Abstract
Satellite cells, the main source of myoblasts in postnatal muscle, are located beneath the myofiber basal lamina. The myogenic potential of satellite cells was initially documented based on their capacity to produce progeny that fused into myotubes. More recently, molecular markers of resident satellite cells were identified, further contributing to defining these cells as myogenic stem cells that produce differentiating progeny and self-renew. Herein, we discuss aspects of the satellite cell transcriptional milieu that have been intensively investigated in our research. We elaborate on the expression patterns of the paired box (Pax) transcription factors Pax3 and Pax7, and on the myogenic regulatory factors myogenic factor 5 (Myf5), myogenic determination factor 1 (MyoD), and myogenin. We also introduce original data on MyoD upregulation in newly activated satellite cells, which precedes the first round of cell proliferation. Such MyoD upregulation occurred even when parent myofibers with their associated satellite cells were exposed to pharmacological inhibitors of hepatocyte growth factor and fibroblast growth factor receptors, which are typically involved in promoting satellite cell proliferation. These observations support the hypothesis that most satellite cells in adult muscle are committed to rapidly entering myogenesis. We also detected expression of serum response factor in resident satellite cells prior to MyoD expression, which may facilitate the rapid upregulation of MyoD. Aspects of satellite cell self-renewal based on the reemergence of cells expressing Pax7, but not MyoD, in myogenic cultures are discussed further herein. We conclude by describing our recent studies using transgenic mice in which satellite cells are traced and isolated based on their expression of green fluorescence protein driven by regulatory elements of the nestin promoter (nestin-green fluorescence protein). This feature provides us with a novel means of studying satellite cell transcriptional signatures, heterogeneity among muscle groups, and the role of the myogenic niche in directing satellite cell self-renewal.
Keywords: Myf5, MyoD, Pax7, satellite cell, Sox, SRF
INTRODUCTION
Skeletal muscle is composed of multinucleated myofibers that are established during embryogenesis by fusion of myogenic cells (myoblasts). Typically, the myofiber nuclei (myonuclei) are mitotically inactive. Addition of new myonuclei or the formation of new myofibers during postnatal and adult life depends on satellite cells, myogenic stem cells located underneath the myofiber basal lamina (Mauro, 1961; Zammit et al., 2006a). During postnatal growth, satellite cells are proliferative and contribute progeny that fuse with the enlarging myofibers. In mature muscles, satellite cells are typically quiescent, but can be activated in response to muscle damage and provide progeny for myofiber repair (Grounds and Yablonka-Reuveni, 1993; Hawke and Garry, 2001). Satellite cells are considered myogenic stem cells (not merely myogenic progenitors) in view of the findings that, in addition to contributing proliferating and differentiating progeny, they can self-renew, which fulfills the definition of tissue-specific stem cells (Collins et al., 2005; Day et al., 2007). Satellite cells are required throughout life for myofiber growth, repair, and maintenance; therefore, stringent regulatory control is needed to maintain a balance among their quiescence, proliferation, differentiation, and renewal. Myogenesis of satellite cells follows a highly orchestrated genetic program to ensure that transcription of specific genes is temporally induced or repressed in accordance with cell-cycle progression and cues from the surrounding niche (Charge and Rudnicki, 2004; Zammit et al., 2006a; Shefer and Yablonka-Reuveni, 2007). The dynamics of gene expression during the myogenesis of satellite cells are discussed in detail in this paper.
SATELLITE CELLS EXPRESS THE PAIRED BOX TRANSCRIPTION FACTOR, PAIRED BOX GENE 7
The identification of satellite cells in their niche in vivo relied for many years on electron microscopy (Mauro, 1961; Grounds and Yablonka-Reuveni, 1993; Yablonka-Reuveni, 1995; Hawke and Garry, 2001). More recently, several antigens were shown to be expressed by satellite cells and were used to identify satellite cells in vivo, or in isolated myofibers. These antigens include the receptor of hepatocyte growth factor (c-met), hematopoietic progenitor cell antigen CD34, syndecan 4, muscle type cadherin (M-cadherin), intergin α7, and integrin β1 (reviewed in Charge and Rudnicki, 2004; Zammit et al., 2006a; Kuang et al., 2007). However, even within the context of the skeletal muscle tissue, these markers, with the possible exception of M-cadherin, are not exclusively expressed in cells of the myogenic lineage. Nevertheless, the utilization of antibodies against these antigens can assist in identifying satellite cells based on their location underneath the myofiber basal lamina.
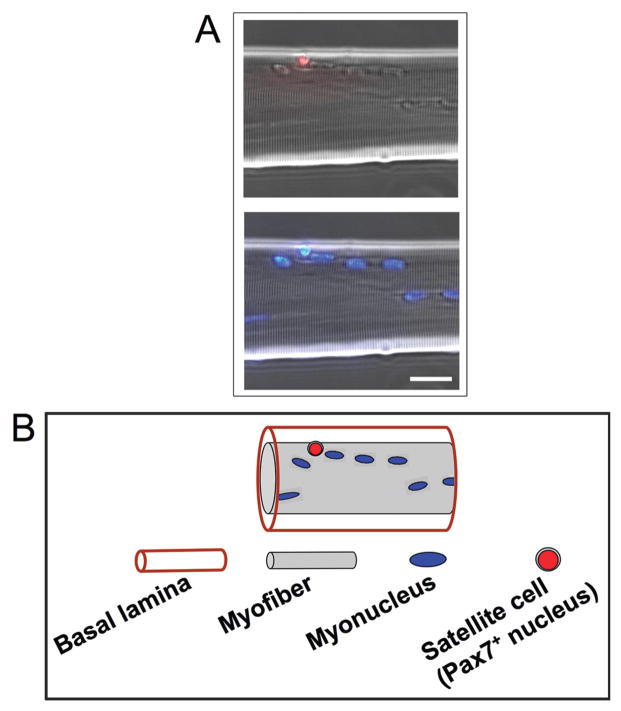
The seminal study by Seale et al. (2000) introduced the paired box (Pax) transcription factor, Pax7, as a transcription factor selectively expressed by satellite cells. This Pax7 expression by satellite cells has permitted their direct identification on myofibers by immunolabeling (Figure 1). We demonstrated both in the chicken and mouse that resident quiescent satellite cells and their proliferating progeny uniformly express Pax7 throughout life (Halevy et al., 2004; Shefer et al., 2006; Zammit et al., 2006a; Day et al., 2007). The factor Pax7 is a member of a larger family of Pax transcription factors involved in cell type and organ determination during embryogenesis of multicellular animals. The Pax proteins are characterized by several conserved elements, including 2 DNA-binding domains, a paired domain, and a homeodomain (Barber et al., 1999; Vorobyov and Horst, 2006). The paralog of Pax7, Pax3, is also expressed by resident satellite cells, but Pax3 transcripts are detected at a relative high level only in certain muscle types, such as the diaphragm (Relaix et al., 2006; Day et al., 2007). Both Pax3 and Pax7 are expressed during muscle development, and they possess both distinct and overlapping roles (Galli et al., 2004; Lamey et al., 2004; Relaix et al., 2004; Buckingham et al., 2006). At first, Pax7 appeared to be required for the specification of satellite cells (Seale et al., 2000). However, satellite cells have been detected in the early postnatal period in Pax7-null mice, and their numbers have been determined to decline rapidly, presumably because of apoptosis, leading to impaired muscle maintenance (Oustanina et al., 2004; Relaix et al., 2006). Thus, Pax7 is likely to be involved in supporting satellite cell survival. The essential role of Pax7 in postnatal muscle cannot be compensated for by Pax3, although embryonic muscle development progresses normally in the absence of Pax7 (Seale et al., 2000; Oustanina et al., 2004; Kuang et al., 2006; Relaix et al., 2006). Paired box transcription factor 7 has also been implicated in the interplay among proliferation, differentiation, and self-renewal, but its exact role as a transcription factor is not well understood (Zammit et al., 2006b; Olguin et al., 2007). Overall, the molecular mechanisms underlying the involvement of Pax7 in the life cycle of satellite cells awaits further study.
Figure 1.
Resident satellite cells can be traced by their expression of the paired box (Pax) transcription factor, Pax7. A) Immunolabeling of a myofiber isolated from the extensor digitorum longus muscle of the adult mouse and reacted with an antibody against Pax7 (obtained from Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). The top panel depicts merged phase and immunostained images; the bottom panel depicts parallel merged phase and 4′,6-diamidino-2-phenylin-dole-stained images, which show myonuclei. Bar = 15 μm. B) Illustration of the myofiber shown in panel A, which schematically depicts the Pax7-positive (Pax7+) satellite cell positioned beneath the basal lamina, corresponding with the immunostained panel above. The continuous basal lamina is formed around myofibers in late stages of embryogenesis, correlating with the appearance of satellite cells (Yablonka-Reuveni, 1995). It is conceivable that unique interactions between the satellite cell and the myofiber basal lamina may establish the satellite cell niche, regulate satellite cell lineage commitment, and control the extent of proliferation, differentiation, and renewal.
MYOGENIC REGULATORY FACTORS ESTABLISH THE BLUEPRINT OF SATELLITE CELL MYOGENESIS
Myogenesis of satellite cells is regulated by a family of muscle-specific transcription factors (i.e., muscle regulatory factors; MRF) that are expressed in a temporally ordered manner (Yablonka-Reuveni and Rivera, 1994; Zammit et al., 2006a). This family includes myogenic determination factor 1 (MyoD), myogenic factor 5 (Myf5), myogenin (also known as myogenic factor 4 when first discovered), and myogenic regulatory factor 4 (MRF4, also known as myogenic factor 6 and herculin when first discovered), which are involved in determining the myogenic fate during early embryogenesis (Ludolph and Konieczny, 1995).
It was originally thought that quiescent satellite cells did not express any of the MRF, but Myf5-driven reporter expression has shown that the Myf5 gene is active in satellite cells (Beauchamp et al., 2000; Zammit et al., 2006a). In accordance with this observation, we documented a relative high-level expression of endogenous Myf5 transcripts in freshly isolated satellite cells (Day et al., 2007). Nevertheless, there appears to be a minor population of satellite cells in adult muscle that do not display Myf5-driven reporter activity (Beauchamp et al., 2000; Day et al., 2007; Kuang et al., 2007). It has further been proposed that satellite cells, which demonstrate Myf5 gene activity, represent committed progenitors, whereas the population in which Myf5 gene is inactive represents the stem cell compartment, which may both produce committed progeny and self-renew (Kuang et al., 2007). Although investigations using the knockin Myf5nLacZ/+ mouse have clearly demonstrated Myf5 promoter activity in resident satellite cells (Beauchamp et al., 2000; Zammit et al., 2006a; Day et al., 2007), it is not known whether the Myf5 protein is expressed in quiescent satellite cells (Yablonka-Reuveni et al., 1999a). Mouse satellite cells and myogenic cell lines exhibited the Myf5 protein in proliferating progeny, whereas the protein was not detected upon differentiation and fusion into myotubes (Lindon et al., 1998; Yablonka-Reuveni et al., 1999a). Additionally, the level of Myf5 protein is modulated in proliferating myoblasts; the phosphorylated form of Myf5 is degraded at the G2/M stage of the cell cycle (Lindon et al., 1998). Although the implication is that Myf5 activity occurs during specific stages of the cell cycle, the exact role of Myf5 may remain elusive until detection of the Myf5 protein is reconciled with transcription of its encoding messenger RNA. To date, there is no evidence for Myf5 protein expression by quiescent satellite cells. During proliferation and differentiation of chicken satellite cells, Myf5 protein expression was observed only within Pax7-positive cells and was not detected in myotubes (K. Day and Z. Yablonka-Reuveni, unpublished results). This leads to the questions of how Myf5 might serve to preserve the continuity of myoblast proliferation and what role it may play in the self-renewal of satellite cells that must assume quiescent status.
Expression of MyoD is observed during satellite cell proliferation, whereas their differentiation is marked by the onset of myogenin expression, with a concomitant decline in Pax7 and Myf5 expression, cell-cycle withdrawal, and subsequent fusion of myoblasts into multinucleated myotubes (Yablonka-Reuveni and Rivera, 1994; Yablonka-Reuveni et al., 1999a; Yablonka-Reuveni and Paterson, 2001; Halevy et al., 2004; Shefer et al., 2006; Zammit et al., 2006a). The myogenic regulatory factor MyoD is also expressed by differentiating progeny of satellite cells, but the level of this expression seems to depend on the extracellular environment. In a serum-rich, mitogen-depleted medium, satellite cells undergo only 1 or 2 rounds of proliferation, after which their progeny enter differentiation and express myogenin, but not MyoD (Yablonka-Reuveni and Rivera, 1994, 1997b; Yablonka-Reuveni et al., 1999a,b). In contrast, the progeny of satellite cells remain proliferative for a long time (more than 1 wk) and express MyoD even upon fusion into myotubes when grown in our standard rich growth medium (Yablonka-Reuveni and Paterson, 2001; Shefer et al., 2006). Satellite cells from MyoD-null mice do not display obvious impairment in myogenesis, but the transition into the differentiation phase is delayed both in culture and in vivo (Yablonka-Reuveni et al., 1999a; White et al., 2000). Thus, the role of MyoD as a regulator of myogenic differentiation is likely compensated for by one or several other MRF (Rudnicki et al., 1993).
Myoblasts are able to proliferate during early phases of the differentiation decision, when cells first transit into the myogenin-expressing state, whereas terminal differentiation requires permanent withdrawal from the cell cycle (Andres and Walsh, 1996; Kitzmann and Fernandez, 2001). Cyclin-dependent kinases, cyclin-dependent kinase inhibitors, cyclin D3, and retinoblastoma protein control terminal myogenic differentiation by regulating cell-cycle withdrawal and expression of genes encoding for structural proteins (Huh et al., 2004; De Falco et al., 2006). It has been suggested that MyoD governs the induction of the latter cell-cycle regulators (Halevy et al., 1995; Cenciarelli et al., 1999), implying that MyoD must be kept in a transcriptionally inactive form until differentiation-inducing signals are conveyed (Song et al., 1998; Kitzmann et al., 1999; Novitch et al., 1999; Perry et al., 2001; Puri et al., 2001). Members of the myocyte enhancer factor 2 transcription factor family act in concert with MRF to direct the late stages of myogenesis by inducing the expression of muscle-specific structural proteins (Molkentin et al., 1995; Black and Olson, 1998). Differentiating progeny of satellite cells exhibit enhanced expression of myocyte enhancer factor 2A in correlation with the onset of myogenin expression (Yablonka-Reuveni and Rivera, 1997a; Kastner et al., 2000; Yablonka-Reuveni and Paterson, 2001; Yablonka-Reuveni and Anderson, 2006).
MyoD EXPRESSION IS RAPIDLY UPREGULATED UPON SATELLITE CELL ACTIVATION
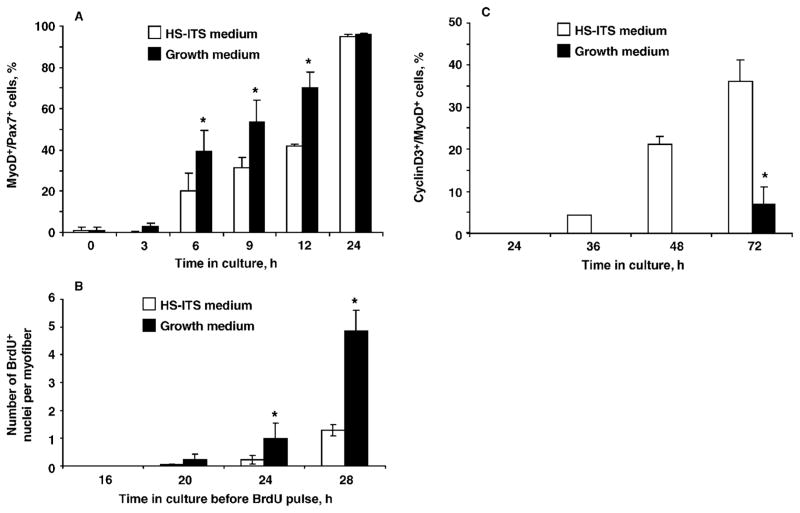
The myogenic regulatory factor MyoD serves as the myogenic master transcription factor that directs the upregulation of differentiation-linked genes (Tapscott, 2005). Although MyoD is expressed in the proliferating progeny of satellite cells (Yablonka-Reuveni and Rivera, 1994; Shefer et al., 2006; Zammit et al., 2006a), its function during proliferation is not well understood (see Wyzykowski et al., 2002, for a proposed role). To gain insight into the role of MyoD in satellite cells prior to differentiation, we investigated the kinetics of MyoD protein expression by satellite cells (Figure 2). Myofibers were isolated from extensor digitorum longus muscles of 4- to 6-mo-old male C57BL/6 mice and cultured individually, as described previously (Shefer et al., 2006). The expression of MyoD in resident satellite cells was compared between myofibers that were supplemented with our standard growth medium [Dulbecco’s modified Eagle’s medium containing 20% fetal bovine serum, 10% horse serum (HS), and 1% chicken embryo extract; referred to in Figure 2 as “growth medium”] and myofibers supplemented with low serum medium (Dulbecco’s modified Eagle’s medium containing 2% HS and supplemented with insulin-transferrin-sodium selenite (ITS, Sigma-Aldrich, St. Louis, MO, 1:100 dilution; referred to in Figure 2 as “HS-ITS medium”). The proportion of satellite cells expressing MyoD at early time points was determined by double immunostaining with antibodies against Pax7 and MyoD (Figure 2A), as described previously (Shefer et al., 2006). The proliferating progeny of satellite cells were identified by tracing cells that incorporated bromodeoxyuridine (Figure 2B), as described previously (Day et al., 2007). Cells positive for bromo-deoxyuridine were either attached to the myofibers or had already migrated away by the time of the analysis. As shown in Figure 2, MyoD protein expression was upregulated in satellite cells prior to the first round of cell proliferation. Essentially all satellite cells expressed MyoD by 24 h in culture (Figure 2A), regardless of the medium in which they were cultured. Nevertheless, the number of MyoD-positive cells, and the subsequent number of proliferating satellite cells in myofiber cultures supplemented with rich growth medium, was greater (Figure 2B). In contrast, differentiation of satellite cells [monitored in the current study by expression of cyclin D3, as described in Yablonka-Reuveni et al. (1999a)], was initiated earlier in myofiber cultures maintained in the 2% HS-ITS medium (Figure 2C).
Figure 2.
Kinetics of satellite cell activation [myogenic determination factor 1 (MyoD) expression; panel A], proliferation [bromodeoxyurine (BrdU) uptake; panel B], and differentiation [cyclin D3 (cyclinD3) expression; panel C] in myofiber cultures isolated from the extensor digitorum longus muscle of the adult mouse and maintained in growth medium or in serum-poor medium containing 2% horse serum (HS) supplemented with insulin-transferrin-sodium selenite (ITS; i.e., HS-ITS medium). Significant differences (P < 0.05) between treatment groups within panels A, B, and C are marked with asterisks. A) Single myofiber cultures were analyzed by double immunofluorescence for Pax7 and MyoD (using mouse and rabbit primary antibodies, respectively), and cells were monitored for the number of cells expressing both MyoD and Pax7 [MyoD-positive (MyoD+)/Pax7-positive (Pax7+)] compared with the total number of satellite cells (i.e., all Pax7+ cells). Data for the various time points were evaluated for statistical differences using the nonparametric Friedman test for repeated measures. Results are from 3 independent experiments and depict the average number (±SD) of MyoD+/Pax7+ cells out of the total number of Pax7+ cells; a minimum of 42 myofibers were analyzed per time point. B) Individual myofiber cultures received a 4-h pulse of 2.5 μM BrdU at the time points indicated on the x-axis and were then fixed and processed for immunofluorescent detection of nuclei that incorporated BrdU (BrdU-positive, BrdU+), thus representing cells in the S phase of the cell cycle during the exposure to BrdU. Data for the various time points were evaluated for statistical differences by using Student’s t-test. Results are from 4 independent experiments and depict the mean (±SD) for a minimum of 47 myofibers per time point. C) Emergence of differentiating myoblasts [cells positive for both cyclinD3 and MyoD (cyclinD3+/MyoD+) out of all MyoD+ cells] in cultures of isolated myofibers. Myofibers were analyzed by double immunofluorescence for MyoD and cyclinD3 (using mouse and rabbit primary antibodies, respectively). Data for the various time points were evaluated for differences by using Student’s t-test. Results are from 3 independent experiments and depict the mean (±SD) for a minimum of 48 myofibers per time point.
Hepatocyte growth factor (HGF) and selective members of the fibroblast growth factor (FGF) family are recognized activators of satellite cell proliferation (Tatsumi et al., 1998; Yablonka-Reuveni et al., 1999b; Kastner et al., 2000; Anderson, 2006; Shefer et al., 2006), illustrated later in the article. In view of our findings that MyoD expression by satellite cells is upregulated before the first round of proliferation, we investigated the hypothesis that inhibition of signaling through HGF and FGF receptors may block MyoD expression by satellite cells in freshly isolated myofibers. We used pharmacological inhibitors of the HGF receptor (i.e., 100 nM PHA665752; a gift from Pfizer, Groton, CT; see Christensen et al., 2003), the FGF receptor (i.e., 40 μM SU5402; Calbiochem, Fontenay sous bois, France; a gift from David Israeli, Genethon, Evry, France; see Mohammadi et al., 1997), or a combination of the 2 inhibitors. Both drugs inhibited cell proliferation in primary myogenic cultures, indicating drug efficacy in our cell culture conditions. However, these drugs failed to prevent our detection of MyoD protein in satellite cells (by 24 h in isolated myofibers) even when added before initiating the cultures (i.e., during enzymatic digestion of the muscle). The average number of Pax7-positive cells in treated and in control myofibers that received only vehicle (i.e., di-methylsulfoxide) did not differ, which eliminated the possibility of cell toxicity. A different pharmacological inhibitor (PD98059, Calbiochem, La Jolla, CA) known to block the activity of extracellular signal-regulated kinases (mitogen-activated protein kinases) Erk1 and Erk2, which are downstream targets of HGF and FGF receptor tyrosine kinases (Yablonka-Reuveni et al., 1999b), also did not affect MyoD expression by satellite cells in freshly isolated myofibers (data not shown).
Collectively, based on our results with the different pharmacological inhibitors, we suggest that MyoD expression is not necessarily regulated by the same growth factors known to induce satellite cell proliferation. It remains possible that MyoD gene upregulation occurs in a very rapid manner during muscle processing and therefore cannot be prohibited by blocking receptor tyrosine kinase-signaling activity. In accordance with these results, we found that freshly isolated satellite cells from hindlimb muscles that were isolated by fluorescent-activated cell sorting, based on their expression of green fluorescence protein (GFP) driven by regulatory elements of the nestin promoter (nestin-GFP; Day et al., 2007), rapidly upregulated MyoD transcription, as shown by quantitative reverse transcription PCR (although the MyoD expression level was only ~20% of that in proliferating satellite cells; K. Day and Z. Yablonka-Reuveni, unpublished results). The rapid upregulation of MyoD transcripts within several hours after muscle injury (Grounds et al., 1992) provides further in vivo support for the hypotheses that satellite cells in adult muscle are committed to rapidly entering the MyoD-expressing state. As previously proposed by Bischoff (1990), it is the actual interaction of satellite cells with their parent myofiber that may restrain them from entering activation. This may be a means for maintaining a proper balance between the quiescent cell pool and the occasional activated daughter cells required for maintaining the adult myofiber. Once the native association is impaired and satellite cells are no longer under the “positional” control of their intact niche, they may rapidly become active, even before growth-promoting agents can contribute to cell-cycle progression.
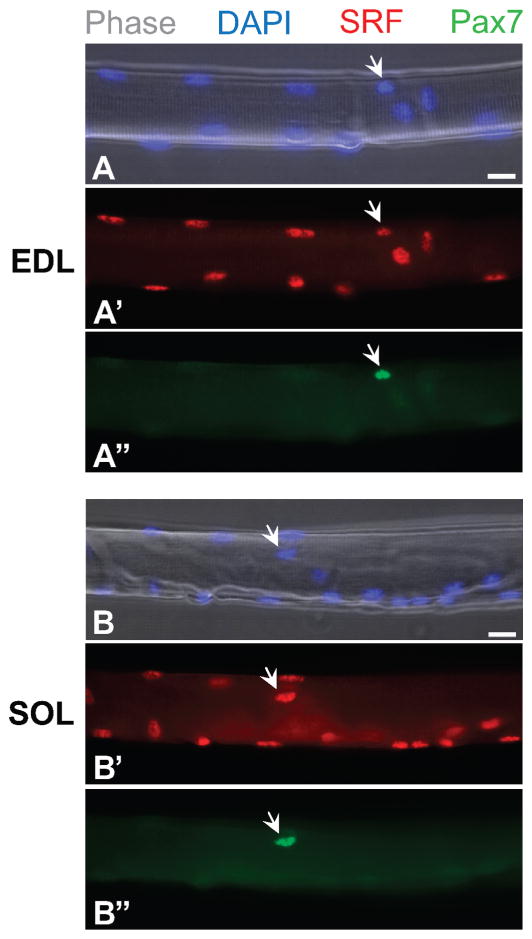
On the basis of results of our immunolabeling studies, we conclude that resident satellite cells express serum response factor (SRF; rabbit anti-SRF antibody was from Santa Cruz Biotechnology, Santa Cruz, CA). Isolated myofibers from extensor digitorum longus and soleus muscles of adult mice exhibited SRF protein expression at a similar level in myofiber nuclei, as well as in satellite cell nuclei; the latter were distinguished from myofiber nuclei based on Pax7 immunostaining (Figure 3). It was previously shown that SRF interacts with the distal regulatory region of the MyoD gene (Soulez et al., 1996; L’Honore et al., 2007). Thus, it is possible that events linked to SRF activity may be involved in the rapid induction of MyoD gene expression in satellite cells.
Figure 3.
Expression of serum response factor (SRF) by satellite cells in freshly isolated myofibers from extensor digitorum longus (EDL, panels A, A′, and A″) and soleus (SOL, panels B, B′, and B″) muscles harvested from the adult mouse. Myofibers were analyzed by double immu-noflurescence for the paired box transcription factor 7 (Pax7) and SRF by using mouse and rabbit primary antibodies, respectively. Myonuclei and satellite cell nuclei are positive for SRF and 4′,6-diamidino-2-phenylindole (DAPI), whereas satellite cells (arrows) are detected by their specific expression of Pax7. Bar = 15 μm.
Pax7-POSITIVE/MyoD-NEGATIVE CELLS REAPPEAR IN SATELLITE CELL CULTURES: EVIDENCE FOR SATELLITE CELL SELF-RENEWAL?
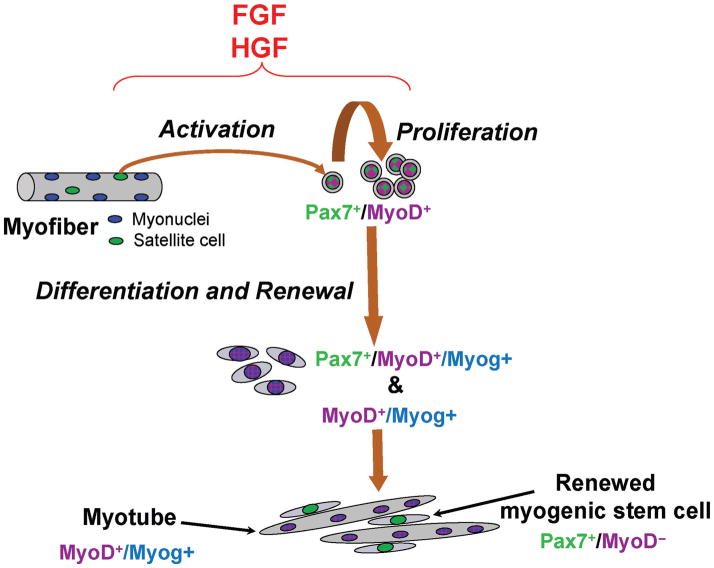
Although quiescent satellite cells commonly express Pax7, proliferating progeny coexpress Pax7 and MyoD. Myoblasts that enter into the differentiation phase feature a decline in Pax7 along with myogenin induction. Based on the consistent reappearance of Pax7-positive/MyoD-negative cells in primary cultures, in single myofiber cultures, and in clones of satellite cells, we suggest that this population represents cells that enter self-renewal, as shown schematically in Figure 4 (Halevy et al., 2004; Zammit et al., 2004; Shefer et al., 2006).
Figure 4.
Our working model of satellite cell activation, proliferation, differentiation, and self-renewal based on the expression patterns of the paired box transcription factor 7 (Pax7), myogenic determination factor 1 (MyoD), and myogenin (Myog). The present model is derived from our reports on the myogenesis of chicken, rat, and mouse satellite cells (Yablonka-Reuveni et al., 1999b; Kastner et al., 2000; Halevy et al., 2004; Shefer et al., 2006; Day et al., 2007). The proliferating myoblast population is represented by the Pax7-positive (Pax7+)/MyoD-positive (MyoD+) mononuclear cells. Nuclei that are MyoD+/Myog-positive (Myog+) (and no longer express Pax7) are found within differentiated mononuclear cells and myotubes, whereas a minor population of Pax7+/MyoD+/Myog+ cells represents a transitional stage within recently differentiated myoblasts; newly formed myotubes occasionally display Pax7+/ MyoD+/Myog+ nuclei as well. Renewed cells [Pax7+/MyoD-negative (MyoD−)] also express green fluorescence protein (GFP) when satellite cell cultures are established from transgenic mice expressing GFP driven by regulatory elements of the nestin gene (nestin-GFP). Nestin-GFP transgene upregulation in mononuclear cells indicates reentry into the satellite cell niche. FGF = fibroblast growth factor; HGF = hepatocyte growth factor.
NESTIN-GFP TRANSGENIC EXPRESSION BY RESIDENT SATELLITE CELLS AND THEIR Pax7-POSITIVE/MyoD-NEGATIVE PROGENY: EVIDENCE FOR SATELLITE CELL SELF-RENEWAL
Our recent studies on the expression of the nestin-GFP transgene by satellite cells provide further support that proliferating progeny of satellite cells give rise to both differentiating myoblasts and a self-renewing population of satellite cells (Day et al., 2007). Nestin is an intermediate filament protein that was originally identified in neural progenitors. However, endogenous nestin expression was subsequently detected in a wide range of progenitor cells, including proliferating and differentiating myoblasts (summarized in Day et al., 2007). Unexpectedly, when investigating transgenic mice expressing GFP driven by regulatory elements of the nestin gene, we identified GFP expression within resident satellite cells, which permitted characterization of these cells in their niche (Day et al., 2007). Sorted nestin-GFP-positive cells exclusively acquired a myogenic fate even when supplemented with media supporting nonmyogenic development. Nestin-GFP expression declined following satellite cell activation and was reacquired by nonproliferating Pax7-positive progeny in highly differentiated myogenic cultures composed mainly of extensive networks of myotubes. Our recent studies demonstrated that sorted nestin-GFP-positive cells isolated from such highly differentiated myogenic cultures give rise to proliferating and differentiating myoblasts as well as to new nestin-GFP-positive expressing cells (K. Day and Z. Yablonka-Reuveni, unpublished results). We propose that the dynamics of the nestin-GFP expression pattern reflect satellite cell self-renewal. Another notable observation was that renewed cells always developed in close association with myotubes, suggesting a role for the myogenic environment generated by myotubes in directing myoblasts to enter the satellite cell compartment. Furthermore, our clonal studies, which showed the re-emergence of nestin-GFP-positive cells in progeny of individual satellite cells (Day et al., 2007), also demonstrated that not all clones gave rise to nestin-GFP-positive cells. The outcome of our clonal analysis may imply that the initial pool of satellite cells (nestin-GFP-positive cells) is heterogeneous and composed of cell subsets that can self-renew (i.e., give rise to new nestin-GFP-positive cells) or produce only differentiating myoblasts. Our hypothesis that satellite cells self-renew in an environment generated by myotubes does not preclude the contribution of intracellular mechanisms, such as differences in proliferative rates (Schultz, 1996) or asymmetrical cell division (Shinin et al., 2006; Kuang et al., 2007) to self-renewal.
NESTIN-GFP TRANSGENE EXPRESSION PERMITS INSIGHT INTO SATELLITE CELL MOLECULAR SIGNATURES
From our recent studies on satellite cells in nestin-GFP transgenic mice, we established a novel direction for analyzing the molecular signature of satellite cells. With the ability to sort freshly isolated satellite cells (GFP-positive), gene expression by satellite cells can be compared across different muscle groups (Day et al., 2007). Indeed, we showed that satellite cells from all muscle groups expressed Pax7 and Myf5, whereas Pax3 was expressed in satellite cells from diaphragm muscles, but not from hindlimb muscles. We also investigated transcription factors that were previously shown to promote nestin expression by binding to the second intron enhancer region of the nestin gene, which we hypothesized could also account for directing the nestin transgene expression in satellite cells (Tanaka et al., 2004). Specifically, expression of mouse brain-2 gene, a POU domain transcription factor, was detected in nestin-GFP-negative, but not in nestin-GFP-positive, cells. Expression of the sex-determining region Y-box (Sox)2 gene was below detection levels in both populations (Day et al., 2007). Therefore, the mouse brain-2 gene and Sox2 may not regulate nestin-GFP in satellite cells. Additionally, we analyzed the expression of Sox8 and Sox9, which were previously reported to be expressed in satellite cells (Schmidt et al., 2003). These 2 Sox genes were expressed in both nestin-GFP-positive and nestin-GFP-negative sorted cells, which indicated that these 2 transcription factors are not exclusive to satellite cells (Day et al., 2007).
SUMMARY
Herein we summarized recent studies carried out in our laboratory, which were aimed at elucidating the transcriptional signature of satellite cells. We attempted to decipher molecular paths that control the decision between myogenic differentiation vs. renewal of satellite cells (Day et al., 2007). We are also interested in elucidating how environmental cues may direct satellite cells into other mesenchymal lineages in disease and aging (Shefer and Yablonka-Reuveni, 2007). In pilot studies, we demonstrated that freshly sorted nestin-GFP-positive satellite cells could be transduced with lentiviruses to deliver reporter genes for investigation of gene function during different stages of myogenesis. By using this approach to over-express or knock down genes of interest (e.g., Pax7, Myf5, MyoD) in satellite cells, we will gain insight into their individual functions during myogenesis. Additionally, understanding the regulation of nestin-GFP transgene expression in satellite cells and their self-renewed progeny will provide new information about transcriptional mechanisms that control self-renewal. Greater understanding of satellite cell genetic programs may contribute to developing strategies for enhancing muscle growth, an important aspect of meat production, myofiber maintenance, and combating the age-dependent decline of muscle mass and strength in humans.
Footnotes
This work was funded by grants to Z. Yablonka-Reuveni from the USDA Cooperative State Research, Education, and Extension Service (National Research Initiative Competitive Grant no. 2003-35206-12843) and from the National Institutes of Health (AG21566 and AG13798). K. Day received support from the Genetic Approaches to Aging Training Grant program. We thank our former team member, Joshua B. Richardson (New York University, New York), for his valuable contributions to the studies described in this paper.
Presented at the Growth and Development symposium at the annual meeting of the American Society of Animal Science, San Antonio, TX, July 8 to 12, 2007.
LITERATURE CITED
- Anderson JE. The satellite cell as a companion in skeletal muscle plasticity: Currency, conveyance, clue, connector and colander. J Exp Biol. 2006;209:2276–2292. doi: 10.1242/jeb.02088. [DOI] [PubMed] [Google Scholar]
- Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber TD, Barber MC, Cloutier TE, Friedman TB. Pax3 gene structure, alternative splicing and evolution. Gene. 1999;237:311–319. doi: 10.1016/s0378-1119(99)00339-x. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DSW, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development. 1990;109:943–952. doi: 10.1242/dev.109.4.943. [DOI] [PubMed] [Google Scholar]
- Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Bajard L, Daubas P, Esner M, Lagha M, Relaix F, Rocancourt D. Myogenic progenitor cells in the mouse embryo are marked by the expression of Pax3/7 genes that regulate their survival and myogenic potential. Anat Embryol (Berl) 2006;211(Suppl 1):51–56. doi: 10.1007/s00429-006-0122-0. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, De Santa F, Puri PL, Mattei E, Ricci L, Bucci F, Felsani A, Caruso M. Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblast differentiation. Mol Cell Biol. 1999;19:5203–5217. doi: 10.1128/mcb.19.7.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, Lipson KE, Ramphal J, Do S, Cui JJ, Cherrington JM, Mendel DB. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Falco G, Comes F, Simone C. pRb: Master of differentiation Coupling irreversible cell cycle withdrawal with induction of muscle-specific transcription. Oncogene. 2006;25:5244–5249. doi: 10.1038/sj.onc.1209623. [DOI] [PubMed] [Google Scholar]
- Galli LM, Willert K, Nusse R, Yablonka-Reuveni Z, Nohno T, Denetclaw W, Burrus LW. A proliferative role for Wnt-3a in chick somites. Dev Biol. 2004;269:489–504. doi: 10.1016/j.ydbio.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. Molecular and cell biology of skeletal muscle regeneration. Mol Cell Biol Hum Dis Ser. 1993;3:210–256. doi: 10.1007/978-94-011-1528-5_9. [DOI] [PubMed] [Google Scholar]
- Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: Physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Huh MS, Parker MH, Scime A, Parks R, Rudnicki MA. Rb is required for progression through myogenic differentiation but not maintenance of terminal differentiation. J Cell Biol. 2004;166:865–876. doi: 10.1083/jcb.200403004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 2000;48:1079–1096. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Fernandez A. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell Mol Life Sci. 2001;58:571–579. doi: 10.1007/PL00000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann M, Vandromme M, Schaeffer V, Carnac G, Labbé JC, Lamb N, Fernandez AM. Cdk1- and cdk2-mediated phosphorylation of MyoD Ser200 in growing C2 myoblasts: Role in modulating MyoD half-life and myogenic activity. Mol Cell Biol. 1999;19:3167–3176. doi: 10.1128/mcb.19.4.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Chargé SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamey TM, Koenders A, Ziman M. Pax genes in myogenesis: Alternate transcripts add complexity. Histol Histopathol. 2004;19:1289–1300. doi: 10.14670/HH-19.1289. [DOI] [PubMed] [Google Scholar]
- Lindon C, Montarras D, Pinset C. Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J Cell Biol. 1998;140:111–118. doi: 10.1083/jcb.140.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Honore A, Rana V, Arsic N, Franckhauser C, Lamb NJ, Fernandez A. Identification of a new hybrid serum response factor and myocyte enhancer factor 2-binding element in MyoD enhancer required for MyoD expression during myogenesis. Mol Biol Cell. 2007;18:1992–2001. doi: 10.1091/mbc.E06-09-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph DC, Konieczny SF. Transcription factor families: Muscling in on the myogenic program. FASEB J. 1995;9:1595–1604. doi: 10.1096/fasebj.9.15.8529839. [DOI] [PubMed] [Google Scholar]
- Mauro AR. Satellite cell of skeletal muscle fibers. J Cell Biol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Spicer DB, Kim PS, Cheung WL, Lassar AB. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr Biol. 1999;9:449–459. doi: 10.1016/s0960-9822(99)80210-3. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Yang Z, Tapscott SJ, Olwin BB. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol. 2007;177:769–779. doi: 10.1083/jcb.200608122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RLS, Parker MH, Rudnicki MA. Activated MEK1 binds the nuclear MyoD transcriptional complex to repress transactivation. Mol Cell. 2001;8:291–301. doi: 10.1016/s1097-2765(01)00302-1. [DOI] [PubMed] [Google Scholar]
- Puri PL, Iezzi S, Stiegler P, Chen TT, Schiltz RL, Muscat GEO, Giordano A, Kedes L, Wang JYJ, Sartorelli V. Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol Cell. 2001;8:885–897. doi: 10.1016/s1097-2765(01)00373-2. [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18:1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PNJ, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Glaser G, Wernig A, Wegner M, Rosorius O. Sox8 is a specific marker for muscle satellite cells and inhibits myogenesis. J Biol Chem. 2003;278:29769–29775. doi: 10.1074/jbc.M301539200. [DOI] [PubMed] [Google Scholar]
- Schultz E. Satellite cell proliferative compartments in growing skeletal muscles. Dev Biol. 1996;175:84–94. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Yablonka-Reuveni Z. Reflections on lineage potential of skeletal muscle satellite cells: Do they sometimes go MAD? Crit Rev Eukaryot Gene Expr. 2007;17:13–29. doi: 10.1615/critreveukargeneexpr.v17.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- Song A, Wang Q, Goebl MG, Harrington MA. Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol Cell Biol. 1998;18:4994–4999. doi: 10.1128/mcb.18.9.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulez M, Rouviere CG, Chafey P, Hentzen D, Vandromme M, Lautredou N, Lamb N, Kahn A, Tuil D. Growth and differentiation of C2 myogenic cells are dependent on serum response factor. Mol Cell Biol. 1996;16:6065–6074. doi: 10.1128/mcb.16.11.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kamachi Y, Tanouchi A, Hamada H, Jing N, Kondoh H. Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol. 2004;24:8834–8846. doi: 10.1128/MCB.24.20.8834-8846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott SJ. The circuitry of a master switch: MyoD and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Vorobyov E, Horst J. Getting the proto-Pax by the tail. J Mol Evol. 2006;63:153–164. doi: 10.1007/s00239-005-0163-7. [DOI] [PubMed] [Google Scholar]
- White JD, Scaffidi A, Davies M, McGeachie J, Rudnicki MA, Grounds MD. Myotube formation is delayed but not prevented in MyoD-deficient skeletal muscle: Studies in regenerating whole muscle grafts of adult mice. J Histochem Cytochem. 2000;48:1531–1544. doi: 10.1177/002215540004801110. [DOI] [PubMed] [Google Scholar]
- Wyzykowski JC, Winata TI, Mitin N, Taparowsky EJ, Konieczny SF. Identification of novel MyoD gene targets in proliferating myogenic stem cells. Mol Cell Biol. 2002;22:6199–6208. doi: 10.1128/MCB.22.17.6199-6208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Development and postnatal regulation of adult myoblasts. Microsc Res Tech. 1995;30:366–380. doi: 10.1002/jemt.1070300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Anderson JE. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn. 2006;235:203–212. doi: 10.1002/dvdy.20602. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Paterson BM. MyoD and myogenin expression patterns in cultures of fetal and adult chicken myoblasts. J Histochem Cytochem. 2001;49:455–462. doi: 10.1177/002215540104900405. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Influence of PDGF-BB on proliferation and transition through the MyoD-myogenin-MEF2A expression program during myogenesis in mouse C2 myoblasts. Growth Factors. 1997a;15:1–27. doi: 10.3109/08977199709002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Proliferative dynamics and the role of FGF2 during myogenesis of rat satellite cells on isolated fibers. Basic Appl Myol. 1997b;7:189–202. [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol. 1999a;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seger R, Rivera AJ. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem. 1999b;47:23–42. doi: 10.1177/002215549904700104. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J Histochem Cytochem. 2006a;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006b;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]