Abstract
Background
Classic galactosemia (CG) is a potentially lethal genetic disorder that results from profound loss of galactose-1-phosphate uridylyltransferase (GALT). CG is detected by newborn screening (NBS) in many countries; however, conclusive diagnosis can be complex due to broad and overlapping ranges of GALT activity. Molecular studies can also be complex due to allelic heterogeneity at the GALT locus.
Methods
We conducted both biochemical and molecular follow-up studies for an infant flagged by NBS for possible galactosemia. To clarify the diagnosis we also conducted biochemical and RNA studies of lymphoblasts prepared from the child and one parent.
Results
We identified a novel noncoding GALT variant, c.377+17C>T, that was homozygous in the child and heterozygous in both parents. The child and both parents also showed diminished GALT activity in red blood cells, and transformed lymphoblasts from the child and one parent further showed diminished GALT activity. However, qRT-PCR studies demonstrated apparently normal GALT mRNA levels in lymphoblasts, and Gal-1P values measured in the child following galactose exposure in infancy and at 1 year were normal.
Conclusions
These results highlight the existence of rare but apparently benign variants in GALT and underscore the need for functional studies to distinguish pathogenic from benign variants.
Keywords: galactosemia, newborn screening, non-coding variant, functional studies, qRT-PCR
1. Introduction
Classic galactosemia (CG, OMIM 230400) is an autosomal recessive disorder that results from profound loss of the enzyme galactose-1-phosphate uridylyltransferase (GALT, EC 2.7.7.12). Loss of GALT disrupts galactose metabolism and leads to an accumulation of galactose and metabolites including galactose-1-phosphate (Gal-1P), galactitol, and galactonate following exposure of an affected infant to breast milk or milk-based formula, which contain relatively large quantities of galactose in the form of lactose (reviewed in [1, 2]). Acute symptoms of CG can present within days following milk exposure and progress rapidly from jaundice, vomiting, and diarrhea to failure to thrive, hepatomegaly, E. coli sepsis, and neonatal death. Fortunately, classic galactosemia may be identified pre-symptomatically by newborn screening (NBS) due to low GALT activity and/or elevated total galactose in blood spots; galactosemia is one of the most common metabolic disorders identified by NBS in the United States [3].
Infants flagged by NBS as potentially affected with galactosemia are switched immediately from milk to a low galactose soy or elemental formula. This simple dietary intervention prevents or reverses the acute symptoms of CG, but long-term complications may still occur (reviewed in [1, 2]). Follow-up testing of an infant identified by NBS as potentially galactosemic may confirm a diagnosis of classic galactosemia, or, more frequently, reveals that the infant has a variant form of galactosemia associated with partial rather than profound loss of GALT, is a carrier for a GALT pathogenic variant, or was simply a "false positive" of the screen [4]. Finally, some NBS programs also identify infants with other forms of galactosemia, such as epimerase (GALE) deficiency or galactokinase (GALK) deficiency (reviewed in [2]).
Establishing the correct diagnosis for an infant with low GALT activity is essential for determining appropriate intervention [5]. Accurate diagnosis also has carrier and recurrence risk implications for the immediate and extended family. The diagnostic process can be complex, however, due to the broad and overlapping ranges of GALT activities seen in infants from different diagnostic categories [4]. Diagnosis can be further complicated by the diet-dependence of galactose metabolite accumulation in patient samples, and by allelic heterogeneity at the GALT locus. More than 260 different GALT variants have been reported (http://arup.utah.edu/database/GALT/GALT_welcome.php); these include predominantly coding and non-coding point substitutions whose functional significance may be subtle or unclear.
Here we report identification and functional studies of a novel variant in GALT intron 4 (c.377+17C>T) found in an infant flagged for possible galactosemia by NBS due to borderline low GALT activity and elevated total galactose. That this variant was not seen in control populations and was found in the homozygous state in the child raised concern that it might be pathogenic. However, studies of both GALT activity and RNA from lymphoblasts from the child and a parent confirmed that while GALT activity was marginally low in this family, the c.377+17C>T variant is unlikely to be causal.
2. Materials and Methods
2.1 Study subjects
The infant, his mother and father, designated here as FKT395, FKT395P1 and FKT395P2, respectively, were ascertained by referral from their metabolic specialist. Informed consent was obtained in accordance with Emory University Institutional Review Board Protocol 00024933 (PI: JL Fridovich-Keil). Positive and negative control samples used in this study were derived from classic galactosemic cases and unaffected controls previously enrolled in this study. The hemolysate biochemical data and GALT genotyping data presented here were generated in clinical labs, as noted.
2.2 In silico analyses of the new variant
The frequency of c.377+17C>T in the general population was determined using dbSNP Build 137, Exome Variant Server version ESP6500 (http://evs.gs.washington.edu), 1000 Genomes (http://browser.1000genomes.org/index.html) and the Exome Aggregation Consortium (http://exac.broadinstitute.org/). The predicted effect of c.377+17C>T on use of splice sites was determined using the Berkeley Drosophila Genome Project (BDGP) human splice site prediction tool (http://www.fruitfly.org/seq_tools/splice.html).
2.3 Lymphoblast culture
Epstein-Barr Virus (EBV)-transformed lymphoblasts were prepared from fresh blood samples from the patient and one parent as described previously [6]; transformation of cells from the other parent was attempted but unsuccessful for unknown reasons. Transformed lymphoblasts were maintained in RPMI-1640 medium (Hyclone) containing glucose (2 g/L) and L-glutamine (0.3g/L) and supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml), 25 mmol/L Hepes, and 10% (v/v) fetal bovine serum (FBS) (Gibco/Invitrogen, Carlsbad, CA, USA). All cultures were maintained at 37°C in a humidified 5% CO2 incubator (NuAire, Plymouth, MN, USA).
2.4 Quantitative RT-PCR (qRT-PCR)
RNA samples were prepared from lymphoblast cells using the RNeasy Mini Kit (Qiagen) as instructed by the manufacturer. RNA was quantified by ultraviolet absorption using a NanoDrop 1000 instrument (Thermo Scientific), then used as a template for cDNA synthesis using the High Capacity cDNA reverse transcription kit from Applied Biosystems, as instructed by the manufacturer. All qRT–PCR reactions were performed as described previously [7]. The GALT-specific primers used were (forward) 5’-AACCCCCACCCCCACTGCC-3’ and (reverse) 5’-ACTGGTTAGGACCAGACGTTCCTT-3’.
2.5 GALT and GALE enzyme assays from lymphoblast cell lysates
Lymphoblast cell cultures were harvested and lysates prepared and analyzed for GALT and GALE activities as described previously [8]. Enzyme activity was defined in units of pmol product produced per minute per microgram of soluble protein.
3. Results
3.1 Case history
The male infant, designated FKT395, was born in Michigan at full term to consanguineous parents of Lebanese ancestry. Both prenatal and birth histories were unremarkable, and there was no reported family history of galactosemia. At 28 days of age, the infant's physical examination was normal, including growth and developmental parameters. Clinical data obtained for this child and his parents are summarized in Table 1.
Table 1.
Clinical test results for FKT395 and his parents.
| Volunteer | Genotype | GALT activitya | Total galactoseb |
Gal-1Pc |
|---|---|---|---|---|
| FKT395 (child) |
c.377+17C>T / c.377+17C>T |
2.9 U/g Hgb (NBS day 1), 1.9 U/g Hgb (NBS day 8), 6.3 µMol/hr/g Hgb (follow- up at 10 days), 5.5 µMol/hr/g Hgb (follow- up at 2.5 months) |
20.8mg/dl (NBS day 1), 26.8mg/dl (NBS day 8) |
0.03 µMol/g Hgb (10 days, breast milk); 0 mg/dl (11 months, soy formula); 0.13 and 0.12 mg/dl (12 months, 2 and 4 weeks post-galactose challenge) |
| FKT395P1 (mother) |
WT/ c.377+17C>T |
12.2 µMol/hr/g Hgb | ||
| FKT395P2 (father) |
WT/ c.377+17C>T |
12.1 µMol/hr/g Hgb |
control ranges for GALT activity: >3.1 U/g Hgb (for dried blood spots) or 17–37µMol/hr/g Hgb (follow-up from red blood cells);
control range for total galactose: <20 mg/dl (from dried blood spots);
control ranges for Gal-1P: ≤0.17 µMol/g Hgb or ≤1.0 mg/dl (from hemolysate)
The infant was flagged for possible galactosemia by newborn screening due to low GALT activity and elevated total galactose; screens conducted on samples collected at one and eight days of life demonstrated GALT activities of 2.9 and 1.9 U/gram Hgb, respectively (normal value >3.1). Total galactose was measured reflexively on these samples per protocol, with resulting values of 20.8 and 26.8 mg/dl, respectively (normal <20). Diagnostic testing of a fresh blood sample collected in follow-up to the abnormal NBS result, at day10 of life, revealed a GALT activity of 6.3 µmol/hr/gram Hgb (normal: 17–37). The red blood cell Gal-1P was well within the normal range at 0.03 µmol/gram Hgb (normal: ≤0.17) although the infant had been breast-fed. A repeat enzyme study conducted at 2.5 months of age showed a GALT activity of 5.5 µmol/hr/gram Hgb (normal: 17–37). Subsequent biochemical testing of parental samples showed GALT activities of 12.1 µmol/gm Hgb (father) and 12.2 µmol/gm Hgb (mother), both judged by the reporting lab to be consistent with carrier status.
Targeted molecular analysis of DNA from FKT395 confirmed the absence of common variants in GALT including c.563A>G (p.Q188R), c.404C>T (p.S135L), c.584T>C (p.L195P), c.855G>T (p.K285N), c.940A>G (p.N314D), and c.652C>T (p.L218L). The absence of N314D contradicted the hypothesis that the child might have Duarte variant galactosemia [9]. However, subsequent full sequence analysis of all eleven GALT exons and surrounding intronic regions revealed a novel variant in intron 4, c.377+17C>T in the homozygous state in the child. Subsequent DNA studies of parental samples confirmed that both were heterozygous for this same variant.
The child’s diet was changed from breast milk to powdered soy formula at 10 days of life; this galactose-restricted diet was maintained for the remainder of the first year of life. At 11 months of age, while still on galactose restriction, the child’s RBC Gal-1P was 0 mg/dl, and at 12 months his ability to metabolize galactose was assessed by a galactose challenge under supervision by a metabolic dietitian. Specifically, the child was given up to 16 ounces of milk, or the equivalent, per day for four weeks, and his RBC Gal-1P was measured at both two and four weeks into the challenge. The resulting Gal-1P levels (0.13 and 0.12 mg/dl, respectively) were both considered within the normal range (≤0.17 mg/dl) so all dietary restrictions were removed and the child was discharged from metabolic follow-up.
3.2 In silico analysis of c.377+17C>T
The c.377+17C>T variant has not been observed in over 120,000 alleles in large population studies (see Materials and Methods). From existing knowledge of splicing signals in humans, this variant is not predicted to disrupt the exon 4/intron 4 splice site (see Materials and Methods); therefore, absent further information c.377+17C>T was classified as a variant of unknown significance.
3.3 Testing GALT enzyme activity in case and control lymphoblasts
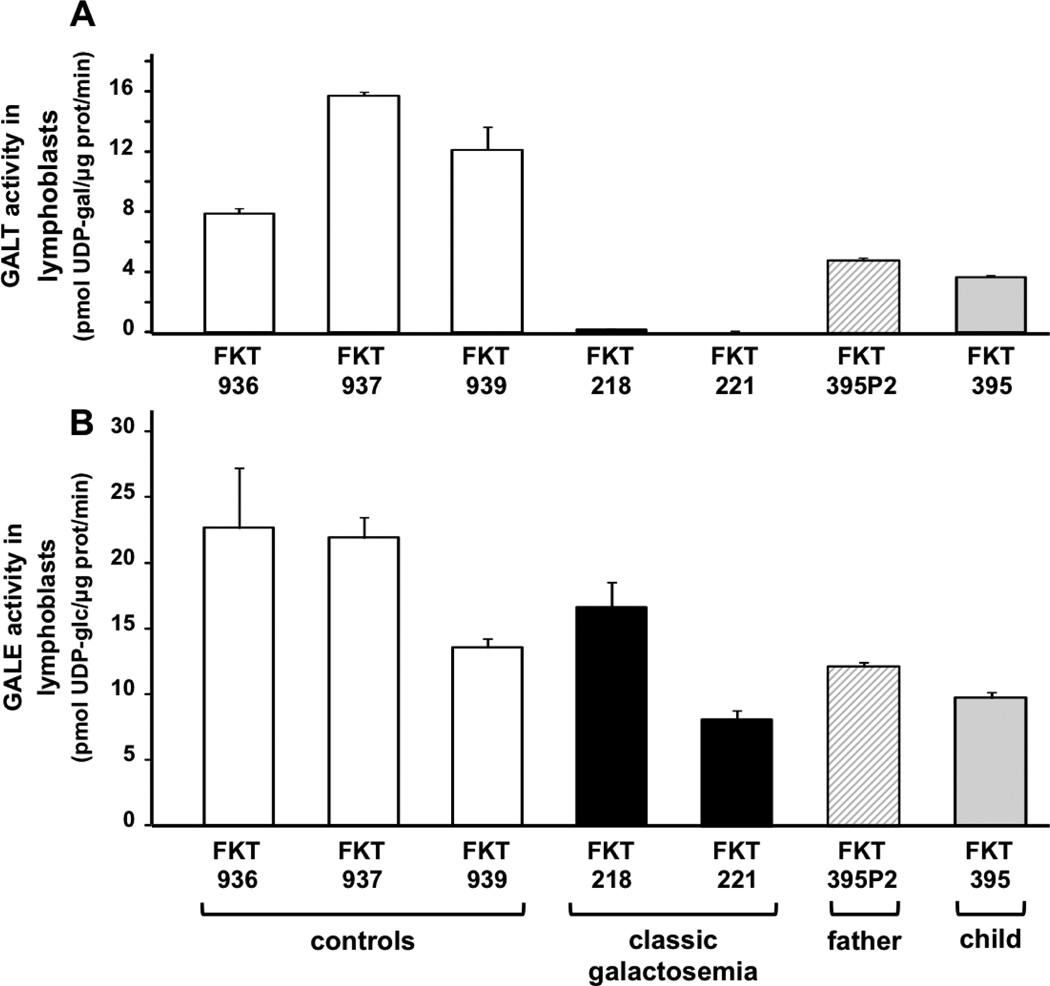
To assess the impact of the c.377+17C>T variant on GALT function in a controlled setting we generated EBV-transformed lymphoblasts using fresh blood samples from both FKT395 and his father (FKT395P2), and tested both GALT and GALE enzymatic activities in lysates from these cultures (Figure 1). Samples prepared from lymphoblasts representing three unrelated controls (FKT936, FKT937, and FKT939), and two unrelated patients with classic galactosemia (FKT218 and FKT221, both Q188R homozygotes) were evaluated in parallel. As expected, GALT activity in the three controls was strong and comparable to levels we have reported previously [8], while in the two classic galactosemia samples GALT activity was essentially undetectable. Consistent with the clinical red blood cell GALT activities presented in Table 1, lymphoblasts from FKT395 and FKT395P2 both exhibited GALT activities below the controls, but clearly well above the classic galactosemics (Figure 1A). As expected, the GALE activity levels in all of these cell lines were similar (Figure 1B), confirming integrity of the samples.
Figure 1. GALT and GALE activity levels in lymphoblast cells.
In vitro assays of GALT (panel A) and GALE (panel B) activities were conducted using lysates from EBV-transformed lymphoblasts from three unaffected controls (open bars), two patients with classic galactosemia (both Q188R homozygotes, black bars), the father (c.377+17C>T heterozygote, cross-hatched bars) and the child (c.377+17C>T homozygote, shaded bars). These results demonstrate both the ranges and variability seen in GALT and GALE activities among controls and patients. The presence of normal GALE activity even in lysates with no detectable GALT activity confirms integrity of the samples.
3.4 Testing GALT cDNA levels in case and control lymphoblasts
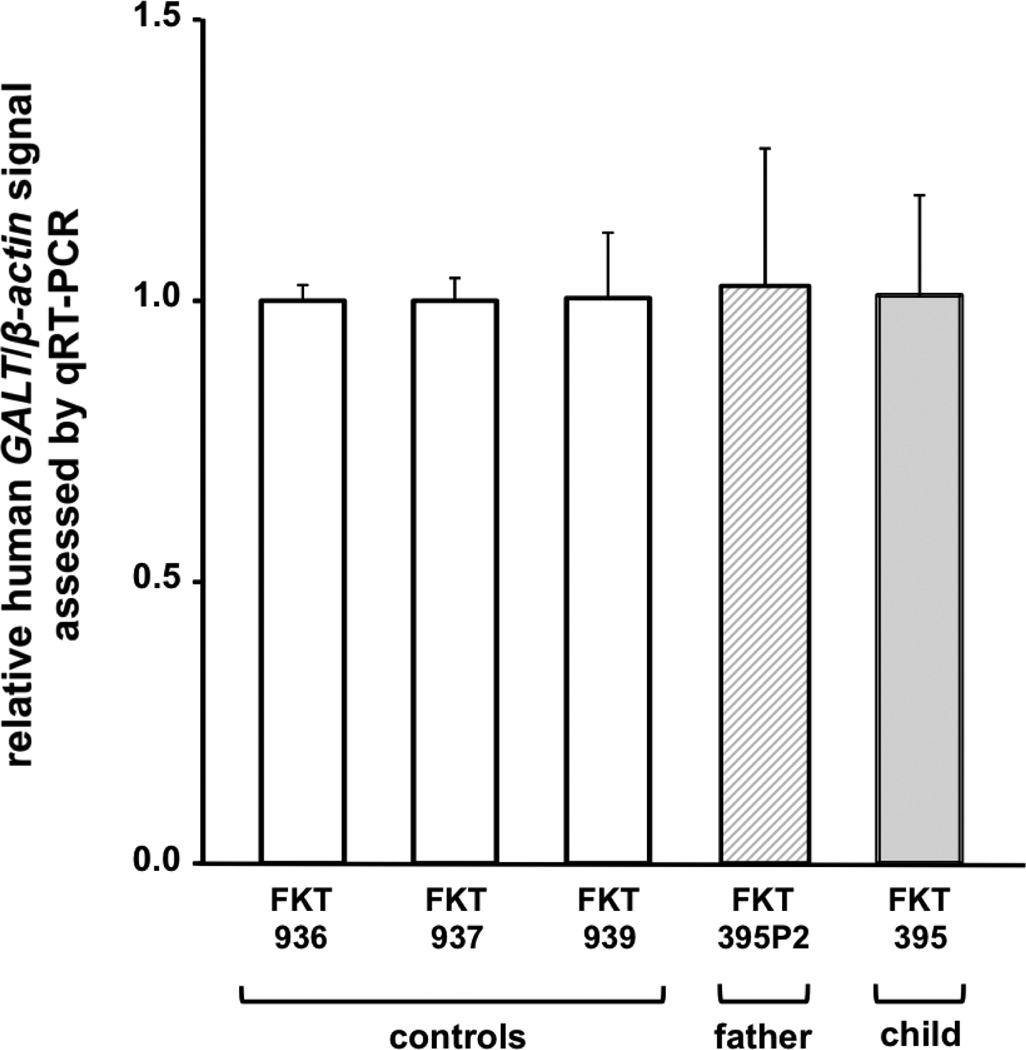
To test whether the c.377+17C>T variant altered GALT message levels we prepared RNA samples from lymphoblasts representing FKT395 and FKT395P2 and performed quantitative RT-PCR. GALT cDNA levels were normalized to β-actin in all samples. As a control we also tested GALT cDNA levels in three lymphoblast cell lines from unrelated, unaffected volunteers (FKT936, FKT937, and FKT939). As presented in Figure 2, the relative GALT cDNA levels in all of the samples were indistinguishable, indicating that the c.377+17C>T variant did not exert any detectable impact on GALT mRNA expression.
Figure 2. GALT expression levels in lymphoblast cells.
Relative expression levels of GALT message, normalized to β-actin, were quantified by qRT-PCR of RNA isolated from EBV-transformed lymphoblasts. Open bars represent signal from control samples; FKT395P2 is heterozygous for the c.377+17C>T variant and FKT395 is homozygous for the c.377+17C>T variant. All GALT/β-actin ratios are presented scaled to a single control result.
4. Discussion
The case study reported here illustrates two important points. First, while biochemical and molecular studies, combined, may be sufficient for clear diagnosis of classic or variant galactosemia in most cases, in some cases even this powerful combination leaves the story unresolved. Second, the case reported here highlights the reality that the biochemical ranges and molecular variant databases currently used to distinguish “normal” from “abnormal” test results in galactosemia, as in other genetic disorders, are broad but still incomplete. Individuals from under-studied populations, such as the family reported here, may therefore be flagged as distinct from controls by biochemical and/or molecular criteria, but they may, nonetheless, be healthy and unaffected.
The child described here was identified by newborn screening as potentially affected with galactosemia on the basis of marginally low GALT activity and marginally elevated total galactose; follow-up biochemical testing confirmed low red blood cell GALT activity in the child and both parents. However, at 10 days of age the child's red blood cell Gal-1P remained normal despite breastfeeding. Further, although the child was found to be homozygous for a novel intronic variant in GALT, studies from lymphoblasts revealed no apparent impact on GALT mRNA levels.
The simplest explanation for the results reported here is that the child, FKT395, does not have galactosemia despite his relatively low GALT activity and his homozygous familial GALT variant. This conclusion is supported by the child's normal Gal-1P level measured at age 10 days while breastfeeding and his normal Gal-1P levels measured at 2 and 4 weeks into a galactose challenge at 12 months. This conclusion is also supported by the similar GALT activity levels detected in the child's and parental lymphoblasts. Finally, this conclusion is supported by studies of GALT cDNA abundance in lymphoblasts from the child, a parent, and three controls, all of which demonstrated very similar levels.
The variant identified in this family, c.377+17C>T, has not been reported previously from studies of galactosemia patients (http://arup.utah.edu/database/GALT/GALT_welcome.php) or from population studies of human genomic diversity (http://evs.gs.washington.edu, http://browser.1000genomes.org/index.html, and http://exac.broadinstitute.org/). It may therefore be rare, or isolated to the ancestral population specific to this family. Since the parents are consanguineous, in the child this rare variant may be identical by decent.
The explanation for the lowered GALT activity in this family remains unclear; similarly, the basis for the >2-fold range of what is considered "normal" GALT activity among controls also remains unclear. The RNA results reported for this family suggest that altered GALT transcription and/or RNA processing are unlikely explanations, at least for this family, but none of the results reported here rule out potentially subtle changes in protein synthesis or stability, possible changes in post-translational covalent modification, or altered saturation of the GALT polypeptide with appropriate metal cofactors [10]. Of course, because our RNA studies were conducted on lymphoblasts, it is also possible that the low GALT activities detected in the child's and parental hemolysates reflected the presence of some other non-coding variant, outside the region sequenced, that might have impacted GALT expression in red blood cells but not lymphoblasts.
Finally, while the data reported here suggest that c.377+17C>T is a benign variant in GALT, that conclusion derives from limited analyses of direct patient samples (blood) and from laboratory studies of lymphoblasts. We therefore cannot rule out the possibility that c.377+17C>T impacts GALT expression or function in other tissues or cell types, or at specific times in development that we did not study.
5. Conclusions
Despite being identified in the homozygous state in an infant flagged for possible galactosemia by newborn screening, a novel non-coding variant of GALT, c.377+17C>T, appears to be a benign variant. This case illustrates the potential complexities of diagnosis in galactosemia despite state-of-the-art biochemical and genetic testing, and underscores the need for functional studies to determine whether a variant is pathogenic.
Highlights.
We conducted follow-up studies of an infant with a positive NBS for galactosemia.
We identified a novel non-coding variant of GALT in the child and both parents.
We demonstrated that this variant does not reduce GALT mRNA levels in the child.
We demonstrated that galactose metabolism in the child remains apparently normal.
Our results illustrate the complexities of diagnosis of galactosemia.
Acknowledgments
We are grateful to the family described in this report for volunteering to participate in this research study, and to Tyler Gleason for confirming the GALT genotype sequences of both parents. This work was supported in part by NIH grant R01DK059904 (to JLFK).
Abbreviations
- CG
Classic galactosemia
- GALT
Galactose 1-phosphate uridylyltransferase
- NBS
newborn screening
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- Gal-1P
galactose-1-phosphate
- GALE
UDP-galactose 4'-epimerase
- GALK
galactokinase
- EBV
Epstein Barr Virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry G. Classic Galactosemia and Clinical Variant Galactosemia. In: Pagon R, Adam M, Ardinger H, et al., editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 2014. [Google Scholar]
- 2.Fridovich-Keil JL, Walter JH. Valle D, Beaudet A, Vogelstein B, Kinzler K, Antonarakis S, Ballabio A, editors. Galactosemia. The Online Metabolic & Molecular Bases of Inherited Disease: McGraw Hill. 2008 http://www.ommbid.com/
- 3.CDC. Morbidity and Mortality Weekly Report (MMWR) Atlanta, GA: Centers for Disease Control and Prevention; 2012. CDC Grand Rounds: Newborn Screening and Improved Outcomes; pp. 390–393. [PubMed] [Google Scholar]
- 4.Pyhtila BM, Shaw KA, Neumann SE, Fridovich-Keil JL. Newborn screening for galactosemia in the United States: looking back, looking around, and looking ahead. JIMD Rep. 2015;15:79–93. doi: 10.1007/8904_2014_302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry G. Galactosemia: when is it a newborn screening emergency? Mol Genet Metab. 2012;106:7–11. doi: 10.1016/j.ymgme.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Human genetics. 1986;73:320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- 7.Jumbo-Lucioni PP, Hopson ML, Hang D, Liang Y, Jones DP, Fridovich-Keil JL. Oxidative stress contributes to outcome severity in a Drosophila melanogaster model of classic galactosemia. Disease models & mechanisms. 2013;6:84–94. doi: 10.1242/dmm.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Bentler K, Coffee B, Chhay JS, Sarafoglou K, Fridovich-Keil JL. A Case Study of Monozygotic Twins Apparently Homozygous for a Novel Variant of UDP-Galactose 4'-epimerase (GALE) : A Complex Case of Variant GALE. JIMD Rep. 2013;7:89–98. doi: 10.1007/8904_2012_153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridovich-Keil J, Gambello M, Singh R, Sharer J. Duarte Variant Galactosemia. In: Pagon R, Adam M, Ardinger H, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 2014. [Google Scholar]
- 10.Ruzicka FJ, Wedekind JE, Kim J, Rayment I, Frey PA. Galactose-1-phosphate uridylyltransferase from Escherichia coli, a zinc and iron metalloenzyme. Biochemistry. 1995;34:5610–5617. doi: 10.1021/bi00016a036. [DOI] [PubMed] [Google Scholar]