Abstract
Age-related Macular Degeneration (AMD) is the leading cause of irreversible and predictable blindness among older adults and creates serious physical and mental health consequences for this population. Visual impairment is associated with negative future outlook and depression and has serious consequences for older adults’ quality of life and, by way of depression, on long-term survival. Psychosocial interventions have the potential to alleviate and prevent depression symptoms among older AMD patients. We describe the protocol of the Macular Degeneration and Aging Study, a randomized clinical trial of a psychosocial Preventive Problem-Solving Intervention. The intervention is aimed at enhancing well-being and future planning among older adults with macular degeneration by increasing preparation for future care. Adequate randomization and therapeutic fidelity were achieved. Current retention rates were acceptable, given the vulnerability of the population. Acceptability (adherence and satisfaction) is high. Given the high public health significance and impact on quality of life among older adults with vision loss, this protocol contributes a valid test of a promising intervention for maintaining mental and physical health in this population.
Keywords: Randomized Controlled Trial, Aging, Macular Degeneration, Future Planning, Preventive Problem-Solving
INTRODUCTION
Age-related Macular Degeneration (AMD) is the leading cause of irreversible and predictable blindness among older adults. About 5% of individuals aged 60–64 have early stages of the disease1; rates of severe AMD double with each decade after age 60.2 AMD is associated with blurring or distortion of central vision, a central blind spot, and loss of detail-, contrast-, and color-vision.3 Common consequences are an inability to drive, read, watch television, recognize people, and engage in other valued, discretionary activities4, especially those outside the home, which are central to well-being.5 Visual impairment often leads to a negative future outlook6,7 and 33% of patients meet diagnostic criteria for depression.8 Thus, AMD has serious consequences for older adults’ quality of life.8–12 Furthermore, Medicare beneficiaries with vision loss incur significantly higher costs than those with normal vision, and approximately 90% of these costs are non–eye related medical costs,13 suggesting a growing public health concern for this vulnerable population.
AMD and Preparation for Future Care
In addition to the potential for negative mental health outcomes, the depression symptoms often associated with AMD can lead to difficulty with problem-solving and planning for the future7,14 and older adults with AMD have lower rates of preparation for future care than their non-vision-impaired peers.15 The progressive and sometimes sudden vision loss that occurs with AMD places older adults with the disease at increased risk for requiring care and/or change of residence. Because of this risk, preparation for future care activities, such as being aware of possible care needs, gathering information and making choices about preferred types of care, and sharing care plans with caregivers, become especially important in creating a future scenario that meets the individual’s needs and preferences. Consistent with theories of active life management,16 and proactive coping,17,18 preparation for future care (PFC), such as discussing preferences and options for long-term care with family members or health care providers, is an adaptive response that improves the management of expected age-related losses for most older adults. Indeed, a study of primary care patients showed that those with more concrete planning exhibited fewer depression and anxiety symptoms after a two year period than those who did not plan.19 In contrast, lack of future care planning may lead to distress during care decisions, poor everyday functioning, and stress to caregivers.20,21 Lack of planning for vision loss and age-related health decline may increase the risk of inappropriate residential or care arrangements22,23 that are not tailored to the patient’s values and preferences. Unnecessary health consequences, such as falls and emergency room visits 24–27 may also be viewed as a consequence of poor planning.
Aims
Because of the negative emotional/mental health effects of Macular Degeneration, several interventions with older AMD patients have been developed in the last decade. These focus on vision rehabilitation,28 adaptive skills training,29 cognitive restructuring or reframing,30 disease knowledge, self-efficacy in using assistive devices and maintaining activities,31,32 problem-solving therapy and behavioral activation to prevent depression.33,34 Although these studies show that both group interventions and individual home-based problem-solving training can significantly improve functioning and mood in patients with AMD, the beneficial effects rarely exceed 6 months and some have been tested only up to 4 months. We postulate that an AMD intervention with an added focus on future care needs will show longer lasting effects on well-being. The aims of this paper are (1) to present a new intervention that that includes preparation for future losses due to vision and aging, (2) to describe an efficacy trial of this intervention, (3) to present evidence for effective randomization in the trial; and (4) to compare this intervention to an enhanced control condition with regard to retention rates, compliance, and satisfaction.
METHODS
The Macular Degeneration and Aging Study (MADAS) is a randomized clinical trial of older adults with AMD in which Preventive Problem-Solving Intervention (PREPSI) is tested against an Enhanced Attention Control condition to examine the effectiveness in improving psychological well-being, mood, and preparation for future care. PREPSI addresses PFC by teaching basic problem-solving and then applying these principles to potential future problems. The intervention has two stages. In stage one all participants receive vision education classes to equalize knowledge across groups. In stage two they are randomized to the intervention or control conditions, which are delivered in-home.
Ethics Approval
The study was approved by the Institutional Review Board of the University of Rochester and is registered with ClinicalTrials.gov (NCT02224963). Recruitment has ended, but follow-up is ongoing.
Recruitment of Subjects
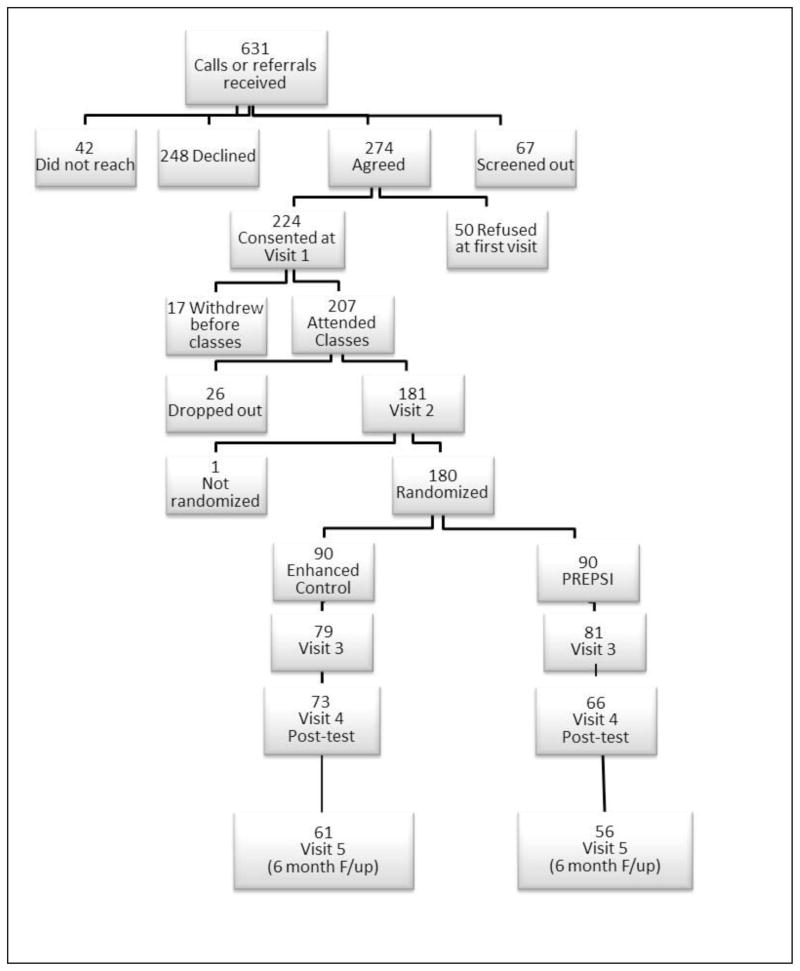
Adults aged 60+ who have received a diagnosis of AMD were invited to participate: 631 contacted us by phone in response to advertisements, or were referred to us, with permission for us to call them, by the Association for the Blind and Visually Impaired or area clinicians. Of these, 67 were not eligible, 42 could not be reached, and 248 declined participation for reasons of timing, general lack of interest, or desire for a medical study. A total of 274 agreed to participate; 50 were unable to start at the time they were asked, had family or health issues, or changed their mind. We consented and completed the baseline interview with 224 participants; 207 attended the vision education classes. Subject flow is shown in Figure 1. Recruitment into the trial began in September of 2009 and ended in February of 2014.
Figure 1.
Inclusion/Exclusion Criteria
Inclusion criteria were (1) diagnosis of AMD (2) 60 years and older; (3) able to communicate in English.
After interview 1, subjects were excluded if they: (1) had significant cognitive impairment at baseline (total score on MMSE(Blind) <18 35 (n=5), equivalent to <21 used in other aging studies36; (2) resided in a nursing home (assisted living facilities were acceptable) (n=0); (3) were acutely suicidal, psychotic (PI and the clinical psychologist/psychiatrist were paged immediately, n=0), or if their homes were deemed unsafe for staff (n=2). Patients were also excluded if they had a terminal illness preventing completion of the intervention (n=6). Four were lost to follow-up after interview 1.
Primary Recruitment Sites and Strategies
Participants were recruited from a number of sites: The Association for the Blind and Visually Impaired (ABVI, 30%), the University of Rochester’s Flaum Eye Institute (FEI, 9%) and local retinal practices (2%). We also used local media (29% of recruited participants), including newspaper advertisements, television appearances. Finally we reached 30% of participants through presentations at local senior centers, professional societies, and faith-based organizations.
Consent
Potential participants were informed during recruitment and over the phone about the purpose, the design, and the duration of the study. The initial phone contact was conducted by a staff member, who ensured that the study was adequately explained. All participants were consented during their first assessment visit in their home. The study was explained again during this visit. Consent was obtained by study staff and a sighted witness.
Study Design, Randomization, and Withdrawal Procedures
The study was a community-based efficacy trial of a psycho-educational intervention to promote problem-solving skills for solving both current and future problems. The entire program, including assessments, lasted 16 weeks. It had a 2 (treatment condition) by 5 (assessment times) repeated measures design. Assessments were conducted at baseline (Interview 1), after the vision education classes (Interview 2), after the first 4 weeks of treatment (Interview 3), at post-treatment (Interview 4), and at 6 months post-trial (Interview 5). Both treatment and control participants met the consenting research assistant at their first assessment visit. Both groups also attended the four Vision Education (VE) classes. Following the last VE class, participants were randomly assigned to an 8-week Preventive Problem-Solving Training Intervention (PREPSI) or an Enhanced Attention Control (EAC) condition (described below).
Participants were randomized using an urn randomization program adjusting for gender, age, living alone, wet or dry AMD, recruitment site, and receipt of low-vision services. Group assignment was communicated to the participants by the project coordinator and randomization was conducted independently of recruitment. Of the 207 who participated in classes, 26 withdrew before Interview 2 and one before randomization. Reasons for withdrawal included health (n=2), too busy or no longer interested (n=6), didn’t like the questions (n=4), loss to follow-up (n=3), died (n=3), other or reason unkown (n=9).
Data Collection
Data were collected at five measurement points: Baseline (Interview 1), after vision education classes (Interview 2), after problem-solving training (Interview 3), after preparation for future care training (Interview 4) and after a 6-month follow-up period (Interview 5). Demographic data about participants were collected at the first home visit, 1–2 weeks before the beginning of the intervention. Characteristics of the participants are shown in Tables 1a.
Table 1a.
Comparison of Participant Characteristics among Participants in Treatment, Control, and Not-Randomized groups (ordinal or continuous variables)
| Variables | PREPSI Mean (SD) (N=90) |
EAC Mean (SD) (N=90) |
Not randomized Mean (SD) (N=33) |
F-statistic (p-value) of 3-way difference | Total Mean (SD) (N=213) |
|---|---|---|---|---|---|
| Age (Years) | 80.81 (7.38) | 80.27 (7.70) | 70.82 (10.77) | .787 (p=.46) | 80.26 (8.09) |
|
| |||||
| Education (Years) | 14.27 (2.61) | 14.29 (2.48) | 15.12 (3.39) | 1.37 (p=.26) | 14.14 (2.70) |
|
| |||||
|
4.68 (2.84) | 5.41 (3.14) | 5.59 (3.60) | 1.650 (p=.20) | 5.12 (3.09) |
|
| |||||
| Financial Adequacy Composite (1= not at all; 5=much more than adequate) | 2.56 (.53) | 2.58 (.48) | 2.61 (.57) | .14 (p=.87) | 2.57 (.51) |
Other data collected included detailed assessments of psychological well-being, depression and anxiety, attitudes toward the future, preparation for future care, valued activities, life satisfaction, and life events. Changes were assessed through repeated measurement at every visit, as well as by asking about recent changes in health, vision, well-being, finances, and family. Phone assessments of satisfaction with the intervention were conducted after the fourth interview. Although we attempted to keep interviewers unaware of group assignment of the participants, by visit 4 41% of participants had unblinded their interviewer inadvertently (51.4 % of the PREPSI and 30.3% of the control group). However, by the 6-month follow-up the proportion of participants with unblinded interviewers was 37% in both groups.
Retention and Tracking
Measures to increase retention included: (1) ensuring subject clarity about the content and commitment of the study; (2) screening out individuals too ill to follow through (acute terminal or life-threatening illness) or too cognitively impaired (MMSE(Blind)<18); (3) providing transportation to classes at ABVI; (4) conducting the problem-solving component of the intervention in the home. The barriers to travel for vision-impaired older adults outweighed the potential variability and privacy violations encountered in the home.
To aid in longitudinal follow-up and retention, we obtained the name of a relative or friend who could be contacted in the case of an unexpected change of address. Some participants were referred by their eye doctor and had not yet received a low vision assessment at ABVI. A make-up visit at ABVI was scheduled for these participants early in the study, ensuring that vision function was assessed subjectively and objectively, and that participants received needed vision rehabilitation services, if indicated.
The Preventive Problem-solving Intervention (PREPSI)
The PREPSI consisted of three components: (1) four Vision Education (VE) classes; (2) four formal problem-solving training visits (PST); and (3) four preparation for future care training visits (PFCT). The Vision Education classes were conducted at the Association for the Blind and Visually Impaired-Goodwill (ABVI); with transportation assistance provided. ABVI physicians, vision rehabilitation team leaders, and rehabilitation specialists co-developed the VE groups with the research team and taught the classes. The eight PST/PFCT sessions and four assessment visits were conducted in participants’ homes, and administered by bachelor’s or master’s level human service professionals who were certified in Problem-Solving Therapy and Preparation for Future Care Training. A given PREPSI trainer was assigned to the same patient throughout the study.
Vision Education (VE)
This component consisted of four group classes and provided general information about the medical aspects of Macular Degeneration, adapting to vision loss, as well as social support and emotion management training. The informational content of the Vision Education groups resembled the information provided to individual clients of the Association for the Blind and Visually Impaired (ABVI). In addition, VE classes created a supportive group setting in which to voice concerns and discuss common issues. Group support has been recognized as an important aspect of AMD interventions.30 The Vision Education classes covered (1) physiological processes of AMD, including how it is diagnosed, risk and preventive factors, and current treatments; (2) in-home adaptations that allow visually impaired adults to remain independent, for example, the use of assistive devices, lenses, and clothing color identification strategies; (3) orientation and mobility skills, for example, how to use public transportation, obtain identification canes, and paratransit; and (4) social and emotional adaptation to vision loss, including a cognitive-behavioral emotion management component.
All subjects received a binder to insert notes from the VE groups, a large-print resource listing with names of local agencies for older adults, and information about care options, and legal and financial issues of caregiving.
Problem-solving Training (PST)
This standardized four-session program for learning a systematic approach to solving self-identified problems was taught using a stepwise approach to solving a problem of the participant’s choice. The steps include (1) fostering positive problem-orientation, or the perspective that having problems is normal and that one can cope with them systematically and effectively; (2) defining problems and setting goals; (3) generating alternative solutions by brainstorming; (4) deciding on preferred solutions by considering pros and cons; (5) implementing solutions or plans for solutions and evaluating solution effectiveness.37 Problems were chosen by the subjects -- they were often, but not always, about vision-related difficulties. Follow-up sessions focus on evaluating progress on the chosen solutions.
Applying PST to Preparation for Future Care
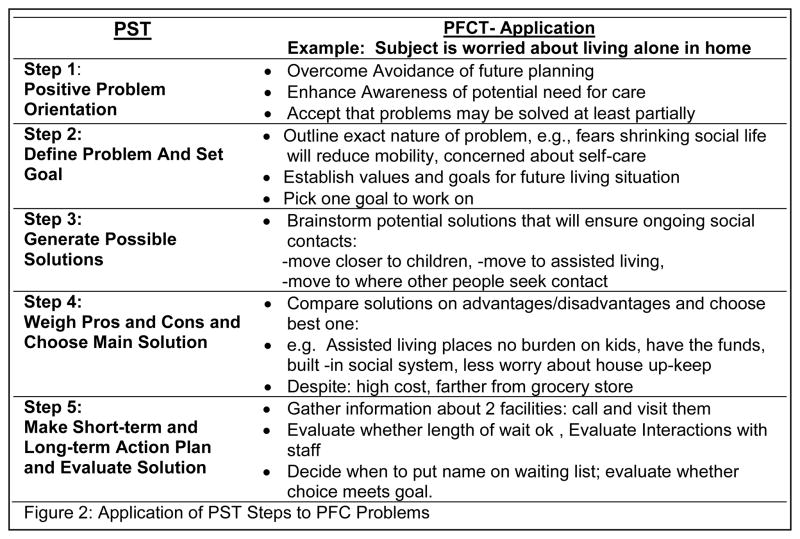
This four session component applied the principles and skills learned in the previous problem-solving sessions to potential future problems. We introduced the principles of “overcoming avoidance” and “becoming aware of future needs and options”, as well as “gathering information,” “decision-making,” and “concrete planning,” which are key actions in preparation for future care38 (Figure 2). The message was conveyed that anticipating and planning for future difficulties can make finding solutions and adapting to vision and health changes easier and result in less distress.
Figure 2.
Application of PST Steps to PFC Problems
Resource Information Modules
Because previous studies have shown that information provided to older AMD patients is often not utilized independently, we devised six Resource Information Modules. These 10 minute mini-lectures were accompanied with large-print written information in a binder, which were presented during the PST and PFCT sessions, and focused on Transportation, Area Agency of Aging Nutrition Sites, Opportunities for Leisure Activities for Seniors, and Senior housing Options, In-home care Options, and Medical Decision-Making & Power of Attorney.
Description of the Enhanced Attention Control (EAC) Condition
The EAC was designed to control for non-specific benefits of social contact and information provision. It consists of two components: (1) four Vision Education (VE) groups and (2) a schedule of home visits and assessments with control subjects that was structurally equivalent to the PREPSI, but did not include the problem-solving content. The EAC was presented as a credible alternative to the PREPSI: It was framed to both trainers and subjects as a “Life and Health Review Intervention.” Trainers were instructed to engage the participant in a structured life review, discussing events from each life stage, based on Erikson’s theory (e.g., childhood, adolescence, young adulthood). Our Life Review procedures manual was adapted from Matteson.39,40 Additionally, EAC trainers interviewed participants about current health concerns and, if appropriate, provided health information (e.g., basic nutrition advice, brochures on osteoporosis, etc.). EAC participants received the same Resource Information Modules and mini-lectures as the treatment group.
Experimental Blinding
Several strategies maximized experimental blinding. Patient blinding occurred, first, by describing the study in the consent as a comparison of two interventions to improve well-being of AMD patients. The stated rationale was that the Life Review Intervention would improve mood as well as access to information. Structural equivalence of the two conditions, provision of health promotion and resource information, audio-taped sessions, and careful record keeping in the EAC contributed to patients’ perceptions of its legitimacy. We administered a treatment expectations questionnaire to detect possible between-group difference in patients’ expectations about treatment progress. Although it was not feasible to achieve trainer-blinding to study hypotheses, we attempted to enhance EAC trainers’ perception of the legitimacy of the Life Review Intervention by structural equivalence of the two conditions, provision of regular social contact, sharing medical and resource information, and the stated rationale enhanced their motivation and helped obscure the experimental assignment for patients.39 EAC Trainers were informed that they were applying a modification of a previously tested depression intervention. EAC trainers audio-taped the session and tracked participant involvement, topics discussed, and quality of rapport with the participant.
Assessor blinding was achieved by separating the tasks of trainers and assessors, who were instructed not to discuss case details in project meetings. The study coordinator was not blinded to group assignment. She informed the patients of the identity of their “trainer” and passed the patient’s contact information to the assessors and trainers. All communications between assessors and trainers about participants were relayed through the coordinator. The assessor conducted the baseline and follow-up interviews and had regular phone contacts with the participants between assessment periods. Patients were reminded repeatedly to avoid disclosing information to assessors that could reveal their treatment assignment. Reminders were made during informed consent, during between-assessment phone calls, and prior to assessment interviews. All instances of broken blind, due to the participants sharing experience or names of trainers, were recorded.
Staff Training and Intervention Fidelity
Staff received ten hours of training in protocol, recruitment strategies, and data entry including a General Procedure Manual. All Staff received five hours of training in sources of vision loss, cultural sensitivity to vision loss, and introduction to visual aids by expert rehabilitation specialists at ABVI. In addition to 5 hours of instruction on medical and psychological issues in aging, aging resources, and effective communication with older adults and 3 hours per year of cultural sensitivity training were provided. Staff members met weekly with the PI and Study Coordinator to ensure uniformity of recruitment, protocol implementation, data collection and entry.
PREPSI Trainers
The three PREPSI trainers received at least 50 hours of training. They participated in a full-day workshop conducted by a PST training specialist, co-author MTH. The workshop included a didactic presentation, viewing and discussing training videotapes, reviewing the treatment manual, role-playing by participants, and discussion. Each PREPSI trainer also completed 5 supervised training cases of 4 consecutive sessions over 4 weeks. Audio files of the first, second, and fourth PREPSI sessions were reviewed and rated by MTH using the PST Adherence and Competence Scale (PST-PAC).41 MTH provided detailed feedback to the trainers in 30-minute phone supervision meetings. Trainers were required to meet satisfactory levels of competence (defined as mean scores ≥ to 3, in a range of 0–5, on the PST-PAC for their last 2 training cases) before being certified to work with the AMD patients. Special attention was focused on adherence to principles and activities outlined in the PREPSI manual, as well as the ability to interact with older adults who have vision impairments. In addition, the PI or project coordinator conducted a training on the PFC modules and the Resource Information Modules. Trainers discussed their progress in supervision with the PI or the study coordinator in weekly ½ hour sessions. One trainer was replaced during the third year of the study.
EAC Trainers
Over the course of the study, a total of ten control group trainers were instructed to do life review. They attended 8 hours of training introducing them to Erikson’s life stages 42, teaching open-ended questioning techniques, and empathetic listening, and discussing the Resource Information Modules. They completed two supervised practice cases of 8 1-hr sessions each. EAC trainers delivering the Life Review received the same sensitivity training protocol as the PREPSI trainers.
Several measures were taken to ensure treatment fidelity:
Qualified Trainers
Although the PST approach does not require extensive psychological training or background knowledge and can be taught to a variety of health professionals41,43,44, trainers were selected for educational background and skills that promise ability to carry out the training protocol.
Manualization
The intervention manual provides clear guidelines for introducing and describing characteristics of the treatment process, and outlining sequencing techniques and what steps to take in effective implementation.45 Manuals diminish variability in treatment adherence.46 The Life Review training was also manualized in order to enhance consistency among the EAC trainers.
High Acceptability of Treatment may facilitate therapist’s ability to follow the intended protocol.45 Because the PREPSI is brief, easily understood, and framed as an educational program (as opposed to psychotherapy) -- thus avoiding a common barrier to treatment – it is perhaps more ‘acceptable’ to older adults. Adherence rates in past studies were relatively high and dropout rates relatively low.47 We reasoned that this would motivate PREPSI trainers to be faithful to the protocol.
Behavior-based Adherence Ratings
Because of social desirability issues with self-report45, treatment fidelity was assessed using the PST-PAC checklist, revised to reflect the PREPSI modifications, to rate randomly selected audio-taped PREPSI sessions. The checklist was developed by MTH for assessing treatment fidelity during PST training on the following dimensions: Defining the Problem; Establishing a Realistic Goal; Brainstorming Solutions; Implementing Decision Making Guidelines; Implementation of Solutions; Processing Tasks; Communication, Personal Effectiveness, and a global rating. MTH rated approximately 10% of the accumulated recorded sessions at 6-month intervals during the intervention period using the PST-PAC checklist. Protocol deviations were discussed in supervision. These procedures prevented treatment drift. Average global ratings for the three primary PREPSI trainers ranged from 4.2 to 4.9 out of 5, with an increasing trend with practice. For processing tasks, the lowest-scoring the dimension, the average rating was across all trainers was 3.96 out of 5.
Supervision of PREPSI Trainers and Treatment Drift Monitoring
All trainers participated in group supervision sessions led by the PI and project coordinator. In these bi-weekly meetings they discussed issues and problems that arose during training and reinforced trainer adherence to their specific protocols. Discussions were used to explore alternate approaches to difficult patients or situations, since these techniques have been shown to increase fidelity.45 A clinical psychologist joined the PREPSI trainer meeting monthly to discuss emergent clinical issues to be available for consultation as needed.
Measurement Strategy
Participants were assessed in weeks 1, 6, 11, and 16, and 6 months post-intervention. At each visit we assessed changes in the participant’s vision functioning, recent use of ABVI or other vision-related services, current diagnosis, treatment, and medication/vitamin use. In assessment 2 we also asked about satisfaction with VE groups, and in assessment 4 we addressed satisfaction with the PST and PFCT components. At the 6-month follow-up we updated demographics, assessed outcome measures and potential interference variables (e.g., changes in social network and health, life events and changes in marital status, health, family relationships, finances, housing). We also asked about the participant’s visual acuity, requested updates from their retina specialist, and about use of low-vision services.
All participant measurements were carried out in interview format. Large print answer cards were used for responding to Likert style items. If the participant was unable to read these, the interviewer repeated the answer options as many times as necessary until the participant was able to use them, or the interviewer applied stepwise questioning, e.g., “do you agree or disagree; do you disagree strongly, moderately, or slightly?”
Interviews lasted 1–3 hours, depending on the participants’ ability to comprehend question and answer formats, and on their talkativeness. Assessors were instructed to end any interview exceeding 2.5 hours and reschedule to continue at another time. They were also trained to recognize fatigue and respond with a break in the interview or by rescheduling.
Instruments
Variables were grouped into the following categories: Emotional Well-being outcomes; Future Outlook and Planning, Functional outcomes. Three outcomes were tested for successful randomization. The first was the Psychological Well-Being scale (PWB),48 a 42-item multidimensional measure assessing 6 dimensions of PWB: Autonomy, Environmental Mastery, Personal Growth, Positive Relations With Others, Purpose In Life, and Self-Acceptance. It is a Likert-scored self-report measure, with anchors of “1-disagree strongly” and “6-agree strongly.”49–52. (2) The second was the Preparation for Future Care Measure (PFCM),53 which has been validated with community-dwelling older adults, and has acceptable internal consistency and retest reliability. It consists of 29 items in five subscales that assess specific behaviors representative of each of five processes observed in PFC: Awareness of care needs: “Talking to other people has made me think about whether I might need help or care in the future.” Gathering Information: “I have gathered information about options for care by talking to friends or family.” Deciding on Preferences: “If I ever need help or care, I can choose between several options that I have considered in some depth.” Making Concrete Plans:” I have explained to someone close to me what my care preferences are.” Avoidance:” I avoid negative topics like future dependence.” The third measure to test for successful randomization was the total score of the Vision Function Questionnaire, a 25-item National Eye Institute-approved Vision Functioning Assessment (NEI-VFQ-25). 54,55
Treatment Compliance and Enactment of Skills
Compliance and resistance were documented by the PREPSI trainer after each session. Trainers record attendance, reluctance to engage in problem-identification or solution generation, content of the problem, completion of the problem-solving process, relevance of the action plan, compliance with homework, success of homework, patient’s understanding and application of PST principles, and extent of problem resolution. In the PFCT section, they also documented whether the problem chosen was future-oriented. Because EAC trainers could not document enactment of skills, participation in all EAC sessions before the visit 4 assessment was used as a measure of compliance.
Data analysis
In order to demonstrate adequate randomization, we compared PREPSI and EAC, as well as non-randomized subjects with regard to baseline scores on the demographic variables. We also checked randomization with regard to the three groups of well-being outcomes that will be the focus of future efficacy analysis: Six psychological well-being (PWB) subscales, five preparation for future care (PFC) subscales, and a measure of vision functioning (NEI-VFQ). We then compared the groups on three process outcomes: (1) Retention rates, (2) compliance (number of sessions attended), and (3) satisfaction ratings. We used Chi-square tests for categorical variables and analysis of variance for ordinal and continuous variables. For data tables with expected values <5 we used Fisher’s exact test. Although the primary hypothesis of this efficacy trial are that the trajectories of PFC and PWB will be significantly different for treatment and control group at visits 4 and 5, this hypothesis will not be tested until the trial is complete, thus the results are not reported here.
Results
Tables 1a and 1b show no differences between treatment, control, and non-randomized subjects on any demographic variables and urn randomization covariates.
Table 1b.
Comparison of Participant Characteristics among Participants in Treatment, Control, and Not-Randomized Groups (categorical variables)
| PREPSI N (%) (N=90) |
EAC N (%) (N=90) |
Not randomized N (%) (N=27) |
χ2-statistic (p-value)a of 3-way difference | Total N (%) (N=213) |
|
|---|---|---|---|---|---|
| Gender | |||||
| Male | 31 (34.4%) | 33 (36.7%) | 14 (42.4%) | .663(p=.72) Df=2 |
78 (36.6%) |
| Female | 59 (65.6%) | 57 (63.3%) | 19 (57.6%) | 135 (63.4%) | |
|
| |||||
| Racea | |||||
| White | 85 (94.4%) | 87 (98.9%) | 33 (100%) | 3.63 (p=.43) Df=4 |
205 (97.2%) |
| Black | 3 (3.3%) | 0 | 0 | 3 (1.4%) | |
| Other | 2 (2.2%) | 1 (1.1%) | 0 | 3 (1.4%) | |
|
| |||||
| Ethnicity a | |||||
| Latino | 1 (1.1%) | 1 (1.1%) | 0 | .596 (p=.99) (Latino versus other) Df=2 |
2(.9%) |
| Not Latino | 88 (98.9%) | 88 (98.9%) | 33 (100%) | 209 (99.1%) | |
| English/Scottish-American | 41 (45.6%) | 30 (33.3%) | 14 (42.4%) | 85 (39.9%) | |
| Irish-American | 25 (27.8%) | 24 (26.7%) | 11 (33.3%) | 60 (28.2%) | |
| German-American | 36(40%) | 38 (42.2%) | 13 (39.4%) | 87 (40.8%) | |
| Italian-American | 7 (7.8%) | 8 (8.9%) | 2 (6.1%) | 17 (8%) | |
| Russian-American | 0 | 2 (2.2%) | 0 | 2 (.9%) | |
| Jewish-American | 5 (5.6%) | 0 | 0 | 5 (2.3%) | |
| (includes individuals who indicated multiple Ethnicities) | |||||
|
| |||||
| Type of AMDa | |||||
| Wet | 25 (32.5%) | 23 (32.4%) | 11 (39.3%) | 3.83 (p=.76) df=6 |
59 (33.5%) |
| Dry | 39 (50.6%) | 34 (47.9%) | 15 (53.6%) | 88 (50%) | |
| Both | 13 (16.9%) | 13 (18.3%) | 2 (7.1%) | 28 (15.9%) | |
| Other Type | 0 | 1 (1.4%) | 0 | 0 | |
|
| |||||
| Living Alone | 47 (52.2%) | 43 (47.8%) | 18 (54.5%) | .586 (p=.75) Df=2 |
108 (50.9%) |
|
| |||||
| Marital Status | |||||
| Married (living with spouse or partner) | 36 (40%) | 33 (36.7%) | 13 (39.4%) | .224 (p=.89) (married versus all others) Df=2 |
82 (38.5%) |
| Not Married | |||||
| Widowed | 42 (46.7%) | 40 (44.4%) | 12 (36.4%) | 94 (44.1%) | |
| Divorced | 5 (5.6%) | 9 (10.0%) | 5(15.2%) | 19 (8.9%) | |
| Single | 5 (5.6%) | 6 (6.7%) | 2 (6.1.%) | 13 (6.1%) | |
| Other | 2 (2.2%) | 2 (2.2%) | 1 (3.0%) | 5 (2.3%) | |
|
| |||||
| Legally Blind | 32 (36.8%) | 25 (30.1%) | 9 (32.1%) | .869 (p=.65) Df=2 |
66 (33.3%) |
|
| |||||
| Received low-vision services | 49 (54.4%) | 49 (54.4%) | 13 (40.6%) | 2.08 (p=.35 Df=2) | 111 (52.4%) |
|
| |||||
| Length of Diagnosis | |||||
| 2 years or less | 19 (23.2%) | 21 (26.6%) | 10 (34.5%) | 7.87 (p=.09) df=4 |
50 (26.3%) |
| 3–9 years | 38 (46.3%) | 33 (41.8%) | 5 (17.2%) | 76 (40.0%) | |
| 10 years or more | 25 (30.5%) | 25 (31.6%) | 14 (48.3%) | 64 (33.7%) | |
Note. Variation in totals is due to missing values.
Fisher’s Exact Test
At baseline, treatment and control groups did not differ in Psychological Well-being, Preparation for Future care, or total vision functioning score (the outcome variables chosen for the efficacy trial, Table 2). Treatment and control also did not differ from the non-randomized group in these variables.
Table 2.
Comparison of Participants in Treatment, Control, and Not-Randomized Groups with Regard to Trial Outcome Variables
| Variables | PREPSI Mean (SD) (N=90) |
EAC Mean (SD) (N=90) |
Not randomized Mean (SD) (N=33) |
F-statistic | p-value |
|---|---|---|---|---|---|
| PWB - Autonomy | 4.72 (.82) | 5.85 (.82) | 4.78 (.75) | .561 | .571 |
| PWB – Environmental Mastery | 4.76 (.88) | 4.83 (.76) | 4.69 (.77) | .399 | .671 |
| PWB – Personal Growth | 4.71 (.84) | 4.80 (.82) | 4.69 (.95) | .307 | .736 |
| PWB – Positive Relationships | 5.00 (.82) | 5.01 (.69) | 4.95 (.92) | .219 | .803 |
| PWB – Purpose in Life | 4.46 (.99) | 4.34 (.87) | 4.18 (.93) | 1.072 | .344 |
| PWB – Self-Acceptance | 4.63 (.89) | 4.59 (.97) | 4.51 (1.087) | .155 | .856 |
| PFC-Awareness | 3.33 (.92) | 3.39 (.82) | 3.02 (1.00) | 1.859 | .159 |
| PFC – Gathering Information | 2.93 (.95) | 2.99 (.94) | 2.99 (1.01) | .138 | .871 |
| PFC – Decision-Making | 3.09 (.98) | 3.07 (.93) | 3.25 (1.02) | .399 | .672 |
| PFC – Concrete Planning | 3.21 (1.05) | 3.31 (.97) | 3.17 (.89) | .333 | .717 |
| PFC – Avoidance | 3.05 (.97) | 2.80 (.97) | 3.09 (.97) | 1.758 | .175 |
| NEI-VFQ Total | 61.39 (17.99 | 62.26 (17.96) | 61.62 (23.19) | .05 | .951 |
Note. All mean values except NEI-VFQ were means across all scale items rather than summary scores. PWB=Psychological Well-Being; PFC=Preparation for Future Care; NEI-VFQ= National Eye Institute Vision Functioning Questionnaire.
Retention rates between randomization to post-intervention (visit 4) for the treatment group (90%) and control group (88%) were comparable in a chi-square test (χ2(1)=.26, p=.16), as were the rates between randomization and 6-month follow-up (visit 5) (χ2(1)=.61, p=.33; treatment group= 62%; control group=68%). The number of sessions attended (7.1 vs. 6.7) were also not significantly different between the two treatment groups.
A random subset of participants (n=73, 53% PREPSI, 47% control) were surveyed about their satisfaction with the program. We used a modified version of the Client Satisfaction Survey.56 We created a satisfaction index (α=.86) from eight items and compared PREPSI and Control group participants with regard to the satisfaction index. In a t-test, the two treatment groups did not differ in satisfaction. Twenty-two percent had an average score of 4 (e.g., “very satisfied”), 67% ranged from 3–3.9 (e.g., “mostly satisfied), and the rest reported an average score of 2.5–2.9 (“indifferent or mildly satisfied”); no one had an average score below 2 (e.g., “not satisfied”). The majority of participants (87%) felt that the trainer listened to them very closely during either condition. None felt that trainers were poor listeners. Also, 98% felt that their rights as an individual were respected.
Among the PREPSI participants, the question “How useful did you find the in-home training sessions?” was answered by 43% with “very useful,” 28% “quite useful” and 28% “somewhat useful.” Nine percent found the in-home training less useful than the classes, 13% found them more useful, and 76% found them equally useful. One quarter of PREPSI respondents found the future planning training more useful than the standard problem-solving training sessions; two thirds found the two equally useful; only 3 % found the future planning training less useful.
DISCUSSION
We offer a detailed research protocol for the Macular Degeneration and Aging Study. This is the first randomized controlled trial to address both Psychological Well-being and Preparation for Future Care in a older population with vision-threatening eye disease. Our analyses suggest that subjects could be successfully randomized, that they were equally satisfied regardless of which group they were randomized to, and that retention rates and compliance were comparable for the treatment versus the control groups. Furthermore, although unblinding was more likely for the treatment than the control group, at follow-up it was equally likely for both groups.
Several considerations were important in developing this protocol. First, we considered using enhanced Treatment as Usual as control – that is, providing only Vision Education and resources instead of social attention visits – but results from the pilot study suggested that the attention that participants receive from weekly visits may enhance their well-being, even if it does not enhance their PFC cognitions and behaviors. The chosen design placed fewer limitations on study conclusions by strengthening the control condition, but increased the risk of non-significant findings.
Participants were urn randomized into the two study groups in order to balance potential confounders. However, unmeasured variables may still influence the results. Also, we found that people with longer time since diagnosis were more likely to drop out before randomization. We will therefore include these potential confounders in more detailed analyses. Since younger patients have had less time to adjust to AMD, we will control for age; because men were less likely to seek out social support, and because living alone increases the risk of having unmet needs, these variables will be included in the analyses and we will assess their potential as effect modifiers (moderators).
Given the public health relevance of late life vision loss due to macular degeneration, the results of this study will have implications for vision care in community settings and for public health policies for reimbursement of psychosocial treatments in vision loss care.
Acknowledgments
The authors wish to thank the National Institute of Aging (R01 AG032032, Sörensen) for funding the study and the National Institute of Mental Health (R49 CE002093, Caine) for staff support and for student and postdoctoral support (T32 MH018911, Lyness; T32 MH020061, Conwell).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Silvia Sörensen, Email: silvia_sorensen@urmc.rochester.edu.
Katherine White, Email: kwhite@abvi-goodwill.org.
Wingyun Mak, Email: WINGYUN.MAK@lehman.cuny.edu.
Katherine Zanibbi, Email: katherine_zanibbi@urmc.rochester.edu.
Wan Tang, Email: wan_Tang@urmc.rochester.edu.
Amanda O’Hearn, Email: Amanda_OHearn@urmc.rochester.edu.
Mark T. Hegel, Email: Mark.T.Hegel@Dartmouth.edu.
References
- 1.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Seddon JM, Chen CA. The epidemiology of age-related macular degeneration. Int Ophthalmol Clin. 2004;44(4):17–39. doi: 10.1097/00004397-200404440-00004. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb JL. Age-related macular degeneration. JAMA. 2002;288(18):2233–2236. doi: 10.1001/jama.288.18.2233. [DOI] [PubMed] [Google Scholar]
- 4.Rovner BW, Casten RJ. Activity loss and depression in age-related macular degeneration. American Journal of Geriatric Psychiatry. 2002;10(3):305–310. [PubMed] [Google Scholar]
- 5.Heyl V, Wahl H, Mollenkopf H. Visual capacity, out-of-home activities and emotional well-being in old age: Basic relations and contextual variation. Soc Indicators Res. 2005;74(1):159–189. [Google Scholar]
- 6.Heyl V, Wahl H. Psychosocial adaptation to age-related vision loss: A six–year perspective. Journal of Visual Impairment & Blindness. 2001;95(12):739–748. [Google Scholar]
- 7.Wahl H, Schilling O, Oswald F, Heyl V. Psychosocial consequences of age-related visual impairment: Comparison with mobility-impaired older adults and long-term outcome. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 1999;54B(5):304–316. doi: 10.1093/geronb/54b.5.p304. [DOI] [PubMed] [Google Scholar]
- 8.Casten RJ, Rovner BW, Tasman W. Age-related macular degeneration and depression: A review of recent research. Curr Opin Ophthalmol. 2004;15(3):181–183. doi: 10.1097/01.icu.0000120710.35941.3f. [DOI] [PubMed] [Google Scholar]
- 9.Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108(10):1893–1900. doi: 10.1016/s0161-6420(01)00754-0. [DOI] [PubMed] [Google Scholar]
- 10.Tasman W, Rovner B. Age-related macular degeneration: Treating the whole patient. Arch Ophthalmol. 2004;122(4):648–649. doi: 10.1001/archopht.122.4.648. [DOI] [PubMed] [Google Scholar]
- 11.Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The psychosocial impact of macular degeneration. Arch Ophthalmol. 1998;116(4):514–520. doi: 10.1001/archopht.116.4.514. [DOI] [PubMed] [Google Scholar]
- 12.Berman K, Brodaty H. Psychosocial effects of age-related macular degeneration. International Psychogeriatrics. 2006;13(3):415–428. doi: 10.1017/S1041610205002905. [DOI] [PubMed] [Google Scholar]
- 13.Javitt JC, Zhou Z, Willke RJ. Association between vision loss and higher medical care costs in Medicare beneficiaries: Costs are greater for those with progressive vision loss. Ophthalmology. 2007;114(2):238–245.e1. doi: 10.1016/j.ophtha.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 14.Fossati P, Ergis AM, Allilaire JF. Executive functioning in unipolar depression: A review. Encephale. 2002;28(2):97–107. [PubMed] [Google Scholar]
- 15.Sörensen S. Predictors of preparation for future care among older adults with macular degeneration. paper presented at. In: Sörensen S, Oliver J, editors. Preference for future care: Patients, providers, and geographical differences; Symposium at the 66th Annual Scientific Meeting of the Gerontological Society of America; New Orleans, LA. 2013. [Google Scholar]
- 16.Rowe JW, Kahn RL. Human aging: Usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 17.Aspinwall LG. Where planning meets coping: Proactive coping and the detection and management of potential stressors. In: Friedman SL, Scholnick EK, editors. The developmental psychology of planning: Why, how, and when do we plan? Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 285–319. [Google Scholar]
- 18.Aspinwall LG, Taylor S. A stitch in time: Self-regulation and proactive coping. Psychol Bull. 1997;121(3):417–436. doi: 10.1037/0033-2909.121.3.417. [DOI] [PubMed] [Google Scholar]
- 19.Sörensen S, Mak W, Chapman B, Duberstein P, Lyness J. The relationship of preparation for future care to depression and anxiety in older primary care patients at 2-year follow-up. Am J Geriatr Psychiatry. 2012;20(10):887–894. doi: 10.1097/JGP.0b013e31822ccd8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maloney SK, Finn J, Bloom D, Andresen J. Personal decision making styles and long-term care choices. Health Care Financ Rev. 1996;18:141–156. [PMC free article] [PubMed] [Google Scholar]
- 21.Barber CE, Pasley BK. Family care of Alzheimer’s patients: The role of gender and generational relationship on caregiver outcomes. Journal of Applied Gerontology. 1995;14(2):172–192. [Google Scholar]
- 22.Liu H, Chia YF, Olin GL. Long-term care arrangement: Personal need and other factors. Abstr Book Assoc Health Serv Res Meet. 1999;16:36–37. [Google Scholar]
- 23.Monagle S. Reducing falls in community dwelling elderly, The role of GP care planning. Aust Fam Physician. 2002;31(12):1111–1115. [PubMed] [Google Scholar]
- 24.Biderman A, Cwikel J, Fried AV, Galinsky D. Depression and falls among community dwelling elderly people: A search for common risk factors. J Epidemiol Community Health. 2002;56(8):631–636. doi: 10.1136/jech.56.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamoureux EL, Chong E, Wang JJ, et al. Visual impairment, causes of vision loss, and falls: The singapore malay eye study. Invest Ophthalmol Vis Sci. 2008;49(2):528–533. doi: 10.1167/iovs.07-1036. [DOI] [PubMed] [Google Scholar]
- 26.Long SK, King J, Coughlin TA. The implications of unmet need for future health care use: Findings for a sample of disabled Medicaid beneficiaries in New York. Inquiry. 2005;42(4):413–420. doi: 10.5034/inquiryjrnl_42.4.413. [DOI] [PubMed] [Google Scholar]
- 27.Owsley C, McGwin G, Jr, Ball K. Vision impairment, eye disease, and injurious motor vehicle crashes in the elderly. Ophthalmic Epidemiology. 1998;5(2):101–113. doi: 10.1076/opep.5.2.101.1574. [DOI] [PubMed] [Google Scholar]
- 28.Dreer LE, Elliott TR, Fletcher DC, Swanson M. Social problem-solving abilities and psychological adjustment of persons in low vision rehabilitation. Rehabilitation Psychology. 2005;50(3):232–238. [Google Scholar]
- 29.Horowitz A, Leonard R, Reinhardt JP. Measuring psychosocial and functional outcomes of a group model of vision rehabilitation services for older adults. Journal of Visual Impairment & Blindness. 2000;94(5):328–337. [Google Scholar]
- 30.Birk T, Hickl S, Wahl H, et al. Development and pilot evaluation of a psychosocial intervention program for patients with age-related macular degeneration. Gerontologist. 2004;44(6):836–843. doi: 10.1093/geront/44.6.836. [DOI] [PubMed] [Google Scholar]
- 31.Brody BL, Roch-Levecq AC, Gamst AC, Maclean K, Kaplan RM, Brown SI. Self-management of age-related macular degeneration and quality of life: A randomized controlled trial. Arch Ophthalmol. 2002;120(11):1477–1483. doi: 10.1001/archopht.120.11.1477. [DOI] [PubMed] [Google Scholar]
- 32.Brody BL, Roch-Levecq AC, Thomas RG, Kaplan RM, Brown SI. Self-management of age-related macular degeneration at the 6-month follow-up: A randomized controlled trial. Arch Ophthalmol. 2005;123(1):46–53. doi: 10.1001/archopht.123.1.46. [DOI] [PubMed] [Google Scholar]
- 33.Rovner BW, Casten RJ, Hegel MT, Leiby BE, Tasman WS. Preventing depression in age-related macular degeneration. Archives of General Psychiatry. 2007;64(8):888–892. doi: 10.1001/archpsyc.64.8.886. [DOI] [PubMed] [Google Scholar]
- 34.Rovner BW, Casten RJ, Hegel MT, et al. Low vision depression prevention trial in age-related macular degeneration: A randomized clinical trial. Ophthalmology. 2014;121(11):2204–2211. doi: 10.1016/j.ophtha.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busse A, Sonntag A, Bischkopf J, Matschinger H, Angermeyer MC. Adaptation of dementia screening for vision-impaired older persons: Administration of the mini-mental state examination (MMSE) J Clin Epidemiol. 2002;55(9):909–915. doi: 10.1016/s0895-4356(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 36.Alexopoulos GS, Katz IR, Bruce ML, et al. Remission in depressed geriatric primary care patients: A report from the PROSPECT study. Am J Psychiatry. 2005;162(4):718–724. doi: 10.1176/appi.ajp.162.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Zurilla TJ, Nezu AM. Social problem-solving in adults. In: Kendall PC, editor. Advances in cognitive-behavioral research and therapy. Vol. 1. New York, NY: Academic Press; 1982. [Google Scholar]
- 38.Sörensen S, Pinquart M. Preparation for future care needs by West and East German older adults. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2000;55B(6):S357–S367. doi: 10.1093/geronb/55.6.s357. [DOI] [PubMed] [Google Scholar]
- 39.Arean PA, Perri MG, Nezu AM, Schein RL, Christopher F, Joseph TX. Comparative effectiveness of social problem-solving therapy and reminiscence therapy as treatments for depression in older adults. Journal of Consulting & Clinical Psychology. 1993;61(6):1003–1010. doi: 10.1037//0022-006x.61.6.1003. [DOI] [PubMed] [Google Scholar]
- 40.Matteson MA. Group reminiscing for depressed insitutionalized elderly. In: Burnside I, editor. Working with the elderly: Group process and techniques. 2. Belmont, CA: Wadsworth; 1984. pp. 287–297. [Google Scholar]
- 41.Hegel MT, Dietrich AJ, Seville JL, Jordan CB. Training residents in problem-solving treatment of depression: A pilot feasibility and impact study. Fam Med. 2004;36(3):204–208. [PubMed] [Google Scholar]
- 42.Erikson EH. Childhood and society. 2. New York, NY: W.W. Norton & CO; 1963. [Google Scholar]
- 43.Mynors-Wallis L, Davies I, Gray A, Barbour F, Gath D. A randomised controlled trial and cost analysis of problem-solving treatment for emotional disorders given by community nurses in primary care. British Journal of Psychiatry. 1997;170:113–119. doi: 10.1192/bjp.170.2.113. [DOI] [PubMed] [Google Scholar]
- 44.Nezu AM, Nezu CM, Felgoise SH, McClure KS, Houts PS. Project genesis: Assessing the efficacy of problem-solving therapy for distressed adult cancer patients. Journal of Consulting & Clinical Psychology. 2003;71(6):1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- 45.Perepletchikova F, Kazdin AE. Treatment integrity and therapeutic change: Issues and research recommendations. Clinical Psychology: Science and Practice. 2005;12(4):365–383. [Google Scholar]
- 46.Miller SJ, Binder JL. The effects of manual-based training on treatment fidelity and outcome: A review of the literature on adult individual psychotherapy. Psychotherapy: Theory, Research, Practice, Training. 2002;39(2):184–198. [Google Scholar]
- 47.Mynors-Wallis L, Gath DH, Lloyd-Thomas AR, Tomlinson D. Randomised controlled trial comparing problem-solving treatment with amitriptyline and placebo for major depression in primary care. British Medical Journal. 1995;310:441–445. doi: 10.1136/bmj.310.6977.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bardi A, Ryff CD. Interactive effects of traits on adjustment to a life transition. J Pers. 2007;75(5):955–984. doi: 10.1111/j.1467-6494.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 49.Ryff CD, Seltzer MM. Family relations and individual development in adulthood and aging. 1995:95–113. [Google Scholar]
- 50.Ryff CD. Psychological well-being in adult life. Current Directions in Psychological Science. 1995;4(4):99–104. [Google Scholar]
- 51.Ryff CD, Singer B. Psychological well-being: Meaning, measurement, and implications for psychotherapy research. Psychother Psychosom. 1996;65(1):14–23. doi: 10.1159/000289026. [DOI] [PubMed] [Google Scholar]
- 52.Poser W, Poser S, Eva-Condemarin P. Mortality in patients with dependence on prescription drugs. Drug Alcohol Depend. 1992;30:49–57. doi: 10.1016/0376-8716(92)90035-b. [DOI] [PubMed] [Google Scholar]
- 53.Sörensen S, Pinquart M. Developing a measure of older adults’ preparation for future care needs. International Journal of Aging and Human Development. 2001;53(2):137–165. doi: 10.2190/1R0D-30TC-F4K1-F0DW. [DOI] [PubMed] [Google Scholar]
- 54.Marella M, Pesudovs K, Keeffe JE, O’Connor PM, Rees G, Lamoureux EL. The psychometric validity of the NEI VFQ-25 for use in a low-vision population. Invest Ophthalmol Vis Sci. 2010;51(6):2878–2884. doi: 10.1167/iovs.09-4494. [DOI] [PubMed] [Google Scholar]
- 55.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 56.Attkisson CC, Greenfield TK. The Client Satisfaction Questionnaire-8 and the service Satisfaction Questionnaire-30. In: Maruish M, editor. The use of psychological testing for treatment planning and outcome assessment. Hillsdale, NJ: Erlbaum; 1994. [Google Scholar]