Abstract
Marine mammals are repeatedly exposed to elevated extra-thoracic pressure and alveolar collapse during diving and readily experience alveolar expansion upon inhalation – a unique capability as compared to terrestrial mammals. How marine mammal lungs overcome the challenges of frequent alveolar collapse and recruitment remains unknown. Recent studies indicate that pinniped lung surfactant has more anti-adhesive components compared to terrestrial mammals, which would aid in alveolar opening. However, pulmonary surfactant composition has not yet been investigated in odontocetes, whose physiology and diving behavior differ from pinnipeds. The aim of this study was to investigate the phosphatidylcholine (PC) composition of lung surfactants from various marine mammals and compare these to a terrestrial mammal. We found an increase in anti-adhesive PC species in harp seal (Pagophilus groenlandicus) and California sea lion (Zalophus californianus) compared to dog (Canus lupus familiaris), as well as an increase in the fluidizing PCs 16:0/14:0 and 16:0/16:1 in pinnipeds compared to odontocetes. The harbor porpoise (a representative of the odontocetes) did not have higher levels of fluidizing PCs compared to dog. Our preliminary results support previous findings that pinnipeds may have adapted unique surfactant compositions that allow them to dive at high pressures for extended periods without adverse effects. Future studies will need to investigate the differences in other surfactant components to fully assess the surfactant composition in odontocetes.
1. Introduction
In marine mammals, alveolar collapse while diving has been suggested to be an important trait that limits elevated blood and tissue N2 levels and prevents N2 narcosis during deep diving and decompression sickness during ascent (Falke et al., 1985; Ridgway and Howard, 1979; Scholander, 1940). Previous studies demonstrated that marine mammals have the capacity to completely collapse their alveoli (Fahlman et al., 2011; Falke et al., 1985; Kooyman and Sinnett, 1982; Meir et al., 2009; McDonald and Ponganis, 2012; Ridgway and Howard, 1979), but the relationship between ambient or intrathoracic pressure, alveolar size, and the extent of gas exchange remains unknown. In a study comparing California sea lions and harbor seals (species known to have different diving habits and respiratory structure) the relationship between diving depth and pulmonary shunt and the estimated alveolar collapse depth were indistinguishable (Kooyman and Sinnett, 1982).
These studies raise the question of how marine mammal lungs overcome the challenges of frequent alveolar collapse and re-inflation. Differences in the lung architecture may be responsible for the pressure-volume characteristics of marine mammal lungs and for the ability of collapsed alveoli to re-inflate. Alternatively, the differences in the pressure-volume relationship seen between odontocetes and phocids (Fahlman et al., 2011), and those reported between dogs and sea lions (Denison et al., 1971) could be related to differences in surfactant composition and alveolar surface properties. For example, an increase in unsaturated, anionic and short chain phospholipids, cholesterol, and surfactant proteins B and C all contribute to a surfactant that has better adsorption and spreading properties (Miller et al., 2005; Notter et al., 1980; Possmayer et al., 2001; Rau et al., 2004; Walters et al., 2000; Wang et al., 1995), which can contribute to an anti-adhesive surfactant and ease the recruitment of alveoli during ascent (Daniels et al., 1998; Miller et al., 2006a, 2006b, 2005).
It is clear from the literature that pulmonary surfactant composition correlates with lung structure, physiology, and development (Bernhard et al., 2001; Daniels et al., 1998; Hunt et al., 1991; Miller et al., 2005; Rau et al., 2004). One of the principle functions of pulmonary surfactant in mammals is to optimize surface tension based on lung volume. Ideally, surface tension is low at low lung volumes to prevent lung collapse and ease breathing and high at high lung volumes to control lung inflation. However, in many non-mammalian vertebrates (e.g., reptiles and amphibians) the respiratory units (e.g., faveolae) are large and compliant, negating the necessity for surfactant that has a high surface activity, i.e., the ability to greatly vary surface tension (Daniels et al., 1998, 1995). Reptiles and amphibians that have body temperatures below 25°C have higher levels of unsaturated phospholipids (e.g., phosphatidylcholine (PC) 16:0/16:1 and PC 16:0/20:4) and lower levels of disaturated phospholipids (e.g., PC 16:0/16:0) compared to those with higher body temperatures and mammals, resulting in surfactant that has a lower surface activity and a more anti-adhesive nature (Daniels et al., 1998, 1996, 1995).
In a variety of mammals, the fluidizing PCs 16:0/14:0 and 16:0/16:1 account for 10–35 mol% of the surfactant composition (Bernhard et al., 2011, 2001, 2000, 1997; Hunt et al., 1991; Miller et al., 2006b; Postle et al., 2001, 1999; Rau et al., 2004; Spragg et al., 2004); however in birds (whose lungs are stiff and do not appear to change volume during respiration), PCs 16:0/14:0 and 16:0/16:1 make up only a small percentage of the lung surfactant composition (Bernhard et al., 2001). The mole percent of PCs 16:0/14:0 and PC 16:0/16:1 correlate with respiratory rate in birds (duck and chicken), pigs, rats, and mice. Additionally, these PCs are higher in full term human infants, when respiratory rate is the fastest, than in developing fetuses (Bernhard et al., 2001), and PCs 16:0/14:0 and 16:0/16:0 increase at the end of gestation in humans, guinea pigs, and rat lung tissue (Hunt et al., 1991).
In addition to changes in PCs 16:0/14:0 and 16:0/16:1 during fetal development, differences were also found for these PCs between neonates and adult animals. Newborn piglets, whose development is similar to humans, had higher levels of PC 16:0/14:0 and PC 16:0/16:1 and lower levels of 16:0/16:0 than adult animals, resulting in a more fluid pulmonary surfactant (Rau et al., 2004). This suggests that the surfactant is adapted to match respiratory needs during development and maturation. In newborn piglets the increased fluidity of the surfactant facilitates rapid respiration rates and lung inflation (Rau et al., 2004).
Data from California sea lions also indicate that the pulmonary surfactant is optimized for physiological needs. Newborn sea lions had lower levels of PC 16:0/14:0 compared to adult sea lions, and neonatal alveoli attained a lower minimum surface tension than adults. These characteristics suggest that newborn sea lion surfactant is adapted for a terrestrial lifestyle, which the sea lion pups maintain for the first six months of life, while the adult sea lion surfactant is more fluid and optimized for a diving lifestyle (Miller et al., 2005).
Previous studies suggested that surfactant properties of broncho-alveolar lavage fluids from marine mammals did not appear to be significantly different from those of terrestrial mammals (Denison et al., 1971). But more recent work suggests that pulmonary surfactants in seals and sea lions have anti-adhesive properties that aid alveolar recruitment following collapse (Foot et al., 2006; Miller et al., 2006a, 2006b; Spragg et al., 2004). However, to the best of our knowledge the surfactant properties in odontocetes have not yet been characterized. We believe that these marine mammal groups may have divergent traits to deal with alveolar recruitment following collapse and hypothesize that the surface tension properties and the biochemical constituents of pulmonary surfactant differ between terrestrial and marine mammals and between pinnipeds and odontocetes. These adaptations may be related to differences in pulmonary anatomy, diving behaviors and physiological condition. Therefore, we investigated the PC composition of surfactant in dogs and several different marine mammals, including pinnipeds and odontocetes. Our results describe both qualitative and quantitative differences in the chemical composition of pulmonary surfactants and how these may reflect differences in the exploitation of disparate ecological habitats by various marine mammals.
2. Material and methods
2.1. Pulmonary lavage and surfactant isolation
Canine lungs were obtained from medium size (20 kg), rabies free dogs (Canus lupus familiaris) with no known preexisting lung diseases from the Corpus Christi Animal Control. Treatment of the lungs with isotonic saline solution was done immediately post-mortem. Two pulmonary lavage collection methods were used. The first method was performed while the lungs were still intact within the dogs. An endotracheal tube (Hudson RCI, Teleflex Medical Inc., Research Triangle Park, NC) was inserted via the larynx, inflated and approximately 75–100 mL of isotonic saline solution was infused into the lungs. The second method was performed after the lungs had been excised from the dogs. The chest wall was incised, the lungs were excised at the tracheal bifurcation, and 75–100 mL of the saline solution was poured directly into primary bronchi. For both methods, the lungs were gently rocked back and forth, and the lavage was collected in large glass containers. This process was repeated three times.
For marine mammals the lavage procedure was the same as for the canine, except that the lavage fluid volume was adjusted to approximately 150 mL per flush for larger animals. The animals obtained were either fishery bycatch (animals that either asphyxiated in gill nets or bottom otter trawls), the harp seal (Pagophilus groenlandicus; ID: IFAW 12 208Pg), or stranded – California sea lion (Zalophus californianus; CSL_10638), common dolphin (Delphinus delphis; ID: D08335Dd), and harbor porpoise (Phocoena phocoena; IDs: D08091Pp, Pp1201_Theo, C-386_Pp) – and were obtained from the National Oceanic and Atmospheric Administration National Marine Fisheries Service Northeast Fisheries Observer Program, the Marine Mammal Rescue and Research Division of the International Fund for Animal Welfare, the Marine Mammal Center (Sausalito, CA), or the Vancouver Aquarium (Vancouver, BC). The time of death was known for the stranded specimens, and the approximate time was recorded for bycaught specimens. All specimens were in good post mortem condition (Smithsonian Institute code 2; Geraci and Lounsbury, 2005) and none were decomposed when the lavage sample was obtained.
The lavage fluid was spun at 200 g for 15 minutes and the supernatant was collected. The supernatant was spun at 39,000 g for 60 minutes and the pellet was resuspended in isotonic saline solution and frozen at −80°C until further analysis. Where indicated, the lavage fluid was frozen prior to surfactant isolation (designated as frozen) or left at 4°C or room temperature for up to two days (timecourse analyses).
2.2. Phospholipid extraction
Aliquots (1 mL) of resuspended surfactant samples were mixed with 2:1 chloroform:methanol to achieve a final solvent ratio of 8:4:3 chloroform:methanol:isotonic saline (Folch 1956). Samples were vortexed for one minute and centrifuged at 930 g for 5 minutes. The bottom chloroform layer was collected and dried under a stream of N2. Samples were stored dessicated at −20°C.
2.3. Mass spectrometry analysis
Immediately prior to analysis samples were reconstituted in 1 mL of methanol. An aliquot of each sample (100 μL) was transferred to an autosampler vial, and methanol diluted (10-fold) or undiluted samples were placed in the autosampler of an Agilent 1200 HPLC (Agilent Technologies, Santa Clara, CA) at 20°C, and 5 μL was injected. Samples were filtered through a column shield (MAC-MOD Analytical, Chadds Ford, PA) onto a Kinetex C18(2), 150 × 3 mm, 2.6 μm, 100 Å column (Phenomenex, Torrance, CA) at 30°C. Phospholipids were separated isocratically at a 0.4 mL/min flow rate using a mobile phase mixture of 95% methanol, 5% water, and 10 mM ammonium acetate for 40 minutes. Samples were analyzed via electrospray ionization in positive ion mode using an Agilent 6410B triple quadrupole mass spectrometer with MassHunter Data Acquisition software (version B.02.01, Agilent Technologies). For qualitative analysis, samples were analyzed in full scan mode with a mass-to-charge (m/z) range of 600–850 and a fragmentation voltage of 200. To identify specific PCs, product ion scans were performed in negative ion mode on the acetate adducts. Additionally, precursor ion scans for m/z 184 were acquired in positive ion mode to confirm the presence of phosphatidylcholine. For quantitative analysis, samples were analyzed via multiple reaction monitoring. Optimal transition ions were determined using standards and MassHunter Optimizer software (ver B.02.01, Agilent Technologies). Fragmentor voltages and transition ions for the quantified PC phospholipids are listed in Supplementary Table 1. Data were analyzed using Qualitative Analysis software (ver. B.03.01, Agilent Technologies). Sodiated adducts were not taken into account for quantitation.
2.4 Identification and Quantitation
Retention time, m/z, and MS/MS of standard PCs were used to confirm the identity of sample PCs. PCs in each sample were quantified by comparing peak area in the samples to PC standard curves obtained by analysis of a mixture of PC phospholipids at seven different concentrations ranging from 0.2 – 20 ng/μL in duplicate or triplicate. Each sample PC identified was compared to the same standard PC with the exception of PC 16:1/18:1, which was quantified using the PC 16:0/18:2 standard, and PC 16:0/16:1, which was quantified using the PC 16:0/16:0 standard. These alternative standards were used as replacements because PC 16:1/18:1 and 16:0/18:2 were not readily obtainable. For a small number of samples (6%), the calculated injected sample concentrations were outside of the 0.2 – 20 ng/μL range of the standard curve. Values were extrapolated from their appropriate standard curve for these samples. For relative quantitation, the peak areas of extracted ion chromatograms from the full scan were compared to the peak area of PC 16:0/16:0 (reported as %DPPC – dipalmitoylphosphatidylcholine).
As determined by the product ion scans, in the California sea lion, harbor porpoise, and common dolphin samples there was a small amount of 18:1/14:0 that co-eluted with 16:0/16:1, and in some marine mammal samples (both odontocetes and pinnipeds) PC 20:1/16:0 co-eluted with PC 18:0/18:1. These were not separated for quantitation. Additionally, quantitation of PC 18:0/18:1 (m/z 758.6) was potentially affected by the presence of a phospholipid at m/z 720.6 + potassium.
2.5 Statistical analysis
Data were statistically analyzed using GraphPad Prism 6.0 (version 6.03, GraphPad Software, Inc., La Jolla, CA). The amount of PCs present in different species/groups (dog versus harbor porpoise; odontocetes versus pinnipeds) or at different times were compared using multiple t-tests followed by the Holm-Sidak method to correct for multiple comparisons. Two- way ordinary ANOVA was used to determine statistical differences in PC amounts when PC saturation in dog and harbor porpoise was compared. Results were considered significantly different when p < 0.05.
3. Results
3.1. Sample collection and preparation
As a first step to investigating marine mammal lung surfactant, we assessed the effects of different sample storage methods. Figure 1 shows the PC composition of pulmonary surfactant isolated from common dolphin pulmonary lavage. A portion of lavage fluid was frozen prior to surfactant isolation, whereas the remaining surfactant was recovered immediately. The percent difference between fresh and frozen for the majority of PCs was ≤ 15%. However, three PCs stood out with high percent differences between the fresh and frozen aliquots – PCs 16:0/18:1 (28%), 16:0/18:2, 16:1/18:1 (22%), and 16:1/16:1 (39%). Furthermore, Figure 1 shows that the total amount of extracted PCs from 1 mL aliquots of the reconstituted surfactant samples was almost five times lower in the frozen sample compared to the fresh sample, which were split from the same original sample. This could be due to differences in recovery and/or reconstitution of the surfactant after isolation, i.e., the surfactant was not recovered/reconstituted quantitatively, or due to differences in PC degradation during freezing. Importantly, the total amount of surfactant extracted does not impact the relative abundance of individual PCs, which were intentionally represented as relative values to overcome differences inherent to the analysis of species of such varying size, e.g., lung volume and lavage fluid volumes introduced and recovered. However, the discrepancy in total PCs extracted from the sample aliquots does indicate a potential for a differential ability to detect certain PC species – i.e., if PC abundance in one sample is very low but is not in another. It also indicates that if absolute quantification is desired, sample preparation, including lavage acquisition and surfactant recovery/reconstitution, will need to be strictly regulated from start to finish. Overall, these results suggest that freezing the sample prior to surfactant isolation may affect the accuracy of the reported composition.
Figure 1.
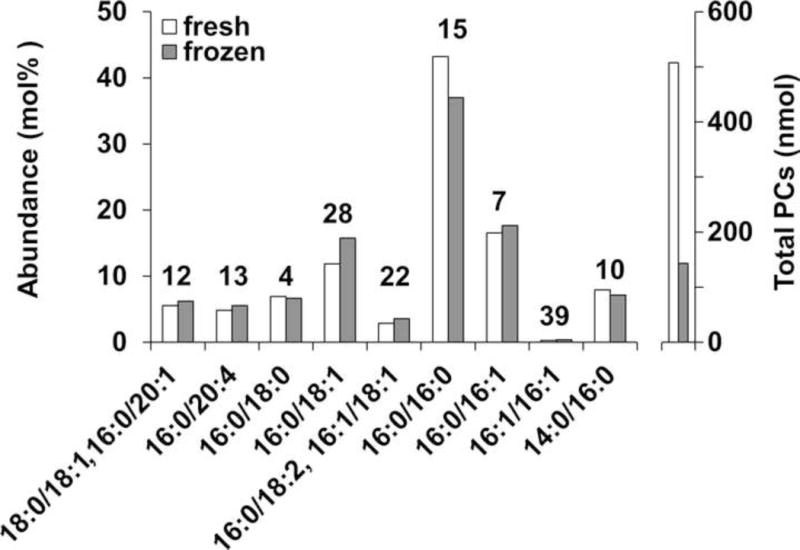
Effects of sample collection and storage on PC composition. The abundance of specific PC species (left axis) and the total abundance of PCs (right axis) determined from an aliquot of surfactant isolated from newly collected (fresh) or frozen common dolphin pulmonary lavage (n=1). The number above each bar represents the percent difference between fresh and frozen samples. The significance and implications of the difference in total amount of PCs extracted from 1 mL aliquots of the reconstituted surfactant samples (right axis) are discussed in the results.
To assess the effect of sampling time on pulmonary lavage fluid PC composition, we collected lavage fluid immediately after euthanasia of a California sea lion and then at intervals of one and two days after incubation at 4°C. The same sampling procedure was used for pulmonary lavage fluid from a euthanized dog. Figure 2 shows the PC composition in dog (Fig. 2A) and California sea lion (Fig. 2B) at the time of death and one or two days following death. The maximum percent difference between these times in dog was less than 15%, and no statistically significant differences were found between the time points (lowest p value > 0.52). The highest percent difference for California sea lion was also less than 15%, indicating that relative abundance of PC species was not altered within two days of death. Figure 2 also shows differences in the total amount of PCs extracted from 1 mL aliquots of the reconstituted surfactant samples of day zero and one or two days following. As mentioned, this could be due to variation in the recovery/reconstitution of the surfactant after isolation and does not affect the relative abundance of the individual PCs in each sample.
Figure 2.
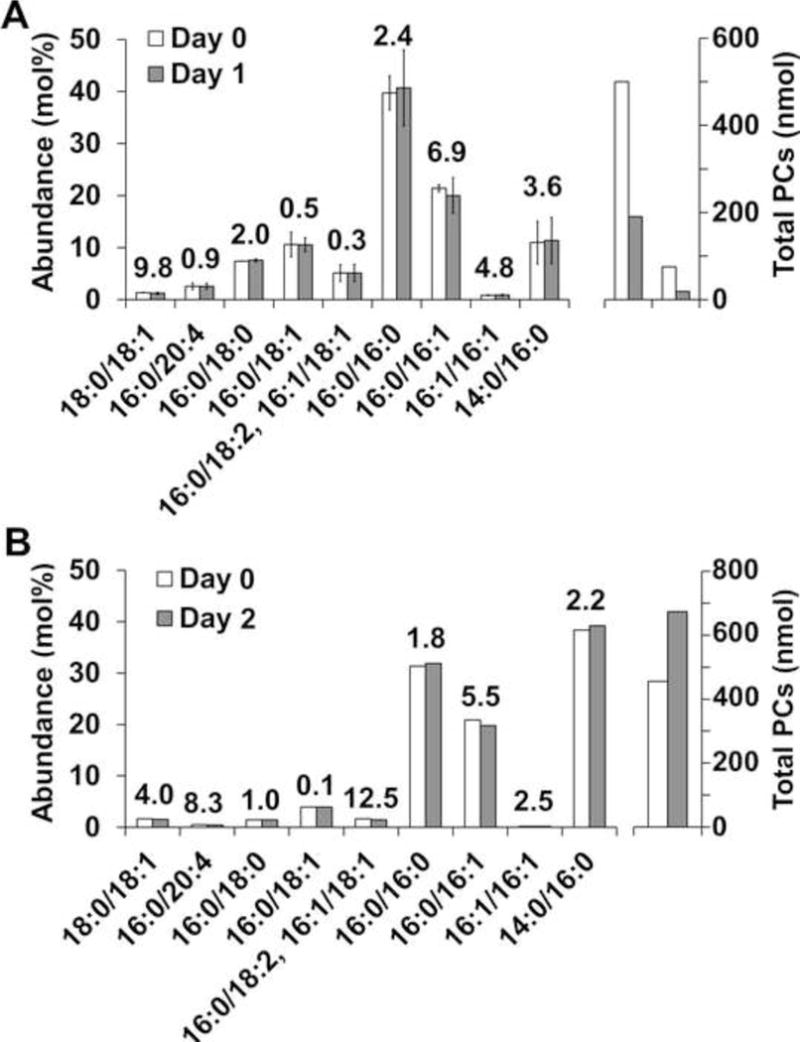
Stability of PC composition regarding postmortem sampling time. The abundance (left axis) of specific PC species in A) dog (n=2) and B) a California sea lion (n=1) is shown for samples collected immediately after death (day 0) or one or two days following death. There were no significant differences (p > 0.05) between day 0 and day 1 PC species for dog (error bars represent standard deviation of the mean, n=2). The number above each bar represents the percent difference between time points. The right axis shows the total PCs extracted from 1 Ml aliquots of the reconstituted surfactant samples; differences between total amounts are discussed in the results.
3.2. Terrestrial and marine mammal quantitative PC comparison
PC composition was examined in dog, harp seal, California sea lion, harbor porpoise, and common dolphin (Fig. 3). The most abundant PCs in all species were 16:0/16:0, 16:0/14:0, 16:0/18:1, and 16:0/16:1. Other quantified PCs included 16:1/16:1 – a minor component in all species analyzed – 16:0/18:0, 16:0/18:2, 16:1/18:1, 16:0/20:4, and 18:0/18:1. Figure 4 shows the comparison of PC composition in dog and harbor porpoise separated from the other species that were not replicated. PCs 16:0/16:1 and 16:1/16:1 were significantly higher (p ≤ 0.001) in dog compared to harbor porpoise (frozen).
Figure 3.
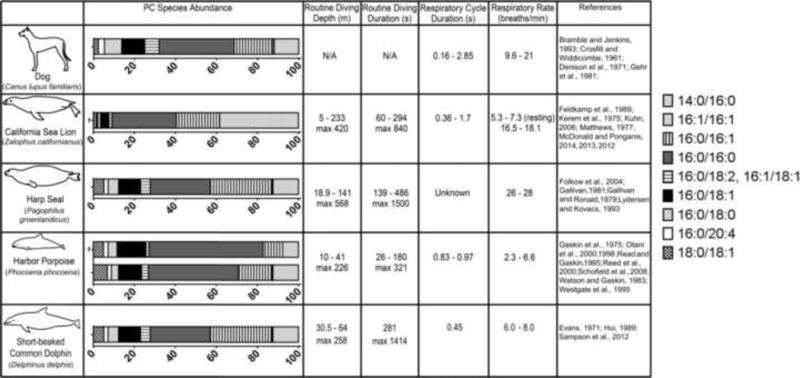
PC abundance in terrestrial and diving marine mammals. The abundance of specific PC species is shown for terrestrial (dog, n=7) and diving marine mammals. PC 18:0/18:1 coeluted with PC 16:0/20:1 in harp seal, harbor porpoise, and common dolphin but was undetermined in California sea lion. Subscript “f” indicates that the sample was frozen prior to surfactant isolation. Cartoon animals are courtesy of Sentiel Rommel.
Figure 4.
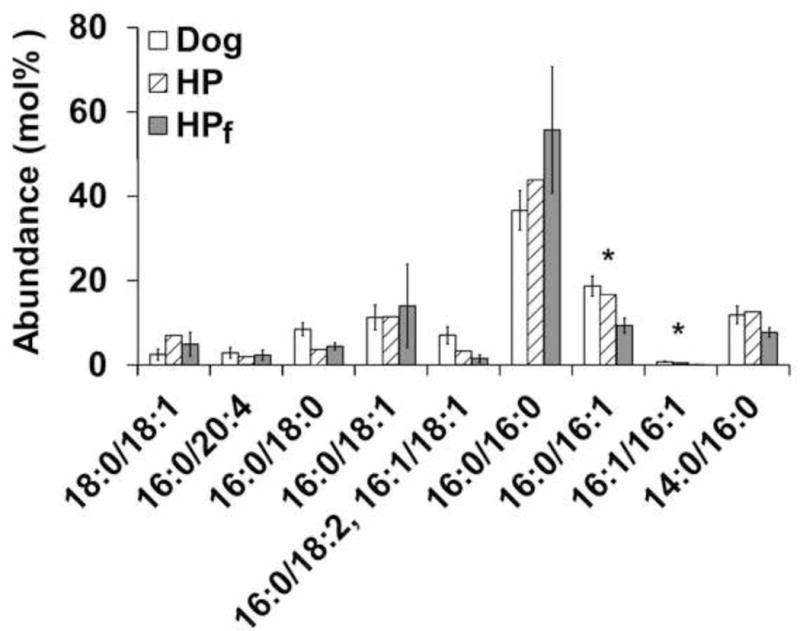
PC abundance in dog (n=7) and harbor porpoise (n=2, frozen; n=1, fresh). Error bars represent standard deviation of the mean. Asterisks represent PC species with statistically significant differences (p < 0.05). PC 18:0/18:1 co-eluted with PC 16:0/20:1 in HP. Subscript “f” indicates that the sample was frozen prior to surfactant isolation. Abbreviations: HP – harbor porpoise.
The amount of individual PCs in odontocetes (common dolphin and harbor porpoise) and pinnipeds (harp seal and California sea lion) were also compared. While there were appreciable differences in the amounts of PCs 16:0/16:0, 16:0/16:1 and 16:0/14:0 no statistically significant differences were found (Fig. 5a). When combined, PC 16:0/16:1 and 16:0/14:0 were higher in pinnipeds (51 ± 12%) compared to odontocetes (22 ± 5.9%; Fig. 5b), but this difference was not statistically significant. PC 16:0/16:0, 16:0/16:1 and 16:0/14:0, combined, were approximately 80 mol% in both groups.
Figure 5.
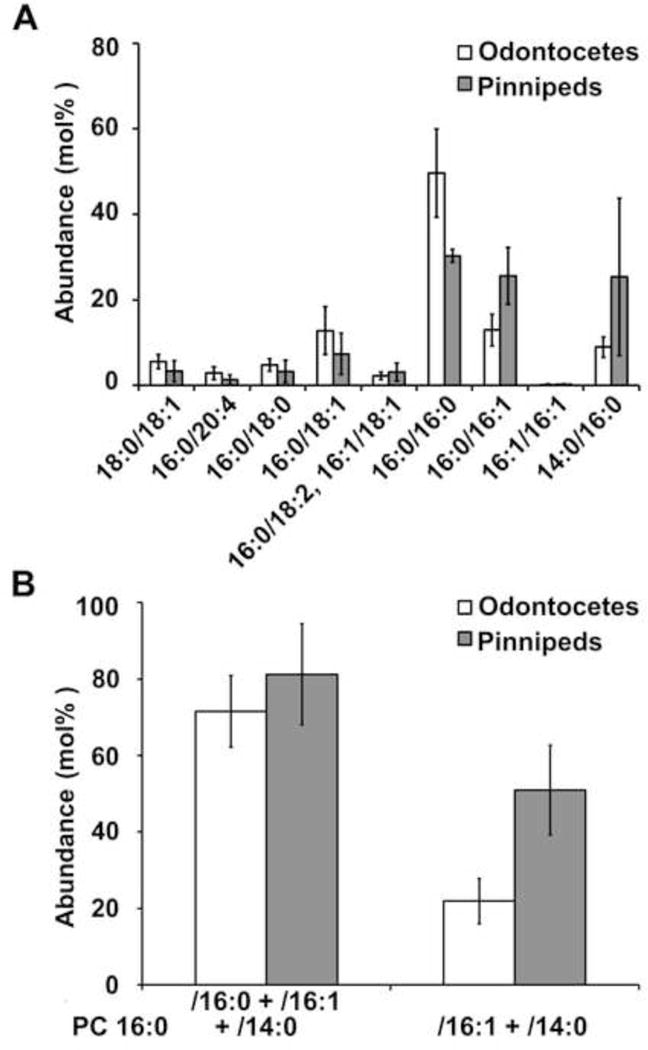
PC abundance in odontocetes versus pinnipeds. The abundance of A) chromatographically separated PC species and B) grouped PC species – PCs 16:0/16:0 + 16:0/16:1 + 16:0/14:0 and PCs 16:0/16:1 + 16:0/14:0 – are compared between odontocetes (common dolphin and harbor porpoise) and pinnipeds (harp seal and California sea lion). PC 18:0/18:1 co-eluted with PC 16:0/20:1 in harp seal, harbor porpoise, and dolphin but was undetermined in CSL. Error bars represent standard deviation of the mean. Harbor porpoise and California sea lion included samples frozen prior to surfactant isolation.
The differences between PC saturation (Fig 6) and chain length were also analyzed. There were no differences between dog PC saturation and that of any of the marine mammal species. These results were consistent even if only the most abundant unsaturated PC species (> 5%) were compared to the saturated PC species and if only PC species with two or more unsaturated fatty acids were considered. Additionally, the abundance of short chain PC species did not change significantly between dog and any of the marine mammal species (data not shown).
Figure 6.
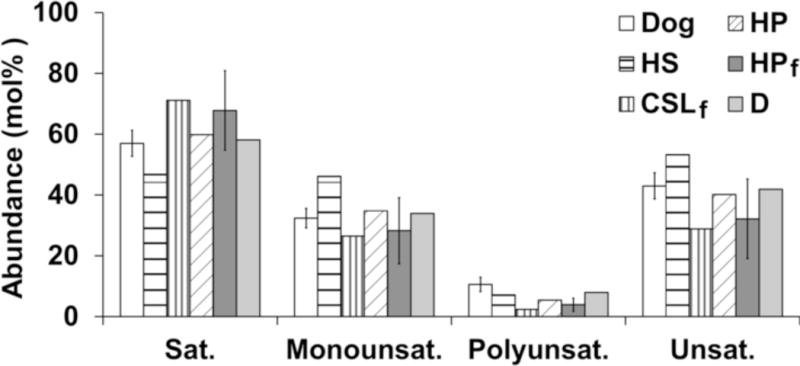
Abundance of saturated and unsaturated PCs among terrestrial and marine mammals. The abundance of saturated (14:0/16:0, 16:0/16:0, and 16:0/18:0), mono-unsaturated, polyunsaturated, and unsaturated PC fatty acids (16:1/16:1, 16:0/16:1, 16:0/18:1, 16:0/18:2, 16:1/18:1, 16:0/20:4, and 18:0/18:1) are shown for dog and marine mammals. Error bars represent standard deviation of the mean. PC 18:0/18:1 co-eluted with PC 16:0/20:1 in HS, HP, and D but was undetermined in CSL. Subscript “f” indicates that the sample was frozen prior to surfactant isolation. Abbreviations: CSL – California sea lion (n=1); D – common dolphin (n=1); HP – harbor porpoise (n=2, frozen; n=1, fresh); HS – harp seal (n=1).
3.3. Qualitative PC analysis
Several PC species were also identified in both dog and marine mammal samples that were not included in the initial MRM analyses. The most interesting differences included m/z 780.6 – identified as PC 16:0/20:5 – and m/z 784.6 – identified as PC 18:1/18:2. Figure 7 shows the relative quantitation of these species as a percent of PC 16:0/16:0. PC 16:0/20:5 was present from 5–16% in the various marine mammal species; however, it was only present in dog at an average of 0.12% – over a tenfold difference in relative abundance and significantly higher in harbor porpoise (frozen) than dog (p = 0.001). PC 18:1/18:2 was present at an average of 3% in dog but was absent or present only from 0.07–0.18% in the marine mammal species. When compared to harbor porpoise (frozen), PC 18:1/18:2 was significantly higher in dog (p < 0.005).
Figure 7.
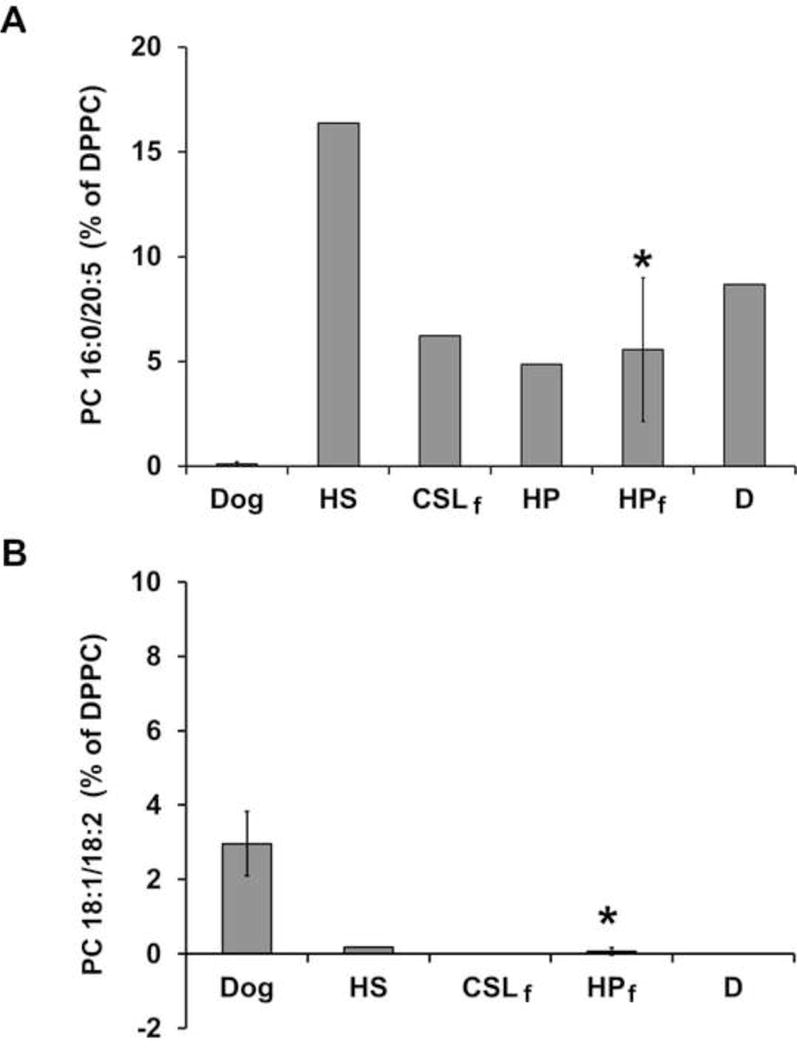
Relative quantitation of two PC species. Differences in the relative abundance of A) PC 16:0/20:5 and B) PC 18:1/18:2 are shown for dog and marine mammal samples. Subscript “f” indicates that the sample was frozen prior to surfactant isolation. Error bars represent standard deviation of the mean. Asterisks indicate PC species with statistically significant differences (p < 0.005) relative to dog. Abbreviations: CSL – California sea lion (n=1); D – common dolphin (n=1); DPPC – dipalmitoylphosphatidylcholine; HP – harbor porpoise (n=2, frozen; n=1, fresh); HS – harp seal (n=1).
4. Discussion
Recent work comparing the composition of pulmonary surfactants between seals and sea lions to terrestrial mammals showed that these species have higher levels of anti-adhesive phospholipids, including PCs 16:0/14:0 and 16:0/16:1, than terrestrial mammals (Miller et al., 2006a, 2006b; Spragg et al., 2004); however, this work did not investigate surfactant composition in odontocetes. PCs represent approximately 70–80% of pulmonary surfactant phospholipids in marine and terrestrial mammals, including humans (Daniels et al., 1998; Miller et al., 2006b; Spragg et al., 2004). Here we have sampled pulmonary lavage from harp seal, sea lion, and odontocetes (common dolphin and harbor porpoise) to compare PC composition.
For logistical reasons, marine mammal tissue/fluids cannot always be processed immediately after collection in the field. The option to freeze pulmonary lavage fluid after collection and before surfactant isolation would increase the number of samples that can be obtained for analysis if the samples are stable. Our data showed that with fresh (code 2) animals, the composition of the identified PCs did change if the lavage was frozen prior to surfactant isolation (Fig. 1); however, another dolphin sample was not obtained to confirm this finding. As such, data from samples frozen prior to surfactant isolation should be interpreted cautiously.
As post mortem change can vary between beach cast marine mammals, sample validation to assess the impact of post mortem interval on sample quality and recovery was initially undertaken. This demonstrated little surfactant degradation over a 24–48 hour post sampling interval at 4°C (Fig. 2). These data indicate that lavage collected from stranded marine mammals up to two days postmortem will provide reliable information with respect to the composition of surfactant PCs relative to each other (i.e., mole percent).
To investigate the differences in pulmonary surfactant composition among marine mammals with various diving adaptations and to compare results to terrestrial mammals, we analyzed pulmonary surfactant from dog, pinnipeds, and odontocetes. Pulmonary surfactant from fur seals, elephant seals, ringed seals, harbor seals, and sea lions have been previously characterized (Miller et al., 2006b, 2005; Spragg et al., 2004) and are discussed here for comparison to previous research. However to the best of our knowledge, pulmonary surfactant from odontocetes has not previously been reported, and the different diving behavior of these animals compared to pinnipeds may offer insight into a relationship between surfactant composition and diving physiology.
The PC values obtained in this study are comparable to previously published PCs from other pinniped species (Miller et al., 2006b; Spragg et al., 2004). Table 1 shows the fold difference of shared PCs identified in harp seal compared to elephant seal, harbor seal, and ringed seal. Most differences were within 2-fold. The greater differences may be due to different sampled species, post mortem interval, intercurrent pulmonary disease, and possibly differences in methodology. In this work, PCs were only included if product ions scans for the fatty acids were able to confirm the specific PC and if the retention time matched the standard (for available standards). Precursor scans for m/z 184 were also used to confirm the PC headgroup.
Table 1.
Fold change in harp seal PC composition
| PC | Elephant seal*ˆ | Harbor seal* | Rinqed sealˆ |
|---|---|---|---|
| 16:0/14:0 | < ±2 | < ±2 | < ±2 |
| 16:0/16:1 | < ±2 | < ±2 | < ±2 |
| 16:0/16:0 | < ±2 | < ±2 | < ±2 |
| 16:0/18:1 | < ±2 | < ±2 | < ±2 |
| 16:0/18:0 | < ±2 | 2.2 | ne |
| 16:0/20:4 | 2.4 | 2.4 | ne |
| 13:0/18:1 | 5 7 | 3.2 | ne |
The notation “< ±2” indicates a less than 2 fold change in harp seal above (+) or below (−) the other species; “ne” – not estimated from the original paper. Comparison made by estimating values from:
When compared to dog, harp seals had a similar surfactant composition with a few exceptions. Unsaturated PCs 16:1/16:1 and 18:1/18:2 were higher in dog (0.75 mol% and 3 %DPPC, respectively) compared to seal (0.33 mol% and 0.18 %DPPC, respectively). The combined amount of PCs 16:1/16:1 and 18:1/18:2 was greater in dog than in harp seal; however, the amount of PC 18:0/18:1, 16:0/20:1 in harp seal, at 5 mol%, was over 2-fold higher than the above PCs in dog. Furthermore, harp seal had a higher relative amount of PC 16:0/20:5 than dog – 16 %DPPC compared to 0.12 %DPPC. These findings suggest the potential for a more anti-adhesive surfactant in the harp seal, in agreement with previous work (Miller et al., 2006b; Spragg et al., 2004).
The values for individual PCs from sea lion presented in Table 2 differed from those previously published (Miller et al., 2006b; Spragg et al., 2004). PC 18:1/18:2 was not found in our case, but was previously reported at 0.2 mol% (Spragg et al., 2004). It is possible that PC 18:1/18:2 is present in sea lion but that the one processed sample was not representative. However, previous misidentification is also a possibility. MS/MS analysis is essential for valid PC identification as PCs of the same molecular weight can co-elute depending on chromatographic conditions. For example, PC standards for 16:0/16:0 and 14:0/18:0 have the same m/z and co-eluted under the conditions in this study. These PCs were only distinguishable by MS/MS analysis of the fatty acids.
Table 2.
Fold change in sea lion PC composition
| PC | Spragg et al., 2004 | Miller et al., 2006b | Miller et al., 2005 |
|---|---|---|---|
| 16:0/14:0 | 3.6 | 4 8 | 3 5 |
| 16:0/16:1 | < ±2 | < ±2 | < ±2 |
| 16:0/16:0 | < ±2 | < ±2 | < ±2 |
| 16:0/18:1 | 0.36 | 0.30 | 0.40 |
| 16:0/18:0 | 0.52 | ne | 0.49 |
| 16:0/20:4 | 0.46 | ne | nl |
| 18:0/18:1 | <±2 | ne | < ±2 |
The notation “< ±2” indicates a less than 2 fold change in sea lion above (+) or below (−) the sea lion data from other studies; “ne” – not estimated from the original paper; “nl” – not listed in the original paper. Note this sample was frozen prior to surfactant isolation.
The PC composition of sea lion lung surfactant was different from dog surfactant. Sea lion surfactant had 3 fold higher levels of PC 16:0/14:0 and 2.8–5.8 fold lower levels of PCs 16:1/16:1, 16:0/18:2, 16:1/18:1, 16:0/18:1, 16:0/18:0, and 16:0/20:4. Additionally, 18:1/18:2 was detected in dog but not in sea lion. The latter PCs make up a combined 8 mol% and that of PC 16:0/14:0 constitutes 38 mol% of the total sea lion PCs. Furthermore, sea lion PC 16:0/20:5 was at 6 %DPPC compared to dog at 0.12 %DPPC. As indicated previously (Miller et al., 2006b; Spragg et al., 2004), the increase in PCs 16:0/14:0 and 16:0/20:5 compared to dog suggests a greater fluidity for surfactant of sea lion.
We also analyzed lung surfactant in odontocetes, specifically in harbor porpoise and common dolphin. The differences between dog and harbor porpoise (frozen) were only significant for two less abundant PCs – 16:0/16:1 and 16:1:16:1, which were higher in dog (Fig. 4). However, the near equal amount of PC16:0/16:1 in fresh harbor porpoise and dog diminishes the significance of this difference between the dog and frozen harbor porpoise. When the odontocetes were compared to pinnipeds, no statistically significant differences were found in PC composition (Fig. 5a); however, PCs 16:0/16:0 and 16:0/16:1 had low unadjusted p-values (p < 0.05). Moreover, the graph shows an appreciable difference in the level of these PCs between the two groups. Although PC 16:0/14:0 exhibits a similar appreciable difference, the error bars overlap and the disparity is most likely due to the high levels of this PC in sea lion (38 mol%). The harp seal had only slightly higher levels of PC 16:0/14:0 (12 mol%) than the odontocetes (8 mol%).
Bernhard et al. (2001) found that PCs 16:0/16:0, 16:0/16:1, and 16:0/14:0 composed around 80% of the lung surfactant in duck, chicken, pigs, rats and mice, and Hunt et al. (1991), reported similar concentrations in human and guinea pig pulmonary lavages. Similar levels of PCs 16:0/16:0, 16:0/16:1, and 16:0/14:0 were found in odontocetes (73 ± 8.5%) and pinnipeds (81 ± 13%; Fig 5b). When PCs 16:0/14:0 and 16:0/16:1 were combined, their abundance was higher in pinnipeds compared to odontocetes (Fig. 5b), suggesting that these surfactants play a greater role in increasing the fluidity of pinniped surfactant compared to odontocetes surfactant. These differences could be related to respiratory physiology (Bernhard et al., 2001), lung structure (Daniels et al., 1998), or diving behavior (Fig. 3). California sea lion and harp seal have deeper and longer routine dives compared to harbor porpoise and common dolphin. These pinnipeds also have a higher respiratory rate than the two odontocetes studied, while respiratory flow-rates are considerably higher in the odontocetes (Kerem et al., 1975; Kooyman and Cornell, 1981; Kooyman and Sinnett 1979).
In conclusion, we have analyzed the PC composition of representative marine mammals from odontocetes and pinniped groups in order to determine if pulmonary surfactant composition is related to differences in diving behaviors and physiology. Our data support the hypothesis that anti-adhesive PCs are increased in seal and sea lion compared to dog, with differences in surfactant composition between odontocetes and pinnipeds. While PCs are the major component in pulmonary surfactant, other compounds such as anionic phospholipids (e.g., phosphatidylglycerol and phosphatidylinositol), surfactant proteins, and cholesterol can all affect surfactant fluidity (Miller et al., 2005; Notter et al., 1980; Rau et al., 2004; Wang et al., 1995), and it is possible that differences in minor components may cause important changes that optimize the surfactant to meet the physiological and diving demands of an organism. In order to further compare fluidity, the composition of other phospholipid classes, surfactant proteins B and C, and cholesterol will need to be considered. This will be especially interesting for odontocetes, which did not have significantly higher levels of the fluidizing PCs analyzed here compared to terrestrial mammals. Future research will need to investigate the functional effects of various surfactant compositions on alveolar collapse/recruitment and surface tension.
Supplementary Material
Highlights.
Fluidizing PCs are increased in pinniped compared to dog pulmonary surfactant
PCs 16:0/14:0 and 16:0/16:1 are more abundant in pinnipeds than odontocetes
Surfactant PC composition of dog and odontocetes are not significantly different
Acknowledgments
Funding for this research included the Texas A&M University Center for Coastal Studies and Office of Research and Commercialization (PZ, DBG), the NSF-NIH/NIES (PZ, R01 ES21968–1), and the Office of Naval Research (AF, award # N000141210269). The lung surfactants obtained in this study were collected from animals that had died from causes unrelated to the study and by a NMFS authorization letter to AF. We are grateful to the Cape Cod stranding network, NE Fisheries Observer Program and the Marine Mammal Center that made it possible to collect bronchoalveolar lavage samples from marine mammals, as well as to the Natural Sciences and Engineering Research Council of Canada (MP) through which some samples were processed. We are also grateful to Ramon Herrera III at Robstown Animal Control that allowed us to collect BAL samples from euthanized dogs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andreas Fahlman, Email: Andreas.Fahlman@tamucc.edu.
Manuela Gardner, Email: Manuela.Gardner@tamucc.edu.
Danielle Kleinhenz, Email: Danielle.Kleinhenz@tamucc.edu.
Marina Piscitelli, Email: piscitellim@gmail.com.
Stephen Raverty, Email: Stephen.Raverty@gov.bc.ca.
Martin Haulena, Email: Martin.Haulena@vanaqua.org.
Paul V. Zimba, Email: Paul.Zimba@tamucc.edu.
References
- Bernhard W, Haagsman HP, Tschernig T, Poets CF, Postle AD, van Eijk ME, von der Hardt H. Conductive airway surfactant: surface-tension function, biochemical composition, and possible alveolar origin. Am J Respir Cell Mol Biol. 1997;17:41–50. doi: 10.1165/ajrcmb.17.1.2594. [DOI] [PubMed] [Google Scholar]
- Bernhard W, Hoffmann S, Dombrowsky H, Rau GA, Kamlage A, Kappler M, Haitsma JJ, Freihorst J, von der Hardt H, Poets CF. Phosphatidylcholine molecular species in lung surfactant: composition in relation to respiratory rate and lung development. Am J Respir Cell Mol Biol. 2001;25:725–731. doi: 10.1165/ajrcmb.25.6.4616. [DOI] [PubMed] [Google Scholar]
- Bernhard W, Mottaghian J, Gebert A, Rau GA, von der Hardt H, Poets CF. Commercial versus native surfactants. Surface activity, molecular components, and the effect of calcium. Am J Respir Crit Care Med. 2000;162:1524–1533. doi: 10.1164/ajrccm.162.4.9908104. [DOI] [PubMed] [Google Scholar]
- Bernhard W, Raith M, Pynn CJ, Gille C, Stichtenoth G, Stoll D, Schleicher E, Poets CF. Increased palmitoyl-myristoyl-phosphatidylcholine in neonatal rat surfactant is lung specific and correlates with oral myristic acid supply. J Appl Physiol. 2011;111:449–457. doi: 10.1152/japplphysiol.00766.2010. [DOI] [PubMed] [Google Scholar]
- Bramble DM, Jenkins FA. Mammalian locomotor-respiratory integration: implications for diaphragmatic and pulmonary design. Science. 1993;262:235–240. doi: 10.1126/science.8211141. [DOI] [PubMed] [Google Scholar]
- Crosfill ML, Widdicombe JG. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol. 1961;158:1–14. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CB, Lopatko OV, Orgeig S. Evolution of surface activity related functions of vertebrate pulmonary surfactant. Clin Exp Pharmacol Physiol. 1998;25:716–721. doi: 10.1111/j.1440-1681.1998.tb02283.x. [DOI] [PubMed] [Google Scholar]
- Daniels CB, Orgeig S, Smits AW. The evolution of the vertebrate pulmonary surfactant system. Physiol Zool. 1995;68:539–566. doi: 10.2307/30166344. [DOI] [Google Scholar]
- Daniels CB, Orgeig S, Smits AW, Miller JD. The influence of temperature, phylogeny, and lung structure on the lipid composition of reptilian pulmonary surfactant. Exp Lung Res. 1996;22:267–281. doi: 10.3109/01902149609031775. [DOI] [PubMed] [Google Scholar]
- Denison DM, Warrell DA, West JB. Airway structure and alveolar emptying in the lungs of sea lions and dogs. Respir Physiol. 1971;13:253–260. doi: 10.1016/0034-5687(71)90029-6. [DOI] [PubMed] [Google Scholar]
- Evans WE. Orientation behavior of Delphinids: radio telemetric studies. Ann N Y Acad Sci. 1971;188:142–160. doi: 10.1111/j.1749-6632.1971.tb13094.x. [DOI] [PubMed] [Google Scholar]
- Fahlman A, Loring SH, Ferrigno M, Moore C, Early G, Niemeyer M, Lentell B, Wenzel F, Joy R, Moore MJ. Static inflation and deflation pressure-volume curves from excised lungs of marine mammals. J Exp Biol. 2011;214:3822–3828. doi: 10.1242/jeb.056366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke KJ, Hill RD, Qvist J, Schneider RC, Guppy M, Liggins GC, Hochachka PW, Elliott RE, Zapol WM. Seal lungs collapse during free diving: evidence from arterial nitrogen tensions. Science. 1985;229:556–558. doi: 10.1126/science.4023700. [DOI] [PubMed] [Google Scholar]
- Feldkamp SD, DeLong RL, Antonelis GA. Diving patterns of California sea lions, Zalophus californianus. Can J Zool. 1989;67:872–883. doi: 10.1139/z89-129. [DOI] [Google Scholar]
- Folkow LP, Nordøy ES, Blix AS. Distribution and diving behaviour of harp seals (Pagophilus groenlandicus) from the Greenland Sea stock. Polar Biol. 2004;27:281–298. doi: 10.1007/s00300-004-0591-7. [DOI] [Google Scholar]
- Foot NJ, Orgeig S, Daniels CB. The evolution of a physiological system: the pulmonary surfactant system in diving mammals. Respir Physiol Neurobiol. 2006;154:118–138. doi: 10.1016/j.resp.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Gallivan GJ. Ventilation and gas exchange in unrestrained harp seals (Phoca groenlandica) Comp Biochem Physiol A Physiol. 1981;69:809–813. doi: 10.1016/0300-9629(81)90175-4. [DOI] [Google Scholar]
- Gallivan GJ, Ronald K. Temperature regulation in freely diving harp seals (Phoca groenlandica) Can J Zool. 1979;57:2256–2263. doi: 10.1139/z79-293. [DOI] [PubMed] [Google Scholar]
- Gaskin DE, Smith GJD, Watson AP. Preliminary study of movements of harbor porpoises (Phocoena phocoena) in the Bay of Fundy using radiotelemetry. Can J Zool. 1975;53:1466–1471. doi: 10.1139/z75-177. [DOI] [Google Scholar]
- Gehr P, Mwangi DK, Ammann A, Maloiy GM, Taylor CR, Weibel ER. Design of the mammalian respiratory system. V. Scaling morphometric pulmonary diffusing capacity to body mass: wild and domestic mammals. Respir Physiol. 1981;44:61–86. doi: 10.1016/0034-5687(81)90077-3. [DOI] [PubMed] [Google Scholar]
- Geraci JR, Lounsbury VJ. Marine mammals ashore: a field guide for strandings. National Aquarium; Baltimore: 2005. [Google Scholar]
- Hui CA. Surfacing behavior and ventilation in free-ranging dolphins. J Mammal. 1989;70:833. doi: 10.2307/1381722. [DOI] [Google Scholar]
- Hunt AN, Kelly FJ, Postle AD. Developmental variation in whole human lung phosphatidylcholine molecular species: a comparison with guinea pig and rat. Early Hum Dev. 1991;25:157–171. doi: 10.1016/0378-3782(91)90112-G. [DOI] [PubMed] [Google Scholar]
- Kerem DH, Kylstra JA, Saltzman HA. Respiratory flow rates in the sea lion. Undersea Biomed Res. 1975;2:20–27. [PubMed] [Google Scholar]
- Kooyman GL, Cornell LH. Flow properties of expiration and inspiration in a trained bottle-nosed porpoise. Physiol Zool. 1981;54:55–61. [Google Scholar]
- Kooyman GL, Kerem DH, Campbell WB, Wright JJ. Pulmonary function in freely diving Weddell seals, Leptonychotes weddelli. Respir Physiol. 1971;12:271–282. doi: 10.1016/0034-5687(71)90069-7. [DOI] [PubMed] [Google Scholar]
- Kooyman GL, Sinnett EE. Pulmonary shunts in harbor seals and sea lions during simulated dives to depth. Physiol Zool. 1982;55:105–111. [Google Scholar]
- Kooyman GL, Sinnett EE. Mechanical properties of the harbor porpoise lung, Phocoena phocoena. Respir Physiol. 1979;36:287–300. doi: 10.1016/0034-5687(79)90042-2. [DOI] [PubMed] [Google Scholar]
- Kuhn CE. Measuring at sea feeding to understand the foraging behavior of pinnipeds. Calif Sea Grant Coll Program 2006 [Google Scholar]
- Lydersen C, Kovacs KM. Diving behaviour of lactating harp seal, Phoca groenlandica, females from the Gulf of St Lawrence, Canada. Anim Behav. 1993;46:1213–1221. doi: 10.1006/anbe.1993.1312. [DOI] [Google Scholar]
- Matthews RC. Pulmonary mechanics of California sea lions. Zalophus californianus 1977 [Google Scholar]
- McDonald B, Ponganis P. Deep-diving sea lions exhibit extreme bradycardia in long-duration dives (879.3) FASEB J. 2014;28:879.3. doi: 10.1242/jeb.098558. [DOI] [PubMed] [Google Scholar]
- McDonald BI, Ponganis PJ. Insights from venous oxygen profiles: oxygen utilization and management in diving California sea lions. J Exp Biol. 2013;216:3332–3341. doi: 10.1242/jeb.085985. [DOI] [PubMed] [Google Scholar]
- McDonald BI, Ponganis PJ. Lung collapse in the diving sea lion: hold the nitrogen and save the oxygen. Biol Lett. 2012;8:1047–1049. doi: 10.1098/rsbl.2012.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir JU, Champagne CD, Costa DP, Williams CL, Ponganis PJ. Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am J Physiol Regul Integr Comp Physiol. 2009;297:R927–939. doi: 10.1152/ajpregu.00247.2009. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Daniels CB, Schürch S, Schoel WM, Orgeig S. The surface activity of pulmonary surfactant from diving mammals. Respir Physiol Neurobiol. 2006a;150:220–232. doi: 10.1016/j.resp.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Postle AD, Orgeig S, Koster G, Daniels CB. The composition of pulmonary surfactant from diving mammals. Respir Physiol Neurobiol. 2006b;152:152–168. doi: 10.1016/j.resp.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Postle AD, Schürch S, Michael Schoel W, Daniels CB, Orgeig S. The development of the pulmonary surfactant system in California sea lions. Comp Biochem Physiol A Mol Integr Physiol. 2005;141:191–199. doi: 10.1016/j.cbpb.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Notter RH, Tabak SA, Mavis RD. Surface properties of binary mixtures of some pulmonary surfactant components. J Lipid Res. 1980;21:10–22. [PubMed] [Google Scholar]
- Otani S, Naito Y, Kato A, Kawamura A. Diving behavior and swimming speed of a free-ranging harbor porpoise, Phocoena phocoena. Mar Mammal Sci. 2000;16:811–814. doi: 10.1111/j.1748-7692.2000.tb00973.x. [DOI] [Google Scholar]
- Otani S, Naito Y, Kawamura A, Kawasaki M, Nishiwaki S, Kato A. Diving behavior and performance of harbor porpoises, Phocoena phocoena, in Funka Bay, Hokkaido, Japan. Mar Mammal Sci. 1998;14:209–220. doi: 10.1111/j.1748-7692.1998.tb00711.x. [DOI] [Google Scholar]
- Possmayer F, Nag K, Rodriguez K, Qanbar R, Schürch S. Surface activity in vitro: role of surfactant proteins. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:209–220. doi: 10.1016/S1095-6433(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Postle AD, Heeley EL, Wilton DC. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:65–73. doi: 10.1016/S1095-6433(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Postle AD, Mander A, Reid KB, Wang JY, Wright SM, Moustaki M, Warner JO. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am J Respir Cell Mol Biol. 1999;20:90–98. doi: 10.1165/ajrcmb.20.1.3253. [DOI] [PubMed] [Google Scholar]
- Rau GA, Vieten G, Haitsma JJ, Freihorst J, Poets C, Ure BM, Bernhard W. Surfactant in newborn compared with adolescent pigs: adaptation to neonatal respiration. Am J Respir Cell Mol Biol. 2004;30:694–701. doi: 10.1165/rcmb.2003-0351OC. [DOI] [PubMed] [Google Scholar]
- Read AJ, Gaskin DE. Radio tracking the movements and activities of harbor porpoises, Phocoena phocoena (L.), in the Bay of Fundy, Canada. Fish Bull US. 1985;83:543–552. [Google Scholar]
- Reed JZ, Chambers C, Hunter CJ, Lockyer C, Kastelein R, Fedak MA, Boutilier RG. Gas exchange and heart rate in the harbour porpoise, Phocoena phocoena. J Comp Physiol [B] 2000;170:1–10. doi: 10.1007/s003600050001. [DOI] [PubMed] [Google Scholar]
- Ridgway SH, Howard R. Dolphin lung collapse and intramuscular circulation during free diving: evidence from nitrogen washout. Science. 1979;206:1182–1183. doi: 10.1126/science.505001. [DOI] [PubMed] [Google Scholar]
- Sampson K, Merigo C, Lagueux K, Rice J, Cooper R, Weber ES, III, Kass P, Mandelman J, Innis C. Clinical assessment and postrelease monitoring of 11 mass stranded dolphins on Cape Cod, Massachusetts. Mar Mammal Sci. 2012;28:E404–E425. doi: 10.1111/j.1748-7692.2011.00547.x. [DOI] [Google Scholar]
- Schofield DT, Early G, Wenzel FW, Matassa K, Perry C, Beekman G, Whitaker B, Gebhard E, Walton W, Swingle M. Rehabilitation and homing behavior of a satellite-tracked harbor porpoise. Aquat Mamm. 2008;34:1–8. doi: 10.1578/AM.34.1.2008.1. [DOI] [Google Scholar]
- Scholander PF. Experimental investigations on the respiratory function in diving mammals and birds. Hvalrådets Skrifter. 1940;22:1–131. [Google Scholar]
- Spragg RG, Ponganis PJ, Marsh JJ, Rau GA, Bernhard W. Surfactant from diving aquatic mammals. J Appl Physiol. 2004;96:1626–1632. doi: 10.1152/japplphysiol.00898.2003. [DOI] [PubMed] [Google Scholar]
- Walters RW, Jenq RR, Hall SB. Distinct steps in the adsorption of pulmonary surfactant to an air-liquid interface. Biophys J. 2000;78:257–266. doi: 10.1016/S0006-3495(00)76589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hall SB, Notter RH. Dynamic surface activity of films of lung surfactant phospholipids, hydrophobic proteins, and neutral lipids. J Lipid Res. 1995;36:1283–1293. [PubMed] [Google Scholar]
- Watson AP, Gaskin DE. Observations on the ventilation cycle of the harbour porpoise Phocoena phocoena (L.) in coastal waters of the Bay of Fundy. Can J Zool. 1983;61:126–132. doi: 10.1139/z83-015. [DOI] [Google Scholar]
- Westgate AJ, Head AJ, Berggren P, Koopman HN, Gaskin DE. Diving behavior of harbour porpoises, Phocoena phocoena. Can J Fish Aquat Sci. 1995;52:1064–1073. doi: 10.1139/f95-104. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


