Abstract
The recent advances in the clinical application of anti-cancer immunotherapeutic agents have opened a new arena for the treatment of advanced cancers. Cancer immunotherapy is associated with a variety of important radiographic features in the assessments of tumor response and immune-related adverse events, which calls for radiologists’ awareness and in-depth knowledge on the topic. This article will provide the state-of-the art review and perspectives of cancer immunotherapy, including its molecular mechanisms, the strategies for immune-related response assessment on imaging and their pitfalls, and the emerging knowledge of radiologic manifestations of immune-related adverse events. The cutting edge clinical and radiologic investigations are presented to provide future directions.
Keywords: Immunothreapy, Cancer, Oncologic imaging, drug toxicity, tumor response assessment
INTRODUCTION
Increasing understanding of regulatory pathways of the immune response to cancer has led to the development and successful application of immunotherapeutic agents1–7. This is best represented by ipilimumab, a cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibody, which significantly improved overall survival in metastatic melanoma patients, leading to the approval of the agent by Food and Drug Administration (FDA) for advanced melanoma8–10. Newer immunotherapeutic agents, such as anti-PD-1 (anti-programmed cell death-1) and anti-PD-L1 antibodies (anti-programmed cell death ligand-1), have been developed and also demonstrated marked activities in patients with advanced cancers11–14. Immunotherapeutic agents have distinct biologic mechanisms of anti-cancer activity, which augment activation and proliferation of T cells and induce tumor infiltration by T cells and tumor regression8, 15–17. These distinct mechanisms result in the unique imaging manifestations in patients receiving immunotherapy, which requires specific attention and knowledge for the accurate radiological interpretations. For example, some of the patients on immunotherapy demonstrate radiologic response patterns that may not be captured by the conventional RECIST and WHO criteria, thus requiring modification in response assessment guidelines as proposed in the immune-related response criteria (irRC)17–19. As the role of immunotherapeutic agents expands in the treatment of advanced cancers, the knowledge of immune-related tumor response will become increasingly important for radiologists to contribute to the state-of-the-art cancer care. Furthermore, the distinct biological mechanism of immunotherapy is also associated with a variety of immune-related adverse events during therapy, where radiologists can contribute significantly in making diagnosis and help clinical decision making20–24.
This article will first review the molecular basis of anti-cancer immunotherapeutic agents and discuss their clinical application in different types of cancers. The article will then provide a detailed review of immune-related response criteria by describing definitions of immune-related response and progression along with the biological background, and discuss their pitfalls. Emerging knowledge of immune-related adverse events and their imaging features will also be described. Finally, future directions will be provided based on the observations in cutting-edge clinical and radiologic investigations. The article will provide with the state-of-the-art knowledge of cancer immunotherapy, which is essential for radiologists to play a role as a key contributor in this new arena of cancer treatment.
I. Molecular basis of cancer immunotherapy
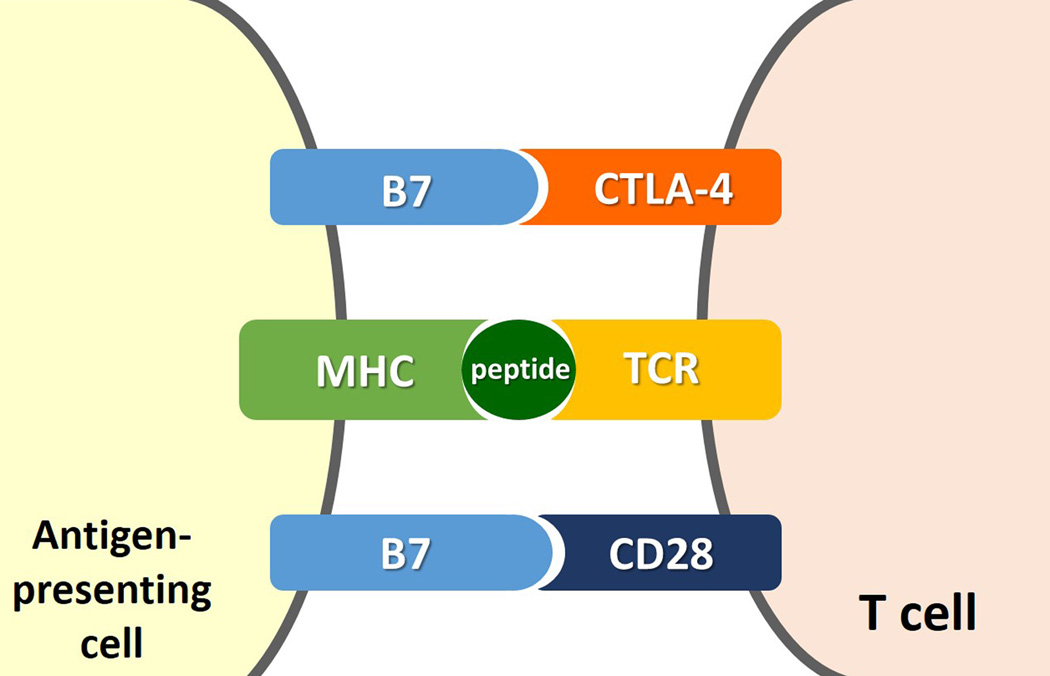
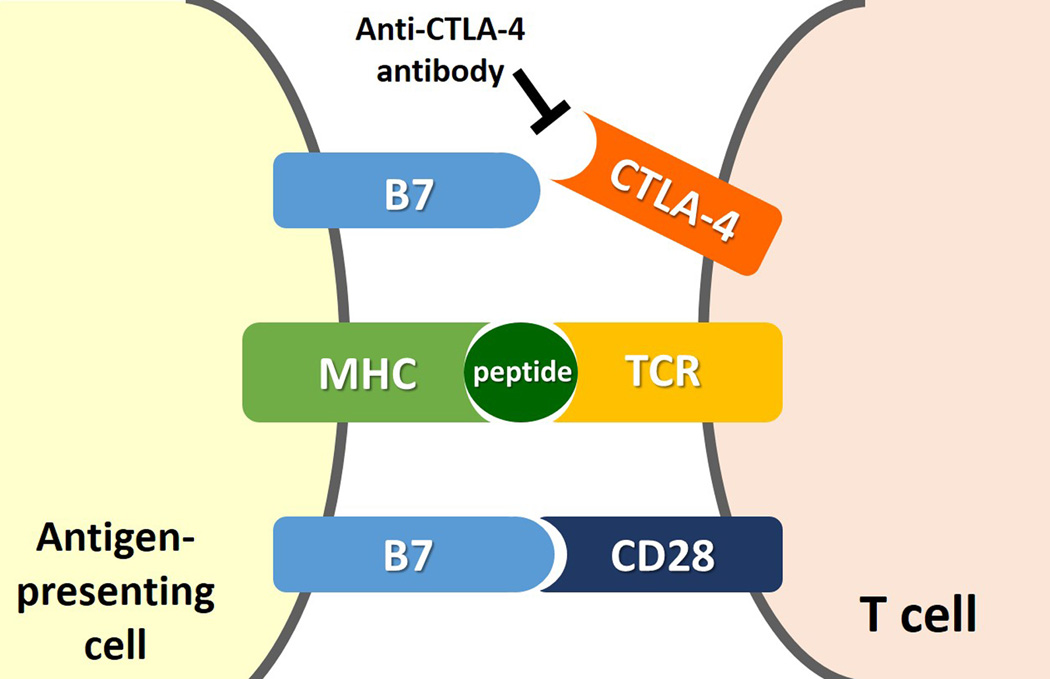
Immunotherapeutic agents such as ipilimumab and anti-PD-1 antibodies exert the anti-tumor activity through the blockade of immunologic inhibitiory pathways and the augmentation of T cell activation and proliferation, as opposed to the direct cytotoxic effects to tumor cells7, 8, 17, 25, 26. For effective anti-tumor immunity, T cells play a major role in the immune defense against cancer. Upon encountering tumor antigens, T cells become activated, circulate and work toward elimination of cancer cells27–29. There are several checkpoints where this response can be modified, with the primary purpose of suppressing immune-attack to self-antigens or autoimmunity. Some cancers also utilize the checkpoint pathways to suppress anti-cancer immune response and escape from T cell immunity of the host29. This biological background of T cell immunity provides the rationale to pursue the blockade of checkpoint molecules as an anti-cancer therapeutic option. CTLA-4 and PD-1 pathways are the two major immune-checkpoint pathways which have been studied, leading to the clinical application of novel agents involved in the pathways29, 30. Both CTLA-4 and PD-1 are expressed on activated T cells, and interact with their ligands on antigen-presenting tumor cells to inhibit the immune response against tumor. Therefore, antibodies against CTLA-4, PD-1 and its ligand (i.e., PD-L1) that can block this interaction results in anti-cancer therapeutic effect by blocking the T cell immune inhibition by tumors and activating the immune response against cancer29, 31–33 (Fig. 1, 2).
Fig 1.
Molecular mechanisms for immune inhibition by tumors and its blockade by anti-CTLA-4 antibody. (Modified from Refs [40, 42]: N Engl J Med 2014;371:2189-99 and N Engl J Med. 2014;371: 2230–2232).
A. Interaction between CTLA-4 on T cell and its ligand (B7) on antigen-presenting cell inhibits the T cell immune response against tumor, allowing tumor cells escape from immune attack.
B. Anti-CTLA-4 antibodies, such as ipilimumab, block the interaction between CTLA-4 and its ligand, causing blockade of the T cell immune inhibition and thus activating immune response against cancer.
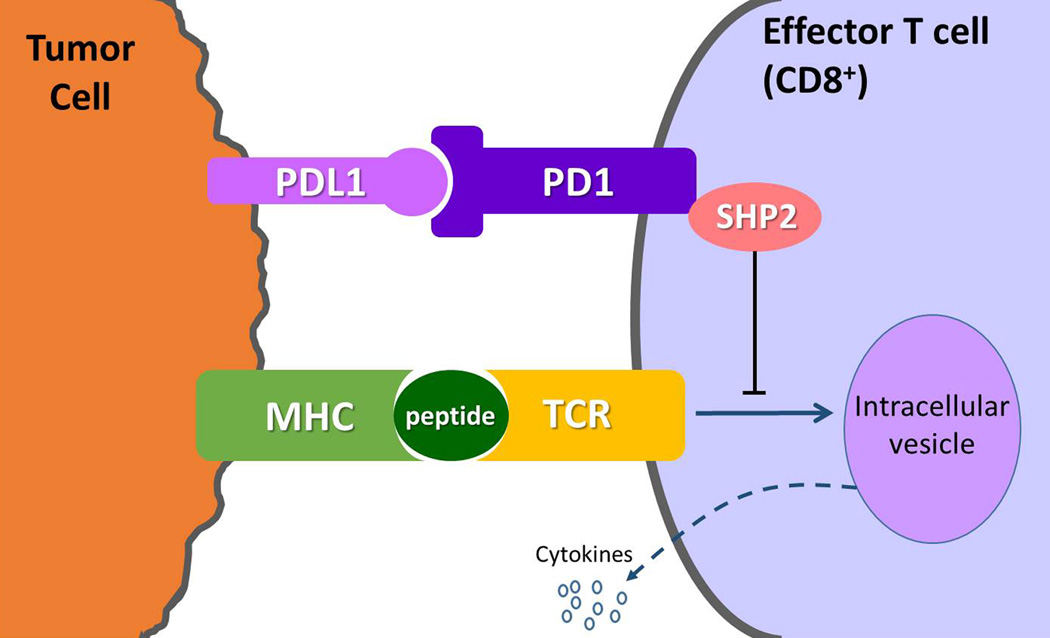
Fig. 2.
Mechanism of PD-1 immunosupression as a target for cancer therapy. (Modified from Refs [31–33]. Clin Cancer Res. 2012;18: 6580–6587, Nat Rev Cancer. 2012;12: 252–264; Nat Immunol. 2013;14: 1212–1218).
PD-1 is expressed on the surface of effector T cells upon activation, and its ligand, PD-L1 is expressed on the tumor cells either by constitutive oncogenic signaling or by the induction in response to inflammatory signals as a response to tumor. The binding of PD-L1 to PD-1 delivers an inhibitory signal, through the phosphatase SHP2, which reduces cytokine production and proliferation of T cells, thus enabling tumor cells to evade the host immune response. Antibodies against PD-1 or PD-L1 prevent the binding and block immune inhibition by tumor, inducing anti-tumor immune response. Multiple additional receptor-ligand interactions that regulate T cell responses in the tumor microenvironment have been identified, such as KIR (killer cell immunoglobulin-like receptor), LAG3 (lymphocyte activation gene 3), and TIM3 (T cell membrane protein 3), and are currently under active investigation as possible targets for cancer immunotherapy.
II. Clinical application of immunotherapeutic agents in cancer treatment
Cancer immunotherapy has rapidly expanded its role in the current clinical oncology practice since the approval of ipilimumab (anti-CTLA-4 antibody) for advanced melanoma in 2011. In a phase 3 trial of ipilimumab, patients with previously treated melanoma who received ipilimumab achieved a significantly extended overall survival (median OS: 10.1 months) compared with patients who received a glycoprotein 100 peptide vaccine (median OS: 6.4 months)8. This was the first phase III trial that demonstrated a substantial improvement in overall survival in patients with metastatic melanoma, which led to the approval of ipilimumab for all patients with metastatic melanoma by United States FDA in 2011. Ipilimumab is currently being evaluated for other tumors such as lung cancer and prostate cancer, and demonstrated improved progression-free survival in non-small cell lung cancer (NSCLC)34–37.
Among the novel agents with different mechanisms for immunotherapy, anti-PD-1 antibody, pembrolizumab, has received accelerated FDA approval for advanced or unresectable melanoma in September 2014, further demonstrating the promise of the field. Pembrolizumab was tested in 173 advanced melanoma patients treated as a randomized dose-comparison cohort of a phase 1 trial, and demonstrated an overall response rate of 26%, with good treatment tolerance and no drug-related deaths12. Another promising anti-PD-1-antibody, nivolumab, was tested in a phase 1 trial and demonstrated the cumulative response rates of 28% in melanoma, 18% in NSCLC, and 27% in RCC, which were the highest rate of anti-tumor activity of the many immunotherapy approaches tested in the clinic for the treatment of cancer during the past 3 decades38. More recent study reported that nivolumab can induce durable response that persists after drug discontinuation11. Nivolumab was also granted accelerated approval for patients with unresectable or metastatic melanoma who no longer respond to other drugs in December of 2014 by US FDA. More recently, in March 2015, FDA also approved nivolumab for the treatment of patients with metastatic squamous lung cancer who have progressed on or after platinum-based chemotherapy39.
The use of anti-PD-1 antibodies are further expanding to hematologic malignancies with the initial results of dramatic anti-cancer activity. In a phase 1 trial of relapsed or refractory Hodgkin lymphoma, the objective response rate was 87% (20/23), including 17% complete response and 70% partial response, and the remaining 13% of the patients had stable disease30. The progression-free survival rate at 24 weeks was 86%, with an acceptable toxicity profile, indicating the wider applicability of immunotherapy in cancer treatment. Given the accumulating evidence of the promise of immunotherapy as a new major player in clinical oncology, the ongoing research efforts also focus on identifying predictive biomarkers for response to therapy25, 40–42. Imaging has a major role in evaluating patients receiving immunotherapy and in defining the efficacy of these novel agents, and therefore needs to advance in parallel with the advances of immunotherapeutic treatment.
III. Assessment of immune-related tumor response to therapy
Immune-related response criteria (irRC): overview and clinical application
Due to the distinct mechanism of anti-cancer activity of immunotherapeutic agents, some patients on anti-cancer immunotherapy demonstrate tumor response patterns that may not be captured by the conventional tumor response criteria such as Response Evaluation Criteria in Solid Tumors (RECIST) and World Health Organization (WHO) criteria17–19. Notably, in patients treated with immunotherapy, tumors may show response after an initial increase in tumor burden, or during/after the appearance of new lesions17–19. To accurately capture these additional response patterns during immunotherapy, a novel set of quantitative imaging criteria was developed and proposed in 2009 as “immune-related response criteria (irRC)”, through the discussion by 200 oncologists, immunotherapists, and regulatory experts17. This was a first attempt to develop a method to describe the pattern of responses observed by clinical investigators, and to provide a basis to apply the method prospectively in clinical investigations.
In addition to the conventional response pattern of tumor burden decrease, irRC describes 2 additional patterns of immune-related response specific to immunotherapy, including 1) responses after an initial increase in total tumor burden (Fig. 3), and 2) reduction in total tumor burden during or after the appearance of new lesions at time points later than 12 weeks since the initiation of therapy17–19. These additional patterns of response are likely due to the activation of T cell immunity caused by ipilimumab. In a case study of an ipilimumab-treated patient with apparent increase of tumor burden at 12 weeks of therapy, histologic analyses showed that the increase in lesion size was due to T-cell infiltration rather than tumor cell proliferation17.
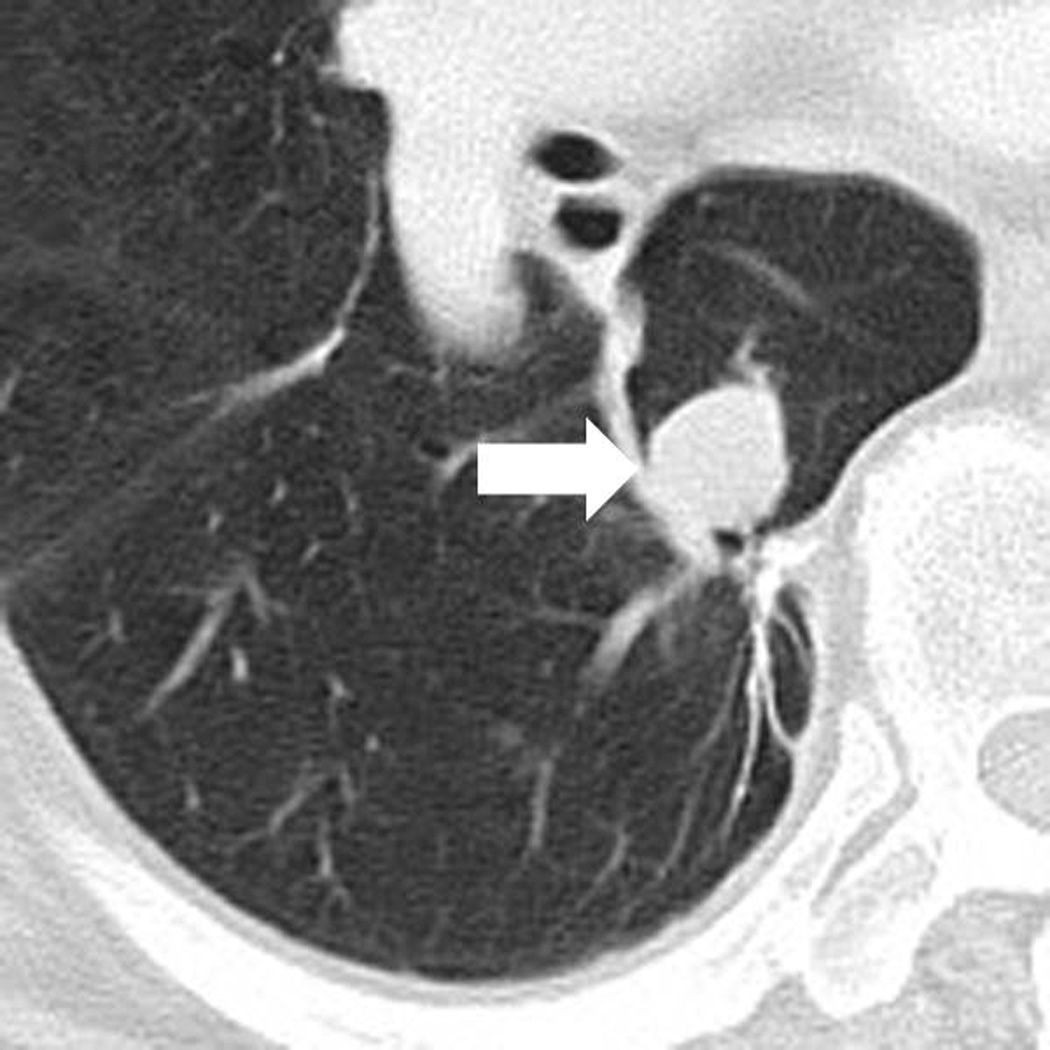
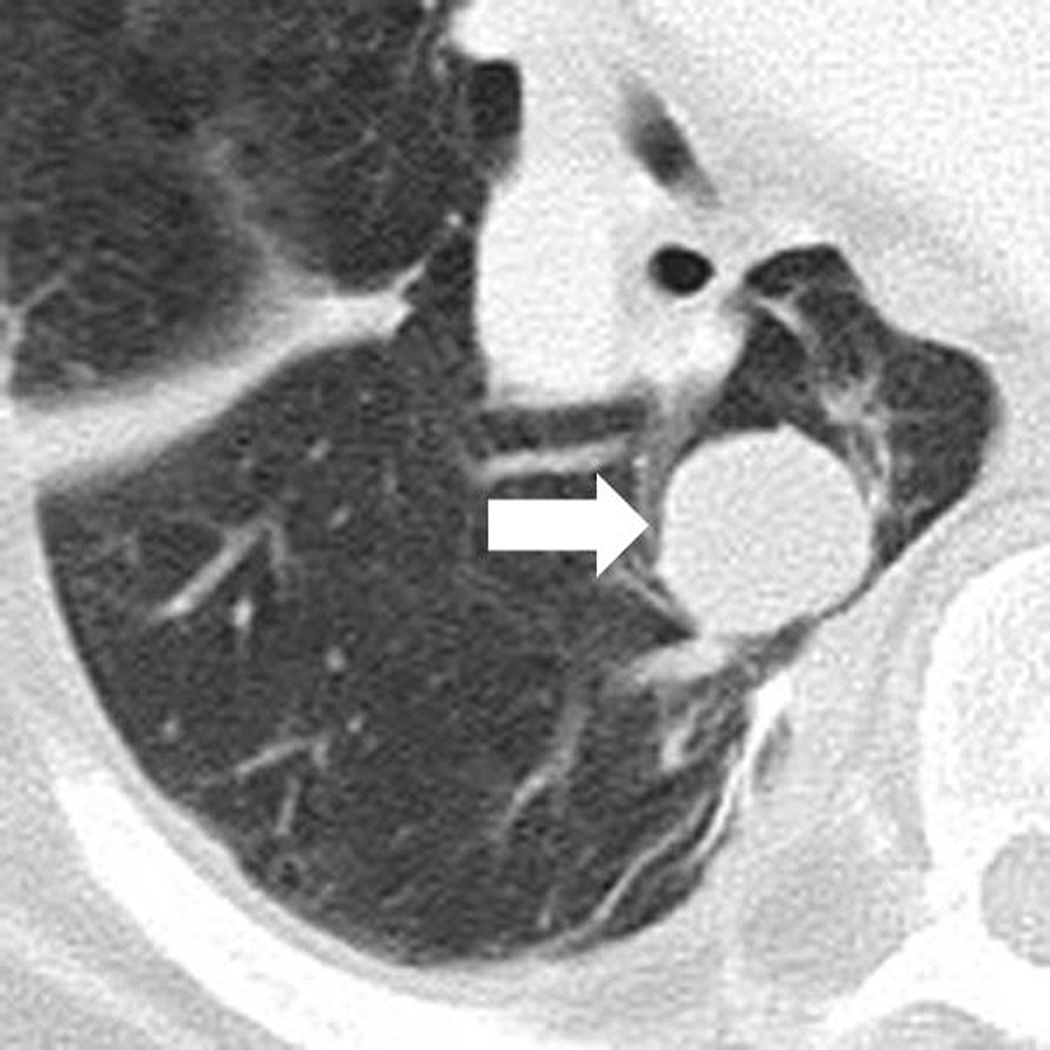
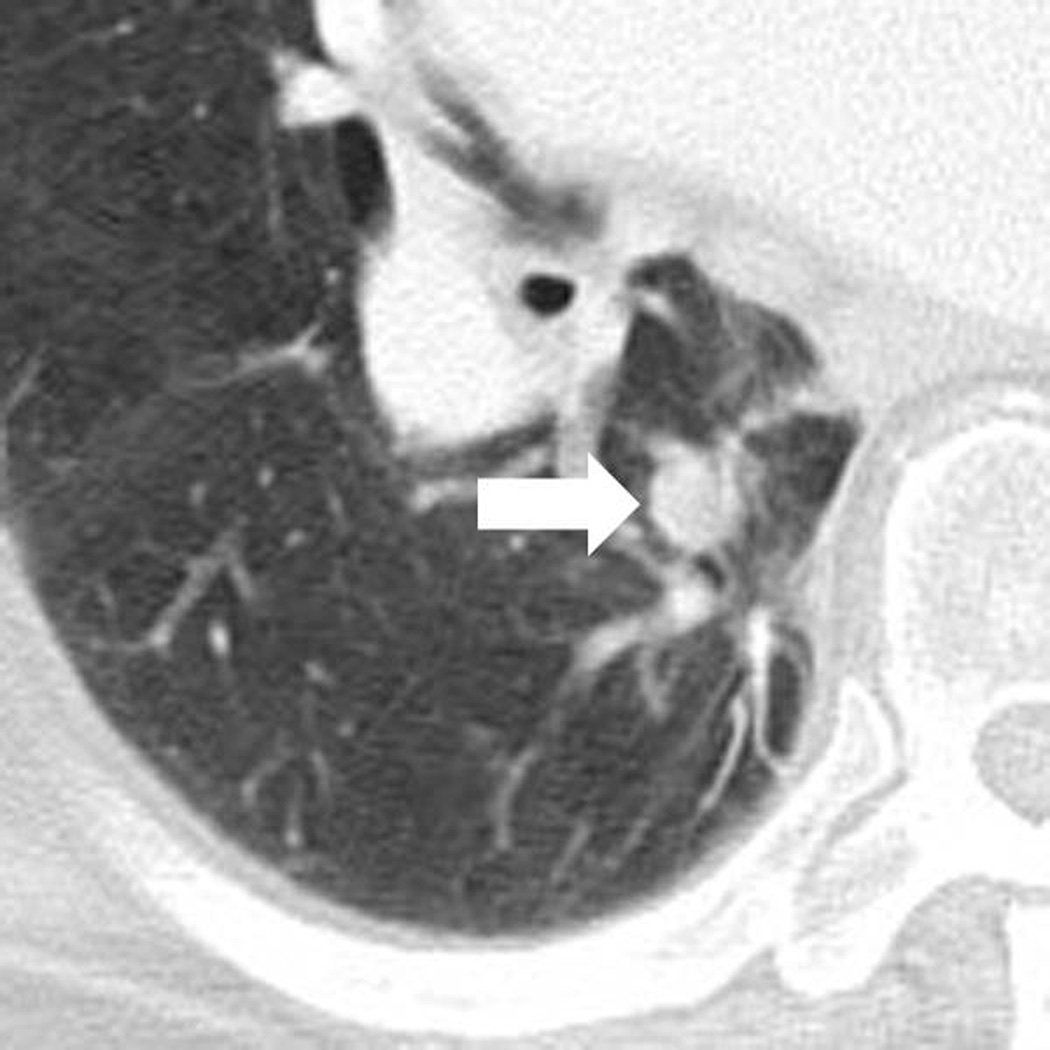
Fig. 3.
Response after an initial increase in total tumor burden in a 77 year-old male with advanced melanoma treated with ipilimumab.
A. The baseline CT scan demonstrated a lung lesion (arrow) measuring 19 mm in the longest diameter.
B. At 12 weeks of therapy, the lesion (arrow) measured 29 mm, demonstrating 53% increase comparing to the baseline, indicating progressive disease by RECIST.
C. The patient remained on therapy and another follow-up CT at 24 weeks showed a reduction of the lesion (arrow), measuring 12 mm, indicating immune-related response to therapy.
To capture these immune-related response patterns, irRC requires confirmation for progressive disease by a repeat consecutive assessment no less than 4 weeks from the first documentation. While the appearance of new lesions define progression by RECIST and WHO criteria43–45, new lesions may appear in the setting of response during immunotherapy, presumably as a result of T cell infiltration in the area of microscopic metastasis that was below the resolution of imaging prior to therapy. Therefore, the new lesions does not define progression by irRC; instead, the measurements of new lesions are included in the sum of the measurements to assess changes of tumor burden. A phase 2 trial of ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV NSCLC utilized irRC to assess response and define endpoints35, indicating the impact of the proposed criteria in the future trial designs. Radiologists need to be aware the distinct response patterns during immunotherapy and the modification described in irRC, to accurately evaluate response and progression in cancer patients treated with immunotherapeutic agents on their follow-up imaging studies.
Pitfalls of immune-related response criteria (irRC)
Although irRC describes an important concept for capturing response patterns specific to immunotherapy, there are several pitfalls and areas of the need for further work to optimize the immune-related response evaluation. A major pitfall of the original irRC includes the use of bidimensional measurements, i.e., the product of the longest diameter and the longest perpendicular diameter, based on the method according to WHO criteria (Fig. 4). However, multiple prior investigations have shown that bidimensional measurements are subject to a larger variability compared to the unidimensional measurements used in RECIST, and therefore cannot accurately capture small changes of tumor burden during therapy46–49. Furthermore, most clinical trials of solid tumors in the past decade utilized unidimensional measurements based on RECIST, which as originally published in 200043–45, and has served as a basis for FDA approval of new anti-cancer agents since then. The use of bidimensional measurements in irRC make it difficult to directly compare the results of immunotherapy trials with the results of the prior trials documented using unidimensional RECIST guidelines. For example, of the two recent reports of the trial results of anti-PD-1 antibodies, one used unidimensional measurements according to RECIST1.1 while the other study used bidimensional measurements according to modified WHO criteria to describe the magnitude of response13, 14. While both trials demonstrated the substantial activity and response of the agents, it requires additional efforts to directly compare the observations, and makes it challenging to understand the similarities and differences between the 2 trial results. The initial step of the further work in this area needs to focus on unifying the strategy of image-based assessment of immune-related response that can serve as a “common language” to describe results of cancer immunotherapy18, 50, 51. Further steps should also address the incorporation of other metrics such as tumor density, volume, metabolic activities and other functional information, while keeping in mind the role as a “common language” and the need for technical standardization.
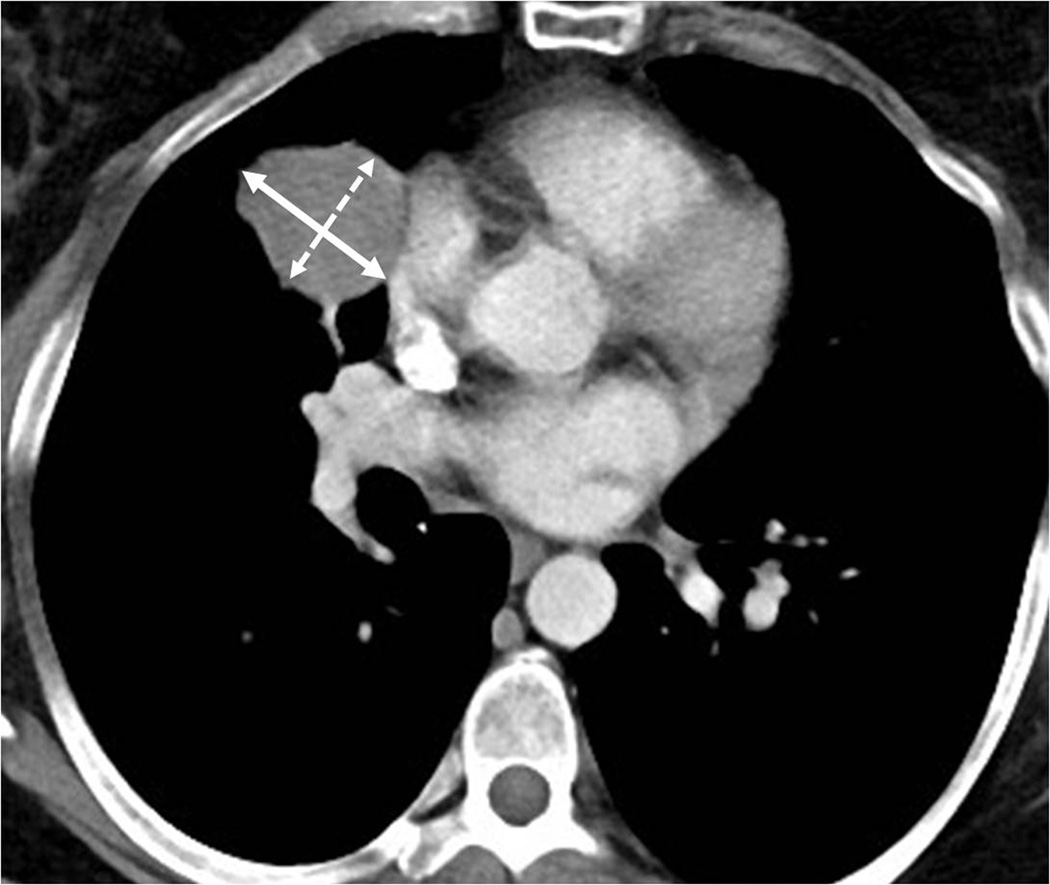
Fig. 4.
Bidimensional versus unidimensional measurements for tumor response assessment. Baseline CT scan prior to ipilimumab therapy in a 51-year-old female with metastatic melanoma demonstrated a target lesion in the lung, measuring 16.4 cm2 (4.2×3.9 cm) by bidimensional measurements and 4.2 cm by uindimensional, longest diameter measurement.
Radiologic investigations to improve immune-related response assessment
Several recent radiologic investigations have attempted to address the issue of unifying strategy for immune-related response assessment (Table 1). A recent study from our group evaluated 57 patients with advanced melanoma treated with ipilimumab in a phase 2 trial, and compared the original irRC using bidimensional measurements with the irRC using unidimensional measurements18. The study demonstrated that unidimensional immune-related response assessment was highly concordant with the bidimensional assessment, with best response by two methods showing almost perfect agreement (κw=0.881). The measurement variability of the unidimensional method was half of that of the bidimensional method, indicating the better reproducibility of the unidimensional approach (Figs. 5, 6)18. Another study from the group has also demonstrated that the unidimensional immune-related response assessment using decreased number of target lesions, maximum 2 per organ and 5 in total simulating RECIST1.1, is highly concordant with the assessment using the number of target lesions according to RECIST1.0, with almost identical measurement variability(Fig. 7)52. These results support the direction toward moving “immune-related RECIST1.1 (irRECIST1.1)” assessment using unidimensional measurements and the number of lesions according to RECIST1.1, while keeping the important unique features (confirmation of progression and inclusion of new lesion measurements) to capture immune-related responses52, 53. The strategy is simple and practical, and provides response assessments that can be directly compared to the results from other trials utilizing RECIST.
Table 1.
Summary of the approaches for immune-related response assessment described in the radiologic investigations[18, 52]
| Bidimensional assessment (the original irRC) |
Unidimensional assessment | ||
|---|---|---|---|
| irRC simulating RECIST1.0 | irRC simulating RECIST1.1 | ||
| Measurable lesions | ≥5×5 mm by bidimensional measurements | ≥10 mm in the longest diameter for all lesions | ≥10 mm in the longest diameter for all lesions
except for lymph nodes ≥15 mm in short axis for nodes |
| Number of target lesions | Up to 5 lesions per organ, up to 10 visceral and 5 cutaneous lesions | Up to 5 per organ, up to 10 in total | Up to 2 per organ, up to 5 in total |
| Measurement of each lesion | The longest diameter × the longest perpendicular diameter (cm2) | The longest diameter for all target lesions | The longest diameter for non-nodal lesions, short axis for lymph nodes |
| The sum of the measurements | The sum of the bidimensional measurements of all target lesions and new lesions if any | The sum of the longest diameters of all target lesions and new lesions if any | The sum of the diameters of all target lesions and new lesions if any |
| Response assessment | PD: ≥25% increase from the
nadir PR: ≥50% decrease from baseline CR: Disappearance of all lesions |
PD: ≥20% increase from the
nadir PR: ≥30% decrease from baseline CR: Disappearance of all lesions |
PD: ≥20% increase from the
nadir PR: ≥30% decrease from baseline CR: Disappearance of all lesions, and all nodes <10 mm in short axis |
| New lesions | The presence of new lesion(s) does not define progression. The measurements of the new lesion(s) are included in the sum of the measurements. | ||
| Confirmation | Confirmation by two consecutive observations not less than 4 weeks apart was required for CR, PR and PD | ||
Fig. 5.
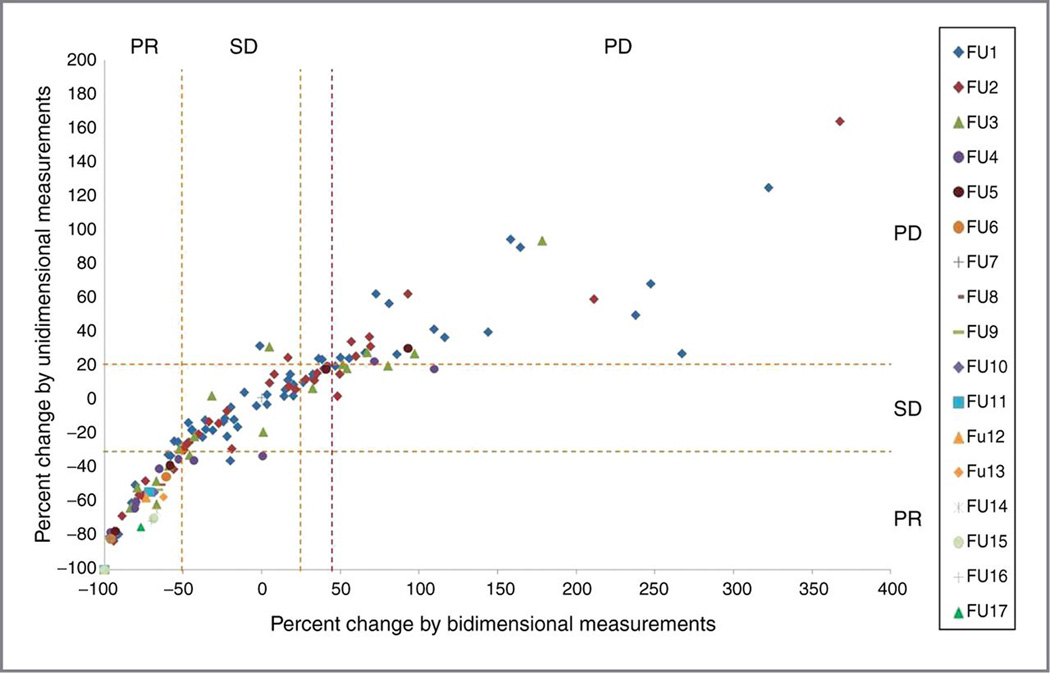
Comparison between irRC using bidimensional measurements and the irRC using unidimensional measurements. (Reprinted with permission from Ref. 18: Clin Cancer Res. 2013;19:3936-43.)
A. The percent changes according to bidimensional and unidimensional measurements at each follow-up scan from the 1st to 17th follow-up scans. The orange dashed lines represent the cut-off values for response and progression (−50% and +25% for bidimensional measurements, −30% and +20% for unidimensional measurements). The observations within the top left, middle center, and top right boxes have concordant assessment between tow measurements, whereas observations in other boxes have discordant assessment. The purple dashed line represents +44% change for bidimensional measurements, which corresponds to +20% change for unidimensional measurements, which was given to visually demonstrate that more observations are concordant if this cut-off value is used. The percent changes presented in the figure are in comparison with baseline measurements when tumors are decreasing to assess response and in comparison with the nadir (the smallest measurement since baseline) when tumors are increasing to assess progression. These values are displayed as they are used to define response/progression in patients at the time of response assessment.
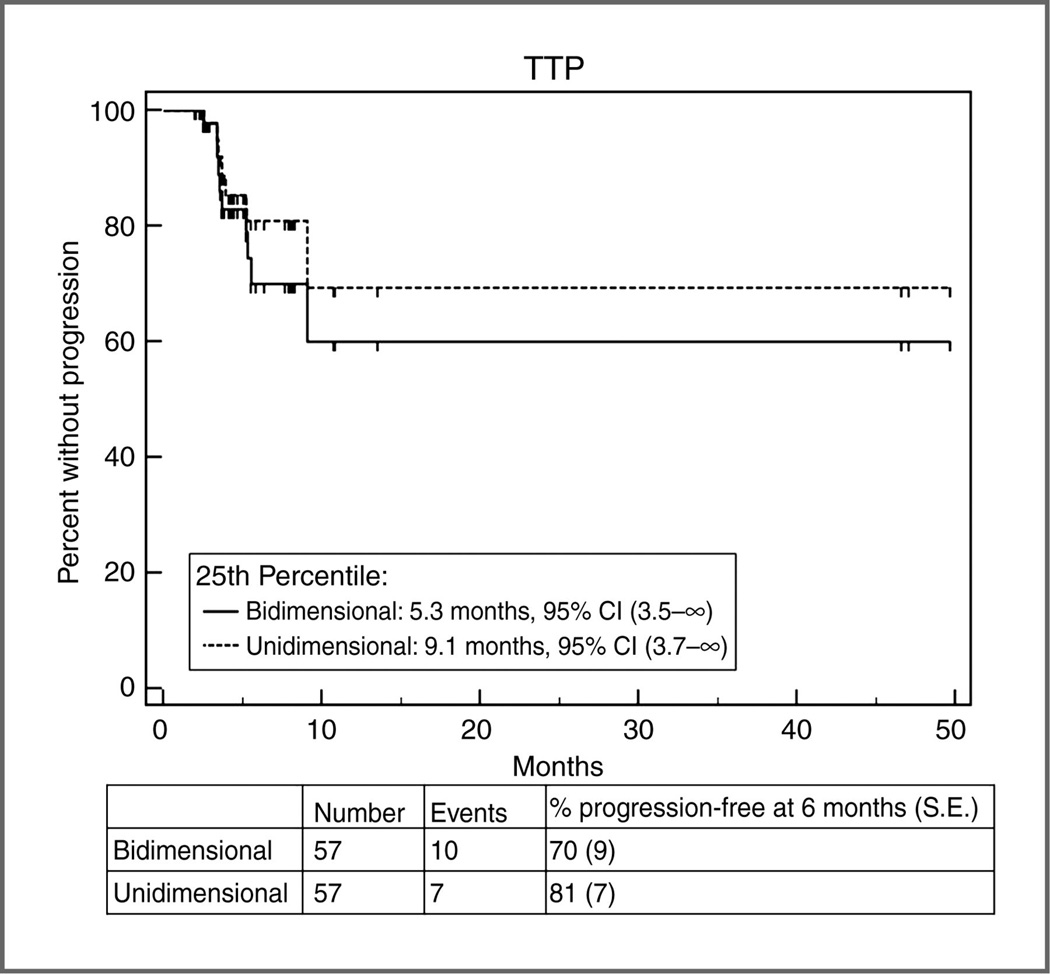
B. TTP according to bidimensional versus unidimensional assessment.
Estimates of the 25th percentile (time point at which 75% are free of progression) were 5.3 months (95% CI, 3.5–∞) by bidimensional assessment versus 9.1 months (95% CI, 3.7–∞) by unidimensional assessment. On the basis of the almost identical confidence intervals for the 25 percentile, there is no evidence of a difference in TTP between the 2 methods of assessment.
Fig. 6.
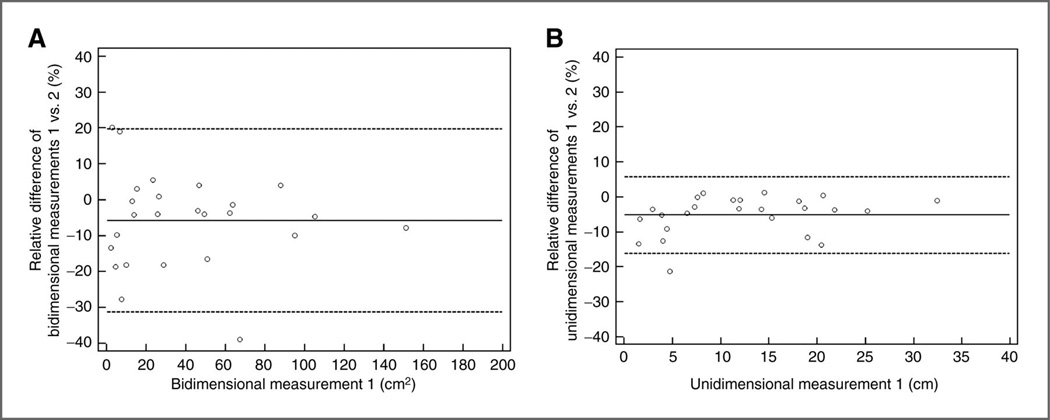
Interobserver variability of bidimensional and unidimensional measurements. (Reprinted with permission from Ref. 18: Clin Cancer Res. 2013;19:3936-43.)
Bland–Altman plots show interobserver variability of bidimensional and unidimensional measurements on baseline scans in 25 patients. The 95% limits of agreement of bidimensional measurements were (−31.3%, 19.7%; A, dashed lines), that were twice wider compared with those of unidimensional measurements (−16.1%, 5.8%; B, dashed lines). The dotted lines represent the mean relative difference (%).
Fig. 7.
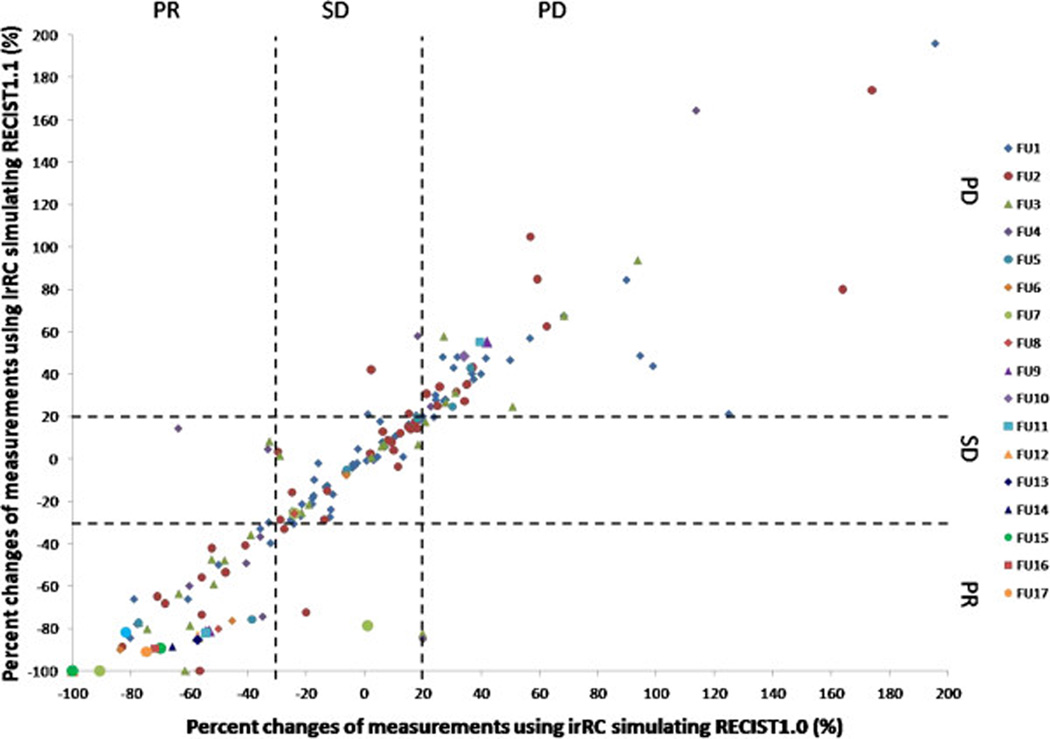
Comparison between irRC simulating RECIST1.0 and irRC simulating RECIST1.1. Reprinted with permission from Ref. 52: J Immunother Cancer. 2014 Jun 18;2:17.)
A. The percent changes of measurements using irRC simulating RECIST1.0 and irRC simulating RECIST1.1 at each follow-up from the 1st to the 17th follow-up scans are shown. Dashed lines at +20% and −30% represent the cut-off values for progressive disease and partial response, respectively. The observations within the lower left, middle center, and upper right boxes have concordant assessment between tow measurements, while observations in other boxes have discordant assessment. One concordant observation (+80% by irRC simulating RECIST1.0, +330% irRC simulating RECIST1.1) is not displayed since it is beyond the range of the Y axis. The percent changes presented in the figure are in comparison with baseline measurements when tumors are decreasing to assess response, and in comparison with the nadir (the smallest measurement since baseline) when tumors are increasing to assess progression. These values are displayed since they are used to define response/progression in patients at the time of response assessment. Please also note that the number of patients decreases as the follow-up proceeds, starting from 71 patients at 1st follow-up, 43 patients at the 2nd follow-up, 27 patients at the 3rd follow-up, and so on. There were 3 patients at the 12th–14th follow-up, 2 patients at 15th and 16th follow-up, and one follow-up at the 17th follow-up.
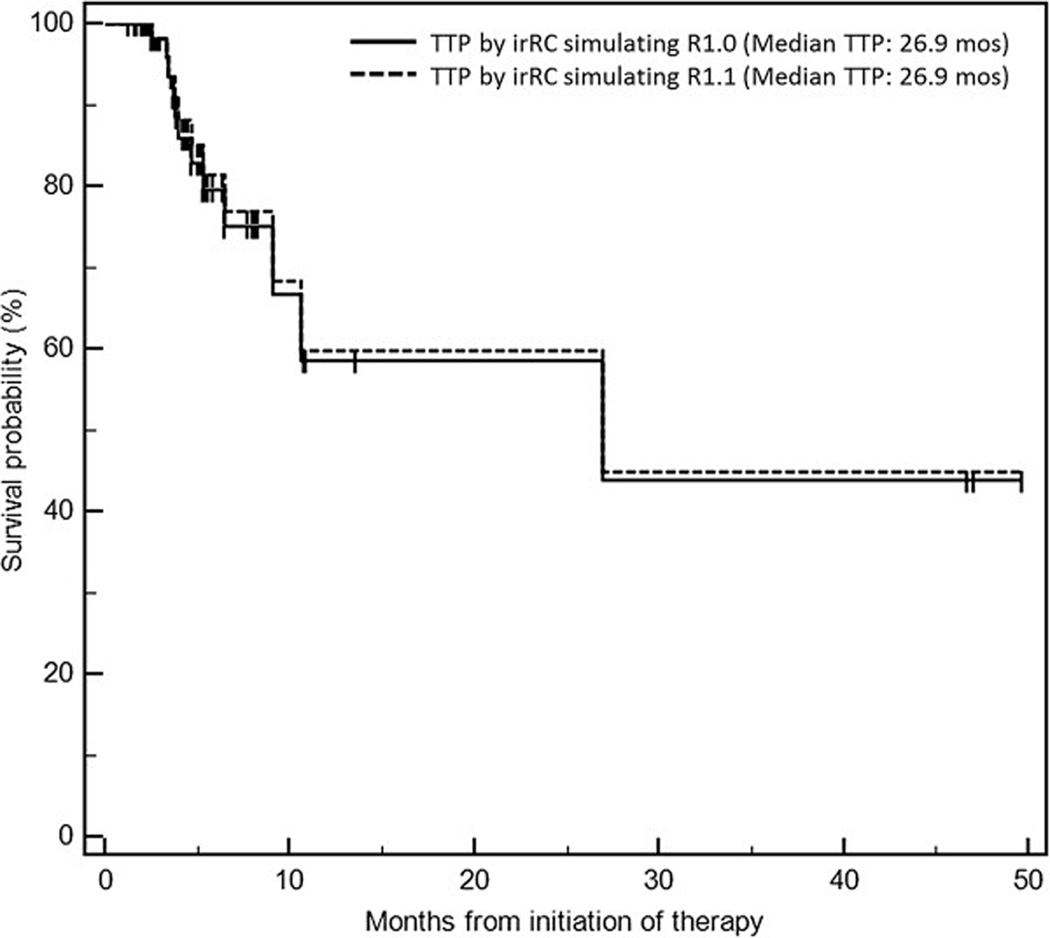
B. Time to progression by irRC simulating RECIST1.1 and irRC simulating RECIST1.0 had a median survival of 26.9 months (95% CI: 9.1–∞), without evidence of difference.
While both irRC and RECIST rely only on tumor size (measured in diameters) as a quantitative marker for tumor burden changes, incorporation of additional quantitative imaging markers may help to further optimize immune-related response evaluation. In several solid tumors such as gastrointestinal stromal tumors (GIST) and RCC treated with targeted therapy, decrease in tumor CT density, measured in Hounsfield Unit (HU), may indicate response even if size changes do not meet the criteria for response19, 54–56. In advanced RCC treated with anti-angiogenic therapy, MASS (morphology, attenuation, size, and structure) criteria propose to define response as ≥20% diameter decrease, or ≥40HU density decrease, or marked central necrosis in predominantly solid enhancing lesion54. Gray et al recently studied 44 metastatic melanoma patients treated with bevacizumab with or without interferon, and demonstrated that the assessment by MASS criteria on the first follow-up CT in combination with baseline serum lactate dehydrogenase (LDH) level accurately predicted progression-free survival and overall survival57. In another study in 21 advanced melanoma patients treated with ipilimumab plus bevacizumab (VEGF inhibitor), one-third of the patients had CT tumor density decrease ≥15% (defined as density response by Choi criteria for GIST55), indicating that CT tumor density decrease is a relatively common phenomenon during immunotherapy, while its role in evaluating anti-cancer activity and therapeutic benefit remain to be determined52.
IV. Immune-related adverse events and their imaging manifestations
Given the unique mechanism of action, immunotherapeutic agents are associated with a wide spectrum of immune-related adverse events, such as enterocolitis, hepatitis, hypophysitis, dermatitis, thyroiditis, and sarcoid-like mediastinal and hilar lymphadenopathy (Fig. 8, 9)20, 22, 24, 58–60. Many of these entities are associated with radiologic manifestations, and radiologists play an essential role in the diagnosis and follow-up. In a series of 119 advanced melanoma patients treated with ipilimumab by Bronstein et al, 20 patients (16.8%) demonstrated radiologic abnormalities potentially explained by immune-related adverse events20. Among them, clinically evident cases of immune-related adverse events included colitis (n=6), hypophysitis (n=2), arthritis (n=4), and thyroiditis (n=1). Clinically silent cases suggestive of immune-related adverse events included benign lymphadenopathy (n=8), most commonly sacroid-like bilateral hilar and mediastinal involvement, abnormal intramuscular hyperenhancing foci suggestive of myositis (n=2), and diffuse retroperitoneal fat stranding (n=2)20. The study also reported an interesting observation between tumor response and immune-related adverse events. The disease control rate, including those who achieved complete response, partial response, or stable disease according to RECIST1.1, was 55% in the group with radiologic manifestations of immune-related adverse events compared to 10% in the group without immune-related adverse events, indicating the association between radiologic manifestations of immune-related adverse events and improved tumor response and disease control. Awareness of this observation and further studies to systematically address this issue are important for radiologists to further contribute to the patient management during cancer immunotherapy20.
Fig. 8.
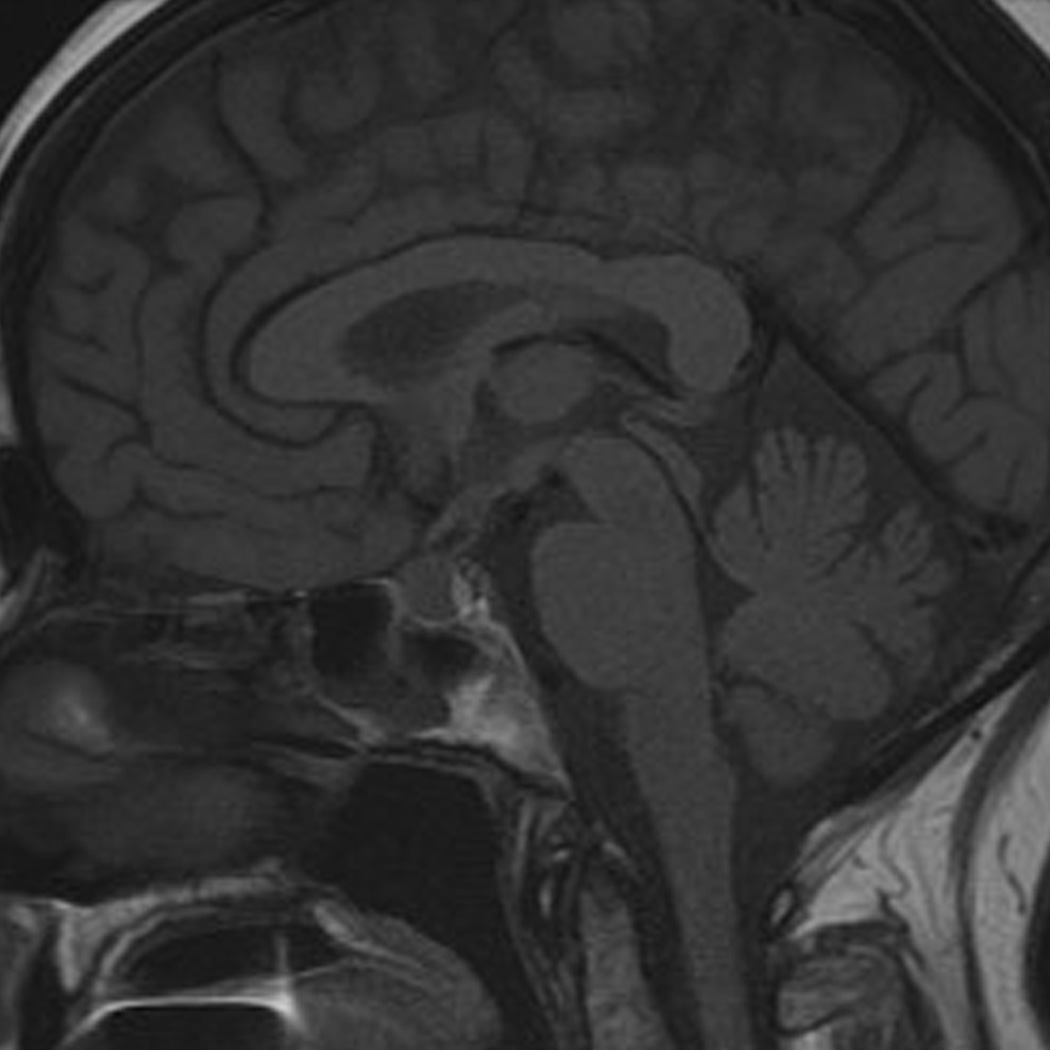
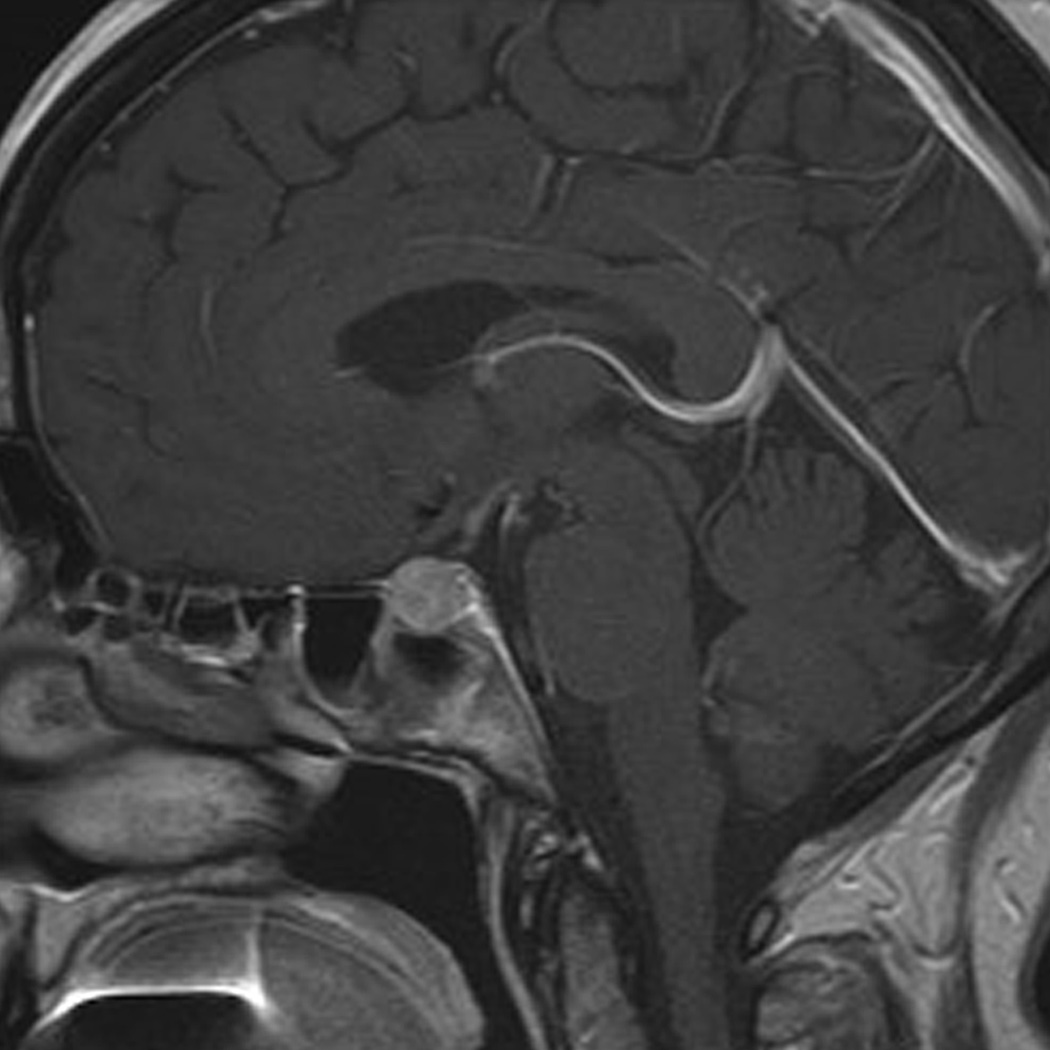
Ipilimumab-associated hypophysitis in a 56-year-old woman with metastatic melanoma. A, B. T1-weighted sagittal MR images of the brain prior to (A) and after (B) the administration of the intravenous contrast agent (gadobutrol) at 7 weeks since the initiation of ipilimumab therapy demonstrated a new marked enlargement of the pituitary gland with enhancement, indicating ipilimumab-associated hypophysitis. The study was negative for brain metastasis. The subsequent endocrinology work-up also revealed hypophysitis-related central hypothyroidism and secondary adrenal insufficiency. The patient was treated with oral predonisone and the follow-up MR imaging after 8 months since the last dose of ipilimumab showed the resolution of pituitary gland enlargement (not shown).
Fig. 9.
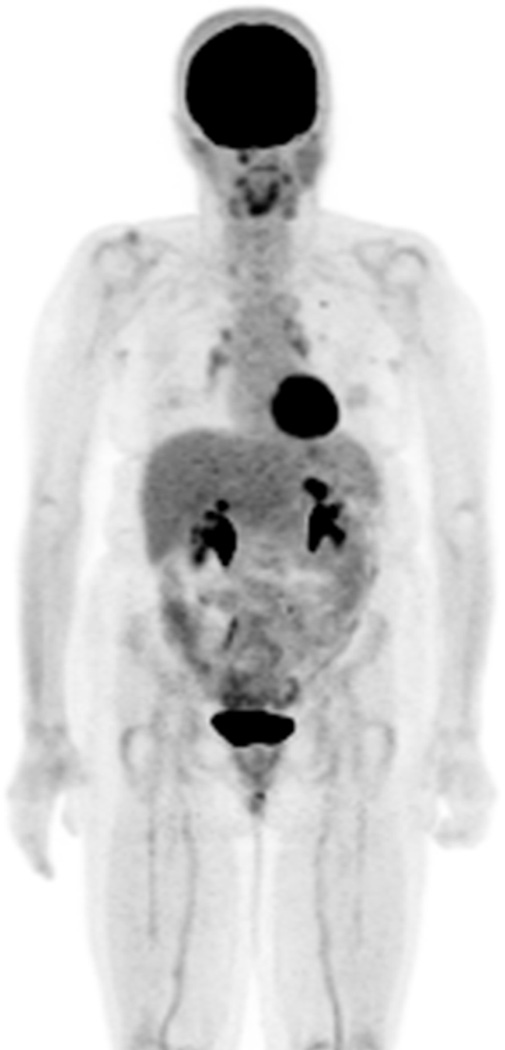
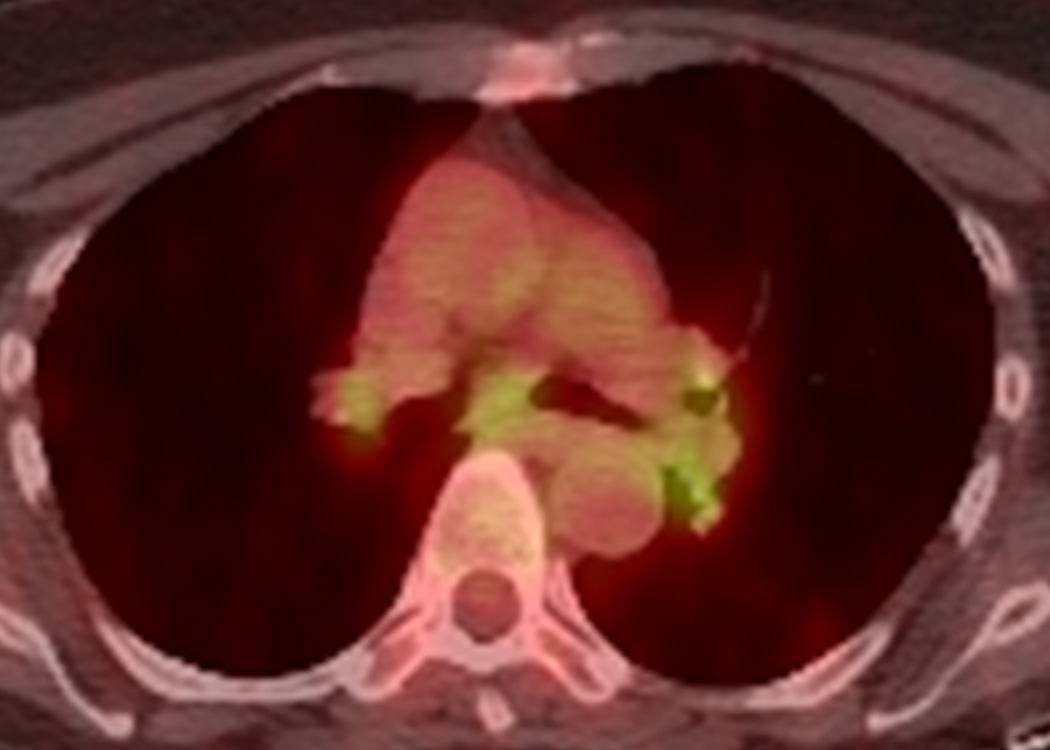
Ipilimumab-associated sarcoid-like mediastinal and hilar lymphadenopathy in a 73-year-old woman with metastatic melanoma.
A, B. Coronal maximum intensity projection (A) and axial fused FDG-PET/CT (B) images 3 months after the initiation of ipilimumab treatment demonstrated new FDG-avid mediastinal and hilar adenopathy mimicking sarcoidosis. Patient was asymptomatic at this time. A follow-up PET/CT 2 months later showed spontaneous resolution of the FDG-avid adenopathy (not shown).
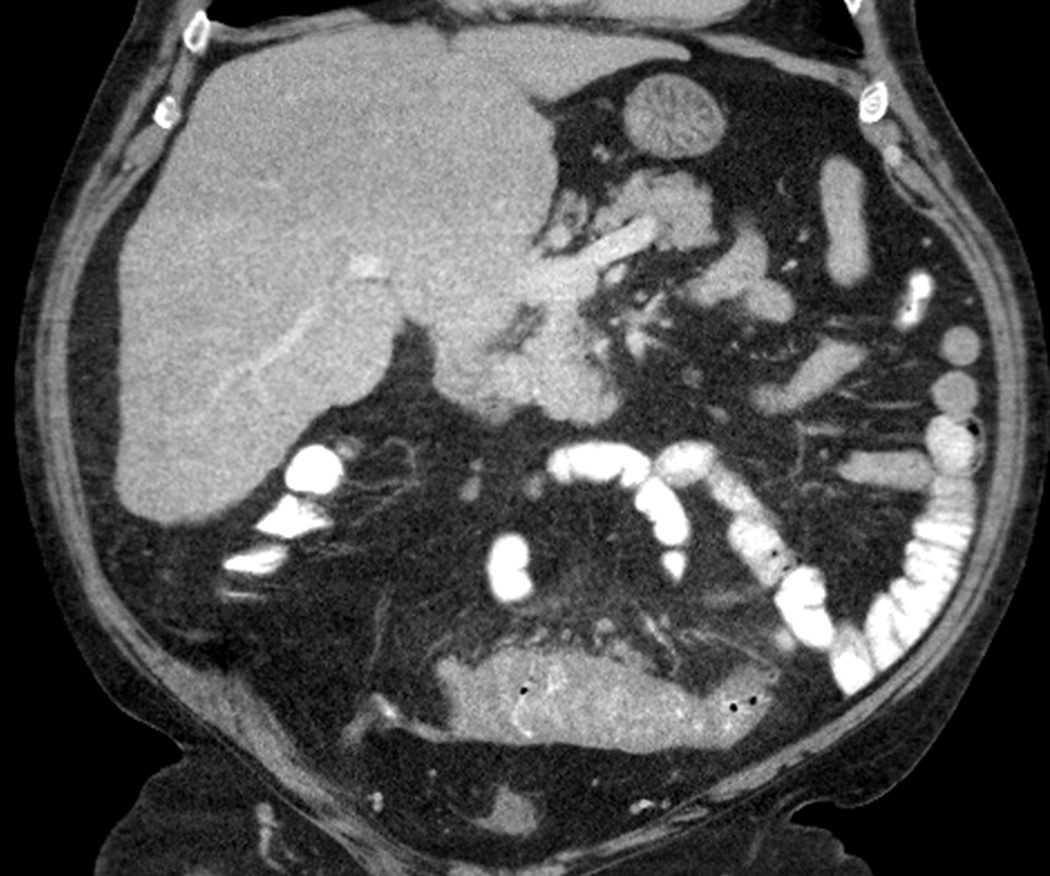
Radiologic manifestations of immune-related adverse events in specific organs have also been described. In a study of 16 patients diagnosed with ipilimumab-associated colitis by Kim et al, two distinct CT patterns were noted (Figs. 10, 11). Diffuse colitis pattern (n=12) demonstrated mesenteric vessel engorgement with mild diffuse bowel wall thickening or fluid-filled distended colon on imaging, and was treated with steroids. The segmental colitis associated with diverticulosis (SCAD) pattern (n = 4) was characterized by segmental moderate wall thickening and associated pericolic fat stranding in a segment of preexisting diverticulosis, and was treated with steroids and antibiotics22. In a study of 6 melanoma patients who were diagnosed as immune-related hepatitis during the ipilimumab therapy, a spectrum of imaging findings that was similar to acute hepatitis of common causes were noted24. Severe cases with systemic symptoms and highly increased level of liver function tests (LFTs) were characterized by mild hepatomegaly, periportal edema, and periportal lymphadenopathy, while mild asymptomatic cases with mildly increased level of LFTs had normal imaging findings. Histologically, ipilimumab associated hepatitis manifested either as a predominant injury to hepatocytes with an acute hepatitis pattern, or as a predominant injury to bile ducts (biliary pattern)24. Further clinical and radiographic characterization of immune-related adverse events of newer immunotherapeutic agents is currently ongoing.
Fig. 10.
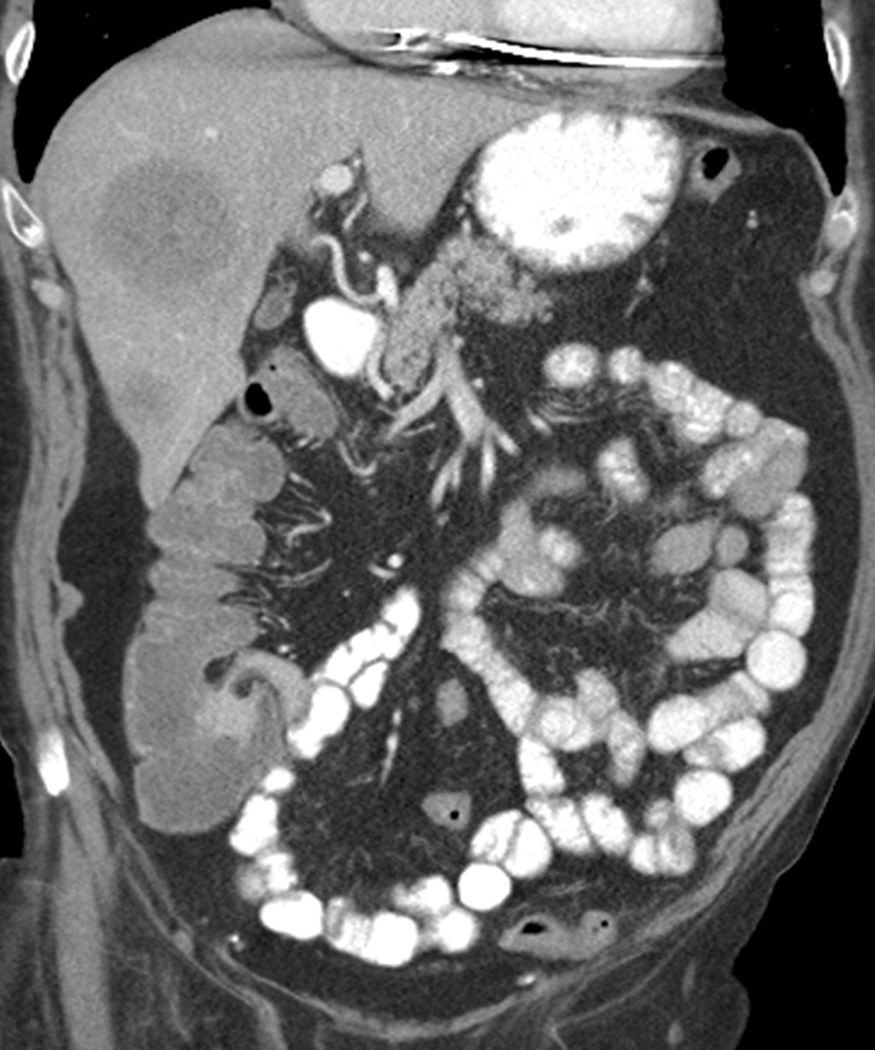
Ipilimumab-associated colitis with a diffuse colitis pattern in an 81-year-old man with watery diarrhea during ipilimumab treatment.
Coronal contrast-enhanced CT images at 3 months since the initiation of ipilimumab therapy demonstrated a new fluid-filled colonic distention with mild mesenteric vessel engorgement consistent with diffuse colitis. Also note metastatic lesions in the liver. Colonoscopic biopsy confirmed colonic inflammation consistent with drug induced mucosal injury.
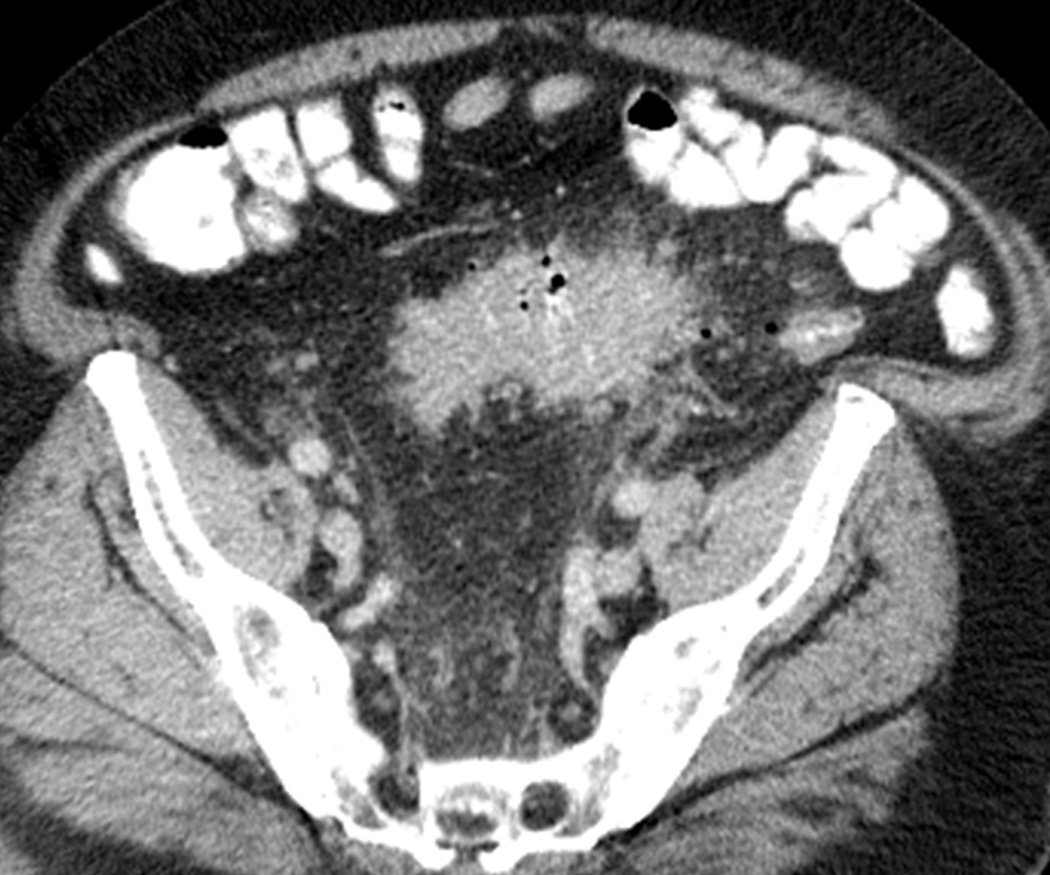
Fig. 11.
Ipilimumab-associated colitis with a segmental colitis associated with diverticulosis (SCAD) pattern in a 55-year-old man who presented with diarrhea during ipilimumab treatment. A, B. Axial (A) and coronal (B) contrast-enhanced CT images performed 3 months after start of treatment with ipilimumab showed segmental colitis associated with diverticulosis (SCAD) pattern, demonstrating severe segmental wall thickening of the sigmoid colon and pericolic fat stranding.
V. Future directions
With the increasing evidence of marked anti-cancer activity of immunotherapeutic agents in both solid and hematologic malignancies, cancer immunotherapy has opened a new promising field in cancer treatment and is rapidly expanding its role and significance. Radiologic assessment of tumor burden is an integral part of evaluating the efficacy and effectiveness of these agents, and the role of radiologists in this new field is also expected to expand. Unifying the strategy for immune-related tumor response assessment that can serve as a “common language” is the first priority, in order to describe the treatment results that can be directly compared across different trials and enable accurate and efficient communication among the investigators, to facilitate further advancement of the field of cancer immunotherapy. With the expertise in quantitative imaging, radiologists need to take an active part in this effort of developing and further optimizing such criteria as well as in the prospective validation of the criteria. Further investigations of the utility of advanced imaging techniques with functional information, which goes along with the translational research efforts of identifying predictive biomarkers for immune-related response, are also needed to complement limitations of size-based approach and to further optimize the strategy for immune-related response assessment.
Highlights.
The successful clinical application of cancer immunotherapy has opened a new arena for the treatment of advanced cancers
Cancer immunotherapy is associated with a variety of important radiographic features in the assessments of tumor response and immune-related adverse events
The state-of-the art knowledge of immunotherapy and the related radiologic manifestations are essential for radiologists
Acknowledgments
Role of the Funding Source
The investigator, M.N., was supported by 1K23CA157631 (NCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Dr. Nishino served as a consultant to Bristol-Myers Squibb. Dr. Hodi has served as a non-paid consultant to Bristol-Myers Squibb and has received clinical trial support from Bristol-Myers Squibb, advisor and clinical trial support from Merck, and advisor and clinical trial support from Genentech. Other authors have nothing to disclose.
REFERENCE
- 1.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 3.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 4.Freeman GJ, Gribben JG, Boussiotis VA, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 5.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, Fisher DE. Adoptive transfer of antigen-specific CD4+ T cells in the treatment of metastatic melanoma. Nat Clin Pract Oncol. 2008;5:696–697. doi: 10.1038/ncponc1259. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 10.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, Dranoff G. The biologic importance of tumor-infiltrating lymphocytes. J Cutan Pathol. 2010;37(Suppl 1):48–53. doi: 10.1111/j.1600-0560.2010.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 18.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936–3943. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–745. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. 2011;197:W992–W1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 21.O'Regan KN, Jagannathan JP, Ramaiya N, Hodi FS. Radiologic aspects of immune-related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapy. AJR Am J Roentgenol. 2011;197:W241–W246. doi: 10.2214/AJR.10.6032. [DOI] [PubMed] [Google Scholar]
- 22.Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab-associated colitis: CT findings. AJR Am J Roentgenol. 2013;200:W468–W474. doi: 10.2214/AJR.12.9751. [DOI] [PubMed] [Google Scholar]
- 23.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs. 2013;31:1071–1077. doi: 10.1007/s10637-013-9939-6. [DOI] [PubMed] [Google Scholar]
- 25.Wolchok J. How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma. Ann Oncol. 2012;23(Suppl 8):viii15–viii21. doi: 10.1093/annonc/mds258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodi FS, Oble DA, Drappatz J, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008;5:557–561. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 27.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 28.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielinski C, Knapp S, Mascaux C, Hirsch F. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann Oncol. 2013;24:1170–1179. doi: 10.1093/annonc/mds647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. N Engl J Med. 2014 doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 32.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 34.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 36.Madan RA MM, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 38.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boussiotis VA. Somatic mutations and immunotherapy outcome with CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2230–2232. doi: 10.1056/NEJMe1413061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 44.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 46.Erasmus JJ, Gladish GW, Broemeling L, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol. 2003;21:2574–2582. doi: 10.1200/JCO.2003.01.144. [DOI] [PubMed] [Google Scholar]
- 47.Nishino M, Guo M, Jackman DM, et al. CT tumor volume measurement in advanced non-small-cell lung cancer: Performance characteristics of an emerging clinical tool. Acad Radiol. 2011;18:54–62. doi: 10.1016/j.acra.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao B, James LP, Moskowitz CS, et al. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology. 2009;252:263–272. doi: 10.1148/radiol.2522081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao B, Tan Y, Bell DJ, et al. Exploring intra- and inter-reader variability in uni-dimensional, bi-dimensional, and volumetric measurements of solid tumors on CT scans reconstructed at different slice intervals. Eur J Radiol. 2013;82:959–968. doi: 10.1016/j.ejrad.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Nishino M, Hatabu H, Johnson BE, McLoud TC. State of the art: Response assessment in lung cancer in the era of genomic medicine. Radiology. 2014;271:6–27. doi: 10.1148/radiol.14122524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishino M, Gargano M, Suda M, Ramaiya NH, Hodi FS. Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer. 2014;2:17. doi: 10.1186/2051-1426-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohnsack OHA, Ludajic K. Adaptation of the immune-related response criteria: irRECIST. ESMO. 2014 Abstract 4958. [Google Scholar]
- 54.Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM. Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol. 2010;194:1470–1478. doi: 10.2214/AJR.09.3456. [DOI] [PubMed] [Google Scholar]
- 55.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 56.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 57.Gray MR, Martin del Campo S, Zhang X, et al. Metastatic melanoma: lactate dehydrogenase levels and CT imaging findings of tumor devascularization allow accurate prediction of survival in patients treated with bevacizumab. Radiology. 2014;270:425–434. doi: 10.1148/radiol.13130776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 59.Berthod G, Lazor R, Letovanec I, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol. 2012;30:e156–e159. doi: 10.1200/JCO.2011.39.3298. [DOI] [PubMed] [Google Scholar]
- 60.Carpenter KJ, Murtagh RD, Lilienfeld H, Weber J, Murtagh FR. Ipilimumab-induced hypophysitis: MR imaging findings. AJNR Am J Neuroradiol. 2009;30:1751–1753. doi: 10.3174/ajnr.A1623. [DOI] [PMC free article] [PubMed] [Google Scholar]