SUMMARY
Coordination of stem cell activity with inflammatory responses is critical for regeneration and homeostasis of barrier epithelia. The temporal sequence of cell interactions during injury-induced regeneration is only beginning to be understood. Here we show that intestinal stem cells (ISCs) are regulated by macrophage-like hemocytes during the early phase of regenerative responses of the Drosophila intestinal epithelium. Upon tissue damage, hemocytes are recruited to the intestine and secrete the TGFβ/BMP homologue Dpp, inducing ISC proliferation by activating the Type I receptor Saxophone and the Smad homologue Smox. Activated ISCs then switch their response to Dpp by inducing expression of Thickveins, a second Type I receptor that has previously been shown to re-establish ISC quiescence by activating Mad. The interaction between hemocytes and ISCs promotes infection resistance, but also contributes to the development of intestinal dysplasia in aging flies. We propose that similar interactions influence pathologies like inflammatory bowel disease and colorectal cancer in humans.
Keywords: Drosophila, intestinal stem cells, regeneration, Dpp signaling
INTRODUCTION
The intestinal epithelium constitutes a regenerative, permeable barrier that interacts with commensal and pathogenic microbes and responds to environmental toxins and to physical stress while allowing selective nutrient resorption1-4. To maintain homeostasis, it is critical that regenerative activity is precisely coordinated with innate immune responses. How this coordination is achieved, however, remains unclear. The Drosophila intestinal epithelium is a powerful model to study epithelial immunity, damage responses and regeneration5. It mounts innate immune responses to control commensal and pathogenic microorganisms and is regenerated by a population of intestinal stem cells (ISCs) that give rise to both enterocytes (ECs) and enteroendocrine cells (EEs)2,5-8.
ISCs exhibit strong proliferative plasticity in the wake of damaging environmental challenges5,9. Regenerative responses are controlled by local and paracrine signals, derived either from damaged enterocytes (ECs) or from the surrounding visceral muscle, that activate a host of pro-proliferative signaling pathways in ISCs5,9. EC-derived interleukin 6-like cytokines called Unpaireds (Upd1-3) promote ISC proliferation either directly by activating JAK/Stat signaling in ISCs, or indirectly by inducing expression of EGF Receptor (EGFR) ligands, such as vein, in the visceral muscle, which then activate EGFR signaling in ISCs9-16. Proliferative activity of ISCs is further controlled by Wingless signaling, Jun-N-terminal Kinase, and insulin signaling, among other signals2,9,17.
The BMP homologue Decapentaplegic (Dpp) plays recurrent and complex roles in the regulation of ISCs18-22. Under homeostatic conditions, Dpp signaling is required for the differentiation of acid-secreting Copper cells in the middle midgut region18,20, while in response to tissue damage, Dpp secreted from visceral muscle engages the type I receptor Thickveins (Tkv) and the Drosophila Smad protein Mad to promote ISC return to quiescence20. In contrast, a second BMP type I receptor, Saxophone (Sax), is required to induce proliferation21. The relationship between Tkv and Sax-mediated regulation of ISC proliferation remains unclear, as does the relevant source of Dpp19-22.
Work in vertebrates has implicated immune cells in the induction of mitotic activity and regeneration in intestinal epithelia23,24,25. In Drosophila, macrophage-like hemocytes constitute a major population of blood cells26 that mediate infection responses and tissue repair27, yet if and how these cells influence regeneration in the intestinal epithelium has not been addressed.
Here we identify a role for Dpp derived from gut-associated hemocytes in the regulation of ISC proliferation. We find that Dpp secreted from these hemocytes induces ISC proliferation through Sax-mediated activation of Smad on X (Smox) in the initial response to Erwinia Carotovora Carotovora 15 (Ecc1513). In the later phase of the regenerative response, ISCs express Tkv, diverting the Dpp signal from Sax/Smox to Mad, restoring ISC quiescence. Differential expression of Sax and Tkv thus allows selective responses of ISCs to Dpp at different timepoints during a regenerative episode. The regulation of ISC proliferation by hemocyte-derived Dpp is critical for host survival during acute intestinal infection, but it also contributes to the development of intestinal dysplasia and the loss of intestinal barrier function in aging flies. Our findings have important implications for our understanding of regenerative processes in barrier epithelia.
RESULTS
Gut-associated hemocytes are required to induce ISC proliferation
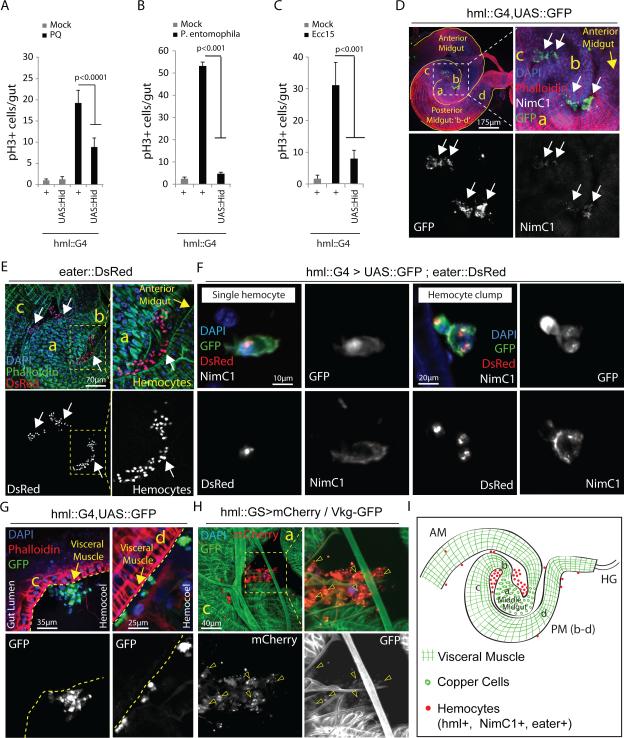
In order to test whether intestinal regeneration in flies is influenced by hemocytes, we expressed pro-apoptotic genes (head involution defective, hid, or reaper, rpr) in developing hemocytes at mid-larval stages, using Gal4 driven by the HmlΔ promoter (Supplementary Fig.1A,B). HmlΔ is active in larval and adult hemocytes28 and in motile extra-epithelial phagocytic cells in the proventriculus and the hindgut29, but is not expressed in the intestinal epithelium (Supplementary Fig.1C) or other adult tissues (Supplementary Fig. 1D,E). The resulting ‘hemoless’ flies emerge as widely normal adults and are viable and reproductively competent, but are sensitive to systemic infection28 (Supplementary Fig. 1B,F). At young ages, the size, structure and cellular composition of the intestinal epithelium of these animals are indistinguishable from wild-type (Supplementary Fig. 1G,H). However, the mitotic response of ISCs to acute intestinal damage by oxidative stress (induced by Paraquat) or infection (by the enteropathogens Ecc1513,15 or Pseudomonas entomophila (PE)10) is impaired (Fig.1A-C: ISCs are the only dividing cells in the intestinal epithelium, quantification of phospho-Histone H3 positive cells is thus commonly used to assess ISC proliferation; the number of Delta+ ISCs remains unchanged; Supplementary Fig.1I). The same effect is observed when hemocytes are ablated specifically in adults (Supplementary Fig.2A).
Figure 1. Gut-associated hemocytes are required for ISC proliferation.
(A-C) Mitotic activity of ISCs (identified by phospho-HistoneH3-positive cells) in intestines of 3 day-old hemoless flies exposed to Paraquat: PQ (A), Pseudomonas entomophila (B) or Ecc15 (C) is compared with wild-type controls.
(D,E) Hemocytes associated with the fly gut under normal rearing conditions can be detected using multiple hemocyte markers: HmlΔ::Gal4 driver and NimC1 (D; white arrows) or eater::DsRed reporter (E; white arrows), while arbitrary midgut regions are identified morphologically using Phalloidin staining that marks Actin (D,E; also see Supplementary Fig.2G).
(F) Hemocytes attach fly intestine as single cells or clumps of irregular shape and express all three hemocyte markers: hml (Hemolectin), eater and NimC1 (Nimrod). Note that hemocyte reporter nuclear eaterDsRed provides efficient quantification for actual number of hemocytes per clump.
(G) Single hemocytes or clumps (white arrows) attach midgut at the outer surface of visceral muscle (marked by Phalloidin staining; yellow arrows); where c and d: posterior midgut regions c and d.
(H) Type IV collagen encoded by Viking (detected as a fused protein with GFP) appears associated with gut-associated hemocytes (arrowheads) at region c of posterior midgut; ‘a’ indicates middle midgut region.
(I) Cartoon showing morphological features of arbitrarily assigned midgut regions (also see Supplementary Fig.2G) where hemocytes usually attach the fly intestine under homeostatic conditions. hml: hemolectin, NimC1: Nimrod, AM: anterior midgut, PM (b-c): posterior midgut regions b-c, HG: hindgut; while ‘a’ identifies middle midgut containing large Copper Cells.
Error bars indicate s.e.m. (A-C: n=12 flies from a representative of 3 independent experiments), p values from Student Ttest. One representative image from 15 flies tested in a single experiment, which was repeated 3 times, is shown in panels D-H.
We tested whether hemocytes interact with the intestinal epithelium, and found hemocytes in the abdominal cavity concentrated in large unstructured aggregates, located within the folds of the midgut (Fig.1D,E,I). Occasionally, individual hemocytes are also seen attached to the intestinal epithelium (Fig.1F,G). Gut-associated immune cells express various hemocyte-specific markers such as eater and Nimrod (NimC1), which identify plasmatocytes, phagocytic cells with similarity to mammalian monocytes and macrophages30,31 (Fig.1F). These cells are closely associated with the intestinal basement membrane (visualized using the BM-specific type IV collagen encoded by Viking11,32 ; Fig.1G,H).
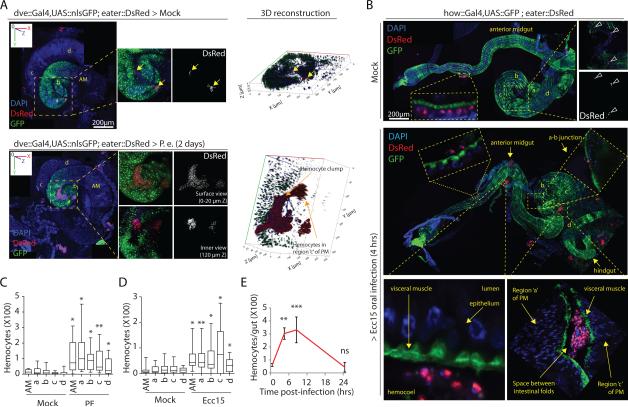
Upon oral infection with PE or Ecc15, hemocytes (expressing GFP under the control of the HmlΔ33 or DsRed under the control of the Eater promoter30) are increasingly found attached to the visceral muscle surrounding the intestinal epithelium throughout the midgut (Fig.2A-D, Supplementary Fig.2B,C, Supplementary Fig.8A; also refer to Figs.1I,2B and Supplementary Fig.3G for identification of arbitrarily assigned morphologically distinguishable midgut regions). Infection also leads to a significant increase in the size of hemocyte clumps located in folds of the middle and posterior midgut (Fig.2A,B and Supplementary Fig.2B). This increased association of hemocytes with the gut is transient, as 24 hours after an infection the number of hemocytes attached to the intestine declines (Fig.2E). We used Mosaic Analysis with a Repressible Cell Marker (MARCM34) to test whether the increased numbers of gut-associated hemocytes result from a proliferative response of hemocytes, but did not find any eater::DsRed+ hemocytes that expressed GFP (as would be expected if mitotic hemocytes or precursors would generate MARCM clones) in any tissue during or after Ecc15 challenge (Supplementary Fig.2E,F). Instead, single hemocytes isolated from hemolymph of Ecc15 challenged flies were bigger in size and more oval in shape than hemocytes from controls, suggesting that circulating hemocytes are activated and change their morphology during intestinal stress (Supplementary Fig.2D).
Figure 2. Hemocytes dynamically recruit to the gut during stress.
(A) Large hemocyte clumps are observed associated with an un-stretched P. entomophila infected, but not mock treated, intestines (also see Supplementary Fig. 2G for dve::Gal4 expression pattern in midgut regions). 3D images are generated using Zeiss LSM700 confocal microscope platform (zeiss.com) to show extent of penetration of hemocyte clumps into midgut folds. AM: anterior midgut, a: middle midgut, and b-d: posterior midgut regions b-d.
(B) Hemocyte recruitment along anterior-to-posterior (AP) axis in partially stretched guts is shown after mock treatment or 4 hours of Ecc15 challenge. Note that eaterDsRed+ hemocytes either attach to the external surface of intestine or appear trapped within midgut folds (see fold between regions a and c) or can be occasionally detected penetrating visceral muscle layer (see a-b junction) in Ecc15-infected flies.
(C-D) EaterDsRed+ hemocytes found in the vicinity of different regions of un-stretched midguts following oral challenge with PE (C; 2 days after infection) or Ecc15 (D; 4 hours after infection) are quantified and compared with those under mock treated conditions. Note that these quantitative studies on region-specific hemocyte recruitment are performed on un-stretched intestines, as stretching of the gastrointestinal tube along the AP axis results in dissociation of hemocytes recruited to the midgut folds (also see B).
(E) Quantification of total eaterDsRed+ hemocytes recruited per midgut during the course of Ecc15 infection episode of 24 hours is shown.
Error bars indicate range (C: n=22 and D: n=15 flies; where boxes show 25-75% percentile and horizontal bar within each box is population median) or s.e.m. (E: n=15 flies) from a representative of 3 experiments), p values from Student Ttest. One representative image from 15 flies tested in a single experiment (replicated 3 times) is shown in panels A and B. ns=non-significant, *p<0.05, **p<0.01, **p<0.001.
Hemocyte-derived Dpp is required for ISC proliferative response
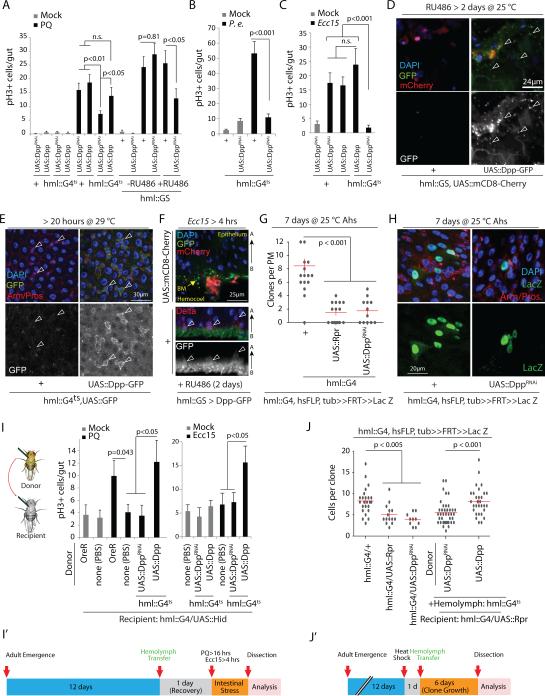
Since these data suggested that local or paracrine signals from hemocytes might modulate ISC function, we performed an RNAi screen targeting putative secreted factors in hemocytes, and identified Decapentaplegic (Dpp) as required in hemocytes to induce ISC proliferation upon oxidative stress (Fig.3A and Supplementary Fig.3A) and pathogen exposure (Fig.3B,C). Knockdown of Dpp in adult hemocytes only (using either a temperature sensitive combination of HmlΔ::Gal4 with tub::Gal80ts, or an RU486-inducible system based on the same promoter, HmlΔ::GS) was sufficient to limit ISC responses (Fig.3A-C and Supplementary Fig.3B). HmlΔ::GS recapitulates the expression profile of HmlΔ::Gal4 in larvae and adults (Supplementary Fig.3F). Knockdown of Dpp was also sufficient to inhibit ISC responses to Ecc15 infection when driven by a separate hemocyte driver, He::Gal435, but not when driven by either a hindgut driver (byn::Gal4) or a proventriculus driver (cardia::Gal4), confirming that the loss of ISC proliferation in Hml::Gal4, UAS::DppRNAi flies is due to knockdown of Dpp specifically in hemocytes and not due to unspecific knockdown in intestinal cells (Supplementary Fig.3C). Accordingly, knockdown of Dpp in ECs (NP1::Gal4), ISCs and EBs (esg::Gal4), trachea (btl::Gal4), or visceral muscle (how::Gal4) did not impact ISC responses to Paraquat (Supplementary Fig.3D). The two DppRNAi lines used in these analyses efficiently reduce Dpp mRNA levels in hemocytes or visceral muscle when knocked down using HmlΔ::Gal4 or how::Gal4, respectively (Supplementary Fig.3E).
Figure 3. Hemocye-derived Dpp acts on ISCs to promote proliferation.
(A-C) ISC proliferation induced by Paraquat (PQ) treatment (A) or bacterial infection (B,C) is significantly reduced when Dpp is knocked down specifically in adult hemocytes either using HmlΔ::Gal4 combined with tub::Gal80ts (hmlG4ts) (A-C) or Mifepristone (RU486) - sensitive hml::GS (A) and a DppRNAi line (Bloomington Stock # 33618). Acute Dpp overexpression in hemocytes neither induces ISC proliferation in the absence of stress (A) nor enhances ISC mitotic activity during tissue damage (A,C). P.e.: P. entomophila.
(D,E) Dpp-GFP fusion protein expressed in adult hemocytes is detected (using anti-GFP antibody; see methods) on the intestinal surface (D: arrowheads) and on ISC/EB doublets identified by their basal location and strong Armadillo expression (E: arrowheads).
(F) During Ecc15 challenge, Dpp-GFP fusion protein expressed in hemoyctes can be seen accumulated at the basal membrane with specific binding to Delta+ ISCs.
(G-J) Lineage tracing from ISCs using FRT recombination of a split Actin-lacZ transgene shows reduced clone number (G) and clone size (H; quantified in J) in hemoless and HDD animals. This defect in clone formation and reduced ISC proliferative response observed in hemoless flies following PQ treatment or Ecc15 infection is rescued upon hemolymph transfer from wild-type flies (I) or from flies overexpressing Dpp specifically in hemocytes, but not upon transfer of DppRNAi-expressing hemocytes (I, J), and experimental timeline is shown in panels I’ and J’. Ahs: after heatshock.
Error bars indicate s.e.m. (A-C: n=10; G: n=12; I (for PQ treatment): Mock: OreR, n=17; none(PBS), n=16; and PQ: OreR, n=17; none(PBS), n=36; UAS::DppRNAi, n=13; UAS::Dpp, n=16; I (for Ecc15 treatment): Mock and Ecc15: n=10; J: n=9 flies; sufficient samples sizes for pH3 analyses in fly gut11,13), p values from Student Ttest. Data shown in panels A-C, G and J are representative of 3 independently performed experiments, while that shown in panel I is a composite from 2 separate experiments.
One representative image from 9 flies tested in a single experiment (repeated 3 times independently) is shown in panels D-F and H, .
We did not observe any differences in number and distribution of Dpp-deficient hemocytes compared to wild-type flies (Supplementary Fig. 3G). Furthermore, Hemocyte-derived Dpp Deficient (HDD) flies are immune-competent to systemic PE infection and recruitment of hemocytes to the intestinal epithelium was still observed in HDD flies (Supplementary Fig.1F and Supplementary Fig.3H).
Dpp is expressed in hemocytes of adult flies, is induced in response to infection, and can modulate the innate immune response36. Recapitulating these observations, we found that Paraquat exposure or Ecc15 infection induced Dpp in hemocytes (Supplementary Fig.3I,J). To assess if hemocyte-derived Dpp signals directly to ISCs, we monitored a Dpp-GFP fusion protein37 expressed specifically in hemocytes (using HmlΔ::GS and HmlΔ::Gal4). Dpp-GFP secreted by gut-associated hemocytes is detected on the intestinal surface (Fig.3D). Dpp-GFP (but not GFP when expressed alone from HmlΔ::Gal4) was also detected within the gut epithelium on diploid progenitor cell nests (basally located cells with strong Armadillo expression, representing doublets of ISCs and EBs) (Fig.3E). Following Ecc15 challenge, accumulation of hemocyte-secreted Dpp-GFP was observed at the basement membrane, specifically close to Delta+ cells in the intestinal epithelium, suggesting that hemocyte-secreted Dpp can penetrate the visceral muscle layer to bind to ISCs (Fig.3F and Supplementary Fig.3K).
Hemocyte-derived Dpp maintains basal stem cell activity
We asked whether Dpp from hemocytes might not only influence acute infection responses, but also modulate basal ISC activity. We used a split-lacZ reconstitution assay to label selected ISCs heritably38, and found that hemocyte-derived Dpp is required for induction (clone numbers per gut) and growth (cells per clone) of ISC-derived cell clones (Fig.3G,H,J and Supplementary Fig.3L). We did not observe signs of apoptosis (using Apoliner39) in flies that co-express DppRNAi directly under the control of the hmlΔ promoter (hmlΔ::DppRNAi), confirming that the reduced clone size in HDD flies is due to decreased ISC proliferation (Supplementary Fig.3M).
Hemocyte transplantation rescues ISC proliferation in hemoless and HDD flies
When wild-type or Dpp over-expressing hemocytes were transplanted into hemoless flies at 12 days of age, the proliferative response of ISCs to Paraquat treatment and Ecc15 infection, as well as clone growth recovered (Fig.3I,J; we used 12 day-old flies as donors, as we found that a substantial number of hemocytes could only be transferred when donor flies were older than 3 days, Supplementary Fig.3N; transplanted hemocytes associated with the intestine, Supplementary Fig.3O). Reduced ISC proliferation in hemoless or HDD flies is thus not caused by unspecific or unidentified defects, but by the lack of hemocyte-derived factors. Accordingly, transplantation of Dpp-deficient hemocytes (from donors expressing DppRNAi under the control of HmlΔ::Gal4) failed to rescue ISC proliferation in hemoless flies (Fig.3I,J).
Hemocyte-derived Dpp acts in parallel to Jak/Stat and EGFR pathways
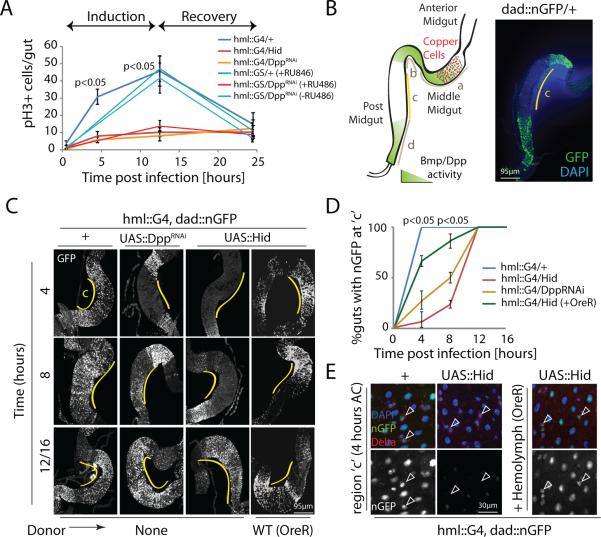
The positive role of hemocyte-derived Dpp in regulating ISC proliferation is in contrast to the established inhibition of ISC proliferation by Tkv and Mad20,22. To explain this paradox, we sought to temporally resolve the response of ISCs to Dpp signaling. ISCs respond to Ecc15 infection by an early burst of proliferative activity (‘induction phase’: 4 to 12 hours post-infection) followed by a return to quiescence when the infection is resolved (‘recovery phase’: 16 to 24 hours post-infection)13 (Fig.4A). Hemoless and HDD flies showed a significantly reduced proliferative response in the inductive phase (Fig.4A).
Figure 4. Hemocyte-derived Dpp activates BMP signaling specifically within ISCs during proliferation.
(A) Temporal dynamics of ISC mitotic activity measured as frequency of phospho-histone H3+ (pH3+) cells per gut are shown upon oral infection with Ecc15, with or without Dpp knockdown in hemocytes using hmlΔ::Gal4 or hmlΔ::GS drivers.
(B) Homeostatic expression of dad::nGFP in posterior midgut regions of wild-type animals is shown (compare with18).
(C-E) Expression of dad::nGFP is rapidly induced at region c of midgut in wild-type animals as early as 4 hours post-Ecc15 challenge (C,D) in all epithelial cells including Delta+ ISCs (E), which is delayed in HDD and hemoless flies and is rescued by transplantation of hemolymph from wild-type flies prior to Ecc15 challenge (C-E).
Error bars indicate s.e.m. (A: n=10; D: n=12 flies; while data shown are from one experiment which was repeated three times), p values from Student Ttest. One representative image from 7 flies tested in a single experiment is shown in panels B,C and E. Experiment was repeated 3 times.
The activation of JAK/Stat and EGFR pathways in guts of infected hemoless and HDD flies is normal (Supplementary Fig.4A-C), suggesting that Dpp from hemocytes is required for ISC proliferation in parallel to the JAK/Stat/EGFR signal. Accordingly, knockdown of hemocyte-derived Dpp also limits the induction of ISC proliferation by Upd2 over-expression in ECs (Supplementary Fig.4D), while acute over-expression of Dpp in hemocytes or in visceral muscle is not sufficient to induce ISC proliferation in the absence of injury (Supplementary Fig.4E), nor shows an additive effect on ISC activity upon tissue damage (Fig. 3A,C). Long-term Dpp over-expression in hemocytes, however, increases proliferation of ISCs both in the anterior and posterior midgut (Supplementary Fig.4F). ISCs thus integrate signals from hemocyte-derived Dpp and EC/muscle-derived Upd/Vein ligands, and require inputs from all three pathways to achieve appropriate proliferative responses to damage. No differences in feeding were observed between wild-type, hemoless and HDD flies, with or without infection (Supplementary Fig.4G,H).
Activation of Dpp signaling in ISCs during inductive phase
To assess if hemocyte-derived Dpp would influence Dpp signaling directly in ISCs, we used the Dpp pathway activity reporter dad::nGFP40. Under basal conditions, this reporter describes two opposing Dpp signaling gradients in the posterior midgut18, with high activity in the distal borders to the gastric region and the hindgut (regions b and d in Fig.4B), and weak reporter activity in the central region ‘c’ (Fig.4B)18. Although hemocyte-derived Dpp induces ISC proliferation throughout the anterior and posterior midgut (Supplementary Fig.4F and Supplementary Fig.5A), we focused our further observations on region ‘c’ of the posterior midgut because of the high concentration of hemocytes present (Fig.1D,E,H,I and Fig.2B,D) and the low levels of basal dad::nGFP in this region (Fig. 4B). During the inductive phase after challenge with Ecc15, dad::nGFP activity increases in region ‘c’ (in both diploid and polyploid cells, including Delta-positive ISCs) in wild-type flies as early as 4 hours after challenge (AC) (Fig.4C-E). This induction was absent in hemoless and HDD flies, and could be restored by hemolymph transfer from wild-type animals (Fig.4C-E). Later induction of Dpp signaling activity in this region (12-16 hours AC), however, was not affected (Fig.4C), suggesting that secretion of Dpp from the visceral muscle, which is observed at 24 hours after challenge20 (Supplementary Fig.5B), is not influenced by hemocytes.
Nuclear translocation of Smox requires hemocyte-derived Dpp
Dpp signals through Thickveins (Tkv) to phosphorylate Mad41. Unexpectedly, Mad phosphorylation (pMad) was not observed in ISCs in region ‘c’ of wild-type flies at 4 hours AC (Supplementary Fig.5C), a stage where ISCs are mitotically active (Fig.4A), but was high in ISCs at 16 hours AC, ruling out a role of Mad activity in the induction of proliferation, and suggesting that phosphorylation of Mad might be limited to the recovery period to promote return of ISCs to a quiescent state (consistent with20). We therefore tested whether another Mad-like nuclear factor, Smox (also called dSmad2)42, may act to regulate the proliferative response of ISCs. A genomic rescue construct encoding a Smox-FLAG fusion protein43 shows high expression specifically in ISCs and EBs of the PM (Fig.5A). Smox-FLAG is localized in the cytoplasm of these cells under homeostatic conditions, yet after 4 hours of Ecc15 infection, nuclear translocation of Smox is observed, and this translocation is dependent on hemocyte-derived Dpp (Fig.5A,B). Consistent with these observations, knockdown of Smox in ISCs prevents induction of dad::nGFP specifically in Delta+ ISCs, but not in other cells, in region ‘c’ of the PM at 4 hours after Ecc15 challenge, indicating that dad is a transcriptional target of Smox in ISCs (Supplementary Fig.5E).
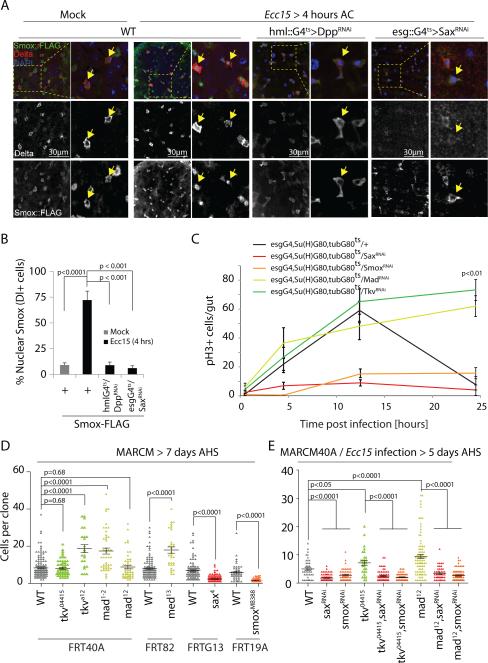
Figure 5. ISC proliferation induced by hemocyte-derived Dpp through Sax/Smox signaling is antagonized by Tkv/Mad signaling.
(A,B) Smox-FLAG is detected in the cytoplasm of Delta+ cells under homeostatic conditions but is rapidly translocated into the nucleus upon Ecc15 infection as early as 4 hours after challenge (AC). This nuclear translocation of Smox::FLAG is significantly prevented in HDD flies and in flies where Sax is knocked down specifically in ISCs (A and B).
(C) ISC-specific knockdown of Sax or Smox inhibits induction of proliferation while that of Tkv or Mad prevents restoration of quiescence in the fly intestine orally infected by Ecc15.
(D) Sax or Smox mutant clones grow smaller in size (measured as number of cells per clone) while Tkv, Med and Mad mutant clones either grow bigger or remain comparable to wild-type controls under unstressed conditions.
(E) Sax or Smox knock down rescues over-growth of Tkv and Mad mutant MARCM following Ecc15 infection.
Error bars indicate s.e.m. (B, n=7; C: n=15 flies; and D: (WTFRT40A, n=132; tkv04415, n=60; tkva12, n=25; mad1-2, n=46; mad12, n=52, WTFRT82, n=105; med13, n=33; WTFRTG13, n=97; sax4, n=116; WTFRT19A, n=46; smoxMB388, n=57); E: (WTFRT40A, n=40; saxRNAi, n=86; smoxRNAi, n=55; tkv04415, n=28; tkv04415,saxRNAi, n=64; tkv04415,smoxRNAi, n=63; mad12, n=90; mad12,saxRNAi, n=47; mad12,smoxRNAi, n=79 mitotic clones tested from 12 flies (sufficient sample size for MARCM analyses in fly gut 11); and each experiment is a representative of three (for panels B-D) or two (for panel E) independent experiments, p values from Student Ttest. One representative image from 10 flies is shown in panels A, and experiment was repeated 3 times independently.
Regulation of Smox activity by hemocyte-derived Dpp in ISCs thus precedes the increase in Mad phosphorylation during an infection, suggesting that Smox may mediate the pro-proliferative effects of hemocyte-derived Dpp. dActivin and Dawdle, TGFβ/Bmp family ligands that have been implicated in Smox regulation in other contexts44,45, are not likely to be required for Smox activation in ISCs, since ubiquitous knockdown of these ligands does not inhibit infection-induced proliferation (Supplementary Fig.5D).
Tkv/Mad signaling antagonizes ISC activity induced upon Sax/Smox pathway stimulation
To gain insight into the selective activation of Smox and Mad by Dpp after an infection, we explored the role of individual Type I receptors. Smox is engaged by the Type I receptor Saxophone, and we found indeed that Sax is required for Smox nuclear translocation after Ecc15 treatment (Fig.5A, B). Sax can be activated both by Dpp46 and Glass Bottom Boat (Gbb)47. Consistent with recent reports, we detected a strong reduction in mitotic activity of ISCs at the ‘inductive’ phase of Ecc15 infection when Gbb was knocked down specifically in ECs, but not in hemocytes, suggesting that both Dpp and Gbb are required for ISC proliferation. However, the functional source for these ligands is different: hemocytes for Dpp and ECs for Gbb21,22 (Supplementary Fig.6A).
Upon binding to Dpp, Sax plays a complex role in regulating Mad activity in conjunction with Tkv46. To dissect the relative contributions of Sax/Smox and Tkv/Mad signaling in the control of ISC proliferation, we knocked down Sax and Smox, as well as Tkv and Mad, specifically in ISCs, and triggered regeneration by exposure to bacteria. Sax/Smox knockdown prevented induction of proliferation, while Tkv/Mad knockdown did not impact the inductive phase, but prevented recovery into quiescence (Fig.5C). These pathways do not seem to crosstalk in ISCs, as Tkv is required and sufficient to phosphorylate Mad in ISCs, while Sax is not (Supplementary Fig.6B,C).
These results provided a model for the dual role of Dpp in the regenerative response: initial engagement of Sax/Smox signaling by hemocyte-derived Dpp results in induction of ISC proliferation, while activation of Tkv/Mad signaling by muscle-derived Dpp (which is induced at later stages) directs recovery to the quiescent state (as previously described in20). To test this model, we performed lineage tracing and epistasis analysis. We used MARCM34 to trace lineages of ISCs mutant for various Dpp signaling components: Sax and Smox mutant ISCs generated smaller MARCM clones than wild-type controls (Fig.5D and Supplementary Fig. 6D), confirming that the basal activity of Sax/Smox signaling is required for normal ISC proliferation. Accordingly, loss of Smox in ISCs suppressed the growth of ISC tumors formed in Notch loss-of-function conditions7,8 (Supplementary Fig.6E). Tkv, Medea (Med, the Drosophila Smad4) and Mad mutant MARCM clones, on the other hand, either grew bigger or were similar to wild-type controls depending on the alleles tested (Fig.5D and Supplementary Fig. S6D). Upon Ecc15 infection, however, Tkv and Mad mutant ISCs consistently generated bigger clones than wild-type controls (Fig.5E, Supplementary Fig.6D), as observed in previous reports showing that Tkv/Mad signaling limits ISC activity following injury20. Loss of Sax or Smox suppressed ISC over-proliferation in Tkv or Mad mutant clones (Fig.5E, Supplementary Fig.6D), further supporting the notion that Sax/Smox signaling is an early event required to induce ISC proliferation, while Tkv/Mad signaling is a late event, required to ensure recovery to stem cell quiescence once the tissue has been repaired.
We further found that the induction of ISC proliferation by constitutively active EGFR or Hop (HopTumL) is significantly reduced upon Smox knockdown (Supplementary Fig.6F). We did not observe enhanced hemocyte recruitment upon constitutive expression of active EGFR in ISCs (Supplementary Fig.6G).
ISC response to Dpp is determined by the relative expression of Sax and Tkv receptors
The results described above are consistent with recent observations linking Sax to ISC proliferation, and Tkv to quiescence20-22, and suggest a model in which the temporal separation of Sax and Tkv-mediated signaling events dynamically controls ISC proliferation during a regenerative episode. To address how the same Dpp ligand could activate two different signaling pathways eliciting opposite proliferative responses within the same stem cells, we assessed the expression of Sax and Tkv in ISCs. We reasoned that the relative expression of these two Type I receptors in ISCs in the inductive and recovery phases might influence the response of ISCs to Dpp. Indeed, Sax, but not Tkv, protein was detected in ISCs under homeostatic conditions (Fig.6A and Supplementary Fig.7A,B). During Ecc15 infection, on the other hand, Sax expression did not change in ISCs, while Tkv expression was low during the inductive phase, but was induced in ISCs at later stages of the regenerative response (Fig.6B). Smox is mostly nuclear during the inductive phase, but translocates to the cytoplasm in the recovery phase, while Tkv induction coincides with Mad phosphorylation at the later stage (Fig.6C and Supplementary Fig.5C). Sax knockdown in ISCs does not prevent the induction of Tkv expression, indicating that Dpp/Sax/Smox signaling is not involved in Tkv regulation (Supplementary Fig.7C).
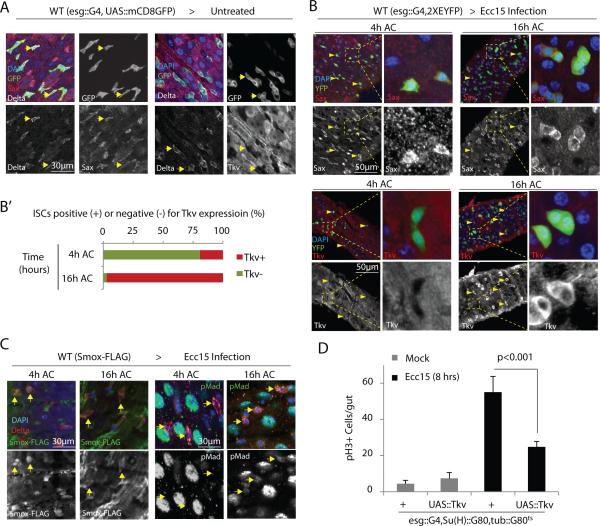
Figure 6. Relative expression of Sax and Tkv receptors determine Smox or Mad activation and proliferative response of ISCs.
(A,B) Sax is highly expressed in ISCs under unstressed conditions (A: arrows) as well as all tested stages of Ecc15 infection (B: arrowheads). Tkv expression remains low in ISCs under basal conditions (A: arrows) as well as at 4 hours of Ecc15 challenge (B: arrowheads) while high Tkv expression is detected in ISCs only at 16 hours after Ecc15 challenge (AC) (B: arrowheads). Panel B’ shows percentage ISCs showing high Tkv expression at 4 and 16 hours of Ecc15 infection.
(C) Nuclear localization of Smox detected in ISCs at 4 hours is lost upon 16 hours of infection, while phosphorylation of Mad is only detected at 16 hours, but not at 4 hours, of Ecc15 challenge (arrows).
(D) Overexpression of wild-type Tkv in ISCs significantly inhibits the proliferative response induced upon Ecc15 challenge.
Error bars in D indicate s.e.m. (data from n=7 flies tested in one experiment, p values from Student Ttest); while experiment was performed 3 times independently. One representative images from 7 flies used in each experiment is shown in panels A-C; while every experiment was reproduced three times independently.
When wild-type Tkv was over-expressed in ISCs 6 hours prior to the bacterial challenge, the induction of ISC proliferation was significantly reduced (Fig.6D), supporting the notion that the induction of Tkv expression in the recovery phase switches the ISC response to Dpp from inducing proliferation to reestablishing quiescence.
Hemocyte / ISC interactions in infection tolerance and epithelial homeostasis
To test the physiological consequences of the identified hemocyte / ISC interaction, we assessed host survival in animals orally infected with Pseudomonas entomophila (PE)10, an enteropathogen that induces tissue damage and strongly activates JAK/Stat signaling (Fig.7B). Flies lacking tissue regeneration rapidly succumbed to PE infection (Fig.7A), indicating that hemocyte-mediated tissue regeneration contributes to host survival.
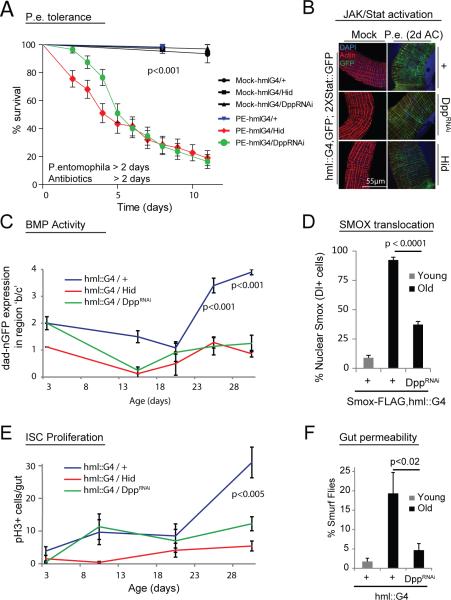
Figure 7. Hemocyte-derived Dpp promotes resistance to acute infection but leads to intestinal dysplasia and increased gut permeability during aging.
(A) Hemoless and HDD flies rapidly succumb to acute intestinal damage. Survival rates of flies were monitored after a prior feeding on PE or Mock for 2 days followed by 2 days antibiotics treatment.
B) Induction of STAT::GFP reporter expression in the gut of hemoless and HDD flies 2 days post PE infection is similar to wild-type controls.
(C-E) Expression of dad::nGFP both at regions ‘b’ and ‘c’ of the PM in shown in aging flies (C) (individual guts were given a score (0-4) for the strength of dad-nGFP gradient at regions b and c of PM: 0 = no GFP signal; 1 = short gradient at region b; 2 = normal gradient at region b; 3 = long gradient at region b that penetrates into region c; 4 = high and evenly expressed GFP signal throughout both regions b and c). D,E). Age-related induction of dad-nGFP expression correlates with enhanced nuclear translocation of Smox in Delta-positive ISCs located in the region ‘c’ (D; arrowheads) as well as with the increased ISC proliferation (E). All of these phenotypes are dependent on hemocyte-derived Dpp (C-E).
(F) HDD flies exhibit improved intestinal epithelial integrity.
p values from log rank test in panel A (calculated using Prism software). Other p values from Student's t-test. Error bars indicate s.e.m. (A: n=40; C: n=8; D: n=7; E: n=18; F: n=150 flies tested in one experiment; while each experiment was reproduced 3 (in panel E) or two times (in panels C,D and F)). One representative image from 10 flies is shown in panel B; and experiment was repeated twice.
In aging flies, ISCs over-proliferate, causing intestinal dysplasia and contributing to the age-related increase in mortality9,48,49. The number of hemocytes attached to the gut in the absence of infection is higher in old than in young flies (Supplementary Fig.8A), and hemocyte-derived Dpp promotes higher dad::nGFP expression in region ‘c’ of the PM in aging animals (Fig.7C, Supplementary Fig. 8B). Accordingly, an age-related increase in Smox nuclear localization, which correlates with increased ISC proliferation and dysplasia, is significantly reduced in HDD flies (Fig.7D,E and Supplementary Figs.1H and 8C,D). These flies have significantly reduced intestinal dysplasia and improved barrier function at 40 days of age (Fig.7F and Supplementary Fig.8D), while over-expression of Tkv in ISCs also reduces the age-related increase in ISC proliferation (Supplementary Fig.8E). The loss of barrier function has been associated with age-related morbidity and mortality50, yet we did not observe a change in lifespan of HDD flies as compared to wild-type controls (Supplementary Fig.8E). Hemoless flies are significantly short lived, likely due to the lack of the cellular immune response (Supplementary Fig.8E).
DISCUSSION
Our results extend the current model for the control of epithelial regeneration in the wake of acute infections in the Drosophila intestine (Fig. 8). We propose that the control of ISC proliferation by hemocyte-derived Dpp integrates with the previously described regulation of ISC proliferation by local signals from the epithelium and the visceral muscle, allowing precise temporal control of ISC proliferation in response to tissue damage, inflammation and infection.
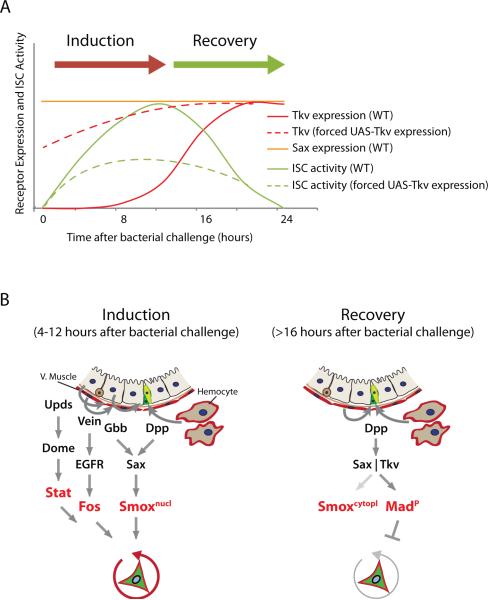
Figure 8. Model.
(A) Proposed relationship between Sax/Tkv expression and ISC proliferation during one regeneration episode.
(B) Model for the dynamic control of ISC activity by hemocyte- and muscle- derived Dpp during the regenerative response. Under basal conditions, Sax, but not Tkv, is expressed in ISCs. In response to infection or damage, hemocyte-derived Dpp and EC-derived Gbb signal through Sax to activate Smox, which requires active Stat and Fos to induce ISC proliferation (‘inductive phase’). Subsequent induction of Tkv expression in ISCs is required for the ‘recovery phase’, where hemocyte- and muscle-derived Dpp and EC-derived Gbb signal through Tkv and Sax to induce Mad signaling to restore stem cell quiescence.
The association of hemocytes with the intestine is extensive, and can be dynamically increased upon infection or damage. In this respect, our observations parallel the invasion of subepithelial layers of the vertebrate intestine by blood cells that induce proliferative responses of crypt stem cells during infection23. A role for macrophages and myeloid cells in promoting tissue repair and regeneration has been described in adult Salamanders51 and in mammals24,25, where TGFβ ligands secreted by these immune cells can inhibit ISC proliferation52,53, but can also contribute to tumor progression54. Our results provide a conceptual framework for immune cell / stem cell interactions in these contexts.
Integration of Dpp into the regenerative signaling network in flies
Our observation that Dpp/Sax/Smox signaling is required for Upd-induced proliferation of ISCs suggests that Sax/Smox signaling cooperates with JAK/Stat and EGFR signaling in the induction of ISC proliferation. Accordingly, while constitutive activation of EGFR/Ras or JAK/Stat signaling in ISCs is sufficient to promote ISC proliferation cell autonomously, we find that this partially depends on Smox. Even in these gain-of-function conditions, ISC proliferation can thus only be fully induced in the presence of basal Smox activity. Since short-term over-expression of Dpp in hemocytes does not induce ISC proliferation, we further propose that Dpp/Sax/Smox signaling can only activate ISCs when JAK/Stat and/or EGFR signaling are activated in parallel (Fig.8). However, long-term over-expression of Dpp in hemocytes results in increased ISC proliferation, suggesting that chronic activation of immune cells disrupts normal signaling mechanisms and results in ISC activation even in the absence of tissue damage.
BMP signal transduction in ISCs
BMP/TGFβ signaling pathways are critical for metazoan growth and development and have been well characterized in flies. Multiple ligands, receptors and transcription factors with highly context-dependent interactions and function have been described46,55-59. This complexity is reflected by the sometimes conflicting studies exploring Dpp/Tkv/Sax signaling in the adult intestine18-21. These studies consistently highlight two important aspects of BMP signaling in the adult Drosophila gut: 1) ISCs can undergo opposite proliferative responses to BMP signals, and 2) there are various sources of Dpp that differentially influence ISC function in specific conditions. By characterizing the temporal regulation of Bmp signaling activity in ISCs, our results resolve some of these conflicts: we propose that early in the regenerative response, hemocyte-derived Dpp triggers ISC proliferation by activating Sax/Smox signaling, while ISC quiescence is reestablished by muscle-derived Dpp as soon as Tkv becomes expressed (Fig.7). Of note, some of the conflicting conclusions described in the literature may have originated from problems with the genetic tools used in some studies (see Materials and Methods). In our study, we have used two independent RNAi lines (BL25782 and BL33618) that effectively decrease Dpp mRNA levels in hemocytes when expressed using hmlΔ::Gal4.
The close association of hemocytes with the type IV collagen Viking suggests that the stimulation of ISC proliferation by hemocyte-derived Dpp may also be controlled at the level of ligand availability, as suggested previously for Dpp from other sources21,60.
The regulation of Sax/Smox signaling by Dpp observed here is surprising, but consistent with earlier reports showing that Sax can respond to Dpp in certain contexts46,56. Biochemical studies have suggested that heterotetrameric complexes between the Type II receptor Punt and the Type I receptors Sax and Tkv can bind Dpp, while complexes with Tkv/Tkv homodimers preferentially bind Dpp, and complexes with Sax/Sax homodimers preferentially bind Gbb58. In the absence of Tkv, Sax has been proposed to sequester Gbb, shaping the Gbb activity gradient, but to fail to signal effectively46. Expression of Gbb in the midgut epithelium has recently been described, and ligand heterodimers between Gbb and Dpp are well established21,57. Consistent with earlier reports21,22, we find that Gbb knockdown in ECs significantly reduces ISC proliferation in response to infection. Complex interactions between hemocyte-derived Dpp, epithelial Gbb, and ISC-expressed Sax, Punt and Tkv thus likely shape the response of ISCs to damage, and will be an interesting area of further study.
Similar complexities exist in the regulation of transcription factors by Sax and Tkv. Canonically, Smox is regulated by Activin ligands (Activin, Dawdle, Myoglianin and maybe more), and the Type I receptor Baboon55. We have tested the role of Activin and Dawdle in ISC regulation, and, in contrast to Dpp, were unable to detect a requirement for these factors in the induction of ISC proliferation after Ecc15 infection. Furthermore, our data establish a requirement for hemocyte-derived Dpp as well as for Sax expression in ISCs in the nuclear translocation of Smox after a challenge. Our study thus indicates that in this context, Sax responds to Dpp and regulates Smox. Regulation of Smox by Sax has been described before61, yet Sax is also known to promote Mad phosphorylation, but only in the presence of Tkv46. Consistent with such observations, we have detected Mad phosphorylation in ISCs only in the late recovery phase upon bacterial infection, when Tkv is simultaneously induced in ISCs. During this recovery phase, ISCs maintain high Sax expression, but Smox nuclear localization is not detected anymore, suggesting that Sax cannot activate Smox in the presence of Tkv, and might actually divert signals towards Mad instead, as previously suggested46. Our data also suggest that Medea (the Drosophila Smad4 homologue) is not required for Smox activity. While surprising, this observation is consistent with recent reports that Smad proteins in mammals can translocate into the nucleus and activate target genes in a Smad4-independent manner62. The specific signaling readouts in ISCs when these cells are exposed to various Bmp ligands and are expressing different combinations of receptors are thus likely to be complex.
Hemocyte / ISC interactions, infection tolerance, and aging
Our findings demonstrate that the control of ISC proliferation by hemocyte-derived Dpp is critical for tolerance against enteropathogens, but contributes to aging-associated epithelial dysfunction, highlighting the importance of tightly controlled interactions between blood cells and stem cells in this tissue. Nevertheless, while hemocytes themselves are required for normal lifespan, loss of hemocyte-derived Dpp does not impact lifespan. One interpretation of this finding is that beneficial (improved gut homeostasis) and deleterious (for example reduced immune competence of the gut epithelium) consequences of reduced hemocyte-derived Dpp cancel each other out over the lifespan of the animal. It will be interesting to test this hypothesis in future studies.
Aging is associated with systemic inflammation, and a role for immune cells in promoting inflammation in aging vertebrates has been proposed63,64. In humans, recruitment of immune cells to the gut is required for proper stem cell proliferation in response to luminal microbes23, and prolonged inflammatory bowel disease (IBD) further contributes to cancer development65. It is thus anticipated that conserved macrophage/stem cell interactions influence the etiology and progression of such diseases. Our data confirm a role for hemocytes in age-related intestinal dysplasia in the fly intestine, and provide mechanistic insight into the causes for this deregulation. It can be anticipated that similar interactions between macrophages and intestinal stem cells may contribute to the development of IBDs, intestinal cancers, and general loss of homeostasis in the aging human intestine.
METHODS
Methods and any associated references are available in the online version of the paper.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Institute on General Medical Sciences (R01 GM100196) and the National Eye Institute (R01 EY018177). We would like to thank Dr. J. Karpac for comments.
Footnotes
AUTHOR CONTRIBUTIONS
A.A. and H.J. designed all experiments. A.A. generated transgenic animals, performed experiments on hemocytes, Dpp, Sax and Smox signaling, interactions of Sax/Smox signaling with Tkv/Mad, EGFR and JAK/Stat pathways and role of Tkv expression in mitosis and on aging, dysplasia and lifespan; H.L. validated specificity of Tkv and Sax antibodies, performed experiments on Tkv expression and on lineage tracing of Mad, Med and Tkv mutant ISCs, and provided additional reagents for other experiments. A.A. and H.J. analyzed the data and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors report no competing financial interests.
REFERENCES
- 1.Ferrandon D. The complementary facets of epithelial host defenses in the genetic model organism Drosophila melanogaster: from resistance to resilience. Current opinion in immunology. 2013;25:59–70. doi: 10.1016/j.coi.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre B, Miguel-Aliaga I. The Digestive Tract of Drosophila melanogaster. Annu Rev Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- 3.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5:1465–83. doi: 10.1002/emmm.201201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiko GE, Stappenbeck TS. Host-microbe interactions shaping the gastrointestinal environment. Trends Immunol. 2014 doi: 10.1016/j.it.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nature reviews. Microbiology. 2013;11:615–26. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 6.Ayyaz A, Jasper H. Intestinal inflammation and stem cell homeostasis in aging. Front Cell Infect Microbiol. 2013;3:98. doi: 10.3389/fcimb.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 8.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 9.Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell stem cell. 2011;9:402–11. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development (Cambridge, England) 2011;138:1045–55. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu N, et al. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Developmental biology. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC biology. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell stem cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes & development. 2009;23:2333–44. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronin SJF, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science (New York, N.Y.) 2009;325:340–3. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordero JB, Sansom OJ. Wnt signalling and its role in stem cell-driven intestinal regeneration and hyperplasia. Acta physiologica (Oxford, England) 2012;204:137–43. doi: 10.1111/j.1748-1716.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Qi Y, Jasper H. Dpp signaling determines regional stem cell identity in the regenerating adult Drosophila gastrointestinal tract. Cell reports. 2013;4:10–8. doi: 10.1016/j.celrep.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Zhang Y, Han L, Shi L, Lin X. Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev Cell. 2013;24:133–43. doi: 10.1016/j.devcel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Driver I, Ohlstein B. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. The Journal of cell biology. 2013;201:945–61. doi: 10.1083/jcb.201302049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian A, Jiang J. Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut. Elife. 2014;3:e01857. doi: 10.7554/eLife.01857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, et al. Dpp/Gbb signaling is required for normal intestinal regeneration during infection. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Skoczek DA, et al. Luminal microbes promote monocyte-stem cell interactions across a healthy colonic epithelium. J Immunol. 2014;193:439–51. doi: 10.4049/jimmunol.1301497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malvin NP, Seno H, Stappenbeck TS. Colonic epithelial response to injury requires Myd88 signaling in myeloid cells. Mucosal Immunol. 2012;5:194–206. doi: 10.1038/mi.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual review of immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 27.Babcock DT, et al. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10017–22. doi: 10.1073/pnas.0709951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charroux B, Royet J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9797–802. doi: 10.1073/pnas.0903971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaidman-Remy A, Regan JC, Brandao AS, Jacinto A. The Drosophila larva as a tool to study gut-associated macrophages: PI3K regulates a discrete hemocyte population at the proventriculus. Dev Comp Immunol. 2012;36:638–47. doi: 10.1016/j.dci.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Tokusumi T, Shoue DA, Tokusumi Y, Stoller JR, Schulz RA. New hemocyte-specific enhancer- reporter transgenes for the analysis of hematopoiesis in Drosophila. Genesis. 2009;47:771–4. doi: 10.1002/dvg.20561. [DOI] [PubMed] [Google Scholar]
- 31.Kurucz E, et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007;17:649–54. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 32.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell stem cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makhijani K, Alexander B, Tanaka T, Rulifson E, Bruckner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–91. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 35.Zettervall CJ, et al. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 2004;101:14192–7. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark RI, Woodcock KJ, Geissmann F, Trouillet C, Dionne MS. Multiple TGF-beta superfamily signals modulate the adult Drosophila immune response. Curr Biol. 2011;21:1672–7. doi: 10.1016/j.cub.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–80. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 38.Harrison DA, Perrimon N. Simple and efficient generation of marked clones in Drosophila. Current biology : CB. 1993;3:424–33. doi: 10.1016/0960-9822(93)90349-s. [DOI] [PubMed] [Google Scholar]
- 39.Bardet PL, et al. A fluorescent reporter of caspase activity for live imaging. Proc Natl Acad Sci U S A. 2008;105:13901–5. doi: 10.1073/pnas.0806983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamaratoglu F, de Lachapelle AM, Pyrowolakis G, Bergmann S, Affolter M. Dpp signaling activity requires Pentagone to scale with tissue size in the growing Drosophila wing imaginal disc. PLoS Biol. 2011;9:e1001182. doi: 10.1371/journal.pbio.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brummel TJ, et al. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell. 1994;78:251–61. doi: 10.1016/0092-8674(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 42.Henderson KD, Andrew DJ. Identification of a novel Drosophila SMAD on the X chromosome. Biochem Biophys Res Commun. 1998;252:195–201. doi: 10.1006/bbrc.1998.9562. [DOI] [PubMed] [Google Scholar]
- 43.Spokony R, White K. Spokony insertions. Flybase; 2013. [Google Scholar]
- 44.Zhu CC, et al. Drosophila Activin- and the Activin-like product Dawdle function redundantly to regulate proliferation in the larval brain. Development. 2008;135:513–21. doi: 10.1242/dev.010876. [DOI] [PubMed] [Google Scholar]
- 45.Kim MJ, O'Connor MB. Anterograde Activin Signaling Regulates Postsynaptic Membrane Potential and GluRIIA/B Abundance at the Drosophila Neuromuscular Junction. PloS one. 2014;9:e107443. doi: 10.1371/journal.pone.0107443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bangi E, Wharton K. Dual function of the Drosophila Alk1/Alk2 ortholog Saxophone shapes the Bmp activity gradient in the wing imaginal disc. Development. 2006;133:3295–303. doi: 10.1242/dev.02513. [DOI] [PubMed] [Google Scholar]
- 47.Haerry TE, Khalsa O, O'Connor MB, Wharton KA. Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development. 1998;125:3977–87. doi: 10.1242/dev.125.20.3977. [DOI] [PubMed] [Google Scholar]
- 48.Biteau B, et al. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS genetics. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo L, Karpac J, Tran SL, Jasper H. PGRP-SC2 Promotes Gut Immune Homeostasis to Limit Commensal Dysbiosis and Extend Lifespan. Cell. 2014;156:109–22. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21528–33. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9415–20. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Assoian RK, et al. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987;84:6020–4. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–13. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakefield LM, Hill CS. Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nat Rev Cancer. 2013;13:328–41. doi: 10.1038/nrc3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson AJ, O'Connor MB. Strategies for exploring TGF-beta signaling in Drosophila. Methods. 2014;68:183–93. doi: 10.1016/j.ymeth.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Twombly V, et al. Functional analysis of saxophone, the Drosophila gene encoding the BMP type I receptor ortholog of human ALK1/ACVRL1 and ACVR1/ALK2. Genetics. 2009;183:563–79. 1SI–8SI. doi: 10.1534/genetics.109.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bangi E, Wharton K. Dpp and Gbb exhibit different effective ranges in the establishment of the BMP activity gradient critical for Drosophila wing patterning. Dev Biol. 2006;295:178–93. doi: 10.1016/j.ydbio.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Haerry TE. The interaction between two TGF-beta type I receptors plays important roles in ligand binding, SMAD activation, and gradient formation. Mech Dev. 2010;127:358–70. doi: 10.1016/j.mod.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Brummel T, et al. The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 1999;13:98–111. doi: 10.1101/gad.13.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–7. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 61.Li CY, Guo Z, Wang Z. TGFbeta receptor saxophone non-autonomously regulates germline proliferation in a Smox/dSmad2-dependent manner in Drosophila testis. Dev Biol. 2007;309:70–7. doi: 10.1016/j.ydbio.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 62.Isogaya K, et al. A Smad3 and TTF-1/NKX2-1 complex regulates Smad4-independent gene expression. Cell Res. 2014;24:994–1008. doi: 10.1038/cr.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–87. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang GC, Casolaro V. Immunologic changes in frail older adults. Transl Med UniSa. 2014;9:1–6. [PMC free article] [PubMed] [Google Scholar]
- 65.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.