Abstract
Pheochromocytomas and paragangliomas are chromaffin cell tumors arising from neuroendocrine cells. At least one third of paragangliomas are related to germline mutations in one of 17 genes. While these tumors can occur throughout the body, cardiac paragangliomas are very rare, accounting for less than 0.3% of mediastinal tumors. The purpose of this study was to determine the clinical characteristics of patients with cardiac paragangliomas, particularly focusing on their genetic backgrounds. A retrospective chart analysis of fifteen patients with cardiac paraganglioma was performed to determine clinical presentation, genetic background, diagnostic work-up, and outcomes. The average age at diagnosis was 41.9 years. Typical symptoms of paraganglioma (e.g., hypertension, sweating, palpitations, headache) were reported at initial presentation in 13 patients (86.7%); the remaining 2, as well as 4 symptomatic patients, initially presented with cardiac-specific symptoms (e.g., chest pain, dyspnea). Genetic testing was done in 13 cases (86.7%); 10 (76.9%) were positive for mutations in succinate dehydrogenase (SDHx) subunits B, C, or D. Thirteen cases (86.7%) underwent surgery to remove the paraganglioma with no intraoperative morbidity or mortality; one additional patient underwent surgical resection but experienced intraoperative complications after removal of the tumor due to comorbities and did not survive. SDHx mutations are known to be associated with mediastinal locations and malignant behavior of paragangliomas. In this report, we extend the locations of predominantly SDHx-related paragangliomas to cardiac tumors. In conclusion, cardiac paragangliomas are frequently associated with underlying SDHx germline mutations, suggesting a need for genetic testing of all patients with this rare tumor.
Keywords: Pheochromocytoma, Paraganglioma, Succinate Dehydrogenase, Cardiac tumors, Genetics
INTRODUCTION
Paragangliomas (PGL) are rare extra-adrenal chromaffin cell tumors arising from neuroendocrine cells. These tumors have the ability to synthesize, secrete, store, and metabolize catecholamines, leading to clinical manifestations such as hypertension, headaches, palpitations, and sweating.1 Biochemical diagnosis is based on the measurement of catecholamines and metanephrines in the plasma and/or urine, and tumors can be classified based on their predominant hormone secretion.2 Currently, 17 genes have been linked to the pathogenesis of PGL, responsible for approximately 35% of cases. Mutations in all 4 subunits (A, B, C, and D) of succinate dehydrogenase (SDH) are some of the most common, particularly SDHB and SDHD. SDH, or mitochondrial complex II, is involved in both the Krebs cycle and electron transport chain, linking important cellular metabolic processes. SDH-related tumors are usually extra-adrenal and commonly multiple in number; SDHB mutations have a higher rate of metastases.3 Cardiac PGLs are extremely rare,4 comprising 1-3% of primary cardiac tumors.5,6 Numerous case reports have been published about cardiac PGLs; however, their genetic background, outcomes, and management have not been studied in a large series of patients. The present study summarizes our extensive experience with cardiac PGLs, focusing particularly on their genetic background.
METHODS
This is a multi-institutional study that included 15 patients with documented cardiac PGLs who presented to the National Institutes of Health, Bethesda, Maryland; Tufts University Medical Center, and Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts. These patients were selected from a large registry of pheochromocytoma (PHEO) and PGL patients available at each institution. Charts were reviewed to determine the genetic backgrounds, biochemical results, imaging studies, surgical pathology, treatments, and outcomes of each patient. The study was reviewed and approved by the Institutional Review Boards of each respective institution. All patients provided written and informed consent.
Blood samples for genetic testing of germline DNA were collected. Genomic DNA was extracted from whole blood, and PCR-based bidirectional sequencing for SDHB, SDHC, and/or SDHD was performed by Mayo Medical Laboratories, Rochester, MN, or by the Division of Molecular Diagnostics at the University of Pittsburgh Medical Center, as previously described.7 Testing for large deletions was done with multiplex ligation-dependent probe amplification and Luminex® FlexMap Technologies.8
Tumors were classified as adrenergic (secreting predominantly epinephrine and/or its metabolite metanephrine), noradrenergic (secreting predominantly norepinephrine and/or its metabolite normetanephrine), or dopaminergic (secreting predominantly dopamine and/or its metabolite methoxytyramine) based on their predominant hormone secretion, as previously described.2 Elevations in biochemistry were defined as any levels above the upper reference limit.
RESULTS
A total of 15 patients were identified with cardiac PGLs. The average age at diagnosis was 41.9 years (range 28-63). Eight patients were male (53.3%). In most patients, symptoms at presentation were typical for catecholamine excess, with 13 (86.7%) presenting with a combination of palpitations, hypertension, headaches, sweating, and anxiety (Table 1). Six patients (40%), including the two patients without catecholamine-related symptoms, also displayed cardiac-related symptoms of chest pain and/or shortness of breath (Table 1). Other less common symptoms seen only in one patient included weakness, flushing, sleep apnea, hot flashes, weight loss, and fatigue.
Table 1. Patient symptoms before the diagnosis of cardiac paraganglioma.
| Patient | Headache | Hypertension | Palpitations | Tachycardia | Anxiety/ Nervousness |
Sweating | Nausea/ Vomiting |
Dyspnea | Chest Pain |
|---|---|---|---|---|---|---|---|---|---|
| 1 | − | + | − | − | + | − | − | − | − |
| 2 | + | + | + | − | − | − | − | − | − |
| 3 | − | − | − | − | − | − | − | + | + |
| 4 | + | + | + | − | + | + | − | − | − |
| 5 | + | + | − | − | − | + | + | − | − |
| 6 | + | + | − | + | + | + | + | + | + |
| 7 | − | − | − | − | + | + | − | − | − |
| 8 | − | − | + | + | + | − | − | − | − |
| 9 | − | + | − | − | − | − | − | − | − |
| 10 | − | − | − | − | − | − | − | + | − |
| 11 | − | − | + | − | + | − | − | + | − |
| 12 | − | + | + | − | − | − | − | + | + |
| 13 | − | + | − | − | − | − | − | − | − |
| 14 | + | + | − | − | − | − | − | − | − |
| 15 | + | + | + | − | − | − | − | − | + |
Patients were diagnosed with a combination of biochemical testing and multiple imaging studies. All 15 patients underwent biochemical testing with plasma and/or urinary catecholamines and/or metanephrines. One patient (6.67%) had normal catecholamines and metanephrines. Of the patients with elevated biochemistry, 13 (92.8%) had noradrenergic phenotypes, with elevations of norepinephrine and/or normetanephrine; 7 of these (53.8%) also had elevated dopamine. The remaining patient (7.1%) only had elevated dopamine. Patients were imaged with both anatomical and functional imaging modalities. Four patients underwent computed tomography (CT) and magnetic resonance imaging (MRI); 3 were positive on each modality. Nine patients underwent cardiac MRI, with all identifying the tumor. Transthoracic echocardiograms were performed with successful tumor localization in 9 patients. The most commonly used functional imaging modality was 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy. Although 11 patients were scanned with this modality, only 6 (54.5%) had positive scans for the cardiac tumor. Ten patients underwent positron emission tomography (PET) scanning with 18F-fluorodeoxyglucose (18F-FDG), and all 10 had positive uptake in the cardiac tumor. Seven patients had PET scans with 18F-fluorodopamine (18F-FDA) and 18F-fluorodopa (18F-FDOPA); 4 were positive on 18F-FDA (57.1%), but all 7 had uptake on 18F-FDOPA. Octreotide (111In-pentetreotide) scintigraphy was also used in a 5 patients; 3 (60%) had positive scans. A summary of each patient’s diagnostic workup and treatment is presented in Table 2.
Table 2. Genetics, imaging, biochemistry, and management of patients with cardiac paraganglioma.
| Pt | Age (yrs) |
Genetics | Biochemistry* | Surgical Resection |
Tumor Size (cm) |
Tumor Location |
Mlt | Mets | Treatment | Died | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDHB | SDHC | SDHD | CgA | DA | MTX | NE | NMN | VMA | |||||||||
| 1 | 36 | + | 0 | 0 | --- | 0 | --- | + (p, u) | + (p, u) | + (u) | + | 2.9 × 2.2 × 2.4 | L atrium | + | 0 | 0 | 0 |
| 2 | 38 | + | 0 | 0 | --- | --- | + (p, u) | + (p, u) | --- | + | 3 × 2 × 2 | R ventricle | + | 0 | 0 | 0 | |
| 3 | 50 | + | 0 | 0 | --- | 0 | --- | 0 | 0 | --- | + | 8 × 7 | L ventricle | 0 | 0 | 0 | 0 |
| 4 | 33 | 0 | + | 0 | --- | + (u) | --- | + (u) | + (u) | --- | + | 5.3 × 5.6 | R AV groove |
+ | 0 | 0 | 0 |
| 5 | 37 | 0 | + | 0 | --- | 0 | --- | + (u) | + (p) | --- | + | 3.6 | R atrium/AV groove |
+ | 0 | cisplatin/ etoposide |
0 |
| 6 | 46 | 0 | + | 0 | + (p) | + (p) | 0 | 0 | 0 | --- | +** | 3.2 × 2.3 | L atrium | 0 | 0 | CVD (pre- operative) |
+ |
| 7 | 28 | 0 | 0 | + | --- | + (u) | + (u) | + (u) | + (u) | --- | + | 7 × 3 × 2 | L AV groove |
+ | 0 | 0 | 0 |
| 8 | 31 | 0 | 0 | + | + (p) | + (u) | --- | + (p, u) | + (p) | --- | + | 2.5 × 1.9 × 2.1 | R AV groove |
+ | 0 | 0 | 0 |
| 9 | 39 | 0 | 0 | + | + (p) | + (p) | --- | + (p) | + (u) | + (u) | ND | L atrium | + | 0 | 0 | 0 | |
| 10 | 63 | 0 | 0 | + | --- | + (p, u) | --- | + (p, u) | + (p, u) | + (u) | + | 3.5 × 3.4 × 4.2 | R atrium | + | 0 | 0 | 0 |
| 11 | 59 | 0 | --- | 0 | --- | + (u) | --- | + (u) | + (p, u) | --- | + | 6.7 | AV groove | 0 | + | CVD | 0 |
| 12 | 33 | 0 | 0 | 0 | + (p) | + (p, u) | + (p) | + (p, u) | + (p, u) | --- | + | 3.4 cm | L atrium | 0 | 0 | 0 | 0 |
| 13 | 63 | 0 | 0 | 0 | + (p) | 0 | --- | 0 | + (p) | --- | + | 4.3 × 4.0 × 3.5 | R atrium/R ventricle |
+ | 0 | 0 | 0 |
| 14 | 32 | --- | --- | --- | --- | 0 | --- | + (p) | 0 | --- | + | --- | 0 | + | 0 | + | |
| 15 | 40 | --- | --- | --- | --- | 0 | --- | + (p) | 0 | --- | + | L atrium | + | + | radiation | + | |
Abbreviations: ---: not done or not available, AV: atrioventricular, CgA: chromogranin A, CVD: cyclophosphamide/vincristine/dacarbazine chemotherapy, DA: dopamine, L: left, Mets: metastases, Mlt: multiple; MTX: methoxytyramine, NE: norepinephrine, NMN: normetanephrine, p: plasma, R: right, SDHB: succinate dehydrogenase subunit B, SDHC: succinate dehydrogenase subunit C, SDHD: succinate dehydrogenase subunit D, u: urine, VMA: vanillylmandelic acid
: Epinephrine and metanephrine values are not listed because they were negative in all tested patients.
: Tumor was successfully resected, but patient suffered intraoperative complications after resection and did not survive.
In 13 patients (86.7%), surgery was the initial treatment for the cardiac tumor. None of the patients experienced intra- or post-operative complications. One additional patient (6.7%) was first treated with 7 cycles of chemotherapy with cyclophosphamide, vincristine, and dacarbazine (CVD) in an attempt to shrink the tumor before surgery; however, the tumor did not respond to this regimen. The patient was then taken to the operating room with successful resection of the tumor; however, post-operatively he bled from the left main coronary artery, leading to ventricular fibrillation arrest and death. The remaining patient did not undergo a surgical procedure or any treatment, but is undergoing careful surveillance. After surgery, one patient (6.7%) had residual tumor upon follow-up imaging. Another patient (6.7%) had a recurrent tumor in the heart, which mandated a second resection. This patient had positive surgical margins at the first operation, for which she underwent 6 cycles of chemotherapy with cisplatin and etoposide. Ten patients (66.7%) had multiple primary tumors, commonly located in the carotid body (4/9, 44.4%) and jugular foramen (4/9, 44.4%), though other locations were also found. One of the 10 patients had a prior history of adrenal PHEO. Three patients (20%) later developed metastases; one of these patients underwent CVD chemotherapy, and a second received external beam radiation for lesions in the spine. Two of the patients who developed metastases died; the third has stable disease on CVD (Table 2).
Thirteen patients underwent genetic testing; 10 (76.9%) had mutations identified in subunits of the SDH complex. Three had SDHB mutations, 3 SDHC, and 4 SDHD. Of the remaining patients, 2 underwent testing for all three of these genes and had no mutations identified; 1 had negative test results for SDHB and SDHD (Table 2).
DISCUSSION
The present study demonstrates an association between cardiac PGLs and mutations in SDHB, SDHC, and SDHD genes. More than three fourths of the patients in this series who underwent genetic testing had a germline mutation in an SDHx gene, which is much higher than previously reported rates of SDHB (8.46%), SDHC (1.85%), and SDHD (6.2%) mutations in sporadic tumors.9 This is also higher than rates of SDHx mutations in extra-adrenal PGLs in our database; of 106 abdominal and thoracic PGLs, 65 (61.3%) had mutations in an SDHx gene. It is important to note that one of the patients who did not have genetic testing had a strong family history of PGL, suggesting an undetected germline mutation, but the patient died before testing could be performed. In addition, one of the patients with no known mutation was only tested for SDHB and SDHD mutations. Although SDHC mutations are rare,9,10 the high prevalence (20%) in this series suggests that this patient may have carried an SDHC mutation. SDHC mutations have previously been associated with head and neck and thoracic PGLs,11,12 as well as one case of a cardiac PGL.13 Further study is needed to elucidate the pathogenic mechanisms by which SDHC mutations cause these rare tumors.
The high percentage of SDHB and SDHD mutations is less surprising, since SDHB and SDHD mutations have already been strongly associated with extra-adrenal PGLs.3 Case reports of patients with cardiac PGLs due to SDHB14-17 and SDHD18,19 mutations have been previously published. These mutations are also common in patients with tumors in the mediastinum.20 SDHx gene mutations are also associated with multiple tumors, which were common in this series, and SDHB mutations are linked to higher rates of metastatic tumors.3 Although 17 genes have been linked to PGL, only SDHx mutations were found in this series.
PGLs are typically biochemically active, leading to their symptomatic presentation. As can be expected for extra-adrenal PGLs, all the functioning tumors secreted norepinephrine and/or normetanephrine. In addition, the secretion of dopamine and its metabolite methoxytyramine are particularly associated with SDHx mutations, as is consistent with the findings in this series.2
Multiple anatomical imaging modalities are available for cardiac tumors. Standard CT and MRI can suggest the presence of a tumor in the region of the heart. Using contrast CT gated to the cardiac cycle for patients with suspected cardiac PGLs significantly improves image quality (Figure 1). In addition, cardiac-specific imaging using cardiac MRI can better delineate cardiac PGLs (Figure 2), as demonstrated by the high rate of positive cardiac MRI in this series. Based on this series, cardiac MRI appears to be the anatomic modality of choice in the evaluation of cardiac PGL.
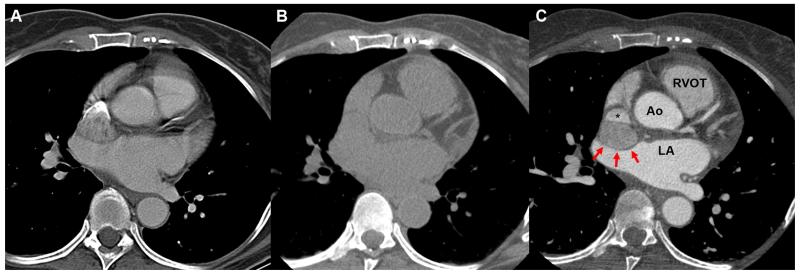
Figure 1.
Different scanning methods with CT. (A) A contrast chest CT that is not gated to the cardiac cycle demonstrates the blurriness of the cardiac silhouette. Contrast can be seen entering into the superior vena cava. (B) Non-contrast gated CT demonstrates the difficulty in seeing the mass next to the superior vena cava. (C) A contrasted gated CT allows better visualization of the mass (red arrows) impinging onto the superior vena cava without blurring of the cardiac silhouette. Ao: Aortic root, LA: left atrium, RVOT: right ventricular outflow tract.
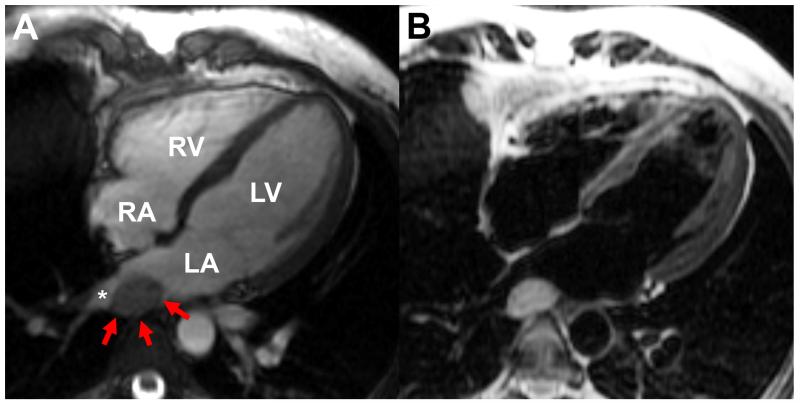
Figure 2.
(A) Cardiac MRI steady state free precession image in the 4-chamber view demonstrating an oval mass (red arrows) posterior to the left atrium (LA) abutting the right lower pulmonary vein (asterisk); (B) with turbo spin echo, the blood signal can be nulled to enhance the appearance of surrounding structures such as the mass (B). LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
Since cardiac PGLs are extremely rare, patients with symptoms of PGL may not routinely undergo cardiac imaging. Functional imaging can assist with initial localization (Figure 3), indicating the tumor’s thoracic location and encouraging more cardiac-specific imaging. 123I-MIBG scintigraphy has long been the gold standard for functional imaging of PGL, but this did not prove to be valuable in this series. This imaging modality is known to have a lower sensitivity for extra-adrenal tumors,21 as well as for SDHx tumors.22 Indeed, 4 out of the 5 patients with false-negative 123I-MIBG scintigraphy in this series had SDHx mutations.
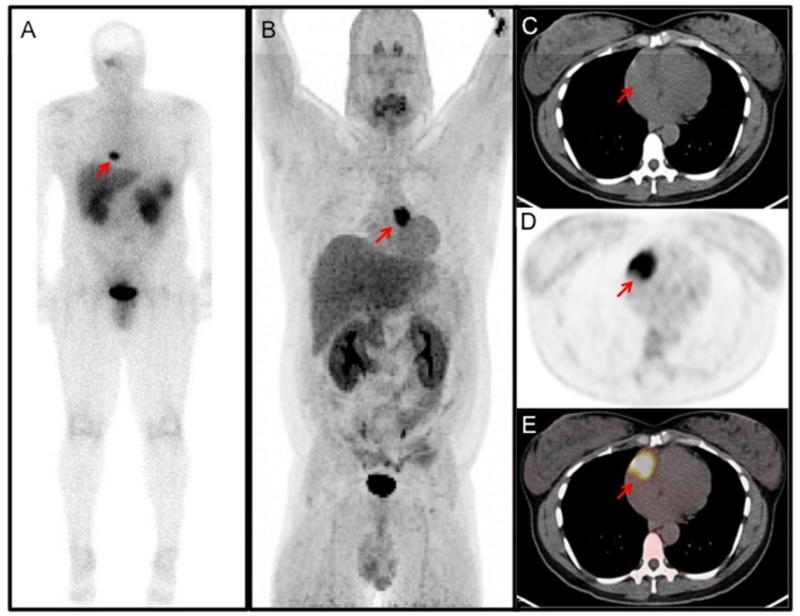
Figure 3.
Functional imaging of cardiac PGLs from different patients. (A) Octreotide (111In-pentetreotide) scan demonstrating increased uptake in the neck and heart. There is also increased uptake in the liver, kidneys, spleen, gallbladder, and urinary bladder, which are physiologic. (B) 18F-FDOPA (18F-fluorodihdyroxyphenylalanine) PET demonstrating a large cardiac paraganglioma at the base of the heart near the root of the great vessels. (C-E) 18F-FDG PET/CT of a mass in the right atrioventricular groove. (C) Non-contrast CT scan for localization; (D) 18F-FDG PET showing an area of high activity; (E) fused PET/CT image.
Octreotide scintigraphy, which targets somatostatin receptors, is also sometimes used in PGL management. Octreotide scintigraphy has recently been shown to be a sensitive technique for screening SDHB mutation carriers23; however, its limited use in this series makes it difficult to draw conclusions about its utility. The preliminary data from this series do not suggest a primary role for 123I-MIBG or octreotide scintigraphy in the evaluation of cardiac PGL.
Due to the availability of multiple tracers and the more precise localization offered by PET, this technique has become more commonly used in the localization of PGL. Of the available PET imaging tracers, 18F-FDG is the most widely available. A high specificity of 18F-FDG PET has been established, particularly in SDHB-related tumors.21,24,25 More specific tracers for PGL have been identified, including 18F-FDOPA and 18F-FDA. However, these tracers are less widely available, so their use in this series was limited. 18F-FDOPA PET has been shown to have a sensitivity similar to 18F-FDG PET, except in the head and neck, where it is superior.21,25 A high sensitivity for 18F-FDOPA PET was also described in SDHx mutation carriers,26 which suggests a possible larger role in the imaging of cardiac PGLs. Overall, based on this study, 18F-FDG PET may be the most valuable functional imaging modality for evaluation of patients with cardiac PGL, especially due to its wide availability.
Because of their location, cardiac PGLs can be difficult to approach surgically, but this is the only curative treatment available. Pre-operative blockade is essential; adequate blood pressure control can be obtained as long as the patient has been appropriately blocked. For patients in whom surgery is not an option due to the location or invasion by the tumor, treatment options are limited. Symptom management may be obtained using anti-hypertensive medications, but there is no strong evidence for pre-operative treatment with chemotherapy.
Further treatment after surgery may also need to be considered in patients who develop metastatic disease. CVD remains one of the most effective treatments for metastatic PGL.27 131I-MIBG therapy is also used as a treatment modality, but its use is limited to patients in whom 123I-MIBG scanning is positive. Another novel treatment option is the use of radiolabeled DOTA peptides, which can be used for patients in whom 68Ga-DOTA PET scanning or Octreotide scintigraphy is positive. Preliminary results with these treatment modalities have been promising, especially in patients with SDHx mutations,28-30 suggesting a possible future role in patients with cardiac PGL.
Cardiac PGLs are extremely rare, accounting for approximately 1-3% of cardiac and pericardiac neoplasms in imaging and autopsy series.5,6 Therefore, accumulating a large enough case series to draw meaningful conclusions is a difficult prospect. While numerous case reports exist in the literature, this article represents the first attempt to gather a larger series to guide the management of these rare tumors. The finding that 76.9% of these tumors are due to underlying mutations in SDHB, SDHC, and SDHD suggests the importance of genetic testing to determine appropriate follow-up and to provide information to family members who may also be affected. Although multiple tumors were fairly common within this series, metastases were relatively rare, suggesting that with appropriate surgical management, patients with cardiac PGL can have successful outcomes. Proper diagnosis, including recognition of symptoms, biochemical testing, and appropriate anatomical and functional imaging, are critical to these successful outcomes.
ACKNOWLEDGMENTS
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Lenders JWM, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 2.Eisenhofer G, Lenders JWM, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, Bornstein SR, Tiebel O, Adams K, Bratslavsky G, Linehan WM, Pacak K. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–420. doi: 10.1373/clinchem.2010.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44:328–333. doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- 4.Whalen RK, Althausen AF, Daniels GH. Extra-adrenal pheochromocytoma. J Urol. 1992;147:1–10. doi: 10.1016/s0022-5347(17)37119-7. [DOI] [PubMed] [Google Scholar]
- 5.Patel J, Sheppard MN. Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovasc Pathol. 2010;19:343–352. doi: 10.1016/j.carpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Beroukhim RS, Prakash A, Buechel ERV, Cava JR, Dorfman AL, Festa P, Hlavacek AM, Johnson TR, Keller MS, Krishnamurthy R, Misra N, Moniotte S, Parks WJ, Powell AJ, Soriano BD, Srichai MB, Yoo SJ, Zhou J, Geva T. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol. 2011;58:1044–1054. doi: 10.1016/j.jacc.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Brouwers FM, Elkahloun AG, Munson PJ, Eisenhofer G, Barb J, Linehan WM, Lenders JWM, de Krijger R, Mannelli M, Udelsman R, Ocal IT, Shulkin BL, Bornstein SR, Breza J, Ksinantova L, Pacak K. Gene expression profiling of benign and malignant pheochromocytoma. Ann NY Acad Sci. 2006;1073:541–556. doi: 10.1196/annals.1353.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monico CG, Rossetti S, Schwanz HA, Olson JB, Lundquist PA, Dawson DB, Harris PC, Milliner DS. Comprehensive mutation screening in 55 probands with type 1 primary hyperoxaluria shows feasibility of a gene-based diagnosis. J Am Soc Nephrol. 2007;18:1905–1914. doi: 10.1681/ASN.2006111230. [DOI] [PubMed] [Google Scholar]
- 9.Buffet A, Venisse A, Nau V, Roncellin I, Boccio V, Le Pottier N, Boussion M, Travers C, Simian C, Burnichon N, Abermil N, Favier J, Jeunemaitre X, Gimenez-Roqueplo AP. A decade (2001-2010) of genetic testing for pheochromocytoma and paraganglioma. Horm Metab Res. 2012;44:359–366. doi: 10.1055/s-0032-1304594. [DOI] [PubMed] [Google Scholar]
- 10.Schiavi F, Boedeker CC, Bausch B, Peczkowska M, Gomez CF, Strassburg T, Pawlu C, Buchta M, Salzmann M, Hoffmann MM, Berlis A, Brink I, Cybulla M, Muresan M, Walter MA, Forrer F, Valimaki M, Kawecki A, Szutkowski Z, Schipper J, Walz MK, Pigny P, Bauters C, Willet-Brozick JE, Baysal BE, Januszewicz A, Eng C, Opocher G, Neumann HPH, European-American Paraganglioma Study Group Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA. 2005;294:2057–2063. doi: 10.1001/jama.294.16.2057. [DOI] [PubMed] [Google Scholar]
- 11.Boedeker CC, Hensen EF, Neumann HPH, Maier W, van Nederveen FH, Suarez C, Kunst HP, Rodrigo JP, Takes RP, Pellitteri PK, Rinaldo A, Ferlito A. Genetics of hereditary head and neck paragangliomas. Head Neck. 2014;36:907–916. doi: 10.1002/hed.23436. [DOI] [PubMed] [Google Scholar]
- 12.Else T, Marvin ML, Everett JN, Gruber SB, Arts HA, Stoffel EM, Auchus RJ, Raymond VM. The clinical phenotype of SDHC-associated hereditary paraganglioma syndrome (PGL3) J Clin Endocrinol Metab. 2014;99:E1482–1486. doi: 10.1210/jc.2013-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Illouz F, Pinaud F, De Brux JL, Mirebeau-Prunier D, Rodien P. Long-delayed localization of a cardiac functional paraganglioma with SDHC mutation. Ann Intern Med. 2012;157:222–223. doi: 10.7326/0003-4819-157-3-201208070-00026. [DOI] [PubMed] [Google Scholar]
- 14.Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peczkowska M, Morrison CD, Lehtonen R, Januszewicz A, Jarvinen H, Juhola M, Mecklin JP, Pukkala E, Herva R, Kiuru M, Nupponen NN, Aaltonen LA, Neumann HPH, Eng C. Early-Onset Renal Cell Carcinoma as a Novel Extraparaganglial Component of SDHB-Associated Heritable Paraganglioma. Am J Hum Genet. 2004;74:153–159. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beroukhim RS, del Nido P, Teot LA, Janeway K, Geva T. Cardiac paraganglioma in an adolescent. Circulation. 2012;125:e322–324. doi: 10.1161/CIRCULATIONAHA.111.043968. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez Lopez MT, Gonzalez SG, Garcia ES, Romero SG, de Loma JG. Surgical excision with left atrial reconstruction of a primary functioning retrocardiac paraganglioma. J Cardiothorac Surg. 2013;8:22. doi: 10.1186/1749-8090-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moline J, Ngeow J, Rajiah P, Eng C. Evil lurks in the heart of man: cardiac paraganglioma presenting as recurrent dyspnoea and chronic cough. BMJ Case Rep. 2011 doi: 10.1136/bcr.11.2011.5170. doi: 10.1136/bcr.11.2011.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Didier D, Meyer P, Philippe J. Multislice gated-computed tomography of cardiac paragangliomas. J Am Coll Cardiol. 2010;55:2509. doi: 10.1016/j.jacc.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 19.Petramala L, Cotesta D, Filetti S, Letizia C. Pigmented ‘black’ cardiac paraganglioma in a patient with a novel germ-line SDHD mutation. Eur J Cardio-Thorac Surg. 2009;35:189. doi: 10.1016/j.ejcts.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Ghayee HK, Havekes B, Corssmit EPM, Eisenhofer G, Hammes SR, Ahmad Z, Tessnow A, Lazurova I, Adams KT, Fojo AT, Pacak K, Auchus RJ. Mediastinal paragangliomas: association with mutations in the succinate dehydrogenase genes and aggressive behavior. Endocr Relat Cancer. 2009;16:291–299. doi: 10.1677/ERC-08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taieb D, Timmers HJ, Hindie E, Guillet BA, Neumann HP, Walz MK, Opocher G, de Herder WW, Boedeker CC, de Krijger RR, Chiti A, Al-Nahhas A, Pacak K, Rubello D, European Association of Nuclear Medicine EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39:1977–1995. doi: 10.1007/s00259-012-2215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonte JS, Robles JF, Chen CC, Reynolds J, Whatley M, Ling A, Mercado-Asis LB, Adams KT, Martucci V, Fojo T, Pacak K. False-negative 123I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocr Relat Cancer. 2012;19:83–93. doi: 10.1530/ERC-11-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimenez-Roqueplo AP, Caumont-Prim A, Houzard C, Hignette C, Hernigou A, Halimi P, Niccoli P, Leboulleux S, Amar L, Borson-Chazot F, Cardot-Bauters C, Delemer B, Chabolle F, Coupier I, Libe R, Peitzsch M, Peyrard S, Tenenbaum F, Plouin PF, Chatellier G, Rohmer V, EVA Investigators Imaging work-up for screening of paraganglioma and pheochromocytoma in SDHx mutation carriers: a multicenter prospective study from the PGL. J Clin Endocrinol Metab. 2013;98:E162–173. doi: 10.1210/jc.2012-2975. [DOI] [PubMed] [Google Scholar]
- 24.Timmers HJLM, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams KT, Pacak K. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104:700–708. doi: 10.1093/jnci/djs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timmers HJLM, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miederer M, Fottner C, Rossmann H, Helisch A, Papaspyrou K, Bartsch O, Mann WJ, Musholt TJ, Weber MM, Lackner KJ, Schreckenberger M. High incidence of extraadrenal paraganglioma in families with SDHx syndromes detected by functional imaging with [18F]fluorodihydroxyphenylalanine PET. Eur J Nucl Med Mol Imaging. 2013;40:889–896. doi: 10.1007/s00259-013-2346-6. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez C, Rohren E, Habra MA, Rich T, Jimenez P, Ayala-Ramirez M, Baudin E. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15:356–371. doi: 10.1007/s11912-013-0320-x. [DOI] [PubMed] [Google Scholar]
- 28.Zovato S, Kumanova A, Dematte S, Sansovini M, Bodei L, Di Sarra D, Casagranda E, Severi S, Ambrosetti A, Schiavi F, Opocher G, Paganelli G. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL) Horm Metab Res. 2012;44:411–414. doi: 10.1055/s-0032-1311637. [DOI] [PubMed] [Google Scholar]
- 29.van Essen M, Krenning EP, Kooij PP, Bakker WH, Feelders RA, de Herder WW, Wolbers JG, Kwekkeboom DJ. Effects of therapy with [177Lu-DOTA, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. 2006;47:1599–606. [PubMed] [Google Scholar]
- 30.Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging. 2008;52:334–340. [PubMed] [Google Scholar]