Abstract
Background
Glycemic index and glycemic load are used to facilitate glucose control among adults with type 2 diabetes, with a low glycemic index diet associated with improved glycemic control.
Objective
To examine long-term longitudinal associations between changes in glycemic index and glycemic load with glycemic and metabolic control among Latino adults with diabetes.
Design
Secondary data from intervention and comparison participants in the Latinos en Control trial (2006–2008) were analyzed.
Participants/setting
Data on dietary intake and metabolic characteristics were from low-income, Latino adults (N=238; 87.7% Puerto Rican) with type 2 diabetes.
Intervention
The Latinos en Control trial was a randomized clinical trial targeting diabetes self-management among Latinos with type 2 diabetes. Participants were randomized to a group-based behavioral intervention or usual care and followed through 12 months.
Main Outcome Measures
Outcomes included hemoglobin A1c (HbA1c) levels, fasting blood glucose, lipid profiles, anthropometrics, and blood pressure.
Statistical Analysis
Glycemic index and load were analyzed using data from three 24-hour dietary recalls conducted at baseline, 4 months, and 12 months. Repeated measures regression models were used to examine change in glycemic index and load associated with metabolic characteristics at 12 months. Covariates included sex, age, body mass index (BMI), blood pressure, total energy intake, medication use and intensity, physical activity, intervention status (intervention vs. usual care), and time.
Results
Increases in glycemic index from baseline to 12 months were associated with increased logarithm of HbA1c levels (β=0.003; p=0.034) and waist circumference (β=0.12; p=0.026)over time, but not with fasting glucose, blood lipids, or BMI. There was modest evidence to support small, positive associations between glycemic load and HbA1c levels and waist circumference.
Conclusions
Lowering glycemic index is associated with improvements in certain metabolic risk factors among Latinos with diabetes. Targeting glycemic index may be an important component of dietary strategies for diabetes self-management.
Keywords: glycemic index, glycemic load, metabolic risk factors, Latinos, type 2 diabetes
Introduction
In 2010–2012, an estimated 12.8% of Latino adults in the United States (U.S.) were diagnosed with type 2 diabetes compared to 7.6% of non-Latino whites.1 Diabetes self-management among Latinos is inadequate,2–4 with over half of those diagnosed with diabetes having uncontrolled glycosylated hemoglobin (HbA1c) levels of 7% or higher.4 The prevalence of type 2 diabetes (diagnosed and undiagnosed) among Latinos is estimated to be 70–80% higher than that of non-Latino whites.5 Latinos also experience disproportionately higher rates of complications and mortality related to diabetes compared to non-Latino whites.2,6,7 Further compounding these health disparities is the common co-occurrence of diabetes with other chronic conditions, such as obesity and hypertension, particularly within this sub-group.8 A study of adults with diabetes living along the U.S.-Mexico border indicated that among Latino adults (including U.S. born Latinos and Mexican immigrants), over 80% were overweight or obese and approximately half were hypertensive (≥140/90 mm Hg).9
Glycemic index and glycemic load may be important factors to investigate for the prevention and management of a variety of chronic conditions, including diabetes, obesity, and hypertension.10 Glycemic index is a relative measure of the increase in blood glucose levels after consuming a specified amount of carbohydrate and represents the quality but not the quantity of carbohydrate.11 Glycemic load represents both the quality and quantity of carbohydrates consumed and may be interpreted as a measure of diet-induced insulin demand.12 Low glycemic index foods provoke a slower, more sustained blood sugar response, with several studies supporting an association between consuming a lower glycemic index diet and improved glycemic control among adults with type 2 diabetes.13–18 A review of epidemiologic studies concluded that a low glycemic load diet is protective against metabolic disease.19 Diabetes-self management interventions targeting Latinos have incorporated glycemic index and glycemic load as part of recommended dietary strategies to facilitate successful management of diabetes.20,21 Less well investigated are the long-term longitudinal effects of lowering glycemic index and/or glycemic load associated with a variety of metabolic risk factors among Latino adults with diabetes.
Understanding the relation between glycemic index, glycemic load, and metabolic risk factors over time is of high importance, particularly among Latinos, an underserved and growing ethnic population at high risk for multiple morbidities. The objective of this study was to conduct a secondary analysis of changes in glycemic index and glycemic load over time associated with metabolic risk factors, including lipid profiles, HbA1c levels, anthropometrics, and blood pressure among low-income Latino adults with type 2 diabetes. We hypothesized that reduced glycemic index and glycemic load would be associated with improved metabolic measures.
Methods
Participants and Procedures
Secondary data from participants in the Latinos en Control trial (2006–2008) were analyzed. The Latinos en Control trial was a randomized clinical trial of a diabetes self-management intervention targeting low-income Latinos with type 2 diabetes.21,22 Screening for eligibility and recruitment were conducted at five urban community health centers in Massachusetts from 2006–2007 and have been previously described.23 Criteria for participant inclusion were: Latino ethnicity; age ≥ 18 years; documented diagnosis of type 2 diabetes; last HbA1c level (previous 7 months) at or above 7.5%; no type 1 diabetes or history of ketoacidosis; no medical contraindications to participation in dietary and physical activity intervention; no use of glucocorticoid therapy within the prior three months; not currently participating in a cardiac rehabilitation or formal weight loss program; access to a telephone; ability and willingness to provide informed consent (English or Spanish); and physician approval to participate. Written informed consent was obtained from all interested and eligible participants.
Intervention and usual care conditions
The Latinos en Control intervention consisted of 12 weekly sessions followed by 8 monthly sessions. Sessions were group-based, took place in community settings, and targeted diabetes-related knowledge, self-efficacy, and self-management behaviors, including blood-glucose self-monitoring, diet, and physical activity. Specific dietary targets of the intervention included decreasing intake and portion size of high glycemic index foods (e.g., starchy vegetables), sodium and saturated fat intake, and increasing fiber intake.
A picture-based food guide that organized foods into traffic stop colors (green=eat often, yellow=limit portion size, and red=avoid) in accordance to their glycemic index, caloric, saturated or trans-fat, sodium, and fiber content.24,25 Foods designated in the green category had a glycemic index (glucose reference) < 55 and were low in calories and fat content. Foods in the yellow category had glycemic index of 55–69 or were high in calorie or fat content. Foods in the red category had glycemic index > 70 or were high in calorie or fat content. A graph of an “ideal” plate targeted glycemic load through its emphasis on reducing portion sizes, in particular foods in the mid and higher end of the glycemic index (foods in the yellow and red categories). Foods in the green category occupied three quarters of the ideal plate, and participants were encouraged to eat foods in this category, such as low-calorie vegetables. Foods in the yellow category occupied one fourth of the plate. Foods in the red category were not included in the plate, and participants were encouraged to eat foods from the red category infrequently and in very small amounts. To facilitate measurement of portions, participants received a set of measuring cups and spoons and were encouraged to use smaller plates.
All group sessions included a video of an educational novella followed by guided group discussions; interactive activities (e.g., taste tests of healthy foods, practice of healthy cooking methods for ethnic foods, label reading, a supermarket tour, measuring skills for cooking and portion control during group meals, strategies to incorporate new cooking methods at home); and a brief coaching segment that included personalized review of progress, problem-solving, and new goal-setting to facilitate learning of skills to reduce glycemic index and reach other behavioral targets. Prior and formative research with members of the target population26 informed the tailoring of intervention materials and activities for the cultural and literacy needs of a low-income Latino population. Additional information regarding the intervention and curriculum materials are available upon request.
Participants in the comparison group received usual care from their health care providers with no additional interventions. Primary care providers of all participants received laboratory results including HbA1c, fasting blood glucose, and lipid profiles obtained at each study assessment and were free to provide care as deemed appropriate or as routinely delivered. Additional details regarding intervention and comparison conditions have been previously reported.21–23 Study protocols, procedures, and measures were approved by the Institutional Review Boards (IRBs) at the University of Massachusetts Medical School and Baystate Health Systems.
Measures
All measures were collected at baseline, 4 months, and 12 months follow-up. Trained bilingual and bicultural research staff blinded to participants’ intervention status conducted assessments on behavioral, clinical, and sociodemographic measures using standardized protocols. Oral administration of survey measures and telephone-administered dietary recalls were available in English and Spanish.
Dietary Measures
Dietary intake was assessed by a registered and trained dietitian via three unannounced telephone-administered 24-hour dietary recalls conducted on randomly selected days within a 3-week period (two weekdays and one weekend). Nutrition Data System for Research (NDS-R) software (versions 2006–2008, University of Minnesota Nutrition Coordinating Center) was used to collect and analyze the dietary recall data, including estimation of glycemic load, glycemic index, and total energy intake (kcal/day).
Metabolic
Measures Metabolic measures included HbA1c levels, fasting blood glucose, lipid profiles, anthropometrics, and blood pressure. Fasting blood samples were drawn by trained medical staff for determination of HbA1c levels, fasting blood glucose (mg/dl), total cholesterol (mg/dl), high density lipoproteins (mg/dl), low density lipoproteins (mg/dl), triglycerides (mg/dl), and ratio of total cholesterol to high density lipoproteins. Participants’ height (inches), weight (pounds), and waist circumference (cm) were measured by trained staff using calibrated instruments, with the mean of two measures used. Height and weight were used to calculate participants’ body mass index (BMI). Two blood pressure measurements were obtained using digital monitors (Dinamap XL Automated Blood Pressure Monitor, Critikon, Tampa, FL) and averaged.
Covariates
Covariates of interest included physical activity, medication usage, and sociodemographics at baseline. Physical activity (MET-hours/day) was assessed via three unannounced telephone-administered 24-hour physical activity recalls.22,27 All medications were recorded including supplements and medications that may cause fluctuations in lipids or weight (either by design or as a side effect). Diabetes medications and dose were recorded directly from the participants’ medication labels and used to determine diabetes medication intensity using a scoring algorithm.22,28 Possible scores ranged from 0–6.5, with each 0.5 unit increase indicating higher intensity of diabetes medication. Socio-demographic data included sex, age (years), education level, and annual income.
Statistical Analysis
The primary analysis was restricted to participants with complete dietary measures at baseline (N=238; 94.4%). All participants had complete data on metabolic characteristics at 12 months follow-up. Descriptive statistics for baseline sociodemographics, glycemic index, glycemic load, and metabolic measures were computed and examined across quartiles of glycemic index and glycemic load. Means and standard deviations are presented as mean (SD). ANOVA and chi-squared tests were used to examine differences in the distribution of continuous and categorical variables by baseline glycemic index and glycemic load quartiles. Mean changes in glycemic index and glycemic load from baseline to 4 months and baseline to 12 months were compared by baseline glycemic index and glycemic load quartiles using chi-squared tests. Repeated measures regression models were used to estimate associations between changes in glycemic index and glycemic load from baseline to 12 months associated with dietary fiber intake at 12 months. Model fit was assessed using a modified coefficient of determination (R2) and a Wald test.
Multivariable regression models with random effects for repeated measures (i.e., panel data) were used to estimate the associations between key predictors (glycemic index and glycemic load from baseline to 12 months modeled as continuous variables) and metabolic outcomes of interest over time. The natural logarithm of HbA1c levels and triglycerides were used, as distributions in these outcomes were skewed. Covariates in the final models included the values at each study period for time (1, 2, 3), mean blood pressure, total energy intake, physical activity, diabetes medication intensity score, medication that may cause fluctuations in lipids (yes/no), medication that may cause fluctuations in weight (yes/no), sex, baseline measures of age and BMI, and intervention status (intervention or usual care). To investigate the potential effect of missing data, two imputed sensitivity analyses were completed. The first analysis was an intention to treat analysis; the second method relied on a multiple imputation algorithm. To assess possible over-fitting in the model from the primary analysis, parse models with predictors that were statistically significant were re-analyzed for each outcome of interest. All analyses were performed using Stata (version 11.0, 2009, StataCorp).
Results
There were no differences between intervention and comparison participants in the Latinos en Control trial (N=252) in sociodemographics, dietary and physical activity measures, and metabolic characteristics at baseline, with the exception of mean diastolic blood pressure (76.3 (9.9) versus 73.4 (8.4) mmHg, comparison versus intervention, respectively; p< 0.011).21 The majority of participants had complete dietary data (94.4%; N=238). No significant differences in baseline characteristics between participants who did and did not have complete dietary data were observed. Additional details regarding intervention and comparison participants at baseline have been previously reported.21
Of the 238 participants with complete dietary data (94.4%), the majority was female (77.3%), identified Puerto Rico as their place of origin (87.7%), and had less than a high school education (76.1%). The mean age was 56.0 years (SD=11.2). As described previously,21,28 most participants were obese (73.9%), had above-target HbA1c levels of 7.0% or higher (89.1%), and had above-target systolic blood pressure (≥ 120 mmHg) (66.8%) at baseline. Participants’ mean baseline glycemic index and glycemic load were 61.0 (5.1) and 126.6 (47.2), respectively. Study attrition was low, with 7% of participants who did not complete at least one study assessment (psychosocial, behavioral, or clinical) at the 12-month follow-up.21,29
Table 1 presents baseline sociodemographic and metabolic risk factor data by glycemic index and glycemic load quartiles. Participants in the lowest quartile of glycemic index had lower mean HbA1c levels (8.2%) compared to participants in the other three quartiles (9.3%, 9.0%, and 9.2%, respectively; p=0.005). With respect to glycemic load, a higher percentage of females were in the lowest quartile (89.8%) than the other quartiles (p=0.033), and higher average daily energy intake was observed from lowest to highest quartile (p<0.001). While significant differences in waist circumference and triglycerides by glycemic load quartiles were also observed, no clear pattern emerged.
Table 1.
Baseline Characteristics By Glycemic Index and Glycemic Load Quartilesa among Latino Adults Participating in a Diabetes Self-Management Intervention (N=238)
|
Glycemic Index Mean (SD) |
Glycemic Load Mean (SD) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 61.0 (5.1) | 126.6 (47.2) | |||||||||
| Glycemic Index Quartiles | Glycemic Load | |||||||||
| 1st N=59 |
2nd N=60 |
3rd N=59 |
4th N=60 |
p-value | 1st N=59 |
2nd N=60 |
3rd N=59 |
4th N=60 |
p-value | |
| Quartile Range | 46.6–58.2 | 58.2–61.0 | 61.0–64.3 | 64.3–76.0 | 30.1–95.4 | 95.4–120.0 | 120.2–151.9 | 151.9–299.4 | ||
| Sociodemographics | ||||||||||
| Age (years) (mean) | 56.6 | 57.2 | 56.4 | 52.6 | 0.101 | 59.1 | 58.0 | 54.2 | 51.4 | <0.001 |
| Sex (% Female) | 76.3 | 80.0 | 81.4 | 71.7 | 0.588 | 89.8 | 78.3 | 72.9 | 68.3 | 0.033 |
| Annual income (% < $10,000) | 50.9 | 64.2 | 54.9 | 52.0 | 0.697 | 52.0 | 64.8 | 54.7 | 50.0 | 0.815 |
| Education (% ≤ high school degree | 76.3 | 81.7 | 76.7 | 70.0 | 0.523 | 74.6 | 80.0 | 78.0 | 71.7 | 0.721 |
| Dietary Measures | ||||||||||
| Glycemic Index | N/A | N/A | N/A | N/A | N/A | 59.1 | 60.6 | 61.6 | 62.6 | 0.001 |
| Glycemic Load | 111.0 | 122.7 | 129.7 | 143.4 | 0.002 | N/A | N/A | N/A | N/A | N/A |
| Total Energy (kcal/day) | 1640 | 1683 | 1711 | 1768 | 0.665 | 1092 | 1514 | 1856 | 2334 | <0.001 |
| Metabolic Measures | ||||||||||
| HbA1c (%) | 8.2 | 9.4 | 9.0 | 9.2 | 0.005 | 8.7 | 8.8 | 9.2 | 9.1 | 0.511 |
| Fasting blood glucose (mg/dl) | 152 | 173 | 172 | 176 | 0.241 | 163 | 164 | 175 | 173 | 0.754 |
| Total cholesterol (mg/dl) | 180.2 | 177.7 | 183.1 | 181.7 | 0.926 | 193.6 | 170.3 | 177.8 | 181.1 | 0.036 |
| HDL cholesterol (mg/dl) | 44 | 43 | 45 | 44 | 0.829 | 47 | 44 | 43 | 43 | 0.086 |
| LDL cholesterol (mg/dl) | 107 | 104 | 104 | 109 | 0.867 | 120 | 99 | 100 | 105 | 0.007 |
| Triglycerides (mg/dl) | 154 | 163 | 163 | 145 | 0.787 | 143 | 141 | 178 | 163 | 0.214 |
| BMI (kg/m2) | 34.6 | 35.1 | 35.3 | 34.2 | 0.847 | 34.9 | 33.1 | 35.0 | 36.2 | 0.100 |
| Waist circumference (cm) | 111 | 112 | 113 | 111 | 0.749 | 111 | 108 | 113 | 116 | 0.012 |
| Mean Arterial Pressure (mm/Hg) | 96 | 99 | 96 | 95 | 0.380 | 97 | 98 | 95 | 95 | 0.391 |
| Covariates | ||||||||||
| Diabetes Medication Intensity Score | 2.9 | 2.9 | 2.7 | 3.1 | 0.660 | 3.0 | 3.1 | 2.8 | 2.8 | 0.804 |
| Total Physical Activity (MET-hours/day) | 12.0 | 13.3 | 11.1 | 14.0 | 0.068 | 12.7 | 11.9 | 12.3 | 13.6 | 0.524 |
1=lowest quartile
Participants in the highest quartiles of glycemic index and glycemic load by definition had the greatest potential for decreasing these values over time though the impact of the intervention by quartile was unknown a priori. We observed that participants in the highest quartile of glycemic index at baseline had the greatest reduction in mean glycemic index compared to all other quartiles at 4 months (−4.8 (5.4) vs. 0.5 (6.8), p<0.001) and 12 months (−5.2 (4.9) vs. 1.5 (6.2), p<0.001). Similarly, participants in the highest glycemic load quartile at baseline had the greatest reduction in mean glycemic load compared to all other quartiles at 4 months (−37.5 (56.3) vs. 4.3 (38.9), p<0.001) and 12 months (−39.7 (48.5) vs. 8.7 (38.2), p<0.001). (Results not shown in tables). Adjusting for intervention status and time, change in glycemic index was negatively associated with dietary fiber intake at 12 months (β=−0.8; p<0.001). No association between change in glycemic load and dietary fiber intake was observed. (Results not shown in tables).
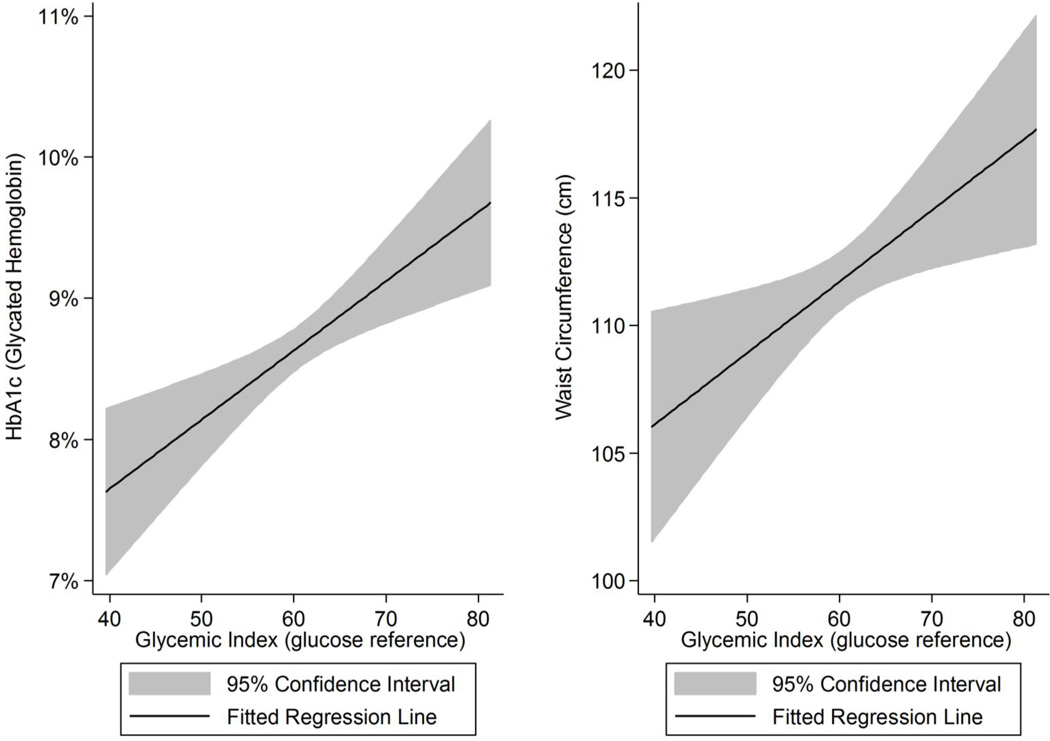
Positive correlations between glycemic index and HbA1c and waist circumference were observed during the study period (Figure 1). The confidence intervals bands illustrate the variability of the data with wider confidence intervals at the extremes of glycemic index due in part to fewer observations at these levels. After adjustment, the positive associations between glycemic index and HbA1c levels and waist circumference over time remained significant, with a one unit change in glycemic index corresponding to a 0.3% change in HbA1c levels (95% CI: 0.00% to 0.06%; p=0.034) and a 0.12 cm change in waist circumference (95% CI: 0.01 to 0.23; p=0.026) (Table 2). Positive associations between glycemic load and HbA1c levels (β= 0.001; p=0.076) and waist circumference (β=0.04; p=0.073) were also observed; no other associations between glycemic load and outcomes of interest over time were observed to be considered clinically or statistically significant (Table 3). Sensitivity analyses and the assessment of the coefficient of glycemic index or glycemic load conducted with parse models yielded findings similar to the data presented in Tables 2 and 3 and are not shown.
Figure 1.
The Unadjusted Relationship between Glycemic Index with Glycated Hemoglobin and Waist Circumference among Latino Adults Participating in a Diabetes Self-Management Intervention (N=238)
Table 2.
Change in Metabolic Measures Over Time by Glycemic Index from Baseline to 12 Months among Latino Adults Participating in a Diabetes Self-Management Intervention (N=238)
| 12 Month Metabolic Outcomesa | Effect estimateb (95% confidence interval) |
p-value |
|---|---|---|
| Model 1. Log Hemoglobin A1c (%) | 0.003 (0.000, 0.006) | 0.034 |
| Model 2. Fasting Blood Glucose (mg/dl) | 0.61 (−0.44 1.65) | 0.255 |
| Model 3. Total Cholesterol (mg/dl) | 0.02 (−0.56, 0.59) | 0.956 |
| Model 4. HDL Cholesterol (mg/dl) | −0.09 (−0.19 to 0.02) | 0.110 |
| Model 5. LDL Cholesterol (mg/dl) | 0.02 (−0.45 to 0.49) | 0.945 |
| Model 6. Log Triglycerides (mg/dl) | 0.00 (−0.00 to 0.01) | 0.396 |
| Model 7. Total Cholesterol:HDL Ratio | −0.00 (−0.00 to 0.00) | 0.810 |
| Model 8. BMI (kg/m2) | −0.02 (−0.06 to 0.01) | 0.191 |
| Model 9. Waist Circumference (cm) | 0.12 (0.01 to 0.23) | 0.026 |
Separate multivariable regression models were used to estimate primary associations of interest for each outcome.
Covariates included: age, sex, baseline BMI, mean blood pressure, energy intake, physical activity, diabetes medication intensity, lipid increasing or decreasing medication (yes/no), weight increasing or decreasing medication (yes/no), intervention status (intervention vs. usual care), and time.
Table 3.
Change in Metabolic Measures Over Time by Glycemic Load From Baseline to 12 Months among Latino Adults Participating in a Diabetes Self-Management Intervention (N=238)
| 12 Month Metabolic Outcomesa | Effect estimateb (95% confidence interval) |
p-value |
|---|---|---|
| Model 1. Log Hemoglobin A1c (%) | 0.001 (−0.000 to 0.002) | 0.076 |
| Model 2. Fasting Blood Glucose (mg/dl) | 0.36 (−0.09 to 0.81) | 0.112 |
| Model 3. Total Cholesterol (mg/dl) | 0.04 (−0.20 to 0.29) | 0.723 |
| Model 4. HDL Cholesterol (mg/dl) | −0.02 (−0.07 to 0.02) | 0.304 |
| Model 5. LDL Cholesterol (mg/dl) | 0.04 (−0.16 to 0.24) | 0.700 |
| Model 6. Log Triglycerides (mg/dl) | 0.00 (−0.00 to 0.00) | 0.376 |
| Model 7. Total Cholesterol:HDL Ratio | −0.00 (−0.00 to 0.00) | 0.633 |
| Model 8. BMI (kg/m2) | −0.01 (−0.02 to 0.01) | 0.345 |
| Model 9. Waist Circumference (cm) | 0.04 (−0.00 to 0.09) | 0.073 |
Separate multivariable regression models were used to estimate primary associations of interest for each outcome.
Covariates included: age, sex, baseline BMI, mean blood pressure, energy intake, physical activity, diabetes medication intensity, lipid increasing or decreasing medication (yes/no), weight increasing or decreasing medication (yes/no), intervention status (intervention vs. usual care), and time.
Discussion
This is the first study to the investigators’ knowledge to assess associations between one-year change in glycemic index and glycemic load and a variety of metabolic measures among Latino adults with type 2 diabetes. Study findings were consistent with previous intervention trials aimed at lowering glycemic index among individuals with diabetes, with a lower glycemic index diet found to be associated with greater improvements in glycemic control.15,16,30–33 However, the literature on long-term associations between glycemic index over time and metabolic measures among a more general population of adults with diabetes have been inconsistent. Two prior studies found no improvement in anthropometrics associated with glycemic index.30,31 Other research indicated improvements in lipid profiles, including high density lipoproteins, low density lipoproteins, and total cholesterol associated with reduced glycemic index,16,31,34 while one study found no improvement with these measures.15 The positive, one-year associations between glycemic index and HbA1c levels and waist circumference observed in this study is notable, particularly as previous studies were conducted with shorter follow-up duration.15,16,30,31
Importantly, this study examined the associations between changes in glycemic index and glycemic load and several metabolic measures, including blood pressure, lipid profiles, and anthropometrics among low-income Latino adults with diabetes, an under-studied population at high risk for multiple morbidities.35 Consistent with our hypothesis and previous research conducted with Latino adults with type 2 diabetes,30 our results indicate that reducing glycemic index is associated with improved glycemic control over time. Jimenez-Cruz and colleagues’ 6-week crossover study30 among 14 overweight and obese Mexicans with type 2 diabetes indicated that participants’ HbA1c levels improved on the low compared with the high glycemic index diet. The current study builds upon existing research with a larger sample of Latinos participating in a more general diabetes self-management intervention with a longer follow-up duration (12 months). Additionally, the observed association between lower glycemic index and lower HbA1c levels after 12 months yields important clinical implications, as a dose-response association exists between HbA1c levels and risk of diabetes-related complications.36 The current study sample was largely comprised of individuals with uncontrolled diabetes, and associations between change in glycemic index and HbA1c levels may differ for populations with controlled diabetes. The lack of a significant association between change in glycemic index and fasting blood glucose at 12 months observed in our study was not unexpected given the greater variability in participants’ fasting blood glucose compared to HbA1c levels.
The current study’s findings of the association between glycemic index and HbA1c should be interpreted with caution. HbA1c was transformed logarithmically due to the skewed distribution of this variable in the study sample. Based on our findings, a one unit in change in in glycemic index corresponds to a 0.3% change in HbA1c levels (95% CI: 0.00% to 0.06%; p=0.034) (note: the lower bound of the confidence interval is positive but is reported as “0.00” due to rounding to two significant digits). A 10 unit change in glycemic index corresponds to a 3.0% change in HbA1c (95% CI: 0.2% to 5.9%). For a participant with a HbA1c level of 8.0%, a 3% decrease in HbA1c (based on a 10 unit change in glycemic index) would be approximately 7.8%. A decrease in HbA1c from 8.0% to 7.8% is the equivalent to decreasing the estimated average glucose (eAG) from 183 mg/dl to 177 mg/dl.
The interpretation of the effect size of glycemic index on waist circumference (0.12 cm change in waist circumference per 1 unit change in glycemic index) is more straightforward. This result implies that a 10 unit decrease in glycemic index would result in a 1.2 cm decrease in waist circumference (95% CI: 0.1 to 2.3 cm). This finding has clinical relevance, as waist circumference is often used as a proxy for abdominal fat mass and is associated with cardiometabolic disease risk.37 Previous research suggests that lowering glycemic index may increase satiety, which in turn would decrease caloric intake and result in weight loss.38 However, our study found no correlations between glycemic index or glycemic load with BMI. Thus, the mechanisms through which changes in glycemic index and load may influence anthropometrics remain unclear. It is possible that weight changes associated with change in glycemic index exist but require longer follow-up duration to be detected.
The negative association between glycemic index and dietary fiber intake observed among study participants emphasizes the need to better understand the role of macronutrients and food groups in relation to glycemic index and in the management of type 2 diabetes, particularly among Latinos, a group largely under-represented in previous trials. Literature on the association between dietary fiber and glycemic control among adults with diabetes is inconclusive. Two systematic reviews found little evidence to support an association between fiber intake and improved glycemic control,33,39 whereas other studies have demonstrated modest improvements of preprandial glucose and HbA1c levels among participants with high fiber intake diets (>50 grams of fiber per day).40,41 Studies examining the association between legumes (among the lowest of glycemic index foods) and glycemic control also yield mixed results. One randomized controlled trial indicated that incorporating legumes (e.g., soybeans, chickpeas, lentils) as part of a low glycemic index diet was associated with improved glycemic control among adults with type 2 diabetes,40 whereas a review indicated that most studies of soy-based supplementation did not support an association between legumes and glycemic control.33 Additional research on dietary fiber and legume intake among Latinos and the role of macronutrients and food groups on glycemic index and glycemic load in the context of diabetes management among this population is needed.
The null findings between change in glycemic load and outcomes of interest are consistent with previous research examining reductions in glycemic load associated with fasting blood glucose, BMI, postprandial blood glucose, and insulin levels among participants (no diagnosis of diabetes) in a 6-month weight loss intervention.42 That study’s authors posited that perhaps greater metabolic dysfunction (i.e., type 2 diabetes) is needed to elicit significant changes in these outcomes. However, results from our study conducted with a sample of overweight and obese individuals with type 2 diabetes do not support this explanation. While epidemiologic literature supports the protective effect of a low glycemic load diet against metabolic disease,19 the longitudinal associations between reducing glycemic load over time among individuals who initially have high glycemic load diets and metabolic measures warrant further investigation. Findings suggest the possibility of small, positive associations between glycemic load and HbA1c levels and waist circumference and warrant further investigation. Within the context of previous findings of the effects of the Latinos en Control intervention on dietary and clinical outcomes,21 the current study’s findings suggest that the integration of information and strategies related to glycemic index within a comprehensive, behavioral intervention may yield additional benefits on HbA1c levels and waist circumference. Future studies with larger sample sizes are needed to more definitively determine the impact of glycemic load on these outcomes.
This study has numerous strengths, including the long-term follow-up, multiple metabolic measures collected, and the focus on low-income Latinos with type 2 diabetes, the majority of whom had elevated HbA1c levels, hypertension, and were overweight or obese. Nevertheless, our findings should be considered in light of study limitations. Glycemic index and glycemic load were derived from dietary intake data, which was collected via self-report and may be subject to inaccurate reporting and/or recall bias in the amount and types of foods reported.43 More objective measures of dietary intake would be less prone to recall bias but may be more difficult to implement in an outpatient setting. Participants were primarily of Puerto Rican descent and were Massachusetts residents; thus, study findings may not be generalizable to other populations.
Conclusion
Lower glycemic index is associated with improvements in glycemic control and waist circumference in our study sample of Latinos with type 2 diabetes. These results may have important implications to inform dietary targets for diabetes self-management. No associations with glycemic index or glycemic control and other metabolic outcome measures were observed. Larger reductions in glycemic index and glycemic load and/or larger study samples may be needed to observe clinically significant improvements in these outcomes. Additional experimental and observational longitudinal studies on how changes in glycemic index and glycemic load as part of medical nutrition therapy for patients with diabetes relate to metabolic measures are needed.
Acknowledgements
Funding/Support Disclosure
This study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant R18-DK-65985 and grants from the Robert Wood Johnson Foundation and Novo Nordisk Pharmaceutical. We acknowledge the contributions of the study staff and are grateful to the patients who participated and made the study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Contributor Information
Monica L. Wang, Email: Mlwang@bu.edu.
Lauren Gellar, Email: lgellar@utk.edu.
Brian Nathanson, Email: brian.h.nathanson@att.net.
Lori Pbert, Email: Lori.Pbert@umassmed.edu.
Yunsheng Ma, Email: Yunsheng.Ma@umassmed.edu.
Ira Ockene, Email: Ira.Ockene@umassmed.edu.
Milagros C. Rosal, Email: Milagros.Rosal@umassmed.edu.
References
- 1.Centers for Disease Control and Prevention (CDC) National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 2.Gonzalez AB, Salas D, Umpierrez GE. Special considerations on the management of Latino patients with type 2 diabetes mellitus. Current medical research and opinion. 2011 May;27(5):969–979. doi: 10.1185/03007995.2011.563505. [DOI] [PubMed] [Google Scholar]
- 3.Kirk JK, Passmore LV, Bell RA, et al. Disparities in A1C levels between Hispanic and non-Hispanic white adults with diabetes: A meta-analysis. Diabetes care. 2008 Feb;31(2):240–246. doi: 10.2337/dc07-0382. [DOI] [PubMed] [Google Scholar]
- 4.Boltri JM, Okosun IS, Davis-Smith M, Vogel RL. Hemoglobin A1c levels in diagnosed and undiagnosed black, Hispanic, and white persons with diabetes: Results from NHANES 1999–2000. Ethnicity & disease. 2005 Autumn;15(4):562–567. [PubMed] [Google Scholar]
- 5.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the 371 U.S. population in 1988–1994 and 2005–2006. Diabetes care. 2009 Feb;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999 Mar;22(3):403–408. doi: 10.2337/diacare.22.3.403. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Age-Adjusted Percentage of Civilian, Noninstitutionalized Population with Diagnosed Diabetes, by Hispanic Origin and Sex, United States, 1997–2011. [Accessed September 9 2014];2013 http://www.cdc.gov/diabetes/statistics/prev/national/fighispanicthsex.htm.
- 8.Suh DC, Choi IS, Plauschinat C, Kwon J, Baron M. Impact of comorbid conditions and race/ethnicity on glycemic control among the US population with type 2 diabetes, 1988–1994 to 1999–2004. Journal of diabetes and its complications. 2010 Nov-Dec;24(6):382–391. doi: 10.1016/j.jdiacomp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Vijayaraghavan M, He G, Stoddard P, Schillinger D. Blood pressure control, hypertension, awareness, and treatment in adults with diabetes in the United States-Mexico border region. Revista panamericana de salud publica = Pan American journal of public health. 2010 Sep;28(3):164–173. doi: 10.1590/s1020-49892010000900006. [DOI] [PubMed] [Google Scholar]
- 10.Flood A, Subar AF, Hull SG, Zimmerman TP, Jenkins DJ, Schatzkin A. Methodology for adding glycemic load values to the National Cancer Institute Diet History Questionnaire database. Journal of the American Dietetic Association. 2006 Mar;106(3):393–402. doi: 10.1016/j.jada.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981 Mar;34(3):362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 12.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002 Jul;76(1):274S–280S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- 13.Brand JC, Colagiuri S, Crossman S, Allen A, Roberts DC, Truswell AS. Low-glycemic index foods improve long-term glycemic control in NIDDM. Diabetes Care. 1991 Feb;14(2):95–101. doi: 10.2337/diacare.14.2.95. [DOI] [PubMed] [Google Scholar]
- 14.Gutschall MD, Miller CK, Mitchell DC, Lawrence FR. A randomized behavioural trial targeting glycaemic index improves dietary, weight and metabolic outcomes in patients with type 2 diabetes. Public Health Nutr. 2009 Jan 23;:1–9. doi: 10.1017/S1368980008004680. [DOI] [PubMed] [Google Scholar]
- 15.Jarvi AE, Karlstrom BE, Granfeldt YE, Bjorck IE, Asp NG, Vessby BO. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care. 1999 Jan;22(1):10–18. doi: 10.2337/diacare.22.1.10. [DOI] [PubMed] [Google Scholar]
- 16.Rizkalla SW, Taghrid L, Laromiguiere M, et al. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care. 2004 Aug;27(8):1866–1872. doi: 10.2337/diacare.27.8.1866. [DOI] [PubMed] [Google Scholar]
- 17.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008 Jan;87(1):114–125. doi: 10.1093/ajcn/87.1.114. [DOI] [PubMed] [Google Scholar]
- 18.Miller CK, Gutshcall MD, Mitchell DC. Change in food choices following a glycemic load intervention in adults with type 2 diabetes. Journal of the American Dietetic Association. 2009 Feb;109(2):319–324. doi: 10.1016/j.jada.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts CK, Liu S. Effects of glycemic load on metabolic health and type 2 diabetes mellitus. Journal of diabetes science and technology. 2009 Jul;3(4):697–704. doi: 10.1177/193229680900300414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller CK, Kristeller JL, Headings A, Nagaraja H, Miser WF. Comparative effectiveness of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: a pilot study. Journal of the Academy of Nutrition and Dietetics. 2012 Nov;112(11):1835–1842. doi: 10.1016/j.jand.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosal MC, Ockene IS, Restrepo A, et al. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income latinos: latinos en control. Diabetes care. 2011 Apr;34(4):838–844. doi: 10.2337/dc10-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosal MC, White MJ, Restrepo A, et al. Design and methods for a randomized clinical trial of a diabetes self-management intervention for low-income Latinos: Latinos en Control. BMC Med Res Methodol. 2009;9:81. doi: 10.1186/1471-2288-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosal MC, White MJ, Borg A, et al. Translational research at community health centers: Challenges and successes in recruiting and retaining low-income Latino patients with type 2 diabetes into a randomized clinical trial. The Diabetes Educator. 2010 Sep-Oct;36(5):733–749. doi: 10.1177/0145721710380146. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes care. 2008 Dec;31(12):2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand-Miller J, Wolever TMS, Foster-Powell KF, Colagiuri S. The new glucose revolution. New York: Marlowe & Company; 2003. [Google Scholar]
- 26.Rosal MC, Olendzki B, Reed GW, Gumieniak O, Scavron J, Ockene I. Diabetes self-management among low-income Spanish-speaking patients: a pilot study. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2005 Jun;29(3):225–235. doi: 10.1207/s15324796abm2903_9. [DOI] [PubMed] [Google Scholar]
- 27.Matthews CE, DuBose KD, LaMonte M, Tudor-Locke C, Ainsworth BE. Evaluation of a computerized 24-hour physical activity recall (24PAR). [abstract] Med Sci Sports Exer. 2002;34(Suppl 5):S41. [Google Scholar]
- 28.Wang ML, Lemon SC, Olendzki B, Rosal MC. Beverage-consumption patterns and associations with metabolic risk factors among low-income Latinos with uncontrolled type 2 diabetes. Journal of the Academy of Nutrition and Dietetics. 2013 Dec;113(12):1695–1703. doi: 10.1016/j.jand.2013.06.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang ML, Lemon SC, Whited MC, Rosal MC. Who Benefits from Diabetes Self-Management Interventions? The Influence of Depression in the Latinos en Control Trial. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2014 Mar 25; doi: 10.1007/s12160-014-9606-y. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez-Cruz A, Bacardi-Gascon M, Turnbull WH, Rosales-Garay P, Severino-Lugo I. A flexible, low-glycemic index mexican-style diet in overweight and obese subjects with type 2 diabetes improves metabolic parameters during a 6-week treatment period. Diabetes Care. 2003 Jul;26(7):1967–1970. doi: 10.2337/diacare.26.7.1967. [DOI] [PubMed] [Google Scholar]
- 31.Luscombe ND, Noakes M, Clifton PM. Diets high and low in glycemic index versus high monounsaturated fat diets: effects on glucose and lipid metabolism in NIDDM. Eur J Clin Nutr. 1999 Jun;53(6):473–478. doi: 10.1038/sj.ejcn.1600779. [DOI] [PubMed] [Google Scholar]
- 32.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. The Cochrane database of systematic reviews. 2009;(1) doi: 10.1002/14651858.CD006296.pub2. CD006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler ML, Dunbar SA, Jaacks LM, et al. Macronutrients, food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes care. 2012 Feb;35(2):434–445. doi: 10.2337/dc11-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heilbronn LK, Noakes M, Clifton PM. The effect of high- and low-glycemic index energy restricted diets on plasma lipid and glucose profiles in type 2 diabetic subjects with varying glycemic control. J Am Coll Nutr. 2002 Apr;21(2):120–127. doi: 10.1080/07315724.2002.10719204. [DOI] [PubMed] [Google Scholar]
- 35.McEwen MM, Pasvogel A, Gallegos G, Barrera L. Type 2 diabetes self-management social support intervention at the U.S.-Mexico border. Public Health Nurs. 2010 Jul-Aug;27(4):310–319. doi: 10.1111/j.1525-1446.2010.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000 Aug 12;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein S, Allison DB, Heymsfield SB, et al. Waist Circumference and Cardiometabolic Risk: a Consensus Statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity (Silver Spring) 2007 May;15(5):1061–1067. doi: 10.1038/oby.2007.632. [DOI] [PubMed] [Google Scholar]
- 38.Bornet FR, Jardy-Gennetier AE, Jacquet N, Stowell J. Glycaemic response to foods: impact on satiety and long-term weight regulation. Appetite. 2007 Nov;49(3):535–553. doi: 10.1016/j.appet.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Franz MJ, Powers MA, Leontos C, et al. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. Journal of the American Dietetic Association. 2010 Dec;110(12):1852–1889. doi: 10.1016/j.jada.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins DJ, Kendall CW, Augustin LS, et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Archives of internal medicine. 2012 Nov 26;172(21):1653–1660. doi: 10.1001/2013.jamainternmed.70. [DOI] [PubMed] [Google Scholar]
- 41.Post RE, Mainous AG, 3rd, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. Journal of the American Board of Family Medicine : JABFM. 2012 Jan-Feb;25(1):16–23. doi: 10.3122/jabfm.2012.01.110148. [DOI] [PubMed] [Google Scholar]
- 42.Pittas AG, Roberts SB, Das SK, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity (Silver Spring) 2006 Dec;14(12):2200–2209. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 43.Olendzki BC, Ma Y, Hebert JR, et al. Underreporting of energy intake and associated factors in a Latino population at risk of developing type 2 diabetes. J Am Diet Assoc. 2008 Jun;108(6):1003–1008. doi: 10.1016/j.jada.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]