Abstract
Background and Objectives
Antiretrovirals (ARVs) are currently used for the treatment and prevention of HIV infection. Poor adherence and low tolerability of some existing oral formulations can hinder their efficacy. Long-acting (LA) injectable nanoformulations could help address these complications by simplifying ARV administration. The aim of this study is to inform the optimisation of intramuscular LA formulations for eight ARVs through physiologically-based pharmacokinetic (PBPK) modelling.
Methods
A whole-body PBPK model was constructed using mathematical descriptions of molecular, physiological and anatomical processes defining pharmacokinetics. These models were validated against available clinical data and subsequently used to predict the pharmacokinetics of injectable LA formulations
Results
The predictions suggest that monthly intramuscular injections are possible for dolutegravir, efavirenz, emtricitabine, raltegravir, rilpivirine and tenofovir provided that technological challenges to control release rate can be addressed.
Conclusions
These data may help inform the target product profiles for LA ARV reformulation strategies.
1 Introduction
The human immunodeficiency virus (HIV) is a global pandemic affecting 75 million people till date, with 2.3 million new cases in 2012 [1]. Modern antiretroviral (ARV) therapy is based on the targeting of specific stages of the viral replication, with optimal suppression obtained through the use of two or more ARV [2] classes in combination. ARVs can also be used for pre-exposure prophylaxis (PrEP) strategies, preventing the transmission of HIV to un-infected individuals who are at high risk of acquiring the infection [3].
Currently available oral formulations necessitate lifelong, daily dosing and subjects often encounter pill fatigue as a consequence. This has the potential of producing suboptimal adherence, which has emerged as one of the main causes of therapeutic failure and low rates of PrEP protection [4]. The introduction of long-acting (LA) formulations could revolutionise treatment and PrEP by enabling administration once-monthly or even less frequently. This can simplify ARV administration and improve adherence [5]. LA formulations are most commonly administered parenterally, decreasing inter- and intra-patient variability in drug exposure. Additional potential advantages include a decrease in overall drug consumption and cost of treatment [6]. LA injectable contraceptives and antipsychotics are widely used and have excellent efficacy and tolerability compared to oral routes of administration [4].
Recently, injectable LA formulations of the ARVs rilpivirine and S/GSK1265744/cabotegravir have been developed with the potential for once monthly dosing, or less frequently [4]. The development of the first injectable LA ARV formulations has led to considerable interest amongst patients; a recent study found that 84% of surveyed patients would definitely or probably try a once-monthly injectable LA ARV [7]. The LA formulations are based on solid drug nanoparticles (SDNs) which release the drug from the depot over a protracted period of time. The optimisation of SDNs for LA administration is complicated by several pharmacokinetic factors and has to take into account the optimal dose and drug release into the circulation to achieve therapeutic or prophylactic plasma concentrations for the entire dosing interval.
The pharmacokinetics of a drug are determined by processes that can be simulated through physiologically-based pharmacokinetic (PBPK) models. PBPK models are based on the mathematical description of anatomical, physiological and molecular processes defining drug distribution, integrating information on drug characteristics and patient-specific factors [8]. This modelling approach can be applied to the development and optimisation of novel formulations to identify optimal pharmacokinetic characteristics [8]. PBPK models have been used extensively in different disease areas for the prediction of pharmacokinetics for novel drugs, optimisation of existing therapies and simulation of different clinical scenarios. Specifically, PBPK can be used to predict pharmacokinetics as a function of formulation and route of administration [9]. These predictions can then inform the design of LA formulations, identifying optimal characteristics in terms of dose and depot release rate. For ARVs, this modelling approach has been applied to identify factors influencing the pharmacokinetics of novel formulations [10, 11] the effect of genetics on drug distribution [11], pharmacokinetics in special populations [12] and drug-drug interactions [13].
The aim of this study was to simulate the pharmacokinetics of ARVs after administration of virtual intramuscular LA formulations using PBPK modelling. The PBPK models were first validated against clinical data for oral counterparts, to assess the accuracy of the simulation. Theoretical target dose and release rate combinations for once weekly and once monthly intramuscular administration were then identified.
2 Methods
Virtual patients between the ages of 18 to 60 years were generated. All organs and tissues were represented as individual compartments. The model was based on the assumptions: 1) well stirred compartments with instant distribution of the drug; 2) no absorption of the drug from the colon; and 3) the model is blood flow limited. The PBPK model was designed using Simbiology v.4.3.1, a product of Matlab v.8.2 (MathWorks, Natick, MA, USA 2013). The PBPK approach has been developed for key ARVs (two nucleoside reverse-transcriptase inhibitors (NRTIs), tenofovir and emtricitabine; three non-nucleoside reverse-transcriptase inhibitors (NNRTIs), efavirenz, rilpivirine and etravirine; two integrase inhibitors (IIs), raltegravir and dolutegravir and an unboosted protease inhibitor (PI), atazanavir.
2.1 Anatomy
The age, body mass index, body surface area, height and weight of the individual were defined as previously described [14]. These initial values were used for the calculation of organ and tissue volumes through allometric equations [14]. The blood circulation was represented by considering the cardiac output and regional blood flows as previously described [14].
2.2 Intestinal absorption
A compartmental absorption and transit model has been integrated to simulate for oral drugs as previously described [15]. Absorption rate was evaluated using the apparent permeability obtained from Caco-2 cells or the polar surface area (PSA) and hydrogen bond donor (HBD) values as previously described [16].
2.3 Intestinal metabolism
The clearance of drugs in the gut (CLgut) was calculated considering the in vitro intrinsic clearance (CLint) and the abundance of cytochrome P450 family 3, subfamily A - CYP3A in the intestinal tissue (AbCYP3A) [16, 17]:
| (1) |
The fraction of the drug transiting to the liver after metabolism in the gut (Fg) was computed using the following equation:
| (2) |
where Qgut represents the blood flow to the gut and fu,gut is the fraction unbound in the gut.
2.4 First pass metabolism
The total intrinsic clearance (TCLint) of an enzyme was calculated as:
| (3) |
where Abundance is the amount of enzyme present in a milligram of microsomal protein and MPPGL is amount of microsomal protein per gram of liver [18]:
| (4) |
Then the sum of intrinsic clearances of all the enzymes that are metabolising the drug in the liver gives the total apparent clearance (CLapp):
| (5) |
The systemic clearance (CL) is calculated from the blood and not the liver and is given by the equation:
| (6) |
Where Qhv is the hepatic flow rate and fu is the fraction unbound in plasma.
The fraction of the active drug that is available after first pass hepatic metabolism (Fh) is given as:
| (7) |
2.5 Systemic distribution
The volume of distribution and systemic circulation was calculated using previously published equations [19, 20].
2.6 Intramuscular depot compartment
A compartment was created to virtually represent the depot with a maximum volume of 6 mL since it is the maximum volume of injection that has previously been used for intramuscular administration, although lower volumes are usually needed for optimal tolerability [21]. The blood flow to this area was calculated accordingly. The release of the drug from the depot to the blood capillaries present in the muscle was given as [9]:
| (8) |
where A is the amount of drug released from the depot and k is the first-order release rate. A variability of ±10% for the release rate values was considered.
Also the highest single dose that has been tested clinically is 1500 mg with Cefuroxime [21]. Therefore, any strategy for LA ARV development needs to address depot volume and dose in order to successfully translate.
2.7 Model validation
The physicochemical properties of ARVs used to build the PBPK models are represented in table 1. The models were primarily validated against clinical data at steady state for oral formulations of the ARVs (table 2). The model was also verified against an existing long-acting formulation of rilpivirine [6].
Table 1.
Physicochemical properties, in vitro and population pharmacokinetic data of antiretrovirals
| Atazanavir | Dolutegravir | Efavirenz | Emtricitabine | Etravirine | Raltegravir | Rilpivirine | Tenofovir | |
|---|---|---|---|---|---|---|---|---|
| Drug Properties | ||||||||
|
| ||||||||
| Molecular weight | 704 | 419 | 316 | 247 | 435 | 444 | 366 | 287 |
| log Po:w | 3.17 | 2.20 | 4.60 | −0.43 | 5.20 | 0.58 | 4.32 | 1.25 |
| Protein binding | 86.0% | 99.3% | 98.0% | 4.0% | 99.0% | 83.0% | 99.7% | 0.7% |
| pKa | 7.4 | 8.3 | 10.2 | 2.65 | 3.75 | 6.67 | 3.26 | 3.75 |
| R | 0.75 [13] | 0.535 [42] | 0.74 [43] | 1.0 [33] | 0.7 [44] | 0.6 [45] | 0.67 [27] | b1 |
| PSA (Å2) | - | - | 38.33 | - | 120.64 | - | - | |
| HBD | - | - | 1 | - | 3 | - | - | |
| Papp (10−6 cm/s) | - | - | 2.5 [43] | - | - | 6.6 [45] | 12 [27] | - |
| ka (h−1) | 1.5 [13] | 1.63 ± 1.03 [46] | - | 1.0 [47] | - | - | - | 1.03 [48] |
| F | 68% | - | - | 93% | - | - | - | 20% |
| Vd (L/kg) | 1.2 [13] | - | 3.6 | 1.4 ± 0.3 | a6.03 | 0.6 | - | 0.813 [38] |
|
| ||||||||
| Metabolism | ||||||||
|
| ||||||||
| CL/F (L/h) | a12.6 (10.5-15.4) [49] | 1 (15%) [31] | - | - | - | 4.62 [50] | - | - |
|
Renal
Clearance (L/h) |
- | - | - | 12.80 ± 5.34 [33] | - | 3.6 [45] | - | 11.3 ± 6.2 [51] |
| CYP2A6 CLint | - | - | 0.08 [43] | - | - | - | - | - |
| CYP2B6 CLint | - | - | 0.55 [43] | - | - | - | - | - |
| CYP1A2 CLint | - | - | 0.07 [43] | - | - | - | - | - |
| CYP3A4 CLint | - | †3.0 [52] | 0.007 [43] | - | 0.012 [53] | - | 2.04 [27] | - |
| M2 CYP3A4 CLint | - | - | - | - | 0.00091 [53] | - | - | - |
| CYP3A5 CLint | - | - | 0.03 [43] | - | - | - | - | - |
| CYP2C19 CLint | - | - | - | - | 0.75 [53] | - | - | - |
| UGT1A1 CLint | - | †3.2 [52] | - | - | - | ‡12.4 [45] | - | - |
|
| ||||||||
| CYPs induction | ||||||||
|
| ||||||||
| CYP3A4 Indmax | - | - | 6.5 [43] | - | 2.5 [53] | - | - | - |
| CYP2B6 Indmax | - | - | 5.7 [43] | - | - | - | - | - |
| CYP3A4 Ind50 | - | - | 3.9 [43] | - | - | - | - | - |
| CYP2B6 Ind50 | - | - | 0.8 [43] | - | - | - | - | - |
An average adult body weight of 70 kg was assumed
Value was an assumption (as the drug is similar to emtricitabine and tenofovir value was not available in the literature)
The units are in μ/min/mg
The units are in μ/min/106 hepatocytes; log Po:w – Partition coefficient between octanol and water; pKa – logarithmic value of the dissociation constant; R – blood-to-plasma drug ratio; PSA – polar surface area; HBD – number of hydrogen bond donors; Papp – drug permeability from apical to basolateral in Caco-2 cell monolayer;; Ka – absorption coefficient; F – absolute bioavailability; Vd – volume of distribution; CL/F – Apparent oral clearance; Clint – intrinsic clearance (μ/min/pmol); CYP – cytochrome P450; UGT – uridine diphosphate glucuronosyltransferase; M2 – Second metabolite; Indmax – induction maximum; Ind50 – 50% induction; ‘-’ – data not available
Table 2.
Validation of antiretrovirals at steady state: Clinical vs. Predicted AUC, Cmax and Ctrough
| Drug | Dose (mg) | AUC (ng×h/mL) | Cmax (ng/mL) | Ctrough (ng/mL) | |||
|---|---|---|---|---|---|---|---|
| Clinical | aPredicted | Clinical | aPredicted | Clinical | aPredicted | ||
| Efavirenz | 600 OD | a57,150 ± 27,300 [34] | 65,006 ± 16,383 | 4,000 ± 1,710 [34] | 3,193 ± 699.7 | 2,380 ± 1,140 [34] | 2,298 ± 671.0 |
| Etravirine | 200 BD | a3,713 ± 2,069 [35] | 3,939 ± 1,249 | 451.3 ± 232.3 [35] | 375.0 ± 106.1 | 235.9 ± 163.1 [35] | 285.4 ± 100.5 |
| Rilpivirine | 25 OD | a2,589 ± 868.8 [27] | 3,590 ± 1,256 | 203.8 ± 75.81 [27] | 214.1 ± 53.9 | 89.85 ± 38.07 [27] | 95.7 ± 49.5 |
| Raltegravir | 400 BD | a7,076 ± 4,071 [36] | 9,584 ± 2,160 | 2,519 ± 1,930 [36] | 2,002 ± 378.9 | 71 ± 50 [36] | 54.6 ± 52.1 |
| Atazanavir | 400 OD | a29,303 ± 8,263 [29] | 24,354 ± 4,307 | 5,358 ± 1,371 [29] | 2,718 ± 392.2 | 218 ± 191 [29] | 326.9 ± 150.0 |
| Tenofovir | 300 OD | b3,324 (41.2%) [38] | 4,707 ± 1,004 | 326 (36.6%) [38] | 580.2 ± 80.6 | 64.4 (39.4%) [38] | 20.7 ± 17.7 |
| Emtricitabine | 200 OD | b10,100 (18%) [23] | 9,747 ± 2,326 | 1,720 (16%) [23] | 1,136 ± 236.3 | 73 (28%) [23] | 52.5 ± 32.2 |
| Dolutegravir | 50 OD | b53,600 (27%) [31] | 63,615 ± 8,802 | 3,670 (20%) [31] | 3,474 ± 519.6 | 1,110 (46%) [31] | 1,867 ± 309.0 |
Arithmetic Mean ± S.D.
Geometric Mean (% CV – coefficient of variation expressed as a percentage), AUC – area under the concentration-time curve, Cmax – maximum plasma concentration, Ctrough – trough plasma concentration, OD – once daily, BD – twice daily.
2.8 Prediction of pharmacokinetics following intramuscular injection
In the prediction of intramuscular pharmacokinetics, the dose of the ARV and release rate were optimised such that the mean trough concentration (Ctrough) was above the IC90, IC95 for wild type HIV virus or protein-binding corrected inhibitory concentration (PBIC95) after 7- or 30-days after injection. A dose cut-off of 1500 mg was considered for intramuscular administration.
2.9 Prediction of intracellular concentrations
The active phosphate metabolite of tenofovir, tenofovir diphosphate (TFV-DP) intracellular concentrations were simulated as previously described [22]. The first order elimination rate of emtricitabine triphosphate (FTC-TP) from the cellular compartment was calculated considering the half-life of FTC-TP [23]. The maximum uptake velocity (Vmax) and maximum velocity of anabolism (km) were fitted to obtain a zero-order dose input rate (kin) such that the mean intracellular concentration of the active metabolite at steady state (Css,avg) was in accordance to available clinical data following this equation:
| (9) |
where C is the concentration in the plasma.
Values of 0.9 ± 0.18 ng×h/mL and 18 ± 3.6 ng/mL for Vmax and km were suitable to obtain Css,avg values comparable to previously published data for oral administration [23]. The optimisation of the dose and release rates for emtricitabine and tenofovir considered not only plasma concentrations but also the intracellular FTC-TP and TFV-DP over the reported in vitro IC50 values of 127 fmol/106 cells and 150 fmol/106 cells [24, 25].
3 Results
The validation was conducted by comparing the mean simulated Ctrough, maximum plasma concentration (Cmax) and area under the concentration-time curve (AUC) with the available clinical data for oral administration and also for an existing LA intramuscular formulation of rilpivirine (600 mg; 100 mg/mL) (shown in Table 2 and Figure 1). For emtricitabine and tenofovir, the Css,avg of FTC-TP and TFV-DP were compared with clinical data [23]. Although the commonly agreeable limits of variability for validation is 2-fold from the mean value, we used a more stringent value of 0.5-fold to enhance the accuracy of the predictions [26].
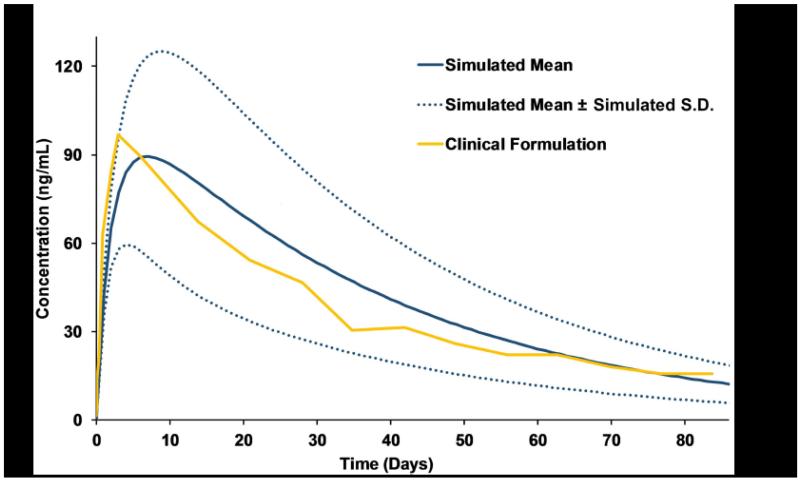
Fig 1.
Validation of the physiologically based pharmacokinetic strategy against clinical data for an existing rilpivirine sustained-release formulation (600 mg; 100 mg/mL) [6]
For intramuscular formulations, multiple dose and release rate combinations were simulated to obtain mean predicted Ctrough greater than the defined cut-off. For tenofovir and emtricitabine, the dose and release rates for intramuscular injection were optimised such that the plasma concentrations were over the IC90 value and also the Css,avg were over the intracellular IC50.
A summary of the predicted AUC, Cmax and Ctrough for eight ARVs following optimal intramuscular injection is shown in Table 3.
Table 3.
Prediction of the dose and release rate of single intramuscular injection of antiretrovirals
| Drug | Intramuscular Dose (mg) |
Release rate (h−1) |
Weekly/ Monthly |
AUC (μg×h/mL)† |
Cmax (ng/mL)† |
Ctrough (ng/mL)† |
Cut-off limit (ng/mL) |
|---|---|---|---|---|---|---|---|
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | |||||||
| Emtricitabine | 1,500 375 |
0.0015 0.009 |
Monthly Weekly |
43.1 ± 15.4 20.5 ± 8.9 |
94.2 ± 32.5 199.4 ± 76.1 |
51.2 ± 41.3 55.6 ± 26.4 |
50.2 [23] (IC90) |
| Tenofovir | 1,300 200 |
0.002 0.008 |
Monthly Weekly |
52.2 ± 15.4 16.6 ± 7.1 |
99.2 ± 28.6 155.6 ± 58.5 |
43.8 ± 17.2 49.1 ±23.0 |
18 [2] (IC90) |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | |||||||
| Efavirenz | 800 200 |
0.007 0.015 |
Monthly Weekly |
333.9 ± 99.3 73.2 ± 22.1 |
850.4 ± 194.6 546.5 ± 139.5 |
140.0 ± 50.8 273.3 ± 81.8 |
126 [30] (PBIC95) |
| Etravirine | 700 | 0.015 | Weekly | 43.4 ± 12.3 | 359.3 ± 80.7 | 126.1 ± 60.7 | 116 [30] (PBIC95) |
| Rilpivirine * | 250 60 |
0.002 0.015 |
Monthly Weekly |
32.9 ± 14.7 10.8 ± 4.8 |
75.4 ± 30.1 97.4 ± 33.3 |
22.6 ± 8.2 26.1 ± 18.7 |
20.3 [28] (PBIC95) |
| Integrase Inhibitors (IIs) | |||||||
| Dolutegravir | 105 20 |
0.002 0.006 |
Monthly Weekly |
95.6 ± 8.5 24.2 ± 2.8 |
212.1 ± 18.1 169.5 ± 18.1 |
65.7 ± 7.1 101.7 ± 14.8 |
64 [32] (PBIC95) |
| Raltegravir | 800 200 |
0.002 0.005 |
Monthly Weekly |
26.1 ± 5.2 8.23 ± 1.47 |
66.9 ± 13.1 69.4 ± 12.1 |
15.7 ± 2.8 30.1 ± 5.4 |
15 [37] (IC95) |
| Protease Inhibitors (PIs) | |||||||
| Atazanavir | 400 | 0.009 | Weekly | 38.9 ± 8.3 | 334.3 ± 70.7 | 114.8 ± 25.4 | 60 [30] (PBIC95) |
Note that this dose is not for the existing rilpivirine formulation
Values are shown as arithmetic mean and standard deviation; AUC – area under the concentration-time curve, Cmax – maximum plasma concentration, Ctrough – trough plasma concentration, IC90 – 90% inhibitory concentration, PBIC95 – 95% protein binding adjusted inhibitory concentration
3.1 Rilpivirine (RPV)
The validation of the PBPK model for rilpivirine was done against available clinical data for the oral formulation. The simulated mean AUC, Cmax and Ctrough values deviated +38.7%, +5.1% and +6.5%, respectively from the clinical data [27]. The PBPK model was additionally validated against an existing LA intramuscular formulation of RPV (600 mg; 100 mg/mL) [6]. The release rate from the intramuscular depot was predicted after matching the model to the clinical pharmacokinetic data. The drug release followed dose-dependent first order kinetics and the release rate was predicted to be between 0.0009 and 0.0012 h−1 (Figure 1). The mean values for AUC were 84.0 ng×h/mL vs. 83.38 ± 33.34 ng×h/mL, Cmax 96.7 ng/mL vs. 86.73 ± 30.51 ng/mL and Ctrough 15.7 vs. 11.81 ± 6.3 ng/mL for clinical versus simulated data. After the validation step with both oral and LA intramuscular formulations, the optimal dose and release rates of both weekly and monthly injection regimens of rilpivirine were identified such that the plasma concentration is over the PBIC95 value of 20.3 ng/mL [28]. A weekly dose of 60 mg with a release rate of 0.015 h−1 and a monthly dose of 250 mg with a release rate of 0.002 h−1 were predicted to maintain the plasma concentrations above the target cut-off.
3.2 Atazanavir (ATV)
The validation at steady-state for the oral administration of unboosted atazanavir resulted in mean predicted AUC, Cmax and Ctrough values which were −16.9%, −49.3% and +50.0% of those observed clinically [29]. An intramuscular dose of 1500 mg atazanavir was predicted to result in plasma concentrations after 30 days below the PBIC95 (60 ng/mL) and was therefore deemed unsuitable for monthly injection. An intramuscular weekly dose of 400 mg however was predicted to achieve mean concentrations in excess of the PBIC95 with a release rate of 0.009 h−1 [30].
3.3 Dolutegravir (DTG)
The predicted variation from the clinical values for mean AUC, Cmax and Ctrough in the validation of oral administration of dolutegravir were +18.7%, −5.3% and +68.2%, respectively [31]. Considering the PBIC95 value of 64 ng/mL as the target concentration, the simulated intramuscular administration requires an estimated weekly dose of 20 mg with a release rate of 0.006 h−1 and a monthly dose of 105 mg with a release rate of 0.002 h−1 [32].
3.4 Emtricitabine (FTC)
The predicted variation from the clinical values in mean AUC, Cmax and Ctrough for oral emtricitabine were −3.5%, −34.0% and −28.1%, respectively [33] and the simulated mean Css,avg of intracellular FTC-TP has a deviation of +2.65% from the clinical value (Table 4). The IC90 value of 50.2 ng/mL and intracellular IC50 of 127 fmol/106 cells were considered as target concentrations [25, 23]. The simulated intramuscular injection required a dose of 375 mg with a release rate of 0.009 h−1 for a weekly administration and 1500 mg with a release rate of 0.0015 h−1 for a monthly administration [23].
Table 4.
Validation and prediction of intracellular concentration of emtricitabine triphosphate and tenofovir diphosphate
| Active Drug | Intracellular Css,avg (fmol/106 cells) | Weekly/ Monthly |
Intramuscular Prediction (fmol/106 cells) |
In vitro IC50 (fmol/106 cells) |
||
|---|---|---|---|---|---|---|
| Clinical | Simulated | Intracellular Ctrough | Intracellular Cmax | |||
| Emtricitabine triphosphate | 1124 ± 592 [23] | 1,154 ± 456 | Monthly Weekly |
163.0 ± 124.6 270.0 ± 130.0 |
228.2 ± 106.1 431.8 ± 198.4 |
127 [25] |
|
| ||||||
| Tenofovir diphosphate | 150.7 ± 92.9 [22] | 156.5 ± 59.5 | Monthly Weekly |
154.2 ± 46.5 163.0 ± 59.0 |
164.6 ± 49.3 174.9 ± 62.8 |
150 [24] |
Css,avg – mean steady state concentration, Ctrough – trough concentration at the end of the duration, Cmax – maximum concentration, IC50 – 50% inhibitory concentration
3.5 Efavirenz (EFV)
The validated PBPK model yielded AUC, Cmax and Ctrough values, which were +13.7%, −20.2% and −3.4% compared to the clinical values [34]. In order to obtain plasma concentrations above the PBIC95 value of 126 ng/mL, a weekly intramuscular dose of 200 mg (release rate - 0.015 h−1) and monthly dose of 800 mg (release rate - 0.007 h−1) were required [30].
3.6 Etravirine (ETV)
The validated PBPK model with oral administration of etravirine resulted in mean AUC, Cmax and Ctrough values which were +6.1%, −16.9% and +21.0% compared to the clinical values [35]. The intramuscular predictions indicate that etravirine may be suitable for weekly administration with a dose of 700 mg and release rate of 0.015 h−1 but not for monthly administration as the drug concentrations fall below the PBIC95 value even for an intramuscular dose of 1500 mg [30].
3.7 Raltegravir (RAL)
The predicted mean AUC, Cmax and Ctrough of orally administered raltegravir were +35.4%, −20.5% and −23.1% compared to the clinical values, respectively [36]. To maintain the plasma concentrations above the IC95 value (15 ng/mL), the prediction suggested a dose of 200 mg (release rate – 0.005 h−1) and 800 mg (release rate – 0.002 h−1) may enable weekly and monthly intramuscular administration of raltegravir, respectively[37].
3.8 Tenofovir disoproxil fumarate (TDF)
The validated PBPK model for TDF predicted mean AUC, Cmax and Ctrough which were +41.6%, +78.0%, −67.9% compared to the clinical data, respectively [38]. The deviation of the intracellular Css,avg was +3.85% compared to the clinical value (Table 4). 200 mg TDF with a release rate of 0.008 h−1 was predicted to provide a sufficient exposure following weekly intramuscular injection and 1300 mg with a release rate of 0.002 h−1 was an adequate dose for a monthly intramuscular administration, maintaining mean plasma concentrations above the IC90 and intracellular TFV-DP above the IC50 [24].
4 Discussion
Imperfect adherence to daily oral ARV formulations continues to hinder the efficacy of HIV therapy and PrEP. Alternative administration strategies are beginning to emerge that provide opportunities for once monthly or even less frequent administration. A greater understanding of formulation characteristics is required to predict dose and release rates that provide adequate pharmacokinetic exposure relative to potency. This information will facilitate development of newer LA ARV formulations.
In this study, PBPK modelling was used to predict the pharmacokinetics of ARVs following intramuscular injection. The PBPK models were based on mathematical equations describing the human anatomy and physiology and the molecular processes regulating drug release and distribution. The PBPK models were initially validated for eight orally administered ARVs against available clinical data for oral formulations. Subsequently, intramuscular administration was simulated by adding an additional compartment to the model to represent the intramuscular depot. For this, a first order kinetics constant was applied to represent the diffusion of ARVs from the depot to the surrounding blood capillaries (as shown in Figure 2). This approach was validated comparing simulated pharmacokinetics of RPV following intramuscular injection versus available clinical data for the LA formulation. The primary objective of this study was to identify whether drug-specific doses and release rates from the depot predicted compatibility for intramuscular administration. To obtain the suppression of the viral replication, ARV Ctrough should reach and be maintained at concentrations above the susceptibility of the virus to that drug. Where possible, dose and release rate were optimised such that the ARV Ctrough remained above previously identified target values. Target concentrations were identified as equal to PBIC95 which represent the ARV concentration that can inhibit 95% of the virus replication in vitro. Since emtricitabine and tenofovir disoproxil fumarate are characterised by very low protein binding (<4% and <0.7% respectively) IC90 values were considered (IC95 data not available). The intracellular concentrations of FTC-TP and TFV-DP were also used as target concentrations in the optimisation of intramuscular dose and release rates for emtricitabine and tenofovir. Although the PBIC95 represents a rational pharmacodynamic cut-off and has been discussed extensively elsewhere, some ARVs do not exert a good relationship between trough concentrations and virologic efficacy [30]. In certain cases the clinical data indicate that the minimum effective concentrations for therapy might be considerably higher than the in vitro IC95.
Fig 2.
The whole body physiologically based pharmacokinetic model with an additional compartment for intramuscular depot and the blood capillaries surrounding the depot. Each of the arrows represents the flow kinetics assumed between tissues in the model. The small intestine is divided into seven parts for effective absorption kinetics. Metabolism occurs in the liver and intestine. Unabsorbed drug is excreted through faeces and excretion occurs through the kidneys. The green line represents the kinetics of the release from the nanoparticulate intramuscular depot. IM – intramuscular, RV and LV - right and left ventricle respectively.
The validation of PBPK models is essential to identify the correct parameters for an accurate simulation of pharmacokinetics for each ARV. Since dissolved molecule rather than nanoparticle is assumed to predominate systemically after intramuscular administration of SDNs, the volume of distribution, metabolism and elimination were fixed irrespective of the route of administration. As such, it should be noted that the presented data are only applicable when this is assumed to be the case, and these models cannot be applied to technologies that change these parameters (e.g. nanocarrier systems such as nanoemulsions or polymeric nanoparticles). Each model prediction was carried out at steady-state to better represent the long term ARV pharmacokinetics and relevant clinical scenarios. The model was considered validated if the mean AUC of the simulation for each ARV was within ±50% compared to clinically reported values. The simulated values from all the included PBPK models were in agreement with available clinical data and were therefore used for prediction of pharmacokinetics after intramuscular administration. The simulations were carried out for weekly and monthly administration and the maximum injectable dose was set to 1500 mg since doses above this have not previously been practical for intramuscular formulations [21]. Table 3 illustrates that the release rate was inversely proportional to the dosage, and a slower release rate was required for monthly administration.
Our PBPK models predicted that two NRTIs, emtricitabine and tenofovir, may be suitable for weekly or monthly administration. Importantly, emtricitabine and tenofovir are widely used in HIV treatment and prevention, and LA formulations of these agents would make possible multi-drug regimens that match current clinical paradigms for therapy. These agents also represent good candidates for monthly PrEP due to their excellent safety and efficacy profiles. Tenofovir alone, or tenofovir combined with emtricitabine, have already been shown to provide good protection against HIV infection in individuals at high risk of infection [3].
Considering NNRTIs, efavirenz and rilpivirine were both predicted to be suitable for monthly administration. Both have long half-lives, which are favourable for development of LA agents. The high systemic clearance of etravirine limits the possibility of a monthly administration format when a dose limit of 1500 mg is applied. Conversely, both integrase inhibitors, dolutegravir and raltegravir, were predicted to be suitable for monthly administration. A low dose of 105 mg was predicted to be sufficient for monthly administration of dolutegravir if a formulation with optimised release rate can be developed. It should be noted that cabotegravir is chemically closely related to dolutegravir, and development of a dolutegravir LA formulation may therefore not be warranted. Our simulation indicated that raltegravir could potentially be developed as a monthly intramuscular injection with a dose of 800 mg. The optimised dose and release rate for unboosted atazanavir predicted feasibility of an injectable weekly option at 600 mg, but doses higher than 1500 mg would be required for monthly administration.
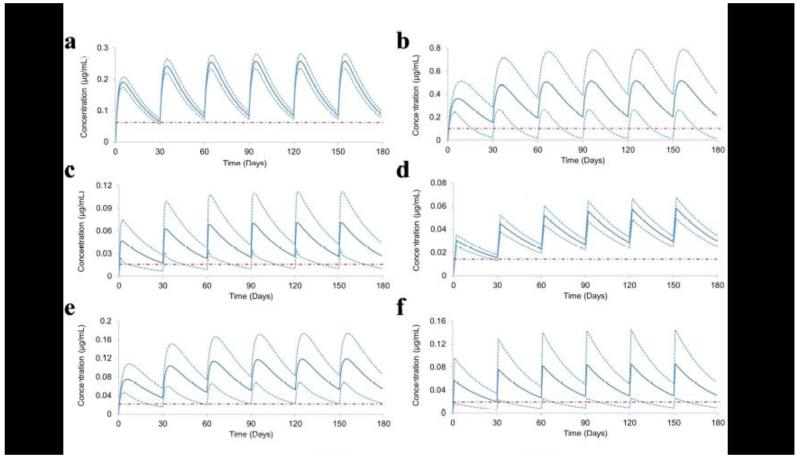
The optimisation of LA strategies should be based on a rational analysis of several pharmacokinetic factors. For many agents, it is likely that the pharmacokinetic variability after intramuscular administration will be reduced compared to oral formulations, due to the lower complexity of the absorption process and avoidance of intestinal transporters and first-pass metabolism. Therapeutic plasma concentrations were predicted to be achieved in approximately eight hours for emtricitabine and tenofovir, and ~24 h for the remaining ARVs after a single intramuscular injection (as shown in Figure 3). Since a day is needed to achieve therapeutic plasma concentrations for the other ARVs, a loading oral dose equivalent to the daily regimen may be necessary. This could represent a minor risk for the development of drug resistance and therapeutic failure, but PBPK modelling may help to inform the ongoing debate regarding some aspects of the need for an oral lead-in with the same drugs. The co-administration of multiple injectable ARVs is possible, but a rational selection of concomitant agents is needed to avoid drug-drug interactions. Studies to validate the utility of co-administration of the two existing LA drugs, rilpivirine and cabotegravir, are underway [39].
Fig 3.
Pharmacokinetics of monthly administration of antiretrovirals over a period of 6 months. a) Dolutegravir, b) Efavirenz, c) Emtricitabine, d) Raltegravir, e) Rilpivirine and f) Tenofovir
The PBPK approach is characterised by some intrinsic limitations due to existing knowledge gaps and the complexity of biological processes underpinning drug distribution. For example, muscle tissues are well connected to the lymphatic circulation, and drugs with high lipophilicity tend to diffuse through lymphatics rather than blood vessels [9]. Consequently, higher accumulation of ARVs in lymph nodes might be possible, but there is a paucity of clinical data in this area for available LA formulations. Similarly, efflux and influx transporters are of clear importance to intestinal absorption, distribution and clearance of drugs, but there is currently very little data on whether these transporters (or metabolic enzymes) are expressed at the depot sites. Gender has been correlated with changes in plasma exposure in a recent clinical study of rilpivirine LA [40]. Although several suggestions have been put forward to explain this, the physiological and anatomical mechanisms characterising the gender differences have not been fully elucidated and consequently have not been included in our simulations.
Although the doses and release rates identified through these simulations set targets for optimal nanoparticle design, it is important to underscore that the technological challenges in achieving these formulation characteristics are not addressed in this work. Long term stability of the ARVs in the interstitial fluid present in the depot site is another important consideration. Since SDN strategies do not involve the use of a carrier with stable size and dissolution rates over time, the nanoparticle properties are likely to change over the dosing interval. Since this will alter the surface to mass ratio, an effect on ARV release kinetics with downstream effect on the overall pharmacokinetics cannot be ruled out. It may also be important to consider other SDN components, such as polymers and surfactants, which will also be gradually released over time [41]. The use of LA injections of ARVs might determine specific complications at the site of injection as described for other treatments; additionally given the prolonged systemic exposition of LA ARV and the inability to interrupt the drug in the event of side effects, a period of oral induction might be considered before initiating LA injections. For patients discontinuing the LA ARV therapy, an oral administration strategy should be considered in order to prevent resistance development due to the diminishing plasma concentrations [4].
5 Conclusion
PBPK models for the simulation of pharmacokinetics after LA administration were successfully developed and validated against clinical data for eight approved ARV drugs. The validated models were applied to identify theoretical optimal dose and release rates required to achieve therapeutic concentrations following intramuscular injection. This approach defines a new paradigm for the rational design of LA formulations incorporating pharmacological properties of candidate ARVs. ARVs with potential for reformulation into intramuscular depots were identified, assuming the technological complexities associated with reformulation can be overcome. Based on their pharmacokinetic properties, dolutegravir, efavirenz, emtricitabine, raltegravir, tenofovir and rilpivirine are potential candidates for once monthly injection. PBPK modelling represents an innovative pharmacological tool to inform drug and formulation design.
Key Points.
This study investigated the feasibility of applying mathematical models to candidate LA formulations for antiretrovirals in order to predict drug concentrations over time.
Six antiretrovirals have been identified as potential therapeutic options for once monthly intramuscular administration, providing theoretical optimal dose and release rates.
This modelling approach constitutes a new paradigm for the rational design of LA formulations, considering a thorough evaluation of the drug pharmacological properties and defining an innovative platform for the optimisation of future nanoformulations.
Acknowledgements
Source of Funding:
This work was supported by the National Institutes of Health (AI 114405-01).
Footnotes
Conflict of Interest:
Andrew Owen has received research funding from Merck, Pfizer and AstraZeneca, consultancy from Merck and Norgine, and is a co-inventor of patents relating to HIV nanomedicines. Marco Siccardi has received research funding from ViiV and Janssen. David J Back is a board member in Abbvie, Boehringer Ingelheim, Gilead, Janssen, Merck and ViiV. He receives consulting or advisor fees from Abbvie, Boehringer Ingelheim, Gilead, Janssen, Merck and ViiV. He also received research funding from Abbvie, Boehringer Ingelheim, BMS, Gilead, Janssen, Merck and ViiV. Charles Flexner received consulting or advisor fees from Abbvie, Boehringer Ingelheim, Bristol Myers-Squibb, Gilead and GlaxoSmithKline, Merck and ViiV. Rajith KR Rajoli, Steve Rannard and Caren Freel Meyers have no conflicts of interest to declare.
References
- 1.UNAIDS [Accessed:20/10/2014];AIDS by the numbers. 2013 Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/JC2571_AIDS_by_the_numbers_en.pdf.
- 2.Palleja S, Ogden R, Hamy F, Vidal V, Klimkait T, Martin D, et al. In Vitro Anti-Hiv Efficacy Of The Chemokine Receptor 5 (CCR5) Antagonist TBR-652 In Combination With Four Other Classes Of Antiretroviral Agents 49th ICAAC. 2009. [Google Scholar]
- 3.Okwundu CI, Uthman OA, Okoromah CAN. Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. 2012;(7) doi: 10.1002/14651858.CD007189.pub3. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007189.pub3/abstract. [DOI] [PMC free article] [PubMed]
- 4.Spreen WR, Margolis DA, Pottage JCJ. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8(6):565–71. doi: 10.1097/COH.0000000000000002. doi:10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boffito M, Jackson A, Owen A, Becker S. New Approaches to Antiretroviral Drug Delivery: Challenges and Opportunities Associated with the Use of Long-Acting Injectable Agents. Drugs. 2014;74(1):7–13. doi: 10.1007/s40265-013-0163-7. doi:10.1007/s40265-013-0163-7. [DOI] [PubMed] [Google Scholar]
- 6.van ‘t Klooster G, Hoeben E, Borghys H, Looszova A, Bouche M-P, van Velsen F, et al. Pharmacokinetics and Disposition of Rilpivirine (TMC278) Nanosuspension as a Long-Acting Injectable Antiretroviral Formulation. Antimicrob Agents Chemother. 2010;54(5):2042–50. doi: 10.1128/AAC.01529-09. doi:10.1128/aac.01529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams J, Sayles HR, Meza JL, Sayre P, Sandkovsky U, Gendelman HE, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond) 2013;8(11):1807–13. doi: 10.2217/nnm.12.214. doi:10.2217/nnm.12.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siccardi M, Rajoli RKR, Curley P, Olagunju A, Moss D, Owen A. Physiologically based pharmacokinetic models for the optimization of antiretroviral therapy: recent progress and future perspective. Future Virol. 2013;8(9):871–90. doi:10.2217/fvl.13.67. [Google Scholar]
- 9.Tegenge M, Mitkus R. A physiologically-based pharmacokinetic (PBPK) model of squalene-containing adjuvant in human vaccines. J Pharmacokinet Pharmacodyn. 2013;40(5):545–56. doi: 10.1007/s10928-013-9328-y. doi:10.1007/s10928-013-9328-y. [DOI] [PubMed] [Google Scholar]
- 10.McDonald TO, Giardiello M, Martin P, Siccardi M, Liptrott NJ, Smith D, et al. Antiretroviral Solid Drug Nanoparticles with Enhanced Oral Bioavailability: Production, Characterization, and In Vitro-In Vivo Correlation. Adv Healthc Mater. 2014;3(3):400–11. doi: 10.1002/adhm.201300280. doi:10.1002/adhm.201300280. [DOI] [PubMed] [Google Scholar]
- 11.Siccardi M, Almond L, Schipani A, Csajka C, Marzolini C, Wyen C, et al. Pharmacokinetic and Pharmacodynamic Analysis of Efavirenz Dose Reduction Using an In Vitro-In Vivo Extrapolation Model. Clin Pharmacol Ther. 2012;92(4):494–502. doi: 10.1038/clpt.2012.61. doi:10.1038/clpt.2012.61. [DOI] [PubMed] [Google Scholar]
- 12.de Roche M, Siccardi M, Stoeckle M, Livio F, Back D, Battegay M, et al. Efavirenz in an obese HIV-infected patient - a report and an in vitro-in vivo extrapolation model indicate risk of underdosing. Antivir Ther. 2012;17(7):1381–4. doi: 10.3851/IMP2107. doi:10.3851/imp2107. [DOI] [PubMed] [Google Scholar]
- 13.Hyland R, Dickins M, Collins C, Jones H, Jones B. Maraviroc: in vitro assessment of drug-drug interaction potential. British Journal of Clinical Pharmacology. 2008;66(4):498–507. doi: 10.1111/j.1365-2125.2008.03198.x. doi:10.1111/j.1365-2125.2008.03198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosgra S, Eijkeren Jv, Bos P, Zeilmaker M, Slob W. An improved model to predict physiologically based model parameters and their inter-individual variability from anthropometry. Crit Rev Toxicol. 2012;42(9):751–67. doi: 10.3109/10408444.2012.709225. doi:doi:10.3109/10408444.2012.709225. [DOI] [PubMed] [Google Scholar]
- 15.Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm. 1999;186(2):119–25. doi: 10.1016/s0378-5173(99)00147-7. doi:10.1016/S0378-5173(99)00147-7. [DOI] [PubMed] [Google Scholar]
- 16.Siccardi M, Olagunju A, Seden K, Ebrahimjee F, Rannard S, Back D, et al. Use of a physiologically-based pharmacokinetic model to simulate artemether dose adjustment for overcoming the drug-drug interaction with efavirenz. In Silico Pharmacol. 2013;1(4) doi: 10.1186/2193-9616-1-4. doi:10.1186/2193-9616-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gertz M, Harrison A, Houston JB, Galetin A. Prediction of Human Intestinal First-Pass Metabolism of 25 CYP3A Substrates from In Vitro Clearance and Permeability Data. Drug Metab Dispos. 2010;38(7):1147–58. doi: 10.1124/dmd.110.032649. doi:10.1124/dmd.110.032649. [DOI] [PubMed] [Google Scholar]
- 18.Barter ZE, Chowdry JE, Harlow JR, Snawder JE, Lipscomb JC, Rostami-Hodjegan A. Covariation of Human Microsomal Protein Per Gram of Liver with Age: Absence of Influence of Operator and Sample Storage May Justify Interlaboratory Data Pooling. Drug Metab Dispos. 2008;36(12):2405–9. doi: 10.1124/dmd.108.021311. doi:10.1124/dmd.108.021311. [DOI] [PubMed] [Google Scholar]
- 19.Poulin P, Theil FP. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. Journal of pharmaceutical sciences. 2002;91(1):129–56. doi: 10.1002/jps.10005. doi:10.1002/jps.10005. [DOI] [PubMed] [Google Scholar]
- 20.Peters S. Evaluation of a Generic Physiologically Based Pharmacokinetic Model for Lineshape Analysis. Clin Pharmacokinet. 2008;47(4):261–75. doi: 10.2165/00003088-200847040-00004. doi:10.2165/00003088-200847040-00004. [DOI] [PubMed] [Google Scholar]
- 21.GlaxoSmithKline [Accessed:17/08/2014];Summary of Product Characterisitics-Zinacef. 1979 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/zinacef_30/WC500127791.pdf.
- 22.Duwal S, Schütte C, von Kleist M. Pharmacokinetics and Pharmacodynamics of the Reverse Transcriptase Inhibitor Tenofovir and Prophylactic Efficacy against HIV-1 Infection. PloS one. 2012;7(7):e40382. doi: 10.1371/journal.pone.0040382. doi:10.1371/journal.pone.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang LH, Begley J, St Claire RL, Harris J, Wakeford C, Rousseau FS. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20(11):1173–82. doi: 10.1089/aid.2004.20.1173. doi:10.1089/0889222042544965. [DOI] [PubMed] [Google Scholar]
- 24.Feng J, Ly J, Myrick F, Goodman D, White K, Svarovskaia E, et al. The triple combination of tenofovir, emtricitabine and efavirenz shows synergistic anti-HIV-1 activity in vitro: a mechanism of action study. Retrovirology. 2009;6(1):1–16. doi: 10.1186/1742-4690-6-44. doi:10.1186/1742-4690-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson JE, Martin JL, Borroto-Esoda K, Hopkins S, Painter G, Liotta DC, et al. The 5′-triphosphates of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolane-5-yl]cytosine equally inhibit human immunodeficiency virus type 1 reverse transcriptase. Antimicrobial agents and chemotherapy. 1993;37(8):1720–2. doi: 10.1128/aac.37.8.1720. doi:10.1128/aac.37.8.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abduljalil K, Cain T, Humphries H, Rostami-Hodjegan A. Deciding on Success Criteria for Predictability of Pharmacokinetic Parameters from In Vitro Studies: An Analysis Based on In Vivo Observations. Drug Metab Dispos. 2014 doi: 10.1124/dmd.114.058099. doi:10.1124/dmd.114.058099. [DOI] [PubMed] [Google Scholar]
- 27.Clinical Pharmacology and Biopharmaceutics Review(s) Center for Drug Evaluation and Research; [Accessed:22/10/2014]. 2011. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202022Orig1s000ClinPharmR.pdf. [Google Scholar]
- 28.Azijn H, Tirry I, Vingerhoets J, de Béthune M-P, Kraus G, Boven K, et al. TMC278, a Next-Generation Nonnucleoside Reverse Transcriptase Inhibitor (NNRTI), Active against Wild-Type and NNRTI-Resistant HIV-1. Antimicrobial agents and chemotherapy. 2010;54(2):718–27. doi: 10.1128/AAC.00986-09. doi:10.1128/aac.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Highlights of Prescribing Information - REYATAZ. Bristol-Myers Squibb Company; [Accessed:13/08/2014]. 2014. Available from: http://packageinserts.bms.com/pi/pi_reyataz.pdf. [Google Scholar]
- 30.Acosta EP, Limoli KL, Trinh L, Parkin NT, King JR, Weidler JM, et al. Novel Method To Assess Antiretroviral Target Trough Concentrations Using In Vitro Susceptibility Data. Antimicrob Agents Chemother. 2012;56(11):5938–45. doi: 10.1128/AAC.00691-12. doi:10.1128/aac.00691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Highlights of Prescribing Information - TIVICAY. Viiv Healthcare; [Accessed:11/10/2014]. 2013. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf. [Google Scholar]
- 32.Castellino S, Moss L, Wagner D, Borland J, Song I, Chen S, et al. Metabolism, Excretion, and Mass Balance of the HIV-1 Integrase Inhibitor, Dolutegravir, in Humans. Antimicrob Agents Chemother. 2013;57:3536–46. doi: 10.1128/AAC.00292-13. doi:10.1128/aac.00292-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Highlights of Prescribing Information - EMTRIVA. Gilead Sciences; [Accessed:12/10/2014]. 2013. Available from: http://www.gilead.com/~/media/Files/pdfs/medicines/hiv/emtriva/emtriva_pi.pdf. [Google Scholar]
- 34.Villani, Regazzi, Castelli, Viale, Torti, Seminari, et al. Pharmacokinetics of efavirenz (EFV) alone and in combination therapy with nelfinavir (NFV) in HIV-1 infected patients. Br J Clin Pharmacol. 1999;48(5):712–5. doi: 10.1046/j.1365-2125.1999.00071.x. doi:10.1046/j.1365-2125.1999.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakuda NT, Schöller-Gyüre Monika, Workman Cassy, Arasteh Keikawus, Pozniak Anton L, De Smedt Goedele, et al. Single- and multiple-dose pharmacokinetics of etravirine administered as two different formulations in HIV-1-infected patients. Antivir Ther. 2008;13(5):655–61. [PubMed] [Google Scholar]
- 36.Fayet Mello A, Buclin T, Franc C, Colombo S, Cruchon S, Guignard N, et al. Cell disposition of raltegravir and newer antiretrovirals in HIV-infected patients: high inter-individual variability in raltegravir cellular penetration. J Antimicrob Chemoth. 2011;66(7):1573–81. doi: 10.1093/jac/dkr151. doi:10.1093/jac/dkr151. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz A, Gisslén M, Spudich S, Lee E, Jayewardene A, Aweeka F, et al. Raltegravir Cerebrospinal Fluid Concentrations in HIV-1 Infection. PloS one. 2009;4(9):e6877. doi: 10.1371/journal.pone.0006877. doi:10.1371/journal.pone.0006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summary of Product Characteristics - VIREAD. Gilead Sciences; [Accessed:15/08/2014]. 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000419/WC500051737.pdf. [Google Scholar]
- 39.Dolgin E. Long-acting HIV drugs advanced to overcome adherence challenge. Nat Med. 2014;20(4):323–4. doi: 10.1038/nm0414-323. doi:10.1038/nm0414-323. [DOI] [PubMed] [Google Scholar]
- 40.Jackson AGA, Else LJ, Mesquita PMM, Egan D, Back DJ, Karolia Z, et al. A Compartmental Pharmacokinetic Evaluation of Long-Acting Rilpivirine in HIV-Negative Volunteers for Pre-Exposure Prophylaxis. Clin Pharmacol Ther. 2014;96(3):314–23. doi: 10.1038/clpt.2014.118. doi:10.1038/clpt.2014.118. [DOI] [PubMed] [Google Scholar]
- 41.Martin P, Giardiello M, McDonald TO, Rannard SP, Owen A. Mediation of in Vitro Cytochrome P450 Activity by Common Pharmaceutical Excipients. Mol Pharm. 2013;10(7):2739–48. doi: 10.1021/mp400175n. doi:10.1021/mp400175n. [DOI] [PubMed] [Google Scholar]
- 42.Product Information - TIVICAY. ViiV Healthcare Pty Ltd; [Accessed:20/10/2014]. 2014. Available from: http://www.medicines.org.au/files/viptivic.pdf. [Google Scholar]
- 43.Siccardi M, Marzolini C, Seden K, Almond L, Kirov A, Khoo S, et al. Prediction of drug-drug Interactions Between Various Antidepressants and Efavirenz or Boosted Protease Inhibitors Using a Physiologically Based Pharmacokinetic Modelling Approach. Clin Pharmacokinet. 2013;52(7):583–92. doi: 10.1007/s40262-013-0056-7. doi:10.1007/s40262-013-0056-7. [DOI] [PubMed] [Google Scholar]
- 44.Schöller-Gyüre M, Kakuda T, Raoof A, Smedt G, Hoetelmans RW. Clinical Pharmacokinetics and Pharmacodynamics of Etravirine. Clin Pharmacokinet. 2009;48(9):561–74. doi: 10.2165/10895940-000000000-00000. doi:10.2165/10895940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Moss DM, Siccardi M, Back DJ, Owen A. Predicting intestinal absorption of raltegravir using a population-based ADME simulation. J Antimicrob Chemother. 2013;68:1627–34. doi: 10.1093/jac/dkt084. doi:10.1093/jac/dkt084. [DOI] [PubMed] [Google Scholar]
- 46.Wajima T, Kubota R. Pharmacokinetic/pharmacodynamic Modeling and Long-term Simulation of Dolutegravir (DTG, S/GSK1349572) in Integrase Resistant Patients with a Simple Viral Dynamic Model; 20th Population Approach Group in Europe; Athens, Greece. 2011. [Google Scholar]
- 47.Valade E, Treluyer J-M, Bouazza N, Ghosn J, Foissac F, Benaboud S, et al. Population Pharmacokinetics of Emtricitabine in HIV-1-Infected Adult Patients. Antimicrob Agents Chemother. 2014;58(4):2256–61. doi: 10.1128/AAC.02058-13. doi:10.1128/aac.02058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baheti G, Kiser JJ, Havens PL, Fletcher CV. Plasma and Intracellular Population Pharmacokinetic Analysis of Tenofovir in HIV-1-Infected Patients. Antimicrob Agents Chemother. 2011;55(11):5294–9. doi: 10.1128/AAC.05317-11. doi:10.1128/aac.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colombo S, Buclin T, Cavassini M, Décosterd LA, Telenti A, Biollaz J, et al. Population Pharmacokinetics of Atazanavir in Patients with Human Immunodeficiency Virus Infection. Antimicrob Agents Chemother. 2006;50(11):3801–8. doi: 10.1128/AAC.00098-06. doi:10.1128/aac.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laufer R, Paz OG, Di Marco A, Bonelli F, Monteagudo E, Summa V, et al. Quantitative Prediction of Human Clearance Guiding the Development of Raltegravir (MK-0518, Isentress) and Related HIV Integrase Inhibitors. Drug Metabolism and Disposition. 2009;37(4):873–83. doi: 10.1124/dmd.108.023804. doi:10.1124/dmd.108.023804. [DOI] [PubMed] [Google Scholar]
- 51.Kiser J, Carten M, Aquilante C, Anderson P, Wolfe P, King T, et al. The Effect of Lopinavir/Ritonavir on the Renal Clearance of Tenofovir in HIV-infected Patients. Clin Pharmacol Ther. 2007;83(2) doi: 10.1038/sj.clpt.6100269. doi:10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 52.Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, et al. In Vitro Investigations into the Roles of Drug Transporters and Metabolizing Enzymes in the Disposition and Drug Interactions of Dolutegravir, a HIV Integrase Inhibitor. Drug Metabolism and Disposition. 2013;41(2):353–61. doi: 10.1124/dmd.112.048918. doi:10.1124/dmd.112.048918. [DOI] [PubMed] [Google Scholar]
- 53.Yanakakis LJ, Bumpus NN. Biotransformation of the Antiretroviral Drug Etravirine: Metabolite Identification, Reaction Phenotyping, and Characterization of Autoinduction of Cytochrome P450-Dependent Metabolism. Drug Metab Dispos. 2012;40(4):803–14. doi: 10.1124/dmd.111.044404. doi:10.1124/dmd.111.044404. [DOI] [PMC free article] [PubMed] [Google Scholar]