Abstract
Background
Single-shot fast spin echo (SSFSE) is particularly appealing in pediatric patients because of its motion robustness. However radiofrequency energy deposition at 3 tesla forces long pauses between slices, leading to longer scans, longer breath-holds and more between-slice motion.
Objective
We sought to learn whether modulation of the SSFSE refocusing flip-angle train could reduce radiofrequency energy deposition without degrading image quality, thereby reducing inter-slice pauses and overall scan times.
Materials and methods
We modulated the refocusing flip-angle train for SSFSE to minimize energy deposition while minimizing blurring and motion-related signal loss. In a cohort of 50 consecutive patients (25 boys, mean age 5.5 years, range 1 month to 17 years) referred for abdominal MRI we obtained standard SSFSE and variable refocusing flip-angle (vrfSSFSE) images and recorded sequence scan times. Two readers independently scored the images in blinded, randomized order for noise, tissue contrast, sharpness, artifacts and left lobe hepatic signal uniformity on a four-point scale. The null hypothesis of no difference between SSFSE and vrfSSFSE image-quality was assessed with a Mann-Whitney U test, and the null hypothesis of no scan time difference was assessed with the paired t-test.
Results
SSFSE and vrfSSFSE mean acquisition times were 54.3 s and 26.2 s, respectively (P-value <0.0001). For each reader, SSFSE and vrfSSFSE noise, tissue contrast, sharpness and artifacts were not significantly different (P-values 0.1810.86). However, SSFSE had better left lobe hepatic signal uniformity (P<0.01, both readers).
Conclusion
vrfSSFSE is twice as fast as SSFSE, with equivalent image quality with the exception of left hepatic lobe signal heterogeneity.
Introduction
MR imaging of the pediatric abdomen has proved to be a powerful diagnostic tool and is increasingly preferred over CT because of ionizing radiation concerns [1] and because MR provides improved soft-tissue characterization. However, acquiring high-quality abdominal MR images can be challenging in children [2]. These patients are frequently unable to stay still or maintain a breath-hold, which, along with irregular respiratory patterns, results in more frequent motion artifacts than imaging in adults. Another challenge is the often small body size and resulting high spatial resolution requirements in these patients.
Because of the need for speed and high spatial resolution, imaging at higher (3-T) field strengths can be very helpful compared to imaging at 1.5 T. Most sequences can leverage the higher intrinsic signal available at 3 T to achieve higher parallel imaging acceleration factors and thereby reduce motion artifacts. Single-shot fast spin-echo sequences (SSFSE and half-Fourier acquisition single-shot turbo spin-echo, or HASTE) are particularly appealing for abdominal MRI [3], especially in pediatric patients, because they result in fewer motion artifacts on individual images than conventional fast spin-echo-based sequences. However, the significantly increased radiofrequency energy deposition (measured in MRI as specific absorption rate, or SAR) at 3 T requires long pauses between acquisition of SSFSE slices (i.e. increased repetition time), leading to longer scan and breath-hold times. This effect can be reduced but not completely mitigated using parallel imaging, and at 3 T acquisition times for SSFSE are often slower than at 1.5 T.
A software solution that does not involve reducing the number of phase encodes or otherwise directly compromising the scan parameters is modulation of the refocusing flip-angle train, which is used in single-shot fast spin-echo imaging. Flip-angle modulation for spin-echo sequences has been reported [4, 5] and is mostly used in the context of volumetric 3-D fast spin-echo imaging (e.g., CUBE [GE Healthcare, Waukesha, WI], SPACE [Sampling Perfection with Application of optimized Contrasts using different flip angle Evolution, Siemens Healthcare, Erlangen, Germany], VISTA [Volumetric Isotropic T2W Acquisition, Philips Healthcare, Best, Netherlands]) [6]. The motivation for its development in the context of volumetric imaging was largely the need to control and diminish T2 decay over the very long spin-echo trains required to achieve reasonable scan times. However, in addition to achieving better maintenance of signal levels to achieve sharper images, flip-angle modulation can be used to reduce specific absorption rate. We describe the use of flip-angle modulation in two-dimensional SSFSE imaging in a pediatric clinical setting and compare its image quality and acquisition time to those of a conventional constant refocusing flip-angle SSFSE sequence.
Materials and methods
This study was approved by the institutional review board and complies with the Health Insurance Portability and Accountability Act. We obtained informed consent in all patients.
We used an algorithm similar to that used in the 3-D fast spin-echo technique described by Busse et al. [6] for computing an echo train with refocusing flip-angle modulation. The sequence is characterized by four flip-angle parameters: finit (initial flip angle), fmin (minimum angle to ramp down to), fcent (flip angle at the center of k-space), fend (ending flip angle). The flip angle over the course of the train varied smoothly among these parameters.
Using volunteers, we varied the four parameters to optimize repetition time (TR) and image quality [7, 8]. To stabilize signal over the initial portion of the echo train (center of k-space) and thereby reduce blurring, fmin was dropped from finit and then slowly ramped up to fcent. Further reductions in fmin led to increasing intermittent dephasing artifacts over the left lobe of the liver from cardiac motion and became unacceptable below an fmin value of 50°. Significant decreases in TR (reflecting SAR reductions) were readily achieved by decreasing flip angles toward the end of the echo train (decreasing fend) [7]. The parameters found empirically to yield an acceptable balance between reducing scan time and maintaining image quality were finit 130°, fmin 90°, fcent 100°, and fend 45°. These values were used for the remainder of this study, yielding a calculated flip-angle train as shown (Fig. 1).
Fig. 1.
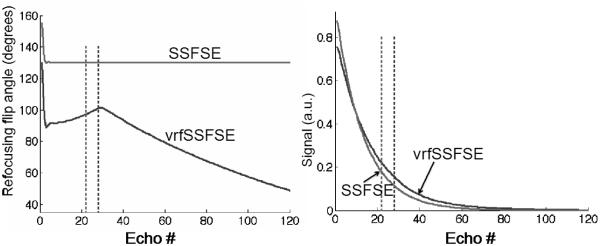
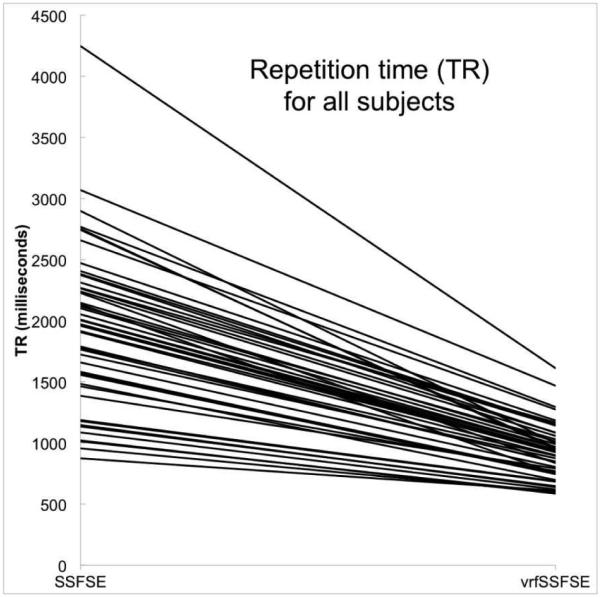
Calculated flip-angle train. a Graphs show refocusing flip-angle train (left) and resulting expected signal intensity (right). The signal intensity curves were derived via simulation as previously described [8]. b Change in repetition time (TR) from SSFSE to vrfSSFSE in all subjects. Each line connects the TR on the left for SSFSE to the TR on the right for vrfSSFSE for a single subject. For each subject, there is a decrease in the TR for vrfSSFSE and the drop in the repetition time is larger for larger SSFSE repetition times. SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
We recruited 50 consecutive children (25 boys and 25 girls) referred to our clinic for abdominal MRI. Each child was scanned on a 3-T MRI scanner (MR750; GE Healthcare, Waukesha, WI). The MR imaging protocol consisted of standard institutional sequences, including conventional SSFSE (constant refocusing flip angle of 130°) and vrfSSFSE, with both sequences using the following parameters: 32-channel cardiac array (38 cases, 76%) or torso array (12 cases, 24%) depending on patient size, ARC (auto-calibrating reconstruction Cartesian sampling) parallel imaging factor 3, half-Fourier k-space filling with homodyne reconstruction, effective TE 100 ms, matrix 416×288 and bandwidth 41.7 kHz. Number of slices (17–41), slice thickness (5–6 mm) and field of view (24–44 cm) were adjusted to each child’s anatomy but were identical between both sequences for a given subject. No respiratory triggering was used, and exams under anesthesia were performed in free-breathing fashion. The interslice gap was 0 mm for conventional SSFSE and for vrfSSFSE, and slices were acquired in an interleaved order to reduce cross-talk between adjacent slices.
Two readers (with 8 years and 1 year of experience interpreting body MRI) independently scored the images in blinded, randomized order for noise, tissue contrast, sharpness, artifacts and left lobe hepatic signal uniformity on a four-point scale (Table 1). A two-tailed Mann-Whitney U test was used to assess the null hypothesis that there was no significant difference in image quality between SSFSE and vrfSSFSE for each image quality feature. A P-value of less than 0.05 was considered statistically significant. Weighted kappa coefficients were used to assess the inter-observer agreements with the following scale: almost perfect (0.8–1.0), substantial (0.6–0.8), moderate (0.4–0.6), fair (0.2–0.4), slight (0.0–0.2) and poor (<0.0). Scan times were also recorded, and the null hypothesis of no significant difference in scan time was assessed with a paired t-test, with a P-value of less than 0.05 considered statistically significant.
Table 1.
Scoring criteria for image assessment when evaluated independently
| Score | Noise | Image contrast | Sharpness | Artifacts | Left lobe hepatic signal uniformity |
|---|---|---|---|---|---|
| 1 (Poor) | No graininess in fat or fluid |
Good contrast between fat-fluid |
Not sharp fluid boundary |
Severe artifacts, non-diagnostic |
Severe non- uniformity, non- diagnostic |
| 2 (Fair) | No graininess in kidneys or spleen |
Good contrast between spleen-fat |
Sharp fluid boundary |
Moderate artifacts, decreased diagnostic ability |
Moderate non- uniformity, decreased diagnostic ability |
| 3 (Good) | No graininess in liver |
Good contrast between renal cortex-medulla or liver-spleen |
Sharp kidney/fat boundary |
Slight artifacts, no decreased diagnostic ability |
Mild non- uniformity, no decreased diagnostic ability |
| 4 (Excellent) | No graininess in muscle |
Good contrast between renal cortex-medulla and liver-spleen |
Sharp right lobe liver boundary |
No significant artifacts |
Good uniformity |
Results
The clinical indications for the study population were diagnosis or follow-up of tumor (27 children, 54%), urinary tract obstruction or renal function (9 children, 18%), abdominal pain, infection or inflammation (6 children, 12%), post liver transplant complication (3 children, 6%) and other conditions (5 children, 10%). Mean age was 5.5+/−4.8 years (+/− SD, range 1 month to 17 years). Forty children (80%) were scanned under general anesthesia and the rest (10 children, 20%) were scanned without sedation. There was a significant reduction in scan time from SSFSE to vrfSSFSE sequences (Fig. 1). For SSFSE, average TR and scan time were 1,957 ms (max 4,250 ms, min 876 ms) and 54.3 s (max 100 s, min 24s); for vrfSSFSE, average TR and scan time were 921 ms (max 1,612 ms, min 587 ms) and 26.2 s (max 43 s, min 15 s) (P<0.01).
Image scores are summarized in Table 2. For both readers, there was no significant difference in image quality between SSFSE and vrfSSFSE in terms of noise, tissue contrast, sharpness and artifacts, with P-values of 0.36, 0.72, 0.30 and 0.86 for reader 1 and 0.75, 0.54, 0.81 and 0.18 for reader 2. Weighted-kappa interobserver agreement in terms of noise, tissue contrast, artifacts and left lobe hepatic signal uniformity for SSFSE were 0.2, 0.4, 0.03 and 1.31 and for vrfSSFSE were 0.17, 0.5, 0.04 and 0.59. For reference, note that Landis-Koch characterize kappa values as: 0–0.2 as slight, 0.2–0.4 as fair, 0.4–0.6 as moderate, 0.6–0.8 as substantial and 0.8–1.0 as near perfect agreement. Agreements were not calculated for sharpness because observed concordances were smaller than mean-chance concordances (Table 3).
Table 2.
Average image-quality scores across five parameters and two-tailed Mann-Whitney U test
| Parameters | Reader 1 average scores |
Reader 1 P- value |
Reader 2 average scores |
Reader 2 P- value |
||
|---|---|---|---|---|---|---|
| SSFSE | vrfSSFSE | SSFSE | vrfSSFSE | |||
| Noise | 3.4 | 3.2 | 0.36 | 2.4 | 2.3 | 0.75 |
| Contrast | 3.8 | 3.8 | 0.72 | 3.5 | 3.6 | 0.54 |
| Sharpness | 3.8 | 3.9 | 0.30 | 3.4 | 3.4 | 0.81 |
| Artifacts | 3.9 | 3.9 | 0.86 | 3.3 | 3.4 | 0.18 |
| Left lobe hepatic signal uniformity |
3.6 | 3.0 | 0.0006* | 3.8 | 3.2 | 0.000002* |
Significant difference (P<0.05)
Highly significant
SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
Table 3.
Weighted-kappa interobserver agreement on image quality between sequences
| Parameter | SSFSE | vrfSSFSE |
|---|---|---|
| Noise | 0.20 | 0.17 |
| Contrast | 0.40 | 0.50 |
| Sharpness | Cannot calculate* | Cannot calculate* |
| Artifacts | 0.03 | 0.04 |
| Left lobe hepatic signal uniformity |
0.31 | 0.59 |
Note: Almost perfect (0.8–1.0), substantial (0.6–0.8), moderate (0.4–0.6), fair (0.2–0.4), slight (0.0–0.2) and poor (<0.0)
Kappa was not calculated for this data set because observed concordance is smaller than mean-chance concordance
SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
SSFSE images had significantly better left lobe hepatic signal uniformity compared to vrfSSFSE, with P-values of <0.001 for both readers (Table 2). Four cases (8%) of SSFSE and 12 cases (24%) of vrfSSFSE for reader 1 and 1 case (2%) of SSFSE and 5 cases (10%) of vrfSSFSE for reader 2 had left lobe hepatic uniformity scores of less than 3, i.e. had a degree of nonuniformity that limited diagnostic evaluation of the left lobe. Figures 2, 3, 4, 5 and 6 show representative examples. Figure 7 shows an example of a particularly nonuniform signal in the left hepatic lobe seen with flip-angle modulation.
Fig. 2.
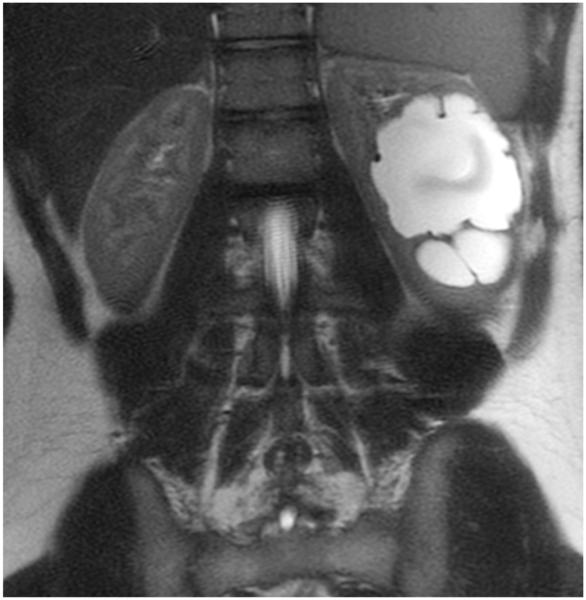
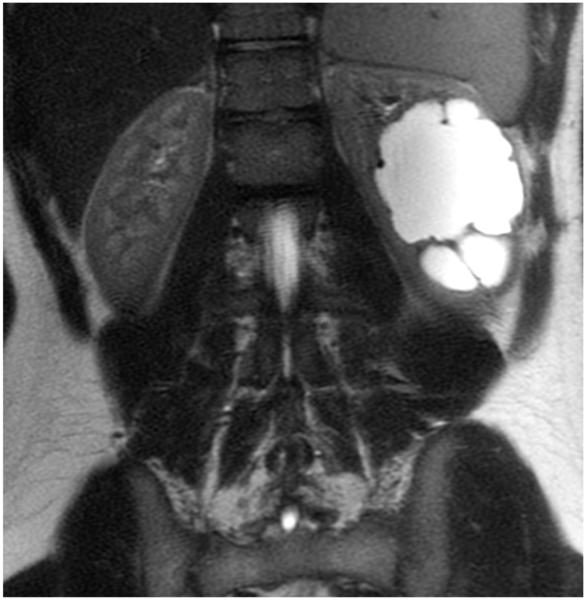
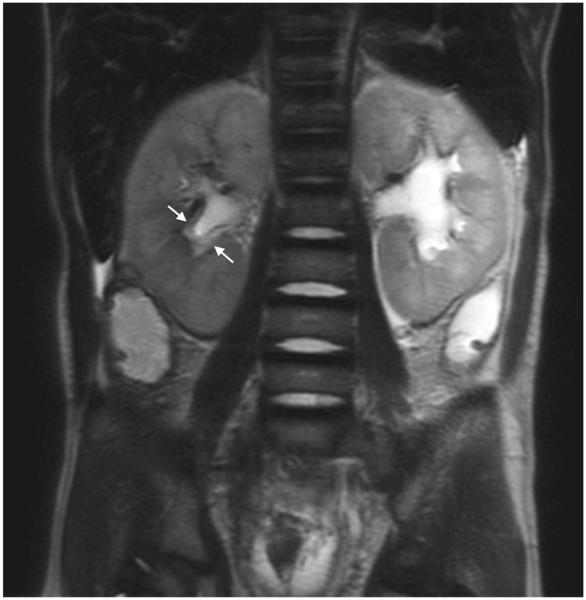
MRI sequence comparison in a 17-year-old boy with hydronephrosis. Equivalent quality is noted between coronal abdominal (a) SSFSE (TR 1,022 ms, total scan time 29 s) and (b) vrfSSFSE (TR 587 ms, total scan time 17 s) sequences for noise, tissue contrast and sharpness. In this example, reader 1 scores for SSFSE/vrfSSFSE were noise 3/3, contrast 4/4, sharpness 4/4, artifacts 4/4, signal void 2/2, artifacts 4/4, and left lobe signal void 2/2 (1 is poor, 4 is excellent). TR repetition time, SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
Fig. 3.
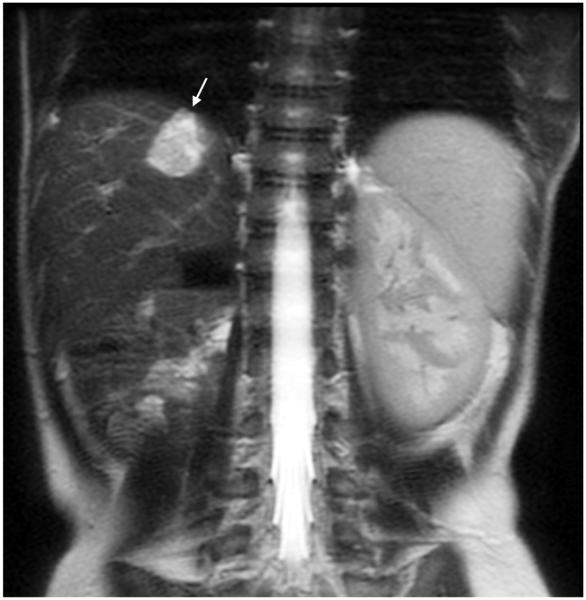
MRI sequence comparison in a 4-year-old boy post right nephrectomy for Wilms tumor. Coronal abdominal (a) SSFSE (TR 1,667 ms, total scan time 45 s) and (b) vrfSSFSE (TR 804 ms, total scan time 22 s) sequences. A hemangioma is well seen on both sequences (arrow). Reader 2 scores for SSFSE/vrfSSFSE were noise 2/2, contrast 4/4, sharpness 4/4 and left lobe signal void 4/3 (1 is poor, 4 is excellent). TR repetition time, SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
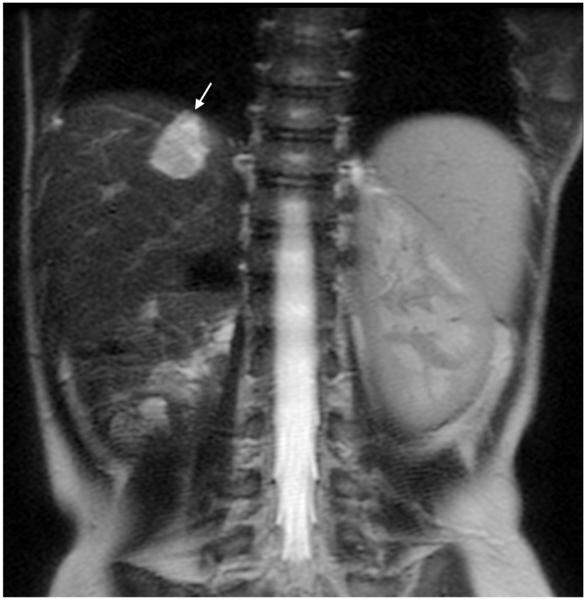
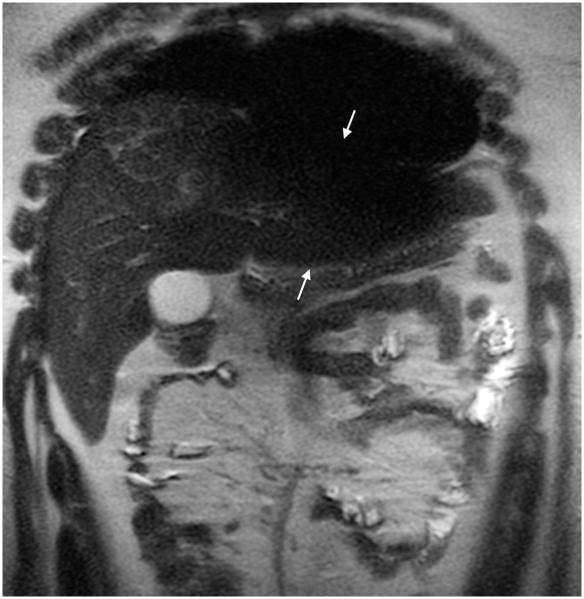
Fig. 4.
MRI sequence comparison in a 4-month-old girl with liver mass. Coronal abdominal (a) SSFSE (TR 2,314 ms, total scan time 54 s) and (b) vrfSSFSE (TR 1,143 ms, total scan time 27 s) sequences. Note the halving of scan time with vrfSSFSE and equivalent image quality. Arrow points to a liver hemangioma. Reader 2 scores for SSFSE/vrfSSFSE were noise 1/1, contrast 2/2, sharpness 3/3, artifacts 2/3 and signal void 4/3 (1 is poor, 4 is excellent). TR repetition time, SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
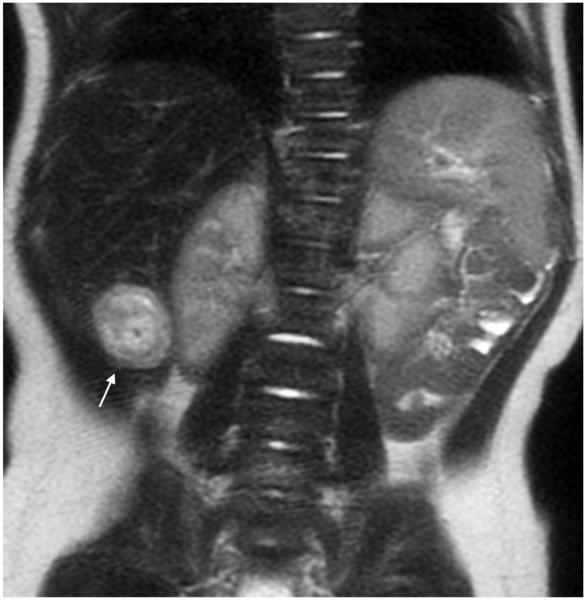
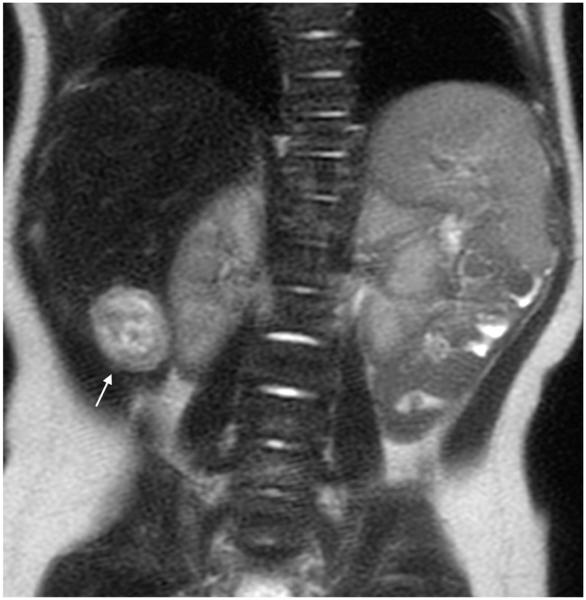
Fig. 5.
MRI sequence comparison in a 5-year-old boy with acute myeloid leukemia. Coronal abdominal (a) SSFSE (TR 2,406 ms, total scan time 82 s) and (b) vrfSSFSE (TR 1,151 ms, total scan time 40 s) sequences. Urothelial thickening (arrows) is well seen with both techniques. TR repetition time, SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
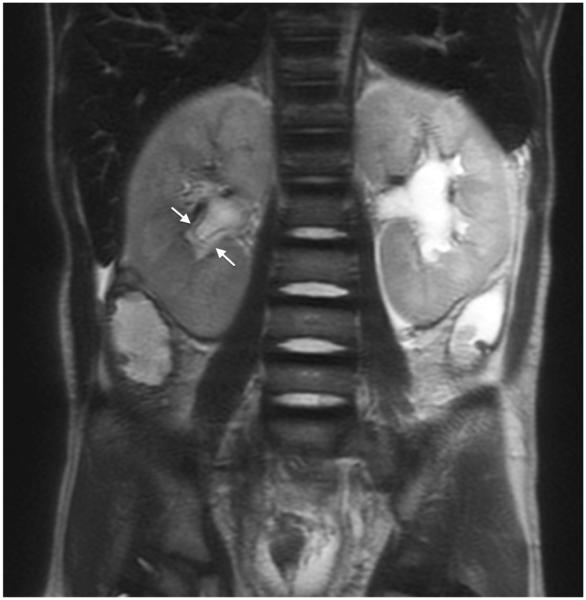
Fig. 6.
MRI sequence comparison in a 10-month-old boy with a history of biliary atresia post liver transplant. Coronal abdominal (a) SSFSE (TR 3,071 ms, total scan time 83 s) and (b) vrfSSFSE (TR 1,469 ms, total scan time 40 s) sequences both show periportal edema (arrows). TR repetition time, SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
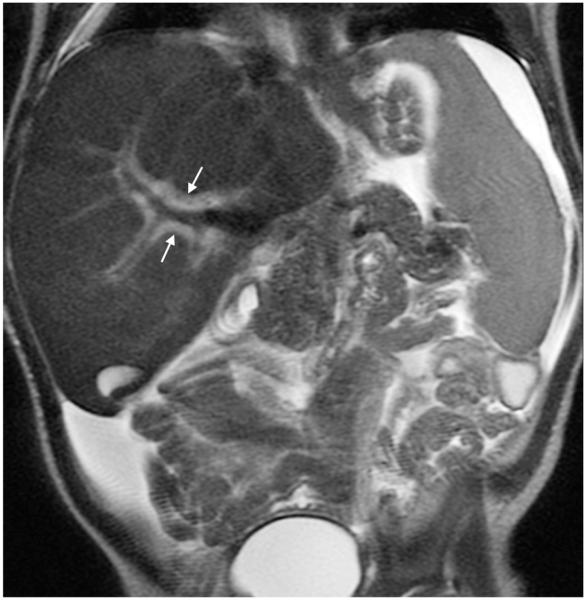
Fig. 7.
Nonuniform signal in the left hepatic lobe in a 16-year-old girl with liver mass (mass not shown). Coronal abdominal (a) SSFSE (TR 876 ms, total scan time 33 s) and (b) vrfSSFSE (TR 611 ms, total scan time 23 s) sequences. Note particularly severe inhomogeneous signal intensity at the left hepatic lobe on vrfSSFSE image (arrows in b). TR repetition time, SSFSE single-shot fast spin echo, vrfSSFSE variable refocusing single-shot fast spin echo
Discussion
Without any other changes, migration of a sequence from 1.5 T to 3 T results in four-fold SAR elevation because of increased tissue radiofrequency power deposition at the doubled Larmor frequency at 3 T. The 2014 U.S. Food and Drug Administration guidance states that the average SAR should not exceed 4 W/kg for whole body and 3.2 W/kg for head, while the dose per gram of tissue should remain under 8 W/kg for head or torso and 12 W/kg for extremities; otherwise MRI exams would be considered significant risk [9].
For gradient echo imaging utilizing small flip angles, increased SAR at 3 T does not typically pose any limitations. However, in spin-echo imaging, the SAR constraint is often encountered. For traditional fast spin-echo imaging, reduced refocusing flip angles as low as 110° have been used, a concept originally referred to as hyperechoes by Hennig and Scheffler [10]. In SSFSE, even at refocusing flip angles as low as 110°, and even with the use of aggressive parallel imaging to shorten the echo train length, SAR still limits imaging speed at 3 T. In order to image within SAR limits, the repetition times between the acquisition of separate SSFSE slices must be lengthened, which leads to longer total scan times. Here, we describe SSFSE imaging using a refocusing flip-angle modulation technique that has been used for 3-D volumetric fast spin-echo imaging. This enabled us to reduce SAR significantly in pediatric abdominal MRI without compromising image quality. In this study the lower minimum TR in vrfSSFSE, (mean 921 ms compared to SSFSE mean 1,957 ms), reduced the average acquisition time by more than a factor of two.
Our study found that despite using such an approach to yield approximately two-fold faster scanning, we obtained equivalent image quality in terms of noise, contrast, sharpness and artifacts. However, there was intermittent loss of left lobe hepatic signal uniformity. The origin of this signal loss is complex and likely in part related to increased sensitivity to high-velocity cardiac-motion-induced shading [11, 12]. Despite this increased sensitivity to motion, uniformity in most cases remained diagnostic, presumably because of the higher flip angles used in the train near the center of k-space. Furthermore, for many clinical exams where the liver is not the organ of interest (e.g., MR urograms, MR enterography, pelvic MRI) the left hepatic lobe signal non-uniformity is less critical.
Our study has several limitations. First, optimized parameters for the variable refocusing flip-angle sequence have only been derived semi-empirically based on simulations and scanning volunteers [7, 8]. The fmin parameter in the flip-angle modulation was chosen entirely empirically based on scanning volunteers. Four parameters are utilized to define the flip-angle train in our approach, and optimal values may vary based on the bandwidth, desired echo time and phase encoding matrix, leading to a very large search space for these parameters. Second, the sample size of the study is somewhat small and interobserver agreement was not high. Perhaps some degree of the interobserver variation is related to the different experience and exposure to pediatric MR exams between the two readers, because the image quality in large adults is often superior to that in children. However for each reader, regardless of the experience, the image-quality scores of vrfSSFSE were nearly the same as scores of SSFSE, with the exception of left lobe hepatic signal uniformity. Third, image evaluation was qualitative rather than quantitative. For example, signal-to-noise measurements were not performed, because accurately obtaining such measurements in the context of in vivo parallel imaging is notoriously difficult [13]. This qualitative evaluation manifests in relatively low interobserver agreement despite the use of defined criteria for scoring of images, probably largely because the two readers in this study had significantly different experience interpreting pediatric body MRI exams. Fourth, sensitivity and specificity for any given pathology were not assessed given the varied clinical indications for the subjects.
In this work, we have only explored coronal imaging of the abdomen. For modern MR systems with at least 32-channel receiver capabilities, abdominal imaging performed in the coronal plane enables leveraging of at least three and often four element columns in phased-array coils in the right-left phase encoding direction, permitting acceleration factors of three or more. For axial and sagittal imaging, phased arrays for abdominal imaging with an anterior and a posterior array only provide two elements in the direction of phase encoding and thus typically have accelerations of two or less. Because the flip-angle modulation optimization depends on the number of refocusing pulses and hence the acceleration, we focused this work on just the coronal plane. Because accelerations in the sagittal and axial planes are lower, the potential benefits of flip-angle modulation may be even higher, and this is the focus of ongoing work.
Another potential approach to SAR reduction is multi-channel radiofrequency transmission. Although multi-channel transmit requires dedicated hardware and may have some uncertainties in predictability of SAR [14], the approach is promising for SAR reduction, particularly in larger subjects. Further, the approach may be synergistic with flip-angle modulation to further reduce SAR, though a thorough investigation of the combination of the two methods would be required. Additionally, in this work B1 variation and its impact on flip-angle variation has not been explored, and though this variation is not as prominent in small children as in larger subjects, parallel transmit techniques can also improve fidelity of the flip-angle modulation train.
The impact of the SAR reduction approach described in this paper is magnified in lighter subjects, such as children, because of a slight nonlinearity in the SAR model used (which is based on measurements of deposited radiofrequency energy as a function of weight). The potential for lower minimum TR and greater breath-held slice coverage is therefore greater in lighter subjects and is ultimately limited by a conservative SAR cutoff imposed for small children and neonates.
SSFSE imaging without flip-angle modulation is relatively fast compared to other MR imaging methods. However we have found that with relatively uncooperative children, slice-to-slice motion can be quite problematic. On the other end of the spectrum with cooperative older children, the time required to obtain a set of single-shot slices that covers the entire abdomen or pelvis in the axial or coronal planes often exceeds breath-holding capability. Thus, the stack of slices has to be divided into two separate breath-holds, and differences in breath-hold position lead to a jittering misregistation artifact when scrolling through slices. With flip-angle modulation the entire MR scan can be shortened for the uncooperative child, and for the older child an entire stack of slices can be obtained in a single breath-hold to eliminate jittering of the slices.
Conclusion
Compared to conventional SSFSE with constant refocusing flip angles, vrfSSFSE is twice as fast and demonstrates largely equivalent image quality. This approach may speed MR urography, MR enterography and pelvic MRI. The major limitation of this approach is that left hepatic lobe signal uniformity for vrfSSFSE can be intermittently compromised by cardiac motion. Further investigation should be directed toward reducing this sensitivity to allow the use of vrfSSFE for magnetic resonance cholangiopancreatography (MRCP) and abdominal oncological applications.
Footnotes
Conflicts of interest D.V. Litwiller is an employee of GE Healthcare.
References
- 1.Brenner DJ. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002;32:228–223. doi: 10.1007/s00247-002-0671-1. discussion 242–244. [DOI] [PubMed] [Google Scholar]
- 2.Olsen OE. Imaging of abdominal tumours: CT or MRI? Pediatr Radiol. 2008;38:S452–458. doi: 10.1007/s00247-008-0846-5. [DOI] [PubMed] [Google Scholar]
- 3.Semelka RC, Kelekis NL, Thomasson D, et al. Abdominal MR imaging using the HASTE sequence; Proceedings of the Society of Magnetic Resonance; New York. 1996. p. 219. [Google Scholar]
- 4.Hennig J, Weigel M, Scheffler K. Multiecho sequences with variable refocusing flip angles: optimization of signal behavior using smooth transitions between pseudo steady states (TRAPS) Magn Reson Med. 2003;49:527–535. doi: 10.1002/mrm.10391. [DOI] [PubMed] [Google Scholar]
- 5.Busse RF. Reduced RF power without blurring: correcting for modulation of refocusing flip angle in FSE sequences. Magn Reson Med. 2004;51:1031–1037. doi: 10.1002/mrm.20056. [DOI] [PubMed] [Google Scholar]
- 6.Busse RF, Brau AC, Vu A, et al. Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med. 2008;60:640–649. doi: 10.1002/mrm.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loening A, Saranathan M, Ruangwattanapaisarn N, et al. Flip angle modulation in single shot fast spin echo imaging greatly increases speed with little change in diagnostic image quality; Proceedings of the joint annual meeting ISMRM-ESMRMB; Milan, Italy. 2014. p. 2174. [Google Scholar]
- 8.Saranathan M, Loening AM, Litwiller DV, et al. Optimized refocusing flip angles for efficient single-shot fast spin echo imaging; Proceedings of the joint annual meeting ISMRM-ESMRMB; Milan, Italy. 2014. p. 2175. [Google Scholar]
- 9.Criteria for Significant risk investigations of magnetic resonance diagnostic devices. U.S. Food and Drug Administration; Silver Spring, MD: 2014. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/Guid anceDocuments/ucm072688.pdf. [Google Scholar]
- 10.Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- 11.Madhuranthakam AJ, Busse RF, Brittain JH, et al. Sensitivity of low flip angle SSFSE of the abdomen to cardiac motion; Proceedings of the ISMRM; Berlin. 2007. p. 15. [Google Scholar]
- 12.Madhuranthakam AJ, Yu H, Shimakawa A, et al. T(2)-weighted 3D fast spin echo imaging with water-fat separation in a single acquisition. J Magn Reson Imaging. 2010;32:745–751. doi: 10.1002/jmri.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeder SB, Wintersperger BJ, Dietrich O, et al. Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med. 2005;54:748–754. doi: 10.1002/mrm.20636. [DOI] [PubMed] [Google Scholar]
- 14.Seifert F, Wubbeler G, Junge S, et al. Patient safety concept for multichannel transmit coils. J Magn Reson Imaging. 2007;26:1315–1321. doi: 10.1002/jmri.21149. [DOI] [PubMed] [Google Scholar]