Abstract
Advanced stage hepatocellular carcinoma (HCC) is a category of disease defined by radiological, clinical and hepatic function parameters, comprehending a wide range of patients with different general conditions. The main therapeutic option is represented by sorafenib treatment, a multi-kinase inhibitor with anti-proliferative and anti-angiogenic effect. Trans-arterial Radio Embolization also represents a promising new approach to intermediate/advanced HCC. Post-marketing clinical studies showed that only a portion of patients actually benefits from sorafenib treatment, and an even smaller percentage of patients treated shows partial/complete response on follow-up examinations, up against relevant costs and an incidence of drug related adverse effects. Although the treatment with sorafenib has shown a significant increase in mean overall survival in different studies, only a part of patients actually shows real benefits, while the incidence of drug related significant adverse effects and the economic costs are relatively high. Moreover, only a small percentage of patients also shows a response in terms of lesion dimensions reduction. Being able to properly differentiate patients who are responding to the therapy from non-responders as early as possible is then still difficult and could be a pivotal challenge for the future; in fact it could spare several patients a therapy often difficult to bear, directing them to other second line treatments (many of which are at the moment still under investigation). For this reason, some supplemental criteria to be added to the standard modified Response Evaluation Criteria in Solid Tumors evaluation are being searched for. In particular, finding some parameters (cellular density, perfusion grade and enhancement rate) able to predict the sensitivity of the lesions to anti-angiogenic agents could help in stratifying patients in terms of treatment responsiveness before the beginning of the therapy itself, or in the first weeks of sorafenib treatment. This would bring a strongly desirable help in clinical managements of these patients.
Keywords: Modified Response Evaluation Criteria in Solid Tumors, Diffusion weighted imaging, Barcelona clinic liver cancer, Advanced hepatocellular carcinoma, Sorafenib, Advanced hepatocellular carcinoma second line therapies, Perfusion weighted imaging, Response evaluation, Hepatocellular carcinoma follow-up, Response Evaluation Criteria in Solid Tumors
Core tip: Advanced stage hepatocellular carcinoma comprehends a wide range of patients with different general conditions. The main therapeutic option is represented by sorafenib. Although the treatment has shown a significant increase in mean overall survival, only a part of patients actually shows benefits. Differentiating responder from non-responder patients is a pivotal challenge for the future. In particular, finding parameters quantitatively describing perfusion grade, and then able to predict the sensitivity of the lesions to anti-angiogenic agents could help stratifying patients in terms of responsiveness before the beginning of the therapy itself. This would bring a great help in management of these patients.
INTRODUCTION
Hepatocellular carcinoma (HCC) represents the fifth most prevalent tumor worldwide and the third cause of cancer related death[1]. The feasibility of treatments and the linked prognosis largely vary because of the tumor characteristics that present wide variability in terms of local and extra-hepatic burden. Moreover, the differences in molecular features and aggressiveness of the tumor significantly influence the natural history of the disease. Finally, the management of HCC is also complicated, in the majority of patients, by its development on a background of a cirrhotic liver, that can compromise the viability of the appropriate treatment[2].
Advanced HCC represents a major problem, as a considerable portion of HCC is diagnosed at this stage despite the wide use of ultrasound for surveillance in patients with increased risk[3]. This stage of disease is related to a poor prognosis and is reported to be associated with a survival rate of about 25% at 1 year[4,5]. Unfortunately, patients with advanced HCC are not suitable for curative therapeutic strategies like surgery, loco-regional treatments or orthotopic liver transplant. Moreover, HCC has a significant resistance to classic radio- or chemotherapy, that represent the standard of care in the majority of advanced tumors. Although the setting changed with the introduction of the multi-kinase inhibitor named sorafenib in 2008 for the treatment of advanced HCC, relevant issues in the management of this disease are still open. In particular, this therapy owns a wide variability in the prolongation of the survival of these patients. Furthermore, sorafenib therapy has some significant side effects and is very expensive.
On this background, the aim of this review is to remind the main problems related to diagnosis, staging and treatment allocation in case of advanced HCC, the principal indications of sorafenib, how to evaluate and to predict the response to treatment and when a second line therapy is suitable.
DIAGNOSIS, STAGING AND TREATMENT ALLOCATION
The development of radiological techniques has radically changed the approach to the diagnosis of HCC in the past decade. According to the American HCC guidelines, in 2005 a diagnosis of HCC without biopsy could be made in presence of a mass > 1 cm showing characteristic arterial enhancement, observed in two different imaging modalities, either biphasic computed tomography (CT) or magnetic resonance (MR)[6]. In the following years the diagnostic accuracy of a single tomographic contrasted technique has been largely validated. The last American guidelines published in 2011 made possible the diagnosis of HCC in a cirrhotic patient when a nodule > 1 cm shows arterial enhancement and portal/delayed phase “washout”, with the use of a single tomographic exam (CT or MR)[7]. Future guidelines may probably include the use of organ-specific contrast agents (CA), that have shown a high sensitivity in the detection of new HCC lesions and of post-surgical disease recurrence as well as a good potential in hypo-vascular HCC diagnosis[8-10]. This additional radiological advancement, which has been included in Japanese guidelines[11] and is currently used in clinical practice, might further reduce the diagnostic role of liver biopsy in HCC in the next years.
Many staging systems have been developed for HCC, and so far there is no international consensus for the use of a favored one. The barcelona clinic liver cancer (BCLC) is the staging system most widely endorsed in HCC evaluation[12]. It was developed in 1999 and refined during the following years[3,5,6,13,14]. Considering different parameters such as the tumor burden, the hepatic function and the presence of disease-related systemic symptoms, the BCLC individuates five different stages of disease and suggests the appropriate first line therapeutic strategy. Moreover, it considers the impact of treatment on overall survival (OS), linking the stage with the prognosis[3].
According to BCLC, advanced stage (BCLC-C) is defined by the presence of unresectable HCC with extra-hepatic spread (metastases or lymph nodes involvement) and/or vascular invasion (portal or segmental invasion) and/or systemic symptoms, defined by an Eastern Cooperative Oncology Group[15] performance status 1 or 2, with a liver function defined by a Child Pugh[16] stage not greater than B. It is easy to understand how advanced stage HCC includes a heterogeneous population of patients, with different prognosis. For instance, the grade of liver function is significantly related to prognosis: patients with a Child Pugh B class have a shorter median survival (5 mo) than patients with more preserved liver function (7 mo)[5,17]. This stage of disease has been considered untreatable until 2008, when sorafenib has proven his efficacy in prolonging the survival of these patients in two different large studies[17,18]. Since then, sorafenib has become the suggested therapy for advanced HCC in the BCLC algorithm (Figure 1).
Figure 1.
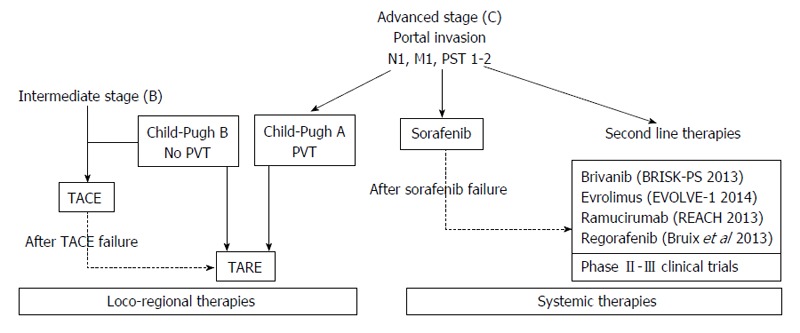
Main therapeutic options for advanced hepatocellular carcinoma treatment. TACE: Trans-arterial chemo-embolization; TARE: Trans-arterial radio embolization; PST: Performance status; PVT: Portal vein thrombosis.
Despite its wide use, the definition of advanced HCC by the BCLC and the allocation of sorafenib show some minor flaws.
The first one is represented by the treatment of intermediate HCC, a stage of disease that includes a heterogeneous group of clinical presentations. Transarterial chemo-embolization (TACE)[3,5] is the recommended primary therapy for this stage, but some authors suggest its use also in selected BCLC-C patients with a better liver function[19,20]. Conversely some others consider TACE not safe in patients with so advanced disease and recommend this treatment only in patients with Child-Pugh A cirrhosis and segmental portal vein thrombosis[21]. Besides, the BCLC does not lead to a clear therapeutic indication for patients who cannot afford or have failed TACE. This problem has been partially solved through the introduction of the concept of “treatment stage migration”: if patients are not candidates for first-line therapy as per stage, they can be shifted to the treatment option for a more progressed BCLC stage[3,5]. Translated in clinical practice, sorafenib should be administered also in intermediate HCC patients who can’t afford or have failed the treatment with TACE. At the same time TACE may be considered a suitable alternative for advanced stage HCC patients who are not compliant with oral therapy or could not have access to sorafenib[22]. In the last years even the combined use of sorafenib and TACE for intermediate and/or advanced HCC has been evaluated in different studies[23-25]. However, data published so far about safety and efficacy of this therapeutic regimen is controversial and a precise validation is still needed.
The second problem is related to the notion that BCLC defines as “advanced HCC” any patient presenting an Eastern Cooperative Oncology Group performance status of 1-2. In clinical practice, it means that patients could be excluded from potentially curative treatments if they are “restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work”[15]. In our judgment, this approach could seriously limit the clinical benefit in this particular kind of patients. It should be stressed that every therapeutic choice, especially in this kind of patients, deserves a multidisciplinary approach, as every disease represents an unique case.
A relatively new promising therapeutic option for intermediate/advanced HCC is represented by trans-arterial radio embolization (TARE). Differently from TACE, its main effect is not related to a mechanic obstruction of the arteries that feed the tumor: by the use of yttrium-loaded glass or resin particles a localized beta radiation of the mass can be obtained[26] (Table 1). Although there are some absolute contraindications, represented by a tumor burden over 75% of liver parenchyma and lung or gastrointestinal uncorrectable shunts[26] (that may lead to development of a radiation induced pneumonia), TARE has emerged as a safe treatment option and showed survival rates similar to TACE and sorafenib in studies published so far[27,28]. In particular this therapeutic option may be considered an interesting alternative to TACE, especially in patients with portal vein thrombosis[29]. However, data from randomized control trials are needed in order to confirm the therapeutic role of TARE for HCC in clinical practice.
Table 1.
Main loco-regional therapies in advanced hepatocellular carcinoma treatment
| Loco-regional therapies |
| TACE is the most common used loco-regional treatment in patients with unresectable HCC, without macrovascular invasion or extrahepatic spreads (BCLC stage B) |
| The use of TACE in advanced HCC is controversial: some authors affirm its better efficacy in term of survival benefit, than the best supportive care in HCC with extrahepatic spreads and macrovascular invasion. Some other ones recommend to be careful and suggest its use only in selected patients with Child A cirrhosis and segmental portal vein thrombosis |
| TACE can be a valid alternative for advanced HCC patients who are not compliant with oral therapies or have severe side effects or could not have access to sorafenib because of health authorities or high cost |
| In advanced HCC, TARE shows survival rates similar to sorafenib and TACE, especially in patients with portal vein thrombosis |
| TARE contraindication: important arterial shunt to gastrointestinal tract or lung, any contraindication to catheterization |
TACE: Trans-arterial chemo-embolization; TARE: Trans-arterial Radio Embolization; HCC: Hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer.
SORAFENIB TREATMENT
Sorafenib still represents the only approved therapy for advanced HCC[5]. It is a multi-kinase inhibitor with anti-angiogenic and anti-proliferative effect. It acts by inhibiting the serine-threonine kinases Raf-1 and B-Raf and the receptor tyrosine kinase activity of vascular endothelial growth factor receptors 1, 2, and 3 and platelet-derived growth factor receptor β[30-32]. Sorafenib, according to technical schedule, can be prescribed in patients with preserved liver function (Child-Pugh A) and it should be orally administered at 800 mg/die (400 mg twice a day). The therapy should be carried on until disease progression or unacceptable adverse effects (AE) occur[33]. Fatigue, diarrhea, hand-foot syndrome, bleeding, arterial hypertension and hepatic toxicity (represented by the elevation of transaminase and/or bilirubin) are some of the most frequent AE observed during treatment, and can compromise the quality of life during a therapy that in any case is palliative[34,35]. Sorafenib treatment cost varies from about 2600 to 5300€ per month, depending on the dose (400 mg/die vs 800 mg/die), with a mean cost about 4079 United States dollars per month[36].
Although sorafenib is the only drug which has indication for advanced HCC, only a few patients obtain a real benefit from this therapy. In general, the outcome and the extent of therapy is also linked to liver function: Child B patients have lower survival than Child A ones[37].
In the two largest studies published so far, “SHARP” and “Asia-Pacific”, the main objective tumor response ratio according to Response Evaluation Criteria in Solid Tumors (RECIST) was only 2%-3% in the sorafenib group patients, and a stable disease was observed in 34%-43% of patients, with an OS only three months longer than placebo group[17,18]. In fact, in the first of these phase III studies conducted comparing sorafenib (at 800 mg/die) and placebo with a double blind fashion on a total of more than 600 patients with advanced HCC[17], this drug showed a significant improvement in terms of OS (median OS 10.7 mo vs 7.9 mo of the placebo control group) and of time to progression, but the number of partial responses in the treatment group was low (7 out of 299)[17,38,39].
The increase in median OS was confirmed also in the second of the two abovementioned studies, conducted in China, Taiwan and South Korea on 226 advanced patients: mean OS was 6.5 mo in the treatment group against a 4.2 mo in the placebo arm[18]. Unfortunately the development of AE can reduce the compliance to therapy and worsen patient prognosis: in the SHARP study the incidence of AE was 70%-85% (vs 43%-60% in the placebo groups) but severe effects were observed in 9.4%-14.6% of patients[17]. The median duration of treatment was 5.3 mo (range, 0.2 to 16.1) and 176 of the patients in the sorafenib arm discontinued the study because of AE[17]. In both studies the most common significant AE causing a drug dose reduction (from 800 to 400 mg/die) were Hand-Foot Syndrome (10%-11% of patients) and diarrhea (5%-7%)[18].
Recent studies suggest that a dose reduced regimen of 400 mg/die could be equally effective in prolonging OS[40]. This data should advise the use of a “softer” regimen in patients who are more likely to develop AE during sorafenib treatment (e.g., Child B7, elder patients). In those cases sorafenib could be started at reduced dose, e.g., 400 mg/die, and “ramped up” to 600 or 800 mg/die if the patient shows a good profile of tolerability. Post-marketing clinical studies showed that only a portion of patients actually benefits from sorafenib treatment (Figure 2), and an even smaller percentage of patients treated shows partial/complete response on follow-up examinations (Figure 3), up against relevant costs and an incidence of drug related AE probably higher (24%-28% of severe AE) than reported in the SHARP and Asia Pacific studies[17,35,41].
Figure 2.
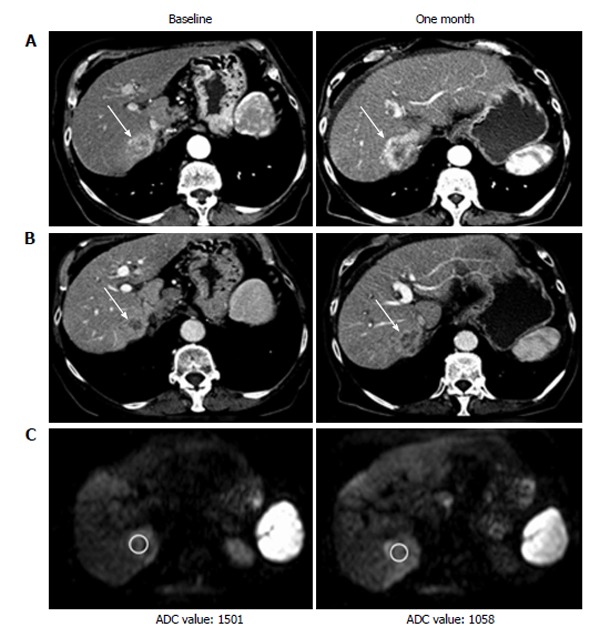
Computed tomography and magnetic resonance imaging examination at baseline and one month after the start of sorafenib therapy of patient showing progressive disease. A: Arterial phase computed tomography (CT); B: Venous phase CT; C: Magnetic resonance imaging diffusion weighted imaging. ADC: Apparent diffusion coefficient.
Figure 3.
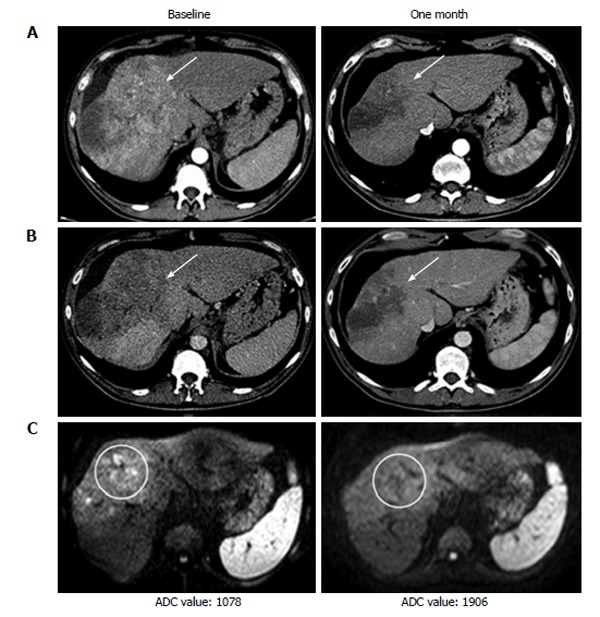
Computed tomography and magnetic resonance imaging examination at baseline and one month after the start of sorafenib therapy of a patient showing partial response. A: Arterial phase computed tomography (CT); B: Venous phase CT; C: Magnetic resonance imaging diffusion weighted imaging. ADC: Apparent diffusion coefficient.
Because of the problems related to the poor effectiveness of sorafenib and because of its cost, many studies tried to compare sorafenib to other commonly used treatments for unresectable HCC. Although, according to BCLC, TACE has no indication for advanced HCC, a study comparing this two different therapeutic options reported similar benefits from TACE and sorafenib in advanced stage HCC[42].
Association therapy of TACE and sorafenib has been investigated in some recent works that showed good results in term of safety and efficacy in BCLC-B patients[24,43], but its therapeutic role in BCLC-C patients is still unclear. In fact, most of the studies have shown that association therapy may improve time to progression, but it does not seem to improve OS if compared to TACE alone[44-47]. Conversely, Bai et al[48] have found some benefits in terms of OS, in patients treated with sorafenib plus TACE. This combination finds its theoretical physiological basis on the anti-angiogenic effect of the drug, in contrast with the physiological release of angiogenic factors consequent to the arterial iatrogenic obstruction[30]. Nevertheless the results about this kind of treatment are still uncertain[44].
In a recent study sorafenib has also been compared to TARE: median OS was similar in the two groups[49]. The extension of portal invasion resulted to be an important prognostic factor for the good result of TARE since patients with partial portal invasion of a branch of the vein had better prognosis than those who had disease extended to the main trunk[50]. The association of TARE and sorafenib has been investigated and showed good results in terms of safety, although data about OS with this combined therapy are still being investigated[51]. The physiological basis to combine these two therapies is that sorafenib seems to decrease the risk to develop a new lesion or distant metastasis, while TARE is more efficient in controlling primary hepatic lesion.
Ravaioli et al[52] reported two cases of advanced disease HCC that became suitable to liver transplantation after TARE treatment. TARE ability to downstage tumor has also been reported by other authors[53].
Over against its apparent simplicity, the treatment with sorafenib owns relevant open issues that can make the management problematic for the clinician. In fact, to reach a real benefit for the patients and to obtain a proper allocation of the money resources, it is crucial to identify a suitable method to evaluate response and hopefully early predictors of response and survival.
BIOCHEMICAL RESPONSE EVALUATION PARAMETERS
According to reported data we deduce that sorafenib therapy does not improve the prognosis in all advanced HCC patients and a part of responders have not such an important benefit to justify an expensive and rich in terms of AE therapy. Therefore, one of the primary objectives is to identify some biomarkers that may predict the efficacy of sorafenib treatment and may help the clinicians to select possible responder patients.
To clarify this point, many studies have focused on serum anti-angiogenic factors concentration; in particular, in the SHARP study, Llovet et al[17] found that low baseline concentration of vascular endothelial growth factor-A (VEGF-A) and high baseline concentration of Ang-2 correlated with a better OS in both arms of the study (sorafenib and placebo group). These data suggest that VEGF-A and Ang-2 are independent prognostic factors, but they have not a straight correlation with sorafenib therapy efficacy[54]. Similar results were shown in another study on patients treated with sorafenib and metronomic tegafur/uracil[55].The possible role of some cytokines [interleukin 6 (IL-6)/IL-8] as predictive biomarker of sorafenib treatment efficacy has also been evaluated, but no significant results have been found[56]. Some interesting, but preliminary results have been found using insulin-like growth factor-1 (IGF-1) baseline serum concentration: high IGF-1 blood levels seem to correlate with a better OS during anti-angiogenic therapy[57]. In the last years, great interest was devoted on serum alpha-fetoprotein (AFP) levels in HCC patients during systemic therapy: high basal levels of AFP generally correlate with a poor prognosis, both in intermediate and advanced HCC[54]. Personeni et al[58] analyzed a cohort of 85 patients treated with sorafenib and individuated a significant association between the decrease of > 20% in AFP in the first 8 wk and OS. Similar results have been found in other studies[59,60]. An important problem in the use of AFP as a biomarker is the difficulty in establishing a reference of percentage decrease (relatively to baseline values) as a cut-off to assess a response to therapy; in fact, an accepted worldwide threshold has not been defined, and the choice of this cut-off differed in the various studies, usually between 20% and 50%. Moreover, measuring the early change in AFP level seems to be a valid predictive factor only for patients who have higher baseline AFP serum level. For this reason, some authors suggest that only patients with pre-treatment AFP level > 200 microg/L are suitable for this analysis[61]. Despite the key role of AFP in diagnosis and follow-up of HCC, the effectiveness in outcome prediction during anti-angiogenic treatment is not clear yet, and needs to be evaluated in future.
In general, countless field-practice studies have analyzed the possible role of other biochemical and clinical parameters in early evaluation of response to sorafenib[40,62-67], i.e., aspartate transaminase, alkaline phosphatase basal and on-going levels, as well as the development of AE such as hand-foot syndrome or diarrhea have been related to a significantly prolonged OS, that represents the ultimate goal of treatment in patients with advanced HCC.
IMAGING RESPONSE EVALUATION PARAMETERS
Evaluation by imaging is another important tool and is usually performed every 2-3 mo during sorafenib treatment[68] by dynamic imaging (CT or MR contrast enhanced scan), applying the modified RECIST (mRECIST)[69].
The introduction of the mRECIST radically changed the approach to treatment response evaluation. While RECIST 1.1 is principally based on lesion dimensions without any consideration for tumoral vitality, mRECIST introduced the evaluation of the actual vital part of the lesions, which is the one that shows contrast enhancement at CT or MR.
Although the efficacy of mRECIST in tumor response evaluation in comparison with old RECIST 1.1 during sorafenib treatment has been recently confirmed by different studies[70,71], these criteria, based on vital lesions size measurements in time, still have some limitations. In fact, since sorafenib mainly operates through an anti-angiogenic effect, considering only the diameter of the vital portion is inadequate for a proper response evaluation. Some other parameters, able to quantitatively assess intralesional vitality or vascularization, are necessary to integrate mRECIST in order to make tumour response evaluation more reliable. It is proven that not all tumour progressions at imaging translate into a decreased OS and some improvements in prognosis have been shown in absence of tumour burden reduction[17,72]. This means that, even considering the increase in median OS, only a part of patients actually shows appreciable benefits, and those whose life expectancy is increased by the treatment are difficult to individuate since they rarely show a decrease in terms of lesion size/conspicuity. In other terms, the response does not correlate, at least initially, with a change in lesion dimension, but more probably it brings some intralesional decrease in cellularity and/or vascularization changes[30,72,73].
In this direction, the analysis of new radiological parameters in evaluation of response to sorafenib has shown promising results, and many attempts to evaluate different tumoral characteristics, such as intralesional perfusion and cellular density, have been performed so far.
Perfusion weighted imaging (PWI) is a relatively new MR/CT technique for qualitative and quantitative evaluation of the delivery of blood to biological tissues[74]. The importance of local changes in blood flow, angiogenesis and capillary permeability in cancer progression and treatment motivate the researchers’ increasing interest in PWI. The primary mechanisms for the cancer lesions enhancement are the filling of the vasculature with CA enhanced blood, and the diffusion of this CA from the blood into the extravascular-extracellular interstitial spaces; these phenomena are increased by tumoral angiogenesis. An increase in blood flow leads to a more rapid CA filling of the vessels, with faster changes in signal intensity/density while a greater blood or extravascular-extracellular volume will increase the fraction of the voxel to be filled with CA[74,75]. In tumoral lesions the level of peak enhancement and the rate of passage of the extravasated CA back to the vessels, with a return of signal intensity/density to its baseline values, is altered. In order to use image signal intensities to track and analyze enhancement dynamics, in PWI it is necessary to form a temporally resolved series of images (multiple acquisitions on the same area) that tracks the signal/density changes in different times after the CA administration, in analogy to tracer studies in nuclear medicine[75,76]. CT PWI parameters evaluation have shown significant changes during sorafenib treatment, in particular with a reduction in intralesional mean transit time as possible consequence of the anti-angiogenic effect of the drug[68,76].
Simple parameters, which indirectly correlate with intralesional vascularization have also been elaborated: Ronot has recently presented that in follow-up during sorafenib treatment the use of CHOI criteria, based on intralesional density on arterial phase CT acquisition, has shown promising potentials in terms of tumor response evaluation, comparable to those of mRECIST, although with minor reproducibility[71].
Studies on perfusion changes during therapy were also developed in ultrasonography. Contrast enhanced ultrasound, a technique which is now available in a large number of centers, and that can be repeated more than once in the first weeks from the beginning of therapy has shown, despite some major limits (such as operator dependency and partial liver volume exploration) some promising results in early response evaluation during sorafenib treatment, since it is able to evidence changes in target lesions enhancement during treatment[77].
The role of MR diffusion weighted imaging (DWI) in response assessment has been evaluated as well with controversial results[78-80]. This MR technique is based on water diffusion, which is the inconsistent and random microscopic motion of molecules caused by thermal energy, also known as Brownian motion. Even the more basic DWI principles description is beyond the aim of this review. It is sufficient to know that DWI indirectly describes the cellular density and the architectural changes of a tissue[81,82]. In fact, if within a tissue or a tumor several cells and many architectural barriers are present (as fibrosis, edema, any type of disorders or derangements), water molecules have difficulties in free movements and so “diffusion” is low (and, in general, signal intensity increases). On the contrary, if the cellular density is low and environment homogeneous, water molecules freely move, “diffusion” is easy and in general signal intensity decreases[81]. DWI technique could then be able to show some intralesional changes that are not evident on standard CT/MR scans. As regards to early assessment by DWI, in general, some studies conducted on different tumoral lesions have shown that apparent diffusion coefficient (ADC) changes in the first few weeks of treatment may precede dimensional reduction since, early after the start of treatment, changes in cellularity and necrosis may occur[83-85]. Conversely to what has been observed in solid cancers during chemotherapy treatment[86], Schraml et al[79] found an unexpected decrease in HCC mean intralesional ADC values in the first 3-4 wk of sorafenib therapy (maybe due to some micro-hemorrhagic intralesional injury), with a subsequent increase at 3 mo evaluation[79].
Also in case of DWI, the main limitation remains the large variability of data (both in different acquisitions and in different centers and scanners), which reduces the reproducibility of this technique[87,88]. However it has also been demonstrated that timing of imaging is relevant: changes in ADC could precede changes in tumor size but may even disappear after a certain time because of repair mechanisms such as edema decrease and necrosis organization[89,90]. The early changes in intralesional ADC described by Schraml et al[79] in advanced HCC could be expression of some intralesional temporarily changes, preceding an eventual dimensional reduction and expressing a possible sensitivity to sorafenib action[79].
Until now, none of the aforementioned radiological technique has been positively tested in a large number of patients, but the good results obtained so far are suggestive for a possible integration of some of these parameters to standard follow-up and response evaluation.
Even more important would be the prediction of the response based on pre-treatment examinations. This continues to be controversial. From a general point of view, tumors with necrotic areas, often surrounded by hypoxic but viable cells, were shown being less sensitive to ionizing radiation[91], more prone to aggressive behavior and probably less sensitive to cytotoxic agents[92]. In case of HCC, on the contrary to what reported for other solid tumors, higher ADC values on DWI baseline images could be related to a minor cellular density and a higher vascularization, and this could be somehow an index of treatment sensitivity (particularly in case of anti-angiogenic drug such as sorafenib itself), while low levels of intralesional ADC could correlate with a worse prognosis a poor response to treatment, as shown by some studies, since they could be expression of a poorly vascular lesion with high cellular density[80]. In these terms also a CT/MR pretreatment evaluation could give some additive information about tumor cellular density and vascularization, and maybe help stratifying patients in terms of anti-angiogenic therapies sensitivity.
Data available in this field are still limited and controversial, but more researches will certainly be made, as being able to identify patients with high probability of response before or shortly after the start of the therapy is strongly desirable.
Even if the first encouraging results will be confirmed in a larger scale, the addition of CT/MR perfusion parameters evaluation to a routinely liver study and then the quantitative evaluation on a per patient basis is not possible yet. The main problems related to perfusion studies are some technical difficulties and the acceptable, but suboptimal reproducibility of these parameters, particularly with MR; while the greatest limitation in DWI use is the mentioned large standard deviation of the measurements and then the low reproducibility[93,94].
SORAFENIB FAILURE AND SECOND-LINE THERAPIES
As already mentioned, no other systemic treatment other than sorafenib have, so far, shown the capability to improve the OS in patients with advanced HCC.
Despite the results in terms of survival during treatment, only a very small percentage of patients actually shows benefits in terms of radiological staging[17,18], so it is still discussed whether sorafenib treatment should actually be prolonged also in case of tumor progression at first follow-up examinations[56,95]. Anyway, even in case of evident benefits from the treatment, most of the patients experience a loss of efficacy of the drug during time[96].
There is then a strong request from clinicians for an established second line therapy to propose to patients when sorafenib cannot be administered or has to be interrupted due to AE or loss of efficacy (Table 2).
Table 2.
Main systemic therapies in advanced hepatocellular carcinoma treatment
| Systemic therapies |
| The only drug approved for the treatment of advanced HCC. Patients treated with sorafenib have longer OS then placebo group in the two largest studies |
| The efficacy of this treatment is linked to liver function: Child B patients have much lower survival than Child A ones (5.5 mo vs 11.3 mo). Child C patients have very poor prognosis and seem not to be suitable for sorafenib therapy (1.6 mo) |
| Patient treated with sorafenib has longer survival than those treated with sunitinib. No difference in OS has been found comparing sorafenib treatment to brivanib |
| Some combination therapies have been proposed, but none of these has shown superiority compared to sorafenib alone |
| At now there is no therapeutic plan approved as second line in advanced HCC pretreated with sorafenib |
| Some drugs as capecitabine, brivanib, sunitinib, everolimus have been tested in monotherapy, moreover some combination therapies as erlotinib with sorafenib, and gemcitabine with oxaliplatin have been evaluated as second line options, but all of them have not given significant results |
| Many studies are still in progress and some interesting, but preliminary results have been obtained in patients with high expression of c-met in treatment with brivanib |
HCC: Hepatocellular carcinoma; OS: Overall survival.
Metronomic capecitabine has been largely used as second line treatment in patients showing progressive disease after sorafenib treatment mainly because of its high tolerability[97].
In the randomized controlled trial that compared brivanib vs placebo as second-line therapy after sorafenib failure[98], the improvement of time to progression observed in brivanib arm did not translated in an increased OS[72]. An interesting phase III trial comparing sunitinib with sorafenib has shown similar results in terms of time to progression between the two drugs, but with worse results for sunitinib in terms of survival[99]. The use of brivanib and the combination of erlotinib with sorafenib have also been tested but failed in phase III trials[72,100-102].
From ongoing studies, the most promising results come from the observation of a significantly better outcome in patient with high expression of c-met treated with tivantinib[103]. From these data, a large phase III trial in second line is currently ongoing.
Although, it has been demonstrated that HCC patients who respond to TACE usually have poor response to a subsequent sorafenib treatment[104], as we mentioned above the possible role of the synchronous use of both therapies is also being investigated[105].
CONCLUSION
Advanced stage HCC is a category of disease defined by clinical, functional and radiological parameters, comprehending a wide range of patients with different general conditions, but with poor prognosis and life expectancy.
Since 2008 the main option for this stage of disease is represented by systemic treatment with sorafenib, that mainly shows an anti-angiogenic effect.
Although the treatment has shown an increase in OS in different studies, only a part of patients actually shows some benefits with a little percentage of partial response, while the incidence of drug related significant AE and the economic costs are high.
Being able to properly differentiate responder from non-responder patients as early as possible is then a pivotal challenge and could spare several patients a therapy often difficult to bear, directing them to some other second line treatment, at now under investigation.
For this reason, some supplemental parameters as biochemical and radiological prognostic factors are being searched for. In particular, finding some parameters quantitatively describing perfusion grade, and then able to predict the sensitivity of the lesions to anti-angiogenic agents could help in stratifying patients in terms of treatment responsiveness before the beginning of the therapy itself.
Footnotes
P- Reviewer: Ferraioli G, Li Q, Maroni L, Plentz RR
S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Supported by Protocollo TESORM by Regione Toscana, Università degli Studi di Firenze and Bayer Health Care s.p.a.
Conflict-of-interest: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 1, 2014
First decision: December 12, 2014
Article in press: February 2, 2015
References
- 1.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44 Suppl 19:96–101. doi: 10.1007/s00535-008-2258-6. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 5.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KA, Kim MJ, Choi JY, Park MS, Lim JS, Chung YE, Kim KW. Detection of recurrent hepatocellular carcinoma on post-operative surveillance: comparison of MDCT and gadoxetic acid-enhanced MRI. Abdom Imaging. 2014;39:291–299. doi: 10.1007/s00261-013-0064-y. [DOI] [PubMed] [Google Scholar]
- 9.Junqiang L, Yinzhong W, Li Z, Shunlin G, Xiaohui W, Yanan Z, Kehu Y. Gadoxetic acid disodium (Gd-EOBDTPA)-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: a meta-analysis. J Magn Reson Imaging. 2014;39:1079–1087. doi: 10.1002/jmri.24354. [DOI] [PubMed] [Google Scholar]
- 10.Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, Choi BI. Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014;271:748–760. doi: 10.1148/radiol.14131996. [DOI] [PubMed] [Google Scholar]
- 11.Bota S, Piscaglia F, Marinelli S, Pecorelli A, Terzi E, Bolondi L. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma. Liver Cancer. 2012;1:190–200. doi: 10.1159/000343833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 16.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 18.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 19.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 21.Tsochatzis EA, Fatourou E, O’Beirne J, Meyer T, Burroughs AK. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20:3069–3077. doi: 10.3748/wjg.v20.i12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duerden M. From a cancer drug fund to value based pricing of drugs. BMJ. 2010;341:c4388. doi: 10.1136/bmj.c4388. [DOI] [PubMed] [Google Scholar]
- 23.Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY, Chao Y. Interim analysis of START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer. 2013;132:2448–2458. doi: 10.1002/ijc.27925. [DOI] [PubMed] [Google Scholar]
- 24.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960–3967. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sieghart W, Pinter M, Reisegger M, Müller C, Ba-Ssalamah A, Lammer J, Peck-Radosavljevic M. Conventional transarterial chemoembolisation in combination with sorafenib for patients with hepatocellular carcinoma: a pilot study. Eur Radiol. 2012;22:1214–1223. doi: 10.1007/s00330-011-2368-z. [DOI] [PubMed] [Google Scholar]
- 26.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology. 2013;58:2188–2197. doi: 10.1002/hep.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 29.Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15 Suppl 4:42–52. doi: 10.1634/theoncologist.2010-S4-42. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 32.Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, Bortolon E, Ichetovkin M, Chen C, McNabola A, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561–574. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 33.EU Summary of Product Characteristics for Nexavar. Berlin, Germany: Bayer Schering Pharma AG: 2010. [Google Scholar]
- 34.Faye E, Bondon-Guitton E, Olivier-Abbal P, Montastruc JL. Spontaneous reporting of serious cutaneous reactions with protein kinase inhibitors. Eur J Clin Pharmacol. 2013;69:1819–1826. doi: 10.1007/s00228-013-1532-6. [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa H, Takeda H, Tsuchiya K, Joko K, Ogawa C, Taniguchi H, Orito E, Uchida Y, Osaki Y, Izumi N. Sorafenib Therapy for BCLC Stage B/C Hepatocellular Carcinoma; Clinical Outcome and Safety in Aged Patients: A Multicenter Study in Japan. J Cancer. 2014;5:499–509. doi: 10.7150/jca.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1739–1746. doi: 10.1111/j.1440-1746.2010.06404.x. [DOI] [PubMed] [Google Scholar]
- 37.Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70–76. doi: 10.1634/theoncologist.2008-0191. [DOI] [PubMed] [Google Scholar]
- 38.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 41.Cammà C, Cabibbo G, Petta S, Enea M, Iavarone M, Grieco A, Gasbarrini A, Villa E, Zavaglia C, Bruno R, et al. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57:1046–1054. doi: 10.1002/hep.26221. [DOI] [PubMed] [Google Scholar]
- 42.Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, Stauber R, Grünberger B, Müller C, Kölblinger C, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590–599. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- 43.Han G, Yang J, Shao G, Teng G, Wang M, Yang J, Liu Z, Feng G, Yang R, Lu L, et al. Sorafenib in combination with transarterial chemoembolization in Chinese patients with hepatocellular carcinoma: a subgroup interim analysis of the START trial. Future Oncol. 2013;9:403–410. doi: 10.2217/fon.13.11. [DOI] [PubMed] [Google Scholar]
- 44.Lencioni R, Llovet JM, Han G, Tak W-Y, Yang J, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. J Clin Onc. 2012;30:Abstr LBA154. [Google Scholar]
- 45.Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17:359–366. doi: 10.1634/theoncologist.2011-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muhammad A, Dhamija M, Vidyarthi G, Amodeo D, Boyd W, Miladinovic B, Kumar A. Comparative effectiveness of traditional chemoembolization with or without sorafenib for hepatocellular carcinoma. World J Hepatol. 2013;5:364–371. doi: 10.4254/wjh.v5.i7.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, Shin YM, Kim KM, Lim YS, Lee HC. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603–611. doi: 10.1148/radiol.13130150. [DOI] [PubMed] [Google Scholar]
- 48.Bai W, Wang YJ, Zhao Y, Qi XS, Yin ZX, He CY, Li RJ, Wu KC, Xia JL, Fan DM, et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis. 2013;14:181–190. doi: 10.1111/1751-2980.12038. [DOI] [PubMed] [Google Scholar]
- 49.Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, Marinelli S, Pettinato C, Erroi V, Fiumana S, Bolondi L, Bernardi M, Trevisani F; BLOG (Bologna Liver Oncology Group) Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int. 2015;35:1036–1047. doi: 10.1111/liv.12574. [DOI] [PubMed] [Google Scholar]
- 50.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–473. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, Schott E, Schütte K, Verslype C, Walecki J, Malfertheiner P; SORAMIC study group. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int. 2015;35:620–626. doi: 10.1111/liv.12622. [DOI] [PubMed] [Google Scholar]
- 52.Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D’Errico Grigioni A, Panzini I, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 53.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 54.Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shao YY, Hsu CH, Huang CC, Cheng AL. Use of plasma angiogenesis-related factors to investigate the association of interleukin 8 and interleukin 6 levels with efficacy of sorafenib-based antiangiogenic therapy in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2011;29 Suppl 4:Abstr 199. [Google Scholar]
- 56.Miyahara K, Nouso K, Tomoda T, Kobayashi S, Hagihara H, Kuwaki K, Toshimori J, Onishi H, Ikeda F, Miyake Y, et al. Predicting the treatment effect of sorafenib using serum angiogenesis markers in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1604–1611. doi: 10.1111/j.1440-1746.2011.06887.x. [DOI] [PubMed] [Google Scholar]
- 57.Shao YY, Huang CC, Lin SD, Hsu CH, Cheng AL. Serum insulin-like growth factor-1 levels predict outcomes of patients with advanced hepatocellular carcinoma receiving antiangiogenic therapy. Clin Cancer Res. 2012;18:3992–3997. doi: 10.1158/1078-0432.CCR-11-2853. [DOI] [PubMed] [Google Scholar]
- 58.Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V, Giordano L, Santoro A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101–107. doi: 10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan ST, Poon RT. The significance of early alpha-fetoprotein level changes in predicting clinical and survival benefits in advanced hepatocellular carcinoma patients receiving sorafenib. Oncologist. 2011;16:1270–1279. doi: 10.1634/theoncologist.2011-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuzuya T, Asahina Y, Tsuchiya K, Tanaka K, Suzuki Y, Hoshioka T, Tamaki S, Kato T, Yasui Y, Hosokawa T, et al. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251–258. doi: 10.1159/000334454. [DOI] [PubMed] [Google Scholar]
- 61.Chen LT, Liu TW, Chao Y, Shiah HS, Chang JY, Juang SH, Chen SC, Chuang TR, Chin YH, Whang-Peng J. alpha-fetoprotein response predicts survival benefits of thalidomide in advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2005;22:217–226. doi: 10.1111/j.1365-2036.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 62.Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, Rimola J, Darnell A, Ríos J, Ayuso C, Bruix J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–324. doi: 10.1016/j.jhep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 63.Faloppi L, Scartozzi M, Bianconi M, Svegliati Baroni G, Toniutto P, Giampieri R, Del Prete M, De Minicis S, Bitetto D, Loretelli C, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer. 2014;14:110. doi: 10.1186/1471-2407-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- 65.Takeda H, Nishikawa H, Osaki Y, Tsuchiya K, Joko K, Ogawa C, Taniguchi H, Orito E, Uchida Y, Izumi N; Japanese Red Cross Liver Study Group. Clinical features associated with radiological response to sorafenib in unresectable hepatocellular carcinoma: a large multicenter study in Japan. Liver Int. 2015;35:1581–1589. doi: 10.1111/liv.12591. [DOI] [PubMed] [Google Scholar]
- 66.Inghilesi AL, Gallori D, Antonuzzo L, Forte P, Tomcikova D, Arena U, Colagrande S, Pradella S, Fani B, Gianni E, et al. Predictors of survival in patients with established cirrhosis and hepatocellular carcinoma treated with sorafenib. World J Gastroenterol. 2014;20:786–794. doi: 10.3748/wjg.v20.i3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koschny R, Gotthardt D, Koehler C, Jaeger D, Stremmel W, Ganten TM. Diarrhea is a positive outcome predictor for sorafenib treatment of advanced hepatocellular carcinoma. Oncology. 2013;84:6–13. doi: 10.1159/000342425. [DOI] [PubMed] [Google Scholar]
- 68.Sacco R, Faggioni L, Bargellini I, Ginanni B, Battaglia V, Romano A, Bertini M, Bresci G, Bartolozzi C. Assessment of response to sorafenib in advanced hepatocellular carcinoma using perfusion computed tomography: results of a pilot study. Dig Liver Dis. 2013;45:776–781. doi: 10.1016/j.dld.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arizumi T, Ueshima K, Takeda H, Osaki Y, Takita M, Inoue T, Kitai S, Yada N, Hagiwara S, Minami Y, et al. Comparison of systems for assessment of post-therapeutic response to sorafenib for hepatocellular carcinoma. J Gastroenterol. 2014;49:1578–1587. doi: 10.1007/s00535-014-0936-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, Castera L, Vilgrain V, Belghiti J, Raymond E, et al. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19:394–402. doi: 10.1634/theoncologist.2013-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 74.Martí-Bonmatí L, Alberich-Bayarri A, Sanz-Requena R, Sánchez-González J. State of the art in liver imaging-MR diffusion/perfusion. Controversies and consensus in imaging and intervention: 2009. Available from: http://www.c2i2.org/web09-05/state_of_the_art_in_liver_imaging.as. [Google Scholar]
- 75.Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661–673. doi: 10.1148/radiol.2343031362. [DOI] [PubMed] [Google Scholar]
- 76.Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007;243:736–743. doi: 10.1148/radiol.2433052020. [DOI] [PubMed] [Google Scholar]
- 77.Sugimoto K, Moriyasu F, Saito K, Rognin N, Kamiyama N, Furuichi Y, Imai Y. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33:605–615. doi: 10.1111/liv.12098. [DOI] [PubMed] [Google Scholar]
- 78.Vouche M, Kulik L, Atassi R, Memon K, Hickey R, Ganger D, Miller FH, Yaghmai V, Abecassis M, Baker T, et al. Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: Imaging analysis from a prospective randomized trial of Y90 ± sorafenib. Hepatology. 2013;58:1655–1666. doi: 10.1002/hep.26487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schraml C, Schwenzer NF, Martirosian P, Bitzer M, Lauer U, Claussen CD, Horger M. Diffusion-weighted MRI of advanced hepatocellular carcinoma during sorafenib treatment: initial results. AJR Am J Roentgenol. 2009;193:W301–W307. doi: 10.2214/AJR.08.2289. [DOI] [PubMed] [Google Scholar]
- 80.Mannelli L, Kim S, Hajdu CH, Babb JS, Taouli B. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: Prediction and assessment of response to transarterial chemoembolization. Preliminary experience. Eur J Radiol. 2013;82:577–582. doi: 10.1016/j.ejrad.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 81.Colagrande S, Carbone SF, Carusi LM, Cova M, Villari N. Magnetic resonance diffusion-weighted imaging: extraneurological applications. Radiol Med. 2006;111:392–419. doi: 10.1007/s11547-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 82.Colagrande S, Belli G, Politi LS, Mannelli L, Pasquinelli F, Villari N. The influence of diffusion- and relaxation-related factors on signal intensity: an introductive guide to magnetic resonance diffusion-weighted imaging studies. J Comput Assist Tomogr. 2008;32:463–474. doi: 10.1097/RCT.0b013e31811ec6d4. [DOI] [PubMed] [Google Scholar]
- 83.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248:894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 84.Theilmann RJ, Borders R, Trouard TP, Xia G, Outwater E, Ranger-Moore J, Gillies RJ, Stopeck A. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6:831–837. doi: 10.1593/neo.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marugami N, Tanaka T, Kitano S, Hirohashi S, Nishiofuku H, Takahashi A, Sakaguchi H, Matsuoka M, Otsuji T, Takahama J, et al. Early detection of therapeutic response to hepatic arterial infusion chemotherapy of liver metastases from colorectal cancer using diffusion-weighted MR imaging. Cardiovasc Intervent Radiol. 2009;32:638–646. doi: 10.1007/s00270-009-9532-8. [DOI] [PubMed] [Google Scholar]
- 86.Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, Husband JE. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188:1001–1008. doi: 10.2214/AJR.06.0601. [DOI] [PubMed] [Google Scholar]
- 87.Kakite S, Dyvorne H, Besa C, Cooper N, Facciuto M, Donnerhack C, Taouli B. Hepatocellular carcinoma: short-term reproducibility of apparent diffusion coefficient and intravoxel incoherent motion parameters at 3.0T. J Magn Reson Imaging. 2015;41:149–156. doi: 10.1002/jmri.24538. [DOI] [PubMed] [Google Scholar]
- 88.Colagrande S, Pasquinelli F, Mazzoni LN, Belli G, Virgili G. MR-diffusion weighted imaging of healthy liver parenchyma: repeatability and reproducibility of apparent diffusion coefficient measurement. J Magn Reson Imaging. 2010;31:912–920. doi: 10.1002/jmri.22117. [DOI] [PubMed] [Google Scholar]
- 89.Mungai F, Pasquinelli F, Mazzoni LN, Virgili G, Ragozzino A, Quaia E, Morana G, Giovagnoni A, Grazioli L, Colagrande S. Diffusion-weighted magnetic resonance imaging in the prediction and assessment of chemotherapy outcome in liver metastases. Radiol Med. 2014;119:625–633. doi: 10.1007/s11547-013-0379-3. [DOI] [PubMed] [Google Scholar]
- 90.Heijmen L, Verstappen MC, Ter Voert EE, Punt CJ, Oyen WJ, de Geus-Oei LF, Hermans JJ, Heerschap A, van Laarhoven HW. Tumour response prediction by diffusion-weighted MR imaging: ready for clinical use? Crit Rev Oncol Hematol. 2012;83:194–207. doi: 10.1016/j.critrevonc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 91.Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9:442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 93.Aronhime S, Calcagno C, Jajamovich GH, Dyvorne HA, Robson P, Dieterich D, Fiel MI, Martel-Laferriere V, Chatterji M, Rusinek H, et al. DCE-MRI of the liver: effect of linear and nonlinear conversions on hepatic perfusion quantification and reproducibility. J Magn Reson Imaging. 2014;40:90–98. doi: 10.1002/jmri.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ippolito D, Fior D, Bonaffini PA, Capraro C, Leni D, Corso R, Sironi S. Quantitative evaluation of CT-perfusion map as indicator of tumor response to transarterial chemoembolization and radiofrequency ablation in HCC patients. Eur J Radiol. 2014;83:1665–1671. doi: 10.1016/j.ejrad.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 95.Miyahara K, Nouso K, Yamamoto K. Chemotherapy for advanced hepatocellular carcinoma in the sorafenib age. World J Gastroenterol. 2014;20:4151–4159. doi: 10.3748/wjg.v20.i15.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frenette C, Gish R. Targeted systemic therapies for hepatocellular carcinoma: clinical perspectives, challenges and implications. World J Gastroenterol. 2012;18:498–506. doi: 10.3748/wjg.v18.i6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, Serra C, Sama C, Golfieri R, Gramenzi A, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist. 2013;18:1256–1257. doi: 10.1634/theoncologist.2013-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 99.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 100.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 101.Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 102.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–2079. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 103.Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
- 104.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 105.Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM, Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R, et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263. doi: 10.1186/1471-2407-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]


