Abstract
Thirteen individual organochlorine compounds at 3 concentrations (80, 400, and 2000 ng/mL culture medium), as well as mixtures, were assayed for the estrogen receptor (ER) activation or inhibition, using a luciferase reporter gene assay (RGA). None of the PCB 138, 153, or 180 or their mixture induced a response in the RGA. o,p′-DDT was the most potent xenoestrogen from the DDT group, inducing a response already at 80 ng/mL. From the HCH and HCB group, only β-HCH (at 400 and 2000 ng/mL) and δ-HCH (at 2000 ng/mL) displayed estrogenic activities. These 13 organochlorines were determined by GC-MS in 12 samples of North Sea harbor porpoise blubber. The PCBs were the main contaminants. Within each group, PCB 153 (6.0 × 102~4.2 × 104 μg/kg), p,p′-DDE (5.1 × 102~8.6 × 103 μg/kg), and HCB (7.6 × 101~1.5 × 103 μg/kg) were the compounds found in highest concentrations. The hormonal activity of the porpoise blubber samples was also assayed in RGA, where two samples showed estrogenic activity, seven samples showed antiestrogenic activity, and one sample showed both estrogenic and antiestrogenic activity. Our results suggest that the 13 POPs measured by GC-MS in the samples cannot explain alone the estrogenicity of the extracts.
1. Introduction
The harbour porpoise (Phocoena phocoena) is the most common cetacean species in the North Sea [1], and there is a growing concern about the adverse effects of persistent environmental contaminants on this and other marine mammal species [2]. Since 1998, the southern region of the North Sea has been characterised by an increased number of stranded marine mammals, in particular the harbour porpoise. However, a temporary increase in the porpoise population in the southern North Sea may have been responsible [3, 4]. Since marine mammals are at the top of the aquatic food chain and have rather long lifespans, they are an important tool to check the long-term effects concerning marine environment pollution and can be studied as global pollution indicators as well [5].
Not all POPs released into the environment have the same bioaccumulation pattern in different species [6]. In this study, we investigated three nondioxin-like polychlorinated biphenyls (NDL-PCBs), which do not share the dioxin's toxic mechanism, and organochlorine pesticides (OCPs) such as dichlorodiphenyltrichloroethane (DDT) and its metabolites and hexachlorocyclohexane (HCH) and its isomers. In marine mammals, POPs enter the body almost exclusively through the diet and since they are lipophilic compounds, they tend to accumulate in the lipid-rich blubber [7]. Moreover, because of the very low enzyme activities in marine mammals as compared to terrestrial animals, cetaceans have a low capacity to metabolise some persistent organic pollutants (POPs), and retain much higher concentrations of these chemicals in their fat [8, 9]. For example, out of the NDL-PCBs, congener 153 is hardly metabolised by the cytochrome P450 (CYP) enzymes, which makes this molecule extremely persistent [10]. In addition, the ability to metabolize PCB congeners is related to the levels of different families of CYP enzymes, which differ between cetaceans and other species [11]. DDT is metabolised by reductive dechlorination catalyzed by the microsomal CYP system. The principal metabolites generated are dichlorodiphenyldichloroethane (DDD) and dichlorodiphenyldichloroethylene (DDE), the latter being the most persistent and stable [12, 13]. Among HCH isomers, β-HCH seems to be more resistant to microbial degradation and thus more persistent in the environment than the other isomers [14]. While concentrations of OCPs (DDTs, HCB, and HCHs) in harbour porpoises stranded on the Belgian North Sea coast are low, relatively high concentrations of PCB are present [15].
Public concern about environmental contamination by POPs increased recently because of many evidences showing that some of these compounds are xenoestrogens and interact with the endocrine system, resulting in numerous biological effects that may affect the health of humans and animals [7, 16–20], as demonstrated in recent studies involving polar bears (Ursus maritimus) [21, 22]. It has been reported that PCBs are associated with reproductive, estrogenic and antiandrogenic, effects [23]. DDT and its metabolites bind the estrogen receptor [24], disrupting the endocrine and reproductive systems. Furthermore, HCH may also affect the reproductive system, possibly through endocrine-mediated mechanisms [25]. In the case of harbour porpoises, several studies have provided consistent support for the hypothesis of a PCB exposure induced immunosuppression contributing to infectious disease mortality [26, 27]. Furthermore, contaminants such as PCBs, DDT, and DDE may interfere with harbour porpoise thyroid functions leading to severe interfollicular fibrosis [28].
Currently, the United States Environmental Protection Agency (US-EPA) estimates that there are more than 87000 potential endocrine disrupters in the world. Nevertheless, developing methods to detect so many chemicals would take a massive financial mobilization. In this way, research on screening methods using short term bioassays to assess the risk of exposure to endocrine disrupting chemicals is needed [29, 30].
In this context, the aim of this study was to measure the hormonal activity of North Sea harbour porpoise blubber samples and to identify the compounds that contribute to the hormonal activity of these samples. In order to associate hormonal activities to xenoestrogen contamination levels, the samples were analysed both by cell-based assays (reporter gene assays) and chemical analysis (mass spectrometry coupled to gas chromatography [GC-MS]). GC-MS combines high separation power with good identification capabilities, while reporter gene assays allow the measurement of the hormonal potency of samples [31, 32], as they are based on the activation of the nuclear estrogen hormone receptor [33]. In this study, we decided to focus on the evaluation of the (anti-) estrogenic effect of xenoestrogens, as it is associated with disruption of the endocrine and reproductive systems. Studies combining biological and chemical analysis have proved to be valuable in uncovering the presence and the impact of hormone-like compounds in the wildlife [34]. Reporter gene assays were largely used to analyse samples such as harbour sediment and wasted waters [35, 36], but only few studies were conducted to assess the hormonal activity of biological tissues [37–39].
2. Material and Methods
2.1. Equipment, Chemicals, and Reagents
The following equipment and materials have been used in this study: carbon dioxide (Air Liquide, Liège, Belgium); helium (Air Products, Brussels, Belgium); acetonitrile (Biosolve, Valkenswaard, Netherlands); luminometer Orion II (BRS, Drogenbos, Belgium); adenosine triphosphate (ATP), Dulbecco's modified Eagle's medium (DMEM), DMEM without red phenol, and fetal bovine serum and trypsin (Fisher Bioblock Scientific, Tournai, Belgium); Focus gas chromatograph and Polaris Q mass spectrometer (Interscience, Louvain-la-Neuve, Belgium); charcoal, dextran, o,p′-DDD, p,p′-DDD, p,p′-DDE, o,p′-DDT, p,p′-DDT, 17β-estradiol, HCB, α-HCH, β-HCH, γ-HCH, δ-HCH, nonane, PCB 80 13C, PCB 138 (2,2′,3,4,4′,5′-hexachlorobiphenyl), PCB 153 (2,2′,4,4′,5,5′-hexachlorobiphenyl), and PCB 180 (2,2′,3,4,4′,5,5′-heptachlorobiphenyl) (Sigma-Aldrich, Bornem, Belgium); D-luciferin (potassium salt) (Synchem OHG, Kassel, Germany); HT8 column (VWR, Leuven, Belgium).
2.2. Stable Reporter Cell Lines and Culture
To obtain an estrogen-responsive cell line, MCF-7 human mammary tumour cells were stably transformed with a reporter vector containing the firefly luciferase gene under control of the vitellogenin promoter [24, 40]. These cells were grown in 75 cm2 culture flasks in DMEM, supplemented by 10% heat-inactivated foetal bovine serum (FBS), at 37°C under 5% CO2.
2.3. Samples
Blubber samples were obtained from 12 juvenile harbour porpoises (8 males and 4 females) stranded on the Belgian and French North Sea coasts between 2000 and 2003. These animals were necropsied by the Laboratory for Oceanology of the University of Liège according to standard procedures detailed elsewhere [3]. Blubber samples were identified by an alphanumeric code and kept apart at −20°C until analysis.
2.4. Extraction Procedure
To separate POPs from blubber samples, we applied a solid-liquid extraction to extract fat with the POPs. This step was followed by an acid silica column chromatography to eliminate the fat. Using this method, we destroyed endogenous steroid hormones and their eventual conjugates, which were hydrolysed in presence of inorganic acids [41]. The advantage of this method is that natural hormones present in the samples could be eliminated from the extracts and would not interfere in the estrogen-like activity elicited by samples as pointed out by some authors [42].
The extraction was performed as follows. One g of harbour porpoise blubber was homogenized in a test tube containing 2 mL of hexane using a glass stirring rod. The organic phase was separated and the hexane evaporated under nitrogen until dryness. Then, 0.25 g or 0.1 g of the extracted fat was solubilised in two different tubes containing 2 mL of hexane for the estrogen receptor- (ER-) mediated activity assays and GC-MS analyses, respectively. The fat solubilised in hexane was applied to a glass column, prewashed with hexane, filled (from the bottom to top) with 5 g of acid silica (40% H2SO4 w/w), 1 g of deactivated alumina, and 1 g of Na2SO4. POPs were eluted with a mixture of dichloromethane and hexane (1 : 3), the eluent was evaporated to dryness. The residue of the first tube was recovered in 25 μL of acetonitrile for the estrogen receptor- (ER-) mediated activity assays, and the residue of the second tube was recovered in 100 μL of nonane containing 10 μL of an internal standard (PCB 80 13C 1.0 ng/μL) for GC-MS analysis. In order to measure the possible loss of the estrogenic activity due to the extraction and purification procedure, a solution containing 13 POPs (see below) was submitted to the extraction/purification procedure and the extracts were then analysed by reporter gene assay. A procedure blank was performed to measure the background response in the reporter gene assay.
2.5. Cell-Based Assays to Test Estrogen Receptor- (ER-) Mediated Activity
The cell-based assays for estrogen receptor ER-mediated activity were carried out as follows. Ninety % confluent MCF-7-ERE cells were cultured at least 24 h in DMEM without phenol red (supplemented by 10% of FBS previously treated with charcoal-dextran), and they were released from the culture flask using 1.5 mL of trypsin (0.5 g/L). Then, cells were suspended in 10 mL of fresh culture medium and this suspension was diluted two times. One hundred μL of diluted cells was seeded in 96-well culture plates, which were incubated overnight at 37°C under 5% CO2. Afterwards, cells were incubated with the standards or the extracts of blubber samples to be tested for 24 hours. The final volume in one well was 200 μL. A 17β-estradiol (E2) calibration curve was performed on each plate (7.0 × 10−5~2.0 × 10−1 ng E2/mL culture medium containing 0.8% acetonitrile). To study agonistic activity of the standards or the extracts of blubber samples (ability to mimic endogenous hormones), cells were exposed, one at a time, to a selection of 13 individual POPs (o,p′-DDD, p,p′-DDD, p,p′-DDE, o,p′-DDT, p,p′-DDT, HCB, α-HCH, β-HCH, γ-HCH, δ-HCH, PCB 138, PCB 153, and PCB 180) or blubber extracts diluted in acetonitrile. Each POP was tested individually at three different concentrations: 80, 400, and 2000 ng/mL culture medium. Then, seven mixtures containing the same weight proportion of each POP (to reach final global concentrations of 80, 400, and 2000 ng/mL culture medium) were tested. The final proportion of acetonitrile in the medium in the agonistic tests was 0.4%. To study the antiestrogenic activity of the tested standards or the extracts of blubber samples (ability to inhibit the binding of a hormone to its receptor), the cells were exposed to increasing concentrations (80, 400, or 2000 ng/mL culture medium) of a selection of POPs, alone or within mixtures, or to blubber extracts diluted in acetonitrile in the presence of the reference ligand (E2) at a concentration near its EC50 (≈5.0 × 10−3 ng E2/mL culture medium). The final proportion of acetonitrile in the medium in this case was 0.8%. After incubation, the cell viability was checked under a microscope. Subsequently, the medium was removed and the cells were lysed with 50 μL of lysis solution containing 25 mM of Tris, 2 mM of 1,4-dithiothreitol, 2 mM of 1,2-diaminocyclohexanetetraacetic acid, 10% of glycerol, and 1% of Triton X-100. After the addition of luciferin and ATP, the luciferase activity was determined using a luminometer and reported as relative light units (RLU). The bend points (beginning and end of the essentially linear region of the sigmoid dose-response curves) were defined as published by Sebaugh and McCray [43]. The minimal relative response was set to 17.6% to consider an accurate measurement.
2.6. Chemical Analyses by GC-MS
Analyses of extracts were performed using a Focus gas chromatograph coupled to a Polaris Q ion trap mass spectrometer. Helium was used as carrier gas at a flow rate of 1 mL/min. A volume of 2.0 μL was injected. Separation of the target analytes was performed on a HT8 column (25 m × 0.22 mm × film thickness 0.25 μm). Injector and ion source temperatures were 250°C and 220°C, respectively. The GC conditions were the following: 2 min at 120°C, ramped to 169°C (30°C/min), 13 min at 169°C, ramped to 170°C (5°C/min), 9 min at 170°C, ramped to 247°C (30°C/min), 2 min at 247°C, ramped to 320°C (20°C/min), and 2 min at 320°C. The total run time was 36 min. Spectra were acquired in single ion monitoring (SIM) mode. The m/z ratios scanned (with corresponding elution times) can be found in Table 1: 181, 183, 284, and 285 (11.5–16.0 min); 181 and 183 (16.0–28.0 min); 302 and 304 (28.0–30.0 min); 235, 237, 316, and 318 (30.0–31.3 min); 235, 237, 360, and 362 (31.4–33.3 min); 235, 237, 360, and 362 (31.4–33.3 min); 394 and 396 (33.3–36.0 min). For each compound, 5 calibration solutions (20, 60, 120, 240, and 400 pg/μL), including the internal standard, were injected in parallel to samples extracts. The limit of detection was 1 μg/kg fat.
Table 1.
m/z ratios scanned and corresponding windows of elution times for each target compound.
| m/z ratios | Elution time (min) | Target compound |
|---|---|---|
| 181/183 | 11.5–16.0 | α-HCH |
| 284/285 | 11.5–16.0 | HCB |
| 181/183 | 16.0–28.0 | γ-HCH, β-HCH, and δ-HCH |
| 302/304 | 28.0–30.0 | PCB 80 13C |
| 235/237 | 30.0–31.4 | o,p′-DDD |
| 316/318 | 30.0–31.4 | p,p′-DDE |
| 235/237 | 31.4–33.3 | o,p′-DDT, p,p′-DDD, and p,p′-DDT |
| 360/362 | 31.4–33.3 | PCB 153 and PCB 138 |
| 394/396 | 33.3–36.0 | PCB 180 |
2.7. Data Analysis
Cell-based assays for estrogen receptor- (ER-) mediated activity data were processed with Slide Write V6 software. Reference curves were fitted using the sigmoid dose-response curve equation: Y = a 0/(1 + (x/a 1)a2), where x is the concentration of E2, Y is the relative response ((RLUsample − RLUblank solvent)/(RLU17β-estradiol maximal dose − RLUblank solvent)), a 1 is the concentration of half-maximal response (EC50), and a 2 is the slope of the linear part of the curve. This equation was used to convert relative responses obtained for tested compounds or blubber samples extracts into estradiol equivalents (EEQ) expressed in ng/mL culture medium, for POPs standards and mixtures, and in μg/kg fat, for blubber samples. Chemical analyses by GC-MS were processed using Xcalibur software (InterScience).
Student's t-tests were performed to determine which compounds and blubber samples produced a response significantly different from the blank in the case of agonistic tests and which compounds and blubber samples produced a response significantly different from the reference ligand (E2 at 5.0 × 10−3 ng/mL culture medium) in the case of antagonistic tests.
3. Results
3.1. Characterization of the MCF-7-ERE Cell Line
After exposing MCF-7-ERE cells to increasing concentrations of E2, sigmoid dose-response curves were obtained with an average coefficient of determination (R 2) of 0.99 ± 0.0049 (n = 10). The MCF-7-ERE cell line responded specifically to its reference ligand E2 and concentrations as low as 1.1 × 10−3 ng/mL of this hormone resulted in a reproducible signal, suggesting that this cell line is able to detect estrogen-like activity. The half maximal effective concentration (EC50) of E2 was 4.4 × 10−3 ± 1.5 × 10−3 ng/mL (Figure 1).
Figure 1.

Dose-response curve of increasing concentrations of E2 (ng/mL culture medium). Data represent the mean ± S.D (n = 10). The maximal response observed for E2 was arbitrarily set to 100%. The dotted line represents the minimal relative response to consider an estrogenic activity (17.6% of response) and the dashed line represents EC50.
3.2. Extraction and Purification of Samples
When applying the extraction and purification procedure to a mixture of 13 POPs, we noticed that 76 ± 3% of the initial estrogenic activity was recovered. As expected, a solution containing E2 showed no estrogenic activity after being submitted to the extraction/purification procedure, indicating that E2 was undoubtedly degraded by the acidified silica of the column. The procedure blank (negative control) showed a relative response of 7%, below the threshold of 17.6%, needed to evidence an estrogenic activity. This indicates that the extraction/purification method does not induce any estrogenic activity and is thus compatible with the reporter gene assay. Likewise, when E2 was added to the extract obtained from the procedure blank, MCF-7-ERE cells responded positively and in the expected intensity, confirming that the extraction and purification methods do not bring any inhibiting effect to the cells (data not shown).
3.3. Detection of Estrogen Receptor- (ER-) Mediated Activity of POPs in MCF-7-ERE Cells
For each selected compound, the maximum relative response was obtained at the concentration of 2000 ng/mL culture medium (Table 2), with no apparent cytotoxicity when observing the cells under the microscope. None of the tested PCBs was able to induce a cell response above 17.6%. The five compounds belonging to the group of DDT and its metabolites presented a strong estrogenic activity, especially o,p′-DDT and o,p′-DDD, which produced a maximum relative response higher than 60%. Among the hexachloro compounds, β-HCH and δ-HCH were the compounds with the strongest estrogenic activity, with responses of 64 and 20%, respectively, at the concentration of 2000 ng/mL culture medium (Table 2).
Table 2.
Estrogen receptor-mediated luciferase expression (measured as light emission) observed in MCF-7-ERE cells exposed during 24 hours to single compounds or mixtures of selected POPs. All mixtures contained the same weight proportion of the constituents to achieve the concentration of 80 ng/mL, 400 ng/mL, or 2000 ng/mL medium (n = 3).
| Relative response (%)a | EEQ (ng/mL)b | |||||
|---|---|---|---|---|---|---|
| 80 ng/mL | 400 ng/mL | 2000 ng/mL | 80 ng/mL | 400 ng/mL | 2000 ng/mL | |
| PCB 138 | <LOQ | <LOQ | <LOQ | n/a | n/a | n/a |
| PCB 153 | <LOD | <LOD | <LOQ | n/a | n/a | n/a |
| PCB 180 | <LOD | <LOD | <LOD | n/a | n/a | n/a |
| Mixture of PCB congeners (1) | <LOD | <LOD | <LOQ | n/a | n/a | n/a |
|
| ||||||
| o,p′-DDT | 28 ± 0 | 67 ± 3 | 72 ± 7 | 2.1 ± 0.0 × 10−3 | 7.6 ± 0.8 × 10−3 | 9.4 ± 2.4 × 10−3 |
| o,p′-DDD | <LOD | 29 ± 3 | 66 ± 3 | n/a | 2.1 ± 0.2 × 10−3 | 7.3 ± 0.6 × 10−3 |
| p,p′-DDT | <LOD | <LOQ | 36 ± 2 | n/a | n/a | 2.8 ± 0.2 × 10−3 |
| p,p′-DDD | <LOD | <LOQ | 32 ± 2 | n/a | n/a | 2.4 ± 0.2 × 10−3 |
| p,p′-DDE | <LOD | <LOQ | 21 ± 1 | n/a | n/a | 1.5 ± 0.0 × 10−3 |
| Mixture of DDT and its metabolites (2) | <LOQ | 26 ± 1 | 49 ± 4 | n/a | 1.9 ± 0.1 × 10−3 | 4.3 ± 0.5 × 10−3 |
|
| ||||||
| β-HCH | <LOQ | 35 ± 2 | 64 ± 4 | n/a | 2.7 ± 0.1 × 10−3 | 6.8 ± 1.0 × 10−3 |
| δ-HCH | <LOQ | <LOQ | 20 ± 3 | n/a | n/a | 1.4 ± 0.2 × 10−3 |
| γ-HCH | <LOD | <LOQ | <LOQ | n/a | n/a | n/a |
| α-HCH | <LOD | <LOQ | <LOQ | n/a | n/a | n/a |
| HCB | <LOD | <LOD | <LOD | n/a | n/a | n/a |
| Mixture of HCH isomers and HCB (3) | <LOQ | <LOQ | 23 ± 1 | n/a | n/a | 1.7 ± 0.1 × 10−3 |
|
| ||||||
| Mixture (1) + (2) | <LOQ | 32 ± 1 | 52 ± 2 | n/a | 2.4 ± 0.1 × 10−3 | 4.7 ± 0.3 × 10−3 |
| Mixture (1) + (3) | <LOQ | <LOQ | 28 ± 2 | n/a | n/a | 2.1 ± 0.2 × 10−3 |
| Mixture (2) + (3) | <LOQ | 20 ± 7 | 54 ± 2 | n/a | 1.5 ± 0.5 × 10−3 | 5.0 ± 0.3 × 10−3 |
| Mixture (1) + (2) + (3) | <LOQ | 27 ± 1 | 54 ± 2 | n/a | 2.0 ± 0.1 × 10−3 | 5.0 ± 0.3 × 10−3 |
aThe maximal response observed for E2 was arbitrarily set to 100% and the responses observed for the chemicals and mixtures are expressed in percentage of the maximal response (relative response).
bEstradiol equivalents were determined by linear extrapolation from calibration curves obtained after exposure to E2 and are expressed in ng/mL culture medium.
n/a: not applicable.
In order to detect a possible interaction between the chemicals mentioned before, MCF-7-ERE cells were also exposed to mixtures containing the same weight proportion of each molecule included in the mixture to reach a final concentration of 80 ng/mL, 400 ng/mL, or 2000 ng/mL culture medium for the sum of all compounds. The mixture of the 3 PCBs (mixture #1) did not activate the estrogen receptor. The most concentrated mixture of DDT and its metabolites (mixture #2, 2000 ng/mL) contains each congener at a concentration of 400 ng/mL, at which only o,p′-DDT and o,p′-DDD showed an activity (relative responses of 67% and 29% for o,p′-DDT and o,p′-DDD, resp.). However, the response observed for this mixture remains below the response of o,p′-DDT alone. Similar observation can be made for the mixture of HCH isomers and HCB (mixture #3), the response of the mixture (containing each compound at a concentration of 400 ng/mL) being lower (23%) than the response of the only compound (β-HCH) giving a response (35%) at the concentration of 400 ng/mL. When mixing the mixtures (binary or ternary mixtures, see the four last lines of Table 2), the response obtained corresponds to the response of the most potent mixture included in the “mixture of mixtures.”
None of the selected chemicals showed antiestrogenic activity. Conversely, o,p′-DDT, o,p′-DDD, β-HCH, δ-HCH, and HCB were able to increase the cellular response when exposed simultaneously to E2 (p < 0.05). In this case the predicted theoretical responses for the exposure of MCF-7-ERE cells to E2 (5.0 × 10−3 ng/mL culture medium) simultaneously with o,p′-DDT, o,p′-DDD, or β-HCH (2000 ng/mL culture medium) would reach the plateau of the cell response. However, the measured responses were below 100% (data not shown).
3.4. Organochlorine Pollutants Determination in North Sea Harbour Porpoise Blubber by Chemical Analyses
Three NDL-PCB congeners (PCB 138, PCB 153, and PCB 180), four HCH isomers (α-HCH, β-HCH, γ-HCH, and δ-HCH), HCB, and five components of the DDT group (o,p′-DDD, p,p′-DDD, p,p′-DDE, o,p′-DDT, and p,p′-DDT) were quantified in North Sea harbour porpoise blubber samples. After optimisation of GC-MS parameters, the obtained chromatograms presented separated and resolved peaks (Figure 2), which permitted the identification and quantification of the target compounds.
Figure 2.
Chromatogram of a mixture of 13 standard solutions of target pollutants.
Among the chemicals analysed, the PCBs were the main contaminants in North Sea harbour porpoise blubber, followed by the DDT group and finally by the isomers of HCH and HCB (Table 3). Within each group, PCB 153 (6.0 × 102 to 4.2 × 104 μg/kg fat), p,p′-DDE (5.1 × 102 to 8.6 × 103 μg/kg fat), γ-HCH (8.0 × 101 to 4.8 × 102 μg/kg fat), and HCB (7.6 × 101 to 1.5 × 103 μg/kg fat) were the compounds found in largest quantities.
Table 3.
Gender, length (cm), weight (kg), blubber thickness (cm), and blubber levels (μg/kg) of 13 organochlorine compounds of 12 North Sea harbour porpoise individuals. Chemicals were divided in three groups and the sum of concentrations for each group is presented for the different samples.
| 03/1521 | 01/1196 | 03/1238 | A00/1140 | A00/258 | A03/1517 | 01/847 | 01/805 | 01/1219 | A00/600 | 01/887 | A00/974 | Average | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | M | M | F | F | M | M | F | M | M | M | M | F | — |
| Length | 117 | 104 | 127 | 112 | 108 | 128 | 114 | 99 | 112 | 103 | 110 | 114 | 112 ± 9 |
| Weight | 30 | n/a | 26 | 20 | 18 | 22 | 29 | 14 | 19 | 27 | 17 | 22 | 22 ± 5 |
| Blubber thickness | 20 | 22 | 10 | 13 | 10 | 8 | 20 | 5 | 8 | 40 | 6 | 20 | 15 ± 10 |
| Emaciation | n/a | n/a | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | — |
| Parasites | n/a | n/a | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | No | — |
|
| |||||||||||||
| PCB 138 | 9.9 × 102 | 1.3 × 103 | 8.3 × 103 | 3.8 × 103 | 3.7 × 103 | 3.1 × 104 | 2.7 × 103 | 3.7 × 103 | 7.7 × 103 | 3.6 × 103 | 2.3 × 103 | 5.9 × 102 | 5.8 ± 8.3 × 103 |
| PCB 153 | 1.4 × 103 | 1.7 × 103 | 1.2 × 104 | 6.3 × 103 | 5.5 × 103 | 4.2 × 104 | 4.0 × 103 | 5.5 × 103 | 1.1 × 104 | 5.1 × 103 | 3.2 × 103 | 6.0 × 102 | 0.8 ± 1.1 × 104 |
| PCB 180 | 3.3 × 102 | 3.2 × 102 | 2.5 × 103 | 1.8 × 103 | 1.2 × 103 | 7.4 × 103 | 8.0 × 102 | 1.2 × 103 | 1.9 × 103 | 9.3 × 102 | 7.9 × 102 | 1.1 × 102 | 1.6 ± 1.9 × 103 |
| Σ (3) PCBs | 2.7 × 103 | 3.3 × 103 | 2.3 × 104 | 1.2 × 104 | 1.0 × 104 | 8.1 × 104 | 7.5 × 103 | 1.0 × 104 | 2.1 × 104 | 9.6 × 103 | 6.3 × 103 | 1.3 × 103 | 1.6 ± 2.2 × 104 |
|
| |||||||||||||
| o,p′-DDT | 4.8 × 101 | 1.0 × 102 | 3.4 × 102 | 9.8 × 101 | 7.4 × 101 | 2.1 × 102 | 6.0 × 101 | 8.2 × 101 | 8.6 × 102 | 5.4 × 101 | 1.7 × 102 | 7.1 × 101 | 1.8 ± 2.3 × 102 |
| o,p′-DDD | n/d | 7.2 × 101 | 1.8 × 102 | 7.8 × 101 | 6.8 × 101 | 4.1 × 102 | n/d | 8.6 × 101 | 5.2 × 102 | n/d | 6.9 × 101 | 4.0 × 101 | 1.7 ± 1.8 × 102 |
| p,p′-DDT | 1.8 × 102 | 3.2 × 102 | 7.9 × 102 | 5.6 × 102 | 4.7 × 102 | 7.1 × 102 | 1.9 × 102 | 3.7 × 102 | 1.4 × 103 | 2.0 × 102 | 5.8 × 102 | 1.1 × 102 | 4.9 ± 3.6 × 102 |
| p,p′-DDD | 3.4 × 102 | 5.3 × 102 | 8.9 × 102 | 9.2 × 102 | 8.5 × 102 | 1.2 × 103 | 2.4 × 102 | 7.3 × 102 | 2.6 × 103 | 4.1 × 102 | 6.0 × 102 | 1.4 × 102 | 7.9 ± 6.6 × 102 |
| p,p′-DDE | 1.0 × 103 | 1.2 × 103 | 3.1 × 103 | 2.6 × 103 | 2.9 × 103 | 4.1 × 103 | 9.1 × 102 | 2.4 × 103 | 8.6 × 103 | 1.4 × 103 | 2.3 × 103 | 5.1 × 102 | 2.6 ± 2.2 × 103 |
| Σ (5) DDTs | 1.6 × 103 | 2.3 × 103 | 5.3 × 103 | 4.3 × 103 | 4.4 × 103 | 6.6 × 103 | 1.4 × 103 | 3.7 × 103 | 1.4 × 104 | 2.1 × 103 | 3.7 × 103 | 8.6 × 102 | 4.2 ± 3.5 × 103 |
|
| |||||||||||||
| β-HCH | n/d | n/d | 9.7 × 101 | n/d | 8.8 × 101 | 8.0 × 101 | n/d | 8.3 × 101 | 1.3 × 102 | 4.2 × 101 | 7.4 × 101 | 4.7 × 101 | 8.0 ± 2.7 × 101 |
| δ-HCH | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | — |
| γ-HCH | 9.8 × 101 | 1.4 × 102 | 1.3 × 102 | 1.3 × 102 | 2.4 × 102 | 2.0 × 102 | 8.0 × 101 | 1.7 × 102 | 4.8 × 102 | 1.7 × 102 | 1.5 × 102 | 1.2 × 102 | 1.8 ± 1.1 × 102 |
| α-HCH | 7.0 × 101 | n/d | 6.8 × 101 | 6.6 × 101 | n/d | n/d | n/d | 8.0 × 101 | 9.8 × 101 | 7.1 × 101 | 1.0 × 102 | 7.4 × 101 | 7.9 ± 1.4 × 101 |
| HCB | 1.4 × 102 | 2.1 × 102 | 5.4 × 102 | 2.2 × 102 | 3.1 × 102 | 4.9 × 102 | 1.3 × 102 | 3.4 × 102 | 1.5 × 103 | 9.8 × 101 | 6.0 × 102 | 7.6 × 101 | 3.9 ± 4.0 × 102 |
| Σ (5) HCHs + HCB | 3.1 × 102 | 3.5 × 102 | 8.4 × 102 | 4.2 × 102 | 6.3 × 102 | 7.7 × 102 | 2.1 × 102 | 6.7 × 102 | 2.2 × 103 | 3.9 × 102 | 9.3 × 102 | 3.1 × 102 | 6.7 ± 5.4 × 102 |
|
| |||||||||||||
| Σ (13) total | 4.6 × 103 | 5.9 × 103 | 2.9 × 104 | 1.7 × 104 | 1.5 × 104 | 8.8 × 104 | 9.1 × 103 | 1.5 × 104 | 3.7 × 104 | 1.2 × 104 | 1.1 × 104 | 2.5 × 103 | 2.1 ± 2.4 × 104 |
n/d: not detected; n/a: not available.
3.5. Detection of Estrogen Receptor- (ER-) Mediated Activity of North Sea Harbour Porpoise Blubber Samples
The agonistic and antagonistic activity mediated by the estrogen receptor (ER) elicited by North Sea harbour porpoise blubber samples in MCF-7-ERE cells is reported in Figures 3 and 4, respectively. Two samples showed significant agonistic ER-mediated responses: A00/258 and 01/1219 (response significantly different from procedure blank, with p < 0.05). The highest response measured was 24% of the activity induced by the reference E2 (sample 01/1219), corresponding to 0.045 μg EEQ/kg fat (Figure 3). When exposed simultaneously to E2, eight samples inhibited significantly (p < 0.05) the activity of the E2 reference ligand: 01/1169, 03/1238, A00/1140, A03/1517, 01/847, 01/1219, A00/600, and A00/974. The highest antiestrogenic effect was observed for samples 03/1238, which decreased the cellular response of 16% (Figure 4).
Figure 3.

Estrogen receptor- (ER-) mediated agonistic activity elicited by 12 North Sea harbour porpoise blubber samples in MCF-7-ERE cells. The horizontal dashed line represents the response of the procedure blank. Results are expressed as percent of the maximal response induced by E2 (columns) or as μg EEQ/kg fat (dots), only for the two samples showing a response significantly different from the procedure blank (p < 0.05).
Figure 4.
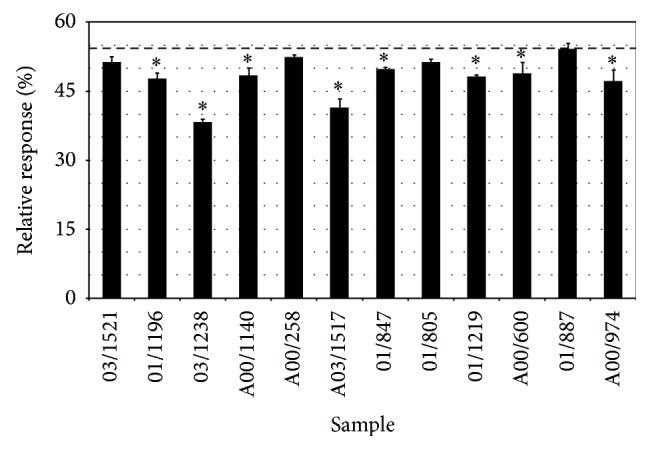
Estrogen receptor- (ER-) mediated antagonistic activity elicited by North Sea harbour porpoise blubber samples (n = 12) in MCF-7-ERE cells when exposed simultaneously to E2 (5.0 × 10−3 ng/mL). The horizontal dashed line represents the response of the cells exposed to 5.0 × 10−3 ng/mL of E2. Results are expressed as percent of the maximal response induced by E2. Asterisks indicate that the response is significantly different from the response of 5.0 × 10−3 ng/mL of E2 (p < 0.05).
3.6. Correlation between Xenoestrogens in North Sea Harbour Porpoise Blubber and Estrogen Receptor- (ER-) Mediated Activity
From GC-MS data of sample contamination (Table 3), the amount of organochlorine contained in culture medium at the moment of the cell-based assay of blubber samples was calculated (Table 4). When comparing this result to the estrogenic activity of the individual compounds and their mixtures (Table 2), it appeared that none of the samples contained enough of the 13 measured organochlorines to elicit a positive response in the reporter gene assay, either an agonistic or antagonistic, making it not possible to predict the ER-mediated activity of the samples. Indeed, if we look to the levels of HCH isomers and HCB, they are all below 400 ng/mL culture medium, which is the smallest concentration tested for individual compounds and at which no response was recorded for these organochlorines in the cell-based assay. From the group of DDT, the most potent substances (o,p′-DDT and o,p′-DDD) represented only a minor part of the organochlorine contamination, resulting in levels below 80 ng/mL culture medium in the cell-based assay. However, one sample showed estrogenic activity, seven samples showed antiestrogenic activity, and one sample showed both estrogenic and antiestrogenic activity.
Table 4.
Concentrations (ng/mL) of 13 organochlorine compounds in the extracts of 12 samples of North Sea harbour porpoise blubber, in culture medium solution to which the ER sensitive cells were exposed in the cell based assay (calculated from GC-MS data from Table 3; according to that 1 mL of culture medium contains the contaminants extracted from 0.04 g of fat). For the samples displaying an estrogenic activity, the percentage of relative response compared to the maximal response induced by E2 is indicated between brackets.
| 03/1521 | 01/1196 | 03/1238 | A00/1140 | A00/258 | A03/1517 | 01/847 | 01/805 | 01/1219 | A00/600 | 01/887 | A00/974 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB 138 | 4.0 × 101 | 5.2 × 101 | 3.3 × 102 | 1.5 × 101 | 1.5 × 102 | 1.3 × 103 | 1.1 × 102 | 1.5 × 102 | 3.1 × 102 | 1.4 × 102 | 9.1 × 101 | 2.4 × 101 |
| PCB 153 | 5.4 × 101 | 6.7 × 101 | 4.9 × 102 | 2.5 × 102 | 2.2 × 102 | 1.7 × 103 | 1.6 × 102 | 2.2 × 102 | 4.5 × 102 | 2.1 × 102 | 1.3 × 102 | 2.4 × 101 |
| PCB 180 | 1.3 × 101 | 1.3 × 101 | 1.0 × 102 | 7.3 × 101 | 4.7 × 101 | 3.0 × 102 | 3.2 × 101 | 4.9 × 101 | 7.5 × 101 | 3.7 × 101 | 3.1 × 101 | 4.5 × 100 |
| Σ (3) PCBs | 1.1 × 102 | 1.3 × 102 | 9.2 × 102 | 4.8 × 102 | 4.2 × 102 | 3.2 × 103 | 3.0 × 102 | 4.2 × 102 | 8.4 × 102 | 3.9 × 102 | 2.5 × 102 | 5.2 × 101 |
|
| ||||||||||||
| o,p′-DDT | 1.9 × 100 | 4.2 × 100 | 1.4 × 101 | 3.9 × 100 | 3.0 × 100 | 8.5 × 100 | 2.4 × 100 | 3.3 × 100 | 3.4 × 101 | 2.2 × 100 | 6.7 × 100 | 2.8 × 100 |
| o,p′-DDD | n/a | 2.9 × 100 | 7.2 × 100 | 3.1 × 100 | 2.7 × 100 | 1.7 × 101 | n/a | 3.4 × 100 | 2.1 × 101 | n/a | 2.8 × 100 | 1.6 × 100 |
| p,p′-DDT | 7.2 × 100 | 1.3 × 101 | 3.2 × 101 | 2.2 × 101 | 1.9 × 101 | 2.8 × 101 | 7.5 × 100 | 1.5 × 101 | 5.6 × 101 | 8.0 × 100 | 2.3 × 101 | 4.3 × 100 |
| p,p′-DDD | 1.4 × 101 | 2.1 × 101 | 3.5 × 101 | 3.7 × 101 | 3.4 × 101 | 5.0 × 101 | 9.5 × 100 | 2.9 × 101 | 1.0 × 102 | 1.6 × 101 | 2.4 × 101 | 5.6 × 100 |
| p,p′-DDE | 4.2 × 101 | 5.0 × 101 | 1.2 × 102 | 1.1 × 102 | 1.2 × 102 | 1.6 × 102 | 3.6 × 101 | 9.6 × 101 | 3.4 × 102 | 5.6 × 101 | 9.3 × 101 | 2.0 × 101 |
| Σ (5) DDTs | 6.4 × 101 | 9.1 × 101 | 2.1 × 102 | 1.7 × 102 | 1.8 × 102 | 2.7 × 102 | 5.6 × 101 | 1.5 × 102 | 5.6 × 102 | 8.2 × 101 | 1.5 × 102 | 3.5 × 101 |
|
| ||||||||||||
| β-HCH | n/a | n/a | 3.9 × 100 | n/a | 3.5 × 100 | 3.2 × 100 | n/a | 3.3 × 100 | 5.0 × 100 | 1.7 × 100 | 3.0 × 100 | 1.9 × 100 |
| δ-HCH | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| γ-HCH | 3.9 × 100 | 5.8 × 100 | 5.3 × 100 | 5.1 × 100 | 9.4 × 100 | 7.8 × 100 | 3.2 × 100 | 6.9 × 100 | 1.9 × 101 | 7.0 × 100 | 6.1 × 100 | 4.6 × 100 |
| α-HCH | 2.8 × 100 | n/a | 2.7 × 100 | 2.6 × 100 | n/a | n/a | n/a | 3.2 × 100 | 3.9 × 100 | 2.8 × 100 | 4.2 × 100 | 3.0 × 100 |
| HCB | 5.7 × 100 | 8.4 × 100 | 2.1 × 101 | 8.9 × 100 | 1.2 × 101 | 2.0 × 101 | 5.4 × 100 | 1.3 × 101 | 6.1 × 101 | 3.9 × 100 | 2.4 × 101 | 3.0 × 100 |
| Σ (5) HCHs + HCB | 1.2 × 101 | 1.4 × 101 | 3.3 × 101 | 1.7 × 101 | 2.5 × 101 | 3.1 × 101 | 8.6 × 100 | 2.7 × 101 | 8.9 × 101 | 1.5 × 101 | 3.7 × 101 | 1.3 × 101 |
|
| ||||||||||||
| Σ (13) total | 1.9 × 102 | 2.4 × 102 | 1.2 × 103 | 6.7 × 102 | 6.2 × 102 | 3.5 × 103 | 3.7 × 102 | 5.9 × 102 | 1.5 × 103 | 4.8 × 102 | 4.4 × 102 | 9.9 × 101 |
|
| ||||||||||||
| Activity recorded in the ER reporter gene assay (% of relative response) | None | Antiestrogenic (7%) | Antiestrogenic (16%) | Antiestrogenic (6%) | Estrogenic (22%) | Antiestrogenic (13%) | Antiestrogenic (5%) | None | Estrogenic (24%) and antiestrogenic (6%) | Antiestrogenic (5%) | None | Antiestrogenic (7%) |
n/a: not applicable.
As previously shown, pollutants from the DDT group presented higher estrogenic activities than the other POPs assessed. Moreover, the most potent agonist blubber samples (A00/258 and 01/1219) presented lower ΣPCB/ΣDDT ratios than the sample that inhibited the activity of the natural hormone the most (03/1238). This fact may explain the hormonal activity of samples A00/258 and 01/1219.
The most contaminated sample (A03/1517), containing a total of more than 88 mg organochlorine/kg fat, displayed a slight antiestrogenic effect. This can be easily explained by the high contribution of PCBs (more than 81 mg/kg), which were not inducing any response in the estrogen-responsive cells.
4. Discussion
The study of estrogen receptor- (ER-) mediated activity of POPs in MCF-7-ERE cells showed that several organochlorine pollutants (DDT and metabolites and HCH isomers) present an estrogenic activity, which was also confirmed by other authors [24, 44–47]. The fact that luciferase gene expression was not induced by PCB 153 and PCB 180 confirms the study of Plíšková et al. [48], where it was observed that higher-chlorinated PCB congeners present low estrogenic activity. It seems that the activity of ER agonism of a PCB is linked to its structure, as larger estrogenic potencies have been reported for low chlorinated compounds [49]. These assays also point out how complex the prediction of the effect of a POP mixture can be. In fact, the approach to investigate endocrine disrupters within mixtures is a new tool that provides clear evidence that POPs have different effects when they are not alone, suggesting that risk assessment should take into consideration the effect of these chemicals within mixture rather than their individual effects [50–54].
Concentrations of organochlorine chemicals found in North Sea harbour porpoise blubber samples are comparable with data formerly published [5, 6, 55, 56]. Percentage distribution of PCB congeners was constant between samples and the importance of single congeners was as follows: 153 > 138 > 180. Similar profiles were found in various harbour porpoise tissues in previous studies [10, 15, 57, 58]. The distribution pattern of PCBs can be explained by differences in structural characteristics within PCB congeners that determine whether a molecule can be easily metabolised by cytochrome P450 enzymes or not.
Metabolites play a dominant role if their persistence exceeds that of the parent product. Even though DDT was banned from utilisation in North America and Western Europe in the 1970s with no new input in the southern North Sea during the last decades [59], the breakdown products of DDT were still detected in the analyzed samples (DDE showing the highest concentrations). In fact, DDT is rapidly metabolised to DDD and slowly metabolised to DDE. Furthermore, DDD has a significantly higher elimination rate than DDE and, subsequently, aquatic animals retain more DDE in their bodies [60, 61]. Thus, the detected contents of DDD and DDE determined in our samples are possibly due to the breakdown of DDT in the environment.
The high concentration of γ-HCH in samples is surprising as γ-HCH is relatively rapidly phototransformed to α-HCH in the environment [62]. This same profile was found in other species of the North Sea [63]. The high concentration of γ-HCH found in blubber samples may indicate a current utilization of this pesticide in some countries, with a subsequent global scale pollution since both the atmosphere and the ocean are important transport media for HCH isomers [64, 65]. Also, HCH isomers and HCB were detected in the samples in lower concentration than PCB congeners and pollutants from the DDT group, possibly because these compounds are easily metabolized and are more water soluble [66].
The large difference of contamination level between samples is probably due to a possible difference in health conditions (e.g., disease or parasitic infection) of the animals, as reported by Pierce et al. [7], maternal transfer, age, and diet. As shown in Table 3, samples from animals that did not present emaciation or parasitic infection figured among the least contaminated. In addition, it was reported that POP concentration in juvenile marine mammals is much higher than in the mothers, since a large portion of the mother's POP burden, transferred to the calf during gestation and lactation, is assimilated into its blubber and other tissues [67], placing juveniles at higher risk [55].
The analyses of blubber samples by cell-based bioassays suggest that POPs measured by GC-MS in this study cannot justify alone the estrogenicity of the extracts and that other endocrine disrupters contaminate the porpoises. Interestingly, the sample showing the highest estrogenic activity and the two samples displaying the highest ER antagonistic activities are among the most contaminated samples. It can be expected that these samples, with a high load of contaminants, also contain other POPs than the 13 organochlorine measured in this study, among which some are agonist or antagonist of the estrogen receptor, such as dioxins and furans [68].
5. Conclusions
Our study permitted the analysis of harbour porpoise blubber samples both by chemical analysis and cell-based assays, providing consistent data including the level of contamination and the (anti-) estrogenic activity of these samples. Within each group of studied substances, PCB 153, p,p′-DDE, and HCB were the compounds found in highest concentrations. Two samples showed estrogenic activity, seven samples showed antiestrogenic activity, and one sample showed both estrogenic and antiestrogenic activity. However, our results suggest that the 13 POPs measured by GC-MS in the samples cannot explain alone the estrogenicity of the extracts and that other EDCs contaminate the porpoises.
High load persistent organic pollutants in porpoise blubber samples indicate that these pollutants can also be found in food from the North Sea, and the hormonal activity measured in some samples confirms the presence of endocrine disrupting chemicals in the marine environment.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Hammond P. S., Berggren P., Benke H., et al. Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. Journal of Applied Ecology. 2002;39(2):361–376. doi: 10.1046/j.1365-2664.2002.00713.x. [DOI] [Google Scholar]
- 2.Beineke A., Siebert U., McLachlan M., et al. Investigations of the potential influence of environmental contaminants on the thymus and spleen of harbor porpoises (Phocoena phocoena) Environmental Science & Technology. 2005;39(11):3933–3938. doi: 10.1021/es048709j. [DOI] [PubMed] [Google Scholar]
- 3.Jauniaux T., Petitjean D., Brenez C., et al. Post-mortem findings and causes of death of harbour porpoises (Phocoena phocoena) stranded from 1990 to 2000 along the coastlines of Belgium and Northern France. Journal of Comparative Pathology. 2002;126(4):243–253. doi: 10.1053/jcpa.2001.0547. [DOI] [PubMed] [Google Scholar]
- 4.Peltier H., Baagøe H. J., Camphuysen K. C. J., et al. The stranding anomaly as population indicator: the case of harbour porpoise Phocoena phocoena in north-western europe. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0062180.e62180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mössner S., Ballschmiter K. Marine mammals as global pollution indicators for organochlorines. Chemosphere. 1997;34(5–7):1285–1296. doi: 10.1016/s0045-6535(97)00426-8. [DOI] [PubMed] [Google Scholar]
- 6.Law R. J., Bersuder P., Barry J., Deaville R., Reid R. J., Jepson P. D. Chlorobiphenyls in the blubber of harbour porpoises (Phocoena phocoena) from the UK: levels and trends 1991–2005. Marine Pollution Bulletin. 2010;60(3):470–473. doi: 10.1016/j.marpolbul.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Pierce G. J., Santos M. B., Murphy S., et al. Bioaccumulation of persistent organic pollutants in female common dolphins (Delphinus delphis) and harbour porpoises (Phocoena phocoena) from western European seas: geographical trends, causal factors and effects on reproduction and mortality. Environmental Pollution. 2008;153(2):401–415. doi: 10.1016/j.envpol.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe S. Contamination and toxic effects of persistent endocrine disrupters in marine mammals and birds. Marine Pollution Bulletin. 2002;45(1-12):69–77. doi: 10.1016/S0025-326X(02)00175-3. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe S., Sung J.-K., Choi D.-Y., et al. Persistent organochlorine residues in northern fur seal from the Pacific coast of Japan since 1971. Environmental Pollution. 1994;85(3):305–314. doi: 10.1016/0269-7491(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 10.Bruhn R., Kannan N., Petrick G., Schulz-Bull D. E., Duinker J. C. CB pattern in the harbour porpoise: bioaccumulation, metabolism and evidence for cytochrome p450 IIB activity. Chemosphere. 1995;31(7):3721–3732. doi: 10.1016/0045-6535(95)00221-s. [DOI] [PubMed] [Google Scholar]
- 11.Boon J. P., van der Meer J., Allchin C. R., et al. Concentration-dependent changes of PCB patterns in fish-eating mammals: structural evidence for induction of cytochrome P450. Archives of Environmental Contamination and Toxicology. 1997;33(3):298–311. doi: 10.1007/s002449900257. [DOI] [PubMed] [Google Scholar]
- 12.D'Amato C., Torres J. P. M., Malm O. DDT (Dichlorodiphenyltrichloroethane): toxicity and environmental contamnation—a review. Química Nova. 2002;25(6A):995–1002. doi: 10.1590/s0100-40422002000600017. [DOI] [Google Scholar]
- 13.Alegría-Torres J. A., Díaz-Barriga F., Gandolfi A. J., Pérez-Maldonado I. N. Mechanisms of p, p′-DDE-induced apoptosis in human peripheral blood mononuclear cells. Toxicology In Vitro. 2009;23(6):1000–1006. doi: 10.1016/j.tiv.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Abhilash P. C., Singh N. Distribution of hexachlorocyclohexane isomers in soil samples from a small scale industrial area of Lucknow, North India, associated with lindane production. Chemosphere. 2008;73(6):1011–1015. doi: 10.1016/j.chemosphere.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 15.Covaci A., Van De Vijver K., DeCoen W., et al. Determination of organohalogenated contaminants in liver of harbour porpoises (Phocoena phocoena) stranded on the Belgian North Sea coast. Marine Pollution Bulletin. 2002;44(10):1157–1165. doi: 10.1016/s0025-326x(02)00147-9. [DOI] [PubMed] [Google Scholar]
- 16.Safe S. H. Endocrine disruptors and human health—is there a problem? An update. Environmental Health Perspectives. 2000;108(6):487–493. doi: 10.1289/ehp.00108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz-de-Toro M., Beldoménico H. R., García S. R., et al. Organochlorine levels in adipose tissue of women from a littoral region of Argentina. Environmental Research. 2006;102(1):107–112. doi: 10.1016/j.envres.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Porte C., Janer G., Lorusso L. C., et al. Endocrine disruptors in marine organisms: approaches and perspectives. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2006;143(3):303–315. doi: 10.1016/j.cbpc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Bila D. M., Dezotti M. Endocrine disrupters in the environment: part 1—effects and consequences. Química Nova. 2007;30(3):651–666. doi: 10.1590/s0100-40422007000300027. [DOI] [Google Scholar]
- 20.Ross P. S., Couillard C. M., Ikonomou M. G., et al. Large and growing environmental reservoirs of Deca-BDE present an emerging health risk for fish and marine mammals. Marine Pollution Bulletin. 2009;58(1):7–10. doi: 10.1016/j.marpolbul.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Gustavson L., Ciesielski T. M., Bytingsvik J., et al. Hydroxylated polychlorinated biphenyls decrease circulating steroids in female polar bears (Ursus maritimus) Environmental Research. 2015;138:191–201. doi: 10.1016/j.envres.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Sonne C., Letcher R. J., Bechshøft T. Ø., et al. Two decades of biomonitoring polar bear health in Greenland: a review. Acta Veterinaria Scandinavica. 2012;54(supplement 1):p. S15. doi: 10.1186/1751-0147-54-s1-s15. [DOI] [Google Scholar]
- 23.Smith A. G., Gangolli S. D. Organochlorine chemicals in seafood: occurrence and health concerns. Food and Chemical Toxicology. 2002;40(6):767–779. doi: 10.1016/s0278-6915(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 24.Willemsen P., Scippo M.-L., Kausel G., et al. Use of reporter cell lines for detection of endocrine-disrupter activity. Analytical and Bioanalytical Chemistry. 2004;378(3):655–663. doi: 10.1007/s00216-003-2217-2. [DOI] [PubMed] [Google Scholar]
- 25.Maranghi F., Rescia M., Macrì C., et al. Lindane may modulate the female reproductive development through the interaction with ER-β: an in vivo-in vitro approach. Chemico-Biological Interactions. 2007;169(1):1–14. doi: 10.1016/j.cbi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Jepson P. D., Bennett P. M., Deaville R., Allchin C. R., Baker J. R., Law R. J. Relationships between polychlorinated biphenyls and health status in harbor porpoises (Phocoena phocoena) stranded in the United Kingdom. Environmental Toxicology and Chemistry. 2005;24(1):238–248. doi: 10.1897/03-663.1. [DOI] [PubMed] [Google Scholar]
- 27.Hall A. J., Hugunin K., Deaville R., Law R. J., Allchin C. R., Jepson P. D. The risk of infection from polychlorinated biphenyl exposure in the harbor porpoise (Phocoena phocoena): a case-control approach. Environmental Health Perspectives. 2006;114(5):704–711. doi: 10.1289/ehp.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das K., Vossen A., Tolley K., et al. Interfollicular fibrosis in the thyroid of the harbour porpoise: an endocrine disruption? Archives of Environmental Contamination and Toxicology. 2006;51(4):720–729. doi: 10.1007/s00244-005-0098-4. [DOI] [PubMed] [Google Scholar]
- 29.Garritano S., Pinto B., Calderisi M., Cirillo T., Amodio-Cocchieri R., Reali D. Estrogen-like activity of seafood related to environmental chemical contaminants. Environmental Health: A Global Access Science Source. 2006;5, article 9 doi: 10.1186/1476-069x-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guéguen M., Amiard J.-C., Arnich N., et al. Shellfish and residual chemical contaminants: hazards, monitoring, and health risk assessment along French coasts. Reviews of Environmental Contamination and Toxicology. 2011;213:55–111. doi: 10.1007/978-1-4419-9860-6_3. [DOI] [PubMed] [Google Scholar]
- 31.Scippo M.-L., Maghuin-Rogister G. Endocrine disruptors in food: potential impact on human health. Annales de Medecine Veterinaire. 2007;151(1):44–54. [Google Scholar]
- 32.Nadzialek S., Vanparys C., Van der Heiden E., et al. Understanding the gap between the estrogenicity of an effluent and its real impact into the wild. Science of the Total Environment. 2010;408(4):812–821. doi: 10.1016/j.scitotenv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Willemsen P., Scippo M.-L., Maghuin-Rogister G., Martial J. A., Muller M. Use of specific bioluminescent cell lines for the detection of steroid hormone (ant)agonists in meat producing animals. Analytica Chimica Acta. 2002;473(1-2):119–126. doi: 10.1016/S0003-2670(02)00772-9. [DOI] [Google Scholar]
- 34.Streck G. Chemical and biological analysis of estrogenic, progestagenic and androgenic steroids in the environment. Trends in Analytical Chemistry. 2009;28(6):635–652. doi: 10.1016/j.trac.2009.03.006. [DOI] [Google Scholar]
- 35.Houtman C. J., Booij P., Jover E., et al. Estrogenic and dioxin-like compounds in sediment from Zierikzee harbour identified with CALUX assay-directed fractionation combined with one and two dimensional gas chromatography analyses. Chemosphere. 2006;65(11):2244–2252. doi: 10.1016/j.chemosphere.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 36.Cai K., Elliott C. T., Phillips D. H., Scippo M.-L., Muller M., Connolly L. Treatment of estrogens and androgens in dairy wastewater by a constructed wetland system. Water Research. 2012;46(7):2333–2343. doi: 10.1016/j.watres.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 37.Houtman C. J., Van Oostveen A. M., Brouwer A., Lamoree M. H., Legler J. Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environmental Science & Technology. 2004;38(23):6415–6423. doi: 10.1021/es049750p. [DOI] [PubMed] [Google Scholar]
- 38.Simon E., Lamoree M. H., Hamers T., et al. Testing endocrine disruption in biota samples: a method to remove interfering lipids and natural hormones. Environmental Science and Technology. 2010;44(21):8322–8329. doi: 10.1021/es101912z. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki G., Tue N. M., van der Linden S., et al. Identification of major dioxin-like compounds and androgen receptor antagonist in acid-treated tissue extracts of high trophic-level animals. Environmental Science & Technology. 2011;45(23):10203–10211. doi: 10.1021/es2024274. [DOI] [PubMed] [Google Scholar]
- 40.Willemsen P., Scippo M.-L., Maghuin-Rogister G., Martial J. A., Muller M. Enhancement of steroid receptor-mediated transcription for the development of highly responsive bioassays. Analytical and Bioanalytical Chemistry. 2005;382(4):894–905. doi: 10.1007/s00216-005-3253-x. [DOI] [PubMed] [Google Scholar]
- 41.Jayle M.-F., Crépy O. Hydrolyse, extraction et purification des stéroïdes. In: Jayle M.-F., editor. Analyses des Stéroïdes Hormonaux: Tome I. Paris, France: Masson et Cie Editeurs; 1961. pp. 61–113. [Google Scholar]
- 42.Rivas A., Olea N., Olea-Serrano F. Human exposure to endocrine-disrupting chemicals: assessing the total estrogenic xenobiotic burden. TrAC: Trends in Analytical Chemistry. 1997;16(10):613–619. doi: 10.1016/s0165-9936(97)00101-5. [DOI] [Google Scholar]
- 43.Sebaugh J. L., McCray P. D. Defining the linear portion of a sigmoid-shaped curve: bend points. Pharmaceutical Statistics. 2003;2(3):167–174. doi: 10.1002/pst.62. [DOI] [Google Scholar]
- 44.Payne J., Jones C., Lakhani S., Kortenkamp A. Improving the reproducibility of the MCF-7 cell proliferation assay for the detection of xenoestrogens. Science of the Total Environment. 2000;248(1):51–62. doi: 10.1016/S0048-9697(99)00479-9. [DOI] [PubMed] [Google Scholar]
- 45.Hatakeyama M., Tessier D. M., Dunlap D. Y., Zou E., Matsumura F. Estrogenic action of β-HCH through activation of c-Neu in MCF-7 breast carcinoma cells. Environmental Toxicology and Pharmacology. 2002;11(1):27–38. doi: 10.1016/s1382-6689(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 46.Hatakeyama M., Zou E., Matsumura F. Comparison of the characteristic of estrogenic action patterns of β-HCH and heregulin β1 in MCF-7 human breast cancer cells. Journal of Biochemical and Molecular Toxicology. 2002;16(5):209–219. doi: 10.1002/jbt.10047. [DOI] [PubMed] [Google Scholar]
- 47.Marabini L., Chiesara E., Radice S. Possible estrogenic effects of some highly bioaccumulated polychlorinated biphenyls (PCB 101, 118,138,153) alone and in mixture in MCF-7 breast cancer cells. Toxicology Letters. 2007;172:S49–S50. [Google Scholar]
- 48.Plíšková M., Vondráček J., Canton R. F., et al. Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environmental Health Perspectives. 2005;113(10):1277–1284. doi: 10.1289/ehp.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamers T., Kamstra J. H., Cenijn P. H., et al. In vitro toxicity profiling of ultrapure non-dioxin-like polychlorinated biphenyl congeners and their relative toxic contribution to PCB mixtures in humans. Toxicological Sciences. 2011;121(1):88–100. doi: 10.1093/toxsci/kfr043. [DOI] [PubMed] [Google Scholar]
- 50.Payne J., Rajapakse N., Wilkins M., Kortenkamp A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Environmental Health Perspectives. 2000;108(10):983–987. doi: 10.1289/ehp.00108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daston G. P., Cook J. C., Kavlock R. J. Uncertainties for endocrine disrupters: our view on progress. Toxicological Sciences. 2003;74(2):245–252. doi: 10.1093/toxsci/kfg105. [DOI] [PubMed] [Google Scholar]
- 52.Tinwell H., Ashby J. Sensitivity of the immature rat uterotrophic assay to mixtures of estrogens. Environmental Health Perspectives. 2004;112(5):575–582. doi: 10.1289/ehp.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Meeuwen J. A., van den Berg M., Sanderson J. T., Verhoef A., Piersma A. H. Estrogenic effects of mixtures of phyto- and synthetic chemicals on uterine growth of prepubertal rats. Toxicology Letters. 2007;170(2):165–176. doi: 10.1016/j.toxlet.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Habibi H., Jeffries K., Nelson E., Jackson L. Risk assessment for endocrine disrupting chemical mixtures. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2008;148(4):p. 455. doi: 10.1016/j.cbpc.2008.10.031. [DOI] [Google Scholar]
- 55.Weijs L., van Elk C., Das K., Blust R., Covaci A. Persistent organic pollutants and methoxylated PBDEs in harbour porpoises from the North Sea from 1990 until 2008: Young wildlife at risk? Science of the Total Environment. 2010;409(1):228–237. doi: 10.1016/j.scitotenv.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 56.Law R. J., Bolam T., James D., et al. Butyltin compounds in liver of harbour porpoises (Phocoena phocoena) from the UK prior to and following the ban on the use of tributyltin in antifouling paints (1992–2005 & 2009) Marine Pollution Bulletin. 2012;64(11):2576–2580. doi: 10.1016/j.marpolbul.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Weijs L., Das K., Siebert U., et al. Concentrations of chlorinated and brominated contaminants and their metabolites in serum of harbour seals and harbour porpoises. Environment International. 2009;35(6):842–850. doi: 10.1016/j.envint.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Weijs L., Dirtu A. C., Das K., et al. Inter-species differences for polychlorinated biphenyls and polybrominated diphenyl ethers in marine top predators from the Southern North Sea. Part 1. Accumulation patterns in harbour seals and harbour porpoises. Environmental Pollution. 2009;157(2):437–444. doi: 10.1016/j.envpol.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Mahfouz C., Henry F., Jauniaux T., Khalaf G., Amara R. Organochlorines in harbour porpoises (Phocoena phocoena) stranded along the southern North Sea between 2010 and 2013. nvironmental Science: Processes & Impacts. 2014;16(12):2774–2781. doi: 10.1039/c4em00490f. [DOI] [PubMed] [Google Scholar]
- 60.Kwong R. W. M., Yu P. K. N., Lam P. K. S., Wang W.-X. Uptake, elimination, and biotransformation of aqueous and dietary DDT in marine fish. Environmental Toxicology and Chemistry. 2008;27(10):2053–2063. doi: 10.1897/07-608.1. [DOI] [PubMed] [Google Scholar]
- 61.Kwong R. W. M., Yu P. K. N., Lam P. K. S., Wang W.-X. Biokinetics and biotransformation of DDTs in the marine green mussels Perna viridis . Aquatic Toxicology. 2009;93(4):196–204. doi: 10.1016/j.aquatox.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Benezet H. J., Matsumura F. Isomerization of γ-BHC to α-BHC in the environment. Nature. 1973;243(5407):480–481. doi: 10.1038/244480a0. [DOI] [Google Scholar]
- 63.Voorspoels S., Covaci A., Maervoet J., De Meester I., Schepens P. Levels and profiles of PCBs and OCPs in marine benthic species from the Belgian North Sea and the Western Scheldt Estuary. Marine Pollution Bulletin. 2004;49(5-6):393–404. doi: 10.1016/j.marpolbul.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 64.Barrie L. A., Gregor D., Hargrave B., et al. Arctic contaminants: sources, occurrence and pathways. Science of the Total Environment. 1992;122(1-2):1–74. doi: 10.1016/0048-9697(92)90245-n. [DOI] [PubMed] [Google Scholar]
- 65.Saadati N., Abdullah M. P., Zakaria Z., Rezayi M., Hosseinizare N. Distribution and fate of HCH isomers and DDT metabolites in a tropical environment-case study Cameron Highlands-Malaysia. Chemistry Central Journal. 2012;6(1, article 130) doi: 10.1186/1752-153x-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleivane L., Skaare J. U., Bjørge A., de Ruiter E., Reijnders P. J. H. Organochlorine pesticide residue and PCBs in harbour porpoise (Phocoena phocoena) incidentally caught in Scandinavian waters. Environmental Pollution. 1995;89(2):137–146. doi: 10.1016/0269-7491(94)00066-m. [DOI] [PubMed] [Google Scholar]
- 67.Krahn M. M., Hanson M. B., Schorr G. S., et al. Effects of age, sex and reproductive status on persistent organic pollutant concentrations in ‘Southern Resident’ killer whales. Marine Pollution Bulletin. 2009;58:1522–1529. doi: 10.1016/j.marpolbul.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Navas J. M., Segner H. Antiestrogenic activity of anthropogenic and natural chemicals. Environmental Science and Pollution Research International. 1998;5(2):75–82. doi: 10.1007/bf02986390. [DOI] [PubMed] [Google Scholar]



