Abstract
Objective
To investigate the prevalence and distribution of paraseptal emphysema on chest CT images in the Framingham Heart Study (FHS) population, and assess its impact on pulmonary function. Also pursued was the association with interstitial lung abnormalities.
Materials and Methods
We assessed 2633 participants in the FHS for paraseptal emphysema on chest CT. Characteristics of participants, including age, sex, smoking status, clinical symptoms, and results of pulmonary function tests, were compared between those with and without paraseptal emphysema. The association between paraseptal emphysema and interstitial lung abnormalities was investigated.
Results
Of the 2633 participants, 86 (3%) had pure paraseptal emphysema (defined as paraseptal emphysema with no other subtypes of emphysema other than paraseptal emphysema or a very few centrilobular emphysema involved) in at least one lung zone. The upper zone of the lungs was almost always involved. Compared to the participants without paraseptal emphysema, those with pure paraseptal emphysema were significantly older, and were more frequently male and smokers (mean 64 years, 71% male, mean 36 pack-years, p<0.001) and had significantly decreased FEV1/FVC% (p=0.002), and diffusion capacity of carbon monoxide (DLCO) (p=0.002). There was a significant association between pure paraseptal emphysema and interstitial lung abnormalities (p<0.001).
Conclusions
The prevalence of pure paraseptal emphysema was 3% in the FHS population, predominantly affects the upper lung zone, and contributes to decreased pulmonary function. Cigarette smoking, aging, and male gender were the factors associated with the presence of paraseptal emphysema. Significant association between paraseptal emphysema and interstitial lung abnormalities was observed.
Keywords: Paraseptal emphysema, Interstitial lung abnormalities, CT
INTRODUCTION
Pulmonary emphysema is categorized into three major subtypes according to the disease distribution in the secondary pulmonary lobules: centrilobular, paraseptal, and panlobular emphysema [1–3]. Paraseptal emphysema, also known as distal acinar emphysema, is characterized by the predominant involvement of the distal alveoli including their ducts and sacs, bounded by any pleural surface and the interlobular septa [4]. If it occurs alone or is the predominant type, paraseptal emphysema tends not to cause any respiratory symptoms, and therefore it is often underrecognized clinically [5, 6]. Although it may present with spontaneous pneumothrax [7], paraseptal emphysema has not been associated with airflow obstruction in prior studies [1]. The clinical significance of paraseptal emphysema and its effects on pulmonary function are not well understood [8]. With increased use of CT in clinical practice as well as in lung cancer screening [9], incidental detection of emphysema in asymptomatic patients is becoming more common, and high-resolution CT (HRCT) allows detailed assessment and subtyping of emphysema without invasive pathological procedures [6, 9–11]. Cigarette smoking is assumed to play an important role in developing emphysema [12].
Interstitial lung abnormalities are defined as areas of increased lung density on CT and also can result from cigarette smoking [13]. Interstitial lung abnormalities may precede the development of clinically significant pulmonary fibrosis [14]. The previous study by Washko et al revealed that interstitial lung abnormalities were commonly seen in smokers with the prevalence of 8%, predominantly in the subpleural lung area [13]. In a study by Jin et al, the prevalence of interstitial lung abnormalities in smokers was 9.7%, and 37% of the cases with fibrotic features progressed in a two-year follow-up [15]. Hunninghake et al described the prevalence of interstitial lung abnormalities of 7% in a community-dwelling sample of the Framingham Heart Study (FHS) including both smokers and nonsmokers and its association with a particular genotype (MUC5B) [14]. In the previous study by Washko et al, interstitial lung abnormalities were associated with reduced total lung capacity and a lesser amount of emphysema based on the result from low attenuation analysis on CT images at the thresholds of −950 and −910 Hounsfield units (HU) [13]. A previous study of smokers suggested that interstitial lung abnormalities were associated with paraseptal emphysema [16]. However, it has not been investigated in the general population. We hypothesize that paraseptal emphysema, in contrast to centrilobular emphysema, and interstitial lung abnormalities are positively associated with each other, because both conditions have a similar subpleural distribution.
The purpose of this study is to describe clinical and imaging characteristics of paraseptal emphysema and investigate its association with interstitial lung abnormalities in the FHS population.
MATERIALS AND METHODS
Study population
In 1948, the Framingham Heart Study (FHS) was initiated to identify epidemiologic risk factors of cardiovascular disease, recruiting the participants regardless of their health condition. From 2009 to 2011, CT exams were performed in 2764 participants of the FHS (the third-generation and offspring cohorts, FHS-MDCT2) in the supine position with no administration of contrast using the 64-detector-row CT scanner (Discovery, GE Healthcare, Waukesha, WI) with 120 kV, 300–350 mA (optimized with body weight), gantry rotation time of 0.35 seconds. CT images were reconstructed with sharp lung algorithm and section thickness of 0.63 mm. Of these, 131 were missing image data and excluded from the study. Therefore, we evaluated data from 2633 participants (1325 female, mean age 59.2) with chest CT scans. This study was approved by the institutional review boards at Boston University and Brigham and Women’s Hospital. All participants provided written informed consent.
Evaluation of chest CT scans
For the visual assessment of chest CT scans, paraseptal emphysema was defined as relatively reduced CT attenuation at subpleural or peribronchovascular areas with or without intact interlobular septa [4]. Cysts, air-trapping lesions, and honeycomb lesions [17, 18] are not regarded as paraseptal emphysema. To investigate the location of paraseptal emphysema, the lungs were divided into three zones: upper, middle, and lower. The upper zone is above the level of the carina, the middle zone is between the level of the carina and the level of the right inferior pulmonary vein, and the lower zone is below the right inferior pulmonary vein [19]. Each zone was evaluated for the existence of paraseptal emphysema (Score 0 for no paraseptal emphysema and 1 for paraseptal emphysema).
All chest CT images were uploaded to a Picture Archiving and Communication System (PACS) workstation (Virtual Place Raijin, AZE Ltd., Tokyo, Japan) and visually evaluated for paraseptal emphysema by three board-certified radiologists specialized in thoracic imaging (TA, MN, HH) using a modified sequential reading method, as previously described [13, 14, 20]. In this reading method, the first reader reviewed all cases and provided a diagnosis of paraseptal emphysema (Score 0 or 1 for each lung zone). The second reader, who was blinded to the initial reading, reviewed all cases diagnosed with paraseptal emphysema (Score 1 in at least one lung zone) and a random selection of 20% of the cases diagnosed as no paraseptal emphysema (Score 0 in all three lung zones) by the first reader. Finally, the third reader, who was blinded to the diagnosis of previous readers, provided a majority opinion on the discordant cases between the first and second readers. Cases with discrepant diagnosis of disease location were also forwarded to the third reader for final evaluation. Window level and width were fixed with −700 HU and 1500 HU respectively during the review sessions. After the sequential reading, all cases with paraseptal emphysema in at least one lung zone were re-evaluated by two radiologists (TA, HH) for consensus. As a results, participants were categorized into three groups: (a) No paraseptal emphysema: all three zones have no paraseptal emphysema; (b) Mixed paraseptal emphysema: overlap with centrilobular emphysema or areas of paraseptal emphysematous lesions are less than 5% of the whole lungs; and (c) Pure paraseptal emphysema: no other subtypes of emphysema other than paraseptal emphysema or a very few centrilobular emphysema are involved.
Information on the identification of interstitial lung abnormalities in the FHS was published previously [14]. In brief, interstitial lung abnormalities were defined as nondependent changes affecting more than 5% of any lung zone, including ground glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, or traction bronchiectasis. CT images showing either focal or unilateral ground-glass attenuation, focal or unilateral reticulation, or patchy ground-glass abnormality (<5% of the lung) were considered to be indeterminate [14]. As a result, interstitial lung abnormalities were categorized into three groups as defined above: (a) No interstitial lung abnormalities, (b) Indeterminate interstitial lung abnormalities status, and (c) Interstitial lung abnormalities.
Statistical analysis
All statistical analyses used mixed effect models (continuous traits) or generalized estimating equations (binary traits) to account for familial correlations in our cohort [21]. Characteristics of the participants were compared between the groups according to the result of visual evaluation for paraseptal emphysema using R (version 3.1.1, The R Foundation for Statistical Computing, Vienna, Austria). The association between paraseptal emphysema and interstitial lung abnormalities was analyzed using SAS (version 9.3, SAS Institute Inc., Cary, NC). All P values were two-sided and P values less than 0.05 were regarded as statistically significant. Interobserver agreement was indicated with κ values, classified as follows: poor, κ = 0–0.20; fair, κ = 0.21–0.40; moderate, κ = 0.41–0.60; good, κ = 0.61–0.80; excellent, κ = 0.81–1 [15, 18].
RESULTS
Prevalence and distribution of paraseptal emphysema
Of the 2633 participants, 86 (3%) showed pure paraseptal emphysema in at least one lung zone (Figure 1), 214 (8%) had mixed, and 2333 (89%) did not have paraseptal emphysema. Of these 86 participants with paraseptal emphysema, 23 (27%) cases involved only the upper lung zone and 1 (1%) involved only the lower zone (one lung zone); 40 cases (47%) involved the upper and middle zones (two lung zones); and 22 cases (26%) involved all three zones (Table 1). Interobserver agreement regarding the presence and location of PSE between the first and second readers was good (κ =0.60; 95% CI: 0.56 – 0.65). In 20% of the cases that the first reader diagnosed with no PSE (Score 0 in all three lung zones), which were forwarded to the subsequent reader(s), 98% of diagnoses by the first reader coincided with the final results of sequential reading, and an agreement rate between the first and second readers was 93%.
Figure 1.
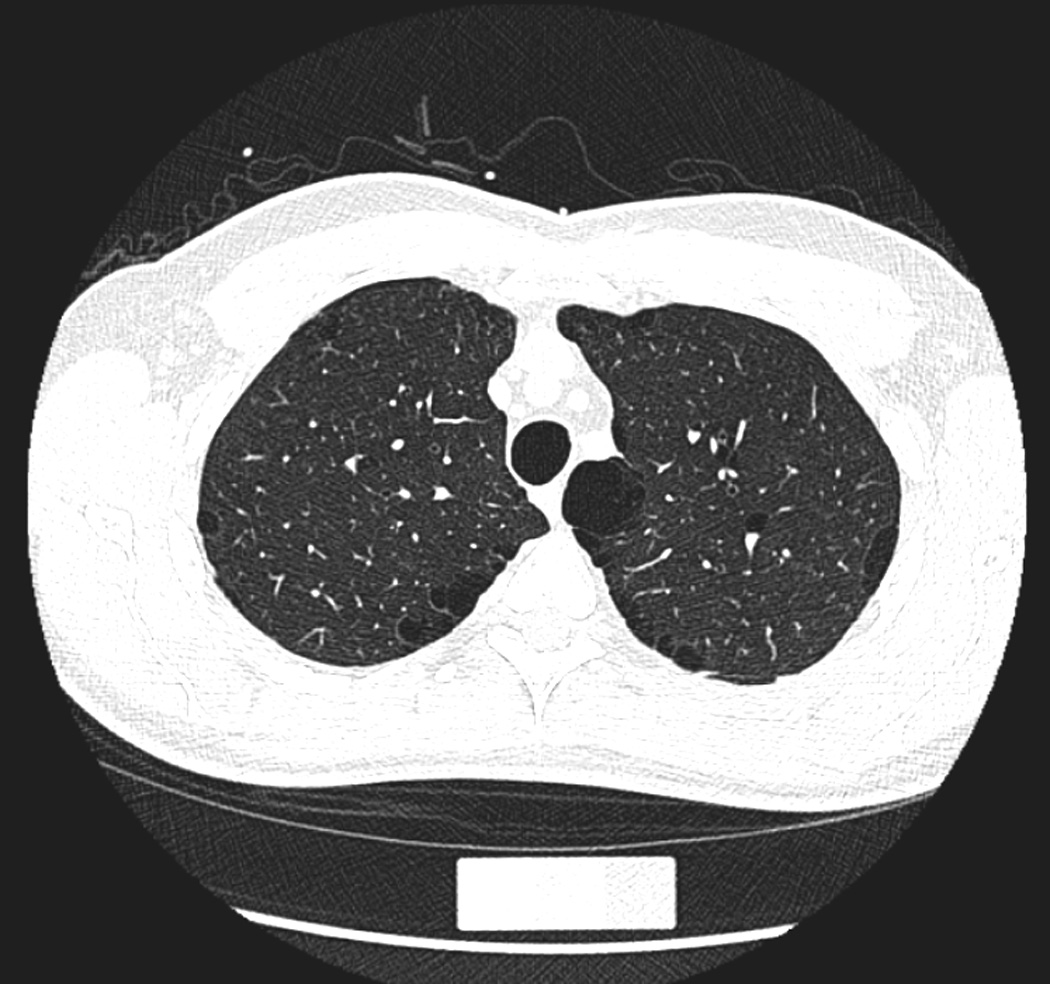
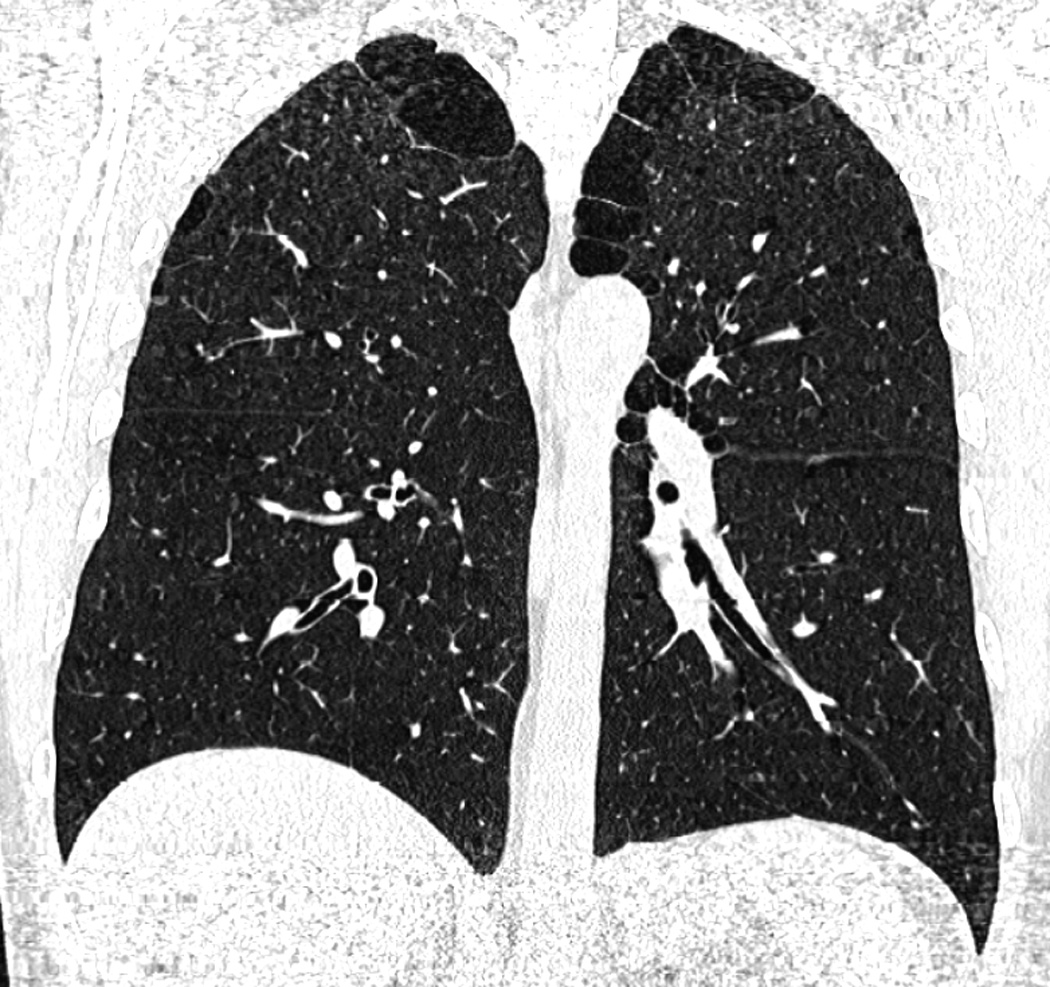
Paraseptal emphysema in a 54-year-old male smoker. (a) A trans-axial CT image shows subpleural low-attenuation areas predominantly in medial to posterior margin of lung parenchyma with interlobular septa. (b) A coronal reconstructed image shows the involvement of upper and middle lung zones with paraseptal emphysema. The participant reported no respiratory symptoms.
Table 1.
Involvement of Lung Zones with Paraseptal Emphysema
| Involved lung zones | No. of participants (%) |
|---|---|
| One lung zone | |
| Upper | 23 (27%) |
| Lower | 1 (1%) |
| Two lung zones | |
| Upper and middle | 40 (47%) |
| All three lung zones | 22 (26%) |
| Total | 86 |
Paraseptal emphysema and characteristics of participants
Characteristics of the participants based on paraseptal emphysema status are shown in Table 2. Compared with the group without paraseptal emphysema, the group with pure paraseptal emphysema was significantly older (63.9 years), more frequently male (71%), and more frequently reported respiratory symptoms such as chronic cough (17%) and shortness of breath (22%, P <0.001 for all categories). The group with paraseptal emphysema was more likely to report a history of cigarette smoking: 64% were former smokers with significantly higher pack-years (mean 36) and 34% were current smokers. Only 2 participants (2%) with paraseptal emphysema had never smoked cigarettes. BMI was not significantly different between the groups with pure paraseptal emphysema and no paraseptal emphysema (P=0.41). Although mean values of pulmonary function test in the group with paraseptal emphysema were still within normal range, FEV1/FVC% and diffusion capacity of carbon monoxide (DLCO) showed slightly but significantly lower values compared with those in the group without paraseptal emphysema (P=0.002 for both, Table 3).
Table 2.
Characteristics of the Participants Based on Paraseptal Emphysema Status
| Characteristic | No PSE (N=2333) |
Mixed PSE (N=214) |
Pure PSE (N=86) |
P Value*1 | |
|---|---|---|---|---|---|
| All three groups |
Pure PSE vs. No PSE |
||||
| Age — year | 58.6 ± 12.0 | 64.3 ± 11.6 | 63.9 ± 10.8 | <0.001 | <0.001 |
| Female sex — no. (%) | 1205 (52) | 95 (44) | 25 (29) | <0.001 | <0.001 |
| Body-mass index*2 | 28.6 ± 5.5 (N=2321) |
27.6 ± 4.5 (N=213) |
28.4 ± 4.6 (N=85) |
0.01 | 0.41 |
| Smoking status —no. (%) | |||||
| Never | 1235 (53) | 25 (12) | 2 (2) | <0.001 | <0.001 |
| Former | 988 (43) | 142 (67) | 54 (64) | <0.001 | <0.001 |
| Current | 88 (4) (N=2311) |
45 (21) (N=212) |
29 (34) (N=85) |
<0.001 | <0.001 |
| Pack-year | 15.0 ± 12.4 (N=995) |
33.7 ± 23.3 (N=183) |
36.0 ± 21.1 (N=80) |
<0.001 | <0.001 |
| Respiratory symptoms — no. (%)*3 | |||||
| Chronic cough | 141 (6) | 20 (9) | 15 (17) | <0.001 | <0.001 |
| Shortness of breath with minor exertion | 234 (10) | 38 (18) | 19 (22) | <0.001 | <0.001 |
Plus-minus values are mean ± standard deviation.
PSE: paraseptal emphysema
P values for the comparison among all three groups and for the comparison between participants with and without pure paraseptal emphysema were calculated with the use of linear mixed effect models to account for familial relationships in the Framingham Heart Study, as described previously [21].
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The P values for respiratory symptoms were adjusted by age, smoking status (current, former or never) and pack-years.
Table 3.
Results of Pulmonary Function Test of the Participants Based on Paraseptal Emphysema Status
| Parameters | No PSE (N=2333) |
Mixed PSE (N=214) |
Pure PSE (N=86) |
P Value*1,2 | |
|---|---|---|---|---|---|
| All three groups |
Pure PSE vs. No PSE |
||||
| FEV1 — % of predicted value*3 | 98.7 ± 14.4 (N=2307) |
91.6 ± 18.8 (N=185) |
90.2 ± 16.7 (N=75) |
<0.001 | 0.15 |
| FVC — % of predicted value*3 | 101.9 ± 13.1 (N=2307) |
101.7 ± 15.1 (N=185) |
98.5 ± 14.0 (N=75) |
0.80 | 0.69 |
| FEV1 / FVC — % of predicted value*3 | 96.4 ± 8.2 (N=2308) |
89.5 ± 12.2 (N=185) |
91.1 ± 10.6 (N=75) |
<0.001 | 0.002 |
| Spirometric restriction — no./total no. (%)*4 | 70 (3) (N=2207) |
5 (3) (N=185) |
4 (5) (N=75) |
0.51 | 0.28 |
| Airflow obstruction — no./total no. (%)*5 | 85 (4) (N=2207) |
26 (14) (N=185) |
12 (16) (N=75) |
<0.001 | <0.001 |
| DLCO — % of predicted value*6 | 97.9 ± 15.1 (N=2049) |
87.4 ± 17.7 (N=152) |
87.0 ± 14.0 (N=61) |
<0.001 | 0.002 |
| Total lung capacity — % of predicted value*7 | 83.6 ± 14.9 (N=2154) |
89.6 ± 16.6 (N=196) |
89.5 ± 14.5 (N=78) |
0.09 | 0.08 |
Plus-minus values are mean ± standard deviation.
PSE: paraseptal emphysema, FEV: forced expiratory volume, FEV1: forced expiratory volume in 1 second, FVC: forced vital capacity, DLCO: diffusing capacity of the lung for carbon monoxide.
P values for the comparison among all three groups and for the comparison between participants with and without pure paraseptal emphysema were calculated with the use of linear mixed effect models to account for familial relationships in the Framingham Heart Study, as described previously [21].
All the P values for variables of pulmonary function test were adjusted by age, smoking status (current, former or never), and pack-years.
Predicted values for FEV1 and FVC are derived from Hankinson et al [30].
Spirometric restriction is defined as an FVC of less than 80% of the predicted value with an FEV1/ FVC ratio that is more than the lower limit of the normal range [30].
Airflow obstruction is defined as an FEV1 and an FEV1/ FVC ratio that are both less than the lower limit of the normal range [30].
Predicted values for DLCO are derived from Miller et al [31].
Quantitative values for total lung capacity were calculated using Airway Inspector (www.airwayinspector.org). Predicted values for total lung capacity are derived from the guidelines of the American Thoracic Society and European Respiratory Society [32].
Paraseptal emphysema and interstitial lung abnormalities
Of the 2633 participants, 177 (7%) showed interstitial lung abnormalities, 1086 (41%) were indeterminate, and 1370 (52%) did not have evidence of interstitial lung abnormalities [14]. The detailed characteristics of the participants in association with interstitial lung abnormalities were described in a previous article [14]. The association between paraseptal emphysema and interstitial lung abnormalities is given in Table 4. Of 86 participants with paraseptal emphysema, 21 (24%) had interstitial lung abnormalities (Figure 2), which is more frequent compared to 130 of 2333 (6%) without paraseptal emphysema. F test showed a statistically significant association between paraseptal emphysema and interstitial lung abnormalities (F test statistic = 16.57, P<0.001).
Table 4.
Association between Paraseptal Emphysema and Interstitial Lung Abnormalities
| ILA Status | |||||
|---|---|---|---|---|---|
| None | Indeterminate | ILA | Total | ||
| PSE Status | None | 1262 | 941 | 130 | 2333 |
| Mixed PSE | 82 | 106 | 26 | 214 | |
| Pure PSE | 26 | 39 | 21 | 86 | |
| Total | 1370 | 1086 | 177 | 2633 | |
PSE: paraseptal emphysema, ILA: interstitial lung abnormalities
F test statistic = 16.57, P<0.0001
Figure 2.
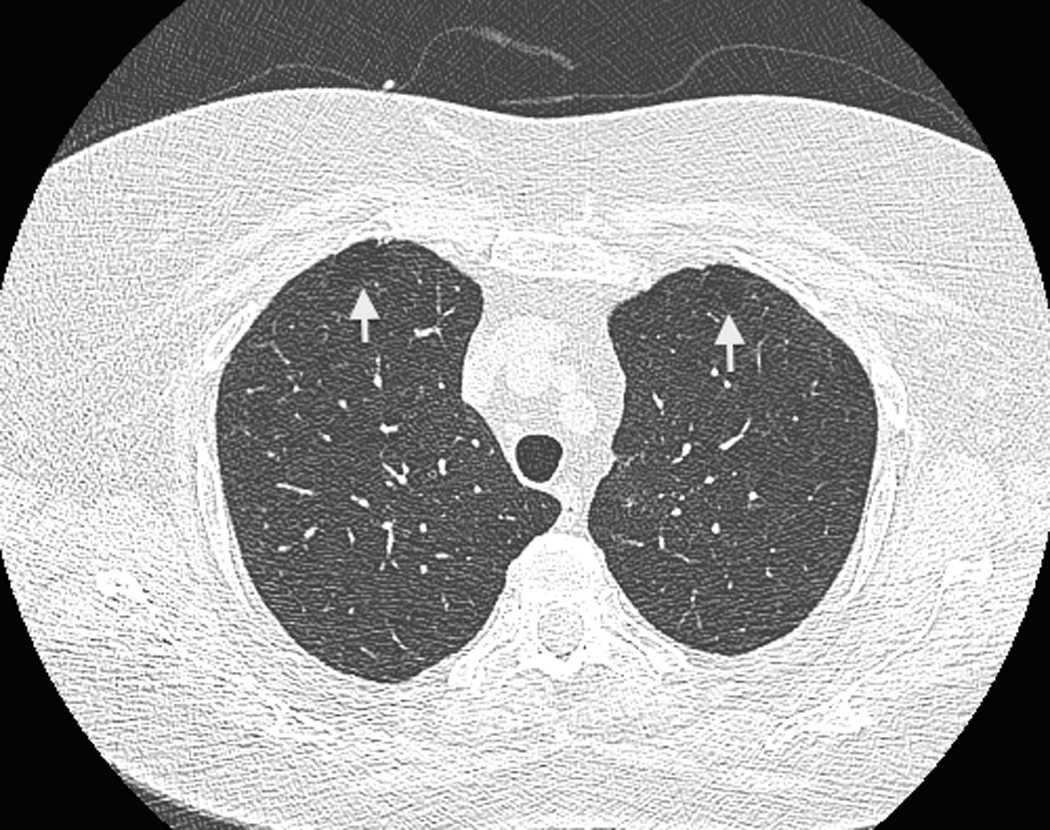
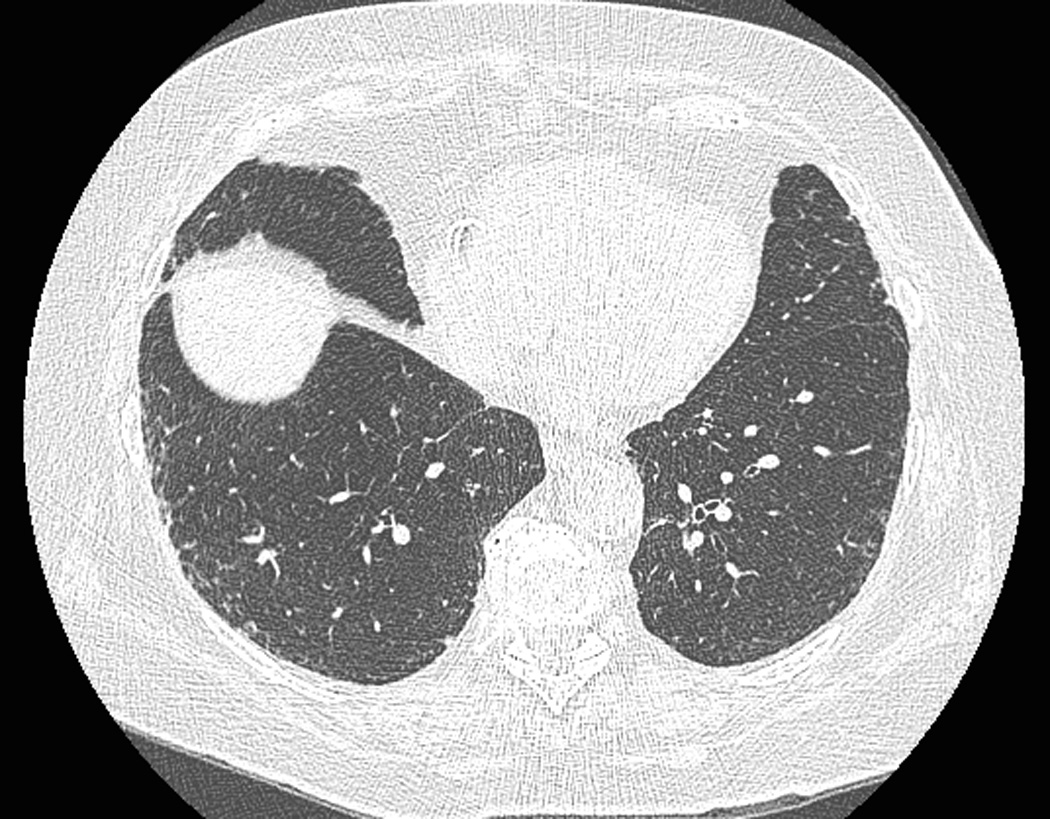
Coexistence of paraseptal emphysema and interstitial lung abnormalities in a 75-year-old female participant. (a) A trans-axial CT image at the upper zone shows subpleural low-attenuation lesions anteriorly (arrows), which is compatible to mild paraseptal emphysema. (b) Another trans-axial CT image at the lower lung zone shows subpleural ground-glass and reticular opacities extending lateral to anterior areas. The participant reported no respiratory symptoms but pulmonary function test revealed airflow obstruction.
DISCUSSION
Paraseptal emphysema is known as a subtype of emphysema [1–3] that may be detected incidentally by thoracic CT scans in patients without any respiratory symptoms [6]. Therefore, it does not usually draw a physician’s attention compared with other subtypes of emphysema such as centrilobular or panlobular. According to the report from the Centers for Disease Control and Prevention (CDC) in 2011, 2% of adults aged 18 or older had been diagnosed with emphysema [22]. Our study reveals that the prevalence of pure paraseptal emphysema defined by chest CT imaging in a community-dwelling sample of the FHS population is 3%, which suggests that the incidence of paraseptal emphysema has been underestimated. Furthermore, because we characterized paraseptal emphysema superimposed with centrilobular emphysema as mixed and not included in the pure paraseptal emphysema group, the actual incidence of paraseptal emphysema in the general population could be higher than 3%. In addition, patients with paraseptal emphysema report respiratory symptoms more frequently than those without paraseptal emphysema although the clinical significance of these findings is still unclear.
The present study shows that pure paraseptal emphysema almost always involves the upper lung zone with or without the involvement of the other lung zones. Paraseptal emphysema affecting the middle and/or lower zones is less common. This characteristic distribution of paraseptal emphysema may suggest the vulnerability of the upper lung zone or a gravity effect. However, the exact mechanism of lung parenchymal damage from cigarette smoking and how it affects lung areas differently is not clearly understood; the upper lung zones tend to be involved with paraseptal emphysema but the lower lung zones, especially posterior areas, are more likely to be involved with interstitial lung abnormalities when fibrosis is present. Gurney et al reported the correlation of regional distribution of emphysema on HRCT with pulmonary function tests in smokers. Although the upper lung zones tend to be more severely affected, the degree of emphysema in the lower lung zones showed stronger association with deterioration of pulmonary function tests [11]. Although we also conducted statistical analysis on the distribution of paraseptal emphysema, there was no significant difference found in participants’ demographics or pulmonary functions (Supplementary Tables 1 and 2). We have not assessed the distribution among lobes, but lung zones, because paraseptal emphysema seems to not respect the boundaries of the lobes for its distribution. However, upper lung zone predominance of paraseptal emphysema indirectly indicates that paraseptal emphysema is more common in the upper lobes compared to middle and lower lobes.
The results of pulmonary function tests in the group with paraseptal emphysema were slightly worse than those without paraseptal emphysema, with relatively lower FEV1/FVC% and DCLO, although these values still stayed within a normal range. These results suggest that paraseptal emphysema contributes to obstructive lung dysfunction in spite of the fact that paraseptal emphysema usually affects a smaller volume of lung parenchyma than centrilobular emphysema does. However, studies to compare participants’ characteristics and pulmonary function results between different subtypes is needed since the present study focused on pure paraseptal emphysema, excluding associations with other subtypes of emphysema and tried to minimize the confounding effect of centrilobular emphysema. Recently, Smith et al investigated 318 smokers with chronic obstructive pulmonary disease (COPD) and reported that 27% had emphysema on CT [8]. They subclassified emphysema into three groups: predominantly centrilobular (14%), paraseptal (9%), and panlobular (4%) emphysema. They reported that paraseptal-predominant emphysema was significantly more common in males, unlike other subtypes of emphysema, and that the severity of paraseptal emphysema as well as centrilobular emphysema was greater in the upper lung zones [8], which is compatible with our results. However, in contrast to our results, Smith et al detected no significant increase in respiratory symptoms and pulmonary function impairment in the group with paraseptal emphysema. The difference in participant demographics and the definition of subclassifications of emphysema should be taken into account: our cohort was recruited to represent the general population, including both smokers and non-smokers from broader age groups, and more strict diagnostic criteria were used for paraseptal emphysema. Perhaps, the influence of paraseptal emphysema was too small to be detected in their study. In fact, our result shows that the values of the pulmonary function test in participants with paraseptal emphysema were still within normal limits, even though there was significant impairment compared to those without paraseptal emphysema.
Our findings demonstrate a positive association between paraseptal emphysema and interstitial lung abnormalities. Although absence of pulmonary fibrosis was historically an additional criterion for the diagnosis of emphysema [23], our current understanding is that pulmonary fibrosis may occur with emphysema in smokers [24–26]. CT imaging has increased the frequency of detecting the simultaneous occurrence of emphysema and pulmonary fibrosis. Cottin et al first described combined pulmonary fibrosis and emphysema (CPFE) in 2005 [27]. CPFE is characterized by the coexistence of emphysema in the upper lobes and pulmonary fibrosis in the lower lobes and typically occurs in men in their 60s and 70s [27, 28]. Interstitial lung abnormalities may precede the development of clinically relevant pulmonary fibrosis [13, 14, 29]. Therefore, our result may suggest that the combination of paraseptal emphysema and interstitial lung abnormalities might be a preceding condition of CPFE; nevertheless, the progression of paraseptal emphysema and interstitial lung abnormalities has not been confirmed and longitudinal investigations with long-term follow-up are necessary. In the previous report, Washko et al revealed that interstitial lung abnormalities in smokers are negatively associated with emphysema in general based on low attenuation analysis on CT images [13]. Their results seem to conflict with our result; however, since they did not subclassify emphysema in the study, it is possible that paraseptal emphysema positively associates with interstitial lung abnormalities in contrast to centrilobular emphysema, which may have different pathology from paraseptal emphysema.
There are several limitations to our study. First, the present study focused on the evaluation of cross-sectional data including CT scans, participants’ information at the exam closest to the CT scan, and the result of pulmonary function tests at one time point. Further studies, including longitudinal analyses, are needed to demonstrate the effect of paraseptal emphysema progression on clinically significant outcomes. Second, we assessed CT images visually since CT is the gold standard for the diagnosis of emphysema and interstitial lung abnormalities [8], rather than using quantitative methods such as low attenuation analysis or lung density analysis. Therefore, misclassification of CT scans by visual evaluation could also be present. However, we used a sequential reading method, which requires evaluation by up to three readers, and comparisons were made between the groups with pure and no paraseptal emphysema excluding the mixed emphysema group. These evaluation processes were intended to reduce the influence of visual misclassification as much as possible. Qualitative analysis of paraseptal emphysema remains an issue for future investigations. Third, our cohort possibly included participants with diseases other than paraseptal emphysema and interstitial lung abnormalities that may also cause respiratory symptoms or affect pulmonary function tests, such as asthma or chronic bronchitis. Therefore, we adjusted the influence of aging and cigarette smoking for the statistical analysis since those are known to be strongly associated with respiratory symptoms and chronic bronchitis. Fourth, this is an observational study of the FHS cohort rather than a hypothesis-driven study. We hope hypothesis-driven investigations will be performed based upon our observational results. Finally, while the FHS is supposed to be a well-characterized general population cohort, most of participants are of European descent, and it is unknown if these findings could be generalized to populations with different racial and ethnic backgrounds.
In summary, our study shows that the prevalence of pure paraseptal emphysema is 3% in the FHS population, predominantly affects the upper lung zone, and contributes to a slight increase in evidence for airway obstruction and an increase in respiratory symptoms. A significant association between paraseptal emphysema and interstitial lung abnormalities was observed and reported for the first time in the literature.
Supplementary Material
Highlights of the manuscript.
The prevalence of pure paraseptal emphysema was 3% (85/2633) in the Framingham Heart Study population, predominantly affects the upper lung zone, and contributes to slightly decreased pulmonary function.
There was significant association between paraseptal emphysema and interstitial lung abnormalities, which is a novel finding.
Prevalence of paraseptal emphysema and its impact on pulmonary function could have been underestimated in the previous reports.
Acknowledgement
Authors acknowledge Alba Cid M.S. for editorial work on the manuscript.
Dr. Nishino is supported by NCI Grant Number: 1K23CA157631. Dr. Rosas is supported by NIH Grant Number: U01 HL105371 and P01 HL114501. Dr. Washko is supported by NIH Grant Number: R01 HL116473, R01 HL107246 and P01 HL114501. Dr. Hunninghake is supported by NIH Grant Number: K08 HL092222, U01 HL105371, P01 HL114501, and R01 HL111024. Dr. Hatabu is supported by NIH Grant Number: K25 HL104085 and R01 HL116473. This work was partially supported by the NHLBI’s Framingham Heart Study contract: N01-HC-25195 and R01 HL111024.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stern EJ, Frank MS. CT of the lung in patients with pulmonary emphysema: diagnosis, quantification, and correlation with pathologic and physiologic findings. American Journal of Roentgenology. 1994;162(4):791–798. doi: 10.2214/ajr.162.4.8140992. [DOI] [PubMed] [Google Scholar]
- 2.Thurlbeck WM, Muller NL. Emphysema: definition, imaging, and quantification. American Journal of Roentgenology. 1994;163(5):1017–1025. doi: 10.2214/ajr.163.5.7976869. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi M, Fukuoka J, Nitta N, Takazakura R, Nagatani Y, Murakami Y, et al. Imaging of pulmonary emphysema: a pictorial review. International Journal of Chronic Obstructive Pulmonary Disease. 2008;3(2):193–204. doi: 10.2147/copd.s2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 5.Edge J, Simon G, Reid L. Peri-acinar (paraseptal) emphysema: its clinical, radiological, and physiological features. British Journal of Diseases of the Chest. 1966;60(1):10–18. doi: 10.1016/s0007-0971(66)80016-5. [DOI] [PubMed] [Google Scholar]
- 6.Tsushima K, Sone S, Fujimoto K, Kubo K, Morita S, Takegami M, et al. Identification of occult parechymal disease such as emphysema or airway disease using screening computed tomography. COPD. 2010;7(2):117–125. doi: 10.3109/15412551003631717. [DOI] [PubMed] [Google Scholar]
- 7.Lesur O, Delorme N, Fromaget JM, Bernadac P, Polu JM. Computed tomography in the etiologic assessment of idiopathic spontaneous pneumothorax. Chest. 1990;98(2):341–347. doi: 10.1378/chest.98.2.341. [DOI] [PubMed] [Google Scholar]
- 8.Smith BM, Austin JH, Newell JD, Jr, D'Souza BM, Rozenshtein A, Hoffman EA, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. The American Journal of Medicine. 2014;127(1):94 e7–94 e23. doi: 10.1016/j.amjmed.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, et al. Results of initial low-dose computed tomographic screening for lung cancer. The New England Journal of Medicine. 2013;368(21):1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein JS, Gamsu G, Webb WR, Golden JA, Muller NL. High-resolution CT diagnosis of emphysema in symptomatic patients with normal chest radiographs and isolated low diffusing capacity. Radiology. 1992;182(3):817–821. doi: 10.1148/radiology.182.3.1535900. [DOI] [PubMed] [Google Scholar]
- 11.Gurney J, Jones K, Robbins R, Gossman G, Nelson K, Daughton D, et al. Regional distribution of emphysema: correlation of high-resolution CT with pulmonary function tests in unselected smokers. Radiology. 1992;183(2):457–463. doi: 10.1148/radiology.183.2.1561350. [DOI] [PubMed] [Google Scholar]
- 12.Hogg JC, Senior RM. Chronic obstructive pulmonary disease - part 2: pathology and biochemistry of emphysema. Thorax. 2002;57(9):830–834. doi: 10.1136/thorax.57.9.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. New England Journal of Medicine. 2011;364(10):897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. The New England Journal of Medicine. 2013;368(23):2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268(2):563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okajima Y, Hunninghake GM, Rosas IO, Crapo JD, Washko GR, Hatabu H. Paraseptal Emphysema and Interstitial Lung Abnormalities in the COPD Gene Study. RSNA. 2011 [abstract] [Google Scholar]
- 17.Johkoh T, Sakai F, Noma S, Akira M, Fujimoto K, Watadani T, et al. Honeycombing on CT; its definition, pathologic correlation, and future direction of its diagnosis. European Journal of Radiology. 2014;83(1):27–31. doi: 10.1016/j.ejrad.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266(3):936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 19.Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, Bowler RP, et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9(2):151–159. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Academic Radiology. 2010;17(1):48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uh HW, Wijk HJ, Houwing-Duistermaat JJ. Testing for genetic association taking into account phenotypic information of relatives. BMC proceedings. 2009;3(Suppl 7):S123. doi: 10.1186/1753-6561-3-s7-s123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams PE, Martinez ME, Vickerie JL, Kirzinger WK. Summary health statistics for the U.S. population: National Health Interview Survey, 2010. Vital and health statistics Series 10, Data from the National Health Survey. 2011;(251):1–117. [PubMed] [Google Scholar]
- 23.The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132(1):182–185. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso WV, Sekhon HS, Hyde DM, Thurlbeck WM. Collagen and elastin in human pulmonary emphysema. Am Rev Respir Dis. 1993;147(4):975–981. doi: 10.1164/ajrccm/147.4.975. [DOI] [PubMed] [Google Scholar]
- 25.Lang MR, Fiaux GW, Gillooly M, Stewart JA, Hulmes DJ, Lamb D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax. 1994;49(4):319–326. doi: 10.1136/thx.49.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katzenstein AL, Mukhopadhyay S, Zanardi C, Dexter E. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Human Pathology. 2010;41(3):316–325. doi: 10.1016/j.humpath.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. The European Respiratory Journal. 2005;26(4):586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 28.Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Myers JL, Martinez FJ. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics. 2008;28(5):1383–1396. doi: 10.1148/rg.285075223. discussion 96-8. [DOI] [PubMed] [Google Scholar]
- 29.Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. Interstitial lung abnormalities and reduced exercise capacity. American Journal of Respiratory and Critical Care Medicine. 2012;185(7):756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American Journal of Respiratory and Critical Care Medicine. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 31.Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127(3):270–277. doi: 10.1164/arrd.1983.127.3.270. [DOI] [PubMed] [Google Scholar]
- 32.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. The European Respiratory Journal. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


