Abstract
Hepatocellular carcinoma is the third leading cause of cancer-related deaths in the world. It is associated with an important mortality rate and the incidence is increasing. Patients showing metabolic syndrome seem to have higher incidence and mortality rates from hepatocellular carcinoma than healthy subjects, especially those with type 2 diabetes mellitus and obesity. Thus, metformin and statins, both to treat features of metabolic syndrome, have been proposed to decrease the risk of hepatocellular carcinoma. Otherwise, liver cancer is the result of a complex process which impairs several signaling cascades, such as RAS/RAF/mitogen-activated protein kinase kinase (MEK)/extracellular-signal-regulated kinase (ERK), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) and Wnt/β-catenin signaling. Metformin (through 5′-adenosine monophosphate-activated protein kinase pathway activation) and statins (through 3-hydroxy-3-methylglutaryl coenzyme A inhibition) show anti-tumoral properties modifying several steps of RAS/RAF/MEK/ERK, PI3K/AKT/mTOR and Wnt/β-catenin signaling cascades. On the other hand, metformin and statins have been found to reduce the risk of hepatocellular carcinoma up to 50% and 60%, respectively. Furthermore, both drugs have shown a dose-dependent protective effect. However, information about chemopreventive role of metformin and statins is mainly obtained of observational studies, which could not take into account some bias. In conclusion, given the rising of incidence of hepatocellular carcinoma and the important morbidity and mortality rates associated with this cancer, looking for chemopreventive strategies is an essential task. Randomized controlled trials are needed to determine the definite role of metformin and statins on the prevention of hepatocellular carcinoma.
Keywords: Hepatocellular carcinoma, Metformin, Metabolic syndrome, Mammalian target of rapamycin, Statin
Core tip: Hepatocellular carcinoma is the result of a complex process which impairs several pathways, such as RAS/RAF/mitogen-activated protein kinase kinase/extracellular-signal-regulated kinase, phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT/mammalian target of rapamycin and Wnt/β-catenin signaling. Patients showing metabolic syndrome seem to have higher incidence and mortality rates from hepatocellular carcinoma than healthy subjects, especially those with type 2 diabetes mellitus and obesity. Thus, metformin and statins, both to treat features of metabolic syndrome, have been proposed to decrease the risk of hepatocellular carcinoma. Metformin (by decreasing hyperglycemia state through 5′-adenosine monophosphate-activated protein kinase pathway activation) and statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) show anti-tumoral properties modifying several steps of the crucial signaling cascades.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths in the world[1]. In recent years, a significant increase in HCC incidence and mortality rates has been observed in Western countries. Given that primary liver cancer shows a poor prognosis due to its infiltrating and malignancy power, we should closely assess those risk factors that could be preventable. Although the main risk factors for HCC are hepatitis C virus (HCV), hepatitis B virus (HBV) and chronic alcohol abuse, many individuals who have been exposed to these factors never develop HCC, while 15%-50% of cases occur among those without exposure, suggesting that further risk factors could be responsible for the increased incidence of HCC[2].
Patients showing metabolic syndrome seem to have higher incidence and mortality rates from HCC than healthy subjects, especially those with type 2 diabetes mellitus (T2DM) and obesity[3]. T2DM is an emerging risk factor of many chronic liver diseases, such as chronic hepatitis, non-alcoholic fatty liver disease and cirrhosis. Furthermore, DM has been proposed as a risk factor for HCC[4]. On the other hand, previous studies have demonstrated that cirrhosis and HCV increase the susceptibility to diabetes mellitus[5]. Nevertheless, exact pathophysiological mechanisms of these significant associations are still unclear. Otherwise, metformin and statins, both to treat features of metabolic syndrome, have been proposed to decrease the risk of HCC[6]. Therefore, in this review, we aim to evaluate the role of some of possible intermediary mechanisms that could be associated with the onset and progression of HCC development, as well as the impact of metformin and statins on the appearance of the liver tumor.
CELL SURVIVAL, PROLIFERATION AND DIFFERENTIATION SIGNALING PATHWAYS
HCC is a kind of tumor based on inflammation. As a result, there are an incessant cell injury, necrosis and regeneration that, ultimately, lead to activate mutations in key genes (especially, oncogenes and tumor suppressor genes)[7]. This complex process results in impairment of several signaling cascades. In this review, we focus on RAS/RAF/mitogen-activated protein kinase kinase (MEK)/extracellular-signal-regulated kinase (ERK) (cell proliferation signaling pathway), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) (cell survival signaling pathway) and Wnt/β-catenin (cell differentiation signaling pathway) signaling cascades (Figure 1). Furthermore, we revise the main pathways of DM associated with HCC.
Figure 1.
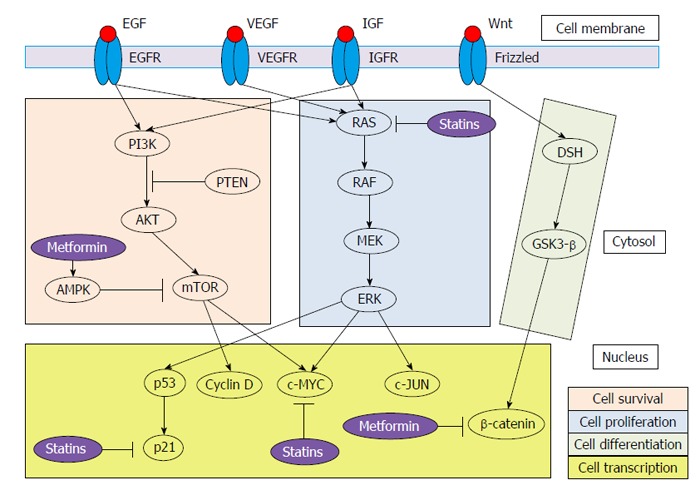
Pathogenic pathways of hepatocellular carcinoma and targets for metformin and statins. PI3K: Phosphatidylinositol-4,5-bisphosphate 3-kinase; PTEN: Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase; AMPK: Adenosine monophosphate-activated protein kinase; mTOR: Mammalian target of rapamycin; MEK: Mitogen-activated protein kinase kinase; ERK: Extracellular-signal-regulated kinase; GSK3-β: Glycogen synthase kinase 3 beta; EGF: Epidermal growth factor; VEGF: Vascular EGF; IGF: Insulin-growth factor; EGFR: EGF receptor; VEGFR: VEGF receptor; IGFR: IGF receptor.
The RAF/MEK/ERK via is one of the most powerful pathway that regulates crucial cellular processes[8]. It is triggered by growth factors [epidermal growth factor (EGF), platelet-derived growth factor, Vascular endothelial growth factor, and insulin-growth factor (IGF)] and activating mutations of major oncogenic proteins, being RAS the key molecular signal regulator[9]. Importantly, RAS also plays a regulatory role in other signaling pathways, especially the PI3K/AKT/mTOR pathway. RAS cascade is one of the main targets of sorafenib, the only currently effective therapy for advanced HCC[10].
Activation of the PI3K-AKT signalling pathway is promoted by binding of growth factors (especially, IGF and EGF) to their receptors, resulting in disruption of the mTOR pathway[11]. PI3K/AKT/mTOR axis has linked to angiogenesis and survival[12]. Therefore, mTOR has emerged as an exciting target for cancer therapy. The mTOR complex comprises two forms: (1) mTOR complex 1 (mTORC1), closely implicated in protein translation; and (2) mTORC2, which is the primary responsible for the phosphorylation of AKT and could be necessary to sustain the oncogenic phenotype related to loss of Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase (PTEN)[13]. PTEN negatively regulates the PI3K-AKT signaling pathway and has been associated with tumor grade, advanced disease stage and reduced overall survival in patients with HCC[14]. In 40%-50% of HCC, dysregulated expression of effectors of mTOR has been observed[15]. On the other hand, mTORC1 activation shows prognostic implications in terms of patient tumor recurrence after surgery[16].
Wnt/β-catenin signaling pathway has a close relationship with cancer[17]. It consists of a large number of proteins that interact with each other. Mutations in β-catenin, which activate the Wnt signalling pathway, occur in one-third of HCCs[18]. Wnt pathway regulates the expression of many genes (c-Myc, c-Jun and cyclin D1) via interaction with Frizzled receptors[19]. In particular, the MYC proto-oncogene family contributes carcinogenesis by unrestricted cell proliferation and inhibiting cell differentiation[20]. Accumulation of β-catenin induces transcription of several genes related to cell differentiation and proliferation. In fact, studies have shown that the expression of β-catenin was higher in HCC than in non-tumor tissues[21], and Wnt-1 is a survival factor for HCC cells[22]. On the other hand, mTOR regulates the expression level of β-catenin[23]. Thus, this pathway is critical for tissue and liver regeneration.
On the other hand, patients showing features of metabolic syndrome may have higher incidence of HCC and mortality rates than those without it[24]. In fact, DM and obesity increase the risk of appearance of HCC. Therefore, one hypothesis for this fact could be that patients with features of metabolic syndrome have more aggressive tumor characteristics, such as increased vascular invasion and metastasis. DM has been proposed as an independent risk factor for HCC[25,26]. Mechanisms proposed for diabetes-induced liver cancer include: (1) hyperinsulinemia state, caused by insulin resistance, increases levels of IGF-1, which is one of the most powerful activators of cellular proliferation. This fact leads to elevated binding and consequently downstream signaling through the RAF/MEK/ERK and PI3K/AKT/mTOR pathways[27]; (2) insulin activates the intrinsic tyrosine kinase of insulin receptor, by phosphorylation of insulin-receptor substrate-1. This latter, together with IGF-1, are overexpressed in tumor cells, generating inhibition of apoptosis[28]; (3) insulin resistance leads to increase the releasing of multiple proinflammatory cytokines, including tumor necrosis factor alpha (TNFα) and interleukin 6, which promote the development of hepatic steatosis, inflammation and subsequent HCC[29]; and (4) reactive oxygen species are also produced, impairing mitochondrial respiration and causing oxidative damage to the mitochondrial genome by activation of the apoptosis cascade[30].
ANTITUMORAL EFFECTS OF METFORMIN AND STATINS
Metformin is an insulin-sensitizer drug frequently used in the first-line oral treatment of T2DM patients. Antioxidant, anti-inflammatory, growth inhibitory and antiangiogenic effects of metformin have been associated to reduce the risk of some solid tumors, such as prostate, colorectal, breast and pancreas[31]. Metformin mainly works by decreasing hyperglycemia state through 5′-adenosine monophosphate-activated protein kinase (AMPK) pathway activation. Proposed anti-tumoral mechanisms of metformin include: (1) activated AMPK has growth inhibition effects on human cancer cell lines, via inhibition of mTOR[32]; (2) metformin has demonstrated to limit cell growth through cell cycle G0/G1 arrest in hepatoma cell lines, by inhibiting cyclin D1 expression[33]; (3) it can also inhibit carcinogenesis by downregulating c-Myc and upregulation miR-33a, which require activation of AMPK[34]; (4) metformin is able to modulate the expression of cytokines, such as TNFα, and oxidative stress[35]; (5) metformin, through AMPK, decreased β-catenin protein levels leading to suppression of Wnt/β-catenin signaling[36]; and (6) metformin is taken up in hepatocytes by the organic cation transporter-1 (OCT-1), which is an essential step for the glucose-lowering effect[37,38]. Interestingly, OCT-1 and OCT-3 expression has been found downregulated in HCC patients and associated with impaired prognosis[39].
Statins are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Additionally to the effect on cholesterol biosynthesis, statins also have antineoplastic properties. Antitumoral effects of statins are related to the following mechanisms: (1) they can effectively downregulate the RAF/MEK/ERK pathway, contributing to the apoptotic response[40]; (2) statins limit the degradation of the cyclin-dependent kinase inhibitors p21 and p27. These molecules show growth-inhibitory effects; (3) HMG-CoA reductase is a crucial regulator of MYC phosphorylation and activation. Consequently, inhibition of HMG-CoA reductase prevents from both c-Myc phosphorylation and activation[41]; and (4) anti-inflammatory and antioxidant effects of statins may be partly mediated by the PI3K/AKT pathway[42], causing a decline in toll-like receptor 4 expression on blood monocytes and TNFα plasma concentration[43].
CHEMOPREVENTIVE ROLE OF METFORMIN AND STATINS
Metformin use seems to decrease the risk of HCC in diabetic patients in several observational studies. Hassan et al[44] compared 420 diabetic patients with 1104 healthy controls [DM was related to HCC (OR = 4.2; P < 0.05)]. They analyzed different treatments, showing metformin and thiazolidinediones (TZD) as protective agents (OR = 0.3; P < 0.05) and sulphonylureas (OR = 7.1; P < 0.05) and insulin therapy (OR = 1.9; P < 0.05) as negative factors[44]. Donadon et al[45] obtained similar results, assessing 610 patients with HCC, 618 cirrhotic patients without HCC and 1696 healthy controls. Metformin was shown as protective therapy (OR = 0.33; P < 0.05), opposite to sulphonylureas and insulin exogenous (OR = 3.06; P < 0.05). Nkontchou et al[46] observed prospectively a reduced incidence of HCC in diabetic HCV-related cirrhotic patients treated with metformin (HR = 0.19; P < 0.05). Lai et al[47] confirmed that T2DM was associated with HCC and that the HCC risk reduction was greater for diabetics taking metformin than those taking TZD (51% vs 44% reduction). Recently, Chen et al[48] concluded that metformin use was related to lower risk of HCC in diabetic patients in a dose-dependent manner. Similar results have been reported in meta-analysis. Zhang et al[49] included three cohort studies and four case-control studies, concluding that metformin treatment was associated with reduced risk of HCC in diabetic patients. Singh et al[50] performed a systematic review and a meta-analysis to evaluate the effect of antidiabetic therapy on the risk of HCC, including ten studies reporting 22650 cases of HCC in 334307 patients with T2DM. Meta-analysis showed a 50% of reduction in HCC incidence with metformin use, a 62% and a 161% increase in HCC incidence with sulfonylurea and insulin use, respectively, while TZD did not modify the risk of developing.
Statins may decrease the risk of HCC in patients with other underlying liver diseases, according to observational studies. Tsan et al[51] reported a dose-dependent association between statin use and decreased risk of HCC development in patients with HCV (HR = 0.33; P < 0.05) taking higher daily doses of statins. The same group performed a similar study in HBV patients, and they observed a risk reduced up to 66% in patients which received more than one year cumulative treatment compared to those never treated. Furthermore, they observed that the reduction in HCC risk was a class effect[52]. A recent meta-analysis evaluated 4298 cases of HCC in 1459417 patients. Authors found a 37% overall reduction in HCC risk with the use of statins. Interestingly, the risk reduction was higher in Asian people (OR = 0.52; P < 0.05), although this effect was also present in Western populations (OR = 0.67; P < 0.05), maybe due to interactions between statins and HBV[53]. Furthermore, statins have been associated with decreased HCC recurrence after resection[54]. In contrast to observational studies, randomized controlled trials have failed to show such association. In a post-hoc analysis from the Cholesterol Treatment Trialists’ collaboration, there was no difference in the risk of appearance of HCC regardless the consumption of statins[55]. However, randomized controlled trials were performed for cardiovascular endpoints, showing limitations: (1) patients enrolled were at low risk for development of HCC, limiting the power to detect a significant difference to development of HCC; (2) the follow-up was shorter than expected to evaluate the developing of HCC; and (3) statin nonusers in these groups had a elevated risk of cardiovascular mortality[56].
Information about chemopreventive role of metformin and statins is mainly obtained of observational studies. However, best level of evidence comes from randomized clinical trials, so the current available data of these drugs show a lack of randomization necessary to control cofounders[57]. The heterogeneity of the studies, the lack of randomization and the increased risk of reporting bias should indicate caution. A main concern about metformin use is the safety profile in patients with advanced liver disease, as metformin has been associated with serious adverse effects. However, there are studies in which well-compensated cirrhotic patients have taken metformin without adverse effects, beyond an increased prevalence of diarrhea[58]. In addition, there is a concern about the safety of using metformin and statins in cirrhotic patients (who show the highest risk of HCC), which could introduce a selection bias at the moment of indicating the treatment. On the other hand, most of studies do not take into account to adjust for concomitant medications. Thus, the protective effect of metformin or statins could be enhanced by the other one, as they are relatively common in patients with metabolic syndrome. Interestingly, etiology of cirrhosis could influence on the antitumoral effect of these drugs, especially the closely relationship between HCV infection and metabolic syndrome[59]. In fact, HCV directly affects the host lipid metabolism, favoring its own replication[60], so inhibitors of lipid synthesis, such as statins, could decrease viral replication. Lastly, the influence of environmental, like aflatoxin, or genetic factors, like PNPLA3[61], could impact and mask the conclusions.
CONCLUSION
In this review, we have summarized the intermediary mechanisms responsible for the association between some features of metabolic syndrome and HCC development. Given the rising of incidence of HCC, especially in the Western countries, and the important morbidity and mortality rates associated with this cancer, looking for chemopreventive strategies is an essential task. Identifying who will benefit, optimal duration of treatment and relevant biomarkers will be crucial to design the appropriate strategy. Non-etiology-specific medications, such as statins and metformin, are cheap, have a favorable safety profile and could have metabolic effects in additional organs. However, further studies are needed to establish the definitive role of metformin and statins on the prevention of HCC. Randomized clinical trials, controlling comedications and genetic factors, are required for this purpose. Therefore, prevention through surveillance of risk populations is the best current option in day-to-day clinical practice to improve the prognostic of patients with HCC.
Footnotes
P- Reviewer: Anty R, Rodriguez-Peralvarez M, Saracco G
S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: No conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 18, 2014
First decision: September 16, 2014
Article in press: February 2, 2015
References
- 1.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Kanwal F. Obesity and hepatocellular carcinoma: hype and reality. Hepatology. 2014;60:779–781. doi: 10.1002/hep.27172. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 5.Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18:1642–1651. doi: 10.3748/wjg.v18.i14.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyoshi H, Kato K, Iwama H, Maeda E, Sakamoto T, Fujita K, Toyota Y, Tani J, Nomura T, Mimura S, et al. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int J Oncol. 2014;45:322–332. doi: 10.3892/ijo.2014.2419. [DOI] [PubMed] [Google Scholar]
- 7.Nishida N, Kudo M. Recent advancements in comprehensive genetic analyses for human hepatocellular carcinoma. Oncology. 2013;84 Suppl 1:93–97. doi: 10.1159/000345897. [DOI] [PubMed] [Google Scholar]
- 8.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 9.Neuzillet C, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141:160–171. doi: 10.1016/j.pharmthera.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Moeini A, Cornellà H, Villanueva A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer. 2012;1:83–93. doi: 10.1159/000342405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn RS. Current and Future Treatment Strategies for Patients with Advanced Hepatocellular Carcinoma: Role of mTOR Inhibition. Liver Cancer. 2012;1:247–256. doi: 10.1159/000343839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou W, Liu J, Chen P, Wang H, Ye BC, Qiang F. Mutation analysis of key genes in RAS/RAF and PI3K/PTEN pathways in Chinese patients with hepatocellular carcinoma. Oncol Lett. 2014;8:1249–1254. doi: 10.3892/ol.2014.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zender L, Villanueva A, Tovar V, Sia D, Chiang DY, Llovet JM. Cancer gene discovery in hepatocellular carcinoma. J Hepatol. 2010;52:921–929. doi: 10.1016/j.jhep.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu TH, Huang CC, Lin PR, Chang HW, Ger LP, Lin YW, Changchien CS, Lee CM, Tai MH. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer. 2003;97:1929–1940. doi: 10.1002/cncr.11266. [DOI] [PubMed] [Google Scholar]
- 15.Sieghart W, Fuereder T, Schmid K, Cejka D, Werzowa J, Wrba F, Wang X, Gruber D, Rasoul-Rockenschaub S, Peck-Radosavljevic M, et al. Mammalian target of rapamycin pathway activity in hepatocellular carcinomas of patients undergoing liver transplantation. Transplantation. 2007;83:425–432. doi: 10.1097/01.tp.0000252780.42104.95. [DOI] [PubMed] [Google Scholar]
- 16.Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis. 2010;30:35–51. doi: 10.1055/s-0030-1247131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 18.Wands JR, Kim M. WNT/β-catenin signaling and hepatocellular carcinoma. Hepatology. 2014;60:452–454. doi: 10.1002/hep.27081. [DOI] [PubMed] [Google Scholar]
- 19.Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107–1117. doi: 10.1016/j.jhep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Kaposi-Novak P, Libbrecht L, Woo HG, Lee YH, Sears NC, Coulouarn C, Conner EA, Factor VM, Roskams T, Thorgeirsson SS. Central role of c-Myc during malignant conversion in human hepatocarcinogenesis. Cancer Res. 2009;69:2775–2782. doi: 10.1158/0008-5472.CAN-08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Xue L, Cao Q, Lin Y, Ding Y, Yang P, Che L. Expression of Notch1, Jagged1 and beta-catenin and their clinicopathological significance in hepatocellular carcinoma. Neoplasma. 2009;56:533–541. doi: 10.4149/neo_2009_06_533. [DOI] [PubMed] [Google Scholar]
- 22.Wei W, Chua MS, Grepper S, So SK. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol Cancer. 2009;8:76. doi: 10.1186/1476-4598-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Giugliano D. The association between metabolic syndrome and hepatocellular carcinoma: a missed meta-analysis. J Clin Gastroenterol. 2014;48:742–743. doi: 10.1097/MCG.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 25.Donadon V, Balbi M, Casarin P, Vario A, Alberti A. Association between hepatocellular carcinoma and type 2 diabetes mellitus in Italy: potential role of insulin. World J Gastroenterol. 2008;14:5695–5700. doi: 10.3748/wjg.14.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Qin L, Wang Y, Tao L, Wang Z. AKT down-regulates insulin-like growth factor-1 receptor as a negative feedback. J Biochem. 2011;150:151–156. doi: 10.1093/jb/mvr066. [DOI] [PubMed] [Google Scholar]
- 28.Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, Gardenal R, Dal Mas M, Casarin P, Zanette G, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddique A, Kowdley KV. Insulin resistance and other metabolic risk factors in the pathogenesis of hepatocellular carcinoma. Clin Liver Dis. 2011;15:281–296, vii-x. doi: 10.1016/j.cld.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D. Metformin as an antitumor agent in cancer prevention and treatment. J Diabetes. 2011;3:320–327. doi: 10.1111/j.1753-0407.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 33.Fang Z, Xu X, Zhou Z, Xu Z, Liu Z. Effect of metformin on apoptosis, cell cycle arrest migration and invasion of A498 cells. Mol Med Rep. 2014;9:2251–2256. doi: 10.3892/mmr.2014.2097. [DOI] [PubMed] [Google Scholar]
- 34.Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, Sacconi A, Biagioni F, Cortese G, Galanti S, et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun. 2012;3:865. doi: 10.1038/ncomms1859. [DOI] [PubMed] [Google Scholar]
- 35.Hyun B, Shin S, Lee A, Lee S, Song Y, Ha NJ, Cho KH, Kim K. Metformin Down-regulates TNF-α Secretion via Suppression of Scavenger Receptors in Macrophages. Immune Netw. 2013;13:123–132. doi: 10.4110/in.2013.13.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takatani T, Minagawa M, Takatani R, Kinoshita K, Kohno Y. AMP-activated protein kinase attenuates Wnt/β-catenin signaling in human osteoblastic Saos-2 cells. Mol Cell Endocrinol. 2011;339:114–119. doi: 10.1016/j.mce.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009;58:745–749. doi: 10.2337/db08-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heise M, Lautem A, Knapstein J, Schattenberg JM, Hoppe-Lotichius M, Foltys D, Weiler N, Zimmermann A, Schad A, Gründemann D, et al. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer. 2012;12:109. doi: 10.1186/1471-2407-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 2004;64:6461–6468. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- 41.Cao Z, Fan-Minogue H, Bellovin DI, Yevtodiyenko A, Arzeno J, Yang Q, Gambhir SS, Felsher DW. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 2011;71:2286–2297. doi: 10.1158/0008-5472.CAN-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Chen Z, Zou Y, Ge J. Atorvastatin represses the angiotensin 2-induced oxidative stress and inflammatory response in dendritic cells via the PI3K/Akt/Nrf 2 pathway. Oxid Med Cell Longev. 2014;2014:148798. doi: 10.1155/2014/148798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuire TR, Kalil AC, Dobesh PP, Klepser DG, Olsen KM. Anti-inflammatory effects of rosuvastatin in healthy subjects: a prospective longitudinal study. Curr Pharm Des. 2014;20:1156–1160. doi: 10.2174/1381612820666140127163313. [DOI] [PubMed] [Google Scholar]
- 44.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 46.Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, Ganne-Carrie N, Grando-Lemaire V, Vicaut E, Trinchet JC, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601–2608. doi: 10.1210/jc.2010-2415. [DOI] [PubMed] [Google Scholar]
- 47.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 48.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 49.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 50.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–891; quiz 892. doi: 10.1038/ajg.2013.5. [DOI] [PubMed] [Google Scholar]
- 51.Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514–1521. doi: 10.1200/JCO.2012.44.6831. [DOI] [PubMed] [Google Scholar]
- 52.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 53.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 55.Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, Bhala N, Holland L, Peto R, Keech A, Collins R, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh S, Singh PP. Statins for prevention of hepatocellular cancer: one step closer? Hepatology. 2014;59:724–726. doi: 10.1002/hep.26614. [DOI] [PubMed] [Google Scholar]
- 57.Carrat F. Statin and aspirin for prevention of hepatocellular carcinoma: what are the levels of evidence? Clin Res Hepatol Gastroenterol. 2014;38:9–11. doi: 10.1016/j.clinre.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Ampuero J, Ranchal I, Nuñez D, Díaz-Herrero Mdel M, Maraver M, del Campo JA, Rojas Á, Camacho I, Figueruela B, Bautista JD, et al. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PLoS One. 2012;7:e49279. doi: 10.1371/journal.pone.0049279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ampuero J, Romero-Gómez M, Reddy KR. Review article: HCV genotype 3 - the new treatment challenge. Aliment Pharmacol Ther. 2014;39:686–698. doi: 10.1111/apt.12646. [DOI] [PubMed] [Google Scholar]
- 60.Clément S, Peyrou M, Foti M, Negro F. Statins may protect against hepatocellular carcinoma development in patients infected with hepatitis C virus, but what are the mechanisms? J Clin Oncol. 2013;31:4160–4161. doi: 10.1200/JCO.2013.51.0354. [DOI] [PubMed] [Google Scholar]
- 61.Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, Waljee AK. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109:325–334. doi: 10.1038/ajg.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]


