Abstract
We recently validated a method of quantifying systemic to pulmonary arterial collateral flow using phase-contrast magnetic resonance imaging velocity mapping (PC-MRI). Cross-sectional data suggest decreased collateral flow in patients with total cavopulmonary connections (TCPC) compared to those with superior cavopulmonary connections (SCPC). However, no studies have examined serial changes in collateral flow from SCPC to TCPC in the same patients. We sought to examine differences in collateral flow between patients with superior cavopulmonary connections (SCPC) and total cavopulmonary connections (TCPC). We quantified collateral flow by two independent measures from 250 single ventricle studies in 219 different patients, (115 SCPC and 135 TCPC studies, 31 patients with both) and 18 controls, during routine studies using through-plane PC-MRI. Collateral flow was indexed to body surface area, aortic flow and pulmonary venous flow. Regardless of indexing method, SCPC patients had significantly higher collateral flow than TCPC patients (1.64±0.8 vs. 1.03±0.8 L/min/m2, p<0.001). In 31 patients who had serial exams, collateral flow as a fraction of aortic flow increased early after TCPC completion. In TCPC patients, indexed collateral flow demonstrated a significant negative correlation with time from TCPC. In conclusion, both SCPC and TCPC patients demonstrate substantial collateral flow, with SCPC patients having higher collateral flow than TCPC patients overall. Based on the paired subset analysis, collateral flow does not decrease in the short term after TCPC completion and trends toward increasing. In the long term, however, collateral flow decreases over time after TCPC completion.
Keywords: single ventricle, collateral circulation, magnetic resonance imaging, blood flow, Fontan
INTRODUCTION
Systemic to pulmonary collateral flow has been implicated as an important factor in the outcome of patients with cavopulmonary palliation for single ventricle physiology 1,2, though considerable controversy exists over its role 1–5. Collateral flow has been difficult to study systematically because there has previously been no reliable way to measure it. Several groups, including the authors, have recently demonstrated that collateral flow can be accurately measured in patients with superior cavopulmonary connections (SCPC) using phase contrast velocity mapping by magnetic resonance imaging (PC-MRI).6,7 Our group and others have used this method to demonstrate an association between collateral flow and longer hospitalization and duration of pleural effusions following Fontan completion.8–10 The current study reports the largest series to date of single ventricle patients both in cross-section as well as serially who underwent the previously described non-invasive method to test the hypotheses that collateral flow is different between SCPC and TCPC patients and represents a significant hemodynamic burden, and that collateral flow regresses with increasing age in TCPC patients.
METHODS
In a prospectively enrolled cohort, we investigated 285 consecutive studies in patients with SCPC or TCPC physiology at the Children’s Hospital of Philadelphia who underwent CMR from April 2008 to December 2012. 35 studies were excluded for significant accessory pulmonary blood flow (antegrade ventricle to pulmonary artery or residual aortopulmonary shunt flow), significant stent or coil artifacts that severely degraded the required PC-MRI sequences, or limited exams that did not obtain complete PC-MRI. In the resultant 250 studies, 115 with SCPC and 135 with TCPC were included in the analysis. Thirty one patients had CMR studies performed both before and after TCPC completion, allowing for a longitudinal paired analysis in a small subset. To establish a control group, we enrolled 18 two-ventricle patients (2 patients without heart disease, 8 with minor aortic arch anomalies and no prior surgery as well as 8 post-operative 2-ventricle repair patients with no known residual shunts) who had complete pulmonary vein flow and branch pulmonary artery measurements. Eleven of these patients also had vena cavae PC-MRI.
All patients underwent CMR imaging consisting of routine anatomical assessment as well as comprehensive through-plane PC-MRI as part of their routine clinical evaluation or research protocol. Retrospectively gated, through-plane PC-MRI cines were performed in the aorta (native and/or neo-aorta), superior and inferior vena cavae (SVC and IVC), right and left pulmonary arteries (RPA and LPA), and right and left pulmonary veins (RPV and LPV). We previously described the typical protocol with parameters and positions used (see Figure 1).6,11 When two outflows were present (aorta and neo-aorta), they were measured separately and summed for the total aortic flow. When possible (based on whether all the pulmonary veins on one side formed a common vein of sufficient length), all the pulmonary veins on one side were measured in one acquisition. In a subset of patients, descending aortic flow (DAo) at the level of the diaphragm was measured and compared to IVC flow. PC-MRI sequences were analyzed using Argus flow analysis software on a Leonardo workstation (Siemens Medical Solutions, Malvern PA) to obtain the Aortic, SVC, IVC, RPA, LPA, RPV and LPV flows (Q). All contours and values were reviewed by a single observer (KKW). To account for baseline offset in the PC-MRI, a static reference region was contoured on all aortic PC-MRI as close to the vessel of interest as possible. If correction made > 10% difference in the aortic flow, the corrected value was used and static reference regions were checked on the remaining PC-MRI sequences. The following calculations were then made for each patient:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6a) |
| (6b) |
| (7a) |
| (7b) |
where Qcoll-syst and Qcoll-pulm represent the estimated collateral flow by comparing supply and return of the systemic and pulmonary systems respectively. Qcoll is the mean estimator of collateral flow. QP and QS are the total pulmonary venous and systemic venous blood flows respectively. QVR is the total venous return to the heart and should equal the aortic flow leaving the heart. QRL is the right to left shunt, which is equivalent to IVC flow in a SCPC and fenestration flow in a Fontan. In two SCPC patients in whom there was an LSVC to coronary sinus, the LSVC was included in the IVC flow for calculation purposes as the LSVC flow represented systemic venous return not connected directly to the pulmonary arterial system.
Figure 1. Imaging Positions for PC-MRI.
Schematic showing the imaging planes used to calculate collateral flow for TCPC. The yellow bars represent velocity map locations. SVC and IVC are superior and inferior vena cava, RPA and LPA (shown in both left panels) are right and left pulmonary artery, RUPV, RLPV, LUPV, and LLPV are the right and left upper and lower pulmonary veins, LPV is the left common pulmonary vein, Ao is ascending aorta.
Collateral flow was normalized to a) body surface area to obtain an indexed flow, b) aortic flow as a percentage of the entire cardiac output and c) total pulmonary vein flow (QRPV + QLPV) to determine the percent of pulmonary flow from collateral flow. As a further check of internal consistency, total venous return to the heart was calculated (Equation 6) to compare to the aortic flow in the SCPC patients. Ventricular volumes, (indexed to body surface area) were also obtained when available. Estimated indexed collateral flow was compared to indexed end diastolic ventricular volumes by linear regression. The two methods of calculating collateral flow, as well as aortic outflow and venous return to the heart, were tested for internal consistency using linear regression, Bland-Altman analysis, and intra-class correlation. Collateral flow parameters among control, TCPC and SCPC patients were compared initially using single factor ANOVA. When a significant difference across groups was detected by ANOVA, individual pairwise comparisons were tested using unpaired Student t-tests with Bonferroni correction for multiple comparisons. For the 31 patients who had both SCPC and TCPC exams, their SCPC and TCPC exams were compared using paired Student t-tests. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written. The study was approved by the institutional review board and informed consent was obtained.
RESULTS
A summary of the demographics is presented in Table 1. Excellent agreement was demonstrated between Qcoll-syst and Qcoll-pulm for both SCPC and TCPC, with negligible bias at 0.02 L/min, a pooled 2 S.D. range of −0.6 to 0.6 L/min (Figure 2), and an intra-class correlation coefficient of 0.84 (p<0.001). Table 2 summarizes the comparison between SCPC, TCPC and controls. Whether collateral flow is normalized to BSA, aortic flow, or total pulmonary flow, SCPC patients on average demonstrated significantly higher collateral flow than TCPC patients. The pulmonary to systemic flow ratio (QP/QS, see Equations 4 and 5), was significantly higher for TCPC than for SCPC. This appeared to be secondary to a significant decrease in QS, as QP was not significantly different in SCPC and TCPC patients.
Table 1.
Demographics of SCPC and TCPC patients.
| SCPC | TCPC | |
|---|---|---|
| n | 115 | 135 |
| Age at SCPC (years) | 0.51±0.21 | 0.76±0.96 |
| Age at TCPC (years) | N/A | 3.2±2.4 |
| Time from SCPC | 2.1±1.2 | 9.0±6.5 |
| Time from TCPC | N/A | 8.9±8.2 |
| Right Ventricle morphology | 79 (69%) | 76 (56%) |
| Left Ventricle morphology | 29 (25%) | 54 (40%) |
| Mixed morphology | 7 (6%) | 5 (4%) |
| Hypoplastic Left Heart Syndrome | 56 (49%) | 47 (35%) |
| Heterotaxy Syndrome | 16 (14%) | 9 (7%) |
| Tricuspid atresia | 11 (10%) | 25 (19%) |
| Double Inlet Left Ventricle | 7 (6%) | 11 (8%) |
| Pulmonary atresia with intact ventricular septum | 5 (4%) | 1 (1%) |
Figure 2. Comparison of two collateral estimators for all patients.
Top: Qcoll-pulm vs. Qcoll-syst demonstrating excellent correlation between the two methods of estimating systemic to pulmonary collateral flow for both SCPC and TCPC. Bottom: Bland-Altman plot of the difference between the two systemic to pulmonary collateral flow estimators (Qcoll-pulm − Qcoll-syst) vs. the average of the two estimators (both in L/min). The thin line represents the mean difference between the estimators and the thick lines represent two standard deviations around the mean difference.
Table 2. SCPC vs. TCPC patients vs. controls.
Values listed are mean ±S.D., p-values are unpaired t-test with Bonferroni correction for multiple measures.
| SCPC | TCPC | p-value SCPC vs. TCPC | Controls | p-value SCPC vs. Ctrl | p-value TCPC vs. Ctrl | |
|---|---|---|---|---|---|---|
| n | 115 | 135 | 18 | |||
| Age (years) | 2.6±1.2 | 12.2±8.8 | <0.001 | 10.1±7.4 | <0.001 | 1.0 |
| Body surface area (m2) | 0.53±0.10 | 1.16±0.51 | <0.001 | 1.05±0.49 | <0.001 | 1.0 |
| QAo (L/min/m2) | 4.85±1.1 | 3.61±1.0 | <0.001 | 3.9±0.9 | 0.007 | 1.0 |
| Qcoll (L/min/m2) | 1.64±0.8 | 1.03±0.8 | <0.001 | 0.21±0.27 | <0.001 | <0.001 |
| 100xQcoll/QAo (%) | 34%±12% | 26%±15% | <0.001 | 5%±6 % | <0.001 | <0.001 |
| 100xQcoll/QP (%) | 48%±17% | 29%±17% | <0.001 | 5%±5 % | <0.001 | <0.001 |
| QP (L/min/m2) | 3.4±0.9 | 3.3±0.8 | 1.0 | 4.1±1.0 | 0.006 | 0.005 |
| QS (L/min/m2) | 3.2±0.9 | 2.6±0.6 | <0.001 | 3.7±0.8 | 0.11 | <0.001 |
| QPA (L/min/m2) | 1.7±0.6 | 2.3±0.6 | <0.001 | 3.9±0.9 | <0.001 | <0.001 |
| QP/QS | 1.10±0.3 | 1.31±0.40 | <0.001 | 1.13±0.2 | 1.0 | 0.20 |
The data for the 31 patients who had collateral flow quantified serially pre-Fontan and post-Fontan are summarized in Table 3. In contrast to the larger cohort of TCPC patients, these subjects were generally much younger at the time of their post-Fontan exam. Twenty six Fontan completions were fenestrated and five were not. There was a trend toward an increase in collateral flow after Fontan that did not attain statistical significance (p=0.09). However, when collateral flow was expressed as a percentage of aortic flow, the increase reached significance (p=0.02). Note that QS decreased significantly from SCPC to TCPC in the face of no significant change in aortic flow. The right to left shunt decreased significantly as expected, but was still sizeable because the majority of the patients had fenestrations. Two patients had flow reversal in the superior Fontan baffle (between the fenestration and pulmonary arteries).
Table 3. Comparison of collateral flow measurements in 31 pts who had both pre and post TCPC MRI’s and no other interval interventions.
A significant decrease was seen in collateral flow indexed to aortic flow from SCPC to TCPC. There were also significant increases in QP and QP/QS, as well significant decreases in QS and right to left shunt.
| SCPC | TCPC | p-value | |
|---|---|---|---|
| n | 31 | 31 | |
| Age (years) | 2.9±1.3 | 4.0±1.3 | |
| Time from TCPC (years) | −0.37±0.5 | 0.62±0.3 | |
| QAo (L/min/m2) | 4.6±0.7 | 4.4±1.0 | 0.13 |
| Qcoll (L/min/m2) | 1.5±0.7 | 1.7±1.0 | 0.09 |
| Qcoll/QAo x100 (%) | 31±13 | 37±17 | 0.02 |
| Qcoll/QP x100 (%) | 45±17 | 41±18 | 0.15 |
| QP (L/min/m2) | 3.2±0.7 | 4.0±1.0 | <0.001 |
| QS (L/min/m2) | 3.2±0.7 | 2.8±0.8 | 0.002 |
| QPA (L/min/m2) | 1.7±0.6 | 2.2±0.8 | 0.001 |
| QP/QS | 1.1±0.4 | 1.5±0.6 | <0.001 |
| Right to Left Shunt (L/min/m2) | 1.5±0.5 | 0.5±0.4 | <0.001 |
The TCPC population demonstrated a significant negative correlation (r=−0.56, p<0.001) between time from TCPC and indexed collateral flow (Figure 3). We were unable to identify a significant relation between indexed collateral flow for SCPC patients and time from SCPC surgery.
Figure 3. Effect of time from TCPC completion on collateral flow.
TCPC patients tend to have lower indexed collateral flow (Qcoll) with increased time from TCPC completion. No relationship was identified between collateral flow (Qcoll) in SCPC pts indexed to body surface area vs. time from SCPC.
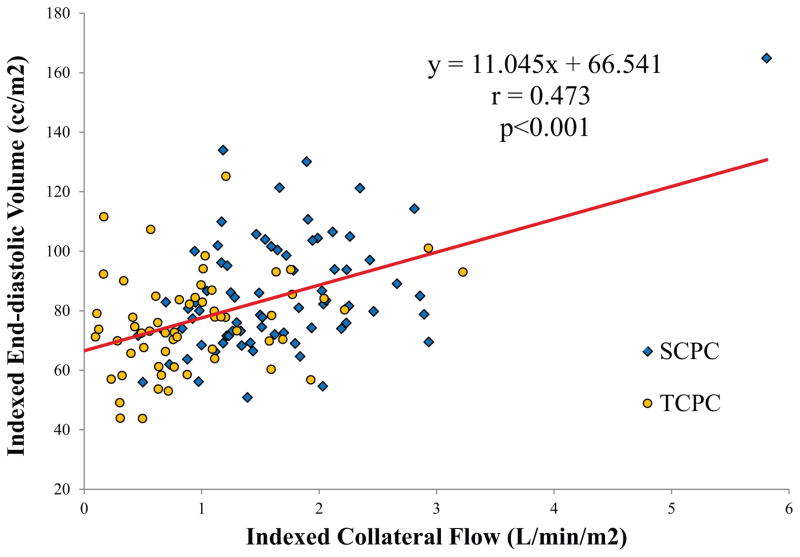
In assessing the relationship between collateral flow and ventricular volumes, patients with significant valve regurgitation or ventricular dysfunction (defined as total regurgitant fraction of greater than 25% or ejection fraction of less than 45%) were excluded. 36 patients with TCPC did not have ventricular volume imaging. In the remaining 190 patients (100 SCPC, 90 TCPC), indexed end-diastolic volume correlated modestly but significantly with indexed collateral flow (Figure 4) (r=0.47, p<0.001).
Figure 4. Effect of collateral flow on ventricular size.
Indexed end-diastolic volume of the ventricle compared to the amount of indexed collateral flow demonstrates a significant correlation. Note that patients who had other reasons for significant RV dilation (at least moderate valve regurgitation and/or ventricular dysfunction) were excluded.
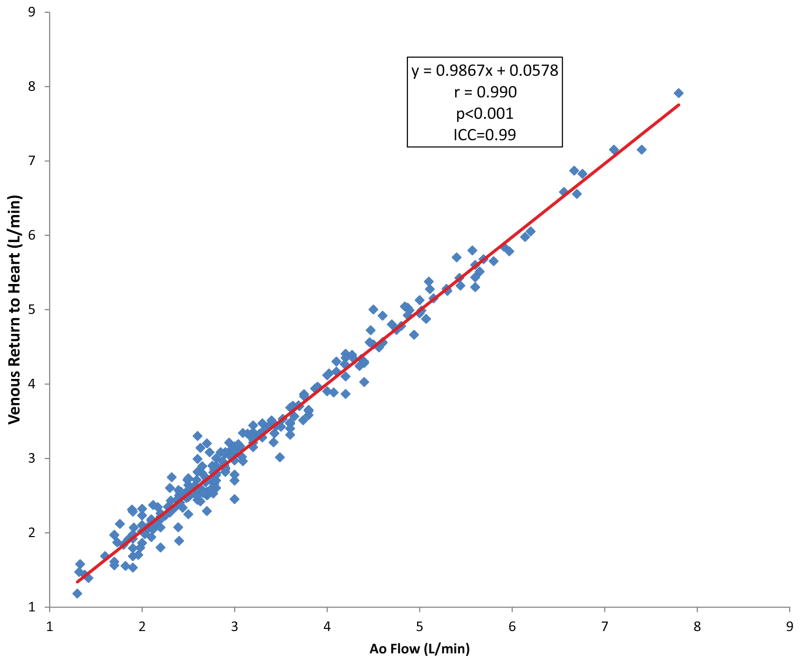
Some institutions advocate descending aorta flow as a surrogate to IVC flow, as it is easier to obtain. Both were obtained in 116 pts, of which 47 were SCPC. In general there was excellent linear correlation between the descending aorta and IVC flows (r=0.97, y=1.05x−0.001). The mean difference in descending aorta and IVC flow for all patients was 0.08±0.28 L/min. In the SCPC population, there were 5 patients in which IVC flow exceeded descending aorta flow by greater than 0.3 L/min. In 3 of these patients, there was clear CMR angiographic evidence of venous decompressing vessels from the cavopulmonary circulation to the lower body which would account for the difference. In the two SCPC pts in which descending aorta flow exceeded IVC flow by greater than 0.3 L/min, both had CMR angiographic evidence of arterial collaterals arising from the abdominal aorta. As a check of internal consistency, there was excellent agreement between the venous return to the heart and the aortic flow (Figure 5). The mean difference was 0.014±0.18 L/min.
Figure 5. Venous return vs. aortic flow.
Venous return to the heart compared to aortic flow demonstrates excellent internal consistency in the flow measurements.
DISCUSSION
While the TCPC group on average demonstrated significantly lower collateral flow than the SCPC group, the paired analysis suggests a more complex relationship. The significant increase in collateral flow as a fraction of aortic flow and the trend toward an increase in indexed collateral flow demonstrates that early after TCPC completion, collateral flow may actually increase. This is in the context of a significantly reduced right to left shunt after Fontan completion and increased Qp/Qs. One might speculate that this is due to more flow through existing collateral connections as a result of increased systemic vascular resistance, growth of new collateral connections as a result of post-operative inflammation, or both. The decrease in QS supports the notion that the increase in collateral flow is at least in part related to increases in systemic vascular resistance. Because total aortic output did not decrease significantly, the decrease in corrected QS is probably not related to changes in preload. The negative correlation between time from TCPC surgery and collateral flow in the TCPC group suggests that these collateral vessels tend to regress over time after Fontan completion, though the process appears to take years or decades. While a prospective longitudinal study may be needed to confirm these relationships, they are not surprising in light of experimental data on collateral formation. In animal models, decreased pulmonary artery flow results in dramatic increases in systemic to pulmonary flow via bronchial and other systemic arteries. Furthermore, in these models it has been demonstrated that reestablishment of normal pulmonary blood flow results in collateral involution.12,13
The study also demonstrates that the previously described method of quantifying collateral flow in SCPC patients extends to the TCPC population with good agreement between the two methods of calculating collateral flow. Both TCPC and SCPC populations demonstrate a significant hemodynamic burden from collateral flow, evidenced by the significant correlation between indexed collateral flow and ventricular volume in patients with no other identifiable source of volume load.
Few studies have focused on quantifying systemic to pulmonary collateral flow in Fontan patients. Grosse-Wortmann and colleagues described a method similar to the one presented here to quantify collateral flow in patients with both SCPC and TCPC. They reported results for 8 TCPC patients, and were thus not powered to show any trends in TCPC collateral flow. There were two important methodological differences: 1) use of descending aortic flow as a surrogate for IVC flow and 2) measured aortic flow distal to the aortopulmonary anastomosis in patients with aortic reconstructions. Using descending aorta instead of IVC flow may introduce errors in patients with SCPC because of both decompressing veins and collateral vessels originating from the abdominal aorta. There was evidence of both of these scenarios in our SCPC cohort. The excellent agreement between descending aorta flow and IVC flow in general suggests either approach is usually acceptable, but using IVC flow will avoid these potential error sources in specific patients. The lack of significant systematic error between the two estimators in our present study, in contrast to the methodologies reported previously, provides strong evidence that our described technique is more reliable. While previous studies have attempted to correct the DAo flow by directly measuring decompressing flow, our experience has been that decompressing vessels can be a network of small vessels which often cannot be measured directly.
A separate study on fenestration flow by Grosse-Wortmann et al found that in 23 patients studied 12 months after Fontan surgery, fenestration flow constituted 31% of aortic flow, and that there was flow reversal above the fenestration in 14 of the 23 patients.14 In contrast, in our paired cohort, on average 6 months out from Fontan surgery, fenestration flow only accounted for 11% of aortic flow, and only 2 of 27 patients with fenestrations had flow reversal in the Fontan baffle. Despite our group being closer to surgery, the groups had similar age at time of exam and fenestration sizes were comparable (all 4 mm in our group, range of 3–6 mm, average 4.2 mm in the Grosse-Wortmann study). Despite having a lower right to left shunt, our patients had similar collateral flow (37% vs. 34% of aortic flow). There were more patients with systemic right ventricle in our group (18 vs. 9). Given the assertion in that manuscript that fenestration flow is associated with untwisting, the lower fenestration flow could be associated with decreased diastolic function in our cohort. However, one of the two patients with Fontan flow reversal was a patient with HLHS. Finally, Prakash and colleagues reviewed their CMR database and found 78 TCPC and 38 SCPC patients in whom collateral flow could be retrospectively calculated, consisting generally of older TCPC patients who had not undergone modern staged palliation. They were able to demonstrate that collateral flow was significantly lower in the TCPC group.15
There are significant limitations to the study. There is no gold standard with which to compare the current method of quantifying collateral flow. However, PC-MRI is a well-validated method over a wide range of flow rates for both venous and aortic flows.11,16–18 It should be noted that there are known errors which can occur in PC-MRI measurements due to intravoxel velocity dispersion and baseline offsets. Attempts to control for these factors are imperfect and while errors are generally less than 5%, the errors can be additive when calculating the differences from multiple flow measures. We attempted to minimize these errors by averaging the two collateral flow estimators, which should reduce the estimator error by since there appears to be little systematic error between the two estimators. 19 While the control population is well matched to the TCPC group, it is not age-matched to the SCPC group. However, there were no significant differences in collateral flow measurements between the 6 control patients less than 5 years of age and the 12 greater than 5 years, which should largely mitigate these concerns. The cross-sectional design of the larger dataset in our study imposes limitations on conclusions regarding the relationship between time from surgery and collateral flow. Because evidence of decreasing collateral flow over time from Fontan completion is drawn from a cohort and not from serial evaluations, they could represent evolving differences in patient care, selection bias, or patient attrition rather than true time effects. Similarly, the lack of a relationship between time and collateral flow in SCPC patients may be confounded by referral bias toward more sick early SCPC patients, as young SCPC patients tend to be referred for problems whereas pre-TCPC studies are generally performed routinely. A longitudinal study will be required to confirm these time-related effects.
Acknowledgments
K.K.W. was supported in part by NIH K23 Grant HL089647 and M.A.F. was supported in part by NIH R01 Grant HL090615, both from the National Heart, Lung and Blood Institute.
The authors would like to acknowledge Veronica O’Connor, BSN for her invaluable help in patient recruitment. Supported in part by NIH grants K23 HL089647 and R01 HL090615 from the NHLBI.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McElhinney DB, Reddy VM, Tworetzky W, Petrossian E, Hanley FL, Moore P. Incidence and implications of systemic to pulmonary collaterals after bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2000;69:1222–1228. doi: 10.1016/s0003-4975(99)01088-7. [DOI] [PubMed] [Google Scholar]
- 2.Triedman JK, Bridges ND, Mayer JE, Lock JE. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993;22:207–215. doi: 10.1016/0735-1097(93)90836-p. [DOI] [PubMed] [Google Scholar]
- 3.Bradley SM, McCall MM, Sistino JJ, Radtke WAK. Aortopulmonary collateral flow in the Fontan patient: does it matter? Ann Thorac Surg. 2001;72:408–415. doi: 10.1016/s0003-4975(01)02813-2. [DOI] [PubMed] [Google Scholar]
- 4.Kanter KR, Vincent RN, Raviele AA. Importance of acquired systemic-to-pulmonary collaterals in the Fontan operation. Ann Thorac Surg. 1999;68:969–974. doi: 10.1016/s0003-4975(99)00782-1. [DOI] [PubMed] [Google Scholar]
- 5.Spicer RL, Uzark KC, Moore JW, Mainwaring RD, Lamberti JJ. Aortopulmonary collateral vessels and prolonged pleural effusions after modified Fontan procedures. Am Heart J. 1996;131:1164–1168. doi: 10.1016/s0002-8703(96)90092-7. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead KK, Gillespie MJ, Harris MA, Fogel MA, Rome JJ. Noninvasive Quantification of Systemic-to-Pulmonary Collateral Flow: A Major Source of Inefficiency in Patients With Superior Cavopulmonary Connections. Circ Cardiovasc Imaging. 2009;2:405–411. doi: 10.1161/CIRCIMAGING.108.832113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary Collaterals After Bidirectional Cavopulmonary Connection or Fontan Completion: Quantification With MRI. Circ Cardiovasc Imaging. 2009;2:219–225. doi: 10.1161/CIRCIMAGING.108.834192. [DOI] [PubMed] [Google Scholar]
- 8.Glatz AC, Rome JJ, Small AJ, Gillespie MJ, Dori Y, Harris MA, Keller MS, Fogel MA, Whitehead KK. Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging. 2012;5:218–225. doi: 10.1161/CIRCIMAGING.111.966986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grosse-Wortmann L, Drolet C, Dragulescu A, Kotani Y, Chaturvedi R, Lee K-J, Mertens L, Taylor K, La Rotta G, van Arsdell G, Redington A, Yoo S-J. Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: A multimodality study. [Accessed April 22, 2012];J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.03.032. Available at: http://proxy.library.upenn.edu:2135/science/article/pii/S0022522312003479. [DOI] [PubMed]
- 10.Odenwald T, Quail MA, Giardini A, Khambadkone S, Hughes M, Tann O, Hsia T-Y, Muthurangu V, Taylor AM. Systemic to pulmonary collateral blood flow influences early outcomes following the total cavopulmonary connection. Heart. 2012;98:934–940. doi: 10.1136/heartjnl-2011-301599. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead KK, Sundareswaran KS, Parks WJ, Harris MA, Yoganathan AP, Fogel MA. Blood flow distribution in a large series of patients having the Fontan operation: A cardiac magnetic resonance velocity mapping study. J Thorac Cardiovasc Surg. 2009;138:96–102. doi: 10.1016/j.jtcvs.2008.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitzner W, Lee W, Georgakopoulos D, Wagner E. Angiogenesis in the Mouse Lung. Am J Pathol. 2000;157:93–101. doi: 10.1016/S0002-9440(10)64521-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadel E, Wijtenburg E, Michel R, Mazoit J-X, Bernatchez R, Decante B, Sage E, Mazmanian M, Herve P. Regression of the Systemic Vasculature to the Lung after Removal of Pulmonary Artery Obstruction. Am J Respir Crit Care Med. 2006;173:345–349. doi: 10.1164/rccm.200506-894OC. [DOI] [PubMed] [Google Scholar]
- 14.Grosse-Wortmann L, Dragulescu A, Drolet C, Chaturvedi R, Kotani Y, Mertens L, Taylor K, La Rotta G, van Arsdell G, Redington A, Yoo S-J. Determinants and clinical significance of flow via the fenestration in the Fontan pathway: A multimodality study. Int J Cardiol. 2013;168:811–817. doi: 10.1016/j.ijcard.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Prakash A, Rathod RH, Powell AJ, McElhinney DB, Banka P, Geva T. Relation of systemic-to-pulmonary artery collateral flow in single ventricle physiology to palliative stage and clinical status. Am J Cardiol. 2012;109:1038–1045. doi: 10.1016/j.amjcard.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beerbaum P, Korperich H, Barth P, Esdorn H, Gieseke J, Meyer H. Noninvasive Quantification of Left-to-Right Shunt in Pediatric Patients: Phase-Contrast Cine Magnetic Resonance Imaging Compared With Invasive Oximetry. Circulation. 2001;103:2476–2482. doi: 10.1161/01.cir.103.20.2476. [DOI] [PubMed] [Google Scholar]
- 17.Kuzo RS, Pooley RA, Crook JE, Heckman MG, Gerber TC. Measurement of caval blood flow with MRI during respiratory maneuvers: implications for vascular contrast opacification on pulmonary CT angiographic studies. Am J Roentgenol. 2007;188:839. doi: 10.2214/AJR.06.5035. [DOI] [PubMed] [Google Scholar]
- 18.Powell AJ, Geva T. Blood flow measurement by magnetic resonance imaging in congenital heart disease. Pediatr Cardiol. 2000;21:47–58. doi: 10.1007/s002469910007. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich CF. Uncertainty, calibration, and probability: the statistics of scientific and industrial measurement. CRC Press; 1991. [Google Scholar]