Abstract
This report examined the most frequently reported bothersome tics among individuals with chronic tic disorders and evaluated the improvement and remission of tics and their associated characteristics. Youths and adults (N = 240) were randomly assigned to receive the comprehensive behavioral intervention for tics (CBIT) or psychoeducation and supportive therapy (PST). At baseline, motor tics and tics with an urge were rated as more bothersome relative to vocal tics and tics without premonitory urges. The five most common bothersome tics included eye blinking, head jerks, sniffing, throat clearing, and other complex motor tics. While CBIT outperformed PST across tic type and urge presence, tics preceded by premonitory urges at baseline had higher severity at posttreatment across treatment condition. Six individual tic types had lower severity at posttreatment following CBIT relative to PST. Baseline urge presence was associated with tic remission for CBIT but not PST. Specific bothersome tics were more likely to remit with CBIT relative to PST. Findings suggest that individual tics respond and remit differently to CBIT relative to PST, with implications highlighting the negative reinforcement hypothesis in tic symptom maintenance.
Keywords: Tourette’s Disorder, Behavior Therapy, Habit Reversal Training, Comprehensive Behavioral Intervention for Tics, Treatment Outcome, Individualized Treatment Response
Introduction
Tics are sudden, nonrhythmic motor movements or vocalizations that are relatively common among school-age youth for brief periods of time (Cubo et al., 2011). Tourette’s Disorder and chronic tic disorders (collectively referred to as CTD) are neurodevelopmental disorders characterized by the presence of motor and/or vocal tics that persist more than a year (American Psychiatric Association, 2000). Symptoms of CTDs typically emerge in early childhood (Bloch & Leckman, 2009), with most youths experiencing a waxing and waning in tic symptom severity (Lin et al., 2002). Although tic symptoms remit for some youth, a considerable portion of adolescents have tics that persist into adulthood (Bloch et al., 2006). Tics can vary broadly across and within individuals according to type (motor or vocal), and complexity (simple or complex). Tic presentation varies little between youth and adults with CTDs. In both groups, common tics including eye blinking, head jerking, mouth movements, and simple vocalizations (McGuire et al., 2013). Likewise, across the age spectrum, those with CTDs may experience considerable impairment (Conelea et al., 2011; Conelea et al., 2013) and a reduced quality of life (Jalenques et al., 2012; Storch et al., 2007).
Historically, tics have been managed with antipsychotic and alpha-2 agonist medications (Scahill et al., 2006). Although effective (Weisman, Qureshi, Leckman, Scahill, & Bloch, 2012), these medications can be accompanied by side effects that can limit tolerability (Scahill et al., 2006). Additionally, in related conditions (e.g., obsessive-compulsive disorder, anxiety disorders), patients and/or parents often express a preference for nonpharmacological treatments (Brown, Deacon, Abramowitz, Dammann, & Whiteside, 2007; Lewin, McGuire, Murphy, & Storch, 2014; McHugh, Whitton, Peckham, Welge, & Otto, 2013; Patel & Simpson, 2010). Behavioral interventions such as habit reversal training (HRT) and its successor, the comprehensive behavioral intervention for tics (CBIT; Woods et al., 2008), offer an alternative. Although there is some variation between HRT and CBIT due to the incorporation of specific therapeutic components (e.g., relaxation training, functional analysis), both include the core therapeutic components of awareness training and competing response training (Peterson, 2007; Woods, Miltenberger, & Lumley, 1996). Randomized controlled trials consistently demonstrate the acceptability, efficacy, and safety of these behavioral interventions (Deckersbach, Rauch, Buhlmann, & Wilhelm, 2006; Piacentini et al., 2010; Wilhelm et al., 2003; Wilhelm et al., 2012), with a recent meta-analysis identifying moderate-to-large treatment effects (McGuire, Piacentini, et al., 2014). Indeed, treatment guidelines recommend behavior therapy as a first-line intervention for youths with CTDs (Murphy, Lewin, Storch, & Stock, 2013; Steeves et al., 2012; Verdellen, van de Griendt, Hartmann, & Murphy, 2011).
Although HRT and CBIT are efficacious in reducing tic severity, not all tics reduce and/or remit after behavioral treatment. Thus, it is possible that specific characteristics of tics (e.g., premonitory urge, motor/vocal, anatomical location) are associated with differential treatment response to this intervention. For instance, clinical experience suggests that some tics (e.g., eye blinking) may be more difficult to manage with behavioral interventions relative to other tics (e.g., arm movements). This treatment disparity may be attributed to the automaticity of the tic (e.g., a simple tic versus a complex tic) or to tic typology, or it may be influenced by the presence of a premonitory urge. Premonitory urges are unpleasant and distressing sensory phenomena that often precede tics and are relieved by tic expression (Leckman, Walker, & Cohen, 1993; Woods, Piacentini, Himle, & Chang, 2005). Indeed, youths and adults often identify the urge as the most distressing aspect of illness relative to tic symptoms (Leckman et al., 1993). As premonitory urges precede many tics, the presence of an urge may facilitate tic awareness training exercises and the timely implementation of competing responses, subsequently leading to better HRT/CBIT response relative to tics without premonitory urges. Contrary to anecdotal clinical experience, the literature has been mixed regarding the relationship between premonitory urges and behavioral control of tics (Ganos et al., 2012; Specht et al., 2013).
Understanding factors associated with individual tic outcome is important for several reasons. First, given the limited number and availability of trained behavioral treatment providers (Woods, Conelea, & Himle, 2010), identifying tics that respond well to behavior therapy can facilitate allocation of scarce therapeutic resources. Second, identification of tics that respond well to behavioral treatment can help clinicians successfully target tics early in the treatment process. Successful treatment of a tic can promote patient confidence and therapeutic alliance, and it can reinforce patient participation. Third, identification of tics that respond poorly to behavior therapy can highlight the need for better competing responses or augmentative approaches to enhance existent behavioral techniques. Despite the importance of this investigation for research and clinical practice, there has been no systematic examination of behavioral treatment efficacy for individual bothersome tics, largely due to small treatment samples and heterogeneous presentation of tics.
The current study examined the nature of bothersome tics and explored the efficacy of CBIT for common bothersome tics relative to a nonbehavioral treatment (e.g., psychoeducation and supportive therapy; PST). First, we characterized the most common bothersome tics reported by participants. Second, we investigated whether specific tic characteristics (e.g., motor/vocal tic type, premonitory urge) were associated with differential efficacy for CBIT relative to PST. Given that the presence of a premonitory urge may facilitate awareness training exercises, we hypothesized that tics with a premonitory urge would respond better to CBIT relative to PST. Third, we examined whether specific bothersome tics had differential efficacy for CBIT relative to PST. We hypothesized that bothersome tics would exhibited a greater reduction with CBIT compared to PST. Finally, we explored whether specific bothersome tics were more likely to remit among individuals receiving CBIT relative to PST. We hypothesized that bothersome tics would more likely remit to among individuals receiving CBIT relative to PST.
Methods
Participants
Two hundred forty-eight participants were enrolled in two randomized multisite clinical trials that examined the efficacy of CBIT for reducing tic symptom severity relative to PST (Piacentini et al., 2010; Wilhelm et al., 2012). Although differing in target age, the two studies employed similar inclusion/exclusion criteria as follows: (1) a diagnosis of CTD of moderate or greater severity; (2) English fluency; (3) an estimated IQ > 80; and (4) a stable dose of psychiatric medication for at least six weeks with no planned changes (if applicable). Exclusion criteria included: (1) an unstable medical condition; (2) current diagnosis of substance abuse/dependence; (3) lifetime diagnosis of pervasive developmental disorder, mania, or psychosis; and (4) four or more previous sessions of HRT. Although 248 participants were included in primary trials (Piacentini et al., 2010; Wilhelm et al., 2012), eight participants had unusable baseline data for bothersome tics (e.g., illegible, inconsistent, or missing). Those participants who discontinued study participation prematurely and/or did not complete the posttreatment assessment were excluded from posttreatment analyses (n = 41). Complete treatment data on participant’s most bothersome tics from baseline to endpoint was available for 207 participants. Table 1 details demographic and clinical characteristics of the sample.
Table 1.
Baseline sample demographic and clinical characteristics (N = 240)
| Characteristic | N (%) |
|---|---|
| Male | 171 (71%) |
| Co-occurring OCD | 44 (18%) |
| Co-occurring ADHD | 65 (27%) |
| Taking tic medication | 76 (32%) |
| Randomized to CBIT/HRT therapy | 122 (51%) |
| Mean | SD (Range) | |
|---|---|---|
| Age (in years) | 21.57 | 14.02 (9-70) |
| Child/adolescent participants (n = 140) | 12.37 | 2.72 (9-17) |
| Adult participants (n = 100) | 34.44 | 13.31 (18-70) |
| YGTSS total score | 23.73 | 6.41 (10-45) |
| YGTSS impairment score | 23.84 | 7.73 (5-50) |
| Number bothersome tics on HM/VTS | 6.22 | 2.02 (1-10) |
Note. OCD = Obsessive-Compulsive Disorder; ADHD = Attention-Deficit/Hyperactivity Disorder; YGTSS = Yale Global Tic Severity Scale, HM/VTS = Hopkins Motor/Vocal Tic Scale
Measures
Diagnostic interviews
Age-appropriate structured diagnostic interviews were used to assess tic and relevant co-occurring diagnoses at baseline. Youth enrolled in the study were administered the Anxiety Disorders Interview Schedule (ADIS) for DSM-IV-TR: Child Version (Silverman & Albano, 1996), which has demonstrated strong psychometric properties (Silverman, Saavedra, & Pina, 2001; Wood, Piacentini, Bergman, McCracken, & Barrios, 2002). Adult participants received the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2002). Given the focus on bothersome tics in the context of behavioral treatment, only co-occurring attention-deficit/hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) are reported here.
Yale Global Tic Severity Scale
(YGTSS; Leckman, Riddle, Hardin, & Ort, 1989). Tic severity was assessed at baseline and posttreatment using YGTSS, a clinician-rated scale with demonstrated reliability and validity (Leckman et al., 1989; Storch et al., 2005). The YGTSS includes a symptom checklist of commonly reported motor and vocal tics and yields four tic severity scores: Total Motor Tic Score (range: 0-25); Total Vocal Score (range 0-25); Total Tic Score (range 0-50): and Impairment Score (range 0-50).
Hopkins Motor/Vocal Tic Scale
(HM/VTS; Walkup, Rosenberg, Brown, & Singer, 1992). Participants nominated up to five motor and five vocal tics they deemed most bothersome at baseline using a modified version of the HM/VTS. These tics were then rated by a clinician on a 5-point scale that ranged as follows: 0 (none), 1 (mild), 2 (moderate), 3 (moderately severe), and 4 (severe). These ratings incorporated frequency, forcefulness, interference, and subject distress. For example, an arm jerking tic that is frequent and forceful, occurs in extended bouts, and interferes with handwriting or other everyday activities would probably warrant a rating of at least moderately severe. The same tic that was lesser in frequency and interference might be properly rated as mild. Participants also reported whether a premonitory urge was associated with each bothersome tic. The same bothersome tics nominated at baseline were reevaluated at midtreatment (Week 5) and posttreatment (Week 10) assessments on the 5-point scale by a treatment-blind clinician. Participants could also nominate new tics that either developed or increased in bothersomeness at the midtreatment and posttreatment assessments. Once nominated, these new bothersome tics tracked in a similar fashion, but are not included in the present report because they did not receive a full course of behavior therapy.
Treatment
Comprehensive Behavioral Intervention for Tics (CBIT) included strategies such as psychoeducation about tics, relaxation training, function-based interventions to minimize factors that worsen tics, with the emphasis of treatment placed on tic awareness training and competing response training (Woods et al., 2008). Tic awareness training involved the identification of premonitory urges that precede tics (Leckman et al., 1993) and/or early tic movements. Awareness training helped individuals recognize and intervene before fully engaging in a tic. Competing response training involved developing a behavior that was physically incompatible with the performance of the tic to be implemented contingent upon the premonitory urge or early tic movement. The competing response helped the individual respond to the urge to tic in a new manner, and attempted to break the negative reinforcement cycle between the premonitory urge and the relief following the tic. Psychoeducation and supportive therapy (PST) served as the comparison treatment condition. In this treatment, individuals received psychoeducation about the course, genetics and underlying neurobiology of CTD, as well as the rationale for current treatments. Although individuals were allowed to discuss tics and tic-related issues as part of supportive therapy, therapists were prohibited from providing advice on strategies for tic management. This treatment was designed to parallel recommended adjunctive components of psychopharmacologic treatment (Goetz & Horn, 2005).
Procedures
Child study recruitment took place at the Johns Hopkins School of Medicine, the University of California Los Angeles, and the University of Wisconsin-Milwaukee. Massachusetts General Hospital/Harvard Medical School, the University of Texas Health Science Center at San Antonio, and Yale University’s Child Study Center served as adult study recruitment sites. Both studies were approved by the Institutional Review Boards at each of these sites. All participants provided written informed consent (assent and parental permission for minors). Clinical assessments were completed by a treatment-blind independent evaluator with a master’s degree or higher in a mental health field. All independent evaluators were trained to reliability and supervised using a structured protocol (Piacentini et al., 2010; Wilhelm et al., 2012). Participants were randomly assigned to receive eight sessions of CBIT or PST over 10 weeks. At posttreatment (Week 10), the independent evaluator reassessed participant’s current tic symptoms and severity (YGTSS), the original bothersome tic symptoms (HM/VTS), and any new bothersome tics that emerged over the course of treatment (HM/VTS). Further methodological details can be found in Piacentini et al. (2010) and Wilhelm et al. (2012).
After trial completion, nominated bothersome tics were coded and grouped into 49 distinct tic type categories using the YGTSS symptom checklist to guide categorization by two raters. There was excellent agreement between the two raters (kappa = 0.91, p < .001), with categorization discrepancies resolved through discussion and consensus. Although every effort was made to ensure complete data collection across all assessments, there were a few instances in which individual bothersome tics were not appropriately tracked at the posttreatment assessment. For these few instances, the following precautionary steps were taken to ensure accurate data entry. If a bothersome tic nominated at baseline was omitted from the HM/VTS at post-treatment, the YGTSS symptom checklist was consulted to determine tic presence. If the tic appeared on the YGTSS symptom checklist at post-treatment, the tic was rated as missing data on the HM/VTS because the tic was assumed to be present without a severity rating. Alternatively, if the bothersome tic nominated at baseline was no longer endorsed on the post-treatment YGTSS symptom checklist, the tic was assumed to be extinguished and received a post-treatment HM/VTS severity rating of zero.
Analytic plan
Among the 240 participants that completed the baseline assessment, there were 27 individual bothersome tic severity ratings that were missing and/or unclear, with baseline premonitory urge data unavailable for 136 bothersome tics. For the 207 participants that completed baseline and posttreatment assessments, there were 19 individual bothersome tic severity ratings that were missing and/or unclear. Little’s Missing Completely At Random (MCAR) evaluated whether a relationship existed between missing data and any values within the dataset (observed or missing), with a significant relationship indicating that data were not MCAR (Little, 1988). Findings suggested that missing bothersome tic severity ratings were MCAR (p = .86). Although this would permit the use of missing data imputation strategies, given the preliminary nature of these analyses, these data were treated as missing and no data imputation strategies were used to minimize unintentional bias.
Descriptive statistics were used to characterize the participant sample, bothersome tic types, and associated premonitory urges (Table 2). A Pearson’s correlation examined the association between the YGTSS Total Tic Severity Score and number of bothersome tics endorsed at baseline. Independent sample t-tests compared baseline severity by tic type (motor/vocal) and premonitory urge presence (absent/present). Chi-square examined the association between tic type and premonitory urge presence. An analysis of covariance (ANCOVA) examined whether tic type, premonitory urge presence, and/or treatment group (CBIT/PST) were associated with a lower severity rating at posttreatment on the HM/VTS, controlling for baseline tic severity on the HM/VTS. Pair-wise comparisons were corrected using Sidak correction (Šidák, 1967). For each bothersome tic that was endorsed by more than 10% of the sample at baseline, an ANCOVA examined the effect of treatment group on bothersome tic severity on the HM/VTS at posttreatment, controlling for baseline bothersome tic severity ratings (Table 3). Pair-wise comparisons were corrected using Sidak corrections (Šidák, 1967). Although Levene’s test for homogeneity of variance was significant in some instances (see Table 3), ANCOVA is robust to violations of this assumption of homogeneity of variances provided the ratio of the largest group variance is not more than three times the smallest group variance (Pendhazur, 1997), which was the case in these analyses. Chi-square examined whether bothersome tic remission (characterized by a severity rating of 0 on the HM/VTS at the posttreatment assessment) differed by tic type, premonitory urge presence, and/or treatment condition. Chi-square also examined whether the remission of individual bothersome tics was more likely to occur with CBIT relative to PST (Table 3). Effect sizes are presented for continuous (Cohen’s d) and categorical (Cramer’s V) comparisons.
Table 2.
Characteristics, frequency, and severity of bothersome tics at baseline (N = 240) and severity ratings at posttreatment (N = 207)
| Tic Symptom Type | Frequency Tic Typea |
# of Participants Endorsing Tic Typea |
Baseline Urge Presentb |
Baseline HM/VTS Rating |
Posttreatment HM/VTS Rating |
|---|---|---|---|---|---|
| Motor Tics | n | n (%) | n (%) | Mean (SD) | Mean (SD) |
| Eye blinking | 98 | 96 (40%) | 39 (40%) | 2.02 (0.99) | 1.19 (1.08) |
| Head jerk/nod movements | 95 | 90 (38%) | 47 (49%) | 2.46 (1.03) | 1.41 (0.95) |
| Shoulder shrugs/movements | 64 | 64 (27%) | 36 (56%) | 1.95 (0.97) | 1.09 (0.95) |
| Mouth/tongue movements | 67 | 61 (25%) | 31 (46%) | 1.77 (0.89) | 0.93 (1.02) |
| Hand movements | 64 | 59 (25%) | 37 (58%) | 1.98 (1.02) | 1.17 (0.98) |
| Other complex motor combinations | 71 | 56 (23%) | 40 (56%) | 2.46 (1.08) | 1.03 (1.16) |
| Eye movements | 57 | 57 (24%) | 20 (35%) | 1.93 (0.95) | 0.85 (1.05) |
| Facial grimace | 52 | 52 (22%) | 26 (50%) | 2.00 (0.99) | 1.10 (0.93) |
| Arm movements | 51 | 49 (20%) | 31 (61%) | 1.94 (0.99) | 0.86 (0.95) |
| Chest/stomach tensing | 46 | 45 (19%) | 28 (61%) | 2.32 (1.18) | 0.81 (0.95) |
| Nose twitches/movements | 42 | 42 (18%) | 22 (52%) | 1.74 (1.06) | 0.91 (0.98) |
| Other motor tics | 36 | 33 (14%) | 23 (64%) | 2.11 (1.06) | 1.06 (1.16) |
| Neck stretches/movements | 34 | 33 (14%) | 24 (71%) | 1.97 (1.00) | 1.41 (0.95) |
| Foot movements | 30 | 30 (13%) | 14 (47%) | 2.10 (1.09) | 1.00 (1.24) |
| Complex head/shoulder/neck tics | 27 | 26 (11%) | 14 (52%) | 2.33 (1.18) | 1.44 (1.36) |
| Hand to face tics | 26 | 23 (10%) | 13 (50%) | 2.35 (1.13) | 1.00 (0.82) |
| Full body tic | 24 | 23 (10%) | 19 (79%) | 2.61 (1.03) | 1.65 (1.23) |
| Torso rotation/twisting/bending | 24 | 21 (9%) | 19 (79%) | 2.29 (0.91) | 1.00 (1.05) |
| Jaw movements | 18 | 18 (8%) | 10 (56%) | 2.06 (0.66) | 1.17 (1.11) |
| Leg tense | 17 | 17 (7%) | 10 (59%) | 1.82 (0.81) | 1.08 (0.73) |
| Leg kick | 14 | 14 (6%) | 8 (57%) | 2.07 (1.21) | 0.86 (1.10) |
| Pelvic tensing/movements | 13 | 13 (5%) | 9 (69%) | 2.00 (0.71) | 1.08 (1.16) |
| Object specific tics | 10 | 9 (4%) | 7 (70%) | 2.1 (0.99) | 0.60 (0.70) |
| Arm/hand movements | 7 | 7 (3%) | 3 (43%) | 2.14 (0.90) | 1.17 (0.75) |
| Complex arm and leg movements | 7 | 7 (3%) | 2 (29%) | 2.43 (0.98) | 1.29 (1.25) |
| Copropraxia | 5 | 5 (2%) | 0 (0%) | 2.60 (0.89) | 0.50 (0.71) |
| Vocal Tics | n | n (%) | n (%) | Mean (SD) | Mean (SD) |
|---|---|---|---|---|---|
| Sniffing | 75 | 75 (31%) | 32 (43%) | 1.64 (1.01) | 0.94 (1.01) |
| Throat clearing | 74 | 72 (30%) | 36 (49%) | 1.78 (1.05) | 1.11 (1.27) |
| Animal noises | 50 | 44 (18%) | 22 (44%) | 2.10 (1.07) | 0.96 (1.06) |
| Coughing | 42 | 42 (18%) | 19 (45%) | 1.98 (0.92) | 0.79 (0.99) |
| Grunting | 41 | 41 (17%) | 21 (51%) | 1.95 (0.90) | 0.91 (1.00) |
| Atypical breathing | 36 | 33 (14%) | 15 (42%) | 1.89 (0.82) | 0.70 (0.99) |
| Blocking/stuttering/pallialia | 26 | 26 (11%) | 13 (50%) | 1.96 (0.93) | 0.86 (0.99) |
| Syllables | 25 | 23 (10%) | 8 (32%) | 1.75 (0.91) | 0.89 (0.96) |
| Humming | 21 | 21 (9%) | 7 (33%) | 2.05 (1.02) | 1.05 (1.12) |
| Snorting | 18 | 18 (8%) | 9 (50%) | 1.72 (1.23) | 1.00 (1.21) |
| Other vocal tics | 17 | 17 (7%) | 9 (53%) | 2.35 (1.06) | 1.07 (1.03) |
| Speech atypicalities | 17 | 16 (7%) | 6 (35%) | 1.63 (1.02) | 1.00 (1.41) |
| Coprolalia | 14 | 12 (5%) | 10 (71%) | 3.00 (1.08) | 2.20 (1.69) |
| Echolalia | 14 | 14 (6%) | 7 (50%) | 2.07 (1.07) | 0.83 (0.94) |
| Mouth noises | 13 | 11 (5%) | 5 (38%) | 1.77 (0.83) | 0.82 (1.17) |
| Whistling | 8 | 7 (3%) | 4 (50%) | 2.25 (1.39) | 0.88 (0.64) |
| Words | 8 | 8 (3%) | 2 (25%) | 1.75 (0.71) | 0.71 (0.95) |
| Gulping/gargling/swallowing/burping | 7 | 7 (3%) | 3 (43%) | 1.86 (0.69) | 0.57 (0.53) |
| Phrases | 6 | 6 (3%) | 3 (50%) | 1.80 (0.84) | 1.40 (1.67) |
| Hiccup | 5 | 5 (2%) | 2 (40%) | 1.40 (1.14) | 1.00 (1.00) |
| Other musical tics | 2 | 2 (1%) | 1 (50%) | 1.50 (0.71) | 1.00 (1.41) |
| Other complex vocal tics | 1 | 1 (<1%) | 1 (100%) | 2.00 (-----) | ----- (------) |
Participants could endorse multiple bothersome tics with the same tic type.
Percentage based on the total frequency of bothersome tics endorsed (not number of participants endorsing the tic).
Table 3.
Symptom response and remission for individual bothersome tics (N = 207)
| Post-Tx Severitya | Tic Remission | |||||||
|---|---|---|---|---|---|---|---|---|
| CBIT | PST | CBIT | PST | |||||
| Motor Tics | Mean (SD) | Mean (SD) | F test | d | n (%) | n (%) | χ 2 | V |
| Eye blinking | 0.98 (0.95) | 1.44 (1.17) | F1,80=5.35* | .43 | 16 (36%) | 11 (28%) | 0.76 | .10 |
| Head jerk/nod movements | 1.17 (1.16) | 1.76 (1.32) | F1,80=5.47* | .48 | 13 (32%) | 11 (26%) | 0.39 | .07 |
| Other complex motor combinations | 0.64 (0.86) | 1.54 (1.30) | F1,56=16.35*** | .84 | 19 (58%) | 7 (23%) | 7.60* | .35 |
| Mouth/tongue movementsb | 0.72 (0.77) | 1.18 (1.26) | F1,51=1.62 | .46 | 14 (42%) | 9 (41%) | 0.01 | .02 |
| Hand movements | 1.08 (0.93) | 1.27 (1.04) | F1,49=0.79 | .19 | 9 (35%) | 7 (27%) | 0.36 | .08 |
| Shoulder shrugs/movements | 1.17 (1.04) | 1.00 (0.94) | F1,50=0.25 | −.18 | 9 (31%) | 7 (28%) | 0.06 | .03 |
| Eye movements | 0.96 (1.07) | 0.76 (1.05) | F1,48=0.25 | −.19 | 9 (39% | 14 (54%) | 1.06 | −.15 |
| Facial grimace | 1.18 (1.01) | 1.00 (0.84) | F1,37=0.34 | −.19 | 6 (27%) | 5 (28%) | c | −.01 |
| Arm movements | 0.83 (0.96) | 0.89 (0.96) | F1,39=0.01 | .06 | 10 (42%) | 8 (44%) | 0.03 | −.03 |
| Chest/stomach tensing | 0.77 (0.97) | 0.86 (0.95) | F1,33=0.13 | .09 | 12 (55%) | 6 (40%) | 0.76 | .14 |
| Nose twitches/movements | 0.82 (1.01) | 1.00 (0.97) | F1,30=0.11 | .18 | 9 (53%) | 6 (38%) | 0.79 | .16 |
| Other motor tics | 1.06 (1.14) | 1.07 (1.22) | F1,29=0.17 | .01 | 7 (42%) | 7 (47%) | 0.10 | −.06 |
| Neck stretches/movements | 1.47 (0.64) | 1.36 (1.22) | F1,26=0.07 | −.11 | 0 (0%) | 4 (29%) | c * | −.41 |
| Foot movements | 0.83 (0.94) | 1.18 (1.54) | F1,20=0.51 | .28 | 5 (42%) | 5 (45%) | c | −.04 |
| Complex head/shoulder/neck tics | 0.63 (0.92) | 1.82 (1.38) | F1,22=6.57* | .95 | 5 (63%) | 3 (18%) | c ^ | .45 |
| Hand to face tics | 0.93 (0.83) | 1.09 (0.83) | F1,22=0.59 | .20 | 5 (36%) | 3 (27%) | c | .09 |
| Full body tic | 1.20 (0.92) | 2.10 (1.37) | F1,17=3.87^ | .77 | 2 (20%) | 2 (18%) | c | .02 |
| Vocal Tics | Mean (SD) | Mean (SD) | F test | d | n (%) | n (%) | χ 2 | V |
|---|---|---|---|---|---|---|---|---|
| Sniffing | 0.63 (0.89) | 1.22 (1.04) | F1,59=7.48** | .60 | 17 (43%) | 11 (34%) | 3.12 ^ | .22 |
| Throat clearingb | 0.73 (0.96) | 1.71 (1.46) | F1,58=8.37** | .83 | 20 (54%) | 7 (27%) | 4.59* | .27 |
| Animal noises | 1.18 (1.22) | 0.76 (0.88) | F1,44=0.95 | −.40 | 9 (41%) | 11 (44%) | 0.05 | −.03 |
| Coughing | 0.74 (0.81) | 0.87 (1.25) | F1,35=0.80 | .15 | 11 (48%) | 8 (53%) | 0.11 | −.05 |
| Grunting | 0.94 (1.06) | 0.88 (0.96) | F1,29=0.07 | −.06 | 7 (44%) | 8 (47%) | 0.04 | −.03 |
| Atypical breathing | 0.52 (0.60) | 1.11 (1.54) | F1,27=0.59 | .61 | 11 (52%) | 5 (56%) | c | −.03 |
| Blocking/stuttering/pallialia | 0.50 (0.76) | 1.07 (1.07) | F1,19=2.01 | .59 | 5 (63%) | 7 (47%) | c | 15 |
| Syllables | 0.50 (0.76) | 1.20 (1.03) | F1,15=2.75 | .76 | 5 (63%) | 5 (38%) | c | .23 |
Analyses controlled for baseline tic severity.
Heterogeneous baseline variances observed by Levene’s Test.
Fisher’s Exact Test.
p < .05,
p < .01,
p < .001
p = .06-0.08
Results
Bothersome tics
Participants reported an average of 6.22 bothersome tics at baseline (SD = 2.02; range: 1-10). A strong relationship was observed between YGTSS total tic severity and the number of bothersome tics nominated at baseline (r = .68, p < .001), which is unsurprising given that YGTSS total tic severity score incorporates the number of tics present and interference caused by the tics. Table 2 provides a list of the most frequently nominated bothersome tics and their baseline severity. The five most frequently endorsed bothersome tics included eye blinking, head jerking or nodding, sniffing, throat clearing, and other complex motor combinations. Bothersome tics with the greatest severity ratings included coprolalia, full body tics, copropraxia, head jerking or nodding, and other complex motor combinations. At baseline, motor tics had greater severity ratings (M = 2.11, SD = 1.03) than vocal tics (M = 1.90, SD = 1.01), t(1490) = 3.68, p < .001, d = .21. Tics associated with a baseline premonitory urge (n = 766, 55%, M = 2.19, SD = 1.01) were rated as more severe than tics without an identified urge (n = 617, 45%, M = 1.86, SD = 1.00), t(1381) = 6.22, p < .001, d = .33. At baseline, bothersome motor tics were more likely to have an associated premonitory urge (58% versus 42%) relative to vocal tics (51% versus 49%), χ2 = 5.23, p < .02, V = .06.
Impact of tic type and premonitory urge on posttreatment tic severity
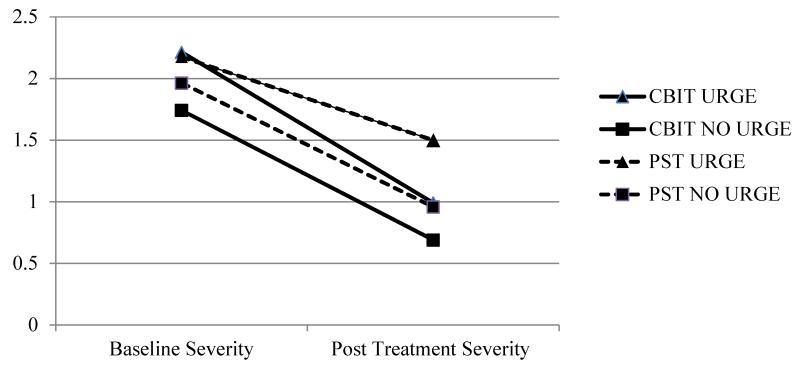
An ANCOVA revealed that baseline tic severity on the HM/VTS positively predicted posttreatment tic severity on the HM/VTS, F(1,167) = 157.45, p < .001. When controlling for baseline tic severity of the individual tics on the HM/VTS, a significant main effect for treatment condition [F(1, 1167) = 40.18, p < .001, η2p = .03] and premonitory urge presence [F(1, 1167) = 20.88, p < .001, η2p = .02] was found at posttreatment, but there was no significant main effect for tic type (i.e., motor vs. vocal; p = .09, η2p < .01). The interaction between treatment condition and baseline urge presence was also significant [F(1,1167) = 5.59, p < .015, η2p = .005]; however, no other interactions were significant (p = .35-.89). Pair-wise comparisons revealed that bothersome tics treated with CBIT had lower severity at posttreatment (M = 0.83, SE = 0.04) relative to bothersome tics treated with PST (M = 1.22, SE = 0.04, p < .001, d = .37). Additionally, tics preceded by a premonitory urge at baseline were rated as more severe at posttreatment (M = 1.17, SE = 0.04) relative to tics without an urge at baseline (M = 0.89, SE = 0.05, p < .001, d = .27). Follow-up analyses for the treatment condition by baseline urge interaction revealed that bothersome tics with a baseline urge had lower severity scores following treatment with CBIT (M = 0.97, SE = 0.05) relative to PST (M = 1.51, SE = 0.06, p < .001, d = .53), F(1,670) = 48.85, p < .001, η2p = .07) (see Figure 1). Similarly, bothersome tics without an urge at baseline were also rated as less severe following CBIT (M = 0.72, SE = 0.06) relative to treatment with PST (M = 0.93, SE = 0.06, p < .015, d = .22), F(1,500) = 5.98, p < .015 η2p = .01).
Figure 1.
Baseline and Post Treatment Severity Ratings on the Hopkins Motor/Vocal Tic Scale by baseline premonitory urge status and treatment condition.
Impact of tic type and premonitory urge on bothersome tic remission
Thirty-nine percent of bothersome tics assessed at posttreatment (n = 1301) met criteria for tic remission at the posttreatment assessment (a severity rating of 0 on the HM/VTS). Bothersome tics of any type were more likely to remit with CBIT relative to PST (44% versus 34%, χ2 = 13.99, p < .001, V = .10), and this finding also held for motor (39% versus 31%, χ2 = 5.52, p < .019, V = .08) and vocal tics (52% versus 38%, χ2 = 8.85, p < .003, V = .14) separately. Tics associated with premonitory urge at baseline (n = 674) were more likely to remit following treatment with CBIT than with PST (37% versus 21%), χ2 = 19.03, p < .001, V = .17. However, there was no statistically significant difference between remission rates for CBIT (55%) and PST (48%) for tics without a premonitory urge at baseline (n = 503), χ2 = 2.48, p = .12, V = .07. Interestingly, bothersome tics without a baseline urge were more likely to remit relative to bothersome tics with a baseline urge in both CBIT (55% versus 37%, χ2 = 19.77, p < .001, V = .18) and PST conditions (48% versus 21%, χ2 = 43.97, p < .001, V = .28). For participants receiving CBIT, vocal tics were more likely to remit compared to motor tics (52% versus 39%, χ2 = 10.09, p < .001, V = .12). However, there was no difference between motor or vocal tic remission rates for PST (32% versus 38%, χ2=2.59, p = .11, V = .06).
Posttreatment tic severity and remission for individualized bothersome tics after treatment
Table 3 presents the results from the ANCOVA for the most commonly reported bothersome tics (those endorsed by 10% or more of the sample). After controlling for baseline tic severity on the HM/VTS, the following bothersome tics had significantly lower tic severity scores at posttreatment with CBIT relative to PST: eye blinking; head jerking or nodding; sniffing; throat clearing; complex head/shoulder/neck tics; and other complex motor tic combinations. Notably, full body tics had lower tic severity ratings that trended toward significance (p = .06) at posttreatment with CBIT relative to PST.
Few specific bothersome tic types demonstrated differential remission rates across the two treatment conditions. Only throat clearing and other complex motor combinations of tics evidenced significantly higher remission with CBIT than PST, although both complex head/shoulder/neck tics (p = .06) and sniffing tics (p = .08) each trended towards greater remission with CBIT compared to PST. Meanwhile, neck stretches/movements were more likely to remit with PST relative to CBIT (see Table 3).
Discussion
This study examined the baseline characteristics of bothersome tics in a large sample of children, adolescents, and adults with CTDs. At baseline, motor tics and tics associated with a premonitory urge were more commonly nominated as bothersome relative to vocal tics and tics without an associated urge, respectively. More specifically, bothersome motor tics were more likely to be preceded by an urge relative to vocal tics. When examining specific tics, the five most frequently nominated bothersome tics included eye blinking, head jerking or nodding, sniffing, throat clearing, and other complex motor combinations. Individual tic severity did not appear to be related to the frequency of nominated tics, as only head jerking or nodding and other complex motor combinations were frequently reported, with coprolalia, copropraxia, and full body tics being severe but less common. From a clinical perspective, these severe tics are commonly reported as highly distressing and interfering due to their negative social impact. Indeed, these severe tics may serve as potential triggers to treatment-seeking behaviors and may prove to be useful early targets for treatment since their resolution could lead to greater reduction in associated distress and impairment.
This study also examined whether specific tic characteristics or specific types of tics were associated with differential reduction tic severity or tic remission with CBIT relative to PST. In general, CBIT outperformed PST in reducing bothersome tic severity, which is consistent with overall findings from the CBIT RCTs (Piacentini et al., 2010; Wilhelm et al., 2012). Across treatment conditions, bothersome tics associated with an urge at baseline had greater severity at posttreatment relative to tics without an urge. These findings are consistent with the conceptualization of premonitory urges as an aversive sensation that is associated with greater symptom severity. Alternatively, it may be that more complex tics possessed baseline urges and caused greater impairment relative to simple tics that infrequently have associated urges.
When examining associations between tic characteristics and tic remission, both motor and vocal tics were more likely to remit with CBIT relative to PST, with a more robust effect observed for vocal tics treated with CBIT. Given that the competing response for most vocal tics is controlled breathing, participants may have been able to implement this response more readily due to its common use with other vocal tics. Additionally, controlled breathing may require more concentration and may be more effective in directing attention away from the premonitory urge relative to muscle tensing responses used for motor tics. Furthermore, controlled breathing may also serve as a calming function that alleviates anxiety that can increase tic presence. While tics with a baseline urge were more likely to remit with CBIT relative to PST, remission rates for tics without a premonitory urge at baseline did not significantly differ by treatment (p = .12). This may suggest that tics without an urge are more transient in nature and have the potential to remit in the absence of targeted interventions. Alternatively, it may be that tic symptoms are less likely to persist in the absence of the negative reinforcement cycle hypothesized to maintain tic behavior (e.g., urge-relief cycle; Capriotti, Brandt, Turkel, Lee, & Woods, 2014; Himle, Woods, Conelea, Bauer, & Rice, 2007; Specht et al., 2013). Taken together, these findings support anecdotal evidence that tics with a premonitory urge are good initial candidates for CBIT. Given that complex tics are more likely than simple tics to be preceded by a premonitory urge, our finding that complex tics with such urges are good targets for CBIT has somewhat counterintuitive treatment implications. More specifically, they support the use of CBIT as first-line intervention for these arguably more severe tics as opposed to the current practice of psychopharmacologic intervention or other more invasive techniques.
When examining individual bothersome tics, eye blinking, head jerking or nodding, complex head/shoulder/neck tics, other complex motor combinations, sniffing, and throat clearing had lower posttreatment severity with CBIT compared to PST. Given that these are many of the same common bothersome tics that participants frequently nominated (e.g., eye blinking, head jerking or nodding, sniffing, throat clearing, and other complex motor combinations), CBIT is an ideal and efficacious treatment to manage bothersome tics. When examining tic remission, an interesting pattern emerged. Complex motor combinations and throat clearing were more likely to remit with CBIT, with complex head/shoulder/neck tics and sniffing tics trending towards significantly greater remission with CBIT. Although future research should examine the transition and evolution of tics in greater detail, taken together, these findings suggest that tics that respond well to CBIT in many cases are likely to remit.
Individual bothersome tics that did not statistically differ between treatments may be accounted for in at least three ways. First, some individual tic comparisons had modest power due to limited tic endorsement within the sample. Thus, differences that did not reach statistical significance may be attributed to low power and should be interpreted with caution. Second, these data focus only on bothersome tics nominated at baseline and do not identify which tics were specifically targeted at which point in the CBIT treatment condition. Tics with premonitory urges are associated with more distress and/or higher severity ratings, and thus, may have served as initial targets in treatment relative to tics without premonitory urges. This may likely account the nonsignificant difference between treatment groups for tics without baseline urges as these tics without urges may likely have not been targeted initially in treatment (p = .12). Finally, some tics may be more challenging to treat with current behavioral approaches due to their specific nature, minimal awareness, and/or lack of an adequate competing response. Future research should examine the differential benefit of specific competing responses for individual tics and explore other pharmacological or behavioral augmentation strategies that have shown promise in related conditions (McGuire, Lewin, & Storch, 2014).
Although novel and noteworthy, several limitations exist. First, the analysis focused on the five most bothersome motor and vocal tics as reported by participants at baseline. While this captured the most relevant tics for treatment, it did not capture all tics that could have been targeted in treatment. Second, we did not assess the effect of CBIT on tics that emerged after baseline. These tics may have served as targets for CBIT due to onset or perceived severity and would not have been captured in the present analysis. While some case reports suggest that behavior therapy may precipitate new tics (Burd & Kerbeshian, 1988), larger studies have failed to find any difference in tic onset in response to behavior therapy (Peterson et al., in submission). Third, the behavioral descriptions of nominated tics were coded and grouped into 49 distinct tic categories using the YGTSS symptom checklist to guide categorization. Although this coding approach was selected to make the findings clinically applicable with YGTSS, some tic categories could have been subsumed into broader tic groupings and may have influenced study findings (e.g., eye blinking and eye movements into a broader category of eye tics). Finally, we did not examine therapeutic factors such as homework compliance (Park et al., 2014) and treatment expectancy (Lewin, Peris, Bergman, McCracken, & Piacentini, 2011) that have been shown to influence treatment outcomes in related interventions.
In summary, this study examined the most bothersome tics in a large sample of individuals with CTDs and explored whether specific tic characteristics or individual tics exhibited a differential reduction in tic severity with CBIT relative to PST. This examination suggested that CBIT outperformed PST on several tic characteristics and individual tics, with baseline urge presence being associated with greater tic remission for CBIT. These findings provide hope for patients and clinicians alike as they demonstrate that some bothersome tics can remit with behavior therapy. Indeed, tic remission in CBIT may likely be attributed to the discontinuation of the negative reinforcement cycle that is implicated in the maintenance of tic symptoms (Capriotti et al., 2014; Himle et al., 2007; Specht et al., 2013). This rationale receives further support by the findings that tics without a baseline urge were more likely to remit in either treatment, as there was no urge-relief cycle to reinforce tic symptoms. Given the robust reduction in tic severity and remission to CBIT for tic characteristics and specific tics, it raises questions as to whether significant improvement observed in prior treatment studies of behavior therapy may be largely driven by the dramatic improvement of individual bothersome tics. While this may be true for some patients that present with one or two severe tics, the broad domain of tic characteristics captured by the YGTSS (number, frequency, intensity, complexity, interference) would not likely be influenced by the reduction/remission of a handful of bothersome tics. However, future research should explore this question in greater detail. From both a research and clinical perspective, these findings strongly encourage a comprehensive assessment of individuals with CTDs that included both individualized tic ratings and standardized global tic rating scales (McGuire et al. 2012). Indeed, it would be interesting to conduct parallel analyses of individualized tic responses to psychiatric medication (e.g., alpha-2 agonists, antipsychotics). These analyses may prove beneficial in identifying the clinical presentation of individuals with CTD that would benefit from pharmacotherapy or behavior therapy.
Highlights.
Nominated motor tics had greater severity relative to nominated phonic tics.
Tics with a premonitory urge had greater severity relative to tics without urges.
CBIT was efficacious relative to PST for tics without and without premonitory urges.
Tics with a baseline premonitory urge were more likely to remit with CBIT.
Individual tics had differential improvement and remission with CBIT relative to PST.
Acknowledgments
This research was supported by the following National Institute of Mental Health grants: R01MH070802 (Dr. Piacentini) with subcontracts to Drs. Woods, Scahill, Wilhelm, Peterson, and Walkup; 5R01MH069877 (Dr. Wilhelm), R01MH069874 (Dr. Scahill), and RO1MH069875 (Dr. Peterson), with subcontracts to Drs. Piacentini and Woods. Dr. Walkup served as a consult on these three grants. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinicaltrials.gov Identifiers: NCT00218777; NCT00231985
References
- American Psychiatric Association . Diagnostic and Statistic Manual of Mental Disorders. 4th ed. American Psychiatric Publishing; Washington, DC: 2000. text rev. [Google Scholar]
- Bloch MH, Leckman JF. Clinical course of Tourette syndrome. Journal of Psychosomatic Research. 2009;67:497–501. doi: 10.1016/j.jpsychores.2009.09.002. http://dx.doi.org/10.1016/j.jpsychores.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, Leckman JF. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Archives of Pediatric and Adolescent Medicine. 2006;160:65–69. doi: 10.1001/archpedi.160.1.65. http://dx.doi.org/10.1001/archpedi.160.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Deacon BJ, Abramowitz JS, Dammann J, Whiteside SP. Parents’ perceptions of pharmacological and cognitive-behavioral treatments for childhood anxiety disorders. Behaviour Research and Therapy. 2007;45:819–828. doi: 10.1016/j.brat.2006.04.010. http://dx.doi.org/10.1016/j.brat.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Burd L, Kerbeshian J. Symptom substitution in Tourette disorder. Lancet. 1988;2:1072. doi: 10.1016/s0140-6736(88)90083-9. http://dx.doi.org/10.1016/s0140-6736(88)90083-9. [DOI] [PubMed] [Google Scholar]
- Capriotti MR, Brandt BC, Turkel JE, Lee H-J, Woods DW. Negative reinforcement and premonitory urges in youth with Tourette syndrome: An experimental evaluation. Behavior Modification. 2014;38:276–296. doi: 10.1177/0145445514531015. [DOI] [PubMed] [Google Scholar]
- Conelea CA, Woods DW, Zinner SH, Budman C, Murphy T, Scahill LD, Compton SN, Walkup J. Exploring the impact of chronic tic disorders on youth: Results from the Tourette Syndrome Impact Survey. Child Psychiatry and Human Development. 2011;42:219–242. doi: 10.1007/s10578-010-0211-4. http://dx.doi.org/10.1007/s10578-010-0211-4. [DOI] [PubMed] [Google Scholar]
- Conelea CA, Woods DW, Zinner SH, Budman CL, Murphy TK, Scahill LD, Compton SN, Walkup JT. The impact of Tourette syndrome in adults: Results from the Tourette Syndrome Impact Survey. Community Mental Health Journal. 2013;49:110–120. doi: 10.1007/s10597-011-9465-y. http://dx.doi.org/10.1007/s10597-011-9465-y. [DOI] [PubMed] [Google Scholar]
- Cubo E, Gabriel y Galan JM, Villaverde VA, Velasco SS, Benito VD, Macarron JV, Guevara JC, Louis ED, Benito-Leon J. Prevalence of tics in schoolchildren in central Spain: a population-based study. Pediatric Neurology. 2011;45:100–108. doi: 10.1016/j.pediatrneurol.2011.03.003. http://dx.doi.org/10.1016/j.pediatrneurol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Rauch S, Buhlmann U, Wilhelm S. Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behaviour Research and Therapy. 2006;44:1079–1090. doi: 10.1016/j.brat.2005.08.007. http://dx.doi.org/10.1016/j.brat.2005.08.007. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Ganos C, Kahl U, Schunke O, Kuhn S, Haggard P, Gerloff C, Roessner V, Thomalla G, Munchau A. Are premonitory urges a prerequisite of tic inhibition in Gilles de la Tourette syndrome? Journal of Neurology, Neurosurgery and Psychiatry. 2012;83:975–978. doi: 10.1136/jnnp-2012-303033. http://dx.doi.org/10.1136/jnnp-2012-303033. [DOI] [PubMed] [Google Scholar]
- Goetz C, Horn S. The Treatment of Tics. In: Kurlan R, editor. Handbook of Tourette’s Syndrome and Related Tic and Behavioral Disorders. 2nd ed Marcel Dekker; New York, NY: 2005. pp. 411–426. [Google Scholar]
- Himle MB, Woods DW, Conelea CA, Bauer CC, Rice KA. Investigating the effects of tic suppression on premonitory urge ratings in children and adolescents with Tourette’s syndrome. Behaviour Research and Therapy. 2007;45:2964–2976. doi: 10.1016/j.brat.2007.08.007. http://dx.doi.org/10.1016/j.brat.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Jalenques I, Galland F, Malet L, Morand D, Legrand G, Auclair C, Hartmann A, Derost P, Durif F. Quality of life in adults with Gilles de la Tourette Syndrome. BMC Psychiatry. 2012;12:109. doi: 10.1186/1471-244X-12-109. http://dx.doi.org/10.1186/1471-244x-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette’s syndrome. American Journal of Psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- Lewin AB, McGuire JF, Murphy TK, Storch EA. Editorial Perspective: Parents want Exposure/Response Prevention Therapy for OCD. Parent preferences and perceptions of behavioral and pharmacological treatments for childhood obsessive-compulsive disorder. Journal of Child Psychology and Psychiatry. 2014;55:1314–1316. doi: 10.1111/jcpp.12344. [DOI] [PubMed] [Google Scholar]
- Lewin AB, Peris TS, Bergman RL, McCracken JT, Piacentini J. The role of treatment expectancy in youth receiving exposure-based CBT for obsessive compulsive disorder. Behaviour Research and Therapy. 2011;49:536–543. doi: 10.1016/j.brat.2011.06.001. http://dx.doi.org/10.1016/j.brat.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Yeh CB, Peterson BS, Scahill L, Grantz H, Findley DB, Katsovich L, Otka J, Lombroso PJ, King RA, Leckman JF. Assessment of symptom exacerbations in a longitudinal study of children with Tourette’s syndrome or obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1070–1077. doi: 10.1097/00004583-200209000-00007. http://dx.doi.org/10.1097/00004583-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- McGuire JF, Kugler BB, Park JM, Horng B, Lewin AB, Murphy TK, Storch EA. Evidence-based assessment of compulsive skin picking, chronic tic disorders and trichotillomania in children. Child Psychiatry and Human Development. 2012;43(6):855–883. doi: 10.1007/s10578-012-0300-7. doi: 10.1007/s10578-012-0300-7. [DOI] [PubMed] [Google Scholar]
- McGuire JF, Lewin AB, Storch EA. Enhancing exposure therapy for anxiety disorders, obsessive-compulsive disorder and post-traumatic stress disorder. Expert Review of Neurotherapeutics. 2014;14:893–910. doi: 10.1586/14737175.2014.934677. http://dx.doi.org/10.1586/14737175.2014.934677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Nyirabahizi E, Kircanski K, Piacentini J, Peterson AL, Woods DW, Wilhelm S, Walkup JT, Scahill L. A cluster analysis of tic symptoms in children and adults with Tourette syndrome: clinical correlates and treatment outcome. Psychiatry Research. 2013;210:1198–1204. doi: 10.1016/j.psychres.2013.09.021. http://dx.doi.org/10.1016/j.psychres.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Brennan EA, Lewin AB, Murphy TK, Small BJ, Storch EA. A meta-analysis of behavior therapy for Tourette Syndrome. Journal of Psychiatric Research. 2014;50:106–112. doi: 10.1016/j.jpsychires.2013.12.009. http://dx.doi.org/10.1016/j.jpsychires.2013.12.009. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: A meta-analytic review. Journal of Clinical Psychiatry. 2013;74:595–602. doi: 10.4088/JCP.12r07757. http://dx.doi.org/10.4088/jcp.12r07757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Lewin AB, Storch EA, Stock S. Practice parameter for the assessment and treatment of children and adolescents with tic disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:1341–1359. doi: 10.1016/j.jaac.2013.09.015. http://dx.doi.org/10.1016/j.jaac.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Park JM, Small BJ, Geller DA, Murphy TK, Lewin AB, Storch EA. Does d-Cycloserine Augmentation of CBT Improve Therapeutic Homework Compliance for Pediatric Obsessive-Compulsive Disorder? Journal of Child and Family Studies. 2014;23:863–871. doi: 10.1007/s10826-013-9742-1. http://dx.doi.org/10.1007/s10826-013-9742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Simpson HB. Patient preferences for obsessive-compulsive disorder treatment. Journal of Clinical Psychiatry. 2010;71:1434–1439. doi: 10.4088/JCP.09m05537blu. http://dx.doi.org/10.4088/jcp.09m05537blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendhazur EJ. Multiple regression in behavioral research. 3rd ed. Hardcourt Brace College Publishers; New York: 1997. [Google Scholar]
- Peterson AL. Psychosocial management of tics and intentional repetitive behaviors associated with Tourette syndrome. In: Woods DW, Piacentini J, Walkup JT, editors. Treating Tourette syndrome and tic disorders: a guide for practitioners. Guilford Press; New York: 2007. pp. 154–184. [Google Scholar]
- Peterson AL, McGuire JF, Wilhelm S, Piacentini JC, Woods DW, Walkup JT, Hatch J, Scahill L. An Empirical Examination of Symptom Substitution Associated with Behavior Therapy. Behavior Therapy. doi: 10.1016/j.beth.2015.09.001. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S, Walkup JT. Behavior therapy for children with Tourette disorder: A randomized controlled trial. Journal of the American Medical Association. 2010;303:1929–1937. doi: 10.1001/jama.2010.607. http://dx.doi.org/10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Erenberg G, Berlin CM, Jr., Budman C, Coffey BJ, Jankovic J, Kiessling L, King RA, Kurlan R, Lang A, Mink J, Murphy T, Zinner S, Walkup J. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx: The Journal of the American Society for Experimental NeuroTherapeutics. 2006;3:192–206. doi: 10.1016/j.nurx.2006.01.009. http://dx.doi.org/10.1016/j.nurx.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šidák Z. Rectangular confidence regions for the means of multivariate normal distributions. Journal of the American Statistical Association. 1967;62:626–633. [Google Scholar]
- Silverman WK, Albano AM. The Anxiety Disorders Interview Schedule for DSM-IV-Child and Parent Versions. Graywinds Publications; San Antonio, TX: 1996. [Google Scholar]
- Silverman WK, Saavedra LM, Pina AA. Test-retest reliability of anxiety symptoms and diagnoses with anxiety disorders interview schedule for DSM-IV: Child and parent versions. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:937–944. doi: 10.1097/00004583-200108000-00016. http://dx.doi.org/10.1097/00004583-200108000-00016. [DOI] [PubMed] [Google Scholar]
- Specht MW, Woods DW, Nicotra CM, Kelly LM, Ricketts EJ, Conelea CA, Grados MA, Ostrander RS, Walkup JT. Effects of tic suppression: ability to suppress, rebound, negative reinforcement, and habituation to the premonitory urge. Behaviour Research and Therapy. 2013;51:24–30. doi: 10.1016/j.brat.2012.09.009. http://dx.doi.org/10.1016/j.brat.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Steeves T, McKinlay BD, Gorman D, Billinghurst L, Day L, Carroll A, Dion Y, Doja A, Luscombe S, Sandor P, Pringsheim T. Canadian guidelines for the evidence-based treatment of tic disorders: Behavioural therapy, deep brain stimulation, and transcranial magnetic stimulation. The Canadian Journal of Psychiatry/La Revue canadienne de psychiatrie. 2012;57:144–151. doi: 10.1177/070674371205700303. Retrieved from http://publications.cpaapc.org/browse/sections/0. [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Lack C, Milsom VA, Geffken GR, Goodman WK, Murphy TK. Quality of life in youth with Tourette’s syndrome and chronic tic disorder. Journal of Clinical Child and Adolescent Psychology. 2007;36:217–227. doi: 10.1080/15374410701279545. http://dx.doi.org/10.1080/15374410701279545. [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Sajid M, Allen P, Roberti JW, Goodman WK. Reliability and validity of the Yale Global Tic Severity Scale. Psychological Assessment. 2005;17:486–491. doi: 10.1037/1040-3590.17.4.486. http://dx.doi.org/10.1037/1040-3590.17.4.486. [DOI] [PubMed] [Google Scholar]
- Verdellen C, van de Griendt J, Hartmann A, Murphy T. European clinical guidelines for Tourette Syndrome and other tic disorders. Part III: Behavioural and psychosocial interventions. European Child & Adolescent Psychiatry. 2011;20:197–207. doi: 10.1007/s00787-011-0167-3. http://dx.doi.org/10.1007/s00787-011-0167-3. [DOI] [PubMed] [Google Scholar]
- Walkup J, Rosenberg LA, Brown J, Singer HS. The validity of instruments measuring tic severity in Tourette’s syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:472–477. doi: 10.1097/00004583-199205000-00013. http://dx.doi.org/10.1097/00004583-199205000-00013. [DOI] [PubMed] [Google Scholar]
- Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: Pharmacological treatment of tic disorders – efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neuroscience and Biobehavioral Reviews. 2012;37:1162–1171. doi: 10.1016/j.neubiorev.2012.09.008. http://dx.doi.org/10.1016/j.neubiorev.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Deckersbach T, Coffey BJ, Bohne A, Peterson AL, Baer L. Habit reversal versus supportive psychotherapy for Tourette’s disorder: a randomized controlled trial. American Journal of Psychiatry. 2003;160:1175–1177. doi: 10.1176/appi.ajp.160.6.1175. http://dx.doi.org/10.1176/appi.ajp.160.6.1175. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, Chang S, Liu H, Dziura J, Walkup JT, Scahill L. Randomized trial of behavior therapy for adults with Tourette syndrome. Archives of General Psychiatry. 2012;69:795–803. doi: 10.1001/archgenpsychiatry.2011.1528. http://dx.doi.org/10.1001/archgenpsychiatry.2011.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Piacentini JC, Bergman RL, McCracken J, Barrios V. Concurrent validity of the anxiety disorders section of the Anxiety Disorders Interview Schedule for DSM-IV: Child and Parent Versions. Journal of Clinical Child and Adolescent Psychology. 2002;31:335–342. doi: 10.1207/S15374424JCCP3103_05. http://dx.doi.org/10.1207/s15374424jccp3103_05. [DOI] [PubMed] [Google Scholar]
- Woods DW, Conelea CA, Himle MB. Behavior therapy for Tourette’s disorder: Utilization in a community sample and an emerging area of practice for psychologists. Professional Psychology: Research and Practice. 2010;41:518–525. http://dx.doi.org/10.1037/a0021709. [Google Scholar]
- Woods DW, Miltenberger RG, Lumley VA. Sequential application of major habit-reversal components to treat motor tics in children. Journal of Applied Behavior Analysis. 1996;29:483–493. doi: 10.1901/jaba.1996.29-483. http://dx.doi.org/10.1901/jaba.1996.29-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DW, Piacentini J, Chang SW, Deckersbach T, Ginsburg GS, Peterson AL, Scahill LD, Walkup JT, Wilhelm S. Managing Tourette Syndrome: A Behavioral Intervention for Children and Adolescents. Oxford University Press; New York, NY: 2008. [Google Scholar]
- Woods DW, Piacentini J, Himle MB, Chang S. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. Journal of Developmental And Behavioral Pediatrics. 2005;26:397–403. doi: 10.1097/00004703-200512000-00001. http://dx.doi.org/10.1097/00004703-200512000-00001. [DOI] [PubMed] [Google Scholar]