controls. Paricalcitol prevented the decrease in Klotho in the kidney, increased expression in the parathyroid (31%), had no effect in the aortic media, but blunted the increase of Klotho in aortic adventitia. We propose that fibroblasts are responsible for expression of Klotho in the adventitia. In hyperplastic human parathyroid tissue from uremic patients, Klotho was higher in oxyphil compared with chief cells. Thus, under our conditions of moderate CKD and mild-to-moderate hyperphosphatemia in rats, the differential expression of Klotho and its regulation by paricalcitol in uremia is tissue-dependent.
Keywords: Klotho, Renal, Aorta, Parathyroid, renal failure, uremic rats, VDRA
Introduction
Alpha-Klotho (Klotho), originally identified as an anti-aging factor, is now recognized as a major player in mineral homeostasis and the pathogenesis of cardiovascular disease in chronic kidney disease (CKD). Klotho is a single-pass transmembrane protein (130 kDa) that is expressed in the kidney (in all major tubular segments along the nephron), the parathyroid gland, and the choroid plexus of the brain [1–5]. There are conflicting reports concerning the expression of Klotho in the vasculature [6–12].
Trans-membrane Klotho is a cofactor that converts fibroblastic growth factor-receptor 1 (FGFR1) into a specific receptor for FGF-23, a bone-derived phosphatonin that induces renal phosphate excretion and decreases 1,25 dihydroxyvitamin D3 (calcitriol) synthesis in the kidney [13–15]. A soluble form of Klotho is also found in the blood, urine and cerebrospinal fluid, arising from proteolytic cleavage of the extracellular domain of the transmembrane form (ectodomain shedding) or by alternative splicing of its transcript [3]. Soluble Klotho is an endocrine factor with a multitude of renal and extrarenal effects and acts independently of FGF-23 [16,17].
The control of calcium and phosphorus homeostasis involves an complex interplay of feedback systems including Klotho, FGF-23, parathyroid hormone (PTH), and calcitriol [18]. Calcitriol stimulates both Klotho and FGF-23, and both FGF-23 and Klotho inhibit renal 1α-hydroxylase, resulting in a decreased conversion of 25-hydroxyvitamin D to calcitriol. FGF-23 also regulates renal calcitriol levels by inducing expression of the catabolic enzyme 24-hydroxylase [19]. Since FGF-23 can decrease the synthesis and secretion of PTH [4,20], renal calcitriol levels may also be regulated by the action of FGF-23 on the parathyroid gland. Therefore, FGF-23 functions as a phosphaturic hormone as well as a counter-regulatory hormone for vitamin D. These functions of FGF-23 are dependent on the presence of the trans-membrane Klotho.
Treatment with paricalcitol, a vitamin D receptor activator (VDRA), is beneficial in patients with CKD, not only for the suppression of serum PTH levels, but also for improved survival [21]. In uremic rats, treatment with paricalcitol ameliorated progression of cardiomyopathy, presumably by preventing a decrease in levels of the vitamin D receptor (VDR) [22]. It has also been reported that the VDR controls expression of the Klotho gene [23]. Therefore, upregulation or restoration of Klotho by paricalcitol may provide a means to slow the progression of CKD and improve cardiovascular disease in these patients.
It is generally accepted that Klotho expression in the kidney is markedly decreased in uremia both in patients with CKD [24–26] and in rat models of CKD [27,28], but there are conflicting reports concerning the regulation of renal Klotho expression by VDRAs [7,23]. There are also varied and contrasting reports, in human and rodent studies, of the effect of uremia on Klotho expression, and its regulation by VDRAs, in parathyroid glands and the vasculature.
Because of the conflicting reports of Klotho expression and its regulation by VDRAs in uremic tissues, we examined the expression of Klotho and its regulation by paricalcitol in renal, vascular and parathyroid tissue of uremic rats. Human parathyroid tissue was also analyzed.
Results
Analytical Determinations
Results of the chemistries for normal, uremic non-treated and paricalcitol-treated rats are shown in Table 1. Serum Cr, TCa, P, and the CaxP product were significantly increased in both groups of uremic rats compared with the normal controls. As expected, compared with controls (31.6 ± 7.1 pg/ml) PTH was significantly higher in the non-treated uremic rats (265.8 ± 62.0 pg/ml; p<0.01). Treatment with paricalcitol prevented the increase in PTH (16.6 ± 4.6 pg/ml; p<0.05 versus uremic control). Parathyroid gland weight was significantly increased 1.7-fold (p≤0.05) in the uremic rat compared with the control rats (0.89 ± 0.05 versus 1.52 ± .13 mg PTG/g body weight; n=4 and 6 for normal and uremic rats, respectively); paricalcitol blunted this increase (1.24 ± .12 mg PTG/g body weight; n=6). The level of FGF-23 in paricalcitol-treated uremic rats was 20-fold higher than normal controls (569 ± 56 versus 11,571 ± 2898 pg/ml for controls and paricalcitol treatment, respectively; p<0.01), and nearly 3-fold higher than non-treated uremic rats (3,998 ± 1,613 pg/ml; p<0.05).
Table 1.
Serum chemistries in normal and uremic rats. Rats were made uremic and treated for 3 months with nothing (UC) or paricalcitol (UP).
| Group | Body Wt (g) | Cr (mg/dl) | ICa++ (mg/dl) | Total Ca (mg/dl) | P (mg/dl) | CaxP (mg2/dl2) | PTH (pg/ml) | FGF-23 (pg/ml) |
|---|---|---|---|---|---|---|---|---|
| NC (n=6) | 263.7 ± 2.9 | 0.48 ± 0.02 | 4.84 ± 0.04 | 9.96 ± 0.08 | 4.23 ± 0.23 | 42.2 ± 2.3 | 31.6 ± 7.1 | 569 ± 56 |
| UC (n=9) | 262.0 ± 5.1 | 1.34 ± 0.14α | 4.92 ± 0.05 | 11.38 ± 0.15α | 6.20 ± 0.53α | 70.7 ± 6.5α | 265.8 ± 62.0α | 3,998 ± 1,613 |
| UP (n=9) | 258.4 ± 3.4 | 1.00 ± 0.07α | 5.41 ± 0.06αβ | 11.99 ± 0.33α | 6.34 ± 0.17α | 76.3 ± 3.6α | 16.6 ± 4.6β | 11,571 ± 2,898αβ |
p≤0.01 vs. NC
p≤0.05 vs. UC. Results are expressed as mean ± SEM.
Klotho Immunostaining
Specificity
The specificity of the Klotho antibody was assessed by immunostaining sections of rat PTG, as shown in Figure 1. The strong staining obtained with the primary antibody (A) was blocked when the primary antibody was pre-absorbed with Klotho antigen (B) or was replaced with goat IgG (C).
Figure 1.
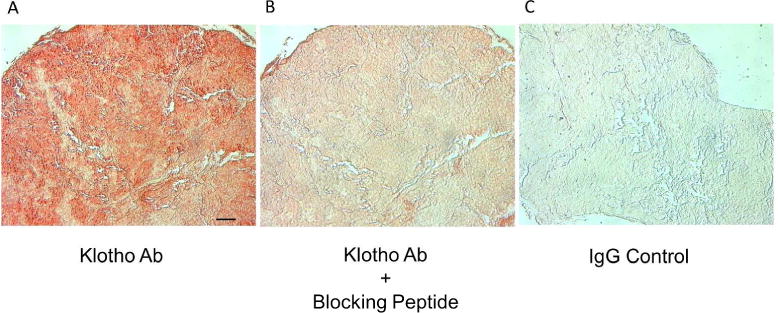
The specificity of the Klotho antibody. Representative images of a rat PTG show that the strong staining obtained with the primary antibody (A) was blocked when the primary antibody was pre-absorbed with Klotho antigen (B) or was replaced with goat IgG (C), 200x; bar represents 50 microns and applies to all figures.
Kidney
Klotho was detected in proximal tubules, distal tubules, and collecting ducts of kidney tissue, which is in agreement with the recent findings of Zhou et al., who showed that Klotho is ubiquitously expressed in all major tubular segments along the nephron in adult kidneys [5]. Klotho immunostaining in the cortex of kidney tissue of normal, uremic and uremic paricalcitol-treated rats is shown in Figure 2A. Quantification of the immunostaining is shown in Figure 2B. Klotho protein, strongly detected in the renal tubules of normal rats, was significantly decreased 65.5% in the uremic rats (p≤0.05); this decrease was blocked by treatment with paricalcitol (n=6 each). Quantification of renal Klotho mRNA (Figure 2C; n=6 each) also showed a significant decrease of 78.3% in Klotho expression in uremic rats (p≤0.05), which was blocked by paricalcitol treatment.
Figure 2.
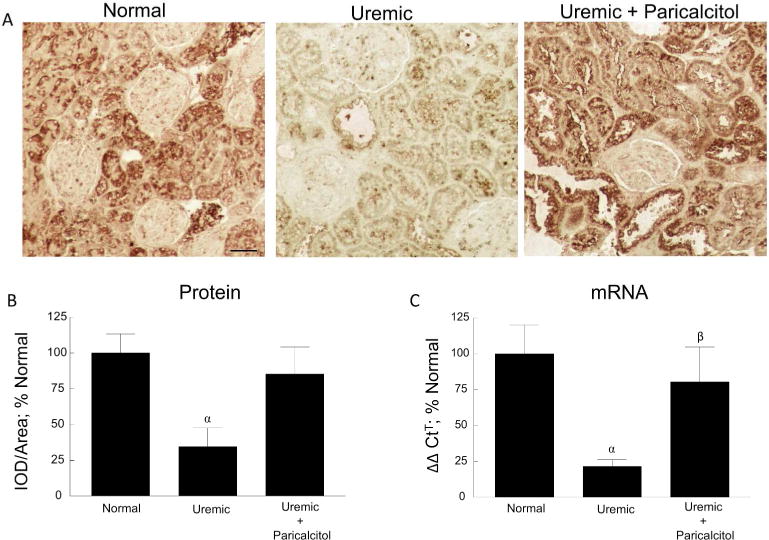
Renal Klotho. (A) Representative images of Klotho immunostaining of normal, uremic, and uremic paricalcitol-treated rat kidney tissue, 200x; bar represents 50 microns and applies to all figures. (B) Quantitation of immunostaining. (C) Quantitation of Klotho mRNA of kidney tissue. αp≤0.01 versus Normal, βp≤0.05 versus Uremic; (n=6 each); Average ± s.e.m.
Aorta
Klotho immunostaining in the aorta of normal, uremic and uremic paricalcitol-treated rats is shown in Figure 3A. Klotho protein was detected in the intimal-medial area of the aortic rings as well as in the adventitia, the outermost layer of the vasculature. The immunostaining was quantitated separately for each area. As seen in the normal tissue, Klotho is expressed in the vascular smooth muscle cells (VSMCs) of intimal-medial area of the aorta. Klotho expression in the intimal-medial area was significantly suppressed by 69.2% in the uremic rats (p≤0.001) compared to normal and was not regulated by treatment with paricalcitol (Figure 3B). Of particular interest, however, immunostaining of the adventitia was significantly increased by 67.0% (p≤0.05) in the uremic rats; paricalcitol treatment blocked this increase in Klotho expression (Figure 3C).
Figure 3.
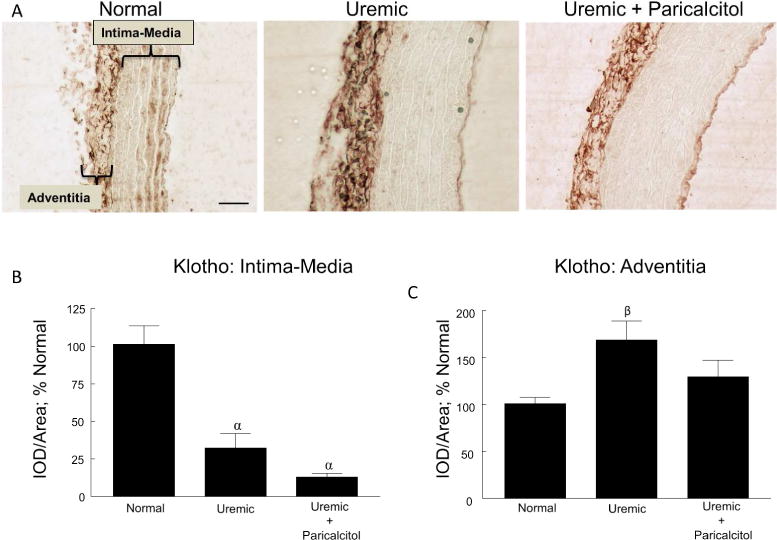
Vascular Klotho. (A) Representative images of Klotho immunostaining of normal, uremic, and uremic paricalcitol-treated rat aorta, 400x; bar represents 50 microns and applies to all figures. The areas of the intima-media and adventitia are indicated in the first panel. (B) Quantitation of immunostaining of the intimal-medial area. (C) Quantitation of immunostaining of the adventitia. αp≤0.001 versus Normal, βp≤0.05 versus Normal; n=6 each; Average ± s.e.m.
To identify the cells responsible for the expression of Klotho in the adventitia, we examined the presence and localization of fibroblasts, using an antibody for vimentin, in relation to areas of Klotho expression. While vimentin is the most frequently found intermediate filament in fibroblasts, which makes it a well-accepted marker for that cell, it is also expressed in other cell types such as VSMCs. Vimentin immunostaining in the aorta of normal, uremic and uremic paricalcitol-treated rats is shown in Figure 4A, with quantitation of the staining shown in Figure 4B. In the intima/media, vimentin expression parallels that of Klotho (see Figure 3B; expression decreases in uremia and is not regulated by paricalcitol). Vimentin expression in the adventitia increased with uremia (paralleling Klotho expression), but was not regulated by paricalcitol.
Figure 4.
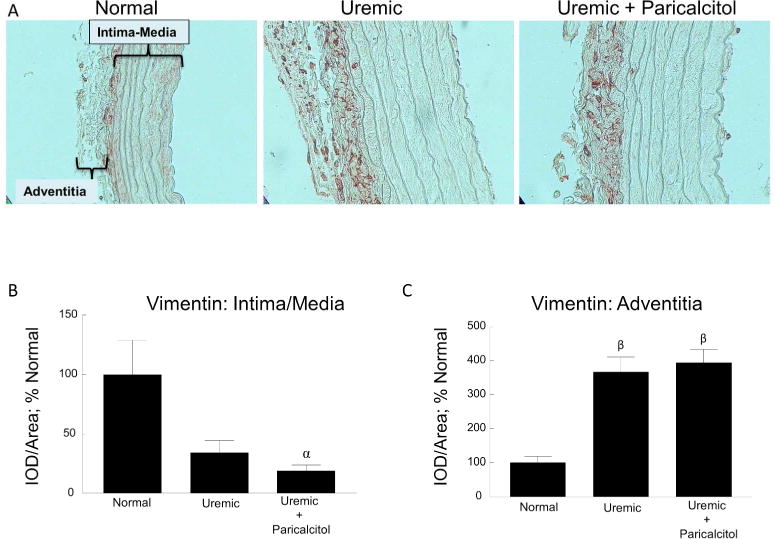
Vascular Vimentin. (A) Representative images of vimentin immunostaining of normal, uremic, and uremic paricalcitol-treated rat aorta, 400x. The areas of the intima-media and adventitia are indicated in the first panel. (B) Quantitation of immunostaining of the intimal-medial area. (C) Quantitation of immunostaining of the adventitia. αp≤0.05 versus Normal, βp≤0.001 versus Normal; n=6 each; Average ± s.e.m.
To examine localization of Klotho expression with different cell types in the adventitia, consecutive sections of rat aorta were immunostained for Klotho, vimentin and CD-34 (Cluster of Differentiation 34), a marker of progenitor cells often used to detect undifferentiated fibroblasts. The upper panels in Figure 5 shows a side-by-side comparison of Klotho, vimentin and CD-34 staining in normal rat aorta. To better visualize this staining, the color images were contrast-enhanced and then inverted so that the staining appeared white against a dark background (lower panels). Figure 6 shows a side-by-side comparison of Klotho, vimentin, CD-34 and CD-68 (a marker of macrophages) in uremic rat aorta (upper panels); contrast-enhanced and inverted images are shown in the lower panels. The areas of staining for fibroblasts (vimentin) and undifferentiated fibroblasts/stem cells (CD-34) were localized to the areas of Klotho expression in the adventitia, suggesting that fibroblasts (mature and immature fibroblasts) may be responsible for the expression of Klotho in the adventitia. Few macrophages were detected.
Figure 5.
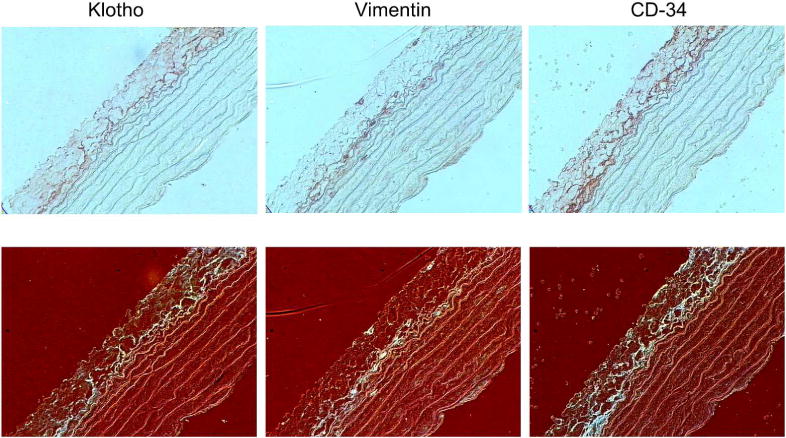
Localization of Klotho, Fibroblasts and Undifferentiated Fibroblasts in Normal Rat Aorta. (Upper panels) Representative images of staining for Klotho, fibroblasts (vimentin) and undifferentiated fibroblasts (CD-34) in normal rat aorta; 400x. (Lower panels) Images were contrast-enhanced and inverted to better visualize staining (seen in white).
Figure 6.
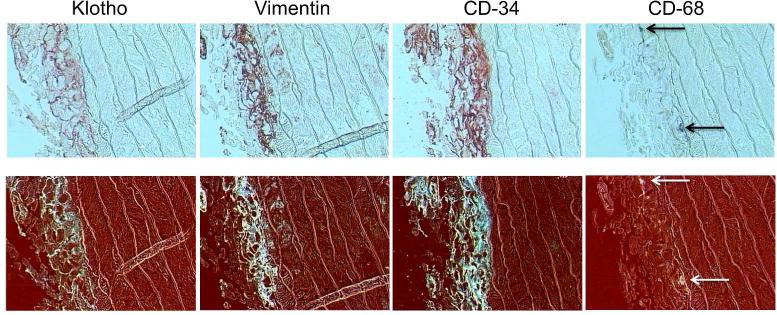
Localization of Klotho, Fibroblasts, Undifferentiated Fibroblasts and Macrophages in Uremic Rat Aorta. (Upper panels) Representative images of staining for Klotho, fibroblasts (vimentin), undifferentiated fibroblasts (CD-34) and macrophages (CD-68) in uremic rat aorta; 400x. (Lower panels) Images were contrast-enhanced and inverted to better visualize staining (seen in white).
No vascular calcification was detected using Von Kossa staining (data not shown).
Parathyroid
Klotho immunostaining in the parathyroid glands of normal, uremic and uremic paricalcitol-treated rats is shown in Figure 7A. Both cytosolic and nuclear staining was observed. Quantitation of the immunostaining (Figure 7B) showed no difference in the expression of Klotho in normal and untreated uremic rats. However, there was a significant increase of 31.0% in Klotho expression in the parathyroid glands of uremic rats treated with paricalcitol (p≤0.05 versus UC). Archived hyperplastic parathyroid glands from 10 patients with secondary hyperparathyroidism and a single normal parathyroid gland were immunostained for Klotho. A serial section was stained with hematoxylin-eosin (H&E) to identify the parathyroid cell type as an oxyphil cell or chief cell. A homogeneous Klotho staining was seen in the reference normal parathyroid gland, which was composed mainly of chief cells. However, a heterogeneous expression of Klotho was observed in the hyperplastic parathyroid tissue, with a higher expression found in oxyphil cells compared with chief cells in all 10 patients. This was a consistent finding that applied to all the oxyphilic areas of the tissues examined. Representative images of the normal tissue and 3 of the hyperplastic glands (each from a different patient) are shown in Figure 8.
Figure 7.
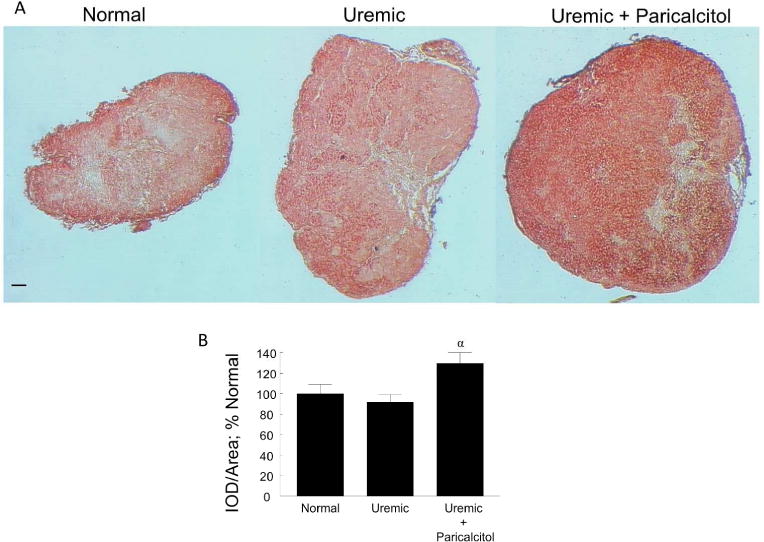
Parathyroid Gland Klotho. (A) Representative images of Klotho immunostaining of normal, uremic, and uremic paricalcitol-treated rat parathyroid glands, 100x; bar represents 50 microns and applies to all figures. (B) Quantitation of immunostaining, (Normal, n=4; Uremic and Uremic+Paricalcitol, n=6 each). αp≤0.05 versus Uremic; Average ± s.e.m.
Figure 8.
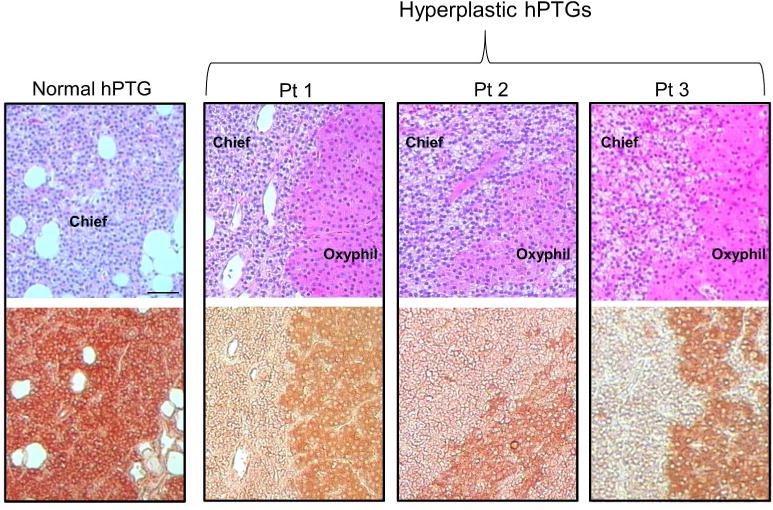
Human Parathyroid Gland Klotho. Representative images of H&E staining and Klotho immunostaining of consecutive sections of normal human parathyroid (hPTG) tissue from 1 patient, and hyperplastic parathyroid tissue from 3 patients with secondary hyperparathyroidism, 200x; bar represents 50 microns and applies to all figures. Oxyphil cells are identified by an intense eosinophilic staining compared to chief cells, as shown in the upper panels. Expression of Klotho in consecutive sections of tissue for each parathyroid gland (lower panels) is highest in the oxyphil cells.
Validation of Klotho immunostaining
To validate the Klotho immunostaining, a second Klotho antibody (ABIN502138; Aviva Systems Biology Corp., San Diego, CA, USA) was tested in a side-by-side comparison with the antibody that used for all of the IHC in the study (sc-22220; Santa Cruz Biotechnology Inc., Dallas, TX, USA). Hyperplastic parathyroid tissue from 3 uremic patients was subjected to H&E staining to identify the oxyphil and chief cells, and then serial sections were immunostained with the 2 Klotho antibodies. As shown in the Figure 9, the immunostaining pattern of Klotho protein was identical for both antibodies, confirming our findings that the oxyphil cells in human parathyroid glands exhibit a more intense staining compared to chief cells. Additionally, consecutive sections of normal rat kidney were immunostained with both antibodies and representative images of 2 different areas of the kidney (A and B) are shown in Figure 10. Staining of the renal tissue is comparable with both antibodies.
Figure 9.
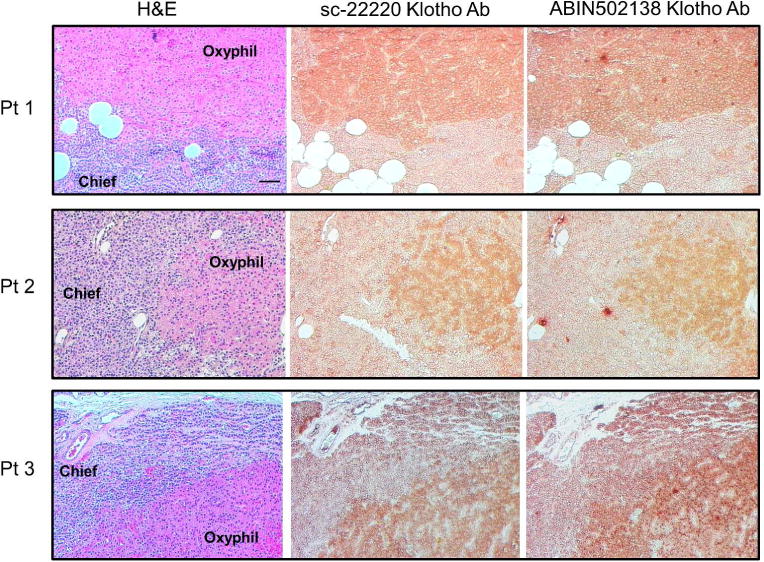
Comparison of Immunostaining of 2 Klotho Antibodies in Human Parathyroid Gland Klotho. Representative images of H&E staining and Klotho immunostaining using 2 different antibodies on consecutive sections of hyperplastic human parathyroid tissue, 200x; bar represents 50 microns and applies to all figures. The immunostaining pattern of both antibodies was nearly identical, with Klotho expression higher in the oxyphil cells compared with chief cells.
Figure 10.
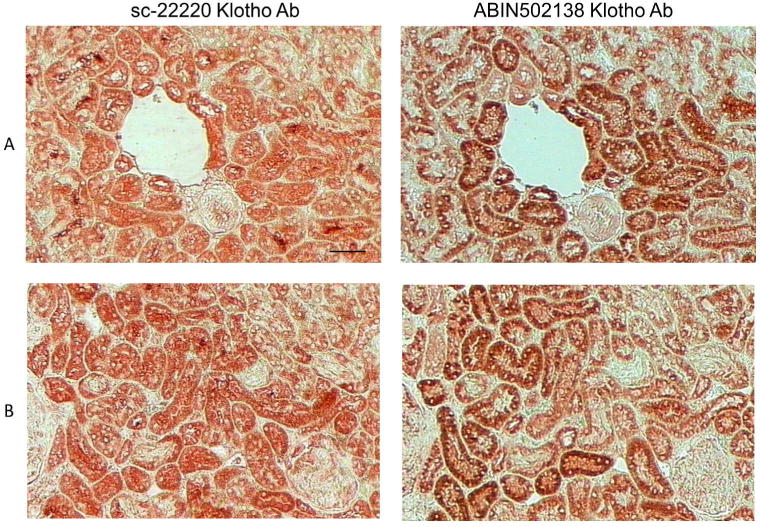
Comparison of Immunostaining of 2 Klotho Antibodies in Rat Kidney. Representative images of H&E staining and Klotho immunostaining using 2 different antibodies on consecutive sections of cortical rat kidney tissue, 200x; bar represents 50 microns and applies to all figures. Staining of the tubules in the cortical areas of the sections was comparable with the 2 antibodies.
Discussion
The subject of the expression and regulation of Klotho in a variety of tissues is not without controversy. In the present study, we show that Klotho is differentially expressed in the kidney, aorta and parathyroid glands of uremic rats, and likewise is differentially regulated in these tissues by treatment with paricalcitol. Our investigation supports certain previous studies, contrasts with others, and introduces new findings.
Kidney
We found that renal Klotho expression (protein and mRNA) is markedly decreased in our uremic rat model, which is consistent with previous reports in patients with CKD [24–26] and in rat and mouse models for CKD [27,28]. Although the exact mechanism of how Klotho is reduced in kidney disease is not known, hyperphosphatemia, hypercalcemia, ischemia, oxidative stress, angiotensin II, TGF-β1, and inflammation are thought to be involved [2,5,27,29,30]. VDRAs have a therapeutic potential in attenuating experimentally induced kidney diseases [31–36]. VDRA treatment attenuates the progression of glomerulosclerosis and albuminuria, and decrease the podocyte loss in subtotally nephrectomized rats. Additionally, clinical studies have shown that VDRAs are associated with decreased morbidity and mortality in patients with CKD [37–39]. Upregulation or restoration of Klotho by paricalcitol or other VDRAs may serve to slow the progression of CKD and improve cardiovascular disease in these patients. Calcitriol has been shown to upregulate Klotho mRNA in a variety of human, murine and simian kidney cell lines [23]. We found that treatment of uremic rats with the VDRA paricalcitol blocked the decrease in renal Klotho expression. This is in contrast with a study by Lau et al. who, in a uremic mouse model, found that while VDRA therapy upregulated serum and urine Klotho levels, renal and parathyroid Klotho were not affected [7]. Since no upregulation of Klotho by VDRA treatment was found in a variety of tissues analyzed, the authors postulated that an increased expression/shedding of Klotho could account for the increase in soluble Klotho. Several factors may explain the different findings between our study and Lau’s study: 1) in our study, the dietary phosphate was 0.4% compared with 1.5% in Lau’s study; 2) paricalcitol decreased PTH to normal levels in our study, but neither paricalcitol nor calcitriol had an effect on PTH levels in Lau’s study; 3) we found no aortic calcification, whereas Lau found marked medial calcification in the aorta; and 4) our study was in rats and Lau’s study was in mice.
Parathyroid
In the parathyroid gland, we found no difference in the expression of Klotho protein in normal and uremic rats. However, treatment of uremic rats with paricalcitol significantly increased Klotho expression. Previous reports of parathyroid Klotho expression are varied. In the parathyroid glands of rats with mild uremia and normal PTH levels (7/8 nephrectomy), Klotho mRNA was not significantly different from that of control rats [40]. However, in parathyroid glands of rats with more severe uremia and marked hyperparathyroidism (adenine/high phosphate diet), Klotho expression was significantly increased at week 1 and 2 of uremia, but was suppressed at week 6 of uremia [41]. Hofman-Bang et al. found that Klotho expression was increased in the parathyoid tissue of uremic rats on a high phosphate diet (5/6 nephrectomy), but not in uremic rats on a standard diet; treatment with calcitriol significantly decreased parathyroid Klotho mRNA and protein in the uremic rats on a high phosphate diet, but had no effect in normal rats [42]. In contrast, Krajisnik et al. showed that calcitriol increased Klotho expression in cultured bovine parathyroid cells, a model for normal parathyroid cells [43]. Thus, there appears to be no concensus as to the effect of uremia on Klotho expression in the parathyroid in animal studies, and no concensus regarding the effect of treatment with VDRAs. These highly variable reports in animals could be the result of a number of factors, including examination of mice versus rats, the type of CKD model used (i.e. different methods of nephrectomy or the use of an adenine diet to induce CKD), the degree of uremia examined, or the level of phosphate in the diet.
Klotho expression has also been examined in human parathyroid tissue. In a study of primary hyperparathyroid patients, expression of Klotho mRNA and protein was decreased or undetectable in parathyroid adenomas, and the expression was inversely correlated to serum calcium levels [44]. In another study, the expression of parathyroid Klotho (but not the FGFR) was decreased in patients with primary hyperparathyroidism compared to normal tissue, but there was no correlation between Klotho expression and serum calcium levels [45]. In uremic patients with secondary hyperparathyroidism, Klotho was detected in nodular parathyroid tissue, but the expression varied in every nodule [46]. Komaba et al. found the expression of Klotho and FGFR1 was significantly decreased in uremic parathyroid hyperplasia compared with normal parathyroid tissue, and the decrease was especially pronounced in nodular hyperplasia compared to diffuse hyperplasia [47]. However, Latus et al. showed that Klotho, but not FGFR-1, was decreased in patients with secondary hyperparathyroidism [48]. Krajisnik et al. showed that Klotho and FGFR1 expression was decreased in hyperplastic parathyroid glands from CKD and kidney transplant patients compared to controls, but the decreased expression was not necessarily lower in nodules [43]. There was no difference in parathyroid Klotho expresssion in patients treated with or without calcitriol, nor did calcitriol have any effect on cultured parathyroid cells from a CKD patient. Consistent with these studies, we found that the chief cells of normal parathyroid tissue exhibited strong Klotho expression. However, we also found a differential expression of Klotho in the parathyroid glands of patients with secondary hyperparathyroidism according to cell type, with greater expression in the oxyphil cells compared with chief cells. Overall, previous studies in humans consistently indicate that the expression of parathyroid Klotho decreases in uremia compared with normal parathyroid tissue. Given our results, we postulate that the decrease in parathyroid Klotho expression observed in uremia is due to a decrease of the protein in chief cells. The finding of the higher expression of Klotho in the oxyphil cells, which are increased in number in patients with uremia and whose function is still unknown, is intriguing. We have previously shown that oxyphil cells have increased expression of parathyroid-specific genes such as PTH, calcium-sensing receptor, glial cells missing-2, parathyroid hormone-related-protein and the 1α-hydroxylase compared to chief cells, [49,50]. Studies that will better define the role of the oxyphil cell in parathroid pathophysiology are warranted. Additionally, correlation of Klotho expression with blood parameters, the expression Klotho in nodular versus diffuse hyperplasia, and regulation of Klotho by VDRAs have yet to be clarified.
It is difficult to correlate our findings in human parathyroid glands to our findings in the rat glands since rat parathyroids “generally” do not exhibit oxyphil cells. When parathyroid glands are removed from patients with CKD, the glands have usually been hyperplastic for years, allowing for transformation of chief cells into oxyphil cells. Would oxyphil cells develop in the parathyroids of rats experiencing uremia over a comparable period of time? A study from 1975 found that Mongolian gerbil parathyroid glands exhibited the presence of oxyphil cells when the glands were cultured in high calcium concentrations for 7 days, suggesting that chief cells can transform into oxyphil cells under these conditions [51]. In addition, while oxyphil cells are not commonly reported in the rat parathyroid, they have been documented under certain circumstances [52,53]. Therefore, rat parathyoid glands may have the potential to develop oxyphilic cells under the proper environment. However, determining whether extended periods of uremia would produce parathyroid oxyphil cells in rats may be problematic because of the high mortality rate seen in rats with long-term renal failure.
Aorta
Cardiovascular disease is responsible for an increased mortality in patients with chronic kidney disease, especially those with end-stage renal disease [54,55]. Arterial calcification is a major contributor to cardiovascular events and mortality in these patients [56–59]. The specific effects of Klotho on vascular calcification are not clear. Lim et al. have reported a high expression of Klotho protein in the arteries of healthy individuals, and this expression is severely decreased in arteries from patients with CKD [6]; VDRA treatment significantly increased Klotho expression in arterial tissue from patients with CKD but not in tissue from healthy individuals.
A vascular deficiency of Klotho has been reported to promote vascular calcification [6,28]. However, a recent report by van Venrooij et al. showed that Klotho expression was detected only in sections of calcified coronary arteries that were positive for FGF-23 and the osteogenic transcription factor DMP1 (dentin matrix protein 1) [60], which contrasts with other reports that Klotho is vital to normal vascular smooth muscle tissue function [6,9,28]. Others have not detected Klotho in vascular smooth muscle cells from uremic humans or mice, or in healthy or calcified mouse aorta [7,10]. Jimbo et al. detected Klotho mRNA and protein in normal rat aorta, but the expression was not decreased in uremia [11]. In isolated, cultured vascular smooth muscle cells (VSMCs), however, Klotho mRNA was detected, but not Klotho protein. We previously demonstrated in uremic rats that paricalcitol ameliorates left ventricular hypertrophy, suppresses myocardial arterial vessel thickness, and myocardial and perivascular fibrosis, possibly by up-regulating the VDR; however, vascular calcification and vascular Klotho were not analyzed [22]. Others have shown that treatment with paricalcitol decreases vascular calcification, but again, either Klotho was not investigated or vascular tissue was analyzed for Klotho and it was not detected [7,61].
Here, we found that the expression of Klotho in uremic rats was decreased in the medial area of the aorta and was not regulated by treatment with paricalcitol. Klotho expression was found in the smooth muscle cells of the media, which corroborates the finding of Lim et.al [6], who was one of the first to show endogenous expression of Klotho in the human vasculature and vascular smooth muscle cells. In contrast, we found an increase in Klotho expression in the adventitia of the aorta, and treatment with paricalcitol blunted this increase. The pattern of Klotho expression in the aorta of uremic rats in our study is similar to FGF-23 expression in mouse aorta as reported by Fang et. al. [8]. They observed FGF-23 immunostaining in the aortic media of normal mice. However, while this medial expression was lost with mild uremia, there was an increase in FGF-23 staining in the adventitia. The authors suggested this increased staining of FGF-23 in the adventitia was possibly due to circulating FGF-23 binding to its receptor, which co-localized to that area. The increased expression of adventitial Klotho in our study could be attributed to the nature of the adventitia itself and its involvement in vascular calcification. It is now acknowledged that the adventitia plays an important role in vascular function and pathophysiology. A recent review by Majesky et al. details the interplay of the cell types in the adventitia and their role in vascular wall growth, remodeling, and disease [62]. The adventitia consists mainly of fibroblasts in addition to perivascular nerves and microvessels embedded in a collagen extracellular matrix. The adventitia is a major site for immune responses, for inflammatory cell trafficking into and out of the vessel wall, and it is considered to be a source of progenitor cells. The adventitia also provides microvascularization, nutrient supply, and mesenchymal progenitors to the outer half of the media [63–65]. In uremic patients with increasing degrees of vascular calcification, it has been shown that areas of calcification progress concentrically from the adventitia to the intima [66]. In diabetic mice, activated myofibroblasts in the adventitia promote vascular calcification of the media by inducing paracrine Wnt signals [67,68]. It should be noted that our detection of Klotho and the detection of FGF-23 by Fang in the adventitia in uremic animals occurred at a stage of kidney failure in which there was no detection of medial calcium deposition. Since Klotho is generally associated with cellular protection, upregulation of Klotho in the adventitia is most likely a protective/compensatory response to counteract the development of calcification in the media. However, it is possible that Klotho upregulation is actually involved in a pathophysiological event, as is suggested in a study that found a link between increased Klotho and the process of retinal degeneration [69].
It should also be pointed out that, given the differential expression in the adventitia and intima/media, care should be taken when analyzing the aorta in future studies. Stripping the adventitia from the media when the tissue is processed could greatly alter the results of an experiment. This could, in part, explain some of the varied results obtained in different laboratories.
Our data suggest that mature and undifferentiated fibroblasts may be responsible for the expression of Klotho in the adventitia. This is supported by recent studies that have demonstrated the expression of Klotho in: 1) normal human skin fibroblasts [70], 2) synovial fibroblasts [71], and 3) MRC-5 human primary fibroblast cell line [72]. Fibroblasts are the most abundant cells in the adventitia and upon activation secrete a number of cytokines, chemokines and growth factors, produce extracellular matrix, and communicate with neural cells and cells of hemopoietic origin. Although macrophages were detected in the adventitia, they were too few in number to account for the degree of Klotho expression.
In the intima/media, vimentin expression parallels that of Klotho (i.e., expression decreases in uremia and is not regulated by paricalcitol). However, interpretation of vimentin expression in the intima/media is more complicated than in the adventitia. VSMCs are different from other smooth muscle cells in that they express vimentin rather than desmin, another intermediate-sized cytoskeletal filament. It is thought that vimentin in VSMCs reflects a differentiation pathway separate from that of other smooth muscle cells and may be related to special functions and pathological disorders of blood vessels [73]. The association of decreased VSMC vimentin and decreased Klotho expression in the uremic media may be reflective of this.
In conclusion, in our model of moderate CKD and mild-to-moderate hyperphosphatemia we found that the differential expression of Klotho and its regulation by paricalcitol in uremia is tissue-dependent. Clearly, given the importance of Klotho in mineral metabolism and the pathogenesis of CKD, the expression of Klotho and its regulation by VDRAs in uremia warrants further investigation in the kidney, parathyroid, and vasculature. The varied results observed in the investigation of Klotho expression and regulation in recent years may be the result of many factors including the method of induction of uremia, the concentration of phosphate in the diet, the degree of uremia, the duration of uremia, the use of mice, rat or human tissue, in vitro or in vivo experiments, the antibody used for Klotho analysis, as well as how tissue is processed. A major task ahead lies in the interpretation of data obtained from diverse studies that utilize a range of experimental design, conditions, and analysis.
Materials and Methods
Experimental Protocol
The Animal Studies Committee at Washington University School of Medicine approved the experimental protocol of the current study in accordance with federal regulations. Uremia was induced in a group of female Sprague-Dawley rats (225–250g) by 5/6 nephrectomy and the rats were divided into a uremic control group (UC, no treatment) and a group that was treated with paricalcitol 3 times per week (UP; 200 ng/rat administered intraperitoneally). Treatment with paricalcitol was started 1 day after surgery. Normal rats served as controls (NC). All rats were fed normal rat chow containing 0.9% calcium and 0.4% phosphorus. The treatment lasted for 3 months. Rats were killed by exsanguination via the dorsal aorta; plasma and serum were collected and frozen. The remnant kidney and aorta were divided into several sections and either fixed in 10% buffered formalin for immunohistochemistry or snap frozen in liquid nitrogen and stored at 80°C until further examination. The parathyroid glands were weighed (CAHN-31, Orion Instruments, Inc., Boston, MA, USA) and fixed in 10% buffered formalin for immunohistochemical analysis.
Human Parathyroid Tissue
Archived parathyroid tissue obtained from 10 patients undergoing parathyroidectomy due to uremic secondary hyperparathyroidism was used for analysis of Klotho expression. Informed consent had been obtained for collection of the tissue. Paraffin sections were chosen for immunostaining based on the presence of a mixed-cell population (chief and oxyphil cells) as determined with H&E staining. For reference, a single normal parathyroid gland obtained from a patient undergoing a total thyroidectomy was immunostained.
Analytical determinations
Serum phosphorus (P) and creatinine (Cr) were measured by an autoanalyzer (COBAS-MIRA Plus, Branchburg, NJ, USA). Total serum calcium (TCa) was measured by atomic absorption spectrophotometry (Perkin-Elmer, model 1100B, Norwalk, CT, USA). Ionized Ca (ICa) was measured using a Nova 8 electrolyte analyzer (Nova Biomedical, Woltham, MA, USA). Serum PTH and FGF-23 were determined using the Rat Bioactive Intact PTH ELISA kit and the Mouse FGF-23 (C-Term) ELISA kit, respectively, from Immutopics (San Clement, CA, USA).
Immunohistochemical evaluation
Formalin-fixed, paraffin sections of kidney, parathyroid and aorta tissue were immunostained using a Klotho antibody (goat) obtained from Santa Cruz Biotechnology, Inc. (sc-22220). This is an affinity purified goat polyclonal antibody raised against a peptide mapping within an internal region of human Klotho between amino acids 500–550. Kidney and aorta tissue sections were deparaffinized, rehydrated, then microwaved at high intensity for 10 min in 10 mM citric acid (pH 6.0) and then allowed to cool for 10 min, after which the sections were quenched for 10 min in 0.6% hydrogen peroxide in methanol; the parathyroid tissue was processed using the same protocol, with the exception that it was not microwaved. Sections were blocked for at least 10 min with 2.5% horse serum (Vector Laboratories, Burlingame, CA, USA), and were incubated overnight (4°C) with the Klotho antibody diluted in 2.5% horse serum (1:50 dilution for kidney and aorta tissue, 1:250 dilution for parathyroid tissue). Incubation with goat IgG in place of the primary antibody (Santa Cruz Biotechnology, Inc.) was used as a negative control. The second antibody (peroxidase-conjugated anti-goat ImmPRESS reagent from Vector Laboratories) was applied for 30 min at room temperature, and the immune complexes were visualized with 3-amino-9-ethylcarbazole substrate-chromogen (AEC) from Vector Laboratories. The specificity of the Klotho antibody was determined by preabsorbing the Klotho antibody with a 10-fold excess of Klotho peptide (Santa Cruz Biotechnology, Inc.; sc-22220-P blocking peptide) for 1 hour before application to the tissue sections. An additional Klotho antibody (Aviva Systems Biology Corp.; #ABIN502138; obtained through AntibodiesOnline.com) was used to validate the Klotho staining in our tissue (rat kidney, 1:250 dilution, with microwave treatment; human parathyroid tissue 1:500 dilution, no microwave treatment). This is a rabbit polyclonal antibody; the immunogen for the antibody was a synthetic peptide directed towards the middle region of human Klotho. Additionally, in sections of aorta, fibroblasts were detected using an antibody to vimentin (mouse pre-diluted Clone V-9; Invitrogen Life Technologies), dedifferentiated fibroblasts (stem cells) were detected using an antibody to CD-34 (1:50 dilution goat polyclonal #sc-7045; Santa Cruz Biotechnology, Inc) and macrophages were detected using an antibody to CD-68 (1:50 dilution mouse anti-rat #MCA341R; Serotec, Oxford, UK); microwave antigen retrieval was used, and antibodies were incubated on the tissue overnight at 4°C. CD-68 was visualized with or 3,3′-diaminobenzidine (DAB) from Vector laboratories.
Quantification of Klotho immunostaining was performed as previously described [49]. Images of each stained tissue section were captured using a Nikon Diaphot-TMD microscope coupled to a camera and an image analysis system. The number of images captured for each tissue per rat was as follows: 5 images (200x) of the kidney cortical area containing glomeruli, 1 image (100x) for each parathyroid gland, and 8 images (400x) for each aortic ring (the areas of the intima-media and adventitia were analyzed separately). The images were converted to grayscale and analyzed using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). The intensity of staining was quantified using the OD (optical density) function of the software; for each image, the integrated OD (IOD) and the area were obtained, and the IOD/area ratio was calculated to express the intensity of staining per unit area. When multiple images were analyzed (for the kidney and aorta), the IOD/area values obtained for each of the multiple images of each tissue were averaged, resulting in 1 value for that tissue for each rat. The IOD/area of the negative control (i.e. IgG) immunostaining was subtracted from the IOD/area for the antibody-stained sections to obtain a “corrected” IOD/area. The average of the corrected values obtained for the normal control animals was considered to be the baseline staining. The corrected IOD/area values for the uremic animals were reported as percentage of the baseline staining. Quantification of vimentin immunostaining was performed by analyzing 10–18 images of each aortic ring (400x). On each color image, an area of interest (AOI) was drawn around the adventitial area, and the color cube function used to highlight the staining. The highlighted areas of the entire adventitia were counted and expressed as a percentage of the total area of the adventitia. This process was then repeated on the intima/media area of each image. The Image-Pro software was also used to better visualize the localization of Klotho, vimentin, CD-34 and CD-68 staining on consecutive sections of tissue. The color images of the staining were contrast-enhanced and then inverted so that the staining appeared white against a dark background.
Formalin-fixed paraffin sections of aorta were examined for vascular calcification using a Von Kossa kit (Polysciences Inc, Warrington, PA, USA).
Real-time PCR
Kidney RNA samples were analyzed by real-time (RT) quantitative PCR. Total RNA was isolated using RNAzol Bee (Tel-Test, Friendswood, TX). Reverse transcription of the RNA was carried out using oligo-dT primer and SMART MMLV reverse transcriptase (CLONTECH Laboratories, Mountain View, CA, USA). RT-PCR was performed using Fast SYBR Green Master Mix (Applied Biosystems; Foster City, CA) in an Applied Biosystems 7900HT Fast Real-Time PCR System. QuantiTect Primer Assays obtained from Qiagen (Valencia, CA) were obtained for quantifying rat Klotho (Klotho; QT00185822) and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH; QT00199633). The ΔΔC technique was used to calculate the ratio of target gene to GAPDH with respect to normal control rats. The results were reported as the percent of normal control values, which represented 100%.
Statistical analysis
All data are expressed as mean ± s.e.m. One-way analysis of variance (ANOVA) with the Tukey’s post-test, unless stated otherwise, was used for comparison between uremic groups; p ≤ 0.05 was considered significant. GraphPad InStat 3 software was used for the statistical analysis (GraphPad Software; La Jolla, CA).
Acknowledgments
This work was supported by a Washington University Research in Renal Diseases Grant (3068-31030A), a Washington University Center for Kidney Disease Research O’Brian Center Grant (P30DK079333), and an AbbVie IIS Program Grant.
Footnotes
Disclosure
Washington University and E. Slatopolsky may receive income based on a license of related technology by the University of Wisconsin.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Experimental nephrology. 2005;101:e67–74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- 3.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for fgf23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of klotho contributes to kidney injury by derepression of wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 7.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM. Vitamin d receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82:1261–1270. doi: 10.1038/ki.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y, Ginsberg C, Sugatani T, Monier-Faugere MC, Malluche H, Hruska KA. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int. 2014;85:142–150. doi: 10.1038/ki.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, Muros-de-Fuentes M, Perez H, Meneses-Perez B, Cazana-Perez V, Navarro-Gonzalez JF. Expression of fgf23/klotho system in human vascular tissue. Int J Cardiol. 2013;165:179–183. doi: 10.1016/j.ijcard.2011.08.850. [DOI] [PubMed] [Google Scholar]
- 10.Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, Chavkin NW, Rahman M, Wahl P, Amaral AP, Hamano T, Master SR, Nessel L, Chai B, Xie D, Kallem RR, Chen J, Lash JP, Kusek JW, Budoff MJ, Giachelli CM, Wolf M, Chronic Renal Insufficiency Cohort Study I Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T, Shimosawa T. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of klotho deficiency. Kidney Int. 2013 doi: 10.1038/ki.2013.332. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg K, Olauson H, Amin R, Ponnusamy A, Goetz R, Taylor RF, Mohammadi M, Canfield A, Kublickiene K, Larsson TE. Arterial klotho expression and fgf23 effects on vascular calcification and function. PloS one. 2013;8:e60658. doi: 10.1371/journal.pone.0060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical fgf receptor into a specific receptor for fgf23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 14.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M. Isolated c-terminal tail of fgf23 alleviates hypophosphatemia by inhibiting fgf23-fgfr-klotho complex formation. Proc Natl Acad Sci U S A. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. Alpha-klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 18.Hu MC, Kuro OM, Moe OW. Renal and extrarenal actions of klotho. Semin Nephrol. 2013;33:118–129. doi: 10.1016/j.semnephrol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. Fgf-23 is a potent regulator of vitamin d metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 20.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 21.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus M, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 22.Mizobuchi M, Nakamura H, Tokumoto M, Finch J, Morrissey J, Liapis H, Slatopolsky E. Myocardial effects of vdr activators in renal failure. J Steroid Biochem Mol Biol. 2010;121:188–192. doi: 10.1016/j.jsbmb.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, Haussler MR, Kerr Whitfield G. Vitamin d receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414:557–562. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 25.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y. Decreased renal alpha-klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81:539–547. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 26.Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, Iwamoto N, Kurumatani N, Iwano M, Nabeshima Y, Konishi N, Saito Y. Reduced renal alpha-klotho expression in ckd patients and its effect on renal phosphate handling and vitamin d metabolism. PloS one. 2014;9:e86301. doi: 10.1371/journal.pone.0086301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Downregulation of the klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 28.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, Nagail R. Molecular cloning of rat klotho cdna: Markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 30.Sugiura H, Yoshida T, Mitobe M, Yoshida S, Shiohira S, Nitta K, Tsuchiya K. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via hsp-70. Nephrol Dial Transplant. 2010;25:60–68. doi: 10.1093/ndt/gfp451. [DOI] [PubMed] [Google Scholar]
- 31.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 32.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin d analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol. 2007;18:1796–1806. doi: 10.1681/ASN.2006091028. [DOI] [PubMed] [Google Scholar]
- 33.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K. 1,25-dihydroxyvitamin d3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol. 2004;286:F526–533. doi: 10.1152/ajprenal.00316.2003. [DOI] [PubMed] [Google Scholar]
- 34.Makibayashi K, Tatematsu M, Hirata M, Fukushima N, Kusano K, Ohashi S, Abe H, Kuze K, Fukatsu A, Kita T, Doi T. A vitamin d analog ameliorates glomerular injury on rat glomerulonephritis. Am J Pathol. 2001;158:1733–1741. doi: 10.1016/S0002-9440(10)64129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panichi V, Migliori M, Taccola D, Filippi C, De Nisco L, Giovannini L, Palla R, Tetta C, Camussi G. Effects of 1,25(oh)2d3 in experimental mesangial proliferative nephritis in rats. Kidney Int. 2001;60:87–95. doi: 10.1046/j.1523-1755.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E. Effect of 1,25 (oh)2 vitamin d3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998;53:1696–1705. doi: 10.1046/j.1523-1755.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 37.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 38.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG, Medical Directors of Dialysis Clinic I Mortality risk among hemodialysis patients receiving different vitamin d analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 39.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 40.Carrillo-Lopez N, Roman-Garcia P, Rodriguez-Rebollar A, Fernandez-Martin JL, Naves-Diaz M, Cannata-Andia JB. Indirect regulation of pth by estrogens may require fgf23. J Am Soc Nephrol. 2009;20:2009–2017. doi: 10.1681/ASN.2008121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–218. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 42.Hofman-Bang J, Martuseviciene G, Santini MA, Olgaard K, Lewin E. Increased parathyroid expression of klotho in uremic rats. Kidney Int. 2010;78:1119–1127. doi: 10.1038/ki.2010.215. [DOI] [PubMed] [Google Scholar]
- 43.Krajisnik T, Olauson H, Mirza MA, Hellman P, Akerstrom G, Westin G, Larsson TE, Bjorklund P. Parathyroid klotho and fgf-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 2010;78:1024–1032. doi: 10.1038/ki.2010.260. [DOI] [PubMed] [Google Scholar]
- 44.Bjorklund P, Krajisnik T, Akerstrom G, Westin G, Larsson TE. Type i membrane klotho expression is decreased and inversely correlated to serum calcium in primary hyperparathyroidism. J Clin Endocrinol Metab. 2008;93:4152–4157. doi: 10.1210/jc.2008-0564. [DOI] [PubMed] [Google Scholar]
- 45.Latus J, Lehmann R, Roesel M, Fritz P, Braun N, Ulmer C, Steurer W, Biegger D, Ott G, Dippon J, Alscher MD, Kimmel M. Involvement of alpha-klotho, fibroblast growth factor-, vitamin-d- and calcium-sensing receptor in 53 patients with primary hyperparathyroidism. Endocrine. 2013;44:255–263. doi: 10.1007/s12020-013-9881-6. [DOI] [PubMed] [Google Scholar]
- 46.Ohkido I, Yokoyama K, Imura A, Utsunomiya Y, Hosoya T, Nabeshima Y. Persistent alpha-klotho (a-kl) expression in the parathyroid glands of patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2010;25:1007–1008. doi: 10.1093/ndt/gfp743. author reply 1008–1009. [DOI] [PubMed] [Google Scholar]
- 47.Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K, Tsugawa N, Okano T, Kitazawa R, Fukagawa M, Kita T. Depressed expression of klotho and fgf receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–238. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 48.Latus J, Lehmann R, Roesel M, Fritz P, Braun N, Ulmer C, Steurer W, Biegger D, Ott G, Dippon J, Alscher MD, Kimmel M. Analysis of alpha-klotho, fibroblast growth factor-, vitamin-d and calcium-sensing receptor in 70 patients with secondary hyperparathyroidism. Kidney Blood Press Res. 2013;37:84–94. doi: 10.1159/000343403. [DOI] [PubMed] [Google Scholar]
- 49.Ritter CS, Haughey BH, Armbrecht HJ, Brown AJ. Distribution and regulation of the 25-hydroxyvitamin d3 1alpha-hydroxylase in human parathyroid glands. J Steroid Biochem Mol Biol. 2012;130:73–80. doi: 10.1016/j.jsbmb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Ritter CS, Haughey BH, Miller B, Brown AJ. Differential gene expression by oxyphil and chief cells of human parathyroid glands. J Clin Endocrinol Metab. 2012;97:E1499–1505. doi: 10.1210/jc.2011-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boquist L. Occurrence of oxyphil cells in suppressed parathyroid glands. Cell Tissue Res. 1975;163:465–470. doi: 10.1007/BF00218492. [DOI] [PubMed] [Google Scholar]
- 52.Barai SR, Suryawanshi SA, Pandey AK. Responses of parathyroid gland, c cells, and plasma calcium and inorganic phosphate levels in rat to sub-lethal heroin administration. Journal of environmental biology / Academy of Environmental Biology, India. 2009;30:917–922. [PubMed] [Google Scholar]
- 53.Rangoonwala SP, Suryawanshi SA, Pandey AK. Responses of serum calcium and inorganic phosphate levels as well as parathyroid gland and calcitonin producing c cells of rattus norvegicus to mipcin administration. Journal of environmental biology / Academy of Environmental Biology, India. 2007;28:475–481. [PubMed] [Google Scholar]
- 54.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 55.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the united states medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 56.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 57.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 58.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 59.Wang AY, Wang M, Woo J, Lam CW, Li PK, Lui SF, Sanderson JE. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: A prospective study. J Am Soc Nephrol. 2003;14:159–168. doi: 10.1097/01.asn.0000038685.95946.83. [DOI] [PubMed] [Google Scholar]
- 60.van Venrooij NA, Pereira RC, Tintut Y, Fishbein MC, Tumber N, Demer LL, Salusky IB, Wesseling-Perry K. Fgf23 protein expression in coronary arteries is associated with impaired kidney function. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gft523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cozzolino M, Mehmeti F, Ciceri P, Volpi E, Stucchi A, Brenna I, Cusi D. The effect of paricalcitol on vascular calcification and cardiovascular disease in uremia: Beyond pth control. International journal of nephrology. 2011;2011:269060. doi: 10.4061/2011/269060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: A progenitor cell niche for the vessel wall. Cells, tissues, organs. 2012;195:73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heistad DD, Marcus ML, Larsen GE, Armstrong ML. Role of vasa vasorum in nourishment of the aortic wall. Am J Physiol. 1981;240:H781–787. doi: 10.1152/ajpheart.1981.240.5.H781. [DOI] [PubMed] [Google Scholar]
- 64.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in apoe-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: From innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 66.Ballanti P, Silvestrini G, Pisano S, De Paolis P, Di Giulio S, Mantella D, Iappelli M, Favaro A, Bonucci E, Coen G. Medial artery calcification of uremic patients: A histological, histochemical and ultrastructural study. Histol Histopathol. 2011;26:191–200. doi: 10.14670/HH-26.191. [DOI] [PubMed] [Google Scholar]
- 67.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: An early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 69.Farinelli P, Arango-Gonzalez B, Volkl J, Alesutan I, Lang F, Zrenner E, Paquet-Durand F, Ekstrom PA. Retinitis pigmentosa: Over-expression of anti-ageing protein klotho in degenerating photoreceptors. J Neurochem. 2013;127:868–879. doi: 10.1111/jnc.12353. [DOI] [PubMed] [Google Scholar]
- 70.Xie H, Liu L, Shi W, Xiao X, Tian L, Jian D, Chen X, Li J. Down regulation of cd147 boosts the premature senescence in human skin fibroblasts by destroying the redox balance and inhibiting klotho. J Dermatol Sci. 2011;64:243–245. doi: 10.1016/j.jdermsci.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 71.Pasztoi M, Nagy G, Geher P, Lakatos T, Toth K, Wellinger K, Pocza P, Gyorgy B, Holub MC, Kittel A, Paloczy K, Mazan M, Nyirkos P, Falus A, Buzas EI. Gene expression and activity of cartilage degrading glycosidases in human rheumatoid arthritis and osteoarthritis synovial fibroblasts. Arthritis research & therapy. 2009;11:R68. doi: 10.1186/ar2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Oliveira RM. Klotho rnai induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS letters. 2006;580:5753–5758. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 73.Gabbiani G, Schmid E, Winter S, Chaponnier C, de Ckhastonay C, Vandekerckhove J, Weber K, Franke WW. Vascular smooth muscle cells differ from other smooth muscle cells: Predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981;78:298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]


