Abstract
Over the last few years, the concept of food addiction has become a common feature in the scientific literature, as well as the popular press. Nevertheless, the use of the term “addiction” to describe pathological aspects of food intake in humans remains controversial, and even among those who affirm the validity of the concept, there is considerable disagreement about its utility for explaining the increasing prevalence of obesity throughout much of the world. An examination of the literature on food addiction indicates that mesolimbic and nigrostriatal dopamine systems often are cited as mechanisms that contribute to the establishment of food addiction. However, in reviewing this literature, it is important to have a detailed consideration of the complex nature of dopaminergic involvement in motivational processes. For example, although it is often stated that mesolimbic dopamine mediates “reward”, there is no standard or consistent technical meaning of this term. Moreover, there is a persistent tendency to link dopamine transmission with pleasure or hedonia, as opposed to other aspects of motivation or learning. The present paper provides a critical discussion of some aspects of the food addiction literature, viewed through the lens of recent findings and current theoretical views of dopaminergic involvement in food motivation. Furthermore, compulsive food intake and binge eating will be considered from an evolutionary perspective, in terms of the motivational subsystems that are involved in adaptive patterns of food consumption and seeking behaviors, and a consideration of how these could be altered in pathological conditions.
Keywords: reinforcement, motivation, behavioral economics, reward, decision making, behavioral activation, aversion, depression, addiction
Introduction
The Emerging Concept of Food Addiction, and the Hypothesized Role of Dopamine
One of the first suggestions that food could have addictive properties was provided by Randolph in 1956 (1). Although this idea was relatively dormant for some time, there has been an explosion of research in this area during the last few years. A Medline search reveals that there were only 9 papers published with “food addiction” as a key phrase from 1956 to 2007; since 2008, there have been more than 65. There have been many reasons for this explosion. Hoebel and colleagues (e.g. 2) have provided a body of animal work that offers a potential rodent model of this phenomenon, with all the associated opportunities for investigating physiological mechanisms. Clinicians have developed rating scales and structured interview techniques that have allowed for the characterization of addiction-like behaviors, and have yielded insights into pathological aspects of eating behavior in humans (3,4). Furthermore, though this is a scientific review, it is hard to ignore the impact of the concept of “food addiction” on the general public. In the midst of a dramatic increase in obesity in the developed world that some would say has reached epidemic proportions, the discussion of food addiction in venues ranging from the internet to television talk shows has also become an epidemic of sorts.
Of course, like most scientific endeavors, research on food addiction and related phenomena such as binge eating disorder is much more complex than the popular media would suggest. Though many researchers argue strongly for the validity of the concept of food addiction (5,6), others urge caution (7). It has been noted in multiple papers that many of the behavioral characteristics of food addiction or binge eating resemble some of the diagnostic criteria established for drug dependence, including the compulsive nature of the food seeking and taking behavior, the escalation of consumption, and the resistance to the effects of consequences (5, 8). Nevertheless, considerable controversy remains. Researchers and clinicians who focus on drug addiction often talk about dependence and withdrawal phenomena; applied to food as a general category, this concept is meaningless, because nutrients are a genuine physiological need. This recognition has led many to consider that, in some general sense, food addiction is an oversimplified term (9), because people and other animals that are said to have this condition are not addicted to food per se, but rather to foods with particular macronutrient contents (2,9,10,11,12), under certain environmental conditions (9). Moreover, the relation between food addiction and binge eating disorder, as well as the role that these conditions play in subtypes of obesity, remains uncertain. For example, it has been suggested that the number of food addicted people is relatively small compared to the number who are obese (13), and that there are sizeable proportions of underweight, normal weight, and overweight individuals who could be classed as food addicted based upon the Yale Food Addiction Scale (4).
In view of the continuing controversies in this area, it seems reasonable to suggest that the scientists and clinicians should exercise caution in interpreting these emerging findings, not only in the scientific literature, but also in terms of how this information is disseminated to the general public (14,15). For example, Rogers and Smit (14) argued that the vast majority of self-reported cases of food addiction should not be viewed as addictive behavior, and note that overweight people attempting to resist consumption of sweets could be mislabeling cravings and desire for more as a sign of addiction. In addition, Esptein and Shaham (15) suggested that food addiction should not be viewed by patients as an excuse for overeating, and that the unhealthy eating associated with food addiction should not be equated with obesity. Certainly, too much exposure to superficial discussion of food addiction in the media, coupled with casual use of the term by health care professionals, could promote these kinds of misconceptions in the general public. Furthermore, one can question whether the general trend of labeling more and more patterns of behavior as “addictive” is causing this term to dilute its meaning. There are drug addictions for a wide array of substances. In addition, terms such as gambling addiction, sex addiction, email addiction, internet addiction, pornography addiction, and video game addiction are becoming every more widely used. There are of course alcoholics, but also workaholics and “chocoholics”. Indeed, one could be forgiven for wondering if neuroscientists and clinicians have become addicted to the term addiction, since the use of this term is escalating so much. But on a very serious note, one must always wonder in psychiatry whether the apparent increase in prevalence of a disorder stems from increased awareness, or sensitivity of the diagnostic tools, or if instead it represents overuse and overdiagnosis. Furthermore, one must exercise caution in the use of labels for disorders (7).
Amidst all this uncertainty and controversy related to food addiction, there is another set of concerns related to the hypothesized involvement of “reward” mechanisms that are said to involve dopamine (DA) systems. A significant fraction of the published studies in this area posit some type of DAergic mediation or regulation of food addiction. In many cases, these studies seem to rely on an uncritical acceptance of a series of tenets, which include the following concepts: a) “reward” is a valid term to describe a psychological or neurobehavioral process, and that there is a substantial uniformity or consensus in the meaning of this term across articles and laboratories, b) there is a “reward system” that mediates this neurobehavioral process, c) DA is a central component, perhaps the linchpin, of the “reward system”, d) that the DAergic “reward system” is a critical mediator of drug addiction, and e) that DAergic involvement in “reward” therefore provides a plausible explanation for the phenomena that scientists are attempting to capture by the use of the term “food addiction”. Yet despite the fact that many investigators uncritically accept the idea of DAergic mediation of “reward” (e.g.16), this hypothesis is no less controversial and contested than the idea of food addiction itself (e.g. 17,18,19). Therefore, the present review will briefly deconstruct these traditional and oft mentioned tenets of the DA hypothesis of reward, highlight the complex findings in the literature that call them into question, and then re-examine the hypothesized involvement of DA in food addiction in light of this revised view of DA function. Finally, food addiction and binge eating will be considered from an evolutionary perspective, in terms of the motivational subsystems that are involved in adaptive patterns of food consumption and seeking behaviors, and a consideration of how these could be altered in pathological conditions.
Conceptual Problems with Dopamine Hypothesis of “Reward”: What is “Reward”, anyway?
Of course, when the term “reward” is used as a noun, essentially as a synonym for “reinforcer”, its meaning is clear (e.g. “The child received a reward at school.”). Also, when it is used as a verb to signify the presentation of a reinforcer, that also has a clear meaning (e.g. “The child was rewarded for good behavior”). However, when someone uses the term “reward” to signify a psychological process that is mediated by neural mechanisms, there are several problems. There is no standard scientific meaning of this term, when used in this way. As noted before (19,20,21,22,23,24), people tend to use the term “reward” in one of three ways: a) as a synonym for pleasure or hedonia, b) as a general term for appetitive motivation, and c) as a synonym for reinforcement. To obfuscate things even more, at times the term “reward” is used to refer to some hybrid process that includes combinations of a, b and c above, and in some cases, it can be used to mean different things in different parts of the same article. One of the difficulties with this is that when articles only use the term “reward”, it is not clear which of these definitions is being employed. It is arguable that the psychological language would be clearer if, when one meant pleasure, one would use words like pleasure or hedonia, rather than “reward”. Similarly, since reinforcement has been the accepted psychological term in the lexicon of instrumental conditioning for almost a century, it is reasonable to suggest that when one is attempting to refer to reinforcement (in the sense of strengthening responses during acquisition and maintenance of instrumental behavior), then that term should be used. The same is true for appetitive motivation, which is arguably a more useful term as a general descriptor of the processes regulating approach or “seeking” behavior (i.e., increasing the proximity, probability or availability of stimuli) as well as consumption or “taking”. The term appetitive motivation does not necessarily or explicitly connote subjective pleasurable emotions, per se, and provides a clear contrast with the term aversive motivation. Is pleasure isomorphic with appetitive motivation and reinforcement? Aren’t these just three different ways of saying exactly the same thing? No. It is clear from the behavioral neuroscience literature that hedonia, appetitive motivation, and positive reinforcement learning are dissociable processes. Indeed, much of the work from Berridge, Robinson and colleagues over the last 20 years serves as a testament to the dissociability of these processes (e.g. 25,26,27,28,29). In view of this research, ambiguities about the definition of “reward” are not merely some esoteric semantic exercise; words are tools as much as any scientific technique, and the term “reward”, when used to refer to a neurobehavioral process, is a very blunt instrument. While one paper may maintain that DA systems mediate “reward” but not hedonia, other papers use “reward” and hedonia synonymously. Exactly what is meant when a paper states that palatable food “activates the reward system” (e.g. 30)? Clearly, one can question the utility of the concept that DA mediates “reward”, and that this involvement has anything to do with food addiction, when one considers that “reward” can be made to mean so many different things.
Does DA Mediate Hedonia Instigated by Food Consumption?
For many years, it was thought that DA, especially mesolimbic DA, mediated pleasure or hedonia. One still can hear this mentioned at scientific meetings, or see it referred to in an article, including one that is focused on phenomena related to food addiction/binge eating (e.g. 31). Nevertheless, evidence has been accumulating for some time indicating that this is a grossly oversimplified view of DAergic function. Although consumption or preference of sucrose solutions is sometimes used as a measure of food-related hedonia (32), there are several reasons why consumption measures per se are not a reliable index of the emotional state (28,33). If sucrose consumption is impaired by a particular manipulation, it could reflect changes in a number of different processes, including oral motor function, approach to the drinking tube, or sensorimotor processes (18,28,33). One of the tests that has become widely accepted as a measure hedonic reactivity to sweet solutions is the taste reactivity paradigm. An enormous body of work from Berridge and colleagues has demonstrated that systemic administration of DA antagonists, as well DA depletions in whole forebrain or nucleus accumbens, do not blunt appetitive taste reactivity for food (25,26,28,34). Moreover, microinjections of amphetamine into nucleus accumbens, which elevates extracellular DA, failed to enhance appetitive taste reactivity for sucrose (29). Sederholm et al. (33) reported that D2 receptors in the nucleus accumbens shell regulate aversive taste reactivity, and that brainstem D2 receptor stimulation suppressed sucrose consumption, but neither population of receptors mediated the hedonic display of taste.
Because of the proposed similarity between food addiction and drug abuse, it is relevant to examine the literature on the effects of DA antagonism on drug-induced hedonic responses in humans. Several years ago, it was reported that DA antagonism could blunt the subjective pleasure induced by amphetamine (35). Since that time, the preponderance of evidence in this area has failed to support this observation. Gawin (36) found that the DA D2 antagonist haloperidol did not reduce the self-reported euphoria produced by cocaine, and several subsequent studies have shown that DA D2 antagonism did not blunt stimulant-induced high or euphoria (37,38). The D1 antagonist ecopipam did not reduce the subjective euphoria induced by cocaine (39). Inhibition of catecholamine synthesis induced by tyrosine/phenylalanine depletion did not blunt the subjective euphoria induced by cocaine (40). In a recent study, catecholamine synthesis inhibition failed to reduce the self-reported craving and hedonic reactions to cigarettes, though it did reduce progressive ratio operant responding for this reinforcer (41). Liggins et al. (42) found that L-DOPA, which enhances catecholamine synthesis, did not have any effect upon self-reported positive mood. Interestingly, a recent imaging paper showed that doses of L-DOPA that enhanced the striatal representation of appetitively motivated actions did not affect the neural representation of reinforcement value (43).
In the literature related to food addiction and binge eating, it is reported that repeated exposure to sucrose in a manner that instigates binge consumption is associated with increases in mesolimbic DA activity (10,44). Moreover, this response does not habituate in animals exposed to the conditions that induce bingeing upon sucrose. To researchers who are not familiar with the specifics of DAergic involvement in aspects of motivation, this finding could be misinterpreted as signifying a simple state of hedonia or pleasure. But as pointed out by Avena et al. (10), this finding is much more complicated, because DA release is involved in many features of appetitive motivation, including “food seeking and reinforcement of learning, incentive motivation, stimulus salience and signaling a stimulus change” (p 22). Indeed, the literature describing the response of mesolimbic DA to appetitive stimuli that act as positive reinforcers is complicated, and needs to be interpreted with caution (45). In a general sense, does food increase DA neuron activity or accumbens DA release? It depends upon the conditions. There are a number of methods that are used to study DA release, which provide measures across various timescales (45–59). Electrophysiology methods record fast phasic activity of putative or identified DA neurons (46, 55–59), while voltammetry methods (e.g. fast cyclic voltammetry) record DA “transients” that are fast phasic changes in extracellular DA that are thought to represent the release from bursts of DA neuron activity (45,47,51). In contrast, microdialysis methods provide a measure of extracellular DA that is integrated over larger units of time (i.e., slow phasic activity) relative to electrophysiology or voltammetry (45,48,49,52–54). Electrophysiology studies have shown that presentation of novel food reinforcers is accompanied by increases in activity of putative ventral tegmental DA neurons, but that this effect goes away with repeated exposure or training (46). In a paper employing voltammetry methods to measure rapid and transient changes in DA release, Roitman et al. (47) showed that, in trained animals, exposure to a conditioned stimulus signaling that lever pressing would result in sucrose delivery was accompanied by an increase in DA transients, however, the actual presentation of the sucrose reinforcer was not. DiChiara and colleagues showed that consumption of a 20% sucrose solution increased prefrontal cortex DA release, but did not increase nucleus accumbens DA release (48). However, exposure to novel palatable snack foods significantly increased extracellular DA in nucleus accumbens as measured by microdialysis (48). This increase in extracellular DA rapidly habituated in the accumbens shell, but persisted in the core if food was presented again 24 hrs after initial exposure (48). A recent microdialysis paper demonstrated that presentation and consumption of high carbohydrate food reinforcers in previously exposed rats (i.e., rats that had consumed the food for several weeks) did not produce any change in extracellular DA in accumbens core or shell (49). In contrast, both the acquisition and maintenance of fixed ratio lever pressing was associated with increases in DA release (49). A similar pattern was shown when markers of DA-related signal transduction (c-Fos and DARPP-32) were measured (50). Thus, the animal literature does not support the widely held belief that food presentation per se, including that of palatable foods, increases accumbens DA release across a broad range of conditions. Rather, the response of accumbens DA to food consumption varies depending upon the type of food, the degree of pre-exposure, the environmental context, and the DA terminal region (51).
In this context, it also is worth noting that enhancement of mesolimbic DA activity is not unique to appetitive conditions; this system also is activated by aversive stimuli. Research with animals has shown that a wide array of aversive conditions (e.g. shock, restraint stress, aversive conditioned stimuli, aversive drugs, social defeat stress) can increase nucleus accumbens DA release as measured by microdialysis (52,53,54). In addition, electrophysiological activity of putative or identified DA neurons has been shown to increase in response to aversive stimuli (46, 55,56,57,58,59). Furthermore, imaging methods in human research have demonstrated that the nucleus accumbens/ventral striatum also responds to stress, aversion and hyperarousal/irritability (60,61,62,63,64,65,66). Putting all this together, the finding that conditions promoting sucrose binging also increase nucleus accumbens DA release clearly are significant, but they do not in themselves serve as an unambiguous marker that DA is specifically mediating some type of hedonic emotional experience. Rather, increased DA transmission could be participating in a number of behavioral functions associated with aspects of learning, motivation, emotion or stress.
What is the Role of Mesolimbic DA in Food Motivation?
A thorough review of the involvement of forebrain DA systems in food motivation is beyond the scope of this paper. Nevertheless, several important points are worth emphasizing for the present discussion. First of all, there are multiple DA systems, and different pathways have distinct functions. Pharmacological studies have demonstrated that hypothalamic DA is involved in appetite modulation, and indicate that blockade of hypothalamic DA receptors stimulates food intake (67), while stimulation of DA receptors, specifically in the perifornical hypothalamus, is associated with a suppression of food intake (68,69). Neostriatal DA, particularly in the lateral neostriatum (70,71,72) is particularly important for sensorimotor and motor aspects of food intake, including food handling and oral motor function. Interestingly, it is often assumed that DA depletions or antagonism in the so-called “reward system” (i.e., nucleus accumbens DA) would exert a powerful effect on food intake. However, this is not supported by the literature. Rather, it has been shown several times over the last few decades that, in contrast to neostriatal DA depletions, interference with nucleus accumbens DA transmission by DA depletions or antagonism has little or no effect on food intake (70–75). In fact, food intake is most greatly affected by DA depletions in ventrolateral neostriatum (70,71,72), but there is no evidence that these impairments are related specifically to motivational dysfunctions. Rather, the effects of ventrolateral neostriatal DA depletions on food intake are related to motoric dysfunctions affecting feeding rate and forepaw usage during feeding, and occur in parallel with the induction of oral tremor that has the characteristics of parkinsonian resting tremor (18,71,76).
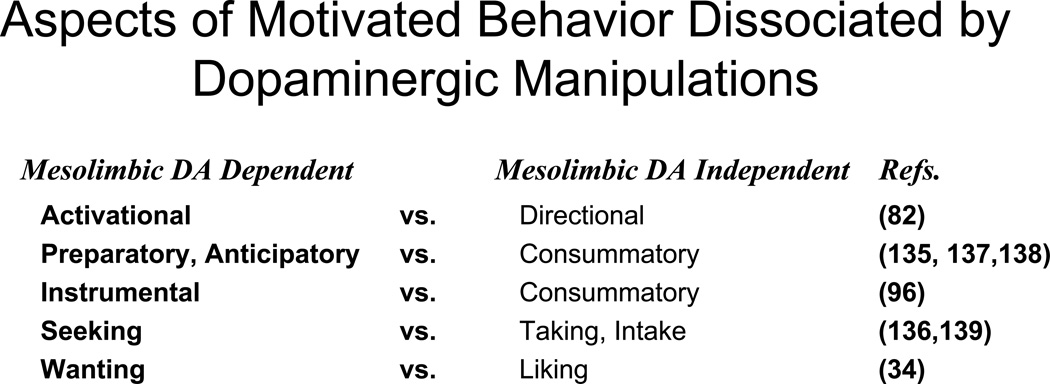
If nucleus accumbens DA does not mediate food-related hedonic reactions, appetite, or food consumption, then what is the nature of its involvement in food motivation? This is a complex question, the study of which has been an area of very intense interest in behavioral neuroscience research over the last few decades. This area is too vast and detailed to cover in the present section, and there have been many reviews published over the last few years that provide a more detailed theoretical discussion (18,19,28,77,78,79,80). Briefly, there is considerable agreement that manipulations affecting nucleus accumbens DA transmission act to dissociate components of food motivation from each other; these manipulations leave core aspects of food-induced hedonia, appetite, or primary food motivation intact, but nevertheless affect critical components of the instrumental (i.e., food seeking) behavior. Within that overall framework, there remains some uncertainty about the best way to characterize the involvement of mesolimbic DA in specific aspects of instrumental behaviors, including food reinforced ones. Investigators have suggested that nucleus accumbens DA is particularly important for behavioral activation (22,24,81,82), pavlovian to instrumental transfer (22,83), flexible approach behavior (79), energy expenditure and regulation (80,84), and responding during delayed reinforcement (85,86). Considerable evidence indicates that accumbens DA is involved in the exertion of effort and the behavioral economics of overcoming work-related response costs in animals (19,77,78, 87,88,89,90), which could be relevant for understanding effort-related motivational symptoms of depression in humans (19,23,91). Pharmacology and imaging studies in humans have shown that striatal areas are involved in making cost/benefit decisions involving effort (92,93,94,95). Several investigators have found it useful to make dichotomous distinctions between aspects of motivation that are impaired by DAergic manipulations and those that are intact (e.g., activational vs. directional aspects of motivation, instrumental vs. consummatory behavior, wanting vs. liking; see Figure 1).
Figure 1.
For several years, researchers have been making distinctions between aspects of motivated behavior that are differentially affected by dopaminergic manipulations. Salamone (82) emphasized the distinction between directional aspects of motivation (i.e., that behavior is directed towards or away from stimuli) and activational aspects of motivation (i.e., that motivated by behavior is characterized by a high degree of activity, vigor and persistence), and suggested that DA antagonists impair activational aspects of food motivation but leave directional aspects intact. Blackburn et al. (135) suggested that DA antagonists impaired preparatory behaviors instigated by a conditioned stimulus at doses that did not impair consummatory behavior. Salamone (96) emphasized the distinction between consummatory behaviors and instrumental responses, and also tried to link this distinction to others by stating that highly active instrumental behaviors elicited and supported by conditioned stimuli are very sensitive to disruption of accumbens DA transmission. Berridge (34) distinguished between the role of DA in “liking” vs. “wanting”, and suggested that interference with striatal and accumbens DA transmission affected incentive motivation (“wanting”) for sucrose, but had little effect on hedonic reactions instigated by sucrose (“liking”). Foltin (136) distinguished between food “seeking” (i.e., instrumental responses reinforced by food) vs. “taking” (consumption), and reported that amphetamine increased food seeking behavior while decreasing food taking behavior. More recently, Ikemoto and Panksepp (137) and Burgdorf and Panksepp (138) stated that it is useful to distinguish between consummatory “reward”, which is relatively unaffected by interference with accumbens DA transmission, and preparatory or anticipatory aspects of “reward”, which are more greatly impaired. Czakowski et al. (139) studied the effects of DA antagonism on ethanol seeking and intake, and reported that the D2 antagonist remoxipride had substantial effects on measures of ethanol seeking behavior (lever pressing) in a dose range that had little effect on ethanol intake.
There is extensive evidence that DA systems, including mesolimbic DA, participate in motivational decision making related to effort, delay and risk (19,88,86). For example, low doses of DA antagonists, as well as accumbens DA antagonism or depletions, bias animals being tested in effort-related choice procedures towards the selection of activities with relatively low work-related response costs (19,78,97,98). These effects do not occur because of changes in appetite or food preference (97,99,100), and are not dependent upon drug effects on delay discounting (86). Knockdown of the DA transporter or administration and amphetamine increase the tendency towards selection of high effort activities (95,101,102).
In view of the studies reviewed in this section, and the vagaries involved in the use of the term “reward”, it appears that any general or unqualified statement that DA mediates “reward” is so oversimplified as to be practically meaningless. Moreover, in view of the frequent connotation of “reward” as referring to subjective pleasure or euphoria, such as statement appears to be largely a misrepresentation of the literature. For these reasons, researchers who are studying food addiction and binge eating in humans need to be careful before assigning any specific meaning to the role that DA may play in this phenomenon.
Dopaminergic Involvement in Motivational Aspects of Food Addiction/Binge Eating: Which aspects?
The complex nature of DAergic involvement in specific aspects of food motivation should present a note of caution for those attempting to determine the role of DA in normal food consumption in humans, as well as food addiction, binge eating, or related phenomena. Clearly, one cannot simply assume that DA directly mediates the pleasure induced by food consumption, and that striatal DA directly controls the emotional basis of the intake of highly palatable foods, which in turn leads to binge eating, and eventually, obesity. Research on the role of striatal and accumbens DA in human food intake appears to be every bit as complex as the animal research described above (103). A review of this emerging area of research is beyond the scope of this paper, but a brief summary can highlight some of the complexities seen in the literature.
O’Doherty et al. (104) reported that DAergic areas of the brain (e.g. midbrain, striatum, orbitofrontal cortex) showed fMRI activation in response to visual cues associated with a sweet taste (glucose), but not to the actual receipt of the reinforcer, a finding similar to much of the animal work described above. Volkow et al. (105) found that an imaging marker of extracellular DA (i.e., displacement of radioactive raclopride binding) was increased in the neostriatum, but not the ventral striatum/nucleus accumbens, of humans who were exposed to a display of food, but did not consume it; this response was correlated with self reports of hunger and desire for food. More recent imaging studies (106) have implicated a wider network of structures in anticipation of the delivery of palatable food, including ventral striatum, amygdala and mediodorsal thalamus, though the most reproducible results were with the amygdala and thalamus. A recent meta-analysis identified the ventral striatum/nucleus accumbens, as well as the amygdala, insula, and orbitofrontal cortex, as being consistently responsive to food-associated cues in fMRI studies (107). The imaging literature related to the neural representation of primary gustatory reinforcement is equivocal about the responsiveness of striatal areas to consumption of palatable foods or sweet tastes. Frontal cortical areas, including orbitofrontal and insula/operculum regions, appear to respond relatively consistently to palatable gustatory stimuli (106,108). In contrast, studies of the responsiveness of ventral or dorsal striatum to palatable foods or sweet tastes have yielded mixed results (104,106,108,109,110). Wang et al. (110) recently used imaging methods to characterize putative markers of DA release in obese subjects. They reported that striatal DA release was not increased in response to food-related stimulation (i.e., seeing, smelling and tasting a palatable food) in obese subjects not diagnosed with binge eating disorder, even if they were treated with methylphenidate, which enhances extracellular levels of DA. Like the O’Doherty et al. (104) study, this is consistent with animal research showing a lack of DAergic responsiveness to palatable foods in organisms with previous experience of that food, and appears to underscore the conclusion that it is difficult to make blanket statements about neostriatal or accumbens DA being released due to food presentation per se.
Interestingly, Wang et al. (110) did observe an increase in striatal DA release in response to food-related stimulation in obese binge eaters who were treated with methylphenidate. Although results can vary depending upon the methods, and this result needs to be replicated to demonstrate its reliability, the fact that these authors directly compared binge eaters and non binge eaters under the same conditions suggests that there may be something different about the striatal DAergic responsiveness of binge eaters. Moreover, these findings are consistent with the microdialysis results showing that increases in accumbens DA release in response to sucrose in binge eating rats does not habituate in the same way as it does in normal rats (6,12). However, interpretation of these results is somewhat complicated by findings from some pharmacology studies. For example, methylphenidate has been shown to decrease appetite in patients diagnosed with binge eating disorder (111). Drugs that facilitate DA transmission have long been known to suppress appetite, and evidence indicates that this effect could be related to actions upon DA in the perifornical hypothalamus (68,69,112). Thus, the specific functional significance of increases in striatal DA release in binge eaters remains uncertain.
It is possible that increased ventral or dorsal striatal DA release in binge eating people does not mediate appetite or food consumption in a general sense, but instead acts to promote food-related learning, modulate quantitative or organizational features of the consummatory response (e.g. speed, compulsiveness, or perseveration), or enhance the tendency to engage in active food seeking behavior. Such a suggestion is consistent with much of the animal work described above (19,24), and is compatible with the observation of Hoebel et al. (2) that mesolimbic DA acts as a kind of “go” mechanism that facilitates appetitive behavior. Moreover, it is consistent with recent research on the involvement of DA in regulating some of the motivational effects of leptin and ghrelin. Leptin is a protein that suppresses food intake, and has actions on a number of brain areas, including mesolimbic DA neurons (113). A recent paper showed that leptin acts on the lateral hypothalamus to regulate body weight, caloric intake, and body fat in rats, but interacts with mesolimbic DA to modulate effort-related responding for food (114). Also, antagonism of DA D1 receptors blocked the ability of ghrelin to stimulate the tendency of rats to work for sucrose on a progressive ratio schedule, but did not affect licking parameters (115). Obesity in itself may be an important factor modulating the tendency to work for food. Berthoud et al. (116) reported that rats made obese by feeding upon a high fat diet showed a reduced tendency to work for food, and that effort-related responding was restored after gastric bypass surgery. Moreover, human studies involving patients with binge eating disorder indicate that the tendency to work for food is a potentially important measure of food motivation in these individuals, over and above any role of obesity. Nasser et al. (117) used progressive ratio performance reinforced by food access to study the motivation to eat across multiple groups of people (binge eating disorder, obese and lean people who were not binge eaters). They observed that prefeeding people who were not binge eaters with a liquid meal before the operant test session reduced their work output on the progressive ratio schedule. In contrast, prefeeding the people who met criteria for binge eating disorder actually led to increased progressive ratio responding for food access. In addition, in the pre-fed condition, people with binge eating disorder worked on the schedule significantly more than obese people without binge eating disorder. Future research should determine if mesolimbic or neostriatal DA is involved in this strong tendency of binge eaters to show enhanced work output in food seeking behavior.
Comparisons between food addiction and drug addiction often are made in the literature, and although a thorough exploration of these similarities and differences is the focus of other papers, it is worthwhile to highlight some of the recent trends in drug addiction research. It often is stated that many of the brain mechanisms involved in aspects of food motivation also are involved in drug addiction. For example, imaging studies have demonstrated that similar brain areas are activated by food-related and drug-related cues (107). Another finding has been that obese people and drug addicts tend to show reduced expression of DA D2 receptors in striatal areas (118,119). Yet despite these findings, it is still not clear what these DA receptor data represent from a functional perspective. “Reward” deficiency has been suggested, but other explanations also are plausible, including psychomotor retardation (120), impaired behavioral flexibility or impulsivity, or reduced tendency to expend energy by exercising (80). Striatal D2 receptors were reported to be decreased in obese rats, and the development of compulsive eating was further enhanced by knockdown of D2 receptors (31). In summarizing their conclusion, the authors suggested that this meant that hedonic mechanisms were being altered, and supported this by pointing out that intracranial self-stimulation thresholds were increased in obese animals. However, it should not be assumed that this measure is an unambiguous marker of “anhedonia”. For example, attaching a ratio work requirement also increases self-stimulation thresholds (121). Moreover, a recent paper has emphasized that multiple factors contribute to self-stimulation thresholds, and has demonstrated that DAergic modulation of self-stimulation thresholds is not due to drug-induced changes in reward value, but instead involves shifts in the tendency to overcome response costs, such as effort or opportunity costs (122).
Emerging Concepts of DA Function and their Relation to Food Addiction
Based upon the literature reviewed above, one can see that there is a behavioral pattern that can be observed in humans and other animals, which involves binge eating and or escalating consumption of food, compulsive patterns of food-related behavior, and resistance to the consequences of this activity. Some have labeled this as food addiction, whereas others are more cautious in the use of this term; some stress the role that this pattern may play in obesity, while others emphasize the myriad of other factors that contribute to obesity. At the very least, this pattern of behavior is present in a subset of people, some of whom are obese, while others are not. Thus, despite some of the ambiguities and concerns about this research, or the specific labels used, a pattern of eating behavior with some of the features of addiction is indeed discernable in some humans, and some of these features can be modeled in animals. The discussion above has focused less on the details of the food addiction literature, and more on the potential role of DA systems. In particular, the review emphasized that the involvement of DA in food motivation is highly selective for particular aspects, and that the effects of DAergic manipulations are dissociative in nature, profoundly affecting some aspects of food motivation while leaving others basically intact (18,19,23). Acknowledging these complexities in DAergic function (123), which is more nuanced than simply painting DA with the broad brush of being a mediator of “reward” or hedonia, can shed light on the potential involvement of DA systems in normal food-related behaviors, as well as obesity, food addiction, binge eating, and related phenomena.
In the last few years, much of the addiction literature has moved beyond the original DA hypothesis of “reward”. There has been a growing emphasis on the idea that addiction should be viewed as a compulsive incentive habit (22,124). According to this view, chronic drug exposure promotes the shift from drug-related action-outcome reinforcement to a compulsive response habit, and this process involves a shift from ventral to dorsal striatal regulation, as well as a transition from prefrontal executive control to striatal habit-related (i.e., stimulus-response) control (125). Viewed in this way, compulsive eating and drug addiction could be seen as belonging to a larger group of impulsive-compulsive syndromes, which also includes attention deficit hyperactivity disorder, obsessive-compulsive disorder, and compulsive gambling (126). This type of disorder is thought to be characterized by a failure of neural mechanisms involved in inhibitory control, which ultimately manifests itself as an escalating pattern of consumption and repeated relapse. Future studies with humans, and in animal models (127), will be necessary to extend some of these emerging concepts from the drug addiction literature into the realm of compulsive eating and food addiction. Additional research in the drug abuse field has identified individual differences in behavioral patterns shown by rats during pavlovian approach conditioning, which are related to the propensity to self-administer drugs. Rats that show greater response to conditioned cues (sign-trackers) display different patterns of DAergic adaptation to training as compared to animals that are more responsive to the primary reinforcer (goal trackers; 128). It has been suggested that these distinct behavioral patterns may be related to the differential vulnerability of some individuals towards drug addiction (129). Moreover, sign trackers are more sensitive to cue-induced reinstatement of food seeking behavior, which may be related to the effect that food-related cues have on people with compulsive eating disorders (130).
As described above, DA systems are involved in several aspects of motivational decision making. In dealing with a complex environment, organisms must assign relative value to, or establish preferences between, different stimuli or activities. This process can be very specific, because aspects of the neural mechanisms involved in selection of food, vs. water, vs. sexual activity, are distinct from each other (indeed, there are multiple types of hunger, and multiple types of thirst). On the other hand, there are neural mechanisms involved in aspects of reinforcement seeking that are more general, and span multiple types of reinforcers; for example, mesolimbic DA is involved in reinforcer-seeking behavior for food, water and sex (18,77,78). Ultimately, organisms must reconcile their preference structure with their behavioral output, and allocate time and effort resources across multiple options. People who are addicts, whether drug addicts or ones addicted to foods, end up showing a radically altered preference structure for motivational stimuli, with selection of the particular drug or food taking a distorted position atop the hierarchy of possible motivational stimuli. Drug addicts, for example, can show markedly reduced behavioral activation in response to natural reinforcers and can display psychomotor retardation during drug withdrawal (120), but they also allocate a disproportionate amount of behavioral and financial resources into drug seeking. This exaggerated effort invested in stimulus seeking in addicts may involve DA, though it also is possible that it is relatively DA independent, relying instead upon the recruitment of other components of the brain circuitry involved in motivation. Nevertheless, these patterns of behavior indicate that the addiction process appears to be characterized by a substantial restructuring of both directional and activational aspects of motivation (131).
Conclusions
An Evolutionary Perspective
This issue of food addiction has vast public policy implications (132), and having considered food addiction, binge eating, and related phenomena from medical, neurobiological and behavioral neuroscience perspectives, it is useful to finish by speculating about the evolutionary significance of this pattern of behavior. First of all, why was this behavioral pattern not selected against by natural selection processes? In contemplating this question, it is useful to remember that only a few thousand years ago, humans were hunter-gatherers. It seems unlikely that the capacity for binge eating in adolescence, for example, would have conveyed enough evolutionary disadvantages such that it would have prevented most people from reaching reproductive age. Perhaps the only disadvantages would have been social, or the ill effects of eating too much after a period of extended starvation. On the other hand, one can argue that the capacity to engage in some type of binge consumption would have had adaptive advantages for hunter-gatherers, who often comb wide areas foraging for food, hunting animals and collecting fruits, nuts and plants for consumption. Under these conditions, periods of relative scarcity of food would have alternated with periods of food excess. Although drying of fruits and smoking of meats or fish was available to humans for several thousand years before the agricultural revolution, it is still true that for many thousands of years before that, there was no way to store food for long periods. Therefore, food had to be consumed when available. In this context, the capacity to engage in overconsumption appears to provide some evolutionary advantages. A group of hunter-gatherers who had been through a period of deprivation could suddenly come upon a herd of game, or a dense patch of berry bushes, and the ability to consume beyond fullness, even to the point of discomfort, would have enabled them make better use of available resources by storing excess calories as fat. Thus, having central and peripheral mechanisms that enable this type of activity to occur would probably have conveyed some advantages. Of course, modern humans do not live this kind of existence; most of us do not burn thousands of calories each day foraging for food, and in the developed world, food is hardly scarce for most people. Thus, a pattern of behavior that may have conveyed some advantages in this evolutionary context has distinct disadvantages in a modern world in which calorically dense foods are available continuously, and people are constantly bombarded by food-related stimuli (see the discussion of what is sometimes called “evolutionary mismatch” in refs. 133, 134). Dieting (in the sense of excessive caloric restriction) may act to stimulate the natural physiological response to food scarcity, and ultimately lead to rebound periods of over eating. Perhaps the tendency to over eat every once in a while is present in virtually all of us; we can struggle to resist gorging ourselves at holiday parties or harvest festivals, and try to rely on inhibitory control mechanisms to limit the impact of this tendency. However, for various reasons, individuals vary in the extent to which these control mechanisms work, and the result is that some people eventually experience an escalating tendency to have consumption of some foods spiral out of control. Ultimately, an understanding of food addiction and related conditions may depend upon our understanding of evolutionary, cultural and anthropological factors as well as neurobiological and psychological ones.
Acknowledgments
Dr. Salamone is supported by grants from the National Institute of Mental Health (MH078023), the National Institute of Drug Abuse (U01DA016194), Pfizer and Hoffman La Roche, and has received an honorarium from Hoffman La Roche. Dr. Correa is supported by a grant from Fundació Bancaixa/ U. Jaume I. (P1.1B2010-43).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
There are no other biomedical financial interests or conflicts to report.
References
- 1.Randolph TG. The descriptive features of food addiction-addictive eating and drinking. Quart J Stud Alcohol. 1956;17:198–224. [PubMed] [Google Scholar]
- 2.Hoebel BG, Avena NM, Bocarsly ME, Rada P. Natural addiction: a behavioral and circuit model based on sugar addiction in rats. J Addict Med. 2009;3:33–41. doi: 10.1097/ADM.0b013e31819aa621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52:430–436. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Meule A. How prevalent is food addiction? Front Psychiat. 2011;2:61. doi: 10.3389/fpsyt.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albayrak O, Wölfle SM, Hebebrand J. Does Food Addiction Exist? A phenomenological discussion based on the psychiatric classification of substance-related disorders and addiction. Obes Facts. 2012;5:165–179. doi: 10.1159/000338310. [DOI] [PubMed] [Google Scholar]
- 6.Avena NM, Gearhardt AN, Gold MS, Wang GJ, Potenza MN. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based upon limited data. Nat Rev Neurosci. 2012;13:514. doi: 10.1038/nrn3212-c1. [DOI] [PubMed] [Google Scholar]
- 7.Ziauddeen H, Farooqi IS, Fletcher PC. Food addiction: is there a baby in the bathwater? Nat Rev Neurosci. 2012;13:514. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- 8.Corsica JA, Pelchat ML. Food addiction: True or False? Curr Opin Gastroenterol. 2010;26:165–169. doi: 10.1097/MOG.0b013e328336528d. [DOI] [PubMed] [Google Scholar]
- 9.Corwin RL. The face of uncertainty eats. Curr Drug Abuse Rev. 2011;4:174–181. doi: 10.2174/1874473711104030174. [DOI] [PubMed] [Google Scholar]
- 10.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bocarsly ME, Berner LA, Hoebel BG, Avena NM. Rats that binge eat fat-rich food do not show somatic signs or anxiety associated with opiate-like withdrawal: implications for nutrient-specific food addiction behaviors. Physiol Behav. 2011;104:865–872. doi: 10.1016/j.physbeh.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zilberter T. Food addiction and obesity: Do macronutrients matter? Front Neuroenerget. 2012;4:7. doi: 10.3389/fnene.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blundell JE, Finlayson G. Food addiction not helpful: the hedonic component - implicit wanting – is important. Addiction. 2011;106:1216–1218. doi: 10.1111/j.1360-0443.2011.03413.x. [DOI] [PubMed] [Google Scholar]
- 14.Rogers PJ, Smit HJ. Food craving and food "addiction": a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav. 2000;66:3–14. doi: 10.1016/s0091-3057(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 15.Epstein DH, Shahan Y. Cheesecake-eating rats and the question of food addiction. Nat Neurosci. 2010;13:529–531. doi: 10.1038/nn0510-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broft A, Shingleton R, Kaufman J, Liu F, Kumar D, Slifstein M, Abi-Dargham A, Schebendach J, Van Heertum R, Attia E, Martinez D, Walsh BT. Striatal dopamine in bulimia nervosa: A PET imaging study. Int J Eat Disord. 2012;45:648–656. doi: 10.1002/eat.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- 18.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 19.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 20.White NM. Reward or reinforcement: what's the difference? Neurosci Biobehav Rev. 1989;13:181–186. doi: 10.1016/s0149-7634(89)80028-4. [DOI] [PubMed] [Google Scholar]
- 21.Stellar JR. Reward. In: Winn P, editor. Dictionary of Biological Psychology. London: Routledge; 2001. p. 679. [Google Scholar]
- 22.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 23.Salamone JD. Will the last person who uses the term 'reward' please turn out the lights? Comments on processes related to reinforcement, learning, motivation, and effort. Addic Biol. 2006;11:43–44. doi: 10.1111/j.1369-1600.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- 24.Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 26.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 27.Berridge KC, Kringlebach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 29.Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erlanson-Albertsson C. How palatable food disrupts appetite regulation. Basic Clin Pharmacol Toxicol. 2005;97:61–73. doi: 10.1111/j.1742-7843.2005.pto_179.x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PM, Kenney PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YW, Rada PV, Bützler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Sederholm F, Johnson AE, Brodin U, Södersten P. Dopamine D(2) receptors and ingestive behavior: brainstem mediates inhibition of intraoral intake and accumbens mediates aversive taste behavior in male rats. Psychopharmacology. 2002;160:161–169. doi: 10.1007/s00213-001-0966-1. [DOI] [PubMed] [Google Scholar]
- 34.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 35.Gunne LM, Anggard E, Jonsson LE. Clinical trials with amphetamine-blocking drugs. Psychiatr Neurol Neurochir. 1972;75:225–226. [PubMed] [Google Scholar]
- 36.Gawin FH. Neuroleptic reduction of cocaine-induced paranoia but not euphoria? Psychopharmacology. 1986;90:142–143. doi: 10.1007/BF00172886. [DOI] [PubMed] [Google Scholar]
- 37.Brauer LH, De Wit H. High dose pimozide does not block amphetamine-induced euphoria in normal volunteers. Pharmacol Biochem Behav. 1997;56:265–272. doi: 10.1016/s0091-3057(96)00240-7. [DOI] [PubMed] [Google Scholar]
- 38.Wachtel SR, Ortengren A, de Wit H. The effects of acute haloperidol or risperidone on subjective responses to methamphetamine in healthy volunteers. Drug Alcohol Depend. 2002;68:23–33. doi: 10.1016/s0376-8716(02)00104-7. [DOI] [PubMed] [Google Scholar]
- 39.Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology. 2001;155:338–347. doi: 10.1007/s002130100724. [DOI] [PubMed] [Google Scholar]
- 40.Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci. 2005;119:1619–1627. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- 41.Venugopalan VV, Casey KF, O'Hara C, O'Loughlin J, Benkelfat C, Fellows LK, Leyton M. Acute phenylalanine/tyrosine depletion reduces motivation to smoke cigarettes across stages of addiction. Neuropsychopharmacology. 2011;36:2469–2476. doi: 10.1038/npp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liggins J, Pihl RO, Benkelfat C, Leyton M. The dopamine augmenter L-DOPA does not affect positive mood in healthy human volunteers. PloS One. 2012;7:e28370. doi: 10.1371/journal.pone.0028370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guitart-Masip M, Huys QJ, Fuentemilla L, Dayan P, Duzel E, Dolan RJ. Go and no-go learning in reward and punishment: Interactions between affect and effect. Neuroimage. 2012;62:154–166. doi: 10.1016/j.neuroimage.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avena NM, Bocarsly ME, Hoebel BG. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol. 2012;829:351–365. doi: 10.1007/978-1-61779-458-2_23. [DOI] [PubMed] [Google Scholar]
- 45.Hauber W. Dopamine release in the prefrontal cortex and striatum: temporal and behavioural aspects. Pharmacopsychiatry. 2010;43:S32–S41. doi: 10.1055/s-0030-1248300. [DOI] [PubMed] [Google Scholar]
- 46.Schulz W. Multiple functions of dopamine. Biol Rep. 2010;2:2. doi: 10.3410/B2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segovia KN, Correa M, Salamone JD. Slow phasic changes in nucleus accumbens dopamine release during fixed ratio acquisition: a microdialysis study. Neuroscience. 2011;96:178–188. doi: 10.1016/j.neuroscience.2011.07.078. [DOI] [PubMed] [Google Scholar]
- 50.Segovia KN, Correa M, Lennington JB, Conover JC, Salamone JD. Changes in nucleus accumbens and neostriatal c-Fos and DARPP-32 immunoreactivity during different stages of food-reinforced instrumental training. Eur J Neurosci. 2012;35:1354–1367. doi: 10.1111/j.1460-9568.2012.08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCullough LD, Sokolowski JD, Salamone JD. A neurochemical and behavioral investigation of the involvement of nucleus accumbens dopamine in instrumental avoidance. Neuroscience. 1993;52:919–925. doi: 10.1016/0306-4522(93)90538-q. [DOI] [PubMed] [Google Scholar]
- 53.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 54.Young AM. Increased extracellular dopamine in nucleus accumbens in response to unconditioned and conditioned aversive stimuli: studies using 1 min microdialysis in rats. J Neurosci Meth. 2004;138:57–63. doi: 10.1016/j.jneumeth.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Anstrom KK, Woodward DJ. Restraint increases dopaminérgica burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- 56.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci. USA. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 61.Pavic L. Alterations in brain activation in posttraumatic stress disorder patients with severe hyperarousal symptoms and impulsive aggressiveness. Eur Arch Psychiatry Clin Neurosci. 2003;253:80–83. doi: 10.1007/s00406-003-0411-z. [DOI] [PubMed] [Google Scholar]
- 62.Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 63.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs. valence during reward prediction in humans. Hum Brain Mapp. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delgado MR, Jou RL, Phelps EA. Neural systems underlying aversive conditioning in humans with primary and secondary reinforcers. Front Neurosci. 2011;5:71. doi: 10.3389/fnins.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baptista T, Lacruz A, Acosta A, Colasante C, de Quijada M, de Mendoza S, Mendoza JM, Hernández L. Naltrexone does not prevent the weight gain and hyperphagia induced by the antipsychotic drug sulpiride in rats. Appetite. 2000;34:77–86. doi: 10.1006/appe.1999.0284. [DOI] [PubMed] [Google Scholar]
- 68.Leibowitz SF. Catecholaminergic mechanisms of the lateral hypothalamus: their role in the mediation of amphetamine anorexia. Brain Res. 1975;98:529–545. doi: 10.1016/0006-8993(75)90371-6. [DOI] [PubMed] [Google Scholar]
- 69.Raimondi L, Alfarano C, Pacini A, Livi S, Ghelardini C, DeSiena G, Pirisino R. Methylamine-dependent release of nitric oxide and dopamine in the CNS modulates food intake in fasting rats. Br J Pharmacol. 2010;150:1003–1010. doi: 10.1038/sj.bjp.0707170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bakshi VP, Kelley AE. Dopaminergic regulation of feeding behavior: I. Differential effects of haloperidol microinjection in three striatal subregions. Psychobiology. 1991;19:223–232. [Google Scholar]
- 71.Jicha GA, Salamone JD. Vacuous jaw movements and feeding deficits in rats with ventrolateral striatal dopamine depletion: possible relation to parkinsonian symptoms. J Neurosci. 1991;11:3822–3829. doi: 10.1523/JNEUROSCI.11-12-03822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- 73.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand. 1971;(Suppl 367):95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 74.Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- 75.Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 76.Collins-Praino LE, Paul NE, Rychalsky KL, Hinman JR, Chrobak JJ, Senatus PB, Salamone JD. Pharmacological and physiological characterization of the tremulous jaw movement model of parkinsonian tremor: Potential insights into the pathophysiology of tremor. Front Sys Neurosci. 2011;5:49. doi: 10.3389/fnsys.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M. The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine and beyond. J Exp Anal Behav. 2012;97:125–146. doi: 10.1901/jeab.2012.97-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30:16585–16600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beeler J, Frazier CRM, Zhuang X. Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front Neurosci. 2012 doi: 10.3389/fnint.2012.00049. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robbins TW, Koob GF. Selective disruption of displacement behaviour by lesions of the mesolimbic dopamine system. Nature. 1980;285:409–412. doi: 10.1038/285409a0. [DOI] [PubMed] [Google Scholar]
- 82.Salamone JD. Dopaminergic involvement in activational aspects of motivation: effects of haloperidol on schedule induced activity, feeding and foraging in rats. Psychobiology. 1988;16:196–206. [Google Scholar]
- 83.Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- 84.Salamone JD. The actions of neuroleptic drugs on appetitive instrumental behaviors. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. New York: Plenum Press; 1987. pp. 575–608. [Google Scholar]
- 85.Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- 86.Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- 87.Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 88.Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 89.Salamone JD, Wisniecki A, Carlson BB, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience. 2001;105:863–870. doi: 10.1016/s0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 90.Mai B, Sommer S, Hauber W. Motivational states influence effort-based decision making in rats: the role of dopamine in the nucleus accumbens. Cogn Affect Behav Neurosci. 2012;12:74–84. doi: 10.3758/s13415-011-0068-4. [DOI] [PubMed] [Google Scholar]
- 91.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Croxson PL, Walton ME, O'Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurniawan IT, Seymour B, Talmi D, Yoshida W, Chater N, Dolan RJ. Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. J Neurophysiol. 2010;104:313–321. doi: 10.1152/jn.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci. 2012;31:16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salamone JD. Behavioral pharmacology of dopamine systems: A new synthesis. In: Willner P, Scheel-Kruger J, editors. The Mesolimbic Dopamine System: From Motivation to Action. Cambridge, England: Cambridge University Press; 1991. pp. 599–613. [Google Scholar]
- 97.Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- 98.Salamone JD, Arizzi M, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: Contrast between the effects of SKF 83566, raclopride and fenfluramine on a concurrent choice task. Psychopharmacology. 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- 99.Koch M, Schmidt A, Schnitzler HU. Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- 100.Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008;196:565–574. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- 102.Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behav Neurosci. 2009;123:463–467. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Egecioglu E, Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Jerlhag E, Engel JA, Dickson SL. Hedonic and incentive signals for body weight control. Rev Endocr Metab Disord. 2011;12:141–151. doi: 10.1007/s11154-011-9166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 105.Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N. "Nonhedonic" food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 106.Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57:786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 108.Felsted JA, Ren X, Chouinard-Decorte F, Small DM. Genetically determined differences in brain response to a primary food reward. J Neurosci. 2010;30:2428–2432. doi: 10.1523/JNEUROSCI.5483-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 110.Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, Wong CT, Fowler JS. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity. 2011;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, Patte K, Kennedy JL. Dopamine transporter gene (DAT1) associated with appetite suppression to methylphenidate in a case-control study of binge eating disorder. Neuropsychopharmacology. 2007;32:2199–2206. doi: 10.1038/sj.npp.1301348. [DOI] [PubMed] [Google Scholar]
- 112.Adamson TW, Corll C, Svec F, Porter J. Role of perifornical hypothalamic monoamine neurotransmitter systems in anorectic effects of endotoxin. Neuroendocrinology. 2010;91:48–55. doi: 10.1159/000262446. [DOI] [PubMed] [Google Scholar]
- 113.DiLeone RJ. The influence of leptin on the dopamine system and implications for ingestive behavior. Int J Obes (Lond) 2009;33(Suppl 2):S25–S29. doi: 10.1038/ijo.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. 2011;69:668–874. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Overduin J, Figlewicz DP, Bennett-Jay J, Kittleson S, Cummings DA ; Ghrelin increases motivation to eat but does not alter palatability. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00488.2011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berthoud HR, Zheng H, Shin AC. Food reward in the obese and after weight loss induced by calorie restriction and bariatric surgery. Ann N Y Acad Sci. 2012 doi: 10.1111/j.1749-6632.2012.06573.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nasser JA, Evans SM, Geliebter A, Pi-Sunyer FX, Foltin RW. Use of an operant task to estimate food reinforcement in adult humans with and without BED. Obesity. 2008;16:1816–1820. doi: 10.1038/oby.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3:8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley J, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 121.Fouriezos G, Bielajew C, Pagotto W. Task difficulty increases thresholds of rewarding brain stimulation. Behav Brain Res. 1990;37:1–7. doi: 10.1016/0166-4328(90)90066-n. [DOI] [PubMed] [Google Scholar]
- 122.Hernandez G, Breton YA, Conover K, Shizgal P. At what stage of neural processing does cocaine act to boost pursuit of rewards? PLoS One. 2010;5:e15081. doi: 10.1371/journal.pone.0015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav. Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 125.Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- 126.Fernando AB, Robbins TW. Animal models of neuropsychiatric disorders. Annu Rev Clin Psychol. 2011;7:39–61. doi: 10.1146/annurev-clinpsy-032210-104454. [DOI] [PubMed] [Google Scholar]
- 127.de Jong JW, Vanderschuren LJ, Adan RA. Towards an animal model of food addiction. Obes Facts. 2012;5:180–195. doi: 10.1159/000338292. [DOI] [PubMed] [Google Scholar]
- 128.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 129.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–34. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salamone JD. Motor function and motivation. In: Koob G, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Vol. 3. Oxford: Academic Press; 2010. pp. 267–276. [Google Scholar]
- 132.Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011;106:1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spinella M. Evolutionary mismatch, neural reward circuits, and pathological gambling. Intern J Neurosci. 2003;113:503–512. doi: 10.1080/00207450390162254. [DOI] [PubMed] [Google Scholar]
- 134.Allen JS. The Lives of the Brain: Human Evolution and the Organ of Mind. Boston: Harvard University Press; 2009. [Google Scholar]
- 135.Blackburn JR, Phillips AG, Fibiger HC. Dopamine and preparatory behavior: III. Effects of metoclopramide and thioridazine. Behav Neurosci. 1989;103:903–906. doi: 10.1037//0735-7044.103.4.903. [DOI] [PubMed] [Google Scholar]
- 136.Foltin RW. Effects of amphetamine, dexfenfluramine, diazepam, and other pharmacological and dietary manipulations on food "seeking" and "taking" behavior in non-human primates. Psychopharmacology. 2001;158:28–38. doi: 10.1007/s002130100865. [DOI] [PubMed] [Google Scholar]
- 137.Ikemoto S, Panksepp J. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci. 1996;110:331–345. doi: 10.1037//0735-7044.110.2.331. [DOI] [PubMed] [Google Scholar]
- 138.Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev. 2006;30:173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 139.Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]