Abstract
Objective
Intraoperative MRI (IoMRI) guided brain biopsy provides a real time visual feedback of the lesion that is sampled during surgery. The objective of the study is to compare the diagnostic yield and safety profiles of ioMRI needle brain biopsy with two traditional brain biopsy methods: frame-based and frameless stereotactic brain biopsies.
Methods
A retrospective analysis from 288 consecutive needle brain biopsies in 277 patients undergoing stereotactic brain biopsy with any of the three biopsy methods at Brigham and Women's Hospital from 2000 to 2008 was performed. Variables such as age, sex, history of radiation and previous surgery, pathology results, complications and postoperative stays were analyzed.
Results
Over the course of eight years, 288 brain biopsies were performed. 253 (87.8%) biopsies yielded positive diagnostic tissue. Young age (<40 years), history of brain radiation or surgery were significant negative predictors for a positive biopsy diagnostic yield. Excluding patients with prior radiation or surgeries, no significant difference in diagnostic yield was detected among the three groups, with frame-based, frameless and ioMRI guided needle biopsies yield 96.9%, 91.8% and 89.9% positive diagnostic yield, respectively. 19 biopsies (6.6%) were complicated by serious adverse events. The ioMRI-guided brain biopsy was associated with less serious adverse events and the shortest postoperative hospital stay.
Conclusions
Frame-based, frameless stereotactic and ioMRI guided brain needle biopsy have comparable diagnostic yield for patients with no prior treatments (either radiation or surgery). IoMRI guided brain biopsy is associated with fewer serious adverse events and shorter hospital stay.
Keywords: brain biopsy, frame-based, frameless stereotactic, intra-operative MRI
Introduction
The continued evolution of image-guided surgical techniques over the past twenty years has provided tremendous advances in the field of neurosurgery. Frame-based techniques have long been considered the gold standard for sampling intracranial lesions, with the rigid frame providing excellent targeting precision (2, 3, 6, 18, 20). However, its use is limited by the frame's bulkiness, patient's discomfort, calculations involved with defining stereotactic entry points, possible prolonged surgical time as well as risk of postoperative infection at the frame's fixture points (17). The development of frameless stereotactic techniques has made it a popular choice among neurosurgeons given its easy to use and comparable diagnostic yield (1, 6).
Both frame-based and frameless stereotactic biopsy techniques utilize preoperative images with a registered probe to access target tissue, hence they both suffer a similar drawback: there is no real-time radiographic feedback confirming that the biopsy needle is in fact in the target tissue. Intraoperative brain shifting and cerebrospinal fluid loss, or technical issues leading to a potential misalignment between the image guide and the actual brain configuration during the operation (5, 7, 11, 14-16, 20). The development of intraoperative MRI (ioMRI) systems has made a real-time radiographic feedback a possibility for brain biopsy. In the ioMRI system used, a frameless three-dimensional optical stereotactic system is combined with intraoperative acquisition of MRI images to provide surgeons with near real-time navigation (15). Using a combination of light-emitting diode (LED)-based optical tracking of biopsy probes with intraoperative manipulation of MRI planes, surgeons are able to modify the preplanned trajectory based on the real-time intraoperative MRI image (4). Intralesional biopsy could be confirmed with the real-time MRI image.
Several reports have been published comparing the effectiveness of the frame-based and frameless stereotactic brain biopsy methods (6, 8, 19, 21). Similar diagnostic yield was found between the two methods (6, 8, 19, 21). However, results comparing the complications and length of hospitalization vary among different studies (8, 19). We have previously demonstrated the feasibility and accuracy of ioMRI brain biopsy technique in a case series of an earlier cohort of 68 patients (15). A separate group from University of Minnesota has also demonstrated that the interventional MRI guided biopsy is a safe and effective method is their case series (9). However, there have not been any studies comparing the safety and effectiveness of ioMRI brain biopsy with the traditional stereotactic biopsy methods.
In the present study we evaluate a series of 288 consecutive brain biopsies over a period of 8 years at the Brigham and Women's Hospital in Boston, Massachusetts. We report our analysis of diagnostic yield, complications and length of postoperative stay between frame-based, frameless and ioMRI-guided brain biopsy procedures.
Patients and Methods
We reviewed a consecutive series of patients who underwent needle-based brain biopsy at the Brigham & Women's Hospital from 2000 to 2008. Open biopsy cases were excluded from the study. One of three biopsy methods (Frame-based, frameless and ioMRI guided stereotactic) was chosen by the attending neurosurgeons. 288 Biopsies were performed in 277 patients. Age, gender, image characteristic, history of prior treatments, duration of hospital stay, post-operative complications were retrospectively collected from electronic medical records. The diagnosis was obtained from the final pathological report.
Frame-based image-guided stereotactic biopsy
For frame-based stereotactic brain biopsy procedures, the surgeon placed CRW stereotactic frame (Integra Burlington MA, Inc. formerly Integra Radionics, Inc. Burlington, MA) preoperatively. CT scan was obtained with the frame and birdcage fiducial in place and the images were then fused with a preoperative MRI scan (T1 post-contrast or T2 weighted images) used to establish the target. Radionics proprietary software was used for image registration, targeting and calculation of offsets and ring and arc settings. The arc system was set up attached to the head ring. A burr hole or twist drill hole was made at the defined stereotactic site and tissues specimens were obtained using a biopsy needle and standard suction-aspiration technique.
Frameless image-guided stereotactic biopsy
For frameless stereotactic brain biopsy, MRI (T1 post-contrast or T2 weighted image) or CT scans were used. One of the two methods was used for surface registration. Either fiducials or surface matching was used for operating room neuronavigation registration. Patients’ heads were fixed in a three-point Mayfield clamp. The surgical plan (the entry point, biopsy target and needle trajectory) was determined using the proprietary GE navigation system software. After accuracy of the neuronavigation system was confirmed using anatomic landmarks, a burr hole was placed and biopsy samples were obtained using standard biopsy needles that are attached to the burr hole fixation needle trajectory guide.
ioMRI-guided biopsy
All procedures were performed in the ioMRI suite at the Brigham and Women's Hospital. A General Electric Signa SP open configuration ioMRI scanner (GE Healthcare, Milwaukee, WI) was used for the ioMRI guided biopsy. The ioMRI suite was a fully functional operating room equipped with a magnetic resonance compatible anesthesia machine and patient monitoring devices. The scanner is based on a 0.5T open configuration superconducing magnet. The MR-compatible Mayfield headholder was used. After positioning, a series of multislice (usually T1-weighted) preoperative images were acquired to assess the adequacy of imaging and to plan the biopsy. Intravenous gadolinium was administered if it was indicated. To determine the site of the burr hole, the surgeon placed a marker on the patient's scalp and acquired a sequence of images. The biopsy needle cannula with its optical tracking sensors was affixed on a Bookwalter arm (Codman, Inc., Burlington, MA) then placed at a proposed angle of entry. Single-slice image acquisition was performed in three separate oblique planes to clearly define the vector to the target and the proposed biopsy site. The sedan side-cutting biopsy needle (Elekta Instruments, Stockholm, Sweden) was then inserted through the cannula and into the brain under image guidance. The biopsy needle used was composed of a titanium alloy. Several further single-slice images were obtained as the needle was passed into the lesion. Biopsies were then obtained using standard technique. When an adequate tissue sample was obtained with the needle in place, a set of volumetric images of the entire brain was obtained. The needle was withdrawn, and then a final set of images of the entire brain was obtained as the incision was closed.
Histopathologic Analysis
Needle biopsy samples were sent for pathology evaluation soon after they were obtained. Definitive diagnoses included pathology of gliomas (GBM, anaplastic astrocytoma, oligodendroglioma, etc.); diffuse large B cell lymphoma; multiple sclerosis or other demyelinating lesion; abscess; metastatic tumors; central neurocytoma; and infarction. Non-definitive diagnoses included pathology of hypercellular tissue; reactive change; gliosis and inflammation (see Table 1).
Table 1.
Risk factor analysis of factors affecting the stereotactic biopsy diagnostic yield
| Diagnostic Yield | OR | CI | p value | ||
|---|---|---|---|---|---|
| Age | <40 | 75.9% (41/54) | 0.33 | 0.15 - 0.70 | < .01 |
| > or = 40 | 90.6% (212/234) | ||||
| Gender | Male | 86.2% (137/159) | 0.70 | 0.34 - 1.45 | > .05 |
| Female | 89.9 % (116/129) | ||||
| Lesion enhancement | non-enhancing | 82.5% (33/40) | 0.54 | 0.22 - 1.35 | > .05 |
| enhancing | 89.7% (218/234) | ||||
| History of brain radiation | with | 69.7% (23/33) | 0.25 | 0.11 - 0.58 | < .001 |
| without | 90.2% (230/255) | ||||
| History of brain surgery | with | 75.9% (44/58) | 0.32 | 0.15 - 0.67 | < .01 |
| without | 90.9% (209/230) | ||||
| Prior non-diagnostic biopsy | with | 55.6% (10/18) | 0.14 | 0.05 - 0.38 | < .001 |
| without | 90.0% (243/270) |
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA). Statistical analysis for biopsy diagnostic yield and complications were performed with Two-tailed Fisher's exact test (for two group comparison) or Chi-Square test (for three group comparison). Age and post-operative hospital stay univariate analysis was performed with two-tailed unpaired t-test. p-values < .05 were considered statistically significant.
Results
Factors affecting needle biopsy yields
The overall diagnostic yield was 87.8%, with a definitive histological diagnosis in 253 of 288 cases. In 35 cases (12.2%) the biopsy yielded non-definitive diagnoses such as atypical cells, inflammation cells, gliosis, etc. We first analyzed possible factors that might affect needle biopsy yields including age, gender, image characteristics, and history of previous treatments.
Table 1 shows a univariate analysis of factors thought to play a role in affecting the diagnostic yield regardless of the brain biopsy technique used.
Age
In our series, the mean age for definite diagnosis is 56.4 +/− 1.0 (mean +/− SEM), and the mean age for non-definite diagnosis is 48.1 +/− 3.1 (mean +/− SEM). Younger age is associated with non-definite diagnosis yield (p < .01). Using age 40 as an arbitrary cut-off, younger patients (<40 years of age) have a diagnostic yield of 75.9% (41/54) while older patients (>40 years of age) have a diagnostic yield of 90.6% (212/234) (p < .01)
Gender
Of patients in the study, 86.2% (137/159) of males had a definitive diagnosis by needle biopsy, compared to 89.9% (116/129) of females. There was no statistical difference (p = .37) between genders.
Image Characteristics
89.7% (218/243) of lesions with T1 contrast enhancement on MRI scan have definitive diagnosis while 82.5% (33/40) of lesions without enhancement have definitive diagnosis. The difference is not statistically significant (p = .18).
Previous treatments
In our study, patients with history of brain irradiation had a diagnostic yield of 69.7% compared to the 90.2% yield of patients without history of brain irradiation (OR: 0.25; 95%-CI: 0.11-0.58, p < .001). Similarly, patients with prior brain surgery had a diagnostic yield of 75.9% compared to the 90.9% yield of patients without history of brain surgery (OR: 0.32; 95%-CI: 0.15-0.67, p < .01). Therefore, stereotactic needle biopsy has a lower yield for patients with recurrent diseases who have been treated in the past, either with surgery or radiation or both. Not surprisingly, patients with history of prior non-diagnostic biopsy only have a 55.6% of diagnostic yield with repeat biopsy, compared to 90.0% yield of patients without history of non-diagnostic biopsy (OR: 0.14; 95%-CI: 0.05-0.38, p < .001).
Diagnostic yield comparison among three biopsy methods
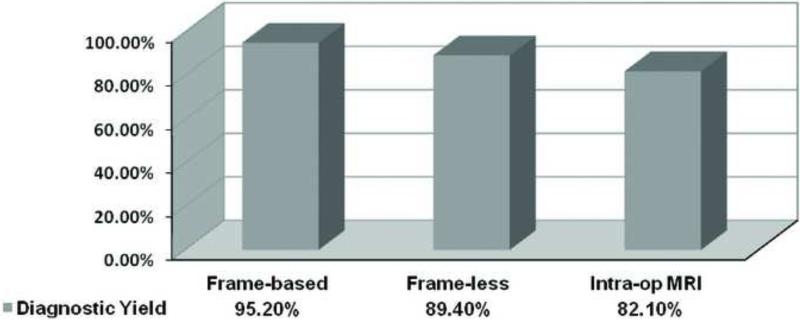
The overall diagnostic yield was 87.8%. In the frame-based group, 60 out of 63 cases (95.2%) obtained a definitive diagnosis. In the frame-less group, 101 out of 113 cases (89.4%) obtained a definitive diagnosis, and in the ioMRI group, 92 out of 112 cases (82.1%) obtained a definitive diagnosis. Table 2 lists the number of cases with various histological diagnoses obtained with each of the three biopsy methods used in this study. Figure 1 is a graphical representation of the percentage of definitive diagnoses according to the brain biopsy technique used. There is a statistical difference for the diagnostic yield among the three groups (p = .03, Chi-Square test). Comparing across groups, no statistically significant difference was found in diagnostic yield between frame-based and frameless biopsies (OR: 2.38; 95%-CI: 0.64 to 8.76, p = .26). Nor was a statistically significant difference was found in diagnostic yield between frameless and ioMRI guided biopsies (OR: 1.83; 95%-CI: 0.85 to 3.95, p= .13). However, comparing the two groups between frame-based and the io-MRI guided biopsies, the diagnostic yield with frame-based biopsy was found to be statistically greater than ioMRI-guided biopsies (OR: 4.35; 95%-CI: 1.24 to 15.28, p = .02) (Table 3).
Table 2.
Histological diagnoses made on tissue samples acquired by stereotactic biopsy
| Diagnosis | Frame-based (n=63) | Frameless (n=113) | IoMRI-guided (n=112) |
|---|---|---|---|
| Diagnostic biopsy* | 60 (95.2%) | 101 (89.4%) | 92 (82.1%) |
| Astrocytoma, Grade I or II | 2 | 1 | 3 |
| Astrocytoma, Grade III or IV | 32 | 71 | 48 |
| Ungraded astrocytoma | 2 | 2 | 8 |
| Oligoastrocytoma | 1 | 3 | 5 |
| Oligodendroglioma | 2 | 1 | 4 |
| Lymphoma | 11 | 9 | 10 |
| Metastasis | 2 | 2 | 0 |
| Abscess | 2 | 3 | 0 |
| Demyelinating lesion | 3 | 6 | 5 |
| Others | 3 | 3 | 9 |
| Non-definitive diagnosis* | 3 (4.8%) | 12 (10.6%) | 20 (17.9%) |
| Atypical cell / Reactive changes | 1 | 4 | 10 |
| Inflammatory cells | 0 | 4 | 1 |
| Hypercellular brain tissue | 1 | 0 | 1 |
| Necrosis | 1 | 1 | 0 |
| Gliosis | 0 | 0 | 5 |
| Normal brain | 0 | 1 | 1 |
| others | 0 | 2 | 2 |
| Total | 63 | 113 | 112 |
P<.05 Frame-based vs. ioMRI-guided
Figure 1.
Comparison of the biopsy yield of the three methods
Table 3.
Statistical analysis of the biopsy yield of the three methods
| Comparison Groups | OR | 95%-CI | P value |
|---|---|---|---|
| Frame-based vs. Frameless | 2.38 | 0.64 - 8.76 | .26 |
| Frameless vs. ioMRI | 1.83 | 0.85 - 3.95 | .13 |
| Frame-based vs. ioMRI | 4.35 | 1.24 - 15.28 | * .02 |
p<.05
Patient characteristic comparison among three biopsy methods
We then examined patient characteristics in the three biopsy groups and the association with diagnostic yield. Table 4 summarizes the patient information related to biopsy method. The analysis revealed a selection bias in which a much higher percentage of patients who underwent ioMRI-guided brain biopsies had prior brain radiation or surgery compared to patients who underwent frame-based or frameless procedures. 22.3% of patients in the ioMRI group had undergone prior brain radiation, compared to 4.8% and 4.4% in the frame-based and frameless groups, respectively (p < .001). Similarly, 33.0% of patients in the ioMRI group had had history of prior brain lesion resection, compared to 12.7% and 11.5% in the frame-based and frameless groups, respectively (p < .001).
Table 4.
Patient characteristic comparison among three biopsy groups
| Patient Characteristic | Frame-based | Frameless | IoMRI |
|---|---|---|---|
| Age (mean +/− SEM) | 54.4 +/− 1.9 | 60.6 +/− 1.4 | 50.7 +/− 1.7 |
| Gender (% male) | 64.5% | 52.6% | 50.4% |
| Nonenhancing lesion | 8.2% | 7.1% | 24.8% |
| Prior brain radiation | 4.8% | 4.4% | 22.3% |
| Prior brain surgeries | 12.7% | 11.5% | 33.0% |
Diagnostic yield comparison among three biopsy methods for patients with no prior treatments
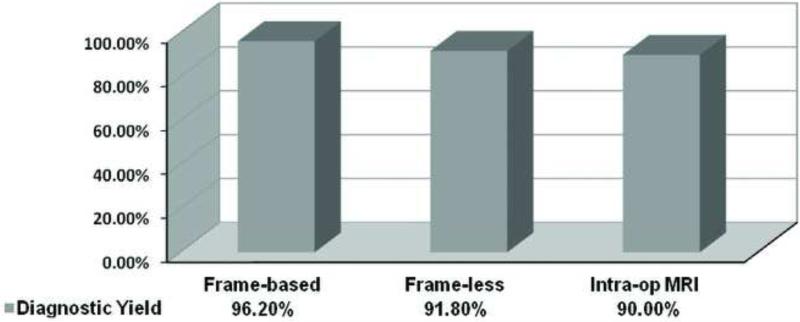
In order to get a more accurate analysis and comparison of the biopsy yield among three methods, we performed further analysis excluding the patients with prior radiation and/or brain surgery. After excluding patients with prior treatments, positive diagnostic yield was 96.2% (51/53) in frame-based biopsies, as compared to 91.8% (89/97) in frameless biopsies and 90.0% (63/70) in ioMRI-guided biopsies. No statistical difference was seen among the three groups (p=.43, Chi-Square test). Comparing across groups, no significant difference was found in diagnostic yield between groups: between frame-based and frameless biopsies (OR: 2.29; 95%-CI: 0.47-11.2, p = .50); between frameless and ioMRI-guided biopsies (OR: 1.24; 95%-CI: 0.43-3.59, p = .79); between frame-based and ioMRI-guided biopsies (OR: 2.83; 95%-CI: 0.56-14.24, p = .30). Figure 2 shows a graphical representation of the percentage of definitive diagnoses according to the brain biopsy technique used, excluding patients who had prior treatments and table 5 summarize the statistical analysis.
Figure 2.
Biopsy yield comparison of the three methods in patients with no prior lesion radiation or surgery.
Table 5.
Statistical analysis of the biopsy yield of the three methods methods in patients with no prior lesion radiation or surgery; no statistical significant difference is seen among three groups.
| Comparison Groups | OR | 95%-CI | P value |
|---|---|---|---|
| Frame-based vs. Frameless | 2.29 | 0.47-11.2 | .50 |
| Frameless vs. ioMRI | 1.24 | 0.43-3.59 | .79 |
| Frame-based vs. ioMRI | 2.83 | 0.56-14.24 | .30 |
Diagnostic yield was also calculated in patients with prior radiation and/or surgeries. The diagnostic yield was 90.0% (9/10), 75.0% (12/16) and 69.0% (29/42), in frame-based, frame-less and ioMRI guided biopsies, respectively. No statistical difference was seen among the three groups (p = .40, Chi-Square test). Comparing across groups, no significant difference was found in diagnostic yield between groups: between frame-based and frameless biopsies, p = .62; between frameless and ioMRI-guided biopsies, p = .76; between frame-based and ioMRI-guided biopsies, p = .25 but this certainly could be related to the small sample size available for comparison.
Complications and duration of hospital stay comparison
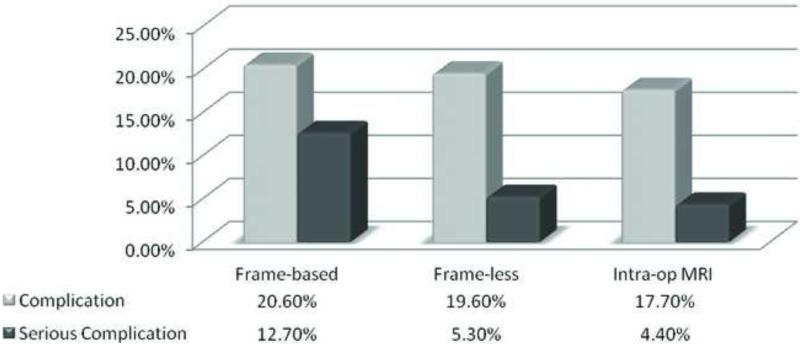
A total of 55 complications happened for the 288 stereotactic biopsy procedures (19.1%). Among them, 13 of 63 (20.6%) of frame-based biopsies have complications, compared to 22 of 112 (19.6%) of frame-less biopsy and 20 of 113 (17.7%) ioMRI guided biopsies. There was no statistical difference for the complication rate among all three biopsy methods (p = .64, Chi-Square test).
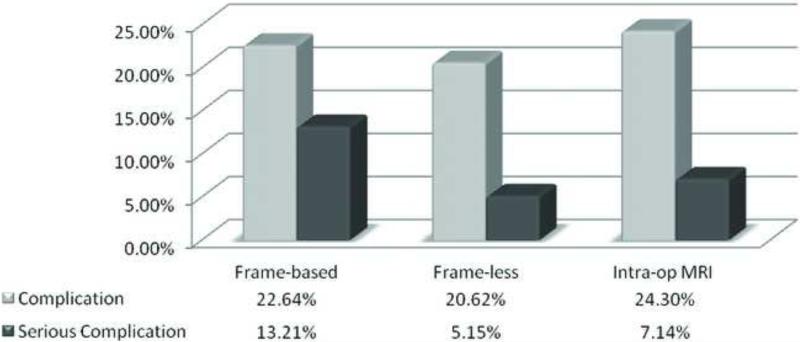
Most of the complications were transient neurological deficit that resolved in a short period of time after surgery. We also studied the occurrence of serious complications such as prolonged neurological deficit, death and/or return to the operating room for emergent clot evacuation. Overall, serious complications occurred after 19 operations (6.6%). Of these, 8 (12.7%) occurred following frame-based brain biopsies, 6 (5.3%) following frameless brain biopsies and 5 (4.4%) following ioMRI-guided procedures. Figure 3 shows a graphical representation of the complication rate across the three brain biopsy methods. There is a trend that the frameless stereotactic biopsies and the ioMRI guided biopsies may have less serious complication rates than the frame-based biopsies (p = .14 and .07, respectively). When this analysis was performed on the subset of cases with no history of prior treatments, similar results were obtained (Fig. 4).
Figure 3.
Comparison of complication rates among three biopsy methods.
Figure 4.
Comparison of complication rates among three biopsy methods with no prior treatments.
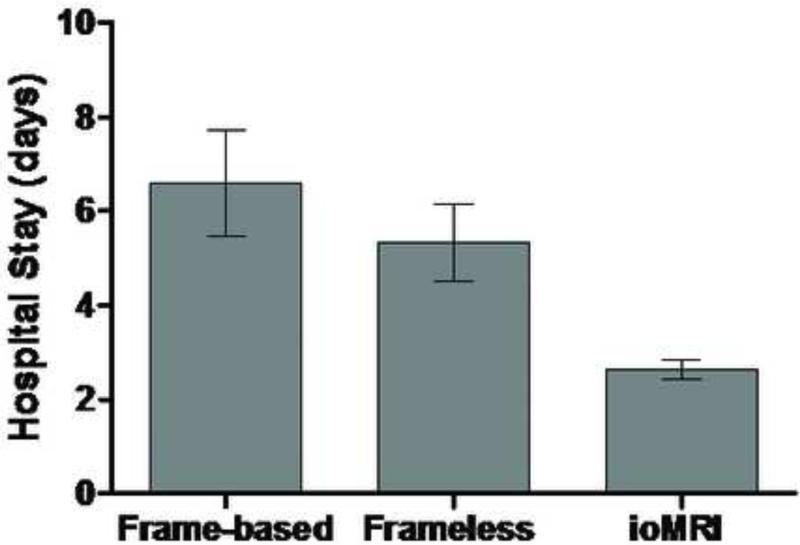
The average post-operative hospital stay was examined across the three brain biopsy methods (fig. 4). Average post-operative hospital stay for patient with frame-based stereotactic biopsy was 6.6 +/− 1.1 days, for frameless stereotactic biopsy was 5.3 +/− 0.8 days and for ioMRI guided biopsy was 2.6 +/− 0.2 days (fig. 5). Patients were discharged earlier from the hospital after frameless brain biopsies compared to the frame-based technique, with an average of 1.2 fewer hospital stay days but this was not statistically significant (p=.41). Length of stay was significantly shorter following ioMRI-guided brain biopsy compared to frameless (p < .05, 2.8 fewer stay days) and frame-based (p<0.05, 4.0 fewer stay days) techniques.
Figure 5.
Comparison of hospital stay length after surgery among three biopsy methods.
The complications have a significant effect on the length of stay. Analyzing all the patients, the patients with complications have an average length of stay of 9.0+/−1.6 days, while the patients without complications have an average stay of 3.5+/−0.3 days (p<0.0001).
Comparing post-operative stay for patients without complications among all three biopsy methods, frame-based stereotactic biopsy was 5.9+/−1.3 days, frameless stereotactic biopsy was 3.4+/−0.4 days and ioMRI guided biopsy was 2.2+/−0.2 days (p=0.0002).
Analysis of non-diagnostic cases
One of the limitations of our study is the definition of the non-definite diagnosis. In our study, we categorize the diagnosis of “reactive changes”, “gliosis”, “inflammatory cells”, “necrosis”, “hypercellular brain tissues”, etc. as non-definitive diagnosis. However, it is possible that those diagnoses may represent the real clinical situations, especially with patients who have received prior radiation. We analyzed the eventual outcomes of our cases with non-definitive diagnosis (Table 6). As we can see from the analysis, half of the patients (7/14) in the ioMRI group with non-definite diagnosis that has follow-ups indeed have stable disease, presumably because that the initial lesions shown on the image studies represent either radiation effect. Indeed, of the 7 patients who have had stable diseases, 5 have received radiation treatments in the past therefore the lesions most likely represents radiation effect. It is unclear what disease process the other 2 cases represent.
Table 6.
Analysis of non-definitive diagnosis cases
| Later diagnosis | Presumptive treatments | Clinical stable disease; no diagnosis | No follow ups | |
|---|---|---|---|---|
|
Frame-based
3 patients (3 non-definitive biopsies) |
0 | 0 | 1 | 2 |
|
Frameless
11 patients (12 non-definitive biopsies) |
5
(1 demyelinating dz, 2 gliomas, 1 lymphoma, 1 stroke) |
3
(1 lymphoma, 1 atypical MS, 1 MS or lymphoma) |
2 | 1 |
|
ioMRI
18 patients (20 non-definitive biopsies) |
5
(2 lymphomas, 2 gliomas, 1 meningoangiomatosis) |
2
(1 lymphoma, 1 brainstem pilocytic astrocytoma) |
7 | 4 |
Discussion
Comparable diagnostic yield among the three needle biopsy methods
In the current study we presented our experience of a large series of consecutive needle brain lesion biopsies using frame-based, frameless, or ioMRI-guided neuro-navigational techniques. When we consider all patients in the series including patients with history of previous treatments, the frame-based needle biopsies were associated with the highest diagnostic yield, while ioMRI-guided needle biopsies were associated with the lowest diagnostic yield. However, examination of patient demographic information revealed a selection bias, with a higher proportion of patients with low yield risk factors especially prior lesion radiation and/or surgery included in the ioMRI group (37.5% for ioMRI vs 14.2% for frameless biopsy, and 15.9% for frame-based biopsy). From our risk factor analysis, it is clear that prior lesion radiation and/or surgery is strongly negatively associated with biopsy diagnostic yield, it is very possible that the apparent lower yield from the ioMRI group is from the overrepresentation of the patients with prior radiation and/or surgeries in this group. In fact, when only the patients with no prior treatments were considered, comparable diagnostic yield across all three methods were found. The disproportional representation of the patients with prior treatments in the ioMRI group might contribute to the decreased biopsy diagnosis yield during initial analysis when all patients are included. Our own analysis indicates that patients with prior treatments (radiation or surgery) had a much lower diagnostic yield compared to the patients without prior treatments (table 1). IoMRI guided biopsy has at least a theoretic advantage of having the ability to confirm the biopsy needle to be within the target real-time. It also allows the surgeon to account for brain shift or to compensate for a deviation that could lead to a non-diagnostic biopsy using the other two stereotactic biopsy methods. We have previously demonstrated the application accuracy of the ioMRI guided biopsy is around 0.2 mm, which is well within the range required for frameless stereotactic neurosurgery (15).
Complications and Hospital stay
In the present study, the overall rate of complications was similar among all three methods. However, the frameless stereotactic biopsy and the ioMRI guided biopsy had a lower rate of serious complications compared to the frame-based biopsy method. In addition, ioMRI guided biopsy is associated with a decreased length of post-operative hospital stay compared to the other two methods. Because needle biopsy is a blind surgical procedure, hemorrhage, despite its small probability, could be a devastating complication with the needle biopsy. IoMRI guided biopsy has an advantage of being able to detect hemorrhage in real time and treatments, if necessary, can be instituted right away. In the frame-based and frameless stereotactic biopsies, hemorrhage caused by biopsy probe may only be detected when the patient deteriorates significantly in the recovery room. Such a delay in diagnosis could lead to irreversible neurologic deficit. We have previously reported a case in which immediate detection of a hemorrhage after the ioMRI guided biopsy allowed evacuation of the clot immediately without any adverse outcomes for the patient (15). In addition, because of the real-time visual feedback that biopsy sample is being taken from the targets, ioMRI guided biopsy increased the surgeon's confidence with the sampling which may have led to decreased number of tissue samples and/or the number of passes with the biopsy needle, which may also lead to decreased complications. It is possible that decreased number and severity of complications in the ioMRI group contributed partially to the shortened hospitalization after the biopsy. However, even for patients without complications, the ioMRI group still has shorter hospital stay. We cannot rule out the possibility of selection bias due to the non-randomized nature of the study. It is possible that that the elective cases (who are generally healthier patients) have a greater chance to receive ioMRI guided biopsy due to the booking logistics with the ioMRI suite, which leads to the apparent shortened hospital stay in the ioMRI group. In addition, the ioMRI biopsy technique was used more frequently in the latter part of the study period. The improvement in the patient's care in ICU and on the floor at our hospital over time could also have contributed to the shorter hospital stay with the patients in the ioMRI guided biopsy group. In our frame-based stereotactic biopsy patients, we routinely use general anesthesia for the procedure. It has been reported that if local anesthesia is used for frame-based stereotactic biopsy, then much less hospital stay duration can be expected after the procedure (8). Compared to frame-based stereotactic biopsy which may only require a stab incision and a twist-drill hole, ioMRI biopsy requires a larger burr hole to compensate for the changing biopsy trajectory on the basis of the information updates from the ioMRI.
Comparison to published biopsy results
A number of previous studies have compared frame-based and frameless stereotaxy for brain biopsy in terms of diagnostic yield, morbidity, mortality and length of hospital stay (6, 8, 19, 21). Our results for diagnostic yield between frame-based and frameless procedures corroborate most of the previous studies (6, 8, 19, 21). For example, Dammers, et al. found no difference in frame-based and frameless stereotactic brain biopsy with a combined 89.4% diagnostic yield and no difference in complication rates comparing the two methods (6). Woodworth, et al. also reported similar findings, showing a 90% combined diagnostic yield with no differences between frame-based or frameless techniques. Total complication rate was 14%, also with no differences between the two methods (21). Smith et al. reported similar biopsy diagnostic yield but reported a significantly less surgical time and hospital stay length with frame-based biopsy methods compared to frameless stereotactic biopsy (19). On the contrary, Dorward et al. reported a significantly less OR time and mean hospital stay time for frameless biopsy compared to frame-based biopsy (8). The differences may be related to the surgeons’ experience with each method and whether general anesthesia or sedation is used for frame-based biopsy. When only local anesthesia is used with frame-based stereotactic biopsy, it is associated with significantly less OR time and post-op hospital stay length (19). In our study, we performed frame-based biopsy mostly under general anesthesia and there is no significant difference in terms of the postoperative hospital stay time compared to frameless methods. The addition of ioMRI-guided brain biopsy group in our study augments the findings of other studies and provides an important comparison of the newest brain needle biopsy method with the traditional methods. In a recent study published by Harrisson, et al., a new frameless, pinless biopsy method using electromagnetic image-guided biopsy was described with a specific diagnosis rate of 96.7% with only 0.7% complication rate but 4.7% patients were noted for postoperative intracranial hemorrhages that are larger than 10mm in diameter (10).
Study Limitation
The main limitation of this study is the non-randomized nature of the study. The decision of which biopsy method to be used was determined by the surgeons depending on equipment availability and the surgeons’ experience and comfort level with each method. Our case series has an overrepresentation of the more difficult cases in the ioMRI guided biopsy group. It is possible the operating surgeon choose a more “difficult” case to get biopsied using the ioMRI suite due to the perceived theoretic advantage with the ioMRI guided biopsy method.
Another confounding factor of our study is the vague definition of positive biopsy diagnosis. It is very possible that a “negative” biopsy sample which carries the pathology diagnosis of “gliosis” or “inflammation cells” could be either a truly negative biopsy of sampling being done at the periphery of the actual pathology or in fact an accurate diagnosis, especially if the biopsy was done in patients with history of previous radiation or surgery.
With the refinement of the ioMRI biopsy techniques, the results of the ioMRI guided biopsy may continue to improve. As a matter of fact, recent years have seen a rapid advancement in the ioMRI technology. The system we used was a 0.5T open MRI system. Current ioMRI systems are typically of higher field strength such as 1.5T or 3T, which have higher resolution of images. The increased resolution of the real time images may lead to higher precision in biopsy targeting. However, the longer image acquisition time with the higher field strength MRI system may prove harder for real time imaging. In addition, current system typically involves performing the surgery outside of the MRI field then sliding the patient into the MRI bore for the intraoperative images. How will that affect the logistics of ioMRI guided biopsies and the results will need to be studied.
Conclusion
Frame-based, frameless and ioMRI-guided brain biopsy techniques are approximately equivalent in their ability to reliably obtain a histopathologic diagnosis following lesion sampling in the group of patients with no prior radiation or surgical treatments. Frame-based brain biopsy has better diagnostic yield than ioMRI guided biopsy when all patients are included in the analysis. The ioMRI-guided technique may prove to be safer and more cost-efficient in the future, suggested by the possible reduction in hospital stay days as well as lower risk of serious complication. Deciding which technique to employ, however, should be specific to individual patients, surgeon's comfort level with each methods and the availability of the surgical equipments. Institution-specific infrastructure also needs to be taken into account, given the large overhead cost of installing and maintaining an ioMRI suite.
List of abbreviations
- ioMRI
intra-operative MRI
- LED
light-emitting diode
References
- 1.Amin DV, Lozanne K, Parry PV, Engh JA, Seelman K, Mintz A. Image-guided frameless stereotactic needle biopsy in awake patients without the use of rigid head fixation. Journal of neurosurgery. doi: 10.3171/2010.7.JNS091493. [DOI] [PubMed] [Google Scholar]
- 2.Apuzzo ML, Chandrasoma PT, Cohen D, Zee CS, Zelman V. Computed imaging stereotaxy: experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930–937. doi: 10.1227/00006123-198706000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Apuzzo ML, Chandrasoma PT, Zelman V, Giannotta SL, Weiss MH. Computed tomographic guidance stereotaxis in the management of lesions of the third ventricular region. Neurosurgery. 1984;15:502–508. doi: 10.1227/00006123-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Black PM, Moriarty T, Alexander E, 3rd, Stieg P, Woodard EJ, Gleason PL, Martin CH, Kikinis R, Schwartz RB, Jolesz FA. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41:831–842. doi: 10.1097/00006123-199710000-00013. discussion 842-835. [DOI] [PubMed] [Google Scholar]
- 5.Chang H, Fitzpatrick JM. A technique for accurate magnetic resonance imaging in the presence of field inhomogeneities. IEEE transactions on medical imaging. 1992;11:319–329. doi: 10.1109/42.158935. [DOI] [PubMed] [Google Scholar]
- 6.Dammers R, Haitsma IK, Schouten JW, Kros JM, Avezaat CJ, Vincent AJ. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta neurochirurgica. 2008;150:23–29. doi: 10.1007/s00701-007-1473-x. [DOI] [PubMed] [Google Scholar]
- 7.Dorward NL, Alberti O, Velani B, Gerritsen FA, Harkness WF, Kitchen ND, Thomas DG. Postimaging brain distortion: magnitude, correlates, and impact on neuronavigation. Journal of neurosurgery. 1998;88:656–662. doi: 10.3171/jns.1998.88.4.0656. [DOI] [PubMed] [Google Scholar]
- 8.Dorward NL, Paleologos TS, Alberti O, Thomas DG. The advantages of frameless stereotactic biopsy over frame-based biopsy. British journal of neurosurgery. 2002;16:110–118. doi: 10.1080/02688690220131705. [DOI] [PubMed] [Google Scholar]
- 9.Hall WA, Martin AJ, Liu H, Nussbaum ES, Maxwell RE, Truwit CL. Brain biopsy using high-field strength interventional magnetic resonance imaging. Neurosurgery. 1999;44:807–813. doi: 10.1097/00006123-199904000-00067. discussion 813-804. [DOI] [PubMed] [Google Scholar]
- 10.Harrisson SE, Shooman D, Grundy PL. A prospective study into the safety and efficacy of frameless, pinless electromagnetic image guided biopsy of cerebral lesions.: Electromagnetic image-guided biopsy of cerebral lesions. Operative Neurosurgery. 2012;70:29–33. doi: 10.1227/NEU.0b013e31822d75af. [DOI] [PubMed] [Google Scholar]
- 11.Jodicke A, Deinsberger W, Erbe H, Kriete A, Boker DK. Intraoperative three-dimensional ultrasonography: an approach to register brain shift using multidimensional image processing. Minim Invasive Neurosurg. 1998;41:13–19. doi: 10.1055/s-2008-1052008. [DOI] [PubMed] [Google Scholar]
- 12.Kim JE, Kim DG, Paek SH, Jung HW. Stereotactic biopsy for intracranial lesions: reliability and its impact on the planning of treatment. Acta neurochirurgica. 2003;145:547–554. doi: 10.1007/s00701-003-0048-8. discussion 554-545. [DOI] [PubMed] [Google Scholar]
- 13.Krieger MD, Chandrasoma PT, Zee CS, Apuzzo ML. Role of stereotactic biopsy in the diagnosis and management of brain tumors. Seminars in surgical oncology. 1998;14:13–25. doi: 10.1002/(sici)1098-2388(199801/02)14:1<13::aid-ssu3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Ludeke KM, Roschmann P, Tischler R. Susceptibility artefacts in NMR imaging. Magnetic resonance imaging. 1985;3:329–343. doi: 10.1016/0730-725x(85)90397-2. [DOI] [PubMed] [Google Scholar]
- 15.Moriarty TM, Quinones-Hinojosa A, Larson PS, Alexander E, 3rd, Gleason PL, Schwartz RB, Jolesz FA, Black PM. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: stereotactic brain biopsy. Neurosurgery. 2000;47:1138–1145. doi: 10.1097/00006123-200011000-00023. discussion 1145-1136. [DOI] [PubMed] [Google Scholar]
- 16.Nauta HJ. Error assessment during “image guided” and “imaging interactive” stereotactic surgery. Comput Med Imaging Graph. 1994;18:279–287. doi: 10.1016/0895-6111(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 17.Owen CM, Linskey ME. Frame-based stereotaxy in a frameless era: current capabilities, relative role, and the positive- and negative predictive values of blood through the needle. Journal of neuro-oncology. 2009;93:139–149. doi: 10.1007/s11060-009-9871-y. [DOI] [PubMed] [Google Scholar]
- 18.Sawin PD, Hitchon PW, Follett KA, Torner JC. Computed imaging-assisted stereotactic brain biopsy: a risk analysis of 225 consecutive cases. Surgical neurology. 1998;49:640–649. doi: 10.1016/s0090-3019(97)00435-7. [DOI] [PubMed] [Google Scholar]
- 19.Smith JS, Quinones-Hinojosa A, Barbaro NM, McDermott MW. Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy. Journal of neuro-oncology. 2005;73:173–179. doi: 10.1007/s11060-004-4208-3. [DOI] [PubMed] [Google Scholar]
- 20.Sumanaweera TS, Adler JR, Jr., Napel S, Glover GH. Characterization of spatial distortion in magnetic resonance imaging and its implications for stereotactic surgery. Neurosurgery. 1994;35:696–703. doi: 10.1227/00006123-199410000-00016. discussion 703-694. [DOI] [PubMed] [Google Scholar]
- 21.Woodworth GF, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD. Frameless image-guided stereotactic brain biopsy procedure: diagnostic yield, surgical morbidity, and comparison with the frame-based technique. Journal of neurosurgery. 2006;104:233–237. doi: 10.3171/jns.2006.104.2.233. [DOI] [PubMed] [Google Scholar]