Abstract
Serum carbamylated albumin (C-Alb) levels are associated with excess mortality in patients with diabetic end stage renal disease. To gain insight into the pathophysiology of carbamylation, we determined associations between C-Alb and causes of death in patients on chronic hemodialysis. The Die Deutsche Diabetes Dialyse Studie (4D study) was a randomized controlled trial testing the effects of atorvastatin on survival in diabetic patients on dialysis during a median follow-up of 4 years. We stratified 1,161 patients by C-Alb to see if differences in carbamylation altered the effects of atorvastatin on survival. Baseline C-Alb significantly correlated with serum cardiac stress markers troponin T and N-terminal pro-B-type-natriuretic peptide, and was associated with history of heart failure and arrhythmia. C-Alb was strongly associated with 1-year adjusted risk of CV mortality, sudden cardiac death and the 4-year risk of death from congestive heart failure (Hazard Ratios of 3.06, 3.78 and 4.64, respectively), but not with myocardial infarction or stroke. Patients with low C-Alb, treated with atorvastatin, experienced a significant improvement in their 4-year survival (Hazard Ratio 0.692). High C-Alb levels are associated with ongoing cardiac damage, risk of congestive heart failure and sudden cardiac death. Thus, carbamylation and uremic cardiomyopathy are associated in patients with diabetes mellitus and kidney disease. Additionally, statins were specifically beneficial to hemodialysis patients with low C-Alb.
Keywords: cardiovascular disease, congestive heart failure, urea, hemodialysis, uremia, uremic toxins
Introduction
One of the consequences of chronically elevated urea levels in patients with kidney disease is the accelerated chemical modification of proteins by urea (carbamylation).(1-3) The accumulation of these modifications on albumin (as well as other serum proteins) has been shown to be proportional to blood urea concentrations, in a fashion analogous to the relationship between hemoglobin A1c levels and time-averaged glucose concentrations.(4, 5) In addition to chronic uremia, amino acid deficiencies are common in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD),(6) and there is evidence that these amino acid deficiencies may further exacerbate protein carbamylation.(3, 5) As a result, the proportion of albumin that is carbamylated (C-Alb) represents a biomarker that simultaneously integrates time-averaged urea concentrations and possibly protein energy wasting in patients on hemodialysis.(5)
Recent work has demonstrated a strong and independent association between serum carbamylated protein levels and risk of death in patients on hemodialysis treatment.(4, 5) However, the mechanisms linking uremia, protein carbamylation, and mortality remain poorly understood. A number of studies have suggested a direct role for protein carbamylation in the cardiovascular pathology associated with CKD. For example, when nephrectomized ApoE-null mice were fed pure urea in their chow, it dramatically accelerated rates of aortic atherogenesis.(7) It has also been shown that protein carbamylation has pro-atherogenic effects on lipoproteins. Animal studies have shown that uremia increases carbamylation of plasma low density lipoproteins (LDL) and high density lipoproteins (HDL), and this modification inhibits receptor mediated uptake and instead promotes foam cell formation and accumulation in atherosclerotic tissues.(8-13) A clinical study of patients undergoing cardiac catheterization and age-matched controls further demonstrated that when cases and controls were stratified by their serum carbamylated protein levels, subjects with higher carbamylated protein levels were affected by CVD in greater proportions. (14) Together these results suggest protein carbamylation may contribute to the cardiovascular disease burden associated with kidney failure.
We recently reported strong independent associations between carbamylated albumin levels and 12 month all-cause mortality in both incident and prevalent hemodialysis patient cohorts, even after adjusting for other significant risk factors (5) In order to test whether C-Alb values are also associated with longer-term outcomes, herein we describe analysis of 4-year risk of death from specific causes associated with C-Alb. Furthermore, in order to search for specific pathologic sequelae associated with carbamylation, we re-analyzed the study database for associations between C-Alb and co-existing comorbidities as well as future risk of death from specific causes. In addition, in light of previous studies suggesting a connection between protein carbamylation and atherogenesis, we investigated the relationship between C-Alb, serum cholesterol levels, and survival benefits from atorvastatin therapy.
Results
Associations between carbamylated albumin levels, clinical laboratory tests, and co-existing comorbidities
Baseline differences in clinical, demographic, and biochemical characteristics were compared in 4D subjects according to their tertile of baseline C-Alb levels (tertile 1: C-Alb <0.72, tertile 2: C-alb between 0.72 and 0.90, tertile 3: C-alb > 0.90). The mean length of follow-up was 3.96 years in the atorvastatin group and 3.91 years in the placebo group. As shown in Table 1, analysis of variation found associations between higher tertile C-Alb levels and higher pre- and post-dialysis blood urea and creatinine levels. High C-Alb was also associated with elevated phosphate and potassium levels and lower urea reduction ratio values. These findings are consistent with the hypothesis that chronic uremia and lower efficiency of urea reduction therapy may be contributing to increased protein carbamylation. High C-Alb was also associated with higher average NT-Pro-BNP, troponin T, intact PTH, alkaline phosphatase, and HDL cholesterol, and with lower average body mass index, lower total cholesterol, lower hemoglobin, and low ferritin. Baseline C-Alb also showed modest correlations with troponin T (r = +0.183, p<0.001) and NT-proBNP (r = +0.227, p<0.001). The data set was further analyzed for associations between high C-Alb levels and co-existing co-morbidities, revealing that high C-Alb was associated with current diagnosis of congestive heart failure, atrial fibrillation, and hypertension. In contrast, there were no significant associations between high C-Alb and history of coronary artery disease, peripheral vascular disease, or cerebrovascular disease.
Table 1.
Baseline clinical and laboratory data for study population categorized by carbamylated albumin tertile
| Baseline characteristic | Carbamylated albumin tertile | P-value | ||
|---|---|---|---|---|
| C-Alb values under 0.72 (n = 387) | C-Alb values 0.72 to 0.90 (n = 387) | C-Alb values over 0.90 (n = 387) | ||
| Age (years) | 65 ± 9 | 66 ± 8 | 66 ± 8 | 0.099 |
| Male | 52 | 57 | 54 | 0.364 |
| Proportion of subjects receiving atorvastatin | 46.0% (178) | 50.6% (196) | 50.9% (197) | 0.307 |
| Duration of dialysis (months) | 9.3 ± 7.1 | 8.3 ± 6.9 | 7.2 ± 6.4 | <0.001 |
| Pre-dialysis BUN (mg/dL) | 102 ± 54 | 121 ± 61 | 139 ± 65 | <0.001 |
| Post-dialysis BUN (mg/dL)* | 38 ± 19 | 51 ± 26 | 54 ± 27 | <0.001 |
| Urea reduction ratio (%)* | 64 ± 9 | 60 ± 14 | 61 ± 10 | 0.011 |
| Creatinine (mg/dL) | 6.6 ± 2.2 | 7.0 ± 2.2 | 7.0 ± 2.4 | 0.004 |
| Systolic blood pressure (mmHg) | 145 ± 22 | 146 ± 22 | 146 ± 22 | 0.850 |
| Diastolic blood pressure (mmHg) | 76 ± 11 | 76 ± 11 | 76 ± 11 | 0.934 |
| BMI (kg/m2) | 28.2 ± 4.8 | 27.2 ± 4.5 | 27.0 ± 4.9 | 0.001 |
| Alkaline phosphatase (IU/L) | 121 ± 50 | 119 ± 50 | 133 ± 80 | 0.003 |
| Troponin T (ng/mL) | 0.07 ± 0.11 | 0.08 ± 0.10 | 0.10 ± 0.12 | 0.006 |
| NT-Pro-BNP (pg/mL) | 5886 ± 9676 | 7776 ± 14697 | 10754 ± 15431 | <0.001 |
| Creatinine kinase (U/L) | 66 ± 70 | 69 ± 55 | 72 ± 51 | 0.336 |
| C-reactive protein (ng/mL) | 10.8 ± 15.1 | 9.4 ± 13.0 | 11.6 ± 22.2 | 0.202 |
| Total cholesterol (mg/dL) | 224 ± 42 | 220 ± 43 | 215 ± 42 | 0.016 |
| LDL cholesterol (mg/dL) | 124 ± 28 | 126 ± 29 | 127 ± 31 | 0.400 |
| HDL cholesterol (mg/dL) | 34 ± 12 | 36 ± 13 | 39 ± 15 | <0.001 |
| Albumin (g/dL) | 3.81 ± 0.3 | 3.84 ± 0.3 | 3.82 ± 0.3 | 0.354 |
| Hemoglobin (g/L) | 11.1 ± 1.3 | 10.9 ± 1.3 | 10.7 ± 1.3 | 0.004 |
| Transferrin saturation (%) | 22.9 ± 12.1 | 22.6 ± 10.9 | 21.1 ± 11.1 | 0.054 |
| Calcium (mmol/L) | 2.33 ± 0.21 | 2.30 ± 0.20 | 2.29 ± 0.24 | 0.007 |
| Ferritin (pmol/L) | 537 ± 461 | 480 ± 429 | 450 ± 384 | 0.016 |
| Potassium (mmol/L) | 5.1 ± 0.9 | 5.1 ± 0.8 | 5.3 ± 0.8 | 0.007 |
| Phosphorus (mg/dL) | 5.6 ± 1.4 | 6.1 ± 1.6 | 6.4 ± 1.7 | <0.001 |
| Platelets (103/μL) | 256 ± 76 | 254 ± 76 | 261 ± 87 | 0.490 |
| Intact parathyroid hormone (ng/L) | 93 ± 122 | 100 ± 101 | 115 ± 139 | 0.041 |
| White blood cells (103/μL) | 8.0 ± 2.3 | 8.0 ± 2.3 | 8.4 ± 2.7 | 0.060 |
| Aspartate aminotransferase (IU/L) | 14 ± 6 | 14 ± 16 | 14 ± 7 | 0.890 |
| HbA1c (%) | 6.7 ± 1.2 | 6.7 ± 1.3 | 6.7 ± 1.3 | 0.847 |
| Duration of diabetes (years) | 17 ± 9 | 18 ± 9 | 19 ± 9 | 0.162 |
| Prevalence of comorbid conditions present at baseline | ||||
| Congestive heart failure | 32.8% (127) | 31.0% (120) | 35.6% (166) | 0.001 |
| Atrial fibrillation | 17.1% (66) | 16.0% (62) | 22.5% (87) | 0.046 |
| Hypertension | 84.8% (328) | 90.4% (350) | 90.7% (351) | 0.013 |
| Coronary artery disease | 27.7% (107) | 28.7% (111) | 33.3% (129) | 0.184 |
| Peripheral vascular disease | 45.7% (177) | 44.4% (172) | 44.7% (173) | 0.930 |
| Cerebrovascular accident | 18.9% (73) | 18.3% (71) | 16.0% (62) | 0.545 |
| Blood pressure medication | ||||
| ACE inhibitors | 45.2% (175) | 48.1% (186) | 49.9% (193) | 0.426 |
| Calcium antagonists | 35.1% (136) | 41.6% (161) | 45.5% (176) | 0.013 |
| Diuretics | 78.8% (305) | 78.0% (302) | 82.9% (321) | 0.186 |
| Beta blockers | 38.5% (149) | 39.5% (153) | 36.2% (140) | 0.615 |
Data are expressed as mean ± SD for continuous variables and percentages (%) with number of cases for categorical data. Analysis of variance was used to test for differences between groups for continuous variables, Chi square testing was used to test for significant differences between proportions for categorical variables.
Post-dialysis urea and urea reduction ratio values were available for 471 of the 1,1161 subjects.
Associations between carbamylated albumin levels and specific 1-year adverse events
4D subjects’ baseline C-Alb values were analyzed for associations with 1-year risk of specific adverse outcomes using multivariable-adjusted Cox proportional hazards models. As shown in Table 2, higher baseline C-Alb values were found to be strongly associated with univariate risk of death from all causes, CV and non-CV mortality. The effect was strongest for CV mortality; in particular for sudden cardiac death. The risks associated with C-Alb were adjusted for potentially confounding variables, including age, time on dialysis therapy (dialysis vintage), systolic and diastolic blood pressure, serum albumin, phosphate, parathyroid hormone, cholesterol, HDL-C, hemoglobin, C-reactive protein, potassium, creatinine, NT-proBNP, troponin T, treatment with ACE inhibitors/calcium antagonists/diuretics, history of hypertension, and history of CVD (including CAD, CHF, PVD). After adjusting for all these variables, it was still found that the risk of sudden cardiac death, all-cause mortality and cardiovascular mortality remained significant. Interestingly, there was no specific association between C-Alb values and risk of myocardial infarction.
Table 2.
Association between baseline carbamylated albumin* and 1-year and 4-year risk of death from specific causes
| Cause of death | Unadjusted | MV-adjusted1 | ||
|---|---|---|---|---|
| HR* (95% CI) | P-value | HR (95% CI) | P-value | |
| 1-year risk of adverse events | ||||
| All-cause mortality (n = 138) | 3.73 (2.00 -6.96) | <0.001 | 2.74 (1.35 – 5.57) | 0.005 |
| Sudden death (n = 45) | 5.26 (1.78 – 15.57) | 0.003 | 3.78 (1.18 – 12.10) | 0.025 |
| Congestive heart failure (n=14) | 5.23 (0.75 – 36.59) | 0.096 | 2.44 (0.24 – 25.27) | 0.455 |
| Myocardial infarction (n = 48) | 1.22 (0.42 – 3.55) | 0.721 | 1.33 (0.41 – 4.37) | 0.637 |
| Cerebrovascular accident (n = 33) | 0.76 (0.21 – 2.77) | 0.672 | 0.52 (0.12 – 2.34) | 0.397 |
| Infection (n = 22) | 2.30 (0.48 – 11.11) | 0.300 | 1.28 (0.19 – 8.38) | 0.801 |
| CV mortality2 (n=84) | 3.97 (1.78-8.82) | 0.001 | 3.06 (1.25-7.46) | 0.014 |
| Non-CV mortality (n=54) | 3.38 (1.24-9.19) | 0.017 | 2.43 (0.76-7.82) | 0.137 |
| 4-year risk of adverse events | ||||
| All-cause mortality (n = 572) | 1.86 (1.35 -2.55) | <0.001 | 1.43 (1.01 -2.05) | 0.047 |
| Congestive heart failure (n=38) | 7.26 (2.18 – 24.16) | 0.001 | 4.64 (1.11-19.51) | 0.036 |
| Sudden death (n = 150) | 2.29 (1.23 -4.24) | 0.009 | 1.78 (0.91-3.50) | 0.095 |
| Myocardial infarction (n = 181) | 1.63 (0.92 – 2.86) | 0.089 | 1.25 (0.66 – 2.35) | 0.494 |
| Cerebrovascular accident (n = 97) | 1.71 (0.80 – 3.69) | 0.169 | 1.37 (0.57 – 3.28) | 0.480 |
| Infection (n = 118) | 1.84 (0.91 – 3.70) | 0.089 | 1.50 (0.69 – 3.26) | 0.307 |
| CV mortality2 (n=286) | 2.47 (1.58-3.87) | <0.001 | 1.90 (1.15-3.13) | 0.013 |
| Non-cardiac mortality (n=286) | 1.39 (0.88-2.18) | 0.154 | 1.07 (0.65-1.77) | 0.797 |
Cox proportional hazards model of association between baseline carbamylated albumin (log-transformed continuous variable) and 1-year risk of adverse events.
MV-adjusted hazards model was adjusted for potential confounders, including age, dialysis vintage, systolic and diastolic blood pressure, body mass index, albumin, cholesterol, HDL-C, hemoglobin, C-reactive protein, potassium, phosphate, creatinine, parathyroid hormone, NT-proBNP, troponin T, treatment with ACE inhibitors/calcium antagonists/diuretics, history of hypertension, and history of CVD (including CAD, CHF, PVD).
CV mortality included sudden cardiac death, death due to congestive heart failure, death due to myocardial infarction, or any other deaths ascribed to coronary heart disease.
We performed a sensitivity analysis in the subset of patients with available URR values (n=471). We compared the hazard ratios for 1 year mortality when a) adjustments were made for all variables mentioned above and b) additional adjustments were made for URR. Of note, in both analyses, the risks were similar with hazard ratios of a) 1.77 (0.92-3.40) and b) 1.78 (0.92-3.42), reassuring that URR is unlikely to act as a confounding parameter in our study.
Associations between carbamylated albumin levels and specific 4-year adverse events
4D subjects’ baseline C-Alb values were analyzed for associations with 4-year risk of specific adverse outcomes using multivariable-adjusted Cox proportional hazards models. As shown in Table 2, higher baseline C-Alb values were found to be strongly associated with univariate risk of CV and all-cause mortality. Notably, there was an especially strong association between initial C-Alb values and risk of death from congestive heart failure (HR 7.26 (2.18 – 24.16), p=0.001) and a significant association with sudden cardiac death (HR 2.29 (CI 1.23-4.24)). HR estimates were again calculated using a Cox proportional hazards model adjusted for the potential confounders mentioned above; the risk of death from congestive heart failure, sudden cardiac death, CV and all-cause mortality remained significant even after accounting for these other risk factors.
Lastly, although our multivariable model was adjusted for the effects of many potential confounders, in order to further exclude the possibility that co-morbid conditions may have confounding effects on the association between C-Alb values and risk of death, we re-analyzed our data after stratifying subjects according to presence or absence of a variety of baseline risk factors (including history of hypertension, CAD, CHF, arrhythmias; see Supplementary Table 1). Furthermore, because of the observed association between high C-Alb values and shorter times on dialysis, we also wanted exclude the possibility that differences in dialysis vintage were confounding the association between C-Alb and mortality. Using these stratified analyses, we observed that C-Alb was still significantly associated with 1-year and 4-year mortality in patients with longer or shorter histories of dialysis therapy and in patients with or without past histories of CHF, CAD, or arrhythmia (Supplementary Table 1), indicating that the risks associated with C-Alb were not simply due to associations with these preexisting conditions. Although the 1-year and 4-year risks in patients with no history of hypertension were not statistically significant (HR 2.89 (0.44 – 18.99, p=0.268) and HR 0.90 (0.37 – 2.18, p=0.813), respectively), this may in part be due to the relatively small size of this subgroup (n=133).
Effects of atorvastatin therapy on survival in subjects stratified by carbamylated albumin levels
In Table 1 an association was noted between high C-Alb levels and lower serum total cholesterol and higher HDL cholesterol concentrations. A similar analysis was performed on study data from 186 incident hemodialysis patients (with and without histories of diabetes mellitus) who participated in the ArMORR study and whose characteristics were previously described.(5) Analysis of serum samples collected at baseline from this independent cohort observed a negative correlation between C-Alb and total serum cholesterol levels (r = -0.29, p<0.001). Given the inverse association between serum carbamylated albumin and total cholesterol and the association between C-Alb and mortality, we speculated whether uremia and carbamylation may be modifying the associations between hypercholesterolemia, response to statin therapy, and cardiovascular risk seen in most populations. In order to test this, we sought to determine whether atorvastatin therapy influenced survival in subjects that were first stratified according to their baseline C-Alb values. Baseline characteristics in subjects grouped by treatment arm and low baseline C-Alb values are shown in Table 3. Although there were no survival benefits associated with atorvastatin therapy in subjects in the upper or middle tertile for carbamylated albumin, there was a statistically significant decrease in mortality in subjects with low baseline carbamylated albumin that were treated with atorvastatin (HR 0.692 (CI 0.505 – 0.947, p = 0.022)) (Table 4). Note that subjects in the lower tertile for C-Alb values that were randomized to atorvastatin therapy had slightly higher average LDL cholesterol levels at baseline compared to placebo controls (122 vs. 128, p<0.033).
Table 3.
Baseline characteristics of study population categorized by baseline C-Alb values and Atorvastatin treatment arm
| All subjects | Subjects in low tertile for C-Alb | |||||
|---|---|---|---|---|---|---|
| Placebo (n=590) |
Statin (n=571) |
P-value | Placebo (n=209) |
Statin (n=178) |
P-value | |
| Age (years) | 66 ± 8 | 66 ± 8 | 0.811 | 65 ± 8 | 65 ± 9 | 0.634 |
| Male | 54 | 55 | 0.800 | 52 | 52 | 0.985 |
| Systolic blood pressure (mmHg) | 145 ± 22 | 146 ± 22 | 0.425 | 144 ± 22 | 146 ± 22 | 0.331 |
| Diastolic blood pressure (mmHg) | 76 ± 11 | 76 ± 11 | 0.718 | 76 ± 11 | 76 ± 11 | 0.830 |
| BMI (kg/m2) | 27.4 ± 5.0 | 27.5 ± 4.6 | 0.921 | 28.3 ± 5.2 | 28.1 ± 4.4 | 0.723 |
| Alkaline phosphatase (IU/L) | 124 ± 57 | 126 ± 67 | 0.591 | 124 ± 54 | 118 ± 44 | 0.222 |
| Troponin T (ng/mL) | 0.09 ± 0.11 | 0.09 ± 0.11 | 0.737 | 0.07 ± 0.08 | 0.08 ± 0.14 | 0.171 |
| NT-Pro-BNP (pg/mL) | 8666 ± 15402 | 7600 ± 11544 | 0.184 | 5502 ± 8854 | 6333 ± 10564 | 0.401 |
| Creatinine kinase (U/L) | 69 ± 59 | 69 ± 60 | 0.941 | 65 ± 70 | 67 ± 69 | 0.817 |
| C-reactive protein (ng/mL) | 10.9 ± 18.0 | 10.3 ± 16.5 | 0.567 | 11.4 ± 16.4 | 10.0 ± 13.6 | 0.376 |
| Total cholesterol (mmol/L) | 220 ± 41 | 219 ± 43 | 0.860 | 220 ± 40 | 228 ± 43 | 0.069 |
| LDL cholesterol (mmol/L) | 127 ± 30 | 125 ± 29 | 0.374 | 122 ± 26 | 128 ± 29 | 0.033 |
| HDL cholesterol (mmol/L) | 37 ± 14 | 36 ± 13 | 0.241 | 34 ± 11 | 35 ± 12 | 0.563 |
| Albumin (g/L) | 3.83 ± 0.3 | 3.80 ± 0.3 | 0.098 | 3.81 ± 0.3 | 3.81 ± 0.3 | 0.977 |
| Hemoglobin (g/L) | 10.9 ± 1.4 | 10.8 ± 1.3 | 0.267 | 11.1 ± 1.4 | 11.0 ± 1.3 | 0.491 |
| Transferrin saturation (%) | 22.4 ± 10.8 | 21.9 ± 12.0 | 0.465 | 22.8 ± 11.4 | 23.1 ± 13.0 | 0.806 |
| Calcium (mmol/L) | 2.3 ± 0.2 | 2.3 ± 0.2 | 0.736 | 2.3 ± 0.2 | 2.4 ± 0.2 | 0.040 |
| Ferritin (pmol/L) | 492 ± 425 | 486 ± 429 | 0.812 | 537 ± 461 | 537 ± 461 | 0.998 |
| Potassium (mmol/L) | 5.1 ± 0.8 | 5.2 ± 0.9 | 0.341 | 5.1 ± 0.8 | 5.1 ± 0.9 | 0.508 |
| Phosphorus (mmol/L) | 6.0 ± 1.6 | 6.0 ± 1.6 | 0.819 | 5.6 ± 1.4 | 5.5 ± 1.4 | 0.644 |
| Platelets (103/μL) | 259 ± 81 | 255 ± 79 | 0.428 | 257 ± 75 | 256 ± 79 | 0.858 |
| Intact parathyroid hormone (ng/L) | 102 ± 126 | 103 ± 119 | 0.887 | 95 ± 141 | 90 ± 95 | 0.684 |
| White blood cells (103/μL) | 8.2 ± 2.5 | 8.1 ± 2.4 | 0.547 | 8.0 ± 2.3 | 7.9 ± 2.2 | 0.640 |
| Urea reduction rate (%) | 62 ± 11 | 62 ± 12 | 0.507 | 64 ± 10 | 64 ± 10 | 0.843 |
| Aspartate aminotransferase (IU/L) | 14 ± 7 | 14 ± 13 | 0.727 | 14 ± 7 | 14 ± 5 | 0.268 |
| HbA1c (%) | 6.7 ± 1.3 | 6.7 ± 1.2 | 0.517 | 6.7 ± 1.3 | 6.8 ± 1.2 | 0.816 |
| Duration of diabetes (years) | 18.8 ± 8.9 | 17.4 ± 8.7 | 0.008 | 17.8 ± 8.6 | 16.9 ± 8.6 | 0.334 |
Data are expressed as mean ± SD for continuous variables and percentages (%) with number of cases for categorical data. Student’s t-test was used to test for differences between groups for continuous variables, Chi square testing was used to test for significant differences between proportions for categorical variable
Table 4.
Effects of atorvastatin therapy on 4-year risk of death from all causes in patients stratified according to baseline C-Alb levels.
| C-Alb tertile | HR | 95% CI | P-value |
|---|---|---|---|
| Bottom | 0.692 | 0.505 – 0.947 | 0.022 |
| Middle | 0.935 | 0.703 – 1.243 | 0.624 |
| Top | 1.054 | 0.808 – 1.373 | 0.700 |
Cox proportional hazards model of association between treatment group (atorvastatin vs. placebo control) and 4-year risk of death from all causes.
Discussion
In this study of patients with diabetic end stage kidney disease receiving maintenance hemodialysis we found that elevated serum protein carbamylation is associated with increased serum markers of cardiac stress, as well as increased CV mortality; in particular short-term risk of sudden death and long-term risk of death from congestive heart failure. High C-Alb levels were strongly associated with 4-year risk of death due to heart failure but not associated with 1-year risk; in contrast, there was significant risk of sudden cardiac death associated with high C-Alb levels during the first year of follow-up, but the estimated risk decreased after 4-years of follow-up. We interpreted the risk of sudden cardiac death to be consistent with the hypothesis that cardiac protein “hypercarbamylation” represents an acute insult to the cardiac conduction system which may be reversible if carbamylation is reduced, and thus the risk diminishes over time. We interpreted the long-term risk of heart failure to be consistent with the hypothesis that carbamylation is contributing to uremic cardiomyopathy and fibrosis which are irreversible but take time to progress before fulminant cardiac failure occurs. There were no associations to history of coronary artery disease or future risk of myocardial infarction, however. Interestingly, low C-Alb values were associated with modestly increased total cholesterol, and when subjects were stratified by C-Alb tertile, subjects with lower, C-Alb values responded to atorvastatin treatment with longer survival times compared to patients given placebo. Together these findings may suggest clues as to the mechanistic connections between uremia, protein carbamylation, and cardiac pathology in patients on hemodialysis. If these findings can be confirmed in other populations, protein carbamylation may represent a viable therapeutic target to modify cardiovascular risk in end-stage kidney disease patients.
The majority of ESRD patients die of cardiac causes, and the two most common causes of death in ESRD patients on hemodialysis are from sudden cardiac death and myocardial infarction (MI). (15, 16) Although atherosclerosis is accelerated in patients with kidney disease (17, 18), the pathogenesis of coronary artery disease in patients with kidney failure differs in important ways from the mechanisms of atherogenesis found in patients with normal kidney function. Coronary artery disease in patients with kidney disease is characterized by a combination of atherosclerotic changes combined with progressive calcification (calcific arteriosclerosis), but cholesterol-lowering therapies have not been shown to reduce the risk of myocardial infarctions in these patients. (19-21) These observations have prompted some to suggest that mechanisms other than hypercholesterolemia are responsible for the accelerated atherosclerosis and arteriosclerosis associated with ESRD—mechanisms including carbamylation (3, 22-24). Our data do not appear to support the hypothesis that accelerated atherosclerosis of uremia may be mediated by carbamylation.
Previous studies have suggested that the pathophysiology of sudden death in dialysis patients is due to fatal arrhythmias unrelated to CAD. (15) The risk factors for sudden death in hemodialysis patients are more commonly associated with dialysis-associated electrolyte imbalances, hyperkalemia, hyperphosphatemia, and “uremic cardiomyopathy,” (25) Uremic cardiomyopathy is thought to be caused by the effects of cardiac toxins that accumulate in renal failure, and is characterized by left ventricular hypertrophy (LVH), cardiac conduction abnormalities, progressive myocardial fibrosis with myocyte dropout and congestive heart failure, and may be reversible by improvements in patients’ dialysis therapy. (22, 25-28) It has been suggested that uremic cardiomyopathy is just as important a contributor to mortality in dialysis patients as atheromatous CAD, and our results suggest that hypercarbamylation is associated with this process.(25) Animal studies are needed to evaluate whether protein carbamylation is causally related to uremic cardiomyopathy and congestive heart failure.
In this study, we observed an association between high C-Alb and low total cholesterol levels, and the association between higher serum carbamylated protein levels and lower serum total cholesterol in patients on hemodialysis has been reported previously. (4) Low cholesterol levels in ESRD patients have been linked to anemia, hypoalbuminemia, low BMI, and protein energy wasting, and are paradoxically associated with increased mortality in ESRD. (29-31) In the current study and our previous report, C-Alb was also associated with anemia, lower BMI, amino acid deficiencies, and increased mortality. Furthermore, the association between low cholesterol and mortality in these patients may be the reason why it is difficult to show any measurable benefits of cholesterol-lowering therapy in ESRD patients. (20, 21) Most interestingly, in contrast to the original findings of the 4D study, when we stratified 4D patients according to C-Alb levels, we found that patients with low C-Alb levels in fact did show a survival benefit in response to atorvastatin. Together these findings suggest that ESRD patients whose protein carbamylation levels are already well controlled may benefit from cholesterol-lowering therapies. The reasons why patients with low C-Alb responded to atorvastatin are unknown, but we speculate that in patients with high C-Alb, the detrimental effects of excessive protein carbamylation overwhelm any benefits of statins in these patients (who already tended to have low cholesterol levels at baseline), whereas patients with low C-Alb values benefited from atorvastatin in the same way as non-uremic patients would. Although additional studies are needed to corroborate these findings, these results suggest that an identifiable subset of hemodialysis patients may benefit from statin therapy. (32)
A number of human and animal model studies have demonstrated pro-atherogenic effects of carbamylated lipoproteins. (7-13) It was somewhat unexpected, therefore, that our study found no significant association between C-Alb and risk of myocardial infarction or stroke. One explanation for this is that subjects with high C-Alb levels had lower total cholesterol and higher HDL cholesterol levels. A second possible explanation is that lipoproteins are carbamylated by a different mechanism than that of albumin, and as a result the risks associated with these two markers are also distinct. Although uremia promotes carbamylation of LDL (cLDL), another potentially important source of lipoprotein carbamylation is myeloperoxidase (MPO) activity within atherosclerotic plaques.(7, 14) Large amounts of MPO are secreted by foam cells, and lipoprotein carbamylation is strongly enriched within atheromas, suggesting that carbamylation of lipoproteins may be occurring locally within the plaques. (12-14) Circulating cLDL levels are correlated with MPO, whereas there is no correlation between MPO and C-Alb3 (5, 33) Together these findings suggest that there are differences between the mechanisms of carbamylation of lipoproteins and other circulating proteins, and these differences may provide insight into different pathophysiologies and risks associated with uremia.
There are several limitations to our study. All study subjects had histories of diabetes mellitus, raising the possibility that the findings apply mostly to patients with diabetic kidney disease. Our study was missing samples for 124 out of 1285 of the subjects; although there was no survival difference between the missing subjects and the cohort as a whole, this introduces the possibility of drop-out bias. Although our results show significant associations between C-Alb and short-term risk of sudden cardiac death and long-term risk of death due to heart failure, our findings are correlational and they do not conclusively demonstrate that carbamylation is the cause of uremic heart disease and death. Although elevated C-Alb values were strongly associated with CV mortality, there was no significant independent association with non-CV mortality, as might be expected if carbamylation contributes to other risks associated with uremia. Furthermore, although we were able to adjust for many possible confounding variables in our hazards model, and could show that C-Alb retained its association to survival risk even after stratifying for histories of CHF, atrial fibrillation, CAD/MI, and hypertension, we cannot fully exclude residual confounding. In particular, elevated C-Alb levels may be a sign of insufficient dialysis, but we could not adjust for Kt/V because these data were not available. However, we performed sensitivity analyses in the subset of 471 patients with available URR and foundno difference in risk after adjusting for URR, making any confounding effects by this variable unlikely. Lastly, although we did not have enough sample for additional analyses, it would be informative to know if other tests for serum protein carbamylation such as protein-bound homocitrulline (4) also demonstrate the same specific associations to cardiac mortality in patients with uremia. The strengths of this study include: (1) relatively large size of the study (n=1,161), (2) detailed longitudinal outcomes data based upon pre-specified criteria and panel adjudication, (3) availability of many clinical covariates, (4) availability of 4 years of follow-up which enabled us to demonstrate both short-term and long-term risks associated with C-Alb.
In summary, a secondary analysis of study data from 1,161 diabetic patients on hemodialysis revealed associations between C-Alb values and risk of future cardiac sequelae; the risks of sudden death and heart failure, and the lack of association to myocardial infarction provide clues to specific mechanisms connecting protein carbamylation and uremic cardiomyopathy. The risk of CV mortality, in particular sudden death and heart failure associated with baseline C-Alb remained strong even 4 years after C-Alb was measured, suggesting long-term prognostic value of this new biomarker. Lastly, we discovered that patients on hemodialysis with low C-Alb values demonstrated a statistically significant prolongation of survival when treated with atorvastatin, suggesting that measurement of protein carbamylation burden may represent a novel way to identify the subset of ESRD patients that would benefit from this well-established therapy.
Materials and Methods
Materials
All chemicals, except when noted, were purchased from Sigma-Aldrich.
Study Populations
Frozen specimens from 1,161 subjects of Die Deutsche Diabetes Dialyze Studie (4D trial) were used for analysis.(20, 34) The 4D study was a prospective randomized controlled trial of the effects of atorvastatin on mortality that included 1,255 patients with type 2 diabetes mellitus, aged 18-80 years, receiving hemodialysis for less than 2 years. Between March 1998 and October 2002, patients were recruited in 178 dialysis centres in Germany. After a period of 4 weeks, patients were randomly assigned to double-blinded treatment with either 20mg atorvastatin (n=619) or placebo (n=636) once daily. Study visits took place three times before randomization (visit 1-3), at randomization (visit 4), and at four weeks (visit 5) and every six months (visit 6 etc.) after randomization until the date of death, censoring, or end of the study in March 2004. 4D specimens analyzed for this study were obtained 1 week prior to treatment randomization, after which subjects were followed every 6 months until the date of death, censoring, or end of the study. 4D specimens were stored at -80°C until analysis. The study was conducted in accordance with ethical standards and approved by the institutional medical ethical committee, and all patients gave written informed consent before inclusion. Information for age, sex, and smoking status was obtained through patient interviews. Comorbid conditions, including the presence of coronary artery disease and congestive heart failure, as well as duration of diabetes mellitus and dialysis treatment, were reported by patients’ nephrologists. Blood pressure was measured in the sitting position during their first study visit. 124 of the original 1,255 subjects had insufficient specimen for analysis, leaving 1,161 samples available for our analysis; the mortality rate of this missing subgroup did not differ from the rest of the cohort (48% vs. 49%, p=0.836). Outcome assessment methods were described in the original 4D study. (20, 34)The study end points and serious adverse events were continuously monitored; every end point was adjudicated by three members of the end-point committee on the basis of predefined criteria detailed in (35). Death from cardiac causes was subdivided into categories including fatal myocardial infarction, sudden death, death due to congestive heart failure, and death due to coronary heart disease within 28 days after an intervention. Patients who died unexpectedly and did not meet criteria for other types of cardiac death were considered to have had sudden death from cardiac causes. For the present analysis, CV mortality, sudden death, MI (fatal and non-fatal), stroke (fatal and non-fatal), all-cause mortality and deaths due to infection were all chosen as separate outcome measures.
Biochemical analyses of carbamylated albumin
Biochemical measurements of C-Alb levels in the 4D cohort were performed and reported previously that analyzed the association between C-Alb and 12 month all-cause mortality; a complete description of the mass spectrometric assay for carbamylated albumin (C-Alb) and its analytical validation are described in this previous report.(5) All carbamylated albumin values are reported in terms of the percentage of carbamylated relative to non-carbamylated albumin. Repeat measurements of a representative patient sample demonstrated a coefficient of variance (CV) of 4.2%.
Other biochemical analyses
All clinical blood tests for troponin T, NT-proBNP, and other biochemical markers were performed during the original 4D study by the 4D central laboratory in blood samples obtained at baseline during study visit 3 (1 week before randomization).(34)
Statistical Analysis
SPSS software, version 21 (IBM), was used for all analyses. Comparisons between characteristics of subject groups were analyzed using Student’s t-test, Analysis of Variation, Mann-Whitney-U tests, or Chi-Squared Tests where appropriate. Correlations between variables were calculated using the Pearson product-moment correlation coefficient. Analysis of sudden cardiac death, myocardial infarction, stroke, death due to heart failure, combined cardiovascular events and infectious deaths were performed with the use of Cox proportional-hazards (HR) models. Proportional hazard assumptions were checked for all HR models. C-Alb values were transformed to their natural log and analyzed as a continuous variable for HR analysis. To analyze for associations between C-Alb levels and benefits of atorvastatin therapy, subjects were stratified into tertiles according to their C-Alb values, and independent HR analyses were performed for each tertile. Two-tailed P-values of <0.05 were considered significant.
Supplementary Material
Fig. 1.
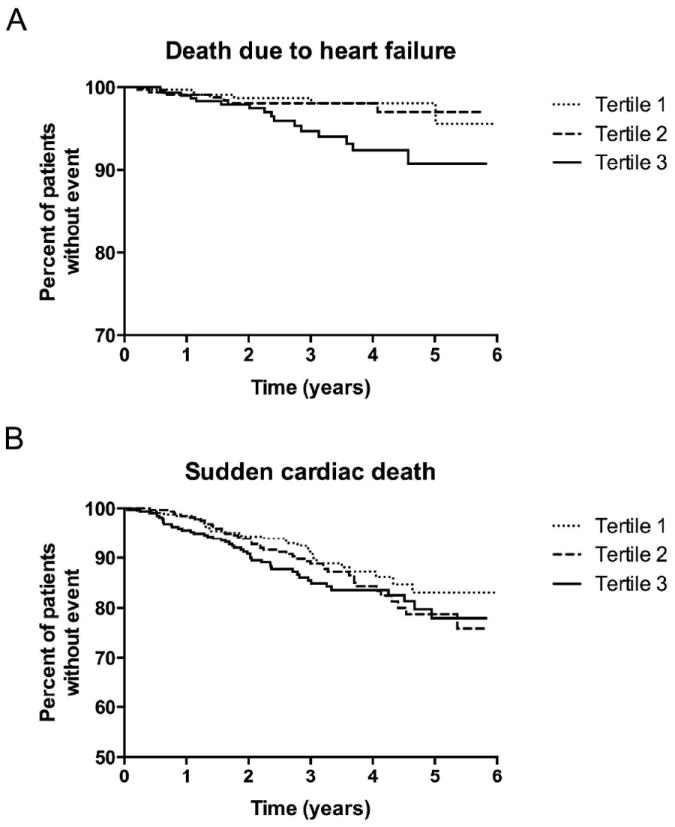
Kaplan-Meier curve estimates of the incidence of sudden cardiac death and death due to congestive heart failure in 4D study patients. Subjects were categorized into upper, middle, and lower tertiles according to serum C-Alb values measured at the outset of the study. (A) Mortality due to sudden cardiac death. (B) Mortality due to congestive heart failure.
Acknowledgments
This work was supported by the NIH KO8 HL121801 (to AHB), Howard Hughes Medical Institute (to SAK), K24 DK094872 (to RT), grants from the German Federal Ministry for Education and Research (BMBF01EO1004) and the University Hospital Wu¨erzberg ‘Gundausstattung’ grant programme (to CD and CW).
Footnotes
Disclosures: Provisional applications for U.S. and International patents related to carbamylated albumin as a biomarker has been filed by AHB, SAK, RT, and their affiliated institutions. RT is a consultant to Fresenius Medical Care North America.
References
- 1.Stark GR. Reactions of cyanate with functional groups of proteins. 3. Reactions with amino and carboxyl groups. Biochemistry. 1965;4:1030–1036. doi: 10.1021/bi00882a008. [DOI] [PubMed] [Google Scholar]
- 2.Kraus LM, Kraus AP., Jr Carbamoylation of amino acids and proteins in uremia. Kidney international Supplement. 2001;78:S102–107. doi: 10.1046/j.1523-1755.2001.59780102.x. [DOI] [PubMed] [Google Scholar]
- 3.Kalim S, Karumanchi SA, Thadhani RI, Berg AH. Protein carbamylation in kidney disease: pathogenesis and clinical implications. American Journal of Kidney Diseases. 2014 doi: 10.1053/j.ajkd.2014.04.034. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koeth RA, Kalantar-Zadeh K, Wang Z, Fu X, et al. Protein carbamylation predicts mortality in ESRD. Journal of the American Society of Nephrology : JASN. 2013;24:853–861. doi: 10.1681/ASN.2012030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg AH, Drechsler C, Wenger J, Buccafusca R, et al. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Science translational medicine. 2013;5:175ra129. doi: 10.1126/scitranslmed.3005218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duranton F, Lundin U, Gayrard N, Mischak H, et al. Plasma and urinary amino acid metabolomic profiling in patients with different levels of kidney function. Clinical journal of the American Society of Nephrology : CJASN. 2013;9:37–45. doi: 10.2215/CJN.06000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolov EO, Ray D, Savenka AV, Shah SV, et al. Chronic uremia stimulates LDL carbamylation and atherosclerosis. Journal of the American Society of Nephrology : JASN. 2010;21:1852–1857. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apostolov EO, Shah SV, Ok E, Basnakian AG. Quantification of carbamylated LDL in human sera by a new sandwich ELISA. Clinical chemistry. 2005;51:719–728. doi: 10.1373/clinchem.2004.044032. [DOI] [PubMed] [Google Scholar]
- 9.Ok E, Basnakian AG, Apostolov EO, Barri YM, et al. Carbamylated low-density lipoprotein induces death of endothelial cells: a link to atherosclerosis in patients with kidney disease. Kidney international. 2005;68:173–178. doi: 10.1111/j.1523-1755.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 10.Apostolov EO, Shah SV, Ok E, Basnakian AG. Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:826–832. doi: 10.1161/01.ATV.0000258795.75121.8a. [DOI] [PubMed] [Google Scholar]
- 11.Apostolov EO, Shah SV, Ray D, Basnakian AG. Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1622–1630. doi: 10.1161/ATVBAHA.109.189795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holzer M, Gauster M, Pfeifer T, Wadsack C, et al. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxidants & redox signaling. 2011;14:2337–2346. doi: 10.1089/ars.2010.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holzer M, Zangger K, El-Gamal D, Binder V, et al. Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: novel pathways generating dysfunctional high-density lipoprotein. Antioxidants & redox signaling. 2012;17:1043–1052. doi: 10.1089/ars.2011.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nature medicine. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 15.Green D, Roberts PR, New DI, Kalra PA. Sudden cardiac death in hemodialysis patients: an in-depth review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57:921–929. doi: 10.1053/j.ajkd.2011.02.376. [DOI] [PubMed] [Google Scholar]
- 16.Rocco MV, Yan G, Gassman J, Lewis JB, et al. Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. The National Institutes of Health-funded Hemodialysis Health Care Financing Administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002;39:146–153. doi: 10.1053/ajkd.2002.29905. [DOI] [PubMed] [Google Scholar]
- 17.Buzello M, Tornig J, Faulhaber J, Ehmke H, et al. The apolipoprotein e knockout mouse: a model documenting accelerated atherogenesis in uremia. Journal of the American Society of Nephrology : JASN. 2003;14:311–316. doi: 10.1097/01.asn.0000045048.71975.fc. [DOI] [PubMed] [Google Scholar]
- 18.Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. The New England journal of medicine. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 19.Lemos MM, Watanabe R, Carvalho AB, Jancikic AD, et al. Effect of rosuvastatin and sevelamer on the progression of coronary artery calcification in chronic kidney disease: a pilot study. Clinical nephrology. 2013;80:1–8. doi: 10.5414/CN107630. [DOI] [PubMed] [Google Scholar]
- 20.Wanner C, Krane V, Marz W, Olschewski M, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. The New England journal of medicine. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 21.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. The New England journal of medicine. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 22.Karumanchi SA, Thadhani R. Kidney complications: why don’t statins always work? Nat Med. 2010;16:38–40. doi: 10.1038/nm0110-38. [DOI] [PubMed] [Google Scholar]
- 23.Duranton F, Depner TA, Argiles A. The Saga of Two Centuries of Urea: Nontoxic Toxin or Vice Versa? Seminars in nephrology. 2014;34:87–96. doi: 10.1016/j.semnephrol.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Massy ZA. The role of lipids and uremic toxins in cardiovascular disease in CKD. Clinical and experimental nephrology. 2014;18:255–256. doi: 10.1007/s10157-013-0864-y. [DOI] [PubMed] [Google Scholar]
- 25.Alhaj E, Alhaj N, Rahman I, Niazi TO, et al. Uremic cardiomyopathy: an underdiagnosed disease. Congestive heart failure. 2013;19:E40–45. doi: 10.1111/chf.12030. [DOI] [PubMed] [Google Scholar]
- 26.Chan CT, Floras JS, Miller JA, Richardson RM, et al. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney international. 2002;61:2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 27.Pauly RP, Chan CT. Reversing the risk factor paradox: is daily nocturnal hemodialysis the solution? Seminars in dialysis. 2007;20:539–543. doi: 10.1111/j.1525-139X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 28.Thomson BK, Huang SH, Chan C, Urquhart B, et al. Nocturnal home hemodialysis associates with improvement of electrocardiographic features linked to sudden cardiac death. ASAIO journal. 2013;60:99–105. doi: 10.1097/MAT.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney international. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Coresh J, Eustace JA, Longenecker JC, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA : the journal of the American Medical Association. 2004;291:451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 31.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 32.Wanner C, Tonelli M Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group M. Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney international. 2014;85:1303–1309. doi: 10.1038/ki.2014.31. [DOI] [PubMed] [Google Scholar]
- 33.Shiu SW, Xiao SM, Wong Y, Chow WS, et al. Carbamylation of LDL and its relationship with myeloperoxidase in type 2 diabetes mellitus. Clinical science. 2013;126:175–181. doi: 10.1042/CS20130369. [DOI] [PubMed] [Google Scholar]
- 34.Wanner C, Krane V, Marz W, Olschewski M, et al. Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): demographic and baseline characteristics. Kidney & blood pressure research. 2004;27:259–266. doi: 10.1159/000080241. [DOI] [PubMed] [Google Scholar]
- 35.Wanner C, Krane V, Marz W, Olschewski M, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


