Abstract
Insulin enhances the compliance of conduit arteries, relaxes resistance arterioles to increase tissue blood flow and dilates precapillary arterioles to expand muscle microvascular blood volume. These actions are impaired in the insulin resistant states. Exercise ameliorates endothelial dysfunction and improves insulin responses in insulin resistant patients, but the precise underlying mechanisms remain unclear. The microvasculature critically regulates insulin action in muscle by modulating insulin delivery to the capillaries nurturing the myocytes and trans-endothelial insulin transport. Recent data suggest that exercise may exert its insulin-sensitizing effect via recruiting muscle microvasculature to increase insulin delivery to and action in muscle. The current review focuses on how the interplay among exercise, insulin action and the vasculature contributes to exercise-mediated insulin sensitization in muscle.
Keywords: muscle contraction, endothelial function, microvasculature, insulin delivery, microvascular blood volume, insulin resistance
Introduction
Blood vessels actively regulate blood pressure and tissue perfusion via synthesis and secretion of vasoactive substances and in response to a variety of vasoactive hormones and neural signals [1-5]. Insulin is a vasoactive hormone. It acts on large conduit artery to increase compliance (see glossary), resistance arterioles to increase overall blood flow to tissue, and precapillary arterioles to increase capillary perfusion. Patients with insulin resistance, as seen in obesity, metabolic syndrome, hypertension, and/or type 2 diabetes mellitus (T2DM) frequently exhibit endothelial dysfunction and are prone to develop arterial atherosclerosis, hypertension and metabolic disarrays such as dysglycemia and elevated plasma free fatty acid levels [6-10]. On the other hand, a large body of evidence has conclusively confirmed that exercise is able to improve endothelial function and insulin’s metabolic actions in these patients. Indeed, lifestyle modification including exercise has been advocated as the cornerstone for diabetes prevention and management [11]. Though the precise mechanisms underlying exercise-induced insulin sensitization remain to be defined, recent studies suggest that the vasculature plays an important role in regulating energy metabolism and insulin action during exercise training. Here, we summarize current knowledge on the interplay among exercise, insulin action and the vasculature, with a focus on the role of muscle microvasculature where the exchanges of nutrients, oxygen, and hormones such as insulin between the plasma and muscle interstitium take place.
Insulin acts on the arterial vasculature to regulate vessel function
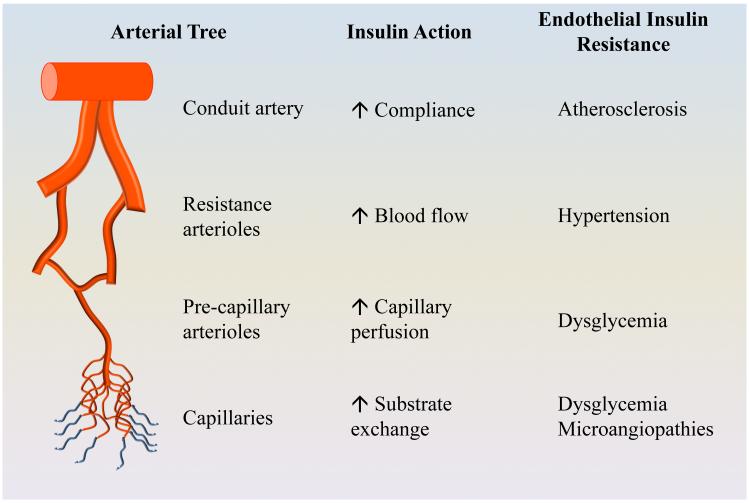
Vascular endothelium expresses abundant insulin receptors as well as the insulin-like growth factor I (IGF-I) receptors and the hybrid insulin/IGF-I receptors [12-15]. In response to insulin stimulation, endothelial cells produce a vasodilator nitric oxide (NO) via the phosphatidylinositol 3-kinase (PI-3 kinase)/protein kinase B (Akt)/endothelial NO synthase (eNOS) signal pathway, and a vasoconstrictor endothelin-1 (ET-1) through the mitogen-activated protein kinase (MAPK) pathway [16, 17]. In the basal state, insulin fine-tunes vascular tone via balancing its signals through these two signalling pathways. When insulin concentrations are raised to high physiological levels as seen during euglycemic hyperinsulinemic clamp or postprandial state, insulin’s vasodilatory effect predominates. Ample evidence has confirmed a direct action of insulin on the conduit, resistance, and microvascular segments of the arterial vasculature. As arterial function varies depending on its size and location, insulin actions on various segments of the arterial tree lead to different outcomes. The conduit arteries mainly regulate arterial plasticity/compliance and blood pressure, the resistance arterioles blood pressure and total blood flow to tissues, the pre-capillary arterioles tissue perfusion and the capillaries exchanges of nutrients, oxygen and hormones between the plasma and tissue interstitium [Figure 1].
Figure 1. Insulin action and resistance in arterial vasculature.
Each segment of the arterial tree has different function and its response to insulin results in various outcomes depending on its size and location. Insulin resistance occurs at all segments of the arterial vasculature in patients with obesity and diabetes.
Conduit arteries are large arteries containing collagen and elastin filaments in the tunica media which enable the arteries to stretch in response to pressure to maintain a relatively constant pressure in the arteries [18]. The ability of conduit arteries to accommodate the volume ejected by the heart is described as arterial compliance. A decrease in arterial compliance, i.e., distensibility, suggests an increase in vessel stiffness and is an independent predictor of coronary and cerebral artery diseases [19]. Arterial stiffness is most frequently assessed in clinical studies by non-invasive measuring pulse wave velocity (PWV) and augmentation index. Insulin relaxes the conduit arteries and increases their compliance. In healthy humans, insulin infusion decreases augmentation index, consistent with increased distensibility or vasodilatation of large arteries [20-22]. Though it is unclear whether insulin does this via the stimulation of NO production, insulin infusion does increase the responsiveness of the femoral artery to methacholine-induced vasodilation in humans [23].
Resistance arterioles, ranging from 400 μm to 100 μm, are the major determinant of vascular resistance [24]. Vascular resistance changes inversely with blood vessel radius and bulk tissue blood flow varies positively with the vessel lumen size. Thus, a small decrease in the lumen (vasoconstriction) can markedly increase vascular resistance and decrease tissue blood flow. On the contrary, increased lumen diameter (vasorelaxation) leads to decreased vascular resistance and increased tissue blood supply. Insulin dilates resistance arterioles and thus is able to decrease vascular resistance and increase total tissue blood flow. This was elegantly demonstrated by Baron and his colleagues in a series of clinical studies using the thermodilution technique to quantify leg blood flow in healthy humans [23, 25-29]. This group of investigators also confirmed a coupling of insulin’s vascular effects and metabolic effects by demonstrating that changes in insulin-mediated leg blood flow were paralleled by changes in insulin-mediated glucose disposal in leg in healthy humans as well as in subjects with obesity [30], type 1 diabetes [31] and type 2 [32] diabetes. Insulin's vasodilatory action on resistance vessels is NO-dependent, as inhibition of NO production with the NO synthase inhibitor L-NG-monomethyl arginine (L-NAME) diminishes insulin-mediated increases in both blood flow and glucose uptake in leg [25, 27, 28].
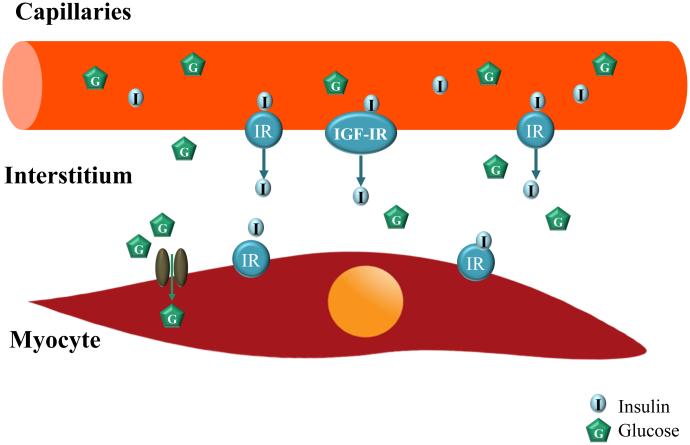
Over the past two decades, much attention has been focused on insulin’s effects on the terminal arterioles and its relation with insulin’s metabolic effects in muscle [4, 10, 33, 34]. Skeletal muscle is a major insulin response organ and accounts for ~ 80% of insulin-stimulated whole body glucose disposal during insulin infusion [35]. To act on its receptors on the myocyte membrane, insulin has to be first delivered into the capillaries nourishing the myocytes and then transported through the capillary endothelium to enter the interstitial space. Insulin delivery to muscle has been confirmed by multiple studies using various methodologies such as lymphatic sampling, microdialysis or radio-labeled insulin uptake techniques to be rate-limiting for insulin action in muscle [36-38]. Muscle microvasculature, including vessels <150 μm in diameter such as small (third and fourth order) arterioles, the capillary network, and small venules, actively regulates insulin delivery to and action in muscle by providing sufficient endothelial surface area for transendothelial transport of insulin from plasma to muscle interstitium [4, 34]. We and others have demonstrated that muscle microvasculature is also an insulin target, and insulin action here is more closely coupled with its metabolic effects in terms of muscle glucose uptake [4, 10, 33, 34]. Indeed, insulin has been repeatedly shown in both laboratory animals and healthy humans to regulate its own delivery to and thus actions in muscle, by recruiting muscle microvasculature [39-43] and facilitating its own trans-endothelial transport [40, 44][Figure 2]. These observations are of particular physiological significance as the major function of muscle microvasculature is to provide exchange surface area to facilitate an adequate delivery of nutrients, oxygen and hormones such as insulin to muscle cells, and the removal of metabolic wastes from the muscle interstitium. In the resting state only ~ 30% of the muscle capillaries are being perfused [45] and the skeletal muscle [leg] basal blood flow averages only ~0.2 L/min [25]. Thus, a relative small increase in capillary perfusion (controlled by the precapillary terminal arterioles, a process termed microvascular or capillary recruitment [34, 45]) could markedly increase substrate (such as glucose, amino acids, and oxygen) and insulin extraction due to an expansion of the endothelial exchange surface area in muscle. Indeed, blockade of insulin’s microvascular action with L-NAME decreases insulin-stimulated steady-state glucose disposal by up to 40% [46, 47]. In addition to insulin, other factors that are capable of increasing muscle microvascular blood volume and blood flow, such as mixed meal, muscle contraction, angiotensin 1-7, angiotensin II type 1 receptor blockers, glucagon-like peptide 1, ranolazine and adiponectin, are also capable of increasing insulin’s metabolic actions in muscle [41, 43, 48-56].
Figure 2. Transendothelial insulin transport.
To act on muscle cell insulin receptors, insulin has to be delivered to the capillaries nurturing the muscle cells and then transported through the endothelial barrier to reach the muscle interstitium. Insulin acts on endothelial cell insulin receptors (IR) and IGF-I receptors (IGF-IR) to facilitate its own transendothelial transport from blood to muscle interstitium.
Metabolic insulin resistance is associated with arterial endothelial dysfunction and insulin resistance
Patients with obesity and diabetes exhibit both arterial endothelial dysfunction and insulin resistance, two abnormalities that usually coexist, mutually perpetuate, and predispose patients to accelerated atherosclerosis and hypertension. Evidence suggests that they are present at all levels of the arterial vasculature. As for the conduit arteries, essentially all disease states such as obesity, metabolic syndrome, diabetes and hypertension that exhibit characteristics of chronic metabolic insulin resistance are associated with increased arterial stiffness and/or impaired NO-mediated vasodilation [21, 57-59]. Plasma free fatty acid levels, an important insulin resistance contributor, are associated with reduced reflection pressure wave magnitude and central blood pressure [60] and insulin’s vasodilatory action on the conduit arteries is impaired by obesity [21, 61]. Endothelial specific knockout of the insulin receptors greatly accelerates the atherosclerotic process in apoE−/− mice [62]. Metabolic insulin resistance is also accompanied by impaired insulin action on the resistance arterioles that regulate skeletal muscle blood flow [25], and endothelial dysfunction in the resistance vessels, as evidenced by abnormal responses to intra-arterial methacholine or acetylcholine infusion [23, 29, 63, 64]. The observation that plasma free fatty acid elevation impairs insulin-mediated vasodilation and NO production lends further support of the NO-dependence of insulin action on the resistance vessels [28]. As insulin resistance is selectively present in the PI3-kinase/Akt/eNOS pathway and insulin signals through the MAPK pathway remain normal or even enhanced, the aggregate consequence is a decreased NO availability and increased ET-1 action [65]. This combination likely contributes to increased vascular tone and explains the predisposition to hypertension and tissue hypoxia in patients with insulin resistance. Insulin resistance is clearly present in the muscle microvasculature in humans and animals with metabolic insulin resistance. Impaired insulin-mediated microvascular recruitment has been demonstrated in obese and diabetic animals [66, 67], obese humans [68] and humans or animals receiving systemic infusions of tumor necrosis factor α or lipid [42, 43, 69, 70]. It is of particular significance to note that vascular insulin resistance occurs before muscle insulin resistance in rodents fed a high fat diet [71]. As all factors causing metabolic insulin resistance appear to be able to decrease insulin responses in the muscle microvasculature and insulin’s vascular actions contribute to the overall insulin’s metabolic actions, muscle microvascular insulin resistance may contribute to the pathogenesis of metabolic insulin resistance and thus muscle microvasculature could be a therapeutic target for the prevention and management of insulin resistance and dysglycemia [65].
Exercise ameliorates endothelial dysfunction and enhances insulin responses in muscle in the insulin resistant states
Given the coexistence of insulin resistance and endothelial dysfunction in patients with obesity and diabetes and that exercise engenders myriad metabolic and cardiovascular benefits, exercise has been used as the cornerstone for diabetes prevention and management. Regular exercise delays the development of T2DM [72-74] in addition to slowing the progression of vascular diseases and reducing the cardiovascular morbidity and mortality associated with insulin resistance syndrome [75]. Even moderate daily exercise can greatly improve insulin sensitivity and a single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese humans [76]. This is not surprising as exercise acutely increases insulin-stimulated muscle glucose uptake and greater phosphorylation of Akt substrate of 160 kDa (AS160) in both insulin sensitive and insulin resistant states [77]. Physical activity reduces the cardiovascular disease risk via both modification of the traditional cardiovascular disease risk factors such as blood pressure, lipids, inflammation and diabetes and other yet to be defined pathways [78-80]. Clearly the vascular effects of exercise are not solely confined within the active muscle bed and ample evidence has confirmed that regular physical activity can alter endothelial phenotype and function in vasculatures perfusing the non-contracting skeletal muscle and non-muscular tissues/organs such as brain, viscera and skin, possibly via hemodynamic forces such as shear stress and cyclic strain and/or circulating factors released from adipose tissue and skeletal muscle during physical activity [80]. It is well documented that exercise increases flow-mediated dilation in response to shear stress stimulus that produce NO-dependent response.
Exercise enhances microvascular perfusion and insulin delivery in muscle
As discussed above, muscle microvasculature provides endothelial surface area for substrate and hormone exchanges and factors that increase muscle microvascular blood volume also enhance muscle glucose uptake and insulin delivery to and action in muscle [4, 10, 34]. At physiologic hyperinsulinemia, plasma insulin concentrations correlates well with the interstitial insulin concentrations which is more directly correlated with insulin-mediated glucose disposal [81, 82]. Among all factors that expand muscle microvascular blood volume, exercise (i.e., muscle contraction) is the most potent inducer of muscle microvascular recruitment. Even light exercise, such as gentle hand grip (at 25% of maximal strength) [48], or electric stimulation at low frequency that does not increase conduit artery blood flow (0.1-Hz contraction) [49, 50] significantly increases muscle microvascular blood volume. Further increase in muscle contraction intensity (from 0.1 Hz to 2 Hz) is able to recruit additional microvascular blood volume [83]. At higher intensity (hand grip at 80% of maximal strength), muscle contraction increases muscle microvascular blood volume to a similar extent but this is accompanied with a marked increase in total muscle microvascular blood flow due to a significant increase in muscle microvascular blood flow velocity [48].
The exercise-induced muscle microvascular recruitment is associated with increased muscle insulin delivery and action [49, 50]. A prior study reported that in insulin sensitive rats receiving insulin infusion at 10 mU/kg/min electric stimulation-induced muscle contraction markedly increased muscle blood flow and interstitial insulin concentrations compared with the non-contracting leg [82]. These findings are consistent with a mathematic model that projects higher interstitial insulin concentrations after exercise in people with or without diabetes [84]. The exercise-mediated muscle insulin uptake do not appear to be dependent on tissue bulk flow as low frequency muscle contraction does not increase muscle bulk flow but significantly increases muscle uptake of insulin [49, 50]. This argues strongly that the changes in microvascular blood volume (i.e., endothelial surface area), not total flow, is critical in muscle delivery and uptake of insulin during exercise/muscle contraction. However, it appears that the effect of muscle contraction on interstitial insulin concentration is not sustained. In insulin resistant oophorectomized female rats exposed to high concentrations of testosterone physical exercise (ad lib wheel running) reversed hyperandrogenicity-induced muscle insulin resistance but did not change the distribution time of muscle interstitial insulin 24 hours after cessation of exercise [85].
Exercise’s microvascular actions in muscle are preserved in the insulin resistant states Evidence thus far has clearly demonstrated that exercise and insulin each increases muscle microvascular recruitment and glucose uptake but via different signaling mechanisms and exercise-mediated muscle microvascular recruitment is preserved in the insulin resistant states. In obese Zucker rats, insulin- but not contraction-mediated glucose uptake in muscle is impaired. Similarly, insulin fails to induce muscle microvascular responses but muscle contraction-mediated capillary recruitment and glucose uptake in muscle are essentially normal in these animals [86]. Neither contraction-mediated muscle microvascular recruitment which precedes increases in total limb blood flow nor glucose uptake is impaired in the high fat diet fed, insulin resistant rats [83]. Furthermore, low frequency contraction-mediated muscle microvascular recruitment and insulin uptake are preserved during lipid infusion in rats [49] and acute systemic administration of tumor necrosis factor α blocks insulin-mediated microvascular recruitment and glucose uptake in muscle but has no effect on contraction (2 Hz, 0.1 ms at 30 V)-induced increases in femoral blood flow, hindleg glucose uptake, and microvascular recruitment [87]. Together with the prior observations that insulin-mediated vascular effects contribute to ~ 25-40% of insulin-stimulated glucose disposal during insulin clamp [27, 39], the microvascular response to muscle contraction may in part explain enhanced insulin action in muscle after episodes of exercise.
Mechanisms underlying exercise-induced microvascular recruitment and insulin sensitization
Though contraction induces a complex molecular signaling response which involves the adenosine monophosphate-activated kinase (AMPK), calcium, and NO synthase in the proximal part of the signaling cascade [88], mounting data suggest that AMPK plays a critical role in exercise-mediated insulin sensitization. Muscle contraction and factors that increase the ratio of AMP to ATP acutely elevate the activity of AMPK, a key regulator of energy metabolism in muscle, and this leads to increased fatty acid oxidation, glucose uptake and glycogenolysis [89]. In addition, AMPK activation improves mitochondria function, and decreases gluconeogenesis, oxidative stress, endoplasmic reticulum stress and inflammation, all factors that are associated with insulin resistance and metabolic syndrome [89]. Activation of AMPK by its activator 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) increases both muscle fatty acid and glucose uptake in white muscle of insulin resistant rats in vivo [90] and enhances muscle and liver insulin action in rats fed a high fat diet [91].
At the vascular level, exercise training ameliorates endothelial dysfunction and arterial remodeling and stiffness and improves redox state and NO availability [75]. Vascular AMPK is clearly activated by exercise training as evidenced by an increase in AMPK and eNOS activities in mouse blood vessels [92, 93]. In cultured endothelial cells activation of AMPK enhances NO production [94] and prevents hyperglycemia-induced apoptosis [95] and mitochondria reactive oxygen species production [96]. However, how AMPK, particularly vascular AMPK component, activation leads to insulin sensitization remains unclear.
The mechanisms underlying exercise-induced muscle microvascular recruitment remain to be defined. While NO plays a critical role in the microvascular recruitment induced by insulin [39, 46], losartan [41, 52, 70], and glucagon-like peptide 1 [53, 54], it does not appear to be involved in muscle contraction-induced microvascular recruitment. Indeed, NO synthase inhibition with simultaneous infusion of L-NAME does not blunt muscle contraction-induced microvascular recruitment [50]. In a different study, local NO synthase inhibition attenuated contraction-stimulated increase in muscle NO synthase activity, femoral blood flow, and skeletal muscle glucose uptake (by ~35%) but had no effect on contraction-mediated muscle AMPK activation and capillary recruitment [97]. Thus, it appears that contraction induces muscle microvascular recruitment via an NO-independent pathway but muscle glucose uptake partly via NO dependent mechanism. These findings clearly reflect the complexity of muscle contraction-mediated vascular and metabolic effects.
Inasmuch as muscle contraction increases AMPK activity and pharmacological activation of AMPK results in increased glucose transport in skeletal muscle, it is unclear whether AMPK activation is required in contraction-induced microvascular recruitment. Though high intensity muscle contraction (8.0 Hz) enhances muscle AMPK phosphorylation and recruits muscle microvasculature [49], low frequency muscle contraction (0.1 Hz) significantly increases muscle microvascular perfusion and insulin uptake without affecting muscle AMPK phosphorylation [49]. However, whether AMPK phosphorylation increases in the vascular endothelium during low frequency muscle contraction has not been examined. Vascular endothelium expresses abundant AMPK and its activation by AICAR increases eNOS activity (as evidenced by Ser1177 phosphorylation) which leads to relaxation of the resistance arteries ex vivo and recruitment of muscle microvasculature in vivo [98]. Thus, it is possible that low intensity muscle contraction may also exert a microvascular recruitment effect via activating endothelial AMPK.
Concluding remarks and future perspectives
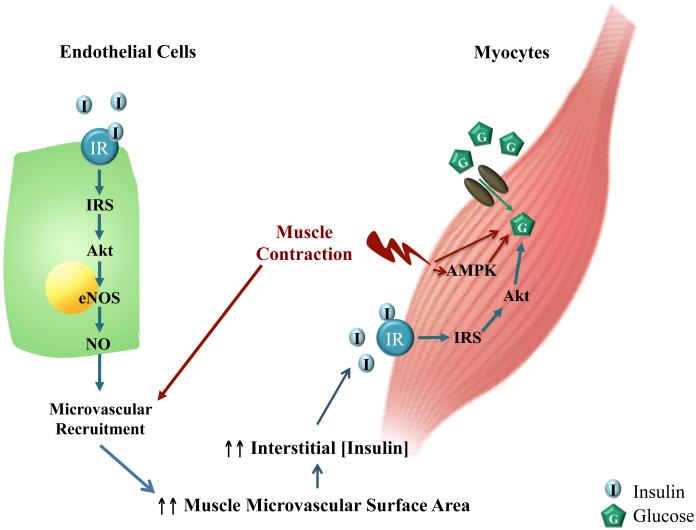
Patients with obesity and T2DM have endothelial dysfunction and vascular insulin resistance in addition to metabolic insulin resistance and are prone to develop hypertension and cardiovascular complications. Exercise ameliorates endothelial dysfunction, improves metabolic insulin responses and reduces the cardiovascular morbidity and mortality associated with obesity and diabetes, and has been used as the corner stone for diabetes prevention and management. Though exercise training induces myriad physiological and signaling responses, mounting evidence has confirmed a critical role of the vasculature in exercise-mediated cardiovascular and metabolic benefits. Of particular interest and significance is the exercise effect on muscle microvasculature which provides the endothelial surface area for the exchanges of nutrients, oxygen and hormones between plasma and muscle interstitium and actively regulates muscle delivery and uptake of insulin. Emerging but strong data have revealed the involvement of muscle microvasculature in exercise-mediated insulin sensitization in muscle in that muscle contraction potently recruits muscle microvasculature and expands the endothelial surface area within muscle via a NO-independent mechanism, leading to increased muscle insulin delivery, uptake and action (Figure 3). These actions are preserved in the insulin resistant states. Further studies are needed to clarify the role of endothelial AMPK in exercise-mediated vasodilation and microvascular recruitment.
Figure 3. Schematic representation of the proposed interplay among exercise, insulin action and muscle microvasculature.
Insulin stimulates endothelial cells to produce NO which causes microvascular recruitment and expands muscle endothelial exchange surface area, leading to increased muscle delivery and action of insulin. Muscle contraction, via AMPK-dependent and independent mechanisms, facilitates glucose uptake. It may also increase muscle delivery of insulin by inducing NO-independent microvascular recruitment.
Highlights.
Exercise ameliorates endothelial dysfunction and improves metabolic insulin responses.
Muscle microvasculature regulates muscle delivery and uptake of insulin.
Muscle contraction recruits microvasculature and increase muscle uptake of insulin.
These actions are preserved in the insulin resistant states.
Acknowledgements
This work was supported by American Diabetes Association grants 1-11-CR-30, 9-09-NOVO-11 and 1-15-CE-32 (to Z.L.), National Institutes of Health grant R01HL094722 (to Z.L.) and Natural Science Foundation of China grant 81200630 (to C.Z.)
Glossary
- Arterial compliance
refers to the action in which arteries yield to pressure or force without disruption and is used as an indication of arterial stiffness. It decreases with aging, an increase in blood pressure, and in menopause.
- Arterial stiffness
describes a decreased compliance or elasticity of the arteries. It occurs with aging and in disease states that are associated with increased cardiovascular risk such as hypertension, diabetes mellitus, obesity, hyperlipidemia, and chronic renal failure.
- Augmentation index
a ratio calculated from the blood pressure waveform, which reflects arterial stiffness and increases with age.
- Euglycemic hyperinsulinemic clamp
the gold standard method of quantifying body insulin sensitivity. During the procedure, plasma insulin concentrations are acutely raised and maintained at a predefined level by a continuous infusion of insulin and plasma glucose concentrations held constant at predefined levels by a variable rate glucose infusion. The higher the glucose infusion rates, which equal glucose uptake by tissues, the higher the insulin sensitivity.
- Insulin resistance
a term that describes a decreased response to the action of insulin in stimulating tissue use of glucose. It typically develops with obesity and plays a pathogenic role in the development of diabetes.
- Insulin sensitivity
a relative term that describes how well a body, organ, tissue or cell responds to insulin in stimulating glucose use. When the response is normal, it is defined as insulin sensitive. The contrary is considered as insulin resistant. Many methods have been developed to estimate body insulin sensitivity with the euglycemic hyperinsulinemic clamp being the gold standard method.
- Microvascular recruitment
a term describes the opening of non-perfused capillaries for perfusion, a result of relaxation of the pre-capillary terminal arterioles.
- Microvasculature
Small blood vessels (<150 μm in diameter) comprising third and fourth order arterioles, capillaries and small venules. It provides endothelial exchange surface area for tissue uptakes of oxygen, nutrients, and hormones from plasma.
- Microvascular blood flow
refers to total blood flow within the microvascular bed, calculated as blood volume x blood flow velocity.
- Microvascular blood volume
refers to total volume of the microvascular bed that is being perfused with blood.
- Transendothelial insulin transport
a process that insulin in the plasma passes through the endothelial layer of the capillary to reach tissue interstitium.
- Vascular resistance
a term describes the resistance to blood flow that must be overcome to push blood through the blood vessels. It changes inversely with blood vessel lumen size. Thus, constriction of blood vessel increases resistance but vasodilation decreases resistance. Vessel length and blood viscosity also increase vascular resistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ko S-H, et al. Hypertension management and microvascular insulin resistance in diabetes. Current Hypertension Reports. 2010;12:243–251. doi: 10.1007/s11906-010-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Hert S. Physiology of hemodynamic homeostasis. Best Practice & Research Clinical Anaesthesiology. 2012;26:409–419. doi: 10.1016/j.bpa.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Wehrwein EA, Joyner MJ. Regulation of blood pressure by the arterial baroreflex and autonomic nervous system. In: Ruud MB, Dick FS, editors. Handbook of Clinical Neurology. Elsevier; 2013. pp. 89–102. [DOI] [PubMed] [Google Scholar]
- 4.Barrett EJ, et al. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011;301:E252–E263. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron AD, et al. Skeletal muscle blood flow. A possible link between insulin resistance and blood pressure. Hypertension. 1993;21:129–135. doi: 10.1161/01.hyp.21.2.129. [DOI] [PubMed] [Google Scholar]
- 6.Muniyappa R, Sowers J. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14:5–12. doi: 10.1007/s11154-012-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natali A, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–1140. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 8.Nigro J, et al. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27:242–259. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 9.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 10.Barrett EJ, Liu Z. The endothelial cell: An "early responder" in the development of insulin resistance. Rev Endocr Metab Disord. 2013;14:21–27. doi: 10.1007/s11154-012-9232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inzucchi SE, et al. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach: Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King GL, et al. Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. J Clin Invest. 1983;71:974–979. doi: 10.1172/JCI110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science. 1985;227:1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- 14.Li G, et al. Insulin and insulin-like growth factor-I receptors differentially mediate insulin-stimulated adhesion molecule production by endothelial cells. Endocrinology. 2009;150:3475–3482. doi: 10.1210/en.2009-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, et al. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology. 2005;146:4690–4696. doi: 10.1210/en.2005-0505. [DOI] [PubMed] [Google Scholar]
- 16.Muniyappa R, et al. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.-a., et al. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 18.Shadwick RE. Mechanical design in arteries. Journal of Experimental Biology. 1999;202:3305–3313. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 19.Mattace-Raso FUS, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 20.Tamminen M, et al. Insulin-Induced Decreases in Aortic Wave Reflection and Central Systolic Pressure Are Impaired in Type 2 Diabetes. Diabetes Care. 2002;25:2314–2319. doi: 10.2337/diacare.25.12.2314. [DOI] [PubMed] [Google Scholar]
- 21.Westerbacka J, et al. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes. 1999;48:821–827. doi: 10.2337/diabetes.48.4.821. [DOI] [PubMed] [Google Scholar]
- 22.Tamminen M, et al. Resistance to Acute Insulin Induced Decreases in Large Artery Stiffness Accompanies the Insulin Resistance Syndrome. J Clin Endocrinol Metab. 2001;86:5262–5268. doi: 10.1210/jcem.86.11.8047. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg HO, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: Role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–318. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- 25.Baron AD. Hemodynamic actions of insulin. Am J Physiol Endocrinol Metab. 1994;267:E187–202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 26.Baron AD, et al. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol Endocrinol Metab. 1994;266:E248–253. doi: 10.1152/ajpendo.1994.266.2.E248. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg HO, et al. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg HO, et al. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg HO, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J. Clin. Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laakso M, et al. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron AD, et al. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab. 1991;73:637–643. doi: 10.1210/jcem-73-3-637. [DOI] [PubMed] [Google Scholar]
- 32.Laakso M, et al. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41:1076–1083. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 33.Clark MG, et al. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 34.Barrett E, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang YJ, et al. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989;84:1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herkner H, et al. Transcapillary insulin transfer in human skeletal muscle. Eur J Clin Invest. 2003;33:141–146. doi: 10.1046/j.1365-2362.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- 38.Holmang A, et al. Interstitial muscle insulin and glucose levels in normal and insulin-resistant Zucker rats. Diabetes. 1997;46:1799–1804. doi: 10.2337/diab.46.11.1799. [DOI] [PubMed] [Google Scholar]
- 39.Vincent MA, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 40.Eggleston EM, et al. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes. 2007;56:2958–2963. doi: 10.2337/db07-0670. [DOI] [PubMed] [Google Scholar]
- 41.Chai W, et al. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension. 2010;55:523–530. doi: 10.1161/HYPERTENSIONAHA.109.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, et al. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab. 2009;94:3543–3549. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, et al. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab. 2011;96:438–446. doi: 10.1210/jc.2010-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, et al. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes. 2008;57:540–547. doi: 10.2337/db07-0967. [DOI] [PubMed] [Google Scholar]
- 45.Honig CR, et al. Active and passive capillary control in red muscle at rest and in exercise. Am J Physiol. 1982;243:H196–206. doi: 10.1152/ajpheart.1982.243.2.H196. [DOI] [PubMed] [Google Scholar]
- 46.Vincent MA, et al. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 47.Vincent MA, et al. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 2002;51:42–48. doi: 10.2337/diabetes.51.1.42. [DOI] [PubMed] [Google Scholar]
- 48.Vincent MA, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290:E1191–1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- 49.Inyard AC, et al. Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes. 2009;58:2457–2463. doi: 10.2337/db08-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inyard AC, et al. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes. 2007;56:2194–2200. doi: 10.2337/db07-0020. [DOI] [PubMed] [Google Scholar]
- 51.Fu Z, et al. Ranolazine recruits muscle microvasculature and enhances insulin action in rats. The Journal of Physiology. 2013;591:5235–5249. doi: 10.1113/jphysiol.2013.257246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chai W, et al. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes. 2011;60:2939–2946. doi: 10.2337/db10-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chai W, et al. Glucagon-like peptide 1 recruits muscle microvasculature and improves insulin’s metabolic action in the presence of insulin resistance. Diabetes. 2014;63:2788–2799. doi: 10.2337/db13-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chai W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu Z, et al. Angiotensin-(1–7) recruits muscle microvasculature and enhances insulin’s metabolic action via Mas receptor. Hypertension. 2014;63:1219–1227. doi: 10.1161/HYPERTENSIONAHA.113.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao L, et al. Adiponectin and insulin cross talk: The microvascular connection. Trends in Cardiovascular Medicine. 2014;24:319–324. doi: 10.1016/j.tcm.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stehouwer CDA, et al. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 58.Vehkavaara S, et al. In vivo endothelial dysfunction characterizes patients with impaired fasting glucose. Diabetes Care. 1999;22:2055–2060. doi: 10.2337/diacare.22.12.2055. [DOI] [PubMed] [Google Scholar]
- 59.Webb DR, et al. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia. 2010;53:1190–1198. doi: 10.1007/s00125-010-1689-9. [DOI] [PubMed] [Google Scholar]
- 60.Tabara Y, et al. Association of serum free fatty acid level with reduced reflection pressure wave magnitude and central blood pressure: The Nagahama Study. Hypertension. 2014;64:1212–1218. doi: 10.1161/HYPERTENSIONAHA.114.04277. [DOI] [PubMed] [Google Scholar]
- 61.Westerbacka J, et al. Resistance to Acute Insulin Induced Decreases in Large Artery Stiffness Accompanies the Insulin Resistance Syndrome. J Clin Endocrinol Metab. 2001;86:5262–5268. doi: 10.1210/jcem.86.11.8047. [DOI] [PubMed] [Google Scholar]
- 62.Rask-Madsen C, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metabolism. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogikyan RV, et al. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. J Clin Endocrinol Metab. 1998;83:1946–1952. doi: 10.1210/jcem.83.6.4907. [DOI] [PubMed] [Google Scholar]
- 64.Preik M, et al. Additive effect of coexistent type 2 diabetes and arterial hypertension on endothelial dysfunction in resistance arteries of human forearm vasculature. Angiology. 2000;51:545–554. doi: 10.1177/000331970005100703. [DOI] [PubMed] [Google Scholar]
- 65.Liu Z. The vascular endothelium in diabetes and its potential as a therapeutic target. Rev Endocr Metab Disord. 2013;14:1–3. doi: 10.1007/s11154-013-9238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clerk LH, et al. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiogensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293:E1804–1809. doi: 10.1152/ajpendo.00498.2007. [DOI] [PubMed] [Google Scholar]
- 67.Wallis MG, et al. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes. 2002;51:3492–3498. doi: 10.2337/diabetes.51.12.3492. [DOI] [PubMed] [Google Scholar]
- 68.Clerk LH, et al. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 69.Youd JM, et al. Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-α. Diabetes. 2000;49:1904–1909. doi: 10.2337/diabetes.49.11.1904. [DOI] [PubMed] [Google Scholar]
- 70.Wang N, et al. Losartan increases muscle insulin delivery and rescues insulin's metabolic action during lipid infusion via microvascular recruitment. Am J Physiol Endocrinol Metab. 2013;304:E538–E545. doi: 10.1152/ajpendo.00537.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim F, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan X-R, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 73.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tuomilehto J, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 75.Roque FR, et al. Exercise training and cardiometabolic diseases: Focus on the vascular system. Current Hypertension Reports. 2013;15:204–214. doi: 10.1007/s11906-013-0336-5. [DOI] [PubMed] [Google Scholar]
- 76.Newsom SA, et al. A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes Care. 2013;36:2516–2522. doi: 10.2337/dc12-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castorena CM, et al. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes. 2014;63:2297–2308. doi: 10.2337/db13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paffenbarger RS, et al. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 79.Mora S, et al. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Padilla J, et al. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology. 2011;26:132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castillo C, et al. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest. 1994;93:10–16. doi: 10.1172/JCI116932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holmäng A, et al. Interstitial muscle insulin and glucose levels in normal and insulin-resistant Zucker rats. Diabetes. 1997;46:1799–1804. doi: 10.2337/diab.46.11.1799. [DOI] [PubMed] [Google Scholar]
- 83.St-Pierre P, et al. Microvascular blood flow responses to muscle contraction are not altered by high-fat feeding in rats. Diabetes, Obesity and Metabolism. 2012;14:753–761. doi: 10.1111/j.1463-1326.2012.01598.x. [DOI] [PubMed] [Google Scholar]
- 84.Derouich M, Boutayeb A. The effect of physical exercise on the dynamics of glucose and insulin. Journal of Biomechanics. 2002;35:911–917. doi: 10.1016/s0021-9290(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 85.Niklasson M, et al. Effects of exercise on insulin distribution and action in testosterone-treated oophorectomized female rats. J Appl Physiol. 2000;88:2116–2122. doi: 10.1152/jappl.2000.88.6.2116. [DOI] [PubMed] [Google Scholar]
- 86.Wheatley CM, et al. Skeletal muscle contraction stimulates capillary recruitment and glucose uptake in insulin-resistant obese Zucker rats. Am J Physiol Endocrinol Metab. 2004;287:E804–809. doi: 10.1152/ajpendo.00077.2004. [DOI] [PubMed] [Google Scholar]
- 87.Zhang L, et al. TNF-α acutely inhibits vascular effects of physiological but not high insulin or contraction. Am J Physiol Endocrinol Metab. 2003;285:E654–660. doi: 10.1152/ajpendo.00119.2003. [DOI] [PubMed] [Google Scholar]
- 88.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 89.Ruderman NB, et al. AMPK, insulin resistance, and the metabolic syndrome. The Journal of Clinical Investigation. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iglesias MA, et al. AMP-activated protein kinase activation by AICAR increases both muscle fatty acid and glucose uptake in white muscle of insulin-resistant rats in vivo. Diabetes. 2004;53:1649–1654. doi: 10.2337/diabetes.53.7.1649. [DOI] [PubMed] [Google Scholar]
- 91.Iglesias MA, et al. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 92.Cacicedo JM, et al. Acute exercise activates AMPK and eNOS in the mouse aorta. Am J Physiol Heart Circ Physiol. 2011;301:H1255–H1265. doi: 10.1152/ajpheart.01279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Q-J, et al. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. The Journal of Physiology. 2009;587:3911–3920. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Z-P, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 95.Ido Y, et al. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 96.Kukidome D, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- 97.Ross RM, et al. Local nitric oxide synthase inhibition reduces skeletal muscle glucose uptake but not capillary blood flow during in situ muscle contraction in rats. Diabetes. 2007;56:2885–2892. doi: 10.2337/db07-0745. [DOI] [PubMed] [Google Scholar]
- 98.Bradley EA, et al. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-{beta}-D-ribofuranoside in the muscle microcirculation increases nitric oxide synthesis and microvascular perfusion. Arterioscler Thromb Vasc Biol. 2010;30:1137–1142. doi: 10.1161/ATVBAHA.110.204404. [DOI] [PubMed] [Google Scholar]