DIFFERENTIAL REGULATION OF IMMUNE RESPONSES BY UVA
In this issue of the PPP Hiramotho’s team (1) presents evidence that irradiation of the eyes by ultraviolet A (UVA) leads to the differentiation of immune responses in the epidermis and colon. UVA had been found to inhibit contact hypersensitivity (CHS) and reduced the number of Langerhans cells in the epidermis of the ear induced by both Th1 and Th2 haptens. In mast cell-deficient mice, this inhibition was only observed for Th2 hapten (1). Interestingly, UVA decreased both the CHS response and IgA expression of the colon only in Th2 hapten-sensitized mice and amplified inflammation in Th1 hapten-sensitized mice, while decreasing the number of histidine decarboxylase (HDC)-positive mast cells in both cases (1). In mast cell-deficient mice, UVA had no effect on inflammation induced by Th2 hapten, while it amplified the Th1 hapten-stimulated inflammation. Thus, UVA can regulate the immune responses through the activation of the pathways originating in the eye and further computed in the brain with neuroendocrine transmission to immune system and peripheral organs.
ULTRAVIOLET RADIATION AS A REGULATOR OF SYSTEMIC HOMEOSTASIS
Overview
When being exposed to electromagnetic energy of solar radiation including visible light (λ >400 nm), ultraviolet (UV)A (λ = 320–400 nm), and UVB (λ = 290–320 nm), the eye is predominantly detecting visible light with the transmission of the signals through the optic nerve to visual cortex or via retinohypothalamic tract to the suprachiasmatic (SCN) nucleus (2). These signaling pathways are involved either in vision or in the central regulation of circadian rhythm (2, 3).
Mammalian eyes also detect UV radiation (UVR) predominantly via UVR receptors, which are involved in the UV vision (2–4). UVR signals detected by the eye are also involved in the activation of other centers in the brain with systemic neuro-endocrine-immune effects (1–5). The mechanism of this neuro-endocrine-immune transmission of UVR signals that is initiated in the eyes and is culminating with the regulation of homeostasis at global and peripheral organ levels represents an enigma.
However, the major recipient of UVR energy is the skin, where depending on the dose, UVA and UVB induce multiple damages at the subcellular, cellular, and tissue levels leading to a variety of skin pathologies (6). UVR also regulates body homeostasis through production of vitamin D (7) and can induce local and systemic immunosuppression (8, 9).
Absorption of UVR by the skin leads to changes in global homeostasis
To protect and/or restore local homeostasis against the UVR-damaging effects (6), the skin is armed with a pigmentary system (10), a local neuroendocrine system (6, 11) encompassing cutaneous melatoninergic (12), steroidogenic (13), hypothalamic and pituitary systems (14) forming local equivalent of the hypothalamus–pituitary–adrenal axis (14). Whereas the role of UVR-induced modulation of immune cells leading to local and systemic immunosuppression has been appreciated for a long time (9), only recent upregulation of the production/release of glucocorticoids (powerful immunosuppressors) by UVB has been established (15–17). Increased production of glucocorticoids on the local level is accompanied by UVB-induced stimulation of corticotropin-releasing hormone (CRH) and CRH-related peptides, increased production of proopiomelanocortin (POMC)-derived peptides including ACTH, α-MSH and β-endorphin (14, 15), and decreased expression of glucocorticoid receptor (GR) in keratinocytes (16). Although CRH is a pro-inflammatory molecule at the periphery, UVB stimulation of POMC peptides production (either by CRH or directly) (14) will lead to net immunosuppressive effects, because of immunosuppressive effects of ACTH, α-MSH, and β-endorphin, and because of the activity of UVB-induced cortisol through following complementary mechanisms: UVB → CRH → ACTH → cortisol (local equivalent of HPA axis) and/or UVB → ACTH → cortisol (local equivalent of pituitary–adrenal axis) and/or UVB → cortisol (local upregulation of expression of steroidogenic enzymes) (14). This UVB-induced multifactorial upregulation of local glucocorticoids production is designed to protect the skin against autoimmune attacks due to exposure of skin antigens. Decreased expression of GR in keratinocytes increases the precision of this protection by attenuation of weakening of epidermal barrier function by glucorticoids, while allowing their suppressive activity on lymphocytes. Interestingly, UVA had no effect on production of ACTH and glucocorticoids; however, it stimulated the CRH and β-endorphin (15), indicating that immunoregulatory functions of UVA do not involve local HPA axis, however, perhaps involving CRH and β-endorphin activities.
UVB has been shown to induce the central HPA axis with increased serum levels of corticosterone; however, this requires an intact pituitary gland (17). This describes the novel mechanism of regulation of body homeostasis induced by UVB and explaining at least in part a systemic immunosuppression as secondary to release into circulation of immunosuppressive corticosteroids and POMC-derived peptides (17). Although the UVB activation of central HPA axis can be multifactorial (Fig. 1), a possible activation of hypothalamic centers via ascending neural roots from the skin, as proposed previously (11), is in striking agreement with Hiramoto et al. (5) who have shown that UVB irradiation of the eye leads to the immunosuppression, also requiring intact pituitary.
Fig. 1.
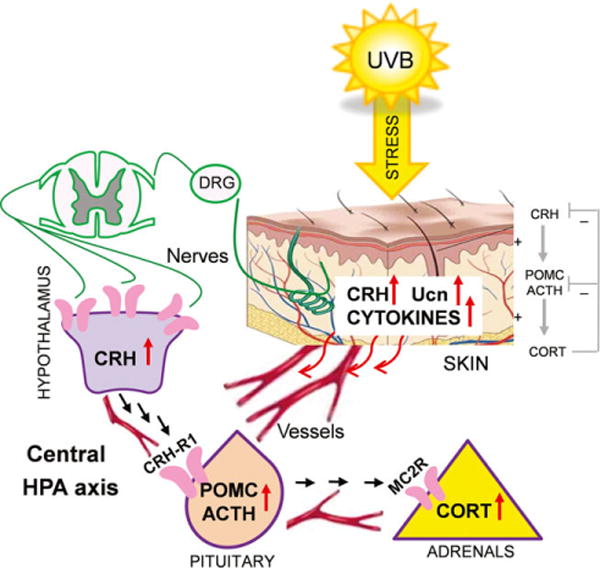
Potential mechanisms involved in an activation of the central HPA axis by UVB. The figure is from the paper by Skobowiat and Slominski (17) and published with permission from the J Invest Dermatol.
Detection of UVR by the eye leads to systemic immunomodulation that is organ specific
Dr. Hiramoto and his collaborators’ work on UVA (1) and UVB (5) immunomodulatory activities has focused on the transmission of the UVR to the brain from the eyes with systemic effects secondary to the activation of neural or endocrine pathways. This transmission of the information is expected, for example, mice have UV vision, and there are photosensitive ganglion cells with routes of activation independent from the vision. However, which nuclei of the hypothalamus are receiving this information (SCN, the superior collicutus, pretectum, paraventricular nucleus) is unclear as well as how this information is transmitted to the immune system. Nevertheless, this effect requires an intact pituitary and is associated with increases in serum levels of POMC-derived α-MSH (5).
This study (1) shows that the exposure of eyes to the UVA can either inhibit, has no effect or it can stimulate immune responses in the organ-specific and context-dependent fashion; it also indicates a difference to UVB-induced pathways that have been exclusively immunoinhibitory (5). This opens new exciting areas to study the mechanisms and routes of UVA-induced immunomodulation which originate in the eye and that are apparently different from those activated by UVB. Interestingly, UVA also shows a differential effect on different element of cutaneous HPA as opposite to the UVB (15, 16).
CONCLUSION
Current (1) and previous (5) studies by Hiramoto’s group on UVR-induced immunomodulation secondary to UVR detection and computing by the eyes show certain similarities as well as differences with parallel studies on UVR-induced changes in systemic homeostasis after its reception and computing by the skin (14, 15, 17). UVB and UVA have differential effects after reception by both the eyes and skin. The future challenge lies in defining which neural routes are activated with translation to neuroimmunoendocrine effects and whether signals from eye and skin reach the same coordinating brain centers. In this context, it is worth mentioning that the eye is also expressing all elements of the HPA axis (reviewed in (14), and it has been proposed that HPA axis and its communication with immune system have developed in the periphery, and this algorithm of the homeostatic regulation has been adopted and perfected by the central neuroendocrine system during evolution (18). Thus, studies on the UVR-induced neuroendocrine pathways secondary to exposure of the eyes or skin that regulate immune system can open unexpected correlations and discover previously unknown mechanisms of homeostatic regulations.
Acknowledgments
We thank Dr Radomir M. Slominski for language corrections proofreading of the manuscript. Writing of the manuscript was supported by NIH grants 2R01AR052190-A6, R21 AR066505-01A1 and 1R01AR056666-01A2 to AS.
Footnotes
Conflicts of interests:
None declared.
References
- 1.Yamate Y, Hiramoto K, Kasahara E, Sato EF. UVA irradiation of the eye modulates the contact hypersensitivity of the skin and intestines by affecting mast cells in mice. Photodermatol Photoimmunol Photomed. 2015;31(3):129–140. doi: 10.1111/phpp.12157. in press. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs GH. The Verriest Lecture 2009: recent progress in understanding mammalian color vision. Ophthalmic Physiol Opt. 2010;30:422–434. doi: 10.1111/j.1475-1313.2010.00719.x. [DOI] [PubMed] [Google Scholar]
- 3.Brainard GC, Barker FM, Hoffman RJ, et al. Ultraviolet regulation of neuroendocrine and circadian physiology in rodents. Vision Res. 1994;34:1521–1533. doi: 10.1016/0042-6989(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Pawelek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol. 1998;16:503–515. doi: 10.1016/s0738-081x(98)00023-6. [DOI] [PubMed] [Google Scholar]
- 5.Hiramoto K, Yanagihara N, Sato EF, Inoue M. Ultraviolet B irradiation of the eye activates a nitric oxide-dependent hypothalamopituitary proopiomelanocortin pathway and modulates functions of alpha-melanocyte-stimulating hormone-responsive cells. J Invest Dermatol. 2003;120:123–127. doi: 10.1046/j.1523-1747.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- 6.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Kripke ML. Ultraviolet radiation and immunology: something new under the sun-presidential address. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- 9.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 10.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 11.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 12.Slominski AT, Kleszczynski K, Semak I, et al. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014;15:17705–17732. doi: 10.3390/ijms151017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–E493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skobowiat C, Slominski AT. UVB activates hypothalamic-pituitary-adrenal axis in C57Bl/6 mice. J Invest Dermatol. 2015;135(6):1638–1648. doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest. 2007;117:3166–3169. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]


