Abstract
Atrial fibrillation (AF) occurs frequently in patients with chronic obstructive pulmonary disease (COPD). Epidemiological studies have found inconsistent associations between lung function and AF, and none have studied pulmonary emphysema, which overlaps only partially with COPD in the general population. In this study, we assessed the relationship between lung function measured by spirometry, the percent of emphysema-like lung on computed tomography and incident AF. The Multi-Ethnic Study of Atherosclerosis (MESA) study is a multicenter cohort study following 6814 subjects free of clinical cardiovascular disease including AF at baseline. Spirometry was performed in a subset of 3965 participants. Percent emphysema was defined on baseline CT scans as lung regions <950 hounsfield units. Incident AF was identified from hospital discharge diagnosis and Medicare claims data. Cox proportional hazards models were used to assess independent associations of lung volumes and percent emphysema with AF. 3811 participants with valid spirometry results were included in this study. The mean age was 64.5±9.8 years and 49.4% were men. AF developed in 149 individuals (3.8%) over a mean follow-up of 4.1 years after spirometry. Lower levels of forced expiratory volume at 1 second and forced vital capacity were associated with a higher risk of AF (HR 1.21 and 1.19 per 500ml respectively; p<0.001) after adjustment of demographic and cardiovascular risk factors. Percentage emphysema was not significantly related to AF. In conclusion, in a multi-ethnic community-based sample of individuals free of cardiovascular disease at baseline, functional airflow limitation was related to a higher risk of AF.
Keywords: Atrial Fibrillation, Lung function, Emphysema
INTRODUCTION
Data from multiple epidemiological studies have identified risk factors for the development of AF including hypertension, smoking, obesity, diabetes, ischemic heart disease, congestive heart disease and valvular heart disease1–6. Atrial arrhythmias occur in increased frequency in patients with chronic obstructive pulmonary disease (COPD)7, 8. Results from previous cohort studies have shown inconsistent relationship between lung function indices and incident AF. Furthermore, the numbers of AF events were relatively small; participants were of limited age groups, ethnically homogenous, and had prevalent cardiac disease at study onset. COPD overlaps partially with emphysema, which is characterized by the destruction of alveolar walls and permanent enlargement of air spaces distal to the terminal bronchioles. In this post hoc analysis, we aimed to examine the relationship between lung function and the percentage of emphysema-like lung (hereafter referred to as percent emphysema) on computed tomography (CT) and incident AF in a multi-ethnic population sample free of cardiovascular disease at baseline.
METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter cohort study designed to investigate the prevalence, correlates and progression of subclinical cardiovascular disease in individuals without previous clinical cardiovascular disease. The details of the study design have been previously described9. The participants of MESA were 6,814 men and women age 45–84 years old who were white, African-American, Hispanic, or Chinese. Exclusion criteria included clinical cardiovascular disease, current atrial fibrillation, pregnancy, active cancer treatment, weight > 300 lbs, any cardiovascular procedure, or any serious medical condition that precluded long-term participation. The MESA-Lung Study enrolled 3,965 participants who were sampled randomly from MESA participants who consented to genetic analyses, underwent baseline measures of endothelial function, and attended an examination during MESA-Lung recruitment in 2004–200610. The protocols of all studies described herein were approved by the Institutional Review Boards of all collaborating institutions and the National Heart, Lung and Blood Institute (NHLBI) and all participants signed informed consent.
The MESA-Lung Study quantitatively assessed percent emphysema on the lung fields of all baseline MESA cardiac CT scans (2000–2002), which included approximately 70% of the lung volume from the carina to the lung bases. Percent emphysema was reported as the percentage of the total voxels in the imaged lung, as well as in the upper and lower lung, with radiodensity below 95011. Spirometry was conducted in 2004 to 2006 in accordance with the American Thoracic Society/European Respiratory Society (ATS/ERS) recommended guidelines.
For the purpose of this study, except spirometry data which was collected in 2004–2006, all covariates including CT parameters were derived from the MESA baseline exam in 2000–2002. Standard questionnaires were used to ascertain smoking. Height was measured to the nearest 0.1 cm with the subject in stocking feet. Weight was measured to the nearest pound with the subject in light clothing using a balanced scale. Resting blood pressure was measured using the Dinamap Monitor PRO 100 (Critikon, Tampa, FL) automated oscillometric device. Serum glucose, total and HDL cholesterol were measured from blood samples after 12-hour fast which were frozen at the time of processing and sent in weekly shipments for assay. The diagnosis of diabetes mellitus was based on the use of insulin or oral hypoglycemic medication or fasting glucose ≥126 mg/dl. Impaired fasting glucose was considered present if fasting glucose was between 100–125 mg/dl. Low-density lipoprotein cholesterol was calculated with the Friedewald equation. Participants were re-examined at 4 subsequent clinic visits after the baseline examination12. In addition, a telephone interviewer contacted each participant every 9 to 12 months to inquire about all interim hospital admissions and cardiovascular outpatient diagnoses. Inpatient medical records were requested for hospitalizations with cardiovascular diagnoses, including AF. In the present study we defined AF by the presence of a hospital discharge International Classification of Diseases, Ninth Revision (ICD9) diagnosis code for AF or atrial flutter (427.31 and 427.32 respectively) in any position. In addition, for those over 65 years of age and enrolled in fee-for-service Medicare, we classified AF as present if the Medicare inpatient claims data included an ICD9 code for AF or atrial flutter in any position. The date of incident AF was assumed to be the date of the earliest hospital admission with an AF or atrial flutter diagnosis.
Participant characteristics were summarized using percentage for discrete variables and means and standard deviations for continuous variables (table 1). Pearson chi square and two sample t-test p-values were obtained separately for each of these covariates. Cox proportional hazard models were used to estimate hazard ratios for the association of risk factors with time to HF with death and loss to follow-up being treated as censored. Lung function and log transformed percent emphysema were assessed in models adjusted for demographic and cardiovascular risk factors. Since lung CTs were performed earlier than spirometry examination, the follow up times used in analyses were different. The mean follow up time from lung CT examinations was 7.9 years and from spirometry measures was 4.1 years. Model 1 was adjusted for age, gender, ethnicity, height, and weight. Model 2 was further adjusted for cigarette smoking status, systolic blood pressure, diabetes status, total and high density lipoprotein cholesterol, heart rate, alcohol use, and exercise. Natural log-transformations were applied to percent emphysema measure due to skewed distribution. We also looked at risk of AF and severity of reduction in percent predicted FEV1; mild (FEV1≥80%), moderate (FEV1 50 to <80%), severe (FEV1 <50%) based on the severity classification by the Global Initiative for Chronic Obstructive Lung disease.
Table 1.
Multi-Ethnic Study of Atherosclerosis participant characteristics at baseline examination in 2000–2002 according to the presence or absence of Atrial Fibrillation first detected after spirometry was defined (n=3811). Data presented as means or percent as appropriate. Spirometry data is from 2004–2006.
| Variable | Atrial Fibrillation | ||
|---|---|---|---|
| Yes (149) | No (3662) | ||
| Age (years) | 72.6±10.0* | 64.4±8.0 | |
| Male | 62.2%* | 46.6% | |
| Race/Ethnicity | |||
| White | 56.8% * | 39.0% | |
| African-American | 20.1% | 27.5% | |
| Hispanic | 16.2% | 21.5% | |
| Asian | 6.9% | 12.0% | |
| Height (cm) | 168.2±10.0* | 166.0±9.9 | |
| Weight (lbs) | 178.3±37.8□ | 172.4±38.3 | |
| Body mass index (kg/m2) | 28.6±5.4 | 28.3±5.5 | |
| Systolic blood pressure (mm per Hg) | 127.0±21.2* | 123.1±20.6 | |
| Diastolic blood pressure (mm per Hg) | 68.5±9.9† | 70.0±10.0 | |
| Any hypertension medicine | 50.8%* | 33.7% | |
| Heart rate (beats/minute) | 61.1±10.2 | 62.9±9.2 | |
| Diabetes mellitus | 16.1% | 15.0% | |
| Impaired fasting glucose | 19.2% | 15.5% | |
| Fasting glucose (mg/dl) | 101.2±35.6 | 98.3±26.3 | |
| Total cholesterol (mg/dl) | 173.5±34.9* | 194.3±34.8 | |
| Low density lipoprotein cholesterol (mg/dl) | 99.4±28.6* | 112.0±31.8 | |
| High density lipoprotein cholesterol (mg/dl) | 49.8±16.0* | 51.8±15.0 | |
| Triglycerides (mg/dl) | 128.3±122.7 | 128.0±85.5 | |
| Any lipid lowering medicine | 19.2%* | 15.3% | |
| FEV1 (ml) | 2212.3±748.9□ | 2389.5±730.0 | |
| FEV6 (ml) | 2887.9±904.4 | 3020.2±890.3 | |
| FVC (ml) | 3099.2±925.4 | 3191.6±956.2 | |
| FEV1 percent predicted | 88.2±21.2* | 94.0±18.0 | |
| FEV6 percent predicted | 89.9±18.0* | 94.7±16.3 | |
| FVC percent predicted | 91.3±17.4* | 95.6±16.2 | |
| FEV1/FVC | 0.72±0.1* | 0.75±0.09 | |
| FEV1/FVC percent predicted | 96.7±13.6† | 98.5±10.8 | |
| FEF 2575 (ml) | 1640.4±970.0* | 2042.6±1026.2 | |
| PEF (ml) | 6455.6±2286.3* | 7004.0±2147.4 | |
| Restrictive defect | 5.7% | 3.8% | |
| Percent emphysema | 4.74±4.95 | 4.27±4.41 | |
| Ever smoker | 62.5.%* | 54.3% | |
| Pack years (in current smokers) | 32.6±34.0* | 22.9±24.2 | |
| Current alcohol use | 61.7% | 55.6% | |
| Exercise (MET-mins/week) | 1397.05±1830.78 | 1582.79±2406.92 | |
:p<0.001,
:p<0.01,
:p<0.05
FEV1: Forced expiratory volume in 1 second
FEV6: Forced expiratory volume in 6 seconds
FVC: Forced vital capacity
FEF2575: Forced expiratory flow at 25–75% of forced vital capacity
PEF: Peak expiratory flow
RESULTS
From the MESA-Lung cohort, 3811 participants with technically valid spirometry results were included in this study. The mean age of this sub-cohort was 64.5±9.8 years and 49.4% were men; 35.6% were white, 25.8% African-American, 22.2% Hispanic and 16.4% were Chinese. In the overall MESA sample, a total of 317 (4.7%) participants developed AF at 7.8 years follow up. In participants who completed spirometry measures, 149 (3.8%) developed AF at a mean follow up of 4.1 years. Demographic, cardiac risk factors and spirometry measures of the included participants are presented in table 1. Participants who developed AF were more likely to be older, male, white, heavier, have higher systolic pressure, lower diastolic pressure, higher pack-years of smoking, lower LDL and HDL cholesterol, were more likely to be ever smokers and on anti-hypertensive and lipid lowering medications. Participants who developed AF also had lower values of spirometry parameters, both absolute and percent predicted; including forced expiratory volume in 1 second (FEV1), forced expiratory volume in 6 seconds (FEV6), ratio of FEV1 to forced vital capacity, peak expiratory flow (PEF) and forced mid expiratory flow rate (FEF2575). Percent emphysematous changes were similar in those who developed AF versus those who did not.
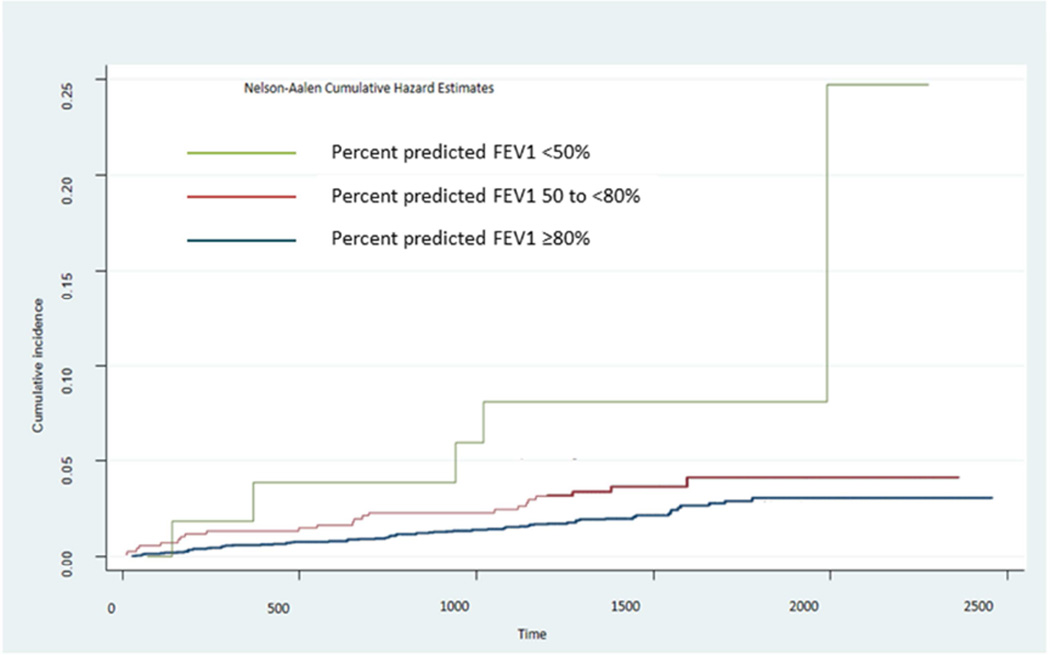
There was an inverse association between FEV1 and incident AF after adjusting for age, sex, ethnicity and body size. For every 500ml lower FEV1, the risk of developing AF increased by 25% (table 2, model 1). This association persisted and remained statistically significant after adjusting for known AF risk factors including systolic blood pressure, heart rate, cigarette smoking, diabetes, total and HDL cholesterol, exercise and alcohol use (table 2, model 2). A similar inverse relationship was seen with FVC and incident AF which was also persistent in model 2. Percent predicted values of FEV1 and FVC also showed significant relationships with AF in both models. FEV1/FVC ratio and percent emphysema were not related with incident AF in age and sex adjusted models. Mild, moderate and severe airflow obstruction showed a stepwise association with AF risk (table 3, figure 1).
Table 2.
Lung Measures and risk of incident Atrial Fibrillation in the Multi-Ethnic Study of Atherosclerosis
| Model 1 | Model 2 | |
|---|---|---|
| Variable | Hazard ratio (95% confidence interval) | |
| FEV1 per 500ml | 1.25 (1.12–1.36) | 1.21 (1.0–1.36) |
| FVC per 500ml | 1.20 (1.08–1.31) | 1.19 (1.02–1.33) |
| FEV1/FVC ratio | 1.65 (0.06–2.02) | 1.51 (0.05–4.9) |
| Percent emphysema (log) | 0.95 (0.83–1.08) | 0.96 (0.84–1.10) |
| Percent predicted FEV1 | 1.02 (1.01–1.03) | 1.01 (1.00–1.02) |
| Percent predicted FVC | 1.02 (1.01–1.03) | 1.01 (1.00–1.03) |
| Percent predicted FEV1/FVC ratio | 1.10 (1.00–1.02) | 1.01 (0.99–1.02) |
FEV1: Forced expiratory volume in 1 second, FVC: Forced vital capacity
Data are presented as decreasing value of lung volumes eg. Row 1, FEV1 per 500ml refers to 500ml lower FEV1
Model 1 adjusted for age, sex, ethnicity, height and weight.
Model 2 further adjusted for cigarette smoking status, log pack years, diabetes status, systolic blood pressure, heart rate, exercise, alcohol use, total and high density cholesterol.
TABLE 3.
Airflow limitation severity and risk of incident atrial fibrillation.
| FEV1 categories | n | Hazard Ratio (95% confidence interval) |
|---|---|---|
| ≥80% | 3034 | Reference category |
| 50 to <80% | 688 | 1.60 (1.08–2.57) |
| <50% | 58 | 4.42 (1.78–10.96) |
Model Adjusted for cigarette smoking, diabetes, log pack years, systolic blood pressure, heart rate, exercise, alcohol use, total and high density cholesterol.
Figure 1.
Cumulative incidence of atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis based on the severity classification for percent predicted forced expiratory volume in 1 second (FEV1) by the Global Initiative for Chronic Obstructive Lung disease.
DISCUSSION
We found that reduced lung function as measured by FEV1 is significantly associated with new onset of clinical AF in a multi-ethnic community-based population sample. This relationship was not attenuated after adjusting for age, sex, body size and several confounding factors. Furthermore, severity of airflow reduction showed a stepwise increment in the risk for AF; with a severe reduction carrying over 4 fold higher risk of developing AF. These findings were similar across different subgroups of age, sex and ethnicity. In contrast, mild emphysematous changes seen on lung CT were not associated with incident AF.
The present study adds to the existing body of publications evaluating the influence of lung dysfunction on the development of AF13–16. Previous epidemiological studies have yielded discordant results. Older studies such as the Framingham and Renfrew Paisley studies showed no association between FEV1 and AF1,6. In both studies; age, male sex, ECG or radiographic evidence of cardiomegaly were the strongest predictors for AF. In contrast, the Cardiovascular Health Study showed a significant inverse relation between FEV1 and AF5. More recently, results from the Copenhagen City Heart Study and The Atherosclerosis Risk in Communities were similar to those of the Cardiovascular Health Study13, 16. These conflicting results may represent temporal trends in population risk factors and/or disease burden along with increased use of anti-hypertensive medications and statins. Our results are in agreement with the more recent studies. One important distinction in older cohorts is the higher prevalence of cardiac risk factors and preexisting cardiovascular disease including valvular disease which has a different pathophysiology than non-valvular AF. The stronger associations with factors related to overt cardiac disease might obscure the relationship between lung function parameters in these cohorts.
The exact mechanism linking reduced lung function and AF is unclear, but there are numerous cardiac sequelae of low lung function. One of the prevailing hypotheses is that acidosis along with hypoxia could lead to development of abnormal ectopic beats from the pulmonic veins17. Another possibility is that common risk factors such as smoking, inflammation and atherosclerotic pathways could underlie both COPD and coronary heart disease. Reduced lung function in COPD has been shown to be related to adverse cardiac remodeling and increased prevalence of ischemic heart disease18–23. However in our analysis the relationship between FEV1 and AF was unchanged after adjusting for several cardiovascular risk factors. COPD is characterized by repeated inflammatory insults to the lungs which eventually lead to small airway remodeling involving sub-epithelial fibrosis and smooth muscle hyperplasia with resulting airflow limitation manifested as reduced FEV124. Lung inflammation that occurs in COPD is accompanied by systemic inflammation involving the heart and pulmonary vasculature25. Atrial fibrosis is a hallmark of arrhythmogenic structural remodeling and has been shown to be a common occurrence in AF26, 27. One of the novel findings of our study was that emphysematous changes in lungs were not related to AF. Tissue remodeling in COPD comprises of two main components; fibrosis which occurs predominantly in airways and extracellular matrix protein degradation mainly involving the parenchyma and leading to emphysema24,28. Although beyond the scope of this analysis, our results would appear to support the inflammation/fibrosis hypothesis in the development of AF.
The present study overcomes several limitations of prior studies. It is the first cohort to show that abnormal lung function is associated with development of AF in a population without clinical cardiovascular disease including valvular heart disease at baseline. The study results are generalizable as the participants were recruited from six geographical regions of the US with even sex distribution comprising 4 major race/ethnic groups. A major limitation of this study is AF event ascertainment; which due to its frequently asymptomatic and paroxysmal nature is especially challenging in population studies and therefore easily missed. In our analysis we only included AF events derived from ICD9 codes from hospital records. Furthermore, the Medicare claims data was available only for those who are older than 65 years and enrolled in fee-forservice Medicare. This might mean that some AF events were incorrectly classified. However, we would argue that correctly classifying more AF events would only have made our results stronger.
ACKNOWLEDGEMENTS
The Multi-Ethnic Study of Atherosclerosis is sponsored by contracts from the National Heart, Lung, and Blood Institute (N01-HC-95159 through N01-HC-95166 and N01-HC95169). This manuscript was reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications, and significant comments were incorporated before submission for publication. We thank the other investigators, staff, and participants of the MESA and MESA-Lung Studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 2.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74:236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 4.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 5.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mapel DW, Dedrick D, Davis K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991–1999. COPD. 2005;2:35–41. doi: 10.1081/copd-200050671. [DOI] [PubMed] [Google Scholar]
- 8.Barrett TW, Self WH, Jenkins CA, Storrow AB, Heavrin BS, McNaughton CD, Collins SP, Goldberger JJ. Predictors of Regional Variations in Hospitalizations Following Emergency Department Visits for Atrial Fibrillation. Am J Cardiol. 2013;112:1410–1416. doi: 10.1016/j.amjcard.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152:201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, Rodriguez J, Stukovsky K, Wong ND, Barr RG. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012–1016. doi: 10.1183/09031936.03.00051502. [DOI] [PubMed] [Google Scholar]
- 14.Kang H, Bae BS, Kim JH, Jang HS, Lee BR, Jung BC. The relationship between chronic atrial fibrillation and reduced pulmonary function in cases of preserved left ventricular systolic function. Korean Circ J. 2009;39:372–377. doi: 10.4070/kcj.2009.39.9.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata Y, Watanabe T, Osaka D, Abe S, Inoue S, Tokairin Y, Igarashi A, Yamauchi K, Kimura T, Kishi H, Aida Y, Nunomiya K, Nemoto T, Sato M, Konta T, Kawata S, Kato T, Kayama T, Kubota I. Impairment of pulmonary function is an independent risk factor for atrial fibrillation: the Takahata study. Int J Med Sci. 2011;8:514–522. doi: 10.7150/ijms.8.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ, Loehr LR, McNeill AM, Poole C, Soliman EZ, Heiss G. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129:971–980. doi: 10.1161/CIRCULATIONAHA.113.004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 18.Anderson WJ, Lipworth BJ, Rekhraj S, Struthers AD, George J. Left ventricular hypertrophy in COPD without hypoxemia: the elephant in the room? Chest. 2013;143:91–97. doi: 10.1378/chest.12-0775. [DOI] [PubMed] [Google Scholar]
- 19.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, Shahar E, Smith LJ, Watson KE. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;62:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640–2646. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD, Heiss G. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158:1171–1181. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 22.Smith BM, Kawut SM, Bluemke DA, Basner RC, Gomes AS, Hoffman E, Kalhan R, Lima JA, Liu CY, Michos ED, Prince MR, Rabbani L, Rabinowitz D, Shimbo D, Shea S, Barr RG. Pulmonary hyperinflation and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation. 2013;127:1503–1511. doi: 10.1161/CIRCULATIONAHA.113.001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 24.Salazar LM, Herrera AM. Fibrotic response of tissue remodeling in COPD. Lung. 2011;189:101–109. doi: 10.1007/s00408-011-9279-2. [DOI] [PubMed] [Google Scholar]
- 25.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of 'overspill' of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- 26.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 27.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 28.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]