Abstract
α,β-Unsaturated γ-amino esters can be synthesized efficiently and stereoselectively through phosphine-catalyzed γ-umpolung additions of sulfonamides to γ-substituted allenoates. The structures of the sulfonamide and γ-substituted allenoate partners can be varied to achieve a range of α,β-unsaturated γ-amino esters with potentially interesting chemical and biological properties.
Keywords: phosphine, γ-umpolung addition, γ-substituted allenoate, stereoselective synthesis
α,β-Unsaturated γ-amino acids are structural motifs within many peptide natural products and related congeners and molecules containing them can display a wide range of biological and pharmaceutical activities.1 For example (Figure 1), syringolin A, isolated from strains of the plant pathogen Pseudomonas syringae pv Syringae (Pss), can induce death of hypersensitive cells colonized by powdery mildew in wheat and other cereals.2 Hemiasterlin, isolated from the marine sponge Hemiasterella minor, is a potent in vitro cytotoxin and antimitotic agent.3 Symplostatin 4, isolated from the genus Symploca exhibits cytotoxic activity against HeLa cervical carcinoma cells and HT-29 colon adenocarcinoma cells.4 In addition, α,β-unsaturated γ-amino acid derivatives are versatile synthons for the preparation of various γ-amino acids.5 They have also been applied as key intermediates in the synthesis of complex peptide natural products.6 Classical procedures for the syntheses of α,β-unsaturated γ-amino acids include Wittig olefination7a and Horner–Wadsworth–Emmons olefination,7b reactions that are not particularly environmentally friendly because they produce stoichiometric amounts of by-products. Consequently, the development of new strategies for the syntheses of α,β-unsaturated γ-amino acids—particularly stereoselective transformations—remains a challenge.
Figure 1.
Representative natural products containing α,β-unsaturated γ-amino acid moieties.
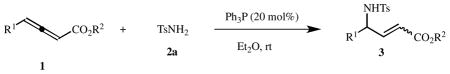
Nucleophilic phosphine organocatalysis has grown significantly over the last 20 years to become one of the most powerful tools for the synthesis of cyclic and acyclic compounds.8 Although phosphine-catalyzed γ-additions of amines to allenoates and alkynoates have been reported sporadically,9 the preparation of α,β-unsaturated γ-amino esters through phosphine-catalyzed γ-additions of sulfonamides to γ-substituted allenoates has not been studied systematically. Herein, we report the results of an investigation into the stereoselective syntheses of α,β-unsaturated γ-amino esters through phosphine-catalyzed γ-umpolung additions of sulfonamides to γ-substituted allenoates under mild conditions.10 We ran these reactions in Et2O at room temperature without any other additives, obtaining the products in high yields and with high stereoselectivity.11
We chose ethyl penta-2,3-dienoate (1a) and p-toluenesulfonamide (2a) as reaction partners in our initial search for suitable conditions for the synthesis of their corresponding α,β-unsaturated γ-amino ester (3a). We were disappointed to observe [thin-layer chromatography (TLC)] no desired product when using either tribenzylphosphine or trimethylphosphine as the catalyst (Table 1, entries 1 and 2). The target product was formed, however, in 93% yield with high E/Z selectivity (93:7) in the presence of 40 mol% of Ph3P (Table 1, entry 3). Tris(4-fluorophenyl)phosphine, tris(4-chlorophenyl)phosphine, and ethyldiphenylphosphine were also effective catalysts for this γ-umpolung addition (Table 1, entries 4, 5, and 8, respectively). In contrast, tris[4-(trifluoromethyl)phenyl]phosphine and tri(2-furyl)phosphine were not useful for this reaction (Table 1, entries 6 and 7). In addition, we did not observe any product when using the nucleophilic amines triethylamine and 4-(N,N-dimethylamino)pyridine (DMAP) as additives (Table 1, entries 9 and 10). Although MeCN, tetrahydrofuran (THF), and toluene were all suitable solvents for this reaction (Table 1, entries 11–13), using Et2O as the solvent (Table 1, entry 14) gave 3a in the highest isolated yield (94%) and with the highest E/Z selectivity (98:2). For a reaction time of 24 h, we found that 20 mol% of Ph3P was superior to 40 mol% (Table 1, entries 14 and 15). With 20 mol% of Ph3P, the reaction was complete within 12 h (Table 1, entry 16). Stopping the reaction at 10 h provided a much lower product yield (Table 1, entry 17). Use of 10 mol% catalyst extended the reaction time (to 48 h) and lowered the product yield (Table 1, entry 18). The efficiency of the reaction decreased dramatically upon increasing of ratio of 2a to 1a (Table 1, entries 20–22). Thus, the optimal conditions for this γ-umpolung addition involved a 1.5:1 ratio of the allenoate to the sulfonamide, with 20 mol% of Ph3P as the catalyst, in Et2O at room temperature.
Table 1.
Evaluation of conditions for the formation of product 3aa.

| |||||
|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | Solvent | Time (h) | Yield of 3a (%)b | E/Zc |
| 1 | (PhCH2)3P (40) | CH2Cl2 | 24 | 0 | – |
| 2 | Me3P (40) | CH2Cl2 | 24 | 0 | – |
| 3 | Ph3P (40) | CH2Cl2 | 14 | 93 | 93:7 |
| 4 | (4-ClPh)3P (40) | CH2Cl2 | 16 | 79 | 94:6 |
| 5 | (4-FPh)3P (40) | CH2Cl2 | 24 | 89 | 93:7 |
| 6 | (4-CF3Ph)3P (40) | CH2Cl2 | 24 | 0 | – |
| 7 | (2-furanyl)3P (40) | CH2Cl2 | 24 | 0 | – |
| 8 | Ph2PEt (40) | CH2Cl2 | 24 | 84 | 93:7 |
| 9 | Et3N (20) | CH2Cl2 | 24 | 0 | – |
| 10 | DMAP (20) | CH2Cl2 | 24 | 0 | – |
| 11 | Ph3P (40) | CH3CN | 24 | 52 | 76:24 |
| 12 | Ph3P (40) | THF | 24 | 77 | 92:8 |
| 13 | Ph3P (40) | toluene | 24 | 74 | 77:23 |
| 14 | Ph3P (40) | Et2O | 24 | 94 | 98:2 |
| 15 | Ph3P (20) | Et2O | 24 | 96 | 93:7 |
| 16 | Ph3P (20) | Et2O | 12 | 95 | 93:7 |
| 17 | Ph3P (20) | Et2O | 10 | 89 | 93:7 |
| 18 | Ph3P (10) | Et2O | 48 | 82 | 93:7 |
| 20d | Ph3P (20) | Et2O | 24 | 75 | 89:11 |
| 21e | Ph3P (20) | Et2O | 24 | 52 | 89:11 |
| 22f | Ph3P (20) | Et2O | 24 | 34 | 87:13 |
Unless noted otherwise, the reaction of 1a (0.3 mmol) and 2a (0.2 mmol) was performed in 2 mL of solvent at room temperature.
Isolated yield based on 2a.
Determined using 1H NMR spectroscopy.
1a (0.2 mmol) and 2a (0.2 mmol).
1a (0.2 mmol) and 2a (0.3 mmol).
1a (0.2 mmol) and 2a (0.4 mmol).
With these conditions in hand, we screened a number of sulfonamides to explore the scope of the reaction (Table 2). In the presence of various electron-withdrawing (Br, Cl, F, NO2) and -donating (Me, MeO) substituents appended to the benzene ring of the sulfonamide, the γ-umpolung additions proceeded efficiently, giving corresponding α,β-unsaturated γ-amino esters (3) with E/Z stereoselectivities ranging from 90:10 to 94:6 (Table 2, entries 1–7). The position of the substituent(s) on the benzene ring affected both the yield and selectivity (Table 2, entries 8–16). For example, when 2-nitrobenzenesulfonamide was the nucleophilic partner, the yield decreased significantly, forming almost entirely the E product (Table 2, entry 12). Moreover, a disubstituted benzenesulfonamide was also a suitable substrate (Table 2, entry 17). Notably, a heteroaryl ring could replace the benzene ring of the substrate; indeed, we obtained a pyridine-containing α,β-unsaturated γ-amino ester in 81% yield (Table 2, entry 18).
Table 2.
Scope of the reaction of the allene 1a with various sulfonamide 2.a

| |||||
|---|---|---|---|---|---|
| Entry | R | Time (h) | 3 | Yield (%)b | E/Zc |
| 1 | H | 12 | 3a | 95 | 93:7 |
| 2 | 4-Me | 12 | 3b | 96 | 93:7 |
| 3 | 4-MeO | 24 | 3c | 93 | 94:6 |
| 4 | 4-Br | 12 | 3d | 95 | 94:6 |
| 5 | 4-Cl | 9 | 3e | 96 | 93:7 |
| 6 | 4-F | 7 | 3f | 98 | 94:6 |
| 7 | 4-NO2 | 30 | 3g | 92 | 90:10 |
| 8 | 3-Me | 10 | 3h | 95 | 94:6 |
| 9 | 3-Br | 8 | 3i | 93 | 93:7 |
| 10 | 3-Cl | 9 | 3j | 88 | 96:4 |
| 11 | 3-NO2 | 24 | 3k | 88 | 89:11 |
| 12 | 2-NO2 | 26 | 3l | 82 | 99:1 |
| 13 | 2-Me | 16 | 3m | 94 | 91:9 |
| 14 | 2-Cl | 10 | 3n | 97 | 96:4 |
| 15 | 2-F | 9 | 3o | 96 | 91:9 |
| 16 | 2-CO2Me | 24 | 3p | 92 | 94:6 |
| 17 | 2,6-di-Cl | 8 | 3q | 91 | 95:5 |
| 18 |
 (2r) |
8 | 3r | 81 | 93:7 |
Reactions of 1a (0.3 mmol) and 2 (0.2 mmol) were performed in Et2O (2 mL) at room temperature.
Isolated yield based on 2.
Determined using 1H NMR spectroscopy.
To further examine the scope of the reaction, we tested the suitability of a variety of γ-substituted allenoates as electrophilic substrates. The allene moieties bearing short straight-chain alkyl units, namely ethyl and n-propyl groups, gave their corresponding products in 81 and 77% yields, respectively (Table 3, entries 2 and 3). The presence of a longer n-hexyl group decreased the reaction efficiency, but resulted in its product forming as only the E isomer. Branched alkyl groups or an aryl group on the allene moiety were not tolerated (Table 3, entries 5–8). The structure of the ester group did not affect the reaction outcome (Table 3, entries 9–11).
Table 3.
Scope of the reaction of various allenes 1 with the sulfonamide 2a.a
| ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | Time (h) | 3 | Yield (%)b | E/Zc |
| 1 | Me | Et | 12 | 3a | 95 | 93:7 |
| 2 | Et | Et | 24 | 3s | 81 | 94:6 |
| 3 | n-Pr | Et | 48 | 3t | 77 | 94:6 |
| 4 | n-Hex | Et | 72 | 3u | 43 | >99:1 |
| 5 | Ph | Et | 72 | – | – | – |
| 6 | t-Bu | Et | 72 | – | – | – |
| 7 | 2-(4,4-dimethyl)pentyl | Et | 72 | – | trace | N.D. |
| 8 | cyclopentyl | Et | 72 | – | trace | N.D. |
| 9 | Me | Me | 10 | 3v | 93 | 93:7 |
| 10 | Me | PhCH2 | 8 | 3w | 91 | 96:4 |
| 11 | Me | t-Bu | 14 | 3x | 96 | 96:4 |
Reactions of 1 (0.3 mmol) and 2a (0.2 mmol) were performed in Et2O (2 mL) at room temperature.
Isolated yield based on 2.
Determined using 1H NMR spectroscopy.
Scheme 1 displays a mechanism that accounts for the formation of the α,β-unsaturated γ-amino ester 3a. Ph3P acted as a nucleophilic promoter that initiated the reaction and produced the zwitterionic intermediate I, which deprotonated p-toluenesulfonamide (2a) to generate the amide anion II and the vinylphosphonium species III. The amide intermediate II underwent γ-umpolung addition to the activated vinylphosphonium carbon–carbon double bond in III to furnish the ylide IV. The thermodynamic stability of the phosphonium ylide IV is presumably the driving force for the γ-umpolung addition, consistent with our observation that amine bases did not facilitate this reaction. Subsequent proton transfer to form the phosphonium enolate V and β-elimination of the phosphine gave the final product 3a.
Scheme 1.
Mechanism of formation of the α,β-unsaturated γ-amino ester 3a.
We have developed an efficient procedure, based on phosphine-catalyzed γ-umpolung additions of sulfonamides to γ-substituted allenoates, for the formation of α,β-unsaturated γ-amino esters in high yields and with high stereoselectivity. The reactions are run at room temperature, require only a catalytic amount of Ph3P, and do not produce any byproducts; thus, the conditions are mild and the transformations are atom-economical and environmentally friendly. This approach further extends the utility of phosphine catalysis in organic synthesis.
Supplementary Material
Acknowledgments
We thank the NIH (GM071779) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Conroy T, Guo JT, Linington RG, Hunt NH, Payne RJ. Chem Eur J. 2011;17:13544–13552. doi: 10.1002/chem.201102538. [DOI] [PubMed] [Google Scholar]; (b) Clerc J, Schellenberg B, Groll M, Bachmann AS, Huber R, Dudler R, Kaiser M. Eur J Org Chem. 2010:3991–4003. [Google Scholar]; (c) Linington RG, Clark BR, Trimble EE, Almanza A, Uren LD, Kyle DE, Gerwick WH. J Nat Prod. 2009;72:14–17. doi: 10.1021/np8003529. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schaschke N. Bioorg Med Chem Lett. 2004;14:855–857. doi: 10.1016/j.bmcl.2003.12.030. [DOI] [PubMed] [Google Scholar]; (e) Nieman JA, Coleman JE, Wallace DJ, Piers E, Lim LY, Roberge M, Andersen RJ. J Nat Prod. 2003;66:183–199. doi: 10.1021/np020375t. [DOI] [PubMed] [Google Scholar]; (f) Coleman JE, de Silva ED, Kong F, Andersen RJ, Allen TM. Tetrahedron. 1995;51:10653–10662. [Google Scholar]; (g) Hagihara M, Schreiber SL. J Am Chem Soc. 1992;114:6570–6571. [Google Scholar]
- 2.(a) Winkler T, Ruedi P, Dudler R. Mol Plant-Microbe Interact. 1998;11:727–733. [Google Scholar]; (b) Coleman CS, Rocetes JP, Park DJ, Wallick CJ, Warn-Cramer BJ, Michel K, Dudler R, Bachmann AS. Cell Proliferation. 2006;39:599–609. doi: 10.1111/j.1365-2184.2006.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Talpir R, Benayahu Y, Kashman Y, Pannell L, Schleyer M. Tetrahedron Lett. 1994;35:4453–4456. [Google Scholar]; (b) Anderson HJ, Coleman JE, Andersen RJ, Roberge M. Cancer Chemother Pharmacol. 1997;39:223–226. doi: 10.1007/s002800050564. [DOI] [PubMed] [Google Scholar]
- 4.Taori K, Liu Y, Paul VJ, Luesch H. ChemBioChem. 2009;10:1634–1639. doi: 10.1002/cbic.200900192. [DOI] [PubMed] [Google Scholar]
- 5.(a) Hintermann T, Gademann K, Jaun B, Seebach D. Helv Chim Acta. 1998;81:983–1002. [Google Scholar]; (b) Plummer JS, Emery LA, Stier MA, Suto MJ. Tetrahedron Lett. 1993;34:7529–7532. [Google Scholar]; (c) Fu Y, Xu B, Zou X, Ma C, Yang X, Mou K, Fu G, Lu Y, Xu P. Bioorg Med Chem Lett. 2007;17:1102–1106. doi: 10.1016/j.bmcl.2006.11.020. [DOI] [PubMed] [Google Scholar]; (d) Reetz MT. Angew Chem, Int Ed. 1991;30:1531–1546. [Google Scholar]
- 6.(a) Conroy T, Guo JT, Hunt NH, Payne RJ. Org Lett. 2010;12:5576–5579. doi: 10.1021/ol1024663. [DOI] [PubMed] [Google Scholar]; (b) Dai C, Stephenson CRJ. Org Lett. 2010;12:3453–3455. doi: 10.1021/ol101252y. [DOI] [PubMed] [Google Scholar]; (c) Nieman JA, Coleman JE, Wallace DJ, Piers E, Lim LY, Roberge M, Andersen RJ. J Nat Prod. 2003;66:183–199. doi: 10.1021/np020375t. [DOI] [PubMed] [Google Scholar]
- 7.(a) Vedejs E, Kongkittingam C. J Org Chem. 2001;66:7355–7364. doi: 10.1021/jo0104882. [DOI] [PubMed] [Google Scholar]; (b) Hoffman TJ, Dash J, Rigby JH, Arseniyadis S, Cossy J. Org Lett. 2009;11:2756–2759. doi: 10.1021/ol900893e. [DOI] [PubMed] [Google Scholar]
- 8.For selected reviews on phosphine catalysis, see: Lu X, Zhang C, Xu Z. Acc Chem Res. 2001;34:535–544. doi: 10.1021/ar000253x.Methot JL, Roush WR. Adv Synth Catal. 2004;346:1035–1050.Nair V, Menon RS, Sreekanth AR, Abhilash N, Biju AT. Acc Chem Res. 2006;39:520–530. doi: 10.1021/ar0502026.Ye LW, Zhou J, Tang Y. Chem Soc Rev. 2008;37:1140–1152. doi: 10.1039/b717758e.Cowen BJ, Miller SJ. Chem Soc Rev. 2009;38:3102–3116. doi: 10.1039/b816700c.Marinetti A, Voituriez A. Synlett. 2010:174–194.Wei Y, Shi M. Acc Chem Res. 2010;43:1005–1018. doi: 10.1021/ar900271g.Wang SX, Han X, Zhong F, Wang Y, Lu Y. Synlett. 2011:2766–2778.Fan YC, Kwon O. Chem Commun. 2013;49:11588–11619. doi: 10.1039/c3cc47368f.Zhao QY, Lian Z, Wei Y, Shi M. Chem Commun. 2012;48:1724–1732. doi: 10.1039/c1cc15793k.Xu S, He Z. RSC Adv. 2013;3:16885–16904.Fan YC, Kwon O. Chem Commun. 2013;49:11588–11619. doi: 10.1039/c3cc47368f.Wang Z, Xu X, Kwon O. Chem Soc Rev. 2014;43:2927–2940. doi: 10.1039/c4cs00054d.Xiao Y, Sun Z, Guo H, Kwon O. Beilstein J Org Chem. 2014;10:2089. doi: 10.3762/bjoc.10.218.
- 9.(a) Trost BM, Dake GR. J Org Chem. 1997;62:5670–5671. [Google Scholar]; (b) Virieux D, Guillouzic AF, Cristau HJ. Tetrahedron. 2006;62:3710–3720. [Google Scholar]; (c) Lundgren JRXX, Wilsily A, Marion N, Ma C, Chung YK, Fu GC. Angew Chem, Int Ed. 2013;52:2525–2528. doi: 10.1002/anie.201208957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For phosphine-catalyzed γ-umpolung additions of carbon-centered nucleophiles, see: Trost BM, Li CJ. J Am Chem Soc. 1994;116:3167–3168.Lu X, Zhang C. Synlett. 1995:645–646.Chen Z, Zhu G, Jiang Q, Xiao D, Cao P, Zhang X. J Org Chem. 1998;63:5631–5635.Alvarez-Ibarra C, Csaky AG, Oliva CG. J Org Chem. 2000;65:3544–3547. doi: 10.1021/jo9918192.Pakulski Z, Demchuk OM, Frelek J, Luboradzki R, Pietrusiewicz KM. Eur J Org Chem. 2004:3913–3918.Smith SW, Fu GC. J Am Chem Soc. 2009;131:14231–14233. doi: 10.1021/ja9061823.Cardoso AL, Beja AM, Silva MR, de los Santos JM, Palacios F, Abreu PE, Pais AACC, Pinho e Melo TMVD. Tetrahedron. 2010;66:7720–7725.Sinisi R, Sun J, Fu GC. Proc Natl Acad Sci USA. 2010;107:20652–20654. doi: 10.1073/pnas.1003597107.Chen JM, Fang YZ, Wei ZL, Liao WW. Synthesis. 2012;44:1849–1853.Pei CK, Jiang Y, Shi M. Eur J Org Chem. 2012:4206–4216.For phosphine-catalyzed γ-umpolung additions of oxygen-centered nucleophiles, see: Trost BM, Li CJ. J Am Chem Soc. 1994;116:10819–10820.Alvarez-Ibarra C, Csaky AG, Oliva CG. Tetrahedron Lett. 1999;40:8465–8467.Chung YK, Fu GC. Angew Chem, Int Ed. 2009;48:2225–2227. doi: 10.1002/anie.200805377.For phosphine-catalyzed γ-umpolung additions of sulfur-centered nucleophiles, see: Sun J, Fu GC. J Am Chem Soc. 2010;132:4568–4569. doi: 10.1021/ja101251d.Fu GC. Chem Sci. 2011;2:2196–2198. doi: 10.1039/c1sc00414j.
- 11.For selected examples of phosphine-catalyzed reactions from our laboratories, see: Zhu X, Lan J, Kwon O. J Am Chem Soc. 2003;125:4716–4717. doi: 10.1021/ja0344009.Dudding T, Kwon O, Mercier E. Org Lett. 2006;8:3643–3646. doi: 10.1021/ol061095y.Zhu XF, Henry CE, Kwon O. J Am Chem Soc. 2007;129:6722–6723. doi: 10.1021/ja071990s.Henry CE, Kwon O. Org Lett. 2007;9:3069–3072. doi: 10.1021/ol071181d.Vardhineedi S, Barcan GA, Kwon O. J Am Chem Soc. 2007;129:12928–12929. doi: 10.1021/ja073754n.Guo H, Xu Q, Kwon O. J Am Chem Soc. 2009;131:6318–6319. doi: 10.1021/ja8097349.Zhiming W, Castellano S, Kinderman SS, Argueta CE, Beshir AB, Fenteany G, Kwon O. Chem Eur J. 2011;17:649–654. doi: 10.1002/chem.201002195.Martin TJ, Tran YS, Vakhshori VG, Kwon O. Org Lett. 2011;13:2586–2589. doi: 10.1021/ol200697m.Szeto J, Sriramurthy V, Kwon O. Org Lett. 2011;13:5420–5423. doi: 10.1021/ol201730q.Andrews IP, Blank BR, Kwon O. Chem Commun. 2012;48:5373–5375. doi: 10.1039/c2cc31347b.Andrews IP, Kwon O. Chem Sci. 2012;3:2510–2514. doi: 10.1039/C2SC20468A.Zhou QF, Chu XP, Ge FF, Wang Y, Lu T. Adv Synth Catal. 2013;355:2787–2792.Zhou QF, Chu XP, Ge FF, Cheng L, Lu T. Mol Divers. 2013;17:563–571. doi: 10.1007/s11030-013-9456-8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




