Abstract
Introduction
Persistent systemic inflammation is associated with mortality among undernourished, HIV-infected adults starting antiretroviral therapy (ART) in sub-Saharan Africa, but the etiology of these deaths is not well understood. We hypothesized that greater systemic inflammation is accompanied by cardiovascular dysfunction over the first 12 weeks of ART.
Methods
In a prospective cohort of 33 undernourished (body mass index <18.5 kg/m2) Zambian adults starting ART, we measured C-reactive protein (CRP), tumor necrosis factor-α receptor 1 (TNF-α R1), and soluble CD163 and CD14 at baseline and 12 weeks. An EndoPAT device measured the reactive hyperemia index (LnRHI; a measure of endothelial responsiveness), peripheral augmentation index (AI; a measure of arterial stiffness), and heart rate variability (HRV; a general marker of autonomic tone and cardiovascular health) at the same time points. We assessed paired changes in inflammation and cardiovascular parameters, and relationships independent of time point (adjusted for age, sex, and CD4+ T-cell count) using linear mixed models.
Results
Serum CRP decreased (median change -3.5 mg/l, p=0.02), as did TNF-α R1 (-0.31 ng/ml, p<0.01), over the first 12 weeks of ART. A reduction in TNF-α R1 over 12 weeks was associated with an increase in LnRHI (p=0.03), and a similar inverse relationship was observed for CRP and LnRHI (p=0.07). AI increased in the cohort as a whole over 12 weeks, and a reduction in sCD163 was associated with a rise in the AI score (p=0.04). In the pooled analysis of baseline and 12 week data, high CRP was associated with lower HRV parameters (RMSSD, p=0.01; triangular index, p<0.01), and higher TNF- α R1 accompanied lower HRV (RMSSD, p=0.07; triangular index, p=0.06).
Conclusions
Persistent inflammation was associated with impaired cardiovascular health over the first 12 weeks of HIV treatment among undernourished adults in Africa, suggesting cardiac events may contribute to high mortality in this population.
Keywords: cardiovascular, HIV, antiretroviral therapy, nutrition, sub-Saharan Africa, inflammation
Introduction
Sub-Saharan Africa is disproportionately affected by the twin epidemics of HIV infection and chronic undernutrition, and a low body mass index (BMI) at the time of antiretroviral (ART) initiation is associated with increased early morbidity and mortality in HIV patients. In the first 90-180 days of ART, the mortality rate among undernourished HIV-infected adults is over 25% in some settings, which is 4 to 6-fold higher than HIV-infected adults with normal BMI and is independent of CD4 T-cell suppression [1-4]. While the etiology of the high early mortality is likely multifactorial, persistent, heightened systemic inflammation is a major risk factor for death in undernourished, HIV-infected adults starting ART in the region [5, 6].
In individuals with normal BMI and without HIV, increased systemic inflammation is known to correlate with reduced heart rate variability, increased cardiovascular event rates, increased carotid intima-media thickness, coronary artery calcification, ischemic and dilated cardiomyopathies, myocardial infarction, and stroke [7-11]. We hypothesized that a similar link between inflammation and cardiovascular dysfunction, possibly compounded by undernutrition-related factors such as reduced muscle mass and bioavailable phosphate stores, is present in malnourished, HIV-infected adults in sub-Saharan Africa and may contribute to the high rate of early ART mortality in these patients. [12-16]. To investigate this question further, we conducted a prospective pilot study to assess relationships between circulating inflammatory markers and cardiovascular function among undernourished adults with advanced HIV over the first 12 weeks of ART.
Methods
The Mechanisms of Early Mortality in Adults Initiating Antiretroviral Therapy (MEMART) study was nested within the Nutritional Support for African Adults Starting Antiretroviral Therapy (NUSTART) study, which was a prospective, double-blinded, randomized placebo-controlled clinical trial designed to test whether the addition of vitamins and minerals to a lipid-based nutritional supplement would reduce early mortality in ART-eligible, undernourished, HIV-infected patients (trial registration #PACTR201106000300631). The NUSTART study was conducted in Zambia and Tanzania from 2011 to 2013, and the MEMART pilot study prospectively enrolled a convenience sample of Zambian participants between August and December 2012 to evaluate the relationship between systemic inflammation and cardiovascular function.
Potentially eligible participants were evaluated by study staff at local health clinics and referred to University Teaching Hospital in Lusaka, Zambia for enrollment in the parent NUSTART trial and all study procedures. Inclusion criteria were a BMI <18.5 kg/m2, at least 18 years of age, ART- naïve (except for prior receipt of short-term Prevention of Mother To Child Transmission regimens), ART-eligible as determined by CD4+ T-cell count <350 cells/μl or Stage 3 or 4 HIV disease, willing to undertake intensive ART follow-up in the study clinic, and able to provide written or thumb print informed consent. Exclusion criteria were participation in a potentially conflicting research protocol, pregnancy by self-report, or the presence of an acute infectious condition aside from tuberculosis (participants with known tuberculosis needed to be on treatment) or oral candidiasis as assessed by study clinicians. All participants received efavirenz, tenofovir, and emtricitabine as a first-line ART regimen.
At baseline (prior to ART initation) and 12 weeks after ART initiation, measurements of systemic inflammation and oxidative stress were obtained. The inflammatory markers C-reactive protein (CRP), soluble CD163, and tumor necrosis factor-α receptor 1 (TNF-α R1, a soluble receptor of TNF-α that changes in relation to the circulating cytokine level) were measured using ELISA (R&D Systems, Minneapolis, MN USA). Soluble CD14 was also measured using ELISA (Hycult Biotech, Uden, Netherlands). Urine samples were collected for assessment of oxidative stress using the 15-F2t-isoprostane/creatinine ratio (Oxford Biomedical Research, Rochester Hills, MI).
Cardiovascular assessments at baseline and after 12 weeks of ART included seated blood pressure and heart rate, ECGs to measure the corrected QT interval, and EndoPAT (Itamar Medical, Caesarea, Israel) assessment of the reactive hyperemia index (LnRHI; natural-log transformed), peripheral augmentation index (AI), and heart rate variability (HRV, comprising the root mean square of the successive differences [RMSSD], triangular index, high and low frequency variation, and power ratio, which is the ratio of low-frequency to high-frequency oscillations) [17]. The EndoPAT is a portable device to assess peripheral arteriolar tonography, or the reactivity of digital vessels in response to a brief period of ischemia (produced by inflation of a blood pressure cuff) [18]. A LnRHI value below ≤0.51 was previously shown to provides a sensitivity of 82% and a specificity of 77% for diagnosing coronary endothelial dysfunction in a large clinical study [18]. Higher AI values indicate increased arterial stiffness, as measured by the magnitude of the reflected arterial pressure wave during systole [19]. HRV, which was recorded for 5 minutes preceding brachial artery occlusion, is a measure of variations of both instantaneous heart rate and beat-to-beat intervals, and lower HRV is thought to reflect poor autonomic tone which confers increased risk of cardiovascular events and mortality [20, 21].
Participant clinical and demographic characteristics were expressed as percentages or as median values with interquartile range. The change in each variable from recruitment to 12 weeks of ART was assessed using the Wilcoxon signed rank test. Our primary analysis evaluated whether changes in cardiovascular markers were associated with changes in inflammatory markers (i.e., within-patient paired comparison) using ordinary least squares regression, adjusted for the effect of baseline values. As a secondary analysis, we assessed the relationships between inflammatory biomarkers and cardiovascular markers irrespective of time point (baseline or 12 weeks) using a linear mixed model with a random effect for the patient to account for correlation that may arise from measurements taken from the same patient, adjusted for age, sex, and pre-treatment CD4+ T-cell count. Inflammatory markers were log-transformed, and cardiovascular markers remained on the unit scale (with the exception of LnRHI). Analyses were performed using R (version 2.12.1; www.r-project.org). Analysis scripts are posted at biostat.mc.vanderbilt.edu/ArchivedAnalyses.
The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the responsibility for the decision to submit for publication.
Results
Sixty ART-naïve adults were recruited for this study and 33 returned at 12 weeks for the clinical assessment and laboratory studies. Of the 27 patients without week 12 data, one-third (n=9) had never started ART, 3 died, 3 withdrew due to relocation, pregnancy, or injury (road traffic accident), and 12 declined to return for week 12 measurements. Among the 3 deceased patients, one died of encephalitis and sepsis, and the other two died at home of unknown causes. Those included and excluded from the analysis were similar in regards to age, sex, and pre-treatment BMI, serum hemoglobin, and CD4+ T cell count (p>0.05 for all). Among the 33 patients in the analysis cohort, 58% were male, the median age was 36 years (interquartile range [IQR] 31-42 years), and the median CD4+ T cell count was 224 cells/μl (IQR 151-273 cells/μl; Table 1). All participants were undernourished according to WHO criteria (i.e. BMI <18.5 kg/m2), with a median BMI of 16.7 kg/m2 (IQR 15.4-17.6 kg/m2). Over twelve weeks of ART, the median weight gain was 2.5 kg (IQR 0.1-6.0 kg) and the median change in BMI was 0.9 kg/m2 (0.0-1.9 kg/m2). Baseline median CRP level was markedly elevated at 18.3 mg/l and declined to 6.4 mg/l by week 12 (p=0.02), and TNF-α R1 declined from 1.3 to 1.0 ng/ml (p<0.01, Table 1 and Figure).
Table 1. Description of the cohort (n=33).
| Demographic and clinical characteristics | ||
|---|---|---|
| Female sex, n (%) | 14 (42%) | |
| Age (median years, IQR) | 36 (31 - 42) | |
| Baseline BMI (median kg/m2, IQR) | 16.7 (15.4 - 17.6) | |
| Week 12 BMI (median kg/m2, IQR) | 17.6 (16.5 – 19.0)a | |
| Baseline CD4+ count (median cells/μL, IQR) | 224 (151 - 273) | |
| Week 12 CD4+ count (median cells/μL, IQR) | 349 (275 - 426)a | |
| Median serum, urine, and cardiovascular values pre-treatment and after 12 weeks of antiretroviral therapy, median (IQR) | ||
| Variable | Recruitment | Week 12 of ART |
| C-reactive protein (mg/l) | 18.3 (4.5-64.8) | 6.4 (3.8-19.7)a |
| TNF-α receptor 1 (ng/ml) | 1.3 (0.8 – 2.4) | 1.0 (0.6 – 1.3)a |
| Soluble CD163 (ng/ml) | 684 (511 - 1022) | 531 (376 - 1040) |
| Soluble CD14 (μg/ml) | 2.4 (2.1-2.7) | 2.5 (2.0-3.1) |
| Isoprostane-to-creatinine ratio | 0.02 (0.01-0.05) | 0.09 (0.05-0.24)a |
| Augmentation Index | 11.9 (1.0-30.0) | 19.0 (0.1-25.9) |
| Reactive Hyperemia Index | 0.6 (0.3-0.8) | 0.6 (0.4-0.7) |
| QTc (ms) | 434 (421-447) | 431 (417-446) |
| RMSSD (ms) | 26.4 (17.5-41.3) | 37.2 (19.2-71.6)a |
| Triangular Index | 6.8 (5.9-10.6) | 9.6 (6.1-14.5) |
| Low frequency variations (ms2) | 130.2 (85.0-151.9) | 112.9 (76.1-151.6) |
| High frequency variations (ms2) | 214.7 (154.7-260.8) | 232.6 (169.7-327.1) |
| Power Ratio | 0.6 (0.4-0.9) | 0.4 (0.3-0.9) |
| Systolic blood pressure (mm Hg) | 100 (93-110) | 110 (100-120)a |
| Diastolic blood pressure (mm Hg) | 60 (60-70) | 70 (60-70) |
| Heart rate (bpm) | 78 (71-89) | 68 (63-82) |
Abbreviation: BMI, body mass index; QTC, corrected QT interval; RMSSD, root mean square of the successive differences; TNF-α, tumor necrosis factor-α.
Reactive hyperemia index is natural log transformed
p<0.05 for change at 12 weeks
Figure. Changes in inflammation biomarkers and cardiovascular parameters over 12 weeks of antiretroviral therapy.
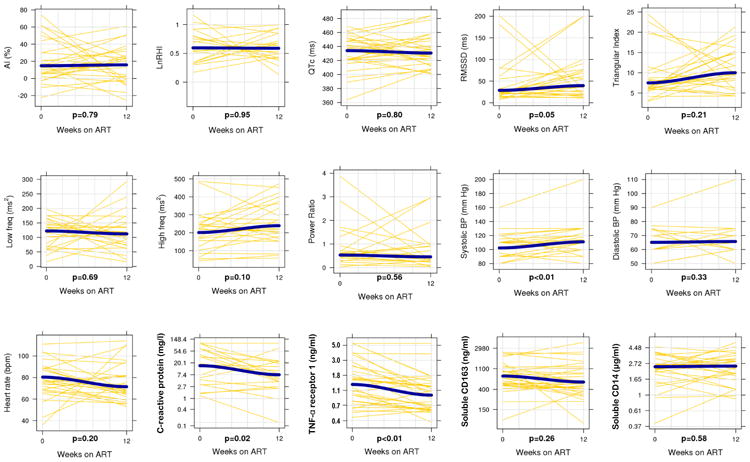
Yellow lines represent paired samples and blue lines represent smoothed (loess) curves fit to the data. As in the statistical models, the inflammation biomarkers are log-transformed and the cardiovascular markers are on the unit scale. Wilcoxon signed rank test was used to assess statistical significance.
In the linear model of paired changes (Table 2), the change in TNF-α R1 over 12 weeks was inversely associated with the LnRHI score (p=0.03), and a similar relationship was observed for CRP (p=0.07), indicating increased endothelial responsiveness as inflammation declined. The change in sCD163 over 12 weeks was also inversely associated with the change in the AI score (p=0.04), indicating increased arterial stiffness was present in those patients with greater reductions in sCD163. Lastly, a reduction in sCD163 and TNF-α R1 over 12 weeks was accompanied by a reduction in the resting heart rate (p=0.06 for both). No other changes in the inflammatory markers were significantly associated with changes in the cardiovascular markers.
Table 2. Linear models of the paired change in cardiovascular markers and inflammation biomarkers over 12 weeks of ART.
| Cardiovascular markers (change in regression outcome) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation biomarkers (change in log- transformed regression predictor) |
Isoprostane-to-Creatinine ratio |
Augmentation Index | Reactive Hyperemia Index | QTc | RMSSD | Triangular Index | Low frequency variations | High frequency variations | Power Ratio | Systolic blood pressure | Diastolic blood pressure | Heart rate |
| C-reactive protein | 0.00, p=0.96 | -2.22, p=0.54 | -0.09, p=0.07 | 1.35, p=0.74 | -5.76, p=0.37 | -1.32, p=0.18 | 5.60, p=0.65 | -22.80, p=0.18 | 0.05, p=0.81 | 1.62, p=0.60 | -0.09, p=0.97 | 3.43, p=0.23 |
| Tumor necrosis factor-α receptor 1 | -0.08, p=0.52 | -6.22, p=0.54 | -0.26, p=0.03 | 9.62, p=0.31 | -10.09, p=0.69 | -2.13, p=0.41 | 12.78, p=0.70 | -38.72, p=0.40 | 0.02, p=0.97 | 0.20, p=0.98 | -3.98, p=0.38 | 14.10, p=0.06 |
| Soluble CD163 | 0.02, p=0.86 | -14.06, p=0.04 | -0.11, p=0.17 | 2.71, p=0.68 | -2.05, p=0.91 | -1.75, p=0.34 | 28.46, p=0.16 | 1.86 p=0.95 | 0.33, p=0.23 | 5.19, p=0.30 | 2.34, p=0.46 | 9.44, p=0.06 |
| Soluble CD14 | -0.03, p=0.83 | -15.37, p=0.25 | -0.05, p=0.77 | -4.08, p=0.73 | 11.25, p=0.76 | 0.41, p=0.91 | 9.91, p=0.79 | -21.07, p=0.72 | -0.03, p=0.96 | -2.51, p=0.78 | 2.45, p=0.67 | 13.31, p=0.17 |
Models estimate the relationship between the change in log-transformed inflammation biomarker and change in cardiovascular marker using ordinary least squares regression, adjusted for baseline values. Bold values represent statistically significant findings.
Abbreviation: QTC, corrected QT interval; RMSSD, root mean square of the successive differences.
Reactive hyperemia index is natural log transformed
The associations between inflammatory markers and cardiovascular markers also were assessed using linear mixed models pooling data from both time points and adjusting for age, sex, and baseline CD4+ T-cell count (Table 3). While this method does not assess whether biomarkers change in conjunction with each other, it detects relationships between biomarkers that may persist over time. CRP was inversely associated with several measurements of HRV: RMSSD (p=0.01), triangular index (p<0.01), and high frequency variations (p=0.02). There was also an inverse relationship between TNF-α R1 and systolic (p=0.05) and diastolic blood pressure (p=0.05), RMSSD (p=0.07), and triangular index (p=0.06). However, TNF-α R1 was positively associated with LnRHI (p=0.01), which was unexpected given the directionality of relationships observed in the linear models of paired changes in inflammatory and cardiovascular variables. Higher heart rate was associated with higher CRP (p<0.01) and soluble CD14 levels (p<0.01). While there was a significant increase in the urine isoprostane/creatinine ratio over 12 weeks, the ratio was not associated with any of the inflammatory biomarkers or with the cardiovascular parameters (data not shown). We did not detect any other significant relationships between inflammatory biomarkers and cardiovascular markers.
Table 3. Regression coefficients estimating the associations between cardiovascular markers and inflammation.
| Cardiovascular markers (regression outcome) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation biomarkers (log- transformed regression predictor) |
Isoprostane-to- Creatinine ratio |
Augmentation Index | Reactive Hyperemia Index | QTc | RMSSD | Triangular Index | Low frequency variations |
High frequency variations |
Power Ratio | Systolic blood pressure |
Diastolic blood pressure |
Heart rate |
| C-reactive protein | 0.01, p=0.95 | -1.36, p=0.06 | 0.02, p=0.37 | -0.77, p=0.90 | -7.03, p=0.01 | -1.73, p<0.01 | -0.32, p=0.86 | -26.35, p=0.02 | 0.18, p=0.18 | -2.51, p=0.28 | -1.93, p=0.16 | 4.90, p<0.01 |
| Tumor necrosis factor-α receptor 1 | -0.07, p=0.51 | 0.44, p=0.95 | 0.14, p=0.01 | 1.30, p=0.77 | -17.83, p=0.07 | -2.12, p=0.06 | -12.02, p=0.34 | -19.54, p=0.40 | -0.04, p=0.96 | -9.40, p=0.05 | -5.12, p=0.05 | 7.70, p=0.11 |
| Soluble CD163 | -0.11, p=0.20 | -6.01, p=0.40 | -0.04, p=0.46 | 0.02, p=0.70 | 5.75, p=0.39 | -0.79, p=0.60 | 11.60, p=0.23 | -20.80, p=0.33 | 0.25, p=0.18 | 2.06, p=0.26 | 0.70, p=0.34 | 4.82, p=0.21 |
| Soluble CD14 | 0.00, p=0.95 | -13.88, p=0.10 | -0.04, p=0.67 | 6.62, p=0.19 | 12.34, p=0.43 | -1.70, p=0.46 | -5.34, p=0.78 | -46.00, p=0.24 | 0.19, p=0.58 | -9.04, p=0.17 | -5.53, p=0.11 | 15.52, p<0.01 |
Results from linear mixed models pooling data across both recruitment and 12 weeks of ART. Models adjusted for age, sex, and baseline CD4+ T-cell count and included a random effect for patient. Bold values represent statistically significant findings.
Abbreviation: QTC, corrected QT interval; RMSSD, root mean square of the successive differences.
Reactive hyperemia index is natural log transformed
Discussion
This pilot study examining relationships between inflammatory and cardiovascular markers in undernourished HIV-infected adults during initiation of ART demonstrated several novel findings that may implicate poor cardiovascular health and function as one of the mechanistic links in the well-described relationship between persistent inflammation and early mortality in this population. We found that higher levels of circulating inflammatory markers were associated with reduced vascular endothelial responsiveness and heart rate variability, both indicative of poor cardiovascular health, at baseline and after 12 weeks of ART. However, we did not observe paired changes in several of these variables, which may have been due in part to our small cohort size. Based on these findings, further studies are warranted to assess the relationship between inflammation and cardiovascular health over time, and to assess whether cardiovascular dysfunction, particularly sudden cardiac death, is a frequent proximate cause of early mortality among malnourished adults starting ART.
Serum CRP levels in our participants were high at baseline (median 18.3 mg/l) and remained elevated, although reduced, at 12 weeks (6.4mg/l). While CRP levels over 3.0 mg/l have been associated with high cardiovascular risk in US and European studies of HIV-infected and uninfected adults, caution is warranted in extrapolating these findings to African HIV patients given differences in populations, background disease risk, and laboratory methodology [22]. During 6 years of observation, Triant et al. found that the odds of an acute myocardial infarction was more than four-fold higher for patients with both an elevated CRP and HIV compared to uninfected individuals with normal CRP [23]. During 29 months of observation, Duprez et al. similarly found that the odds of a cardiovascular event were two-fold higher in HIV patients with elevated CRP (mean CRP 3.3 mg/l) when compared to those with normal CRP (mean CRP 1.7 mg/l) [24]. One explanation for the high mortality rate among malnourished patients in the early post-ART period may be that increased metabolic activity and weight gain accompanying virologic suppression places sudden strain on a cardiovascular system already injured by the effects of advanced HIV disease and chronic inflammation.
Increases in LnRHI, indicating improved arteriolar responsiveness following ischemia, were associated with reductions in circulating TNF-α R1 levels over 12 weeks, and a similar relationship with CRP levels approached significance (p=0.07). TNF-α is known to contribute to the pathogenesis of atherosclerosis, heart failure, and myocardial ischemia, and both CRP and TNF-α are associated with markedly elevated coronary artery calcium scores and carotid intima-media thickness, two surrogate markers of subclinical atherosclerosis [9, 25]. Similarly, LnRHI is significantly associated with carotid intima-media thickness and with coronary artery disease, and it is an independent predictor of major adverse cardiac events [26-28]. The combination of elevated CRP or TNF-α with impaired endothelium-dependent vasodilation correlates with increased risk of cardiac events in patients with coronary artery disease, ischemic cardiomyopathy, nonischemic cardiomyopathy, post-cardiotomy heart failure, and cardiogenic shock [10, 29-31]. However, in our pooled analysis, TNF-α R1 levels were positively associated with LnHRI, which was unexpected and may indicate a more complex relationship.
Heart rate varies with inputs from the autonomic nervous system; therefore, heart rate variability has been used to measure autonomic function [32]. In our cohort, higher CRP was significantly associated with reductions in several markers of HRV (RMSSD, triangular index, and high-frequency variations), and TNF-α R1 was closely related with two of these parameters (RMSSD and triangular index). Low HRV is known to be associated with disease severity in chronic coronary artery disease, heart failure, and atrial fibrillation, and with sudden cardiac death among patients with heart failure, atrial fibrillation, or recent myocardial infarction [33-37].
We observed divergent relationships between the inflammation markers and peripheral AI as compared to LnRHI. The median AI value increased in the cohort as a whole over 12 weeks, indicative of increasing arterial stiffness, and greater gains in AI were associated with larger reductions in circulating CD163 over the same period. A prior study of aortic inflammation in HIV-infected adults in the United States using positron emission tomography found greater aortic arterial inflammation was associated with higher circulating CD163 levels, and we had expected that ART initiation would result in reduced vascular monocyte activation and relaxation of the arteries. [38]. However, two recent studies suggest that both systemic and vascular inflammation are not major determinants of arterial stiffness as measured by AI [39, 40]. Furthermore, the median baseline peripheral AI value of 11.9 in our cohort was approximately half the mean value of 21.4 measured using the EndoPAT device in a cohort of healthy black Americans, and the increase in AI we observed may represent a return to a more normal physiologic state on ART rather than a pathologic stiffening of the vasculature related to cardiovascular disease [19]. The inverse CD163 – AI relationship in our cohort may be a chance finding, and further studies are needed to investigate the etiology and consequences of low arterial stiffness in malnourished adults with HIV.
Lastly, there was a significant increase in the urine isoprostane/creatinine ratio over 12 weeks, but the ratio was not associated with any of the inflammatory biomarkers or with the cardiovascular parameters. We attribute this rise in the peroxidation of essential fatty acids to increased free radicals resulting from accelerated metabolic activity on ART, which was not sufficient to exert a significant modulating effect on the already high levels of circulating inflammatory biomarkers [41]. On the other hand, the increase in the urine isoprostane/creatinine ratio could also be due to a reduction in the urine creatinine concentration, either because ART improved nitrogen retention or participants were less dehydrated at 12 weeks as compared to baseline.
Our pilot study was limited by small sample size and high levels of incomplete follow-up; the latter is common in Sub-Saharan African ART programs. Additionally, the study only included undernourished, HIV-infected adults and did not include normal-BMI controls, which precluded an assessment of whether our findings are specific only to undernourished HIV-infected adults or more broadly characterize patients starting ART in Zambia. Lastly, as the goal of this pilot study was to explore potential links between inflammation and cardiovascular instability in a population not previously studied in detail, we performed a large number of statistical comparisons in an exploratory manner without adjusting for multiple comparisons. This may have led to chance findings, such as the conflicting relationships between TNF-α R1 and LnRHI, and our results need to be confirmed in a large cohort.
Conclusions
In this pilot study, higher circulating levels of systemic inflammatory biomarkers were associated with several indicators of impaired cardiovascular health in undernourished HIV-infected adults starting ART in Zambia. This may implicate disruptions in cardiac or vascular physiology as one of the causal links in the well-described relationship between persistent inflammation and early mortality in this population. At present there is a paucity of data on cardiovascular disease risk factors among HIV-infected individuals in sub-Saharan Africa. Our findings should be explored in a larger cohort to assess the mortality burden of cardiac events in the first months after starting HIV treatment, to examine underlying biological mechanisms, and to identify opportunities for therapeutic intervention.
Acknowledgments
Funding: The authors would like to thank Dr. Abdel Sakr for his assistance with the cardiovascular assessments, the NUSTART participants and study staff, and the Zambian Ministry of Health for consistent support of research in the national HIV care and treatment program. This work was supported by the Vanderbilt Meharry Center for AIDS Research (NIH grant number P30 AI54999); the NIH Fogarty International Center, Office of the Director, National Institutes of Health, National Heart, Blood, and Lung Institute, and National Institute of Mental Health, through the Vanderbilt-Emory-Cornell-Duke Consortium for Global Health Fellows (grant number R25 TW009337); the National Center for Advancing Translational Sciences (CTSA award number UL1TR000445) and the European and Developing Countries Clinical Trials Partnership (grant IP.2009.33011.004).
Grant Support: This work was supported by the Vanderbilt Meharry Center for AIDS Research (NIH grant number P30 AI54999); the NIH Fogarty International Center, Office of the Director, National Institutes of Health, National Heart, Blood, and Lung Institute, and National Institute of Mental Health, through the Vanderbilt-Emory-Cornell-Duke Consortium for Global Health Fellows (grant number R25 TW009337); the National Center for Advancing Translational Sciences (CTSA award number UL1TR000445) and the European and Developing Countries Clinical Trials Partnership (grant IP.2009.33011.004).
Footnotes
Conflicts of Interest Statement: No authors report any conflicts of interest including related consultancies, shareholdings and funding grants.
Competing Interests Statement: No authors report any competing interests including related consultancies, shareholdings and funding grants.
Author contributions: M. Bestawros participated in study design, acquisition of funding and data, as well as drafting and revising the manuscript. TC was involved with data acquisition and supervision of research group as well as manuscript revisions. M. Blevins and BS were involved in analysis and interpretation of data and manuscript revisions. AC was involved with study design, acquisition of funding and data, and manuscript revisions. JB assisted with data acquisition and interpretation. PK, SF, and DH participated in study design, acquisition and interpretation of data, research group supervision, and manuscript revision. JK participated in study design, acquisition, analysis and interpretation of data, as well as drafting and revising the manuscript and supervising the research group. All authors read and approved the final manuscript.
Contributor Information
Michael Bestawros, Email: mbestaw@gmail.com.
Takondwa Chidumayo, Email: takondwa05@yahoo.com.
Meridith Blevins, Email: meridith.blevins@vanderbilt.edu.
Ashley Canipe, Email: canipe.ashley@gmail.com.
Jay Bala, Email: jaybalas@gmail.com.
Paul Kelly, Email: m.p.kelly@qmul.ac.uk.
Suzanne Filteau, Email: suzanne.filteau@lshtm.ac.uk.
Bryan E Shepherd, Email: bryan.shepherd@vanderbilt.edu.
Douglas C. Heimburger, Email: douglas.heimburger@vanderbilt.edu.
John R. Koethe, Email: john.r.koethe@vanderbilt.edu.
References
- 1.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. http://www.ncbi.nlm.nih.gov/pubmed/16905784. [DOI] [PubMed] [Google Scholar]
- 2.Koethe JR, Lukusa A, Giganti MJ, Chi BH, Nyirenda CK, et al. Association between weight gain and clinical outcomes among malnourished adults initiating antiretroviral therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2010;53:507–513. doi: 10.1097/QAI.0b013e3181b32baf. http://www.ncbi.nlm.nih.gov/pubmed/19730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. http://www.ncbi.nlm.nih.gov/pubmed/17117022. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One. 2011;6:e28691. doi: 10.1371/journal.pone.0028691. http://www.ncbi.nlm.nih.gov/pubmed/22220193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koethe JR, Blevins M, Nyirenda C, Kabagambe EK, Shepherd BE, et al. Nutrition and inflammation serum biomarkers are associated with 12-week mortality among malnourished adults initiating antiretroviral therapy in Zambia. J Int AIDS Soc. 2011;14:19. doi: 10.1186/1758-2652-14-19. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21477359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18942885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajiasgharzadeh K, Mirnajafi-Zadeh J, Mani AR. Interleukin-6 impairs chronotropic responsiveness to cholinergic stimulation and decreases heart rate variability in mice. Eur J Pharmacol. 2011;673:70–77. doi: 10.1016/j.ejphar.2011.10.013. http://www.ncbi.nlm.nih.gov/pubmed/22044916. [DOI] [PubMed] [Google Scholar]
- 8.Ikonomidis I, Stamatelopoulos K, Lekakis J, Vamvakou GD, Kremastinos DT. Inflammatory and non-invasive vascular markers: the multimarker approach for risk stratification in coronary artery disease. Atherosclerosis. 2008;199:3–11. doi: 10.1016/j.atherosclerosis.2008.02.019. http://www.ncbi.nlm.nih.gov/pubmed/18378239. [DOI] [PubMed] [Google Scholar]
- 9.Freitas WM, Quaglia LA, Santos SN, Soares AA, Japiassu AV, et al. Association of systemic inflammatory activity with coronary and carotid atherosclerosis in the very elderly. Atherosclerosis. 2011;216:212–216. doi: 10.1016/j.atherosclerosis.2011.01.040. http://www.ncbi.nlm.nih.gov/pubmed/21316055. [DOI] [PubMed] [Google Scholar]
- 10.Tentolouris C, Tousoulis D, Antoniades C, Bosinakou E, Kotsopoulou M, et al. Endothelial function and proinflammatory cytokines in patients with ischemic heart disease and dilated cardiomyopathy. International Journal of Cardiology. 2004;94:301–305. doi: 10.1016/j.ijcard.2003.08.002. http://www.ncbi.nlm.nih.gov/pubmed/15093997. [DOI] [PubMed] [Google Scholar]
- 11.Hansson GK. Atherosclerosis--an immune disease: The Anitschkov Lecture 2007. Atherosclerosis. 2009;202:2–10. doi: 10.1016/j.atherosclerosis.2008.08.039. http://www.ncbi.nlm.nih.gov/pubmed/18951547. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. http://www.ncbi.nlm.nih.gov/pubmed/16246962. [DOI] [PubMed] [Google Scholar]
- 13.Foley RN. Phosphate levels and cardiovascular disease in the general population. Clin J Am Soc Nephrol. 2009;4:1136–1139. doi: 10.2215/CJN.01660309. http://www.ncbi.nlm.nih.gov/pubmed/19423568. [DOI] [PubMed] [Google Scholar]
- 14.Venditti FJ, Marotta C, Panezai FR, Oldewurtel HA, Regan TJ. Hypophosphatemia and cardiac arrhythmias. Miner Electrolyte Metab. 1987;13:19–25. http://www.ncbi.nlm.nih.gov/pubmed/3587180. [PubMed] [Google Scholar]
- 15.Schwartz A, Gurman G, Cohen G, Gilutz H, Brill S, et al. Association between hypophosphatemia and cardiac arrhythmias in the early stages of sepsis. Eur J Intern Med. 2002;13:434. doi: 10.1016/s0953-6205(02)00130-9. http://www.ncbi.nlm.nih.gov/pubmed/12384132. [DOI] [PubMed] [Google Scholar]
- 16.Zazzo JF, Troche G, Ruel P, Maintenant J. High incidence of hypophosphatemia in surgical intensive care patients: efficacy of phosphorus therapy on myocardial function. Intensive Care Med. 1995;21:826–831. doi: 10.1007/BF01700966. http://www.ncbi.nlm.nih.gov/pubmed/8557871. [DOI] [PubMed] [Google Scholar]
- 17.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8598068. [PubMed] [Google Scholar]
- 18.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15582310. [DOI] [PubMed] [Google Scholar]
- 19.Morris AA, Patel RS, Binongo JN, Poole J, Al Mheid I, et al. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2:e002154. doi: 10.1161/JAHA.112.002154. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SA, Kambur T, Chan C, Herrington DM, Liu K, et al. Relation of short-term heart rate variability to incident heart failure (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2013;112:533–540. doi: 10.1016/j.amjcard.2013.04.018. http://www.ncbi.nlm.nih.gov/pubmed/23683953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. http://www.ncbi.nlm.nih.gov/pubmed/10982537. [DOI] [PubMed] [Google Scholar]
- 22.Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation. 2003;107:370–371. doi: 10.1161/01.cir.0000053731.05365.5a. http://www.ncbi.nlm.nih.gov/pubmed/12551854. [DOI] [PubMed] [Google Scholar]
- 23.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273. doi: 10.1097/QAI.0b013e3181a9992c. http://www.ncbi.nlm.nih.gov/pubmed/19387353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. http://www.ncbi.nlm.nih.gov/pubmed/22970224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. http://www.ncbi.nlm.nih.gov/pubmed/20621692. [DOI] [PubMed] [Google Scholar]
- 26.Fitch KV, Stavrou E, Looby SE, Hemphill L, Jaff MR, et al. Associations of cardiovascular risk factors with two surrogate markers of subclinical atherosclerosis: endothelial function and carotid intima media thickness. Atherosclerosis. 2011;217:437–440. doi: 10.1016/j.atherosclerosis.2011.04.009. http://www.ncbi.nlm.nih.gov/pubmed/21570076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuvin JT, Mammen A, Mooney P, Alsheikh-Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–16. doi: 10.1177/1358863X06076227. http://www.ncbi.nlm.nih.gov/pubmed/17451088. [DOI] [PubMed] [Google Scholar]
- 28.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. http://www.ncbi.nlm.nih.gov/pubmed/20181680. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. 2003;115(Suppl 8A):99S–106S. doi: 10.1016/j.amjmed.2003.09.016. http://www.ncbi.nlm.nih.gov/pubmed/14678874. [DOI] [PubMed] [Google Scholar]
- 30.Kang SM, Chung N, Kim JY, Koo BK, Choi D, et al. Relation of vasodilator response of the brachial artery to inflammatory markers in patients with coronary artery disease. Echocardiography. 2002;19:661–667. doi: 10.1046/j.1540-8175.2002.00661.x. http://www.ncbi.nlm.nih.gov/pubmed/12487635. [DOI] [PubMed] [Google Scholar]
- 31.Hermansen SE, Kalstad T, How OJ, Myrmel T. Inflammation and reduced endothelial function in the course of severe acute heart failure. Transl Res. 2011;157:117–127. doi: 10.1016/j.trsl.2010.12.002. http://www.ncbi.nlm.nih.gov/pubmed/21316028. [DOI] [PubMed] [Google Scholar]
- 32.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. http://www.ncbi.nlm.nih.gov/pubmed/15649244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janszky I, Ericson M, Mittleman MA, Wamala S, Al-Khalili F, et al. Heart rate variability in long-term risk assessment in middle-aged women with coronary heart disease: The Stockholm Female Coronary Risk Study. J Intern Med. 2004;255:13–21. doi: 10.1046/j.0954-6820.2003.01250.x. http://www.ncbi.nlm.nih.gov/pubmed/14687234. [DOI] [PubMed] [Google Scholar]
- 34.Kotecha D, New G, Flather MD, Eccleston D, Pepper J, et al. Five-minute heart rate variability can predict obstructive angiographic coronary disease. Heart. 2012;98:395–401. doi: 10.1136/heartjnl-2011-300033. http://www.ncbi.nlm.nih.gov/pubmed/22121069. [DOI] [PubMed] [Google Scholar]
- 35.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. http://www.ncbi.nlm.nih.gov/pubmed/9769304. [DOI] [PubMed] [Google Scholar]
- 36.Lombardi F, Colombo A, Basilico B, Ravaglia R, Garbin M, et al. Heart rate variability and early recurrence of atrial fibrillation after electrical cardioversion. J Am Coll Cardiol. 2001;37:157–162. doi: 10.1016/s0735-1097(00)01039-1. http://www.ncbi.nlm.nih.gov/pubmed/11153731. [DOI] [PubMed] [Google Scholar]
- 37.Yamada A, Hayano J, Sakata S, Okada A, Mukai S, et al. Reduced ventricular response irregularity is associated with increased mortality in patients with chronic atrial fibrillation. Circulation. 2000;102:300–306. doi: 10.1161/01.cir.102.3.300. http://www.ncbi.nlm.nih.gov/pubmed/10899093. [DOI] [PubMed] [Google Scholar]
- 38.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22820791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blann AD, Kuzniatsova N, Lip GY. Inflammation does not influence arterial stiffness and pulse-wave velocity in patients with coronary artery disease. J Hum Hypertens. 2013;27:629–634. doi: 10.1038/jhh.2013.17. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23535991. [DOI] [PubMed] [Google Scholar]
- 40.Heffernan KS, Kuvin JT, Sarnak MJ, Perrone RD, Miskulin DC, et al. Peripheral augmentation index and vascular inflammation in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2011;26:2515–2521. doi: 10.1093/ndt/gfq806. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21292815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2123555. [DOI] [PMC free article] [PubMed] [Google Scholar]


