Abstract
This systematic review intended to combine factors associated with tuberculosis treatment non-adherence and lost to follow up among TB patients with/without HIV in developing countries. Comprehensive remote electronic databases (MEDLINE, (PMC, Pub Med Central), Google scholar and Web of science) search was conducted using the following keywords: Tuberculosis, treatment, compliance, adherence, default, behavioural factors and socioeconomic factors. All types of studies intended to assess TB treatment non-adherence and lost to follow up in developing countries among adult TB patient from 2008 to data extraction date were included. Twenty-six original and one-reviewed articles, which meet inclusion criteria, were reviewed. TB treatment non-adherence and lost to follow up were continued across developing countries. The main factors associated with TB treatment non-adherence and lost to follow up were socioeconomic factors: lack of transportation cost, lack of social support, and patients-health care worker poor communication. Behavioural factors were Feeling better after few weeks of treatments, tobacco and alcohol use, knowledge deficit about duration of treatment and consequences of non-adherence and lost to follow up. TB treatment non-adherence and lost to follow up were continued across developing countries throughout the publication years of reviewed articles. Numerous, socioeconomic and behavioural factors were influencing TB treatment adherence and lost to follow up. Therefore, well understanding and minimizing of the effect of these associated factors is very important to enhance treatment adherence and follow up completion in developing countries.
Keywords: Tuberculosis treatment, Non-adherence, Lost to follow up, TB patients
Introduction
Despite the availability of effective short course regimen first line drug since 1980s (1, 2), TB remains a major global health problem; it causes ill-health and death among millions of people each year and ranks second leading cause of death from an infectious disease worldwide, after human immunodeficiency virus (HIV) (2). Current global estimates indicate that about one in every three people in the world is believed to be infected with Mycobacterium tuberculosis (M. tuberculosis) and at risk of developing the disease (3). According to WHO global TB report of 2012 there are 8.7 million new cases and 1.4 million deaths in 2011; and almost one million death among HIV positive TB patients (2, 4).
The proportion of TB cases co-infected with HIV is highest in African region countries; overall, African region accounted for 79% of TB cases among people living with HIV (PLHIV) (2, 5, 6), because the synergy between TB and HIV is strong; i.e. PLHIV ranged from 20-37 folds at increased risk of active TB development compared to HIV uninfected people depending on the state of HIV epidemic in the area (4, 7, 8).
The burden of TB among low and meddle income countries is fuelled due to HIV pandemic, and smoothen by numerous socioeconomic conditions (9). According to research findings, socioeconomic factors such as homelessness, lack of food, financial limitation, lack of transportation cost, low education level, gender, poor health care worker-patient communication, joblessness, social supports etc. are highly associated with TB treatment non-adherence and lost to follow up (9–11). These conditions are related to each other and form a network of causal pathways against TB patient tolerance ability (9). Additionally, overcrowded living condition (9, 12), HIV related immunological weakness (13) and malnutrition (14, 15) are factors that facilitate transmission of bacilli, treatment non-adherence and lost to follow up at economically disadvantaged settings. In general many social determinants of health (SDH) reinforce social stratification in society. Social stratification in turn to an unequal distribution of the social determinants of health, including material living conditions and psychosocial circumstances as well as behavioural and biological risk factors to health problems including TB; finally influence patients’ treatment adherence tolerance (16).
Beside, socioeconomic factors, patient’s individual behavioural factors, like knowledge about TB disease, duration of treatment, consequences of treatment non-adherence and lost to follow up, feeling better after few weeks of treatment, fear of stigma; attitudes towards treatment and poor communication with health care workers, lack of self-efficacy or motivation to complete treatment are the main behavioural factors that associate with TB treatment non-adherence and lost to follow up (17-19). Furthermore, alcohol consumption (19–22) and cigarette smoking (17, 22) are the two individual behavioural factors that associated with TB treatment non-adherence and lost to follow up that reported so far. TB treatment non-adherence and lost to follow up are continues throughout the nations, and it extended its potential consequences, like initial treatment failure and relapse, which are in turns to prolonging morbidity, mortality, prolonged transmission of bacilli and development of medication resistance types of M. tuberculosis)(23).
The current anti-TB therapies are fraught with problems, predominantly because of the long-term treatment and the increasing occurrence of medication resistance types of M. tuberculosis organism (24, 25), which is most probably due to treatment non-adherence and lost to follow up. The most dangerous thing of drug resistant is formation of Multi drug resistant TB (MDR-TB) (24, 26–28) and extensively drug resistant TB (XDR-TB) (28). According to WHO 2012 global TB report about 3.7% of new TB patients in the world infected with MDR-TB strains. Of 3.7%, 9% is XDR-TB strain (2).
Despite, implementation of internationally recommended strategy (DOTS) in almost all parts of WHO (29–31) regions and many national and international efforts exerted against TB prevention and control, still the patients are failing to complete their treatment to declare cure or complete the treatment. Current WHO report shows considerable TB cases are failed after several treatments, many are relapsing (226, 813) after completion of the treatment, many are inter to retreatment (348, 734) after completion of treatment and many cases are developing MDR-TB among retreatment cases (20%) throughout the world (2). For this, most probability treatment non-adherence and lost to follow up are the main responsible.
Non-adherence to treatment is the patient’s inability or refusal to take TB medications according to prescribed by health professional (32–35). Similarly lost to follow up is a TB patient who did not start treatment or whose treatment was interrupted for 2 consecutive months or more (35). Hence, intensive case notification and observing patients while they are taking the medication only are not sufficient to prevent TB treatment non-adherence and lost to follow up. However, well understanding and intervening of associated factors which are influencing TB patients’ tolerance ability and promote treatment non-adherence and lost to follow up is corner stone to good treatment success. Moreover, well understanding and then intervening of these associated factors at the community, family, individual, health care worker and health care system levels are very important through conducting comprehensive large scale community based interventional study. Therefore, this systematic review was intended to combine the associated factors with TB treatment non-adherence and lost to follow up among TB patients with or without HIV, which previously studied in developing countries since 2008.
Methods
Data base searched
MEDLINE, (PMC, Pub Med Central), Google scholar and Web of science electronic data bases were searched exhaustively using keywords: Tuberculosis, Treatment, compliance, adherence, Default, behavioural factors and socioeconomic factors. All types of studies intended to assess TB treatment non-adherence and lost to follow up as the primary outcome variable in developing countries among TB patients were included. Our search was restricted to articles published since 2008 in English, and conference abstracts were not searched.
Inclusion and exclusion Criteria
Any types of study, which conducted in developing country since 2008, intended to assess the main factors that associated with TB treatment non-adherence or lost to follow up on adult TB patients with or without HIV and treated under DOTS was included. Studies conducted on children, prisoner, home based DOTS and on latent TB were not included.
Article Search and selection methods
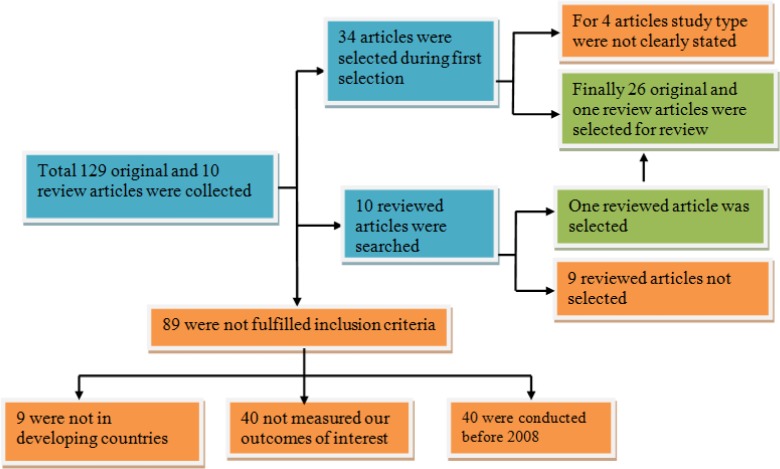
A comprehensive and exhaustive search was executed using Tehran University of Medical Sciences electronic library, to identify all applicable studies published in English. During our search, 129 total original articles which were intended to assess factors associated with TB treatment non-adherence or lost to follow up separately or collectively as primary outcome were identified.
Of these 89 were excluded because they were not conducted in the developing country, not examined factors affect non-adherence or lost to follow up of TB treatment, and conducted before 2008 as clearly indicated on Fig. 1. Finally, 26 original and one review articles were selected for review Fig.1.
Fig. 1.
Procedural demonstration of articles selection and evaluation
Types of outcome measured
The main outcome variables were assessed socioeconomic variables such as age, gender, lack of food, money, transportation cost and social support from family, friends, community and heath care worker, distance from treatment centre and lack of job. Individual behavioural variables were assessed, fear of stigma, feeling better after few weeks of treatments, knowledge about TB treatment (duration, curability, and side effects), and consequences of treatment non-adherence and lost to follow up, alcohol and tobacco use. Additionally, TB patients HIV and antiretroviral status were assessed as the associated factors.
Data extraction: Data extraction was done by first author on pre-developed format based on outcomes intended to be assessed, then it re-checked by second and third authors separately for the quality purpose.
Study characteristics: In this review we describe study population, study settings (health centre, hospital, population based), study area (urban/rural), sample size, treatment methods (DOTS or not), treatment interruption time, HIV status of study participants, factors associated with TB treatment non-adherence and lost to follow up.
Results
A clear summery of countries where the study conducted, number of total articles selected, and factors associated with TB treatment non-adherence and lost to follow up are presented in Table 1.
Table 1.
A summary of socioeconomic and behavioral factors associated with TB treatment non-adherence and lost follow up among TB patients with HIV or without HIV in developing countries
| Country Name | Reference | Main Associated Factors of TB Treatment Non-adherence and lost to follow up |
|---|---|---|
| Moroccan | (36) | Smoking, low education, urban residency, male gender |
| Moroccan | (37) | Feeling better, education, TB treatment knowledge, lack of job, distance from health center, long duration of treatment, side effect, social stigma |
| Uzbekistan | (38) | Lack of education, drug side effects, DOTS weakness, lack of supervision visits during treatment, distance from health centre, lack of knowledge, migration, financial difficulty, long duration of treatment, lack of transportation |
| Uzbekistan | (39) | Incarceration /imprisonment, smoking, complaint, poverty, side effects, HCW-patient relation |
| Bostwana | (40) | Male gender, young age, higher education, distance from HC, alcohol use |
| Indonesia | (41) | Feeling better, lack of money and social support, negative attitude towards HCW, non adherence history, TB treatment knowledge, side effects, stigma, poor patient-HCW relation, lack of health education, getting bored of the pills |
| Ethiopia | (42) | Time-consuming treatment procedure, distance from HC, rigid routines at health centre, lack of money, food, transportation, social support, lack of job, taking drug in empty stomach, health staff attitudes |
| India | (43) | Feeling better, knowledge of treatment, side effects, poor attitude of health workers, negative attitude toward TB patients by HCW, drug abuse, lack of health education, lack of income, job, food, social support, social stigma |
| S. Africa1 | (44) | Poverty, having co-morbidity, alcohol, smoking, male sex, age, education, perception, |
| India | (45) | Male gender, age above 35, side effects |
| S. Africa | (46) | HIV status, being on ART, lack of money, low education, lack of knowledge, smoking, alcohol use, stigma |
| Ethiopia1 | (47) | Distance from HC, poor interaction with health care worker, Social support, stigma, TB treatment knowledge, lack of income, education, food, transportation cost, pills burden, side effects, starting time of ARV |
| Uganda1 | (48) | Poverty, alcohol, ART, transportation, distance from health care, TB treatment knowledge, Alcohol use, smoking, being on ARV, patient — HCW relation |
| Ethiopia1 | (49) | Lack of health education, transportation cost, Patient—health care provider relationship, forgetting, feeling better, side effects |
| China | (50) | Alcohol use, smoking, inadequate knowledge, poor patient provider interaction, side effects, instantly missing doses, DOT effectiveness |
| China | (51) | Male gender, smoking, age above 30, lack of family, high education, lack of income, alcohol use |
| Ethiopia | (52) | Distance from health centre, financial burdens, use of traditional healer, delay in diagnosis, quality of health services, lack of food, lack of transportation, lack of job, communication with HCW and social support |
| Kenya | (53) | TB treatment knowledge, feeling well, worsen of disease, alcohol use, laziness, Drug shortage, service delay, long time treatment, lack of support, discouragement from people, side effects, ;lack of transportation, stress from family, smoking, interruption history |
| S-Saharan African | (54) | Distance from health care centre, lack of repeated smears, unit transfer after the intensive phase, side effects, lack of family support, lack of knowledge, age above 25 years |
| Brazil | (55) | Smoking, incarceration history, symptom complaint score >15, low income, HIV status |
| Kenya | (56) | Employment status, level of education, duration of treatment, distance from treatment area, alcohol abuse, small monthly income, lack of knowledge, stigma, side effects, herbal medication use, waiting time for services |
| Nigeria | (57) | Age above 30, HIV status, distance from health care centre, rural residence |
| Brazil1 | (58) | Lack of job, alcohol use, homelessness, old age, family loss or divorced, HIV infection, migration, side effect |
| Uganda1 | (59) | Distance from health centre, long waiting time for treatment, drug shortage, lack of opportunity to express feelings, TB treatment knowledge, side effects, lack, employment status, forgetting, feelings of lack of family support, fear of stigma |
| Thailand1 | (60) | Alcohol use, feeling better, side effects, work place conflict, Lack of treatment supporter |
| India | (61) | Transportation cost, smoking, alcohol use, male gender, long duration of the treatment, feeling better, distance from HC, drug shortage, lack of self-efficacy, stigma |
| Uganda | (62) | Alcohol use, feeling better, side effects, work place conflict |
1Article conducted on TB patient with HIV, DOT-directly observed therapy, HCW-health care worker, ART-antiretroviral therapy, DOTS-directly observed therapy short-course, HIV-human immunodeficiency virus, HC-health care
Except four countries all selected article were conducted in 22 high TB burden countries (2). Two were from Morocco (36, 37), two from Uzbekistan (38, 39), one from Botswana (40) and one from Indonesia (41). Of 27 articles 13 were conducted to identify associated factors with TB treatment non-adherence (37, 40–50); two treatment compliance (52, 53) and 12 treatment default (lost to follow up) (36, 38, 39, 54-62). Only 7 articles were specified study population HIV status (44, 47-50, 58-60).
The study settings were rural, urban, rural-urban combination, population based in both rural and urban; at hospital and primary health care, and in both public and private sectors. However, two studies conducted in Uzbekistan (38, 39), were not specified study population characteristics and settings.
Treatment strategy and Observer: For almost all studies the treatment strategy was DOTS and the observers were health care worker at health care facility; except for study from Botswana (40) and Indonesia (41) were not DOTS strategy and health care observer used.
Treatment Interruption or lost to follow up time: Majority of the studies were reported the time of treatment interruption or lost to follow up occurred during continuation phase.
Socioeconomic and behavioural factors associated with TB treatment non-adherence and lost to follow up
Financial limitation(40-53, 55, 56, 58-60), lack of food(38, 42, 44, 47, 48, 52, 56, 58, 60), lack of transportation cost(38, 41, 42, 44, 45, 47-49, 51-53, 56, 58-60), lack of job(38, 39, 42-45, 47, 52, 56, 59), lack of social support (support from family, friends, health care worker and community) (38, 39, 42-44, 47, 50, 52-54, 56, 58-61) and lack of basic education(36, 42-44, 46-51, 55, 56, 58, 59, 61) were the main socioeconomic factors that associated with TB treatment non-adherence and lost to follow up (Table 1). Also, numerous individual behavioural factors such as, knowledge shortage about TB treatment and its medications side effects (37, 38, 43, 44, 46-49, 53-56, 59-61); fear of stigma(38, 41, 45-47, 50, 56, 59, 61), feeling better after few weeks of treatments (disappearance of symptom) (37, 38, 41, 42, 44, 46, 59, 61, 62), tobacco smoking (36, 43-46, 48, 53, 56, 58, 60, 61), alcohol consumption(39, 40, 44-46, 48, 53, 55, 56, 58, 61, 62) and medication side effects (37-39, 41, 42, 47-51, 53, 54, 56, 60-62) were associated with TB treatment non-adherence and lost to follow up (Table 1).
Beside, socioeconomic and behavioural factors, patient-health care worker poor communication, lack of information/health education about TB disease and its treatment, lack of support and lack of chance to speak about their worry to health care worker (38, 41, 48, 49, 56, 59-61), distance from health care centre (37, 40-42, 45, 47, 48, 51-57, 59), patient’s HIV status(39, 44, 46-48, 55, 57, 59, 60) and being on antiretroviral therapy (ARV) or pills burden(44, 46–49) were associated with TB treatment non-adherence and lost to follow up. However, in some studies patient’s HIV status (47) and being on antiretroviral therapy (58–60) were reported as protective factors.
Discussion
Socioeconomic, individual behaviour, health care worker and patient’s HIV status were the main factors that associated with TB treatment non-adherence and lost to follow up across developing countries during publication years of articles reviewed. These factors were challenge patient’s tolerance ability to follow their treatment correctly until they declare cure or complete. According to research evidence these factors influence on TB treatment adherence and follow up were demonstrated since many years. For instance, Greene JA reported TB patients were enter to treatment non-adherent due to poverty (63) and other review study reported financial burden was the main cause of treatment interruption (64). These reports were similar with this the results from articles were reviewed. This financial constraint that recognized through lack of money, transportation cost and food has been continued to exert their influence on TB patients tolerance ability to adhere their treatment until declare cure or complete. Studies from Thailand and Ethiopia reported the same factors i.e., lack of income was resulted to lack of food, transportation cost, and then upset TB patients to continue their treatment (52, 60). For TB patients with HIV, financial limitation is double burden; because, many PLHIV are living with single family wife or husband; and caring for their children and themselves, therefore, when they start TB treatment which needs daily follow up, the double burden of financial limitation due to both HIV and TB treatment aggravate the problem, then they obliged to interrupt TB treatment or both (52).
In this review fear of stigma was another main behavioural factor that associated with TB treatment non-adherence and lost to follow up (38, 41, 45-47, 50, 56, 59, 61). Patients were interrupting their treatment because of fear of stigma. This finding was alongside with a study, which indicated that perception of TB as a stigmatizing disease was related to early losing from treatment follow up (64).
Social support, support from family, friends, neighbourhoods, general community and health care worker were identified as extremely important for TB treatment adherence, and for strictly accomplishment of the treatment; because it is to large extent compensation for financial shortage, food, transportation cost and physical support in taking to clinic (42, 52). Moreover, this review revealed that lack of social support obliges to stop or interrupt their treatment, because of lack of money and food compensation. For TB-HIV co-infected patients lack of social support was more difficult; because most of them are living with single family (husband or wife) and the stigma attached to the disease (52).
Beside, a socioeconomic factor, this review revealed that numerous individual behavioural factors were associated with TB treatment non-adherence and lost to follow up. Patient’s knowledge deficit about curability of the disease, availability and duration of the treatment, consequences of non-adherence and lost to follow up, and potential medication side effects were strongly associated with TB treatment non-adherence and lost to follow up (64). Similarly, studies from Morocco (37) and Uzbekistan (38) reported doubt about curability of TB was the main associated factor with TB treatment non-adherent and lost to follow up. This shows that knowledge deficit is continuing to associate with treatment non-adherence and lost to follow up.
It is known reality, as the result of treatment, patients feeling better or the symptoms are resolved after few weeks of treatment. After that, patients inter to interrupt their treatment by considering as they cured, because they do not know the duration of treatment. This fact was reported from Indonesia, Kenya and Uganda as feeling better, which accompany with knowledge deficit about the duration of treatment was associated with treatment interruption (41, 53, 59). This finding was alongside with review article reported in 2007 (64).
Tobacco smoking (36, 43-45, 48, 53, 56, 58, 60, 61) and alcohol consumption (39, 40, 44-46, 48, 53, 55, 56, 58, 61, 62) were the two individual behavioral factors associated with TB treatment non-adherence and lost to follow up. These findings were similar with other studies (65, 66) on tobacco smoking and a study reported on alcohol consumption (67).
Patient-health care worker poor communication was another factor associated with TB treatment non-adherence and lost to follow up (38, 41, 48, 49, 56, 59-61). This was similar with study reported earlier (68–70). Similarly health care worker lack of cooperation to support patients who are physically weak or they practicing DOTS inflexibly without considering patient’s physical ability to come for follow up was obliged patients to interrupt their treatment because of physical inability (42).
Although, WHO reported that DOTS is almost cover 93% population among WHO regions since 2006 (18), however, large volumes of research findings were witnessed as still patients from developing countries were interrupting their treatment due distance from treatment center to their home (37, 40-42, 45, 47, 48, 51-57, 59). Besides, patient’s HIV status (39, 44, 46-48, 55, 57, 59, 60) and being on ART (44, 46–49) were the factors associated with TB treatment non-adherence and lost to follow up. However, few studies reported both HIV status (47) and being on ARV (58–60) as protective factors for TB treatment non-adherence and lost to follow up.
Demographic factors, older age (40, 43, 44, 57, 58) and male gender (36, 39, 40, 44-46, 52, 57, 58) were associated with TB treatment non-adherence and lost to follow up. However, a study from Botswana reported that, young age was associated with non-adherence or lost to follow up (40).
Conclusion
Our review confirmed that, treatment non-adherence and lost to follow up were continued across the studies’ publication years in developing countries. Majority of studies reported non-adherence and lost to follow up were occurred in continuation phase. Socioeconomic factors (age gender, lack of food, transportation cost, social support and job), and individual behavioral factors (fear of stigma, feeling better after few weeks of treatment, tobacco and alcohol use), patient knowledge about TB disease and its treatment, consequences of non-adherence and lost to follow up were associated with non-adherence and lost to follow up. Beside socioeconomic and behavioral factors, patient-health care worker poor communication, distance from treatment center and both TB and HIV medications side effects were associated with TB treatment non-adherence and lost to follow up.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication, redundancy etc.) were strictly controlled by the authors.
Acknowledgment
We would like to acknowledge all staff members of Health Education and Health Promotion Department, School of Public Health Tehran University of Medical Sciences for their unreserved administrative support during this manuscript writing. The authors declare that, there is no conflict of interests.
References
- WHO (2011). Global Tuberculosis Control Report 2010. WHO Press, Geneva, Switzerland, pp-3. [Google Scholar]
- WHO (2012). Global Tuberculosis Control Report of 2011. WHO Press, Geneva, Switzerland, pp-3, 29, 41. [Google Scholar]
- Barsegian V, Mathias KD, Smith PW, Wilde HG, Lindemann M (2008). Prevalence of latent tuberculosis infection in German radiologists. J Hos Infec, 69 (1): 69–76. [DOI] [PubMed] [Google Scholar]
- Glaziou P, Falzon D, Floyd K, Raviglione M (2013). Global Epidemiology of Tuberculosis. Semin Respir Crit Care Med, 34 (1): 3–16. [DOI] [PubMed] [Google Scholar]
- Federal Minister of Health (FMOH) (2007). Implementation Guideline for TB/HIV Collaborative-Activities in Ethiopia. Addis Ababa, Ethiopia. [Google Scholar]
- WHO (2011). WHOXpert MTB/RIF increases timely TB detection among people living with HIV and saves lives Information note. WHO Press, pp-1. [Google Scholar]
- WHO (2003). Guidelines for Implementing Collaborative TB and HIV Programme Activities. WHO Press, Printed in Italy, pp-8. [Google Scholar]
- WHO (2012). Policy on collaborative TB/HIV activities Guidelines for national programmes and other stakeholders. WHO Press, Geneva, p-10. [PubMed] [Google Scholar]
- Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JDH (2011). The Social Determinants of Tuberculosis: From Evidence to Action, Framing Health Matters. Am J Public Health, 101 (4): 654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdoba-DoñaJ A, Novalbos-Ruiz JP, SuárezFarfante J, Andérica-Frías G, Escolar-Pujolar A (2012). Social inequalities in HIV-TB and non-HIV-TB patients in two urban areas in southern Spain: multilevel analysis. Int J Tuberc Lung Dis, 16 (3): 342–7. [DOI] [PubMed] [Google Scholar]
- Suk JE, Manissero D, Büscher G, Semenza JC (2009). Wealth inequality and tuberculosis elimination in Europe. Emerg Infect Dis, 15 (11): 1812–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhan KV, Wilson N, Das A, Satyanarayana S, Chadha S, et al. (2012). Addressing poverty through disease control programmes: examples from Tuberculosis control in India. Int J Equity in Health (11): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun H, Gunneberg C, Granich R, Nunn P (2010). HIV infection-associated tuberculosis: the epidemiology and the response. Clin InfectDis. 50 (S3):s201–7. [DOI] [PubMed] [Google Scholar]
- Miyata S, Tanaka M, Ihaku D (2013). The prognostic significance of nutritional status using malnutrition universal screening tool in patients with pulmonary tuberculosis. Nutr J, (12): 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakasi TA, Karyadi E, Dolmans WM, van der Meer JW, van der Velden K (2009). Malnutrition and socio-demographic factors associated with pulmonary tuberculosis in Timor and Rote Islands, Indonesa. Int J Tuberc Lung Dis, 13 (6): 755–9. [PubMed] [Google Scholar]
- Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M (2009). Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med, 68 (12): 2240–6. [DOI] [PubMed] [Google Scholar]
- E-Din MN, Elhoseeny T, Mohsen AM (2013). Factors affecting defaulting from DOTS therapy under the national programme of tuberculosis control in Alexandria, Egypt. East Mediterr Health J, 19 (2): 107–13. [PubMed] [Google Scholar]
- WHO (2006). Tuberculosis Coalition for Technical Assistance. International Standards for Tuberculosis Care (ISTC). The Hague: Tuberculosis Coalition for Technical Assistance, WHO Pres, pp-29–43. [Google Scholar]
- Méda ZC, Lin YT, Sombié I, Maré D, Morisky DE, Chen YM (2013). Medication adherence predictors among patients with tuberculosis or human immunodeficiency virus infection in Burkina Faso. J Microbiol Immunol Infect, pii: S1684–1182(13) 00080-7. [DOI] [PubMed] [Google Scholar]
- Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lönnroth K et al. (2009). The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health, (9): 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C (2008). Alcohol use as a risk factor for tuberculosis a systematic review. BMC Public Health, (8): 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajalakshmi V, Peto R (2009). Smoking, drinking and incident tuberculosis in rural India: population-based case-control study. Int J Epidemiol, 38 (4): 1018–1025. [DOI] [PubMed] [Google Scholar]
- Pamela Orr (2011). Adherence to tuberculosis care in Canadian Aboriginal populations, Part 2: a comprehensive approach to fostering adherent behaviour. IJ Circumpolar Health, 70 (2): 128–40. [DOI] [PubMed] [Google Scholar]
- Shao Y, Yang D, Xu W, Lu W, Song H, Dai Y, et al. (2011). Epidemiology of anti-tuberculosis drug resistance in a Chinese population: current situation and challenges ahead. BMC Public Health, (11): 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DG, Barry CE, Flynn JL (2010). Tuberculosis: what we don’t know can, and does, hurt us? Science, 328 (5980): 852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S, Maurya AK, Kushwaha RA, Nag VL, Prasad R (2010). Multi-drug resistant tuberculosis: an iatrogenic problem. Biosci Trends, 4 (2): 48–55. [PubMed] [Google Scholar]
- Yew WW, Leung CC (2008). Management of multidrug-resistant tuberculosis: Update 2007. Respirology, 13 (1): 21–46. [DOI] [PubMed] [Google Scholar]
- Minion J, Gallant V, Wolfe J, Jamieson F, Long R (2013). Multidrug and Extensively Drug-resistant Tuberculosis in Canada 1997–2008: Demographic and Disease Characteristics. PLoS ONE, 8 (1): e53466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2008). Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva, WHO Press; p-3. [Google Scholar]
- Obermeyer Z, Abbott-Klafter J, Murray CJL (2008). Has the DOTS Strategy Improved Case Finding or Treatment Success? An Empirical Assessment. PLoS ONE, 3 (3): e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Tu X, Tong Y, Yang R, Wang Y, Cao S, et al. (2012). Development and Validation of a Tuberculosis Medication Adherence Scale. PLoS ONE 7 (12): e50328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2003). Adherence to long-term therapies: evidence for action. WHO Press, Geneva, p–135. [Google Scholar]
- Reichman LB, Lardizabal AA (2013). Adherence to tuberculosis treatment, p-1 Available: http://health.usnews.com/doctors/leereichman-446567.
- Munro S, Lewin S, Swart T, Volmink J (2007). A review of health behaviour theories: how useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS? BMC Public Health, (7): 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2013). Definitions and reporting framework for tuberculosis; revision, WHO Press, Switzerland: pp–4–6. [Google Scholar]
- Tachfouti N, Slama K, Berraho M, Elfakir S, Benjelloun C Nejjari M(2013). Determinants of Tuberculosis treatment default in Morocco: Results from a National Cohort Study. Pan Afr Med J, (14): 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachfouti N, Slama K, Berraho M, Nejjari C (2012). The impact of knowledge and attitudes on adherence to tuberculosis treatment: a case-control study in a Moroccan region. Pan Afr Med J, (12): 52. [PMC free article] [PubMed] [Google Scholar]
- Hasker E, Khodjikhanov M, Sayfiddinova S, Rasulova G, Yuldashova U, Uzakova G et al. (2010). Why do tuberculosis patients default in Tashkent City, Uzbekistan? A qualitative study. Int J Tuberc Lung Dis, 14 (9): 1132–1139. [PubMed] [Google Scholar]
- Hasker E, Khodjikhanov M, Usarova Sh, Asamidinov U, Yuldashova U (2008). Default from tuberculosis treatment in Tashkent, Uzbekistan. Who are these defaulters and why do they default? BMC Infec Dis, (8): 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust AD, Mosimaneotsile B, Mathebula U, Chingapane B, Gaul Z, Pals LS, et al. (2011). Risk Factors for Non-Adherence and Loss to Follow-Up in a Three-Year Clinical Trial in Botswana. PLoS ONE, 6 (4): e18435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjanarko B, Gompelman M, Dijkers M, Werf MJ (2009). Factors that influence treatment adherence of tuberculosis patients living in Java, Indonesia. Patient Prefer Adherence, (3): 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagbakken M, Frich CJ, Bjune G (2008). Barriers and enablers in the management of tuberculosis treatment in Addis Ababa, Ethiopia: a qualitative study. BMC Public, (8): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni PY, Akarte SV, Mankeshwar RM, Bhawalkar JS, Banerjee A, Kulkarni AD (2013). Non-Adherence of New Pulmonary Tuberculosis Patients to Anti-Tuberculosis Treatment. Ann Med Health Sci Res, 3 (1): 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo P, Peltzer K, Louw J, Matseke G, Mchunu G, Tutshana B (2013). Predictors of tuberculosis (TB) and antiretroviral (ARV) medication non-adherence in public primary care patients in South Africa: a cross sectional study. BMC Public Health, (13): 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S, Ambe G, Sathiakumar N (2010). Determinants of Poor Adherence to Anti-Tuberculosis Treatment in Mumbai, India. Int J Prev Med, 1 (4): 223–32. [PMC free article] [PubMed] [Google Scholar]
- Cramm MJ, Finkenflüge H, Møller V, Nieboer AP (2010). TB treatment initiation and adherence in a South African community influenced more by perceptions than by knowledge of tuberculosis. BMC Public Health, (10): 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremariam KM, Bjune GA, Frich JC (2010). Barriers and facilitators of adherence to TB treatment in patients on concomitant TB and HIV treatment: a qualitative study. BMC Public Health, (10): 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuha GM, Kutyabami P, Kitutu FE, OdoiAdome R, Kalyango JN (2009). Non-adherence to anti-TB drugs among TB/HIV co-infected patients in Mbarara Hospital Uganda: Prevalence and associated factors. J Afr Health Scien, 9 (2): 8–15. [PMC free article] [PubMed] [Google Scholar]
- Kebede A, Wabe TN (2012). Medication Adherence and its Determinants among Patients on Concomitant Tuberculosis and Antiretroviral Therapy in South West Ethiopia. N Am J Med Sci, 4 (2): 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lu W, Zhou Y, Zhu L, Shen H, Wang J (2009). Adherence to anti-tuberculosis treatment among pulmonary tuberculosis patients: a qualitative and quantitative study. BMC Health Serv Res, (9): 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Huang W, Hof S., Yang S, Wang X, Chen W et al. (2011). Treatment adherence among sputum smear positive pulmonary tuberculosis patients in mountainous areas in China. BMC Health Serv Res, (11): 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse T, Demissie M, Berhane Y, Kebede Y, Abebe M (2013). Long distance travelling and financial burdens discourage tuberculosis DOTs treatment initiation and compliance in Ethiopia: a qualitative study. BMC Public Health, (13): 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayisi GJ, van’tHoog AH, Agaya JA, Mchembere W, Nyamthimba PO (2011). Care seeking and attitudes towards treatment compliance by newly enrolled tuberculosis patients in the district treatment programme in rural western Kenya: a qualitative study. BMC Public Health, (11): 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelnuovo B (2010). A review of compliance to anti tuberculosis treatment and risk factors for defaulting treatment in Sub Saharan Africa. Afr Health Sci, 10 (4): 320–4. [PMC free article] [PubMed] [Google Scholar]
- GarridoMdS, Penna ML, Perez-Porcuna TM, Souza AB, MarreiroLdS, Albuquerque BC et al. (2012). Factors Associated with Tuberculosis Treatment Default in an Endemic Area of the Brazilian Amazon: A Case Control-Study. PLoS ONE, 7 (6):e39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muture NB, Keraka NM, Kimuu KP, Kabiru WE, Ombeka OV, Oguya F (2011). Factors associated with default from treatment among tuberculosis patients in Nairobi province, Kenya: A case control study. BMC Public Health, (11): 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifebunandu NA, Kingsley Ukwaja N (2012). Tuberculosis treatment default in a large tertiary care hospital in urban Nigeria: Prevalence, trend, timing and predictors. J Infect Public Health, 5 (5): 340–5. [DOI] [PubMed] [Google Scholar]
- Maruza M, Albuquerque MM, Coimbra1 I, Moura LV, Montarroyos UR (2011). Risk factors for default from tuberculosis treatment in HIV-infected individuals in the state of Pernambuco, Brazil: a prospective cohort study. BMC Infec Dis, (11): 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbireer S, Guwatudde D, Mudiope P, NabbuyeSekandi J, Manabe CY (2011). Tuberculosis treatment default among HIV-TB co-infected patients in urban Uganda. Trop Med and Int Health, 16 (8): 981–7. [DOI] [PubMed] [Google Scholar]
- Wanitchaya K, Channawong B, Samroui K, Wanpen W, Chawin S (2009). Factors associated with tuberculosis treatment default among HIV-infected tuberculosis patients in Thailand. Trans R Soc Trop Med Hyg, 103 (1): 59–66. [DOI] [PubMed] [Google Scholar]
- Vijay S, Kumar P, Chauhan LS, Vollepore BH, Kizhakkethil UP, Kizhakkethil UP et al. (2010). Risk Factors Associated with Default among New Smear Positive TB Patients Treated Under DOTS in India. PLoS ONE, 5 (4): e10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendagire I, Schim Van der Loeff M, Kambugu A, Konde-Lule J, Cobelens F (2012). Urban Movement and Alcohol Intake Strongly Predict Defaulting from Tuberculosis Treatment: An Operational Study. PLoS ONE, 7 (5): e35908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JA (2004). An Ethnography of Non-adherence: Culture, Poverty, and Tuberculosis in Urban Bolivia. Cult, Med and Psych (28): 401–25. [DOI] [PubMed] [Google Scholar]
- Munro mail SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J (2007). Patient Adherence to Tuberculosis Treatment: A Systematic Review of Qualitative Research. PLoS Med, 4 (7):e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liefooghe R, Muynck AD (2001). The Dynamics of Tuberculosis Treatment Adherence. JPMA, 51 (1): 3–9. [PubMed] [Google Scholar]
- Balbay O, Annakkaya AN, Arbak P, Bilgin C, Erbas M (2005). Which Patients Are Able To Adhere to Tuberculosis Treatment? A Study in a Rural Area in the Northwest Part of Turkey. Jpn J Infect Dis, 58 (3): 152–8. [PubMed] [Google Scholar]
- Gelmanova IY, Keshavjee S, Golubchikova VT, Berezina VI, Strelis AK, Yanova GV et al. (2007). Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bul WHO, 85 (9): 703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay A, Lancaster J, Holtz TH, Weyer K, Miranda A, Walt M (2012). Patient- and provider-level risk factors associated with default from tuberculosis treatment, South Africa, 2002: a case-control study. BMC Public Health, (12): 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezenega ZS, Gacho YH/M, Tafere TE (2013). Patient satisfaction on tuberculosis treatment service and adherence to treatment in public health facilities of Sidama zone, South Ethiopia. BMC Health Serv Res, 13 (1): 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesfin MM, Newell JN, Walley JD, Gessessew A, Tesfaye T, et al. (2009). Quality of tuberculosis care and its association with patient adherence to treatment in eight Ethiopian districts. Health Policy Plan, 24 (6): 457–66. [DOI] [PubMed] [Google Scholar]