Abstract
Objective
To assess circulating follicular helper-like CD4+ T (cTfh-like) cells in systemic lupus erythematosus (SLE) and determine their relationship to disease activity.
Methods
We analyzed blood samples from SLE patients, and as controls, Behçet’s disease (BD) patients and healthy individuals. We used flow cytometry to enumerate cTfh-like cells using as markers the C-X-C chemokine receptor type 5 (CXCR5), inducible T-cell costimulator (ICOS), programmed cell death protein-1 (PCDC1, PD-1), and secretion of interleukin-21 (IL-21). We compared the frequency of cTfh-like cells with that of circulating plasmablasts (CD19+IgD−CD38+) and evaluated their possible association with disease activity.
Results
cTfh-like T cells, identified as CXCR5hiICOShiPD-1hi, were expanded in the blood of SLE patients compared to BD and healthy controls. Such cells produced IL-21 with lower expression of CCR7, compared to circulating CXCR5hi central memory (Tcm) cells, enabling their distinction. PD-1, not ICOS or CXCR5, expression was significantly elevated in cTfh-like cells from SLE patients compared to controls. PD-1 expression among CXCR5hi cTfh-like cells correlated with disease activity, circulating plasmablasts, and anti-dsDNA antibody positivity, but not disease duration nor past organ injury; rather, it reflected current active disease.
Conclusion
We found that cTfh-like cells are associated with disease activity in SLE, suggesting that their presence indicates abnormal homeostasis of T-B cell collaboration with a causal relationship central to disease pathogenesis. These findings also suggest that cTfh-like cells provide a surrogate for aberrant GC activity in SLE, and that their PD-1 expression offers a tool for following disease activity and response to therapies.
Systemic lupus erythematosus (SLE, lupus) is marked by immune complex-mediated tissue injury in multiple organs. The clinical manifestations and the immunoregulatory factors that contribute to disease are diverse. Identification of common pathogenic pathways and the corresponding biomarkers that link abnormal cellular activity to disease activity are necessary to define therapeutic targets.
Central to antibody production is the collaboration between CD4+ T cells and B cells in germinal centers (GC) of secondary lymphoid organs (SLOs), the site of immunoglobulin (Ig) isotype switching and affinity maturation, with the subsequent genesis of memory B cells and long-lived plasma cells (PCs) (reviewed in (1, 2)). Pathogenic autoantibodies in murine and human lupus are also class-switched and somatically mutated with affinity maturation (3, 4), and arise from autoreactive memory B cells upon restimulation (5-7), features consistent with GC selection. The role of aberrant GC responses in the autoantibody genesis finds support in the observation that spontaneous GCs form in murine lupus (8), with evidence of exuberant GC activity in patients with active lupus nephritis (9). These data indicate that autoreactive B-cell maturation occurs in GCs in SLE.
Follicular B-helper T (Tfh) cells are necessary for T cell-dependent B-cell maturation in the GC (reviewed in (1, 2)). Tfh cells express the transcription factor B-cell lymphoma 6 (Bcl6) that drives a gene program critical for their development and function (10-12). Tfh cells are identified by a combination of markers, including CXCR5 (C-X-C chemokine receptor type 5) that enables their migration along a CXCL13 (C-X-C motif chemokine 13) gradient into B-cell follicles with subsequent GC formation (13, 14); ICOS (inducible T-cell costimulator), necessary for development of nascent Tfh cells upon their activation by dendritic cells (DCs) expressing ICOS ligand (ICOS-L) (15), and for their subsequent expansion upon interactions with ICOS-L expressed on B cells (16, 17); and PD-1 (programmed cell death protein-1; also PCDC1), which provides inhibitory signals to T cells (18), but also regulates GC B-cell selection and survival necessary for formation of long-lived PCs (19) of the type observed in SLE (4, 7). Tfh cells secrete interleukin (IL)-21, critical for GC development and maintenance (20, 21), and for Ig class switching and PC development (22). Aberrant expansion of Tfh cells is causally linked to abundant GCs, autoantibodies, and end-organ damage in murine lupus (23-25). Phenotypically similar T cells (20, 24) drive autoreactive B-cell responses occurring outside of GCs in murine SLOs (26) and in the kidneys of SLE patients (27). Thus, Tfh cells are central to disease in mice and humans.
Although human Tfh cells can be analyzed in spleens and tonsils, their assessment in SLE has been hampered by the inability to routinely sample SLOs. However, cells with a similar CXCR5hiPD-1hi phenotype circulate, potentially providing a window into analysis of Tfh cells in SLOs. For example, a PD-1hi subset of CD4+ CXCR5hi T cells expands transiently following influenza immunization, in conjunction with influenza-specific antibody-secreting cells (28, 29). HIV-infected individuals with neutalizing HIV-specific antibodies also have increased numbers of circulating CD4+ CXCR5hi PD-1hi T cells (30). CXCR5hiPD-1hi cells, some of which are ICOShi, circulate in SLE patients (29, 31) and in patients with juvenile dermatomyositis. Circulating CD4+ CXCR5hi T cells from non-autoimmune individuals drive in vitro differentiation of naïve (30) and memory B cells (28, 30) into Ig-secreting PCs, analagous to tonsillar Tfh cells, findings supporting their functional capability. While the circulating CD4+ CXCR5hi pool does not express the canonical Tfh-cell transcription factor Bcl6 (28-32), these studies nonetheless suggest that blood-borne CD4+ CXCR5hi cells represent a Tfh-cell counterpart and one that reflects the GC response (reviewed in (2)).
Characterization of circulating CD4+ CXCR5hi T cells is confounded by the presence of phenotypically similar blood-borne central memory (Tcm) CD4+ T cells, a portion of which express CXCR5. Yet, Tcm cells can be distinguished from the circulating CD4+CXCR5hiPD-1hi T cells that that expand in autoimmunity and transiently following vaccination as they are PD-1lo and ICOSlo (33). They also express the C-C chemokine receptor type 7 (CCR7) and L-selectin (CD62L) needed by these circulating cells for re-entry into lymph nodes where they become available for antigen rechallenge (14, 34), and differentiation into effector T helper (Th) cell subsets, among them Tfh cells (29, 35, 36). Tcm cells upon re-activation are presumably competent to re-enter B-cell follicles (29) via expression of the CXCR5 receptor interacting with its follicular ligand CXCL13, and to drive Ig secretion by naive and memory B cells, promoting secondary humoral immunity (32, 33).
Patients with SLE have expansion of circulating CD4+ CXCR5hi PD-1hi cells at constant levels that is correlated with autoantibody titers (29, 31). Yet, the relationship of circulating CD4+ CXCR5hi subsets to disease activity and the aberrant B-cell response is uncertain, partly due to the challenge discriminating among CXCR5hi Tfh-like and Tcm populations. Thus, we sought circulating Tfh-like (cTfh-like) cells in lupus patients, correlating them with clinical history, disease activity, and pathological B-cell function. We found that the frequency of cTfh-like cells was increased compared to controls, with expression of PD-1 most strongly associated with disease activity, suggesting the capacity for B-cell help. cTfh-like cells could be distinguished from Tcm cells via PD-1 upregulation and CCR7 downregulation, and IL-21 competence with evidence of recent cell division, suggesting functional capability. These findings suggest that cTfh-like cells provide a surrogate for aberrant GC activity in SLE, and their PD-1 expression offers a tool for following disease activity and therapeutic responsiveness.
PATIENTS AND METHODS
Study populations
We analyzed blood samples from two adult cohorts. The first, from the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, included 49 SLE patients (46 female and 3 male; median age 37 years, range 30-48 years; 32 Caucasian, 17 non-Caucasian), 28 Behçet’s disease (BD) patients (22 female and 6 male; median age 48 years, range 40-56 years; 20 Caucasian, 8 non-Caucasian), and 16 healthy controls (12 female and 4 male; median age 28 years, range 27-32 years; 13 Caucasian, 3 non-Caucasian). Their characteristics are shown in Supplementary Table 1, with phenotypic data summarized in Table 1 and Figures 1-4. We also studied samples from 8 patients with SLE (8 females; median age 40.38 years, range 26 and 60 years; 3 Caucasian, 5 non-Caucasian) from Yale-New Haven Hospital (YNHH), with their data shown in Figure 5. SLE patients fulfilled revised disease criteria of the American College of Rheumatology (ACR) with disease activity assessed by the SLE Disease Activity Index (37), whereas BD patients met criteria set by the International Study Group for Behcet’s Disease (38, 39). Informed consent was obtained from all subjects. This study was approved by the institutional review committees of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo and Yale University.
Table 1.
Mean fluorescence intensity (MFI) of PD-1 staining on CXCR5hi cells and disease features
| Current Disease | Absence of Disease Feature (MFI) |
Presence of Disease Feature (MFI) |
p-value |
|---|---|---|---|
| Proteinuria | 1217 | 1380 | 0.4166 |
| Hematuria | 1211 | 1662 | 0.1086 |
| Proteinuria + Hematuria | 1199 | 1911 | 0.0199 * |
| Pyuria | 1200 | 2590 | 0.0007 *** |
| Arthritis | 1281 | 988.3 | 0.3501 |
| Anti-dsDNA | 1169 | 1891 | 0.0041 ** |
| Low complement | 1173 | 1862 | 0.0064 ** |
|
| |||
| Past Disease | |||
|
| |||
| Skin | 1086 | 1286 | 0.4168 |
| Joint | 1523 | 1197 | 0.1387 |
| Renal | 1297 | 1222 | 0.6627 |
| CNS | 1295 | 1062 | 0.3150 |
| Vasculitis | 1226 | 1343 | 0.5498 |
| Hematological | 1246 | 1269 | 0.8909 |
| Serositis | 1087 | 1551 | 0.0070 ** |
Student’s t-test
p < 0.05,
p < 0.01,
p < 0.001
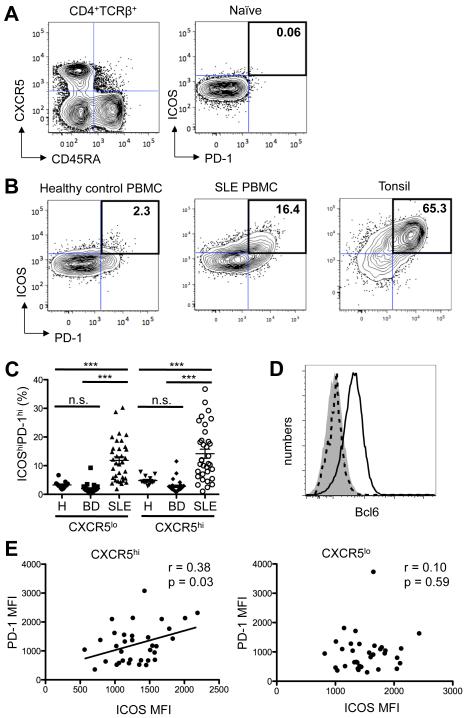
Figure 1.
Circulating CXCR5hiICOShiPD-1hi Tfh-like cells are expanded in the blood of patients with SLE compared to healthy controls and patients with Behçet's disease. A, CD4+TCRβ+ T cells were separated into naïve (CD45RA+) and activated (CD45RA−) pools, with the latter subdivided into CXCR5hi and CXCR5lo subsets (left panel). Gates for the expression of PD-1 and ICOS were set using naïve CD4+ T cells from healthy controls (right panel). B, Contour plots of ICOS and PD-1 expression on CD45RA− CXCR5hi cells from a representative healthy control (left panel) and an SLE patient (middle panel), compared to tonsillar cells from a non-SLE donor (right panel). Values are the percentage of ICOShiPD-1hi cells among CD4+ CXCR5hi cells. C, Percentage of ICOShiPD-1hi lymphocytes among CXCR5hi and CXCR5lo CD4+ T cells in patients with SLE (n = 49), Behçet's disease (BD; n = 28) and healthy controls (H; n = 16) (One-way ANOVA ***p < 0.001). D, Staining of Bcl6 in naïve (shaded) and Tfh cells (black line) from a tonsillar sample, compared to circulating CXCR5hiPD-1hi cells (dotted line) from a representative SLE patient. E, Correlation between the MFI of PD-1 and ICOS on CXCR5hi and CXCR5lo CD4+ T cells (Pearson r = 0.38, p = 0.03, left panel, and Pearson r = 0.10, p = 0.59, right panel, respectively).
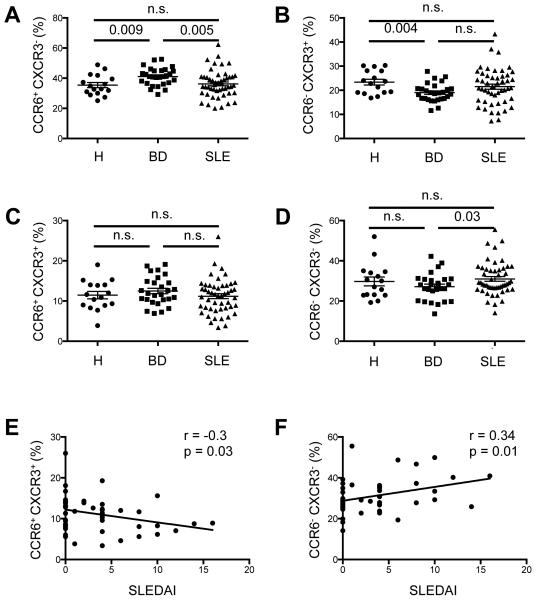
Figure 4. Analysis of CXCR5hi Th-cell subsets and correlation with SLEDAI.
A-D, Percentage of CCR6+CXCR3−(A), CCR6−CXCR3+ (B), CCR6+CXCR3+ (C), and CCR6− CXCR3−(D) cells among the circulating CD45RA−CXCR5hi CD4+ population in SLE patients (n = 49), in comparison to healthy controls (H; n = 16) and BD (n = 28) patients. (Student’s t-test, n.s. = non significant). Horizontal lines represent the mean values with standard error of the mean. E and F, Correlation between percentage of CCR6+CXCR3+ (E), and CCR6−CXCR3−(F) cells among circulating, activated CD45RA−CXCR5hi CD4+ cells and SLEDAI (E, Spearman r = −0.3, p = 0.03; F, Spearman r = 0.34, p = 0.01). Data points represent individual subjects.
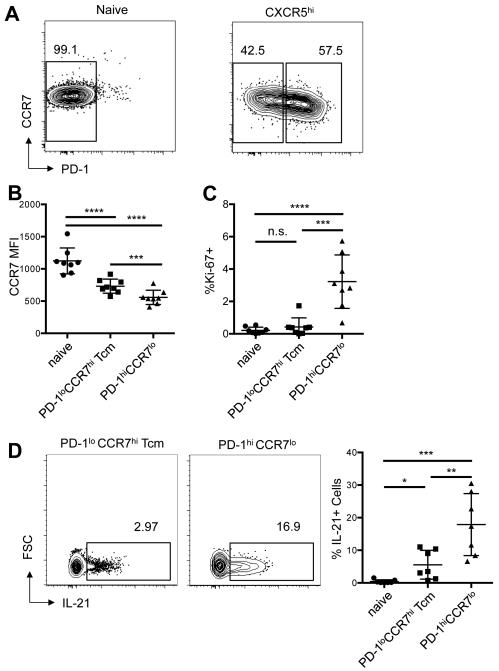
Figure 5.
Circulating CXCR5hiICOShiPD-1hi cTfh-like cells in SLE are dividing and robustly produce IL-21, in comparison to CXCR5hi central memory (Tcm) cells. A, Contour plots of CCR7 and PD-1 expression on naïve (left panel) and activated CD45RA−CXCR5hi (right panel) cells in the blood of a representative patient with SLE, demonstrating the gating strategy to determine PD-1lo Tcm and PD-1hi cells. B and C, The MFI of CCR7 (B) and the percentage of Ki-67+ cells (C) in each of the indicated subsets (Student’s t-test ***p < 0.001, ****p < 0.0001). D, Contour plots of intracellular IL-21 staining in circulating CXCR5hiPD-1lo Tcm populations and CXCR5hiPD-1hi cTfh-like populations from a representative patient with SLE (left and center panels) and the percentage of IL-21+ cells among seven SLE patients (right panel), compared to that among naïve CD45RA+ cells. Horizontal lines represent the mean values with standard deviation of the mean (right panel) (Student’s t-test *p < 0.05; **p < 0.01; ***p < 0.001).
Flow cytometry
We isolated peripheral blood mononuclear cells (PBMC) using density-gradient centrifugation on Ficoll-Paque with single cell suspensions stained with the following antibodies: Alexa Fluor 700-conjugated CD4, V450-conjugated CD45RA and CCR7, PE-conjugated CD4 and CCR6, PE-Cy7-conjugated PD-1, CXCR3, and IgD, Alexa Fluor 647-conjugated CXCR5 and IL-21, fluorescein isothiocyanate (FITC)-conjugated CD62L, APC-Cy7-conjugated CD19, APC-H7-conjugated CD3, PE-Cy5-conjugated CD38, streptavidin-conjugated APC-Cy7 (all from BD PharMingen), and FITC-conjugated ICOS, PE-conjuaged Bcl6, and PE-Cy5 conjugated TCRβ (from eBioscience), and PE-conjugated Ki-67 (from BioLegend). Stained cells were analyzed by multiparameter flow cytometry (LSRFortessa or LSR II, BD), with exclusion of doublets by forward and side scatter, using FlowJo software (Tree Star).
Intracellular IL-21, Bcl6, and Ki-67 staining
Surface-stained PBMC were sorted by flow cytometry (FACSAria, BD Biosciences) into naïve (CD45RA+), cTfh-like (CD45RA−CXCR5hi ICOShiPD-1hi), and Tcm (CD45RA−CXCR5hiICOSloPD-1lo) populations, with the latter distinguished by CCR7 expression (34). Sorted subsets were stimulated with PMA (50 ng/ml) and ionomycin (1 µg/ml) for 2 hours wih Golgi Plug (BD) for an additional 2 hours. Cells were fixed (BD CytoFix/CytoPermTM) and permeabilized (BD PERM/WashTM), and stained with anti-IL-21. For Bcl6 and Ki-67 staining, surface-stained PBMC were fixed and permeabilized without stimulation.
Autoantibody profile and serum complement levels
Antinuclear and anti-double-stranded DNA (anti-dsDNA) antibodies were detected by indirect immunofluorescence (IIF) using as substrates HEp-2 cells or Crithidia luciliae, respectively (INOVA Diagnostics Inc). Serum levels of anti-Ro(SS-A), anti-La(SS-B), anti-RNP, and anti-Sm antibodies were determined by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (INOVA Diagnostics Inc). Total hemolytic complement activity (CH100) was measured by the immunohemolysis assay (normal range 150-350 U/ml).
Statistical analysis
All data are presented as the mean ± SEM. The significance of the difference between groups was analyzed with one-way ANOVA test, with the significance of difference between two groups evaluated by the two-tailed Student’s t-test. Spearman correlation coefficient or Pearson correlation coefficient with two-tailed p value were determined in the analysis of correlations. P values less than 0.05 were considered significant. Data were analyzed with Prism software (version 5.0d for Macintosh; GraphPad Software).
RESULTS
Analysis of cells with a Tfh phenotype in the blood of SLE patients and controls
CXCR5hi Tfh cells in SLOs express ICOS and PD-1, central to their development and helper function in B-cell follicles and GCs (reviewed in (2)). To identify cells with a Tfh-cell phenotype in the blood of lupus patients and controls, we sought CD4+ T cells that were CXCR5hiICOShiPD-1hi, markers that distinguish them from CD4+ CXCR5hiCCR7hiICOSloPD-1lo Tcm cells observed in the circulation of normal individuals.
We analyzed single CD4+ TCRβ+ cells, dividing them into naïve CD45RA+ and activated CD45RA− subsets, with the CD45RA− subset further divided as CXCR5hi or CXCR5lo (Figure 1A, left panel) followed by gating on PD-1 and ICOS (Figure 1B). Flow cytometry gates were set according to the basal expression of PD-1 and ICOS (both < 0.5% positive), or that of the subsequent ICOShiPD-1hi double positive population (< 0.1% positive), on naïve CD45RA+ CD4+ T cells from healthy controls (Figure 1A, right panel). Naïve CD45RA+ T cells, regardless of donor’s health status, were primarily CXCR5lo and largely lacking ICOS and PD-1 expression (Figure 1A, right panel; representative sample from a healthy control). We found only a few circulating activated CD45RA−CXCR5hiICOShiPD-1hi cells, i.e., those bearing a Tfh-cell phenotype, in healthy controls (Figure 1B, left panel and Figure 1C), consistent with previous reports (28-32); CD45RA−CXCR5lo cells from normal donors were also largely PD-1loICOSlo (Figure 1C). By contrast, CD45RA−CXCR5hiICOShiPD-1hi cells were expanded in the blood of lupus patients (Figure 1B, middle panel, representative example, and Figure 1C), with correlation of PD-1 and ICOS expression (Figure 1E, left panel). Expression of both on tonsillar Tfh cells was higher than on circulating CXCR5hiICOShiPD-1hi cells from SLE patients: the mean fluorescence intensity (MFI) ± standard deviation (SD) of PD-1 and ICOS was 10000 ± 1587 and 12210 ± 1481, respectively, on the former (n = 3), compared to an MFI ± SD of PD-1 and ICOS of 4290 ± 543 and 5827 ± 1583, respectively, on the latter cells (n = 21) (Figure 1B, representative examples). tonsillar Tfh cells also expressed Bcl6 (40), the canonical Tfh-cell transcription factor, whereas the circulating CXCR5hi population did not (28-32) (Figure 1D). CXCR5hiICOShiPD-1hi cells were not expanded in blood of patients with BD (Figure 1C), indicating that their presence is not a function of this inflammatory disorder. We noted an increase in the percentage of ICOShiPD-1hi cells within the CXCR5lo subset in lupus patients (Figure 1C), as compared to BD patients and healthy controls; however, PD-1 and ICOS expression was not correlated in this population (Figure 1E, right panel), unlike in the CXCR5hi subset (Figure 1E, left panel). Thus, circulating CD4+CD45RA−CXCR5hi cells in lupus contained a population resembling Tfh cells found in SLOs, albeit with lower PD-1 and ICOS expression, and without Bcl6; we considered these cTfh-like cells.
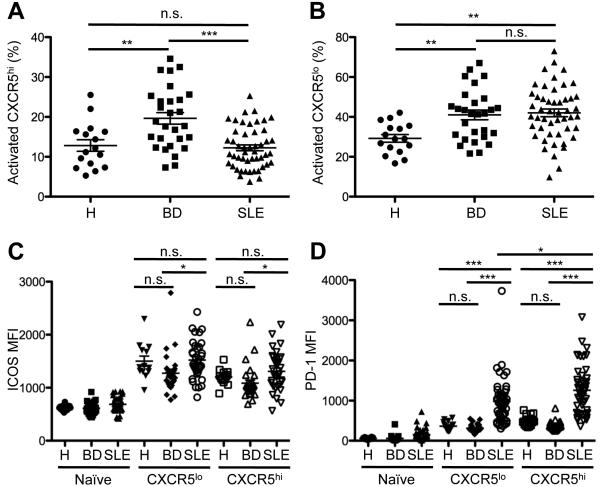
We next asked if individual Tfh-cell markers CXCR5, ICOS, and PD-1 were selectively upregulated on circulating CD4+TCRβ+ cells in lupus. Patients with active disease have increased circulating CD4+ICOShi lymphocytes, although their number varies according to definition of this population and the cohort (41). We did not find that increased percentages of CXCR5hi or CXCR5lo cells per se were associated with SLE (Figure 2A, 2B), or that ICOS expression, as measured by MFI, distinguished circulating CD4+ T cells from patients and controls (Figure 2C). By contrast, expression of PD-1, a molecule integral to GC maturation and PC genesis (19), was increased in the CXCR5hi subset in lupus, compared to those from healthy controls and BD patients (Figure 2D). The increase in PD-1 MFI appeared to account for the increase in CXCR5hi ICOShi PD-1hi cTfh-like cells we observed in the lupus patients (Figure 1C). PD-1 also was upregulated in the CXCR5lo subset in SLE, but less so than in CXCR5hi cells. These data demonstrate that CXCR5hiICOShiPD-1hi cTfh-like cells are expanded in SLE, and PD-1, but not ICOS, is selectively up-regulated on the circulating CXCR5hi subset.
Figure 2.
Expression of the Tfh-cell surface markers CXCR5, PD-1, and ICOS on circulating CD4+ T lymphocytes. A and B, Percentage of activated CD45RA−CXCR5hi (A) and CD45RA−CXCR5lo (B) cells among the circulating CD4+TCRβ+ population in the blood of SLE patients (n = 49), in comparison to healthy controls (H; n = 16) and BD (n = 28) patients. C and D, MFI of ICOS (C) and PD-1 (D) expression on CXCR5hi and CXCR5lo CD4+ T cells from the blood of SLE patients compared to patients with BD and healthy controls. Data points represent individual subjects; horizontal lines represent the mean values with standard error of the mean (One-way ANOVA *p < 0.05; **p < 0.01; ***p < 0.001).
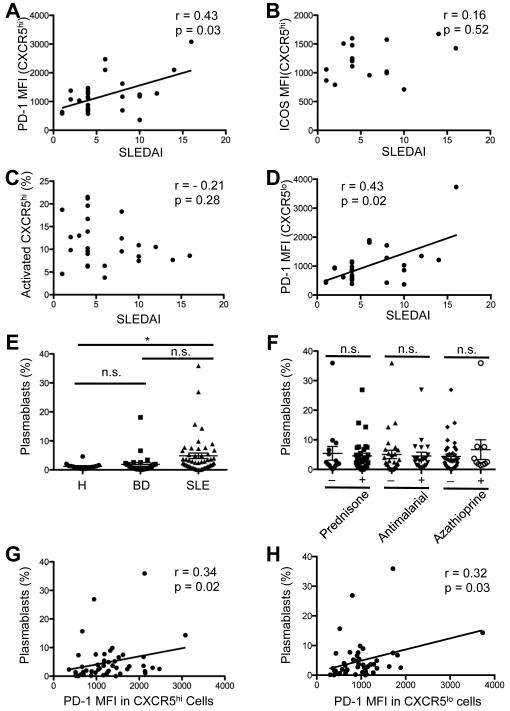
Correlation between cTfh-like cells and disease activity in SLE
We next asked if the expansion of cTfh-like cells in lupus patients was correlated with disease activity, as measured by SLEDAI. We did not find a significant relationship between the percentage of the CXCR5hiICOShiPD-1hi subset and this scale (Spearman’s r = 0.26, p = 0.19; data not shown), and we did not observe a correlation between the former and organ involvement and aberrant B-cell activation (data not shown). Yet, as the cTfh-like markers CXCR5, ICOS, and PD-1 are differentially regulated in cells from patients (Figure 2), we asked if their individual expression correlated with clinical parameters. We found a correlation between the SLEDAI and the MFI of PD-1 (Figure 3A), but not with ICOS MFI and CXCR5hi cells (Figures 3B and C, respectively). We observed a similar correlation between PD-1 expression on the CXCR5lo subset and disease activity (Figure 3D), suggesting possible Th17 and/ or Th1-cell involvement in SLE (42), as these Th subsets are CXCR5lo. Although the elevated PD-1 MFI could reflect T cell activation common in active SLE (41), this did not appear to account for the relationship between it and the SLEDAI, as the latter was not correlated with expression of ICOS, also a marker of activated T cells. Moreover, the percentage of activated CD45RA− CD4 T cells was not related to the SLEDAI (data not shown).
Figure 3.
Association between Tfh-cell surface markers, SLEDAI, and circulating plasmablasts. A and B, Correlation between the MFI of PD-1 (A) and ICOS (B) on circulating CXCR5hi cells and SLEDAI (A, Spearman r = 0.43, p = 0.03 and B, Spearman r = 0.16; p = 0.52). C, Association between percentage of CXCR5hi cells among circulating CD4+TCRβ+ cells and SLEDAI (Spearman r = −0.21, p = 0.28). D, Correlation between MFI of PD-1 on the circulating CXCR5lo subset and SLEDAI (Spearman r = 0.43, p = 0.02). E, Percentage of circulating CD19+IgD−CD38+ plasmablasts among total B cells in the blood of SLE patients (n = 49), healthy controls (H; n =16), and BD (n = 28) patients (One-way ANOVA, *p < 0.05). F, Percentage of circulating plasmablasts in patients with SLE, arranged according to therapy (Student’s t-test, n.s. = non significant). Horizontal lines represent the mean values with standard error of the mean. G and H, Correlation between percentage of circulating plasmablasts among circulating B cells in SLE and the PD-1 MFI on activated CXCR5hi and CXCR5lo cells, respectively (G, Spearman r = 0.34, p = 0.02; H, Spearman r = 0.32, p = 0.03). Data points represent individual subjects.
Given the correlation between PD-1 expression on CXCR5hi cells and disease activity, we asked if the former were linked to organ involvement (Table 1). In patients with proteinuria and hematuria, compared to those without, we found an increase in PD-1 MFI on CXCR5hi cells (1911 vs.1199, respectively; p = 0.02), as we did for patients with ongoing pyuria (2590 vs. 1200; p = 0.0007). By contrast, apart from serositis (1551 vs. 1087, p = 0.007), PD-1 expression was not correlated with organ injury, including in the skin, joints, kidney, CNS, and vasculature. These results suggested that the PD-1 MFI on CXCR5hi cells correlated with disease activity, including renal inflammation. Consistent with these findings, we found that PD-1 MFI on the CD4+ CXCR5hi subset was increased in patients with elevated anti-dsDNA antibodies, compared to patients without such autoantibodies (1891 vs. 1169, respectively; p = 0.004), and was likewise elevated in hypocomplementemic patients (1862 vs. 1173, p = 0.006) as measured by total hemolytic complement activity, with the latter presumably reflecting consumption upon engagement by autoantibody-autoantigen complexes.
Association between PD-1 expression and the expansion of circulating plasmablasts
Considering the pivotal role of PD-1 expressed on Tfh cells in GC maturation and PC genesis (19) and the positive association between PD-1 expression on blood CXCR5hi CD4 cells and anti-dsDNA antibodies with presumed complement consumption, we asked if the elevated expression of PD-1 was correlated with B-cell activation. We purified lymphocytes from patients and controls, staining them with CD19, IgD, and CD38 to assess the pool of the IgD−CD38hi plasmablasts (9). Consistent with previous reports (9, 43), we found an increased percentage of circulating plasmablasts in patients compared to healthy controls (Figure 3E), with their percentage similar regardless of treatment (Figure 3F). We also found that the PD-1 MFI on CXCR5hi, and on CXCR5lo, cells correlated with the plasmablast percentages among circulating B cells (Figure 3G and H, respectively); removal of outliers had no effect on statistical analyses.
Analysis of CXCR5hi Th-cell subsets and correlation with SLEDAI
Differential expression of the chemokine receptors CXCR3 and CCR6 can be used to separate CXCR5hi cells into effector subsets expressing cytokines characteristic of Th1, Th2, and Th17 cells, with production of IFN-γ, IL-4, and IL-17, respectively (32, 44). Patients with SLE have among the CXCR5hi CD4+ population increased frequencies of IL-17-producing CCR6+CXCR3− and IL-4-producing CCR6−CXCR3− subsets, with a decrease in IFNγ-secreting CCR6−CXCR3+ cells (32, 40, 44). In our hands, these CXCR5hi subsets all produced IL-21, while individually producing IL-4, IFN-γ, and IL-17, respectively, in accord with chemokine receptor expression (data not shown). Although we did not observe differences in their relative frequencies in our cohort (Figure 4A-D), we found that the frequency of the CCR6−CXCR3− subset was associated with disease activity, replicating earlier findings (32, 44), while that of the CCR6+CXCR3+ subset was negatively associated (Figure 4E-F).
Relationship of CXCR5hi cTfh-like and CXCR5hi Tcm cells in SLE
CXCR5hi cells in the circulation of healthy individuals display a Tcm-cell phenotype: they are ICOSloPD-1lo with elevated expression of CCR7 and CD62L (14, 34), required for interaction with CCL19 and 21, chemokines abundantly expressed by high endothelial venules and T zone reticular cells, and with vascular addressins, respectively, with CXCR5 aiding follicular homing for B-cell helper function (14). We used these markers to distinguish CXCR5hi cTfh-like and CXCR5hi Tcm cells in the circulation of lupus patients. To establish staining parameters, we analyzed CD4+TCRβ+CD45RA+ naïve cells, which also use CCR7 to home to SLOs, showing they robustly expressed CCR7 and were PD-1lo (Figure 5A, left panel). Activated CD45RA−CXCR5hi cells could be divided into PD-1lo CCR7hi and PD-1hi CCR7lo populations (Figure 5A, right panel), although the former, defined as Tcm cells (29, 34), expressed less CCR7 than did naïve CD4+TCRβ+ cells (Figure 5B, left panel). CXCR5hiPD-1hi cells expressed less CCR7 than did Tcm cells (Figure 5B). A greater percentage of these cells, compared to the Tcm population, also stained for the proliferation marker Ki-67 (Figure 5C), consistent with a proliferation history of cTfh-like cells in contrast to quiescent Tcm cells. Thus, PD-1 expression, with that of CCR7, is a marker for distinguishing cTfh-like from Tcm cells (29).
We next stained for intracellular IL-21, a cytokine critical for the B-cell helper function of Tfh cells, with the idea that cTfh-like cells would have cytokine production upon stimulation. While few if any naïve CD4 cells produced IL-21, activated CXCR5hiPD-1hi cells from the lupus patients expressed this cytokine, producing more in comparison to CXCR5hiPD-1lo Tcm cells (Figure 5D) as expected, since non-Tfh central memory cells, such as those bearing a Th1 phenotype, produce less cytokines than their effector counterparts (34). These data suggest that cTfh-like cells have functional capacity similar to that of Tfh cells found in SLOs, with PD-1 and IL-21 expression.
Discussion
Expansion of Tfh cells is associated with the development of systemic autoimmunity in murine lupus (23, 24), with linkage to heightened GC responses and end-organ damage (25). Patients with SLE also have altered GC homeostasis (4, 7, 9, 45), suggesting that Tfh cells are likewise aberrantly regulated in the human disease. With these ideas in mind, we asked if cells bearing a Tfh-like phenotype circulated in SLE, and if so, did their presence reflect aberrant GC homeostasis.
We found an increase in the number of cTfh-like cells in the blood of lupus patients, compared to controls. In addition to upregulation of PD-1 and ICOS, cTfh-like cells in SLE down-regulated CCR7, a Tcm-cell marker (34), with this combination enabling distinction between these two populations. Expansion of cTfh-like cells was not seen in Behçet’s disease, suggesting that it was not a function of a systemic inflammatory disorder, but a distinctive phenotype in SLE. The cTfh-like cells included those recently proliferating, as determined by Ki-67 positivity, and that secreted IL-21, distinguishing them from functionally “exhausted” CD4+PD-1hi cells found in patients with chronic infections or cancer (46, 47). In particular, the expansion of PD-1hi CXCR5hi cells reflected GC dysfunction in SLE, as evidenced by their correlation with circulating plasmablasts and autoantibodies.
Expansion of circulating IL-21-producing CD4+CXCR5+ T cells has been reported in SLE patients, with such cells expressing the Tfh-cell transcription factor Bcl6 (48). The latter finding is at odds with ours, and those of others, in which circulating cells lack Bcl6 (28-32), differences that perhaps reflect cell isolation or staining, or degree of GC dysfunction. Regardless, these findings indicate that Tfh-like cells circulate in SLE.
While we noted an increase in the frequency of cTfh-like cells in SLE compared to controls, this was not correlated with disease activity measured by SLEDAI. One factor contributing to this lack of correlation was the use of ICOS to identify cTfh-like cells. ICOS is upregulated on all activated CD4+ T cells, and in our hands, its expression was nearly indistinguishable among patient and control groups (Figure 2C), negating its specificity for cTfh-like cell expansion. By contrast, PD-1 expression on CXCR5hi cells was correlated with the SLEDAI, perhaps reflecting the aberrant GC reaction in SLE, as evidenced by a correlation with expansion of circulating plasmablasts and anti-ds-DNA antibodies with hypocomplementemia. Thus, the degree of PD-1 expression on cTfh-like cells, more so than their frequency, is an indicator of pathological B-cell responses and disease activity. It will nonetheless be important to validate the utility of PD-1 expression on blood CXCR5hi cells as a biomarker for disease activity via longitudinal studies.
We also found that PD-1 expression on CD4+ CXCR5lo cells, as for their CXCR5hi counterparts, correlated with disease activity in SLE, as well as with circulating plasmablasts (Figure 3H). These associations may be secondary to the proinflammatory nature of activated CXCR5loPD-1hi cells (42). Consistent with this idea are the observations that such cells upon activation upregulate CXCR5 with B-cell helper function (13), inducing memory B cells to produce class-switched Igs (32).
One contributing factor to elevated levels of PD-1 may be type I interferons (IFNs), as these correlate with disease activity (49, 50). Type I IFNs enhance humoral immunity and promote isotype switching in vivo (51), which in T-cell dependent responses, rely upon Tfh cells, although our recent murine data suggest that effects of these cytokines on Tfh-cell development need dissection in the context of signaling by other cytokines (52). Nonetheless, the heightened PD-1 expression on CXCR5hi cells in SLE is consistent with its induction and maintenance by IFN-α in murine CD4+ T cells (53). We also found that IFN-α enhanced the expression of PD-1 on human Tfh cells (data not shown). Thus, we need in the future to dissect Tfh-cell differentiation and function in a type I IFN-rich environment, common to that in SLE (50, 54). Moreover, since polymorphisms of PDCD1, the gene encoding PD-1, are associated with SLE susceptibility in certain ethnicities (55, 56), understanding of PD-1-pathway regulation in Tfh and cTfh-like cells in SLE will require dissection of genetic and environmental precipitants.
PD-1 provides inhibitory signals to activated T cells, consequently helping maintain peripheral tolerance (18). Yet, engagement of Tfh-cell PD-1 via its ligands on GC B cells is required for appropriate production of T-cell cytokines critical for GC maturation and PC formation, with the absence of PD-1 signaling leading to Tfh cells that are less competent to provide B-cell help (19). Depletion of PD-1 bearing T cells also has a beneficial effect in murine lupus via reduction in autoantibody formation, cytokine production, and renal disease (57). On the other hand, blockade of the PD-1 ligand PD-L1 in murine lupus enhances proinflammatory cytokine and anti-dsDNA antibody production (57). These contrasting observations highlight the fine orchestration of the GC reaction in which PD-1 regulation of Tfh-cell function is necessary for provision of proper B-cell maturation signals at the expense of robust T-cell inflammatory function (19).
Why are cTfh-like cells found in the blood in SLE? They are likely distinct from Tfh cells. They lack expression of the canonical Tfh-cell transcription factor Bcl6 (28-32), critical for induction of the Tfh-cell program of differentiation (10-12). They are also found in mice and humans with genetic deficienciency in SAP, a SLAM (signaling lymphocyte activation molecule)-associated protein, essential for Tfh-cell differentiation and GC formation (29). These findings suggest that cTfh-like cells form, and are released from, SLOs prior to Tfh-cell formation and immigration into the GC. These cells also expand within a few days following flu vaccination, indicative of recall from the Tcm cells (28, 30), as well as arise from naïve cells following immunization of mice (29). They are functional with ability to promote Ig secretion by B cells, and may re-enter GCs to promote B-cell maturation. Thus, it seems likely that they arise from autoreactive naïve precursor and/or memory T cells in SLE, with the capacity to drive pathogenic autoantibody formation, as indicated by the data from others (29) and revealed herein.
In conclusion, we have shown that cTfh-like cells are expanded in the blood of patients with SLE, with linkage to disease activity, suggesting that they play a central role in pathogenesis. These data suggest that PD-1 expression on circulating CD4+ CXCR5hi T cells may be a marker for GC B-cell dysregulation. It also may be useful for monitoring humoral responses in the context of disease activity and therapeutic responsiveness, while providing a basis to better elucidate the dynamics of pathological Tfh-cell activity in human SLE.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by FAPESP 2011/02119-0 (to EB), and by grants provided by AbbVie, the Alliance for Lupus Research and the NIH (AR040072 and AR053495) (to JC). SangTaek Kim was supported by a Scientist Development Award from the American College of Rheumatology Research Foundation and by NIH T32 AR07107.
Footnotes
The authors have no financial conflicts of interest to disclose regarding this work.
REFERENCES
- 1.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nature Reviews Immunology. 2009;9(12):845–57. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 2.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012 doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(24):9150–4. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappione A, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. The Journal of Clinical Investigation. 2005;115(11):3205–16. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anolik J, Sanz I. B cells in human and murine systemic lupus erythematosus. Curr Opin Rheumatol. 2004;16(5):505–12. doi: 10.1097/01.bor.0000133660.52599.f6. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, et al. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis and Rheumatism. 2008;58(6):1762–73. doi: 10.1002/art.23498. [DOI] [PubMed] [Google Scholar]
- 7.Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Research & Therapy. 2011;13(5):243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, et al. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70(4):578–84. [PubMed] [Google Scholar]
- 9.Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003;112(10):1506–20. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–10. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–5. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–68. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of Experimental Medicine. 2000;192(11):1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of Experimental Medicine. 2000;192(11):1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011 doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam K-P, Coyle AJ, et al. ICOS controls the pool size of effector-memory and regulatory T cells. Journal of Immunology. 2008;180(2):774–82. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein JS, Bertino SA, Hernandez SG, Poholek AC, Teplitzky TB, Nowyhed HN, et al. B Cells in T Follicular Helper Cell Development and Function: Separable Roles in Delivery of ICOS Ligand and Antigen. Journal of Immunology. 2014 doi: 10.4049/jimmunol.1302617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunological Reviews. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nature Immunology. 2010;11(6):535–42. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of Experimental Medicine. 2010;207(2):353–63. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell–intrinsic mechanism. The Journal of Experimental Medicine. 2010;207(2):365–78. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ettinger R, Sims GP, Fairhurst A-M, Robbins R, da Silva YS, Spolski R, et al. IL-21 Induces Differentiation of Human Naive and Memory B Cells into Antibody-Secreting Plasma Cells. Journal of Immunology. 2005;175(12):7867–79. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 23.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435(7041):452–8. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 24.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of Experimental Medicine. 2008;205(12):2873–86. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. The Journal of Experimental Medicine. 2009;206(3):561–76. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297(5589):2066–70. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 27.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. Journal of Immunology. 2011;186(3):1849–60. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Science Translational Medicine. 2013;5(176):176ra32. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39(4):770–81. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-(+)1CXCR3(-)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–69. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and Rheumatism. 2010;62(1):234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 32.Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, et al. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity. 2011 doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. Journal of Immunology. 2011;186(10):5556–68. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 35.Pepper M, Pag·n AJ, Igy·rtÛ BZ, Taylor JJ, Jenkins MK. Opposing Signals from the Bcl6 Transcription Factor and the Interleukin-2 Receptor Generate T Helper 1 Central and Effector Memory Cells. Immunity. 2011;35(4):583–95. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38(4):805–17. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petri M. Disease activity assessment in SLE: do we have the right instruments? Ann Rheum Dis. 2007;66(Suppl 3):iii61–4. doi: 10.1136/ard.2007.078477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Disease ISGfBs Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet. 1990;335(8697):1078–80. [PubMed] [Google Scholar]
- 39.Mendes D, Correia M, Barbedo M, Vaio T, Mota M, Goncalves O, et al. Behcet’s disease--a contemporary review. J Autoimmun. 2009;32(3-4):178–88. doi: 10.1016/j.jaut.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Bentebibel S-E, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(33):E488–97. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawamoto M, Harigai M, Hara M, Kawaguchi Y, Tezuka K, Tanaka M, et al. Expression and function of inducible co-stimulator in patients with systemic lupus erythematosus: possible involvement in excessive interferon-gamma and anti-double-stranded DNA antibody production. Arthritis Research & Therapy. 2006;8(3):R62. doi: 10.1186/ar1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah K, Lee WW, Lee SH, Kim SH, Kang SW, Craft J, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Research & Therapy. 2010;12(2):R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorner T, Lipsky PE. Correlation of circulating CD27high plasma cells and disease activity in systemic lupus erythematosus. Lupus. 2004;13(5):283–9. doi: 10.1191/0961203304lu1014oa. [DOI] [PubMed] [Google Scholar]
- 44.Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PloS One. 2013;8(9):e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. Journal of Immunology. 2001;167(4):2361–9. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe T, Bertoletti A, Tanoto TA. PD-1/PD-L1 pathway and T-cell exhaustion in chronic hepatitis virus infection. J Viral Hepat. 2010;17(7):453–8. doi: 10.1111/j.1365-2893.2010.01313.x. [DOI] [PubMed] [Google Scholar]
- 47.Haymaker C, Wu R, Bernatchez C, Radvanyi L. PD-1 and BTLA and CD8(+) T-cell "exhaustion" in cancer: "Exercising" an alternative viewpoint. Oncoimmunology. 2012;1(5):735–8. doi: 10.4161/onci.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terrier B, Costedoat-Chalumeau N, Garrido M, Geri G, Rosenzwajg M, Musset L, et al. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J Rheumatol. 2012;39(9):1819–28. doi: 10.3899/jrheum.120468. [DOI] [PubMed] [Google Scholar]
- 49.Bengtsson AA, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9(9):664–71. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 50.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis and Rheumatism. 2005;52(5):1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 51.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14(4):461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 52.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40(3):367–77. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. Journal of Immunology. 2011;186(5):2772–9. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 54.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreiros-Vidal I, D’Alfonso S, Papasteriades C, Skopouli FN, Marchini M, Scorza R, et al. Bias in association studies of systemic lupus erythematosus susceptibility due to geographical variation in the frequency of a programmed cell death 1 polymorphism across Europe. Genes and Immunity. 2007;8(2):138–46. doi: 10.1038/sj.gene.6364370. [DOI] [PubMed] [Google Scholar]
- 56.Liu JL, Zhang FY, Liang YH, Xiao FL, Zhang SQ, Cheng YL, et al. Association between the PD1.3A/G polymorphism of the PDCD1 gene and systemic lupus erythematosus in European populations: a meta-analysis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2009;23(4):425–32. doi: 10.1111/j.1468-3083.2009.03087.x. [DOI] [PubMed] [Google Scholar]
- 57.Kasagi S, Kawano S, Okazaki T, Honjo T, Morinobu A, Hatachi S, et al. Anti-Programmed Cell Death 1 Antibody Reduces CD4+PD-1+ T Cells and Relieves the Lupus-Like Nephritis of NZB/W F1 Mice. Journal of Immunology. 2010;184(5):2337–47. doi: 10.4049/jimmunol.0901652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.