Abstract
The tumor suppressor p53 is a major regulator of genes important for cell cycle arrest, senescence, apoptosis and innate immunity, and has recently been implicated in retinal aging. In this study we sought to identify the genetic networks that regulate p53 function in the retina using quantitative trait locus (QTL) analysis. First we examined age-associated changes in the activation and expression levels of p53, known p53 target proteins and markers of innate immune system activation in primary retinal pigment epithelial (RPE) cells that were harvested from young and aged human donors. We observed increased expression of p53, activated caspase-1, CDKN1A, CDKN2A (p16INK4a), TLR4, and IFNα in aged primary RPE cell lines. We used the Hamilton Eye Institute (HEI) retinal dataset (www.genenetwork.org) to identify genomic loci that modulate expression of genes in the p53 pathway in recombinant inbred BXD mouse strains using a QTL systems biology based approach. We identified a significant trans-QTL on chromosome 1 (region 172–177Mb) that regulates the expression of Cdkn1a. Many of the genes in this QTL locus are involved in innate immune responses, including Fc-receptors, interferon-inducible family genes and formin 2. Importantly, we found an age-related increase in FCGR3A and FMN2 and a decrease in IFI16 levels in RPE cultures. There is a complex multigenic innate immunity locus that controls expression of genes in the p53 pathway in the RPE, which may play an important role in modulating age-related changes in the retina.
Keywords: p53, CDKN1A, QTL, Genetic networks, Innate immunity, Retina, Aging, Age-related macular degeneration
Introduction
Organismal aging is a complex multi-step-process that is influenced by genetic and environmental factors and results in the progressive decline of cellular and tissue function. Because aging is a risk factor for many diseases including neurodegenerative diseases of the eye like age-related macular degeneration (AMD), considerable research has focused on identifying the cellular and molecular mechanisms inherent in aging. Increased susceptibility to apoptosis and cellular senescence are considered hallmarks of cellular aging along with increased genomic instability, nuclear DNA damage, shortened telomeres, and oxidative stress-induced damage (Campisi 2003). Senescence and apoptosis are thought to contribute to aging and age-related disorders by decreasing the proliferative potential of progenitor stem cells, altering tissue regenerative capacity, decreasing tissue function and by altered tissue architecture and microenvironment caused by altered gene expression and secretion of inflammatory cytokines, growth factors, and proteases (Campisi 2003; Coppe et al. 2008; Garfinkel et al. 1994; Krtolica and Campisi 2002; Kuilman et al. 2008; Novakova et al. 2010; Ohtani and Hara 2013). Increased secretion of cytokines, like interleukin (IL)-1a, IL-6, IFNβ and IFNγ, can also trigger an innate immune response via activation of toll-like receptors (TLRs) (Novakova et al. 2010; Ohtani and Hara 2013). Thus, increased apoptosis, cellular senescence, and inflammation may contribute to neurodegeneration and lead to an aging phenotype.
In the retina, the retina pigment epithelium (RPE) plays an important role in the immune defense of the retina, formation of the blood retina barrier and is also known to express several innate immune receptors, including TLRs and nod-like receptors. Age-related changes in RPE functions, inflammation and apoptosis-driven RPE dysfunction may alter retinal homeostasis and contribute to degenerative retinal diseases, like AMD (Dunaief et al. 2002; Parmeggiani et al. 2012). Aging has been shown to increase expression of genes involved in local inflammation and regulation of the immune system, including the retina (Chen et al. 2010). Altered expression of genes involved in the innate immune system, including genes involved in the alternative complement pathway like the complement factor H gene, are known to play a key role in AMD disease susceptibility and pathogenesis and in advanced stages of neovascular AMD (Whitcup et al. 2013; Zipfel et al. 2010). Secretion of inflammatory cytokines and activation of immunocompetent cells in the retina may play an important role in the loss of blood-retinal barrier function and ultimately to development of neovascular AMD (Parmeggiani et al. 2012). Inflammasome-mediated activation of caspase-1 and cleavage and secretion of IL-1β and IL-18 also appear to be important in AMD and drusen formation (Whitcup et al. 2013). A better understanding of the molecular mechanisms that activate the p53 network (senescence, apoptosis) and the innate immune system (inflammation, para-inflammation) during aging may lead to a better understanding of aging and to better treatment approaches to complex age-related diseases like AMD.
The p53 transcription factor is a master regulator of cell cycle arrest and an initiator of apoptosis and thus plays a major role in the regulation of cellular lifespan. The p53 protein is a sequence-specific transcription factor that regulates the expression of many genes, including its own function (through MDM2, MDM4), cell cycle arrest and DNA repair (CDKN1A [p21CIP1], CDK2, GADD45, TRIM22), senescence (PML, PAI-1), apoptosis (BAX, PUMA, NOXA, survivin), growth factors (IGFBP-3, PTEN), translation (Sestrin), autophagy (Sirtuin1), and innate immune receptors (TLRs) (Barsotti et al. 2012; Hasty and Christy 2013; Kitagawa et al. 2013; Klettner 2012; Purvis et al. 2012; Suzuki et al. 2009; Valente et al. 2013; Valente and Strasser 2013; Vuong et al. 2012). Activation of p53 can also directly initiate apoptosis via down regulation of anti-apoptotic BCL2 family proteins, including BCL2, BCLXL, and induction of mitochondrial outer membrane permeabilization. Importantly, p53 regulates innate immune mediated processes, including increased expression of TLRs, cytokines and chemokines, enhancement and stimulation of IFN signaling, IFN regulatory factor-9, and regulates expression of IFN stimulated genes and IFI family genes, (Gugliesi et al. 2005; Kwak et al. 2003; Menendez et al. 2013, 2011; Munoz-Fontela et al. 2008; Shatz et al. 2012). Aging in the RPE is associated with increased p53 levels and increased p53-mediated apoptosis (Bhattacharya et al. 2012). Thus p53 may play an important role in regulating aging in the RPE and in development of AMD.
Recombinant inbred (RI) strains of mice are a useful tool to study the genetic and molecular networks that contribute to a phenotype, disease or process, like aging in the eye (Geisert et al. 2009). The BXD family of RI mice are the largest panel of RI mice that were generated from a cross between C57BL/6J and DBA/2J mice. The tissue transcriptome profiles from the BXD family were used to generate the Hamilton eye institute (HEI) database and the data can be easily interrogated using the interactive GeneNetwork website (www.genenetwork.org) to identify the genetic networks that regulate phenotypes or diseases (Freeman et al. 2011; Jablonski et al. 2011; Templeton et al. 2013a, 2013b; Whitney et al. 2011). The purpose of this study was to use this systems biology approach to identify genomic loci that regulate the expression of the p53 pathway genes in the retina, and thus may play an important role in the aging retina and in susceptibility to AMD.
Materials and Methods
Interrogation of the HEI Retinal Dataset
In this study we used the HEI retina database to define the genetic loci that regulates expression of the p53 pathway (Trp53, Tlr4, Cdkn2b, Cdkn1A, Ifna2 and Casp1) in the retina. This dataset has been described previously and a description is also available in the GeneNetwork website (Freeman et al. 2011; Geisert et al. 2009). This database contains the eye and retina transcriptome profiles from young (7–16 wks old) mice from 75 BXD strains and 5 control strains (C57BL/6, DBA/2J, both reciprocal F1s and BALB/cByJ). The HEI Retina dataset is comprised primarily of retinal tissue but robust signals for RPE-specific transcripts, including RPE65 and Bestrophin, indicate the presence of RPE in the samples.
When possible we selected ProbeSets within the coding region for each gene (ProbeSets used: 2585183, 2684234, 1227240, 2634083, 1233138 and 1247592). QTL mapping was performed using the WebQTL module on GeneNetwork using simple whole genome analysis without bootstraps or permutations to define QTLs that modulate gene expression levels related to senescence and inflammation. The cis-acting QTLs are located on the same genetic locus as the gene, while the trans-acting QTLs are genetic loci that modulate gene expression in a different location in the genome. This analysis produces a likelihood ratio statistic (LRS) score that indicated the confidence of linkage between the genomic loci and the p53 pathway of interest. A LRS score of over 15 is considered significant.
Primary RPE cell culture
Primary human RPE cells were isolated from postmortem de-identified donor eyes provided by the Midsouth Eye Bank. The Institutional Review Board at the University of Tennessee Health Science Center approved the use of human donor eyes. We used RPE cell lines that were derived from two young donors (age 29 or 40) and from two aged donors (ages 84 and 86) to study the effects of aging on RPE physiology. Inclusion criteria for aged donor eyes included the absence of diagnostic criteria for AMD from the postmortem metadata profile. Exclusion criteria involved known retina dystrophies, diabetic retinopathy, uveitis, trauma, or other proliferative retinal diseases. RPE cells were isolated using procedures described previously (Chaum 2001). Briefly, globes were excised, anterior segment removed, vitreous extracted, and the retina was dissected free. The eyecup was washed with Dulbecco’s Modified Eagles Medium (DMEM) followed by 0.25% trypsin/EDTA for four 15-min digestion cycles. Cells were loosened, transferred to DMEM with FBS, centrifuged at 2,000g for 5mins and the pellet was re-suspended in 1media with 5% Fetal Bovine Serum (FBS) and placed on poly-L-lysine coated 12-well culture ware. The fastest growing cells with cobblestone morphology were used for our studies. Primary cultures were maintained in DMEM and Ham’s F12 medium (1:1 ratio) containing L-glutamine and 10% FBS.
Western blot analysis
The protocol for western blots has been described earlier (Bhattacharya et al. 2009, 2007, 2003). Cell lysates were prepared using mammalian protein extraction buffer (Pierce, Rockford, IL) with 150mM NaCl, 1mM Na2EDTA and a protease inhibitor cocktail followed by SDS-PAGE. Proteins were transferred to Immobilon-P membranes (Millipore Bedford, MA, USA) and probed with primary antibodies against p53, CDKN1A, CDKN2A, TLR4, Caspase1, Actin, FMN2, FCGR3A (Novus Biologicals, San Diego, CA), IFI16 and IFNA2 (Cell Signaling, Beverly, MA) overnight at 4° C in TBS buffer containing 0.1% Tween-20 and 5% nonfat dry milk (Bio-Rad, Hercules, CA), as per manufacturers recommendations. Membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1h and the immunocomplexes were visualized by the ECL detection system (Perkin Elmer, Waltham, MA). Representative Western blots from three experiments are shown. Densitometric analysis of all Western blots was performed using Image J software (National Institutes of Health). All data are expressed as mean ± SE. ANOVA and appropriate post hoc test determined the significance of the differences between means from each group. Values of P<0.05 were considered significant.
RNA isolation and qRT-PCR
Human RPE cell lines from young and aged donor eyes were grown to confluence, RNA was isolated using TRIreagent (Sigma Aldrich) and 1µg of total RNA was reverse transcribed to cDNA (Promega) using random hexamer primers as recommended by the manufacturer. Real-time qPCR was performed using an ABI Prism® 7700 sequence detection system (Applied Biosystems) and 120nM each primer. Each reaction was performed in triplicate from a minimum of 12 samples. Cycle threshold values for each gene are normalized to GAPDH expression in each sample and to expression in young HPE samples using the delta-Ct method. All data are expressed as the mean ± SE. Statistically significant differences were determined using a two-tailed students T-test. Values of P<0.05 were considered significant. Primers used were specific for FCER1γ forward 5`-TGA AGA TCC AAG TGC GAA AG-3`, reverse 5`-GCA TCT ATT CTA AAG CTA CTG TGG-3`; IFI16 forward 5`-GCC AGC GTA ACT CCT AAA ATC-3`, reverse 5`-CCA CTT CCA TCT TCC CTG TA-3`; FMN2 forward 5`-CAG AGA AGT TTT GCT CCC G-3`, reverse 5`-GCA GCC CAG GTA TAA AGT TG-3`; and GAPDH forward 5`-TTC GAC AGT CAG CCG CAT CTT CTT-3`, reverse 5`-ACC AAA TCC GTT GAC TCC GAC CTT-3`.
Results
Aging Activates p53 Signaling and Inflammatory Pathways in Human RPE Cells
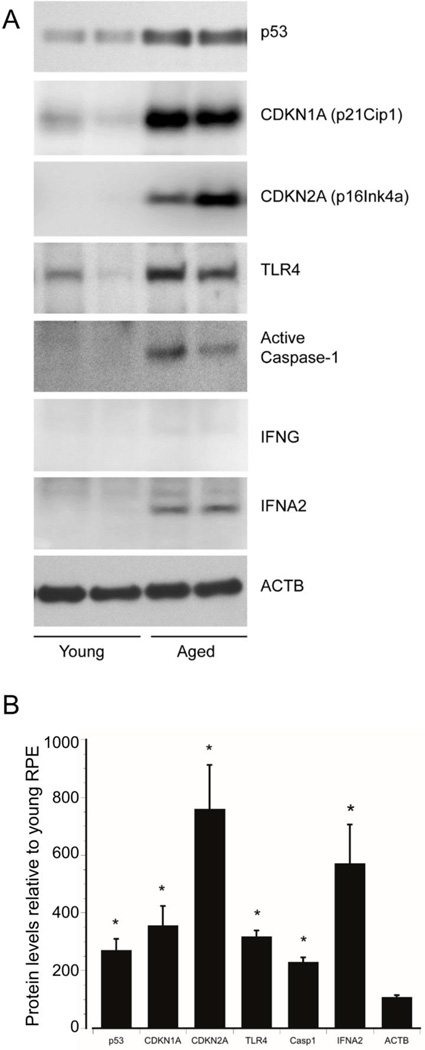
We have previously shown that aging in human RPE cells activates p53-mediated apoptosis through increased level and post-translational modification of p53, increased levels of the pro-apoptotic marker PUMA, activation of caspase-3, increased levels of CDKN1A, a known transcriptional target of p53, and reduced levels of antiapoptotic BCL2, all of which lead to an overall increase in apoptosis (Bhattacharya et al. 2012, 2011). To investigate age-related changes in p53-mediated senescence and inflammation pathways, we measured the levels of p53 and its target proteins in primary RPE cultures from young and aged donors. Consistent with our previous observations, we found that basal levels of p53 were low in RPE cultures from young donors but were significantly increased in RPE from aged donors (Figure 1). To determine if p53 target proteins were also modulated in aged RPE cells, we measured the protein levels of CDKN1A. We observed increased levels of CDKN1A in the aged RPE compared to young RPE (Figure 1). We also examined expression of CDKN2A, which is a biomarker of senescence that is complementary to but independent of p53 activity. Consistent with activation of senescent pathways, aging in the RPE increases expression of CDKN2A (Figure 1).
Figure 1.
Aging activates the p53 pathway in RPE cells. A) Primary cultures of RPE cells obtained from two young and two aged (29, 40, and 84, 86 years, respectively) human donor eyes and were grown to confluence. RPE cell lysates were analyzed by western blot for total-p53, CDKN1A, CDKN2A, TLR4, active caspase-1, IFNG, and IFNA2 using specific antibodies. Beta actin was used as an internal loading control. B) Densitometric values from young RPE cells were set at 100%. Data shows the mean +/− SE. *, significantly different compared with young RPE cells (P<0.05). N=3.
Activation p53 can regulate tissue inflammation including modulation of cellular behavior in response to stressors (Vousden and Prives 2009). Since aging robustly increased p53 levels, we asked if components of the innate immune system that are known to be regulated by p53 (Gupta et al. 2001) were also upregulated in the aged RPE. We found that aging of RPE increases expression of the pro-inflammatory caspase-1 and of TLR4 and its downstream target IFNA2 (IFNα) but not IFNG (Figure 1), suggesting an activation of type I interferon responses. Thus, aging in the RPE is associated with both increased activation of p53 and increased expression of downstream targets that regulate innate immunity and senescence.
Identification of a QTL on Chr1 that Modulates the p53 Pathway in the Retina
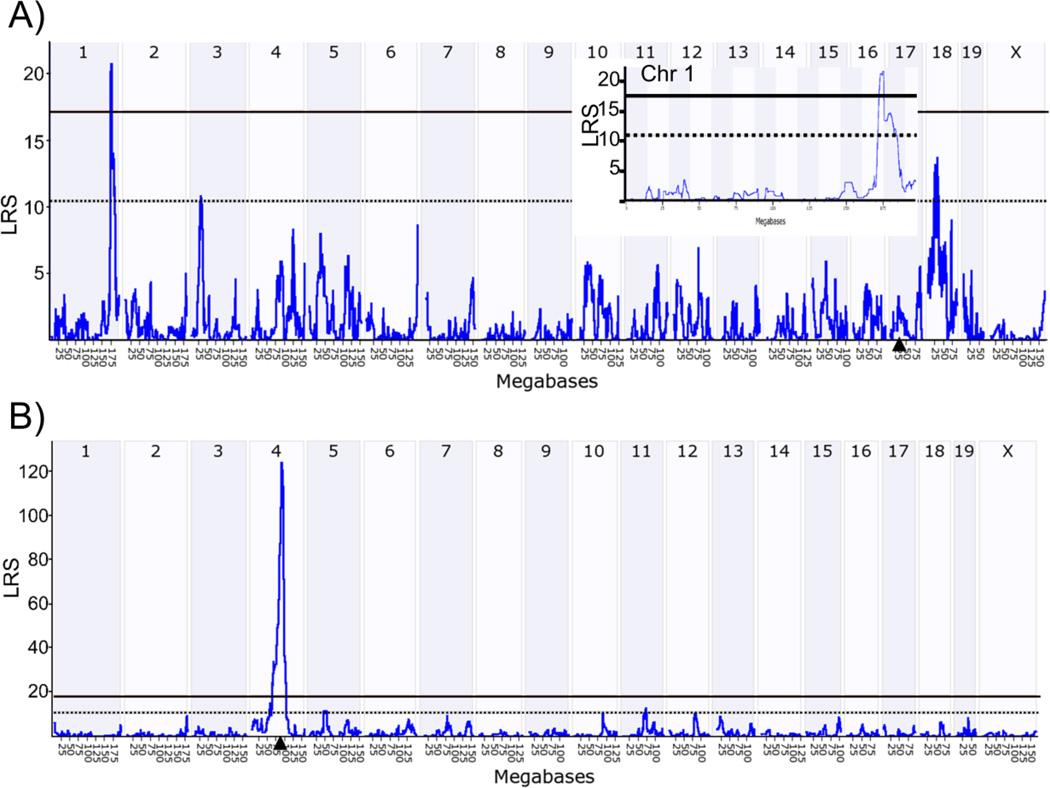
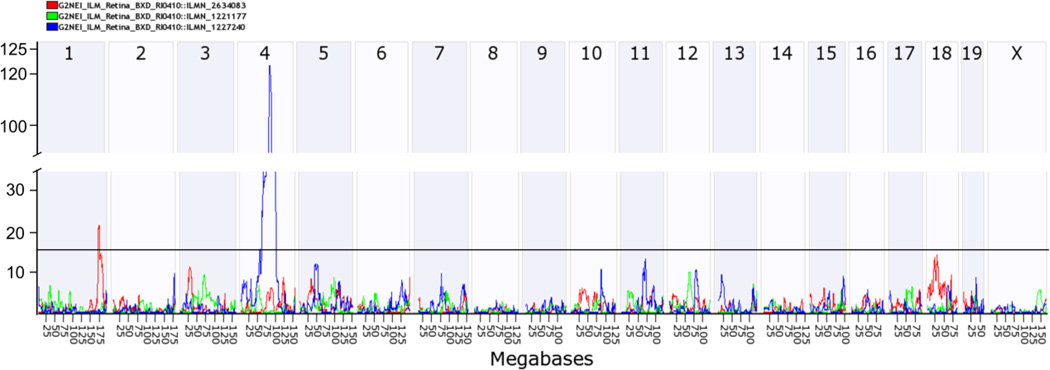
Because increased activation of the p53 pathway appears to play a role in aging in the RPE, we sought to identify genomic loci that regulate p53-induced innate immunity in the retina. The HEI retinal database contains retinal and RPE samples, the latter of which is confirmed by the presence of robust signals for RPE specific transcripts (RPE65 and Bestrophin). We used the HEI retina database and the interactive website GeneNetwork to determine the genetic sources of variation in the expression of the p53-mediated innate immunity genes (Trp53, Tlr4, IFNA2, Cdkn2b, Cdkn2a, Cdkn1a, and Casp1) and to identify genomic regions that control expression of these p53 pathway genes. Of these transcripts, there is sufficient variability in expression of Cdkn2b, Cdkn1a, and Casp1 (fold change 2–2.7) in the BXD mice (Figure S1). We used the QTL mapping tool to identify genomic regions that control expression of the genes in the p53 pathway using a likelihood ratio statistic (LRS), which indicates the confidence of linkage between the QTL and the gene of interest. The regulatory loci can either be a trans-QTL (located at a different genomic locus from the gene), or a cis-QTL (located at the same locus as the gene of interest). We identified a significant trans-QTL for Cdkn1a on Chr 1 (172–177MB, LRS of 21), a cis-QTL for Cdkn2b on Chr 4 (75–100MB, at the Cdkn2b locus, LRS 124), and trans-QTLs for Casp1 on Chr 4 (125–150MB) and on Chr 15 (50–75MB) (Figure 2 A, B). The trans-QTL on Chr 1 has been previously identified as a QTL “hotspot” that is referred to as Qrr1 and exhibits functional and genetic similarities with the orthologous region of human Chr 1q21-q23. (Vogel et al., 2013, 2012). No significant QTLs were identified for the other genes in the p53 pathway. Combined QTL analysis of Cdkn1a, Cdkn2a, and Cdkn2b identify the cis-QTL on Chr 4 and the Qrr1 “hotspot” (Figure 3). Thus we conclude that genes in the Qrr1 locus control expression of Cdkn1a in the mouse retina. Candidate genes in this Qrr1 region may regulate expression and activation of p53, apoptosis and innate immunity, include; FC receptor IgG low affinity (Fcgr) 2b, 3 and 4, FC receptor IgE high affinity γ (Fcer1γ), the interferon activated (Ifi) gene family (Ifi202b, Ifi203-205, Aim2) and Formin 2 (Fmn2) (Table 1). Several candidate genes in the Qrr1 region have variable expression in the retina of BXD mice, including Fcer1γ, Fcgr3, FMN2, and Ifi205 (Figure S2), thus making them strong candidate genes in this region.
Figure 2.
Identification of genomic networks that regulate expression of Cdkn1a, Cdkn2a or Cdkn2b. The data illustrates the likelihood ratio statistic (LRS) scores for expression of (A) Cdkn1a (B) Cdkn2b in the retina of BXD RI mice. QTLs with significant LRS scores are indicated by a horizontal solid black line and regions with suggestive LRS scores are indicated by a dashed black line (~17.2, and ~10.5, respectively). Arrowhead indicates the query gene locus. Insert (A) shows the trans-QTL on Chr 1 (Qrr1).
Figure 3.
Graphic representation of genomic networks that regulate the expression of p53 pathway in the retina. The data illustrates the likelihood ratio statistic (LRS) scores for the combined expression of the Cdkn1a, Cdkn2a and Cdkn2b in the retina of BXD RI mice. A significant cis-QTL peak on mouse chromosome 4 (LRS=124) at the location of the Cdkn2b gene and a trans-QTL on chromosome 1 (LRS= 21) is seen. Horizontal line marks the threshold for significance (LRS=15).
Table1.
Gene-interval analysis of the QTL on chromosome 1 reveals candidate genes involved in innate immunity
| Gene | Description | Mouse Chr |
Mouse Gene ID |
SNP | LRS | Exp. level |
Range (fold) |
Human name |
Human Chr |
Human Gene ID |
|---|---|---|---|---|---|---|---|---|---|---|
| Fcgr2b | Fc receptor, IgG, low affinity IIb | 1, 78.02 | 14130 | 43 | 20.5 | 6.84 | 1.43 | FCGR2B | 1q23 | 2213 |
| Fcgr4 | Fc receptor, IgG, low affinity IV | 1, 78.53 | 246256 | 24 | 20.0 | 7.15 | 2.54 |
FCGR3A FCGR3B |
1q23 | 2214 2215 |
| Fcgr3 | Fc receptor, IgG, low affinity III | 1, 78.80 | 14131 | 59 | 20.0 | 7.31 | 2.44 | FCGR2A | 1q23 | 2212 |
| Fce1γ | Fc receptor, IgE, High affinity gamma | 1, 79.23 | 14127 | 13 | 20.5 | 7.64 | 6.28 | FCER1G | 1q23 | 2207 |
| Aim2 | Absent in melanoma 2 | 1, 80.33 | 383619 | 103 | 19.0 | 6.85 | 1.5 | AIM2 | 1q22 | 9447 |
| Ifi204 | Interferon activated gene 204 | 1, 80.63 | 15951 | 15 | 13.0 | 6.58 | 1.51 | IFI16 | 1q22 | 3428 |
| Ifi203 | Interferon activated gene 203 | 1, 80.76 | 15950 | 31 | 13.0 | 6.67 | 1.3 | IFI16 | 1q22 | 3428 |
| Ifi202b | Interferon activated gene 202B | 1, 80.79 | 26388 | 76 | 13.0 | 6.83 | 1.91 | IFI16 | 1q22 | 3428 |
| Ifi205 | Interferon activated gene 205 | 1, 80.83 | 226695 | 114 | 13.0 | 6.68 | 2.19 | IFI16 | 1q22 | 3428 |
| Fmn2 | Formin 2 | 1, 81.04 | 54418 | 1290 | 13.0 | 9.18 | 2.91 | FMN2 | 1q43 | 56776 |
Exp level, average retinal expression across all BXD mouse strains. LRS, likelihood ratio statistic score
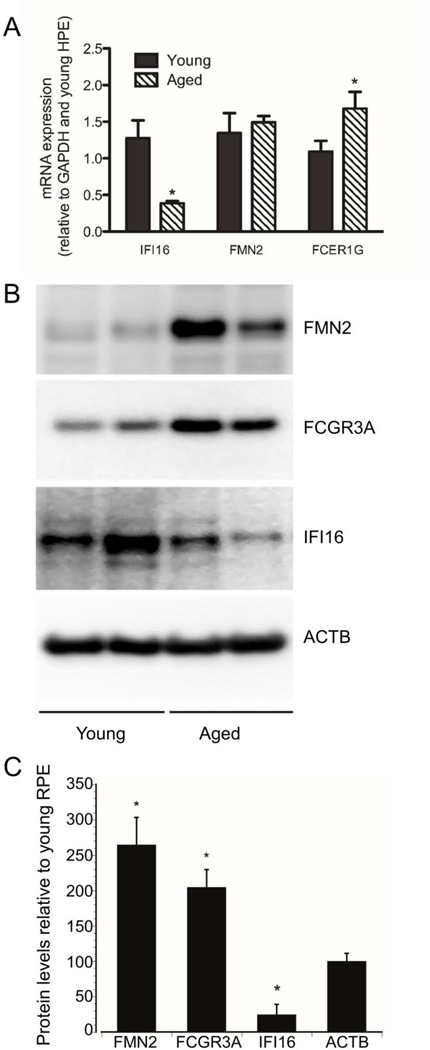
To determine if the levels of candidate genes in the Qrr1 locus were altered in aging, we analyzed mRNA expression using qRT-PCR and performed Western blot analysis of lysates from young and aged human RPE cultures. We found that aging significantly increases FCER1γ mRNA expression, increases FCGR3A and FMN2 levels, and decreases IFI16 (mouse IIF202b, IFI203-205) mRNA and protein levels (Figure 4 A–C). Interestingly, increased FMN2 protein levels did not correlate with increased mRNA expression and may indicate that aging alters FMN2 protein stability or degradation. Importantly, these genes regulate both innate immune system function and expression of p53 (Figure 5). This suggests that the Qrr1 locus may play an important role in regulating age-related expression of Cdkn1a in the RPE, possibly through activation of p53 and local inflammatory pathways.
Figure 4.
Aging alters expression of genes in the Qrr1 locus in RPE cells. A) mRNA expression from primary cultures of young or aged RPE cells were analyzed by qRT-PCR for expression IFI16, FMN2, FCER1γ and GAPDH. Cycle threshold values for each transcript were normalized to GAPDH and young RPE samples using the delta-Ct method. N=12. *, denotes significantly different values (P<0.05). B). Cell lysates from primary cultures of young or aged RPE cells were analyzed by Western blot for expression of IFI16, FMN2, and FCGR3A. Beta actin was used as an internal loading control. C) Densitometry analysis of Western blot bands. Values from young RPE cells were set at 100%. Data shows the mean +/− SE. *, significantly different compared with young RPE cells (P<0.05). N=3
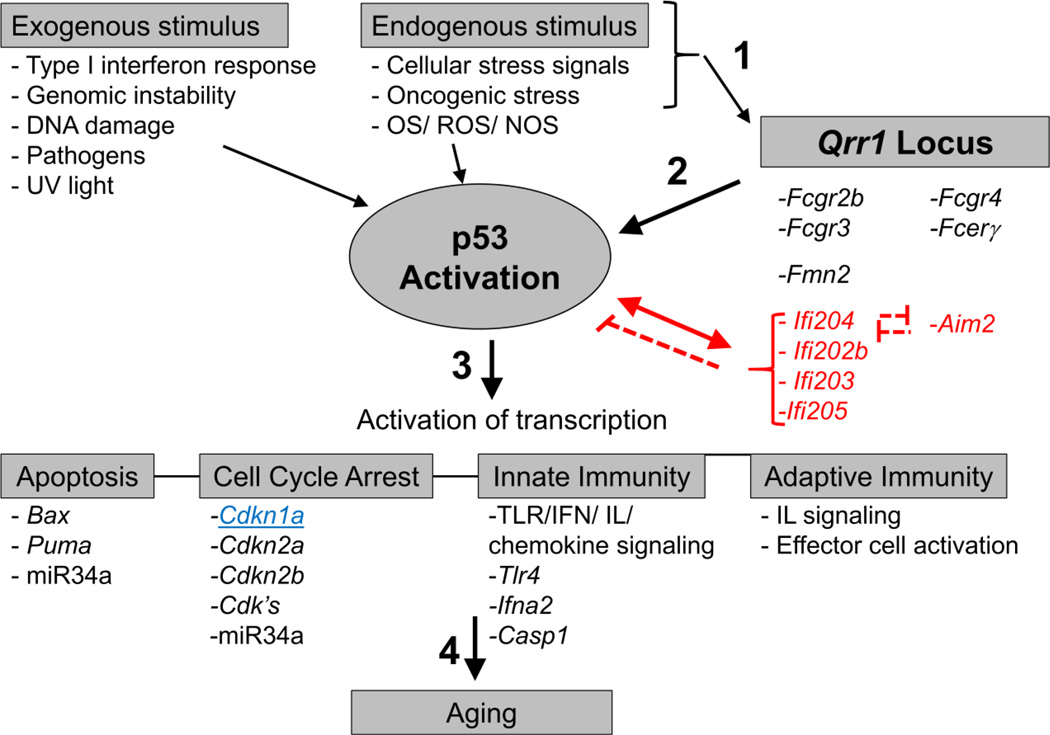
Figure 5.
Schematic showing Qrr1 activation of Cdkn1a and p53 pathways that are important for immunity and aging. 1) Various exogenous and endogenous stimuli, like TLR4 and IFN signaling, DNA damage, oxidative stress and foreign dsDNA, activates proteins in Qrr1 locus and p53. 2) Qrr1 proteins (IFI family) can also increase or decrease p53 transcriptional activity. Importantly, p53 triggers expression of some of innate immunity genes in the Qrr1 locus on chromosome 1. 3) Once activated, p53 initiates expression of genes that play an important role in apoptosis, cell cycle arrest, and innate and adaptive immunity. 4) Misregulation of these pathways are thought to be important for aging and AMD. Cdkn1a, which was used for the QTL analysis, is in blue and underlined. Genes in red indicate a possible negative regulator of p53 activity.
Discussion
Here we demonstrate that aging in the RPE is associated with a significant age-related increase in expression of CDKN1A, CDKN2A, TLR4, IFNA2, activated caspase-1, FMN2 FCGR3A and FCER1γ and a decrease in IFI16 levels (Figure 1, 4). We identified a genomic region on Chr 1 (Qrr1) that controls expression of Cdkn1a in the mouse retina. This Qrr1 hotspot on mouse Chr 1 exhibits functional and genetic similarities with the orthologous region of human Chr1q21-q23. The candidate genes in Qrr1 (Fcgr2b, Fcgr3-4, Fcer1γ, Ifi202b, Ifi203-205, Aim2 and Fmn2) play an important role in innate immune responses and may play a role in aging in the retina. We hypothesize that during aging, increased exposure to harmful environmental factors activate p53 and genes in the Qrr1 locus, which in turn stimulate cell cycle arrest, senescence, innate immunity, and activate the complement cascade, TLR-4, and caspase-1-dependent pro-inflammatory pathways, resulting in abnormal secretion of inflammatory factors including interferons and cytokines (Figure 5). Thus, the Qrr1 locus strongly links multiple genetic loci implicated in senescence, innate immunity, and acquired immunity responses with p53 signaling.
We have previously shown that age is associated with increased expression and post-translational modifications of p53 that leads to disruption of Mdm2 interactions and initiate activation of p53-dependent apoptosis and cell cycle arrest in human RPE cells (Bhattacharya et al., 2012). In this study we identified a genetic network (Qrr1) that regulates CDKN1A expression in the mouse retina and human RPE, thus indicating that Qrr1 may be an important regulator of p53-dependent senescence in the mouse retina and in the aged human RPE. In addition we have demonstrated that aging up-regulates expression of several proteins involved in senescence and inflammation in the human RPE. Together our data indicates the involvement of a genetic mechanism between p53 and several components of the innate immune signaling. The precise molecular mechanisms directing p53, senescence, and pro-inflammatory signaling in response to aging in the RPE are currently unknown and warrant further investigation.
Activation of inflammation and the innate immune system play a key role in aging and age-related disorders, like AMD. As a result of normal retinal physiology and light exposure, the retina is subjected to high levels of oxidative stress, which can activate local innate immunity response (“para-inflammation”) in an effort to restore normal retinal homeostasis (Xu et al. 2009). In the aging retina, RPE cells have increased senescence, increased secretion of chemokines and cytokines, and are exposed to low levels of chronic para-inflammation, all of which may contribute to breakdown of the blood-retinal barrier (Xu et al. 2009). Immune dysfunction is thought to play a key role in the pathogenesis of AMD due to the association of polymorphisms in the complement factor H gene with AMD and due to the presence of elevated retinal- and astrocyte-specific autoantibodies in the serum and retina of AMD patients, which increase with advanced stages of AMD (Morohoshi et al. 2012a, 2012b; Mullins et al. 2001; Patel et al. 2005; Penfold et al. 1990). Activation of the pro-inflammatory complement component 4b, complement factor H and caspase-1, are also found in the aged RPE/choroidal tissue (Cao et al. 2013; Chen et al. 2008). Our observations of increased expression p53, TLR4, IFNA2 and activated caspase-1 in aged RPE cells suggest that this innate immune signaling pathway may also be important during aging in the RPE.
Appropriate regulation of cell cycle progression and senescence is important for the immune system function in aging. CDKN1A is a transcriptional target of p53 but also has p53 independent functions. CDKN1A and p53 are regulators of cell cycle progression, apoptosis, cell survival, differentiation and senescence. IFNγ signaling has been shown to induce cell cycle arrest in part through activation of CDKN1A expression (Chen et al. 2000; Hobeika et al. 1999; Napione et al. 2012; Shen et al. 2008; Xaus et al. 1999). TLR2 can inhibit cancer progression by activating innate immunity signaling cascades that trigger CDKN1A and CDKN2A mediated senescence (Lin et al. 2013). Thus, CDKN1A is an important regulator of innate immune responses.
Our data show that Cdkn1a expression is controlled by the Qrr1 locus in the mouse retina. The Qrr1 locus contains several immunomodulatory genes and has been shown to contain QTLs that regulate obesity, behavior, metabolism, among others, and is syntenic to human chromosome 1q21-23 (Allen et al. 1999; Choubey 2012; Haywood et al. 2000; Hogarth et al. 1998; Kikuchi et al. 2005; Moser et al. 1998; Mozhui et al. 2008; Rozzo et al. 2001; Santiago-Raber et al. 2009; Vogel et al. 2013, 2012; Vyse et al. 1997; Wakeland et al. 2001). Candidate genes in Qrr1 that may modulate p53 activity and Cdkn1a expression include the FC-IgG receptor, low affinity (FCGR) family (Fcgr2b, Fcgr4, Fcgr3), Fc receptor IgE, high affinity receptor 1 γ (Fcer1g), interferon activated gene family (Ifi204, Ifi203, Ifi202b, Ifi205, Aim2), and Formin 2 (Fmn2). These genes are important regulators of the immune system, and we have found that aging in the RPE up-regulates expression of FCRG3A FCER1γ, and FMN2 and down-regulates IFI16 expression (Figure 4).
FCGRs play a role in the regulation of activation of the complement pathway and the immune system in aging. Activation of FCGRs can trigger phagocytosis, expression and secretion of inflammatory cytokines and chemokines, initiation of cell cycle arrest and apoptosis (Gessner et al. 1998; Karsten and Kohl 2012; Murinello et al. 2014; Nimmerjahn and Ravetch 2008; Rittirsch et al. 2009; Schmidt and Gessner 2005; Syed et al. 2009). A linkage between FCGRs and TLR4 has been reported both in vitro and in vivo (Rittirsch et al. 2009), and cross-talk between complement C5a and FCGR signaling pathways generates cytokines and chemokines and modulates FCGR expression (Karsten and Kohl 2012; Kumar et al. 2006; Schmidt and Gessner 2005; Skokowa et al. 2005; Syed et al. 2009). A possible role for FCGRs in AMD was indicated by the observations that their expression is increased in the aged mouse retina and they are present in immune complexes found in the retinas from wet AMD patients (Chen et al. 2010; Murinello et al. 2014). The Fc high affinity IgE gamma (Fcerγ) receptor, also a candidate gene in Qrr1, is a regulator of immune response to allergens and may inhibit activation of immune receptors, like TLRs (Novak et al. 2010). Based on our observations that FCGR3A and IFNA2 levels are increased during aging and because cross-talk between FCGR3 and TLR4 is known, it is possible that increased FCGR3 levels in aged RPE cells increase innate immune responses through activation of TLR4 signaling.
The family of IFN-inducible (IFI) -200 gene family encode structurally related proteins, whose expression is activated by interferons and includes mouse Ifi202b, Ifi203, Ifi204 and absent in melanoma 2 (Aim2) and human IFI16 and AIM2. Expression of IFI16 increases with age and can be activated by p53 (Song et al. 2008; Xin et al. 2004). Depending on the cellular context and its intracellular localization, IFI16 can activate or repress p53 transcriptional activity, activate or repress senescence, increase expression of Cdkn1a, activate cell cycle arrest, regulate autophagy, activate IFNβ, increase expression of interferon stimulated genes, and regulate activation of caspase 1 by AIM2 and NLRP3 inflammasoms and secretion of pro-inflammatory cytokines, chemokines and adhesion molecules (Baggetta et al. 2010; Costa et al. 2011; Datta et al. 1996; Duan et al. 2011; Fujiuchi et al. 2004; Gugliesi et al. 2005; Johnstone et al. 1998; Kwak et al. 2003; Ouchi and Ouchi 2008; Shi et al. 2014; Veeranki et al., 2011; Xin et al. 2004). IFI16 has anti-inflammatory properties through its negative regulation of AIM2-mediated inflammasome formation, and subsequent inhibition of caspase1 and IL1β activation (Veeranki et al. 2011). Our observation of decreased IFI16 levels in aged RPE cells may lead to increased AIM2 levels, AIM2-mediated inflammasome activity, increased activation of caspase-1 and increased levels of activated inflammatory cytokines, like IL-1β. Thus, Aim2 and IFI16 are also candidate genes in the Qrr1 locus.
Fmn2 expression can be activated by conditions that activate p53 including, DNA damage, oncogenic stress and hypoxia, but can also be activated by p53-independent mechanisms involving CDKN2A [p14Arf] and NFκB (Faix and Grosse 2006; Yamada et al. 2013a, 2013b). Intriguingly, FMN2 is required to stabilize CDKN1A levels, by inhibiting its degradation. Given that p53 activation correlates with CDKN1A and FMN2 levels in our aged RPE cells, it is tempting to speculate that FMN2 prevents CDKN1A degradation during aging in the RPE and thereby permits CDKN1A to accumulate to a level where it can promote retinal senescence and activate innate immune responses. Here we show that aging is associated with increased FMN2 protein levels, but not with increased FMN2 mRNA expression. Thus aging may increase FMN2 levels through increased stability of FMN2, possibly through its association with CDKN1A, or through post-translational modifications. Recent analyses indicate that protein levels correlate with the corresponding mRNA by only 20–40% of the time (Tian et al., 2004; Nei et al., 2006). For example, we have shown previously that age-related post-translational modifications of p53 lead to increased stability and accumulation of the protein (Bhattacharya et al., 2012). Therefore, increased protein levels may not always reflect corresponding changes in mRNA expression, and suggest that alterations in protein stability can significantly modulate protein levels.
There are other potential candidate genes in Qrr1 that are involved in adaptive immunity, including signaling lymphocytic activation molecule (SLAM) family genes, ubiquitin-fold modifier conjugating enzyme 1, transformation related protein 53 binding protein 2, toll-like receptor 5, and C-reactive protein. These proteins regulate cytokine production (Th2/ IL4), inflammatory signaling through the NFκB pathway, activate innate immune responses against bacterial infection, activate apoptosis, activate T cells, play an important role in unfolded protein response pathway/ ER stress response pathway and activate the complement cascade (Cannons et al. 2011; Hayashi et al. 2001; Hertel et al. 2013; Komatsu et al. 2004; Ma and Deenick 2011; Means et al. 2003; Schwartzberg et al. 2009; Tordella et al. 2013; Wilson et al. 2013). All of these transcripts may play a role in p53-mediated inflammation during aging and AMD pathogenesis.
Additional studies are needed to demonstrate that Qrr1 regulation of Cdkn1a requires activation of p53, to identify which specific genes in Qrr1 regulate Cdkn1a expression, and to show that Qrr1 modulates aging in the retina. We were not able to identify QTLs for some of the genes in the p53 pathway (Trp53, Cdkn2b and Tlr4) due to the absence of sufficient variation in the expression of these genes across the BXD strains. While the BXD mice used to generate the HEI retinal database were not aged, the expression of the p53 pathway and Qrr1 candidate genes (FCGR3, IFI16, AIM2 and FMN2) suggest a role in the aging RPE and warrant further investigation.
Summary
We have identified a unique genetic locus on mouse Chr 1 (Qrr1) that modulates expression of Cdkn1a, the p53 pathway, and the innate immune system in the RPE. P53 and CDKN1A are important regulators of the cell cycle, apoptosis, and innate immunity, and our data show that the p53 pathway and candidate genes in Qrr1 are modulated in the aged RPE. Several of the genes that map to this region are involved in innate immune responses and, due to the role of p53 in regulation of the innate immune system, are considered among the candidate genes involved in the aging retinal phenotype. We hypothesize that the Qrr1 hotspot plays a role in modulating aging in the RPE through p53-mediated regulation of innate, and possibly acquired, immune responses. Together these genes may act to promote innate immunity, inflammation, and senescence in the aging RPE.
Supplementary Material
Acknowledgements
These studies were supported in part by an unrestricted UTHSC departmental grant from Research to Prevent Blindness, New York, NY, the Plough Foundation, Memphis, TN, The Lions of Arkansas Foundation Inc., and the UTHSC Hamilton Eye Institute NEI Core Grant for Vision Research (P30 EY013080). The authors would also like to thank Dr. Rob Williams for his comments and review of the manuscript.
References
- Allen RD, Dobkins JA, Harper JM, Slayback DL. Genetics of graft-versus-host disease, I. A locus on chromosome 1 influences development of acute graft-versus-host disease in a major histocompatibility complex mismatched murine model. Immunology. 1999;96:254–261. doi: 10.1046/j.1365-2567.1999.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggetta R, De Andrea M, Gariano GR, Mondini M, Ritta M, Caposio P, Cappello P, Giovarelli M, Gariglio M, Landolfo S. The interferon-inducible gene IFI16 secretome of endothelial cells drives the early steps of the inflammatory response. Eur. J. Immunol. 2010;40:2182–2189. doi: 10.1002/eji.200939995. [DOI] [PubMed] [Google Scholar]
- Barsotti AM, Beckerman R, Laptenko O, Huppi K, Caplen NJ, Prives C. p53-Dependent induction of PVT1 and miR-1204. J. Biol. Chem. 2012;287:2509–2519. doi: 10.1074/jbc.M111.322875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Chaum E, Johnson DA, Johnson LR. Age-related susceptibility to apoptosis in human retinal pigment epithelial cells is triggered by disruption of p53-Mdm2 association. Invest Ophthalmol Vis Sci. 2012;53:8350–8366. doi: 10.1167/iovs.12-10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Ray RM, Chaum E, Johnson DA, Johnson LR. Inhibition of Mdm2 sensitizes human retinal pigment epithelial cells to apoptosis. Invest Ophthalmol Vis Sci. 2011;52:3368–3380. doi: 10.1167/iovs.10-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Ray RM, Johnson LR. Role of polyamines in p53-dependent apoptosis of intestinal epithelial cells. Cell. Signal. 2009;21:509–522. doi: 10.1016/j.cellsig.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Guo H, Ray RM, Johnson LR. Basic helix-loop-helix protein E47-mediated p21Waf1/Cip1 gene expression regulates apoptosis of intestinal epithelial cells. Biochem. J. 2007;407:243–254. doi: 10.1042/BJ20070293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Ray RM, Viar MJ, Johnson LR. Polyamines are required for activation of c-Jun NH2-terminal kinase and apoptosis in response to TNF-alpha in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G980–G991. doi: 10.1152/ajpgi.00206.2003. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Exp Gerontol. 2003;38:5–11. doi: 10.1016/s0531-5565(02)00152-3. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- Cao S, Ko A, Partanen M, Pakzad-Vaezi K, Merkur AB, Albiani DA, Kirker AW, Wang A, Cui JZ, Forooghian F, Matsubara JA. Relationship between systemic cytokines and complement factor H Y402H polymorphism in patients with dry age-related macular degeneration. Am. J. Ophthalmol. 2013;156:1176–1183. doi: 10.1016/j.ajo.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaum E. Comparative analysis of the uptake and expression of plasmid vectors in human ciliary and retinal pigment epithelial cells in vitro. J. Cell. Biochem. 2001;83:671–677. doi: 10.1002/jcb.1258. [DOI] [PubMed] [Google Scholar]
- Chen B, He L, Savell VH, Jenkins JJ, Parham DM. Inhibition of the interferon-gamma/signal transducers and activators of transcription (STAT) pathway by hypermethylation at a STAT-binding site in the p21WAF1 promoter region. Cancer Res. 2000;60:3290–3298. [PubMed] [Google Scholar]
- Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PloS one. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Muckersie E, Forrester JV, Xu H. Immune activation in retinal aging: a gene expression study. Invest Ophthalmol Vis Sci. 2010;51:5888–5896. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- Choubey D. DNA-responsive inflammasomes and their regulators in autoimmunity. Clin. Immunol. 2012;142:223–231. doi: 10.1016/j.clim.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa S, Borgogna C, Mondini M, De Andrea M, Meroni PL, Berti E, Gariglio M, Landolfo S. Redistribution of the nuclear protein IFI16 into the cytoplasm of ultraviolet B-exposed keratinocytes as a mechanism of autoantigen processing. Br. J. Dermatol. 2011;164:282–290. doi: 10.1111/j.1365-2133.2010.10097.x. [DOI] [PubMed] [Google Scholar]
- Datta B, Li B, Choubey D, Nallur G, Lengyel P. p202, an interferon-inducible modulator of transcription, inhibits transcriptional activation by the p53 tumor suppressor protein, and a segment from the p53-binding protein 1 that binds to p202 overcomes this inhibition. J. Biol. Chem. 1996;271:27544–27555. doi: 10.1074/jbc.271.44.27544. [DOI] [PubMed] [Google Scholar]
- Duan X, Ponomareva L, Veeranki S, Panchanathan R, Dickerson E, Choubey D. Differential roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts. Mol. Cancer Res. 2011;9:589–602. doi: 10.1158/1541-7786.MCR-10-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Archives of ophthalmology. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- Faix J, Grosse R. Staying in shape with formins. Dev. Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Freeman NE, Templeton JP, Orr WE, Lu L, Williams RW, Geisert EE. Genetic networks in the mouse retina: growth associated protein 43 and phosphatase tensin homolog network. Mol Vis. 2011;17:1355–1372. [PMC free article] [PubMed] [Google Scholar]
- Fujiuchi N, Aglipay JA, Ohtsuka T, Maehara N, Sahin F, Su GH, Lee SW, Ouchi T. Requirement of IFI16 for the maximal activation of p53 induced by ionizing radiation. J. Biol. Chem. 2004;279:20339–20344. doi: 10.1074/jbc.M400344200. [DOI] [PubMed] [Google Scholar]
- Garfinkel S, Brown S, Wessendorf JH, Maciag T. Post-transcriptional regulation of interleukin 1 alpha in various strains of young and senescent human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 1994;91:1559–1563. doi: 10.1073/pnas.91.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisert EE, Lu L, Freeman-Anderson NE, Templeton JP, Nassr M, Wang X, Gu W, Jiao Y, Williams RW. Gene expression in the mouse eye: an online resource for genetics using 103 strains of mice. Mol Vis. 2009;15:1730–1763. [PMC free article] [PubMed] [Google Scholar]
- Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Annals of hematology. 1998;76:231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- Gugliesi F, Mondini M, Ravera R, Robotti A, de Andrea M, Gribaudo G, Gariglio M, Landolfo S. Up-regulation of the interferon-inducible IFI16 gene by oxidative stress triggers p53 transcriptional activity in endothelial cells. J Leukoc Biol. 2005;77:820–829. doi: 10.1189/jlb.0904507. [DOI] [PubMed] [Google Scholar]
- Gupta S, Radha V, Furukawa Y, Swarup G. Direct transcriptional activation of human caspase-1 by tumor suppressor p53. J. Biol. Chem. 2001;276:10585–10588. doi: 10.1074/jbc.C100025200. [DOI] [PubMed] [Google Scholar]
- Hasty P, Christy BA. p53 as an intervention target for cancer and aging. Pathobiol Aging Age Relat Dis. 2013;3 doi: 10.3402/pba.v3i0.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Haywood ME, Hogarth MB, Slingsby JH, Rose SJ, Allen PJ, Thompson EM, Maibaum MA, Chandler P, Davies KA, Simpson E, Walport MJ, Morley BJ. Identification of intervals on chromosomes 1, 3, and 13 linked to the development of lupus in BXSB mice. Arthritis Rheum. 2000;43:349–355. doi: 10.1002/1529-0131(200002)43:2<349::AID-ANR14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hertel P, Daniel J, Stegehake D, Vaupel H, Kailayangiri S, Gruel C, Woltersdorf C, Liebau E. The ubiquitin-fold modifier 1 (Ufm1) cascade of Caenorhabditis elegans. J. Biol. Chem. 2013;288:10661–10671. doi: 10.1074/jbc.M113.458000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobeika AC, Etienne W, Torres BA, Johnson HM, Subramaniam PS. IFN-gamma induction of p21(WAF1) is required for cell cycle inhibition and suppression of apoptosis. J. Interferon Cytokine Res. 1999;19:1351–1361. doi: 10.1089/107999099312812. [DOI] [PubMed] [Google Scholar]
- Hogarth MB, Slingsby JH, Allen PJ, Thompson EM, Chandler P, Davies KA, Simpson E, Morley BJ, Walport MJ. Multiple lupus susceptibility loci map to chromosome 1 in BXSB mice. J. Immunol. 1998;161:2753–2761. [PubMed] [Google Scholar]
- Jablonski MM, Freeman NE, Orr WE, Templeton JP, Lu L, Williams RW, Geisert EE. Genetic pathways regulating glutamate levels in retinal Muller cells. Neurochem. Res. 2011;36:594–603. doi: 10.1007/s11064-010-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RW, Kerry JA, Trapani JA. The human interferon-inducible protein, IFI 16, is a repressor of transcription. J. Biol. Chem. 1998;273:17172–17177. doi: 10.1074/jbc.273.27.17172. [DOI] [PubMed] [Google Scholar]
- Karsten CM, Kohl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology. 2012;217:1067–1079. doi: 10.1016/j.imbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Fossati-Jimack L, Moll T, Amano H, Amano E, Ida A, Ibnou-Zekri N, Laporte C, Santiago-Raber ML, Rozzo SJ, Kotzin BL, Izui S. Differential role of three major New Zealand Black-derived loci linked with Yaa-induced murine lupus nephritis. J. Immunol. 2005;174:1111–1117. doi: 10.4049/jimmunol.174.2.1111. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell. Mol. Life Sci. 2013;70:4785–4794. doi: 10.1007/s00018-013-1423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klettner A. Oxidative stress induced cellular signaling in RPE cells. Front Biosci (Schol Ed) 2012;4:392–411. doi: 10.2741/s275. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Chiba T, Tatsumi K, Iemura S, Tanida I, Okazaki N, Ueno T, Kominami E, Natsume T, Tanaka K. A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO. J. 2004;23:1977–1986. doi: 10.1038/sj.emboj.7600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A, Campisi J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int J Biochem Cell Biol. 2002;34:1401–1414. doi: 10.1016/s1357-2725(02)00053-5. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, Gessner JE. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J. Clin. Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JC, Ongusaha PP, Ouchi T, Lee SW. IFI16 as a negative regulator in the regulation of p53 and p21(Waf1) J. Biol. Chem. 2003;278:40899–40904. doi: 10.1074/jbc.M308012200. [DOI] [PubMed] [Google Scholar]
- Lin H, Yan J, Wang Z, Hua F, Yu J, Sun W, Li K, Liu H, Yang H, Lv Q, Xue J, Hu ZW. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology. 2013;57:171–182. doi: 10.1002/hep.25991. [DOI] [PubMed] [Google Scholar]
- Ma CS, Deenick EK. The role of SAP and SLAM family molecules in the humoral immune response. Ann. N. Y. Acad. Sci. 2011;1217:32–44. doi: 10.1111/j.1749-6632.2010.05824.x. [DOI] [PubMed] [Google Scholar]
- Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- Menendez D, Shatz M, Resnick MA. Interactions between the tumor suppressor p53 and immune responses. Curr Opin Oncol. 2013;25:85–92. doi: 10.1097/CCO.0b013e32835b6386. [DOI] [PubMed] [Google Scholar]
- Menendez D, Shatz M, Azzam K, Garantziotis S, Fessler MB, Resnick MA. The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 2011;7:e1001360. doi: 10.1371/journal.pgen.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi K, Ohbayashi M, Patel N, Chong V, Bird AC, Ono SJ. Identification of anti-retinal antibodies in patients with age-related macular degeneration. Exp Mol Pathol. 2012a;93:193–199. doi: 10.1016/j.yexmp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Morohoshi K, Patel N, Ohbayashi M, Chong V, Grossniklaus HE, Bird AC, Ono SJ. Serum autoantibody biomarkers for age-related macular degeneration and possible regulators of neovascularization. Exp Mol Pathol. 2012b;92:64–73. doi: 10.1016/j.yexmp.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Moser KL, Neas BR, Salmon JE, Yu H, Gray-McGuire C, Asundi N, Bruner GR, Fox J, Kelly J, Henshall S, Bacino D, Dietz M, Hogue R, Koelsch G, Nightingale L, Shaver T, Abdou NI, Albert DA, Carson C, Petri M, Treadwell EL, James JA, Harley JB. Genome scan of human systemic lupus erythematosus: evidence for linkage on chromosome 1q in African-American pedigrees. Proc Natl Acad Sci U S A. 1998;95:14869–14874. doi: 10.1073/pnas.95.25.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhui K, Ciobanu DC, Schikorski T, Wang X, Lu L, Williams RW. Dissection of a QTL hotspot on mouse distal chromosome 1 that modulates neurobehavioral phenotypes and gene expression. PLoS Genet. 2008;4:e1000260. doi: 10.1371/journal.pgen.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Aptsiauri N, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye (Lond) 2001;15:390–395. doi: 10.1038/eye.2001.142. [DOI] [PubMed] [Google Scholar]
- Munoz-Fontela C, Macip S, Martinez-Sobrido L, Brown L, Ashour J, Garcia-Sastre A, Lee SW, Aaronson SA. Transcriptional role of p53 in interferon-mediated antiviral immunity. J. Exp. Med. 2008;205:1929–1938. doi: 10.1084/jem.20080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murinello S, Mullins RF, Lotery AJ, Perry VH, Teeling JL. Fcgamma receptor upregulation is associated with immune complex inflammation in the mouse retina and early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:247–258. doi: 10.1167/iovs.13-11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napione L, Strasly M, Meda C, Mitola S, Alvaro M, Doronzo G, Marchio S, Giraudo E, Primo L, Arese M, Bussolino F. IL-12-dependent innate immunity arrests endothelial cells in G0-G1 phase by a p21(Cip1/Waf1)-mediated mechanism. Angiogenesis. 2012;15:713–725. doi: 10.1007/s10456-012-9286-9. [DOI] [PubMed] [Google Scholar]
- Nie L, Wu G, Zhang W. Correlation between mRNA and protein abundence in Desulfovibrio vulgaris: a multiple regression to identify sources of variations. Biochem Biophys Res Commun. 2006;339:601–610. doi: 10.1016/j.bbrc.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Novak N, Bieber T, Peng WM. The immunoglobulin E-Toll-like receptor network. Int. Arch. Allergy Immunol. 2010;151:1–7. doi: 10.1159/000232565. [DOI] [PubMed] [Google Scholar]
- Novakova Z, Hubackova S, Kosar M, Janderova-Rossmeislova L, Dobrovolna J, Vasicova P, Vancurova M, Horejsi Z, Hozak P, Bartek J, Hodny Z. Cytokine expression and signaling in drug-induced cellular senescence. Oncogene. 2010;29:273–284. doi: 10.1038/onc.2009.318. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Hara E. Roles and mechanisms of cellular senescence in regulation of tissue homeostasis. Cancer Sci. 2013;104:525–530. doi: 10.1111/cas.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi M, Ouchi T. Role of IFI16 in DNA damage and checkpoint. Frontiers in bioscience : a journal and virtual library. 2008;13:236–239. doi: 10.2741/2673. [DOI] [PubMed] [Google Scholar]
- Parmeggiani F, Romano MR, Costagliola C, Semeraro F, Incorvaia C, D'Angelo S, Perri P, De Palma P, De Nadai K, Sebastiani A. Mechanism of inflammation in age-related macular degeneration. Mediators of inflammation. 2012;2012:546786. doi: 10.1155/2012/546786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Ohbayashi M, Nugent AK, Ramchand K, Toda M, Chau KY, Bunce C, Webster A, Bird AC, Ono SJ, Chong V. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115:422–430. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold PL, Provis JM, Furby JH, Gatenby PA, Billson FA. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1990;228:270–274. doi: 10.1007/BF00920033. [DOI] [PubMed] [Google Scholar]
- Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Day DE, Nadeau BA, Zetoune FS, Sarma JV, Werner CM, Wanner GA, Simmen HP, Huber-Lang MS, Ward PA. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 2009;5:e1000464. doi: 10.1371/journal.ppat.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, Kotzin BL. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- Santiago-Raber ML, Amano H, Amano E, Baudino L, Otani M, Lin Q, Nimmerjahn F, Verbeek JS, Ravetch JV, Takasaki Y, Hirose S, Izui S. Fcgamma receptor-dependent expansion of a hyperactive monocyte subset in lupus-prone mice. Arthritis Rheum. 2009;60:2408–2417. doi: 10.1002/art.24787. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Gessner JE. Fc receptors and their interaction with complement in autoimmunity. Immunol. Lett. 2005;100:56–67. doi: 10.1016/j.imlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat. Rev. Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- Shatz M, Menendez D, Resnick MA. The human TLR innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res. 2012;72:3948–3957. doi: 10.1158/0008-5472.CAN-11-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Zheng SS, Park O, Wang H, Sun Z, Gao B. Activation of innate immunity (NK/IFN-gamma) in rat allogeneic liver transplantation: contribution to liver injury and suppression of hepatocyte proliferation. American journal of physiology. Gastrointestinal and liver physiology. 2008;294:G1070–G1077. doi: 10.1152/ajpgi.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Liu J, Liu Q, Li M. IFI16 mis-localization can be a contributing factor to hepatocellular carcinoma progression. Medical hypotheses. 2014;82:398–400. doi: 10.1016/j.mehy.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Skokowa J, Ali SR, Felda O, Kumar V, Konrad S, Shushakova N, Schmidt RE, Piekorz RP, Nurnberg B, Spicher K, Birnbaumer L, Zwirner J, Claassens JW, Verbeek JS, van Rooijen N, Kohl J, Gessner JE. Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation. J. Immunol. 2005;174:3041–3050. doi: 10.4049/jimmunol.174.5.3041. [DOI] [PubMed] [Google Scholar]
- Song LL, Alimirah F, Panchanathan R, Xin H, Choubey D. Expression of an IFN-inducible cellular senescence gene, IFI16, is up-regulated by p53. Mol. Cancer Res. 2008;6:1732–1741. doi: 10.1158/1541-7786.MCR-08-0208. [DOI] [PubMed] [Google Scholar]
- Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- Syed SN, Konrad S, Wiege K, Nieswandt B, Nimmerjahn F, Schmidt RE, Gessner JE. Both FcgammaRIV and FcgammaRIII are essential receptors mediating type II and type III autoimmune responses via FcRgamma-LAT-dependent generation of C5a. Eur. J. Immunol. 2009;39:3343–3356. doi: 10.1002/eji.200939884. [DOI] [PubMed] [Google Scholar]
- Templeton JP, Freeman NE, Nickerson JM, Jablonski MM, Rex TS, Williams RW, Geisert EE. Innate immune network in the retina activated by optic nerve crush. Invest Ophthalmol Vis Sci. 2013a;54:2599–2606. doi: 10.1167/iovs.12-11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton JP, Wang X, Freeman NE, Ma Z, Lu A, Hejtmancik F, Geisert EE. A crystallin gene network in the mouse retina. Exp Eye Res. 2013b;116:129–140. doi: 10.1016/j.exer.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Stepaniants SB, Mao M, Weng L, Feetham MC, Doyle MJ, Yi EC, Dia H, Thorsson V, Eng J, Goodlett D, Berger JP, Gunter B, Linseley PS, Stoughton RB, Aebersold R, Collins SJ, Hanlon WA, Hood LE. Integrated genomic and proteomic analysis of gene expression in Mammalian cells. Mol Cell Proteomics. 2004;3:960–969. doi: 10.1074/mcp.M400055-MCP200. [DOI] [PubMed] [Google Scholar]
- Tordella L, Koch S, Salter V, Pagotto A, Doondeea JB, Feller SM, Ratnayaka I, Zhong S, Goldin RD, Lozano G, McKeon FD, Tavassoli M, Fritzsche F, Huber GF, Rossle M, Moch H, Lu X. ASPP2 suppresses squamous cell carcinoma via RelA/p65-mediated repression of p63. Proc Natl Acad Sci U S A. 2013;110:17969–17974. doi: 10.1073/pnas.1309362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente LJ, Gray DH, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL, Janic A, Strasser A. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep. 2013;3:1339–1345. doi: 10.1016/j.celrep.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Valente LJ, Strasser A. Distinct target genes and effector processes appear to be critical for p53-activated responses to acute DNA damage versus p53-mediated tumor suppression. BioDiscovery. 2013;8:1–16. [Google Scholar]
- Veeranki S, Duan X, Panchanathan R, Liu H, Choubey D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PloS one. 2011;6:e27040. doi: 10.1371/journal.pone.0027040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H, Montag D, Kanzleiter T, Jonas W, Matzke D, Scherneck S, Chadt A, Tole J, Kluge R, Joost HG, Schurmann A. An interval of the obesity QTL Nob3.38 within a QTL hotspot on chromosome 1 modulates behavioral phenotypes. PloS one. 2013;8:e53025. doi: 10.1371/journal.pone.0053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H, Scherneck S, Kanzleiter T, Benz V, Kluge R, Stadion M, Kryvych S, Bluher M, Kloting N, Joost HG, Schurmann A. Loss of function of Ifi202b by a microdeletion on chromosome 1 of C57BL/6J mice suppresses 11beta-hydroxysteroid dehydrogenase type 1 expression and development of obesity. Hum. Mol. Genet. 2012;21:3845–3857. doi: 10.1093/hmg/dds213. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Vuong L, Conley SM, Al-Ubaidi MR. Expression and role of p53 in the retina. Invest Ophthalmol Vis Sci. 2012;53:1362–1371. doi: 10.1167/iovs.11-8909. [DOI] [PubMed] [Google Scholar]
- Vyse TJ, Rozzo SJ, Drake CG, Izui S, Kotzin BL. Control of multiple autoantibodies linked with a lupus nephritis susceptibility locus in New Zealand black mice. J. Immunol. 1997;158:5566–5574. [PubMed] [Google Scholar]
- Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- Whitcup SM, Sodhi A, Atkinson JP, Holers VM, Sinha D, Rohrer B, Dick AD. The role of the immune response in age-related macular degeneration. Int J Inflam. 2013;2013:348092. doi: 10.1155/2013/348092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Lu L, Williams RW, Reese BE. A QTL on chromosome 10 modulates cone photoreceptor number in the mouse retina. Invest Ophthalmol Vis Sci. 2011;52:3228–3236. doi: 10.1167/iovs.10-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, Morquette B, Abdouh M, Unsain N, Barker PA, Feinstein E, Bernier G, Di Polo A. ASPP1/2 regulate p53-dependent death of retinal ganglion cells through PUMA and Fas/CD95 activation in vivo. J. Neurosci. 2013;33:2205–2216. doi: 10.1523/JNEUROSCI.2635-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaus J, Cardo M, Valledor AF, Soler C, Lloberas J, Celada A. Interferon gamma induces the expression of p21waf-1 and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity. 1999;11:103–113. doi: 10.1016/s1074-7613(00)80085-0. [DOI] [PubMed] [Google Scholar]
- Xin H, Pereira-Smith OM, Choubey D. Role of IFI 16 in cellular senescence of human fibroblasts. Oncogene. 2004;23:6209–6217. doi: 10.1038/sj.onc.1207836. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Progress in retinal and eye research. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ono M, Bensaddek D, Lamond AI, Rocha S. FMN2 is a novel regulator of the cyclin-dependent kinase inhibitor p21. Cell Cycle. 2013a;12:2348–2354. doi: 10.4161/cc.25511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Ono M, Perkins ND, Rocha S, Lamond AI. Identification and functional characterization of FMN2, a regulator of the cyclin-dependent kinase inhibitor p21. Mol. Cell. 2013b;49:922–933. doi: 10.1016/j.molcel.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Lauer N, Skerka C. The role of complement in AMD. Adv. Exp. Med. Biol. 2010;703:9–24. doi: 10.1007/978-1-4419-5635-4_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.