Abstract
The endocannabinoid (eCB) system, consisting of eCB ligands and the type 1 cannabinoid receptor (CB1R), subserves retrograde, activity-dependent synaptic plasticity in the brain. eCB signaling occurs “on-demand,” thus the processes regulating synthesis, mobilization and degradation of eCBs are also primary mechanisms for the regulation of CB1R activity. The eCBs, N-arachidonylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), are poorly soluble in water. We hypothesize that their aqueous solubility, and, therefore, their intracellular and transcellular distribution, are facilitated by protein binding. Using in silico docking studies, we have identified the nonspecific lipid binding protein, sterol carrier protein 2 (SCP-2), as a potential AEA binding protein. The docking studies predict that AEA and AM404 associate with SCP-2 at a putative cholesterol binding pocket with ΔG values of −3.6 and −4.6 kcal/mol, respectively. These values are considerably higher than cholesterol (−6.62 kcal/mol) but consistent with a favorable binding interaction. In support of the docking studies, SCP-2-mediated transfer of cholesterol in vitro is inhibited by micromolar concentrations of AEA; and heterologous expression of SCP-2 in HEK 293 cells increases time-related accumulation of AEA in a temperature-dependent fashion. These results suggest that SCP-2 facilitates cellular uptake of AEA. However, there is no effect of SCP-2 transfection on the cellular accumulation of AEA determined at equilibrium or the IC50 values for AEA, AM404 or 2-AG to inhibit steady state accumulation of radiolabelled AEA. We conclude that SCP-2 is a low affinity binding protein for AEA that can facilitate its cellular uptake but does not contribute significantly to intracellular sequestration of AEA.
Keywords: N-Arachidonylethanolamine, 2-Arachidonoylglycerol, AM404, Cholesterol, Uptake, Sequestration, AutoDock
Introduction
The endocannabinoid signaling (ECS) system, consisting of the types 1 and 2 cannabinoid receptors (CB1R and CB2R, respectively) and endocannabinoids (eCBs), 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamine (AEA), modulates cellular responses during a variety of physiological and pathological conditions [1]. Alterations in brain ECS, resulting from changes in either CB1R/CB2R signaling or availability of the eCBs, have been seen in a variety of pathological states including obesity, neurodegeneration, pain, inflammation, and psychiatric disorders.
The eCBs are highly lipophilic and poorly water soluble. Their high lipophilicity allows the eCBs to be released in a non-vesicular manner since they can freely cross the plasma membrane. However, their low aqueous solubility poses a problem for intracellular trafficking and leads to the hypothesis that there are eCB binding proteins that enhance aqueous solubility and movement across aqueous barriers [2, 3]. Indeed, other lipid signaling molecules, such as steroids, bind to intracellular proteins that facilitate their sequestration and trafficking within cells [4]. Findings that the enzymes and substrate for AEA synthesis [5] and degradation [6] are found in intracellular membranes suggest that AEA is trafficked intracellularly between organelles and the plasma membrane. Furthermore, AEA is accumulated within cells far beyond equilibrium, a finding that is consistent with an intracellular binding protein that serves a sequestration role [3]. The enzymes involved in 2-AG synthesis [7] and degradation [8] are present at the plasma membrane, suggesting that 2-AG signalling does not require intracellular trafficking and, thus, not involve an intracellular binding protein.
A number of proteins have been suggested to function as AEA binding proteins, including fatty acid binding proteins (FABP) 5 and 7 [9], albumin and heat shock protein 70 [10]. However, the primary FABP present in adult brain (HFABP, also called FABP3) [11] does not contribute to AEA uptake [9] while neither FABP5 nor FABP7 are expressed in significant amounts in adult neurons [11, 12]. FABP7 (also called BFABP) is highly expressed in radial glial cells and is hypothesized to play an important role in myelination [13]. Albumin and heat shock protein 70 have very limited expression in normal brain, only appearing during stroke or with a compromised blood brain barrier [14]. FLAT, a truncated version of fatty acid amide hydrolase (FAAH), has been recently reported to bind AEA in a saturable manner that also exhibits competition by AEA analogs [15]. However, neurons from FAAH null mice accumulate AEA at wild type amounts [16], suggesting that additional proteins are involved in intracellular sequestration of AEA. Thus, it is likely that additional proteins can serve as AEA carriers.
In this study, we identify sterol carrier protein 2 (SCP-2) as an additional intracellular protein that could contribute to AEA cellular uptake and/or accumulation. SCP-2 is a multi-substrate, lipid binding protein that shuttles cholesterol and other lipids from the endoplasmic reticulum, where they are synthesized, to the cell surface [17]. SCP-2 can bind a variety of structurally diverse lipids, including fatty acids, fatty acyl CoA derivatives, sterols and phospholipids [18]. SCP-2 binds fatty acids and their CoA derivatives with Kd values in the 10−9–10−7 M range [18]. Importantly, SCP-2 is expressed in the brain and is enriched in synaptosomal preparations [19, 20]. The purpose of these studies is to test the hypothesis that SCP-2 binds AEA and contributes to its cellular uptake and/or sequestration.
In support of this hypothesis, we report molecular docking studies which demonstrate a moderate but favorable free energy of binding (ΔG) of AEA to SCP-2. We report further that AEA is predicted to bind within the hydrophobic cavity proposed as the SCP-2 substrate binding site [21]. In addition, AEA inhibits SCP-2-dependent trafficking of cholesterol in vitro and heterologous SCP-2 expression increases the accumulation of AEA in HEK 293 cells at early time points after its addition. However, SCP-2 expression does not affect intracellular AEA concentrations at steady state. Together, these data support the hypothesis that SCP-2 binds AEA with moderate affinity and can increase the amount of AEA taken up by cells but does not affect the extent of accumulation with long incubation times. Thus SCP-2 has the characteristics of an AEA uptake facilitator but not an intracellular sequestration site for AEA.
Materials and Methods
Materials
Human SCP-2 protein expression vectors were purchased from GeneCopoeia (Rockville, MD) and were based upon previous reports [22]. L1210 cells were the kind gift of Dr. Albert Girotti (Medical College of Wisconsin). Human embryonic kidney (HEK 293) cells were obtained from American Type Culture Collection (Manassas, VA, USA). Polyclonal antibodies against SCP-2 were obtained from Santa Cruz Biochemicals; and β-actin from Sigma; secondary antibodies used were goat-anti-rabbit IgG HRP (GE Bio-Sciences, Pittsburg, PA, USA) and goat-anti-mouse IgG HRP (Sigma). [14C]Cholesterol and [3H]AEA (labeled in the arachidonate portion of the molecule) were obtained from American Radiolabeled Chemicals. 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and cholesterol were obtained from Avanti Polar Lipids. AEA, AM404 and 2-AG were purchased from Cayman Chemical. All other buffers and reagents were obtained from common commercial sources.
Computational Docking
AutoDock 4.2 [23] was used to dock cholesterol, AEA, 2-AG, AM-404 and arachidonic acid into the NMR structure of SCP-2 (1qnd, www.pdb.org) [21] in silico, as generally described elsewhere [24]. Briefly, co-crystallized waters and bound ligand (16-doxylstearic acid) were removed and a grid of dimensions 22.5×15×18.75 Å surrounding the hydrophobic binding site was created using GRID. The substrate binding site was compared to a crystal structure of a homologous protein, human peroxisomal multifunctional enzyme type 2 (MFE-2), bound by a lipid substrate, Triton-X (Fig. S1). Molecular structures of cholesterol, AEA, 2-AG, and AM-404 were downloaded from PubChem (pubchem.ncbi.nlm.nih.gov) and converted to mol2 files using Open Babel ([25]; The Open Babel Package, V. 2.0. 2., http://openbabel.org). Arachidonic acid was generated and energy-minimized using Chem3D Pro 12.0 (CambridgeSoft, Inc.). Rigid amino acid side chains were maintained in SCP-2. AutoDock Tools was used to prepare both protein and ligands for docking, including addition of Gasteiger charges. All ligands were docked in their physiologically relevant ionization state (at pH 7.4). Each compound was docked into the hydrophobic binding site using 100 genetic algorithm optimization runs. Results were clustered according to lowest binding energy (ΔG). The lowest mean free energy of binding was then selected as the putative average binding mode.
Preparation of Recombinant SCP-2
SCP-2 was expressed in E. coli and purified according to previously reported protocols [26]. The protein was stored at −20 °C in 50% glycerol (1–6 mg/ml protein) to retain activity over time. Confirmation of purity was determined through Coomassie staining of SDS-PAGE gels.
Assay of SCP-2-Dependent Cholesterol Transfer
Small unilamellar vesicles (SUVs) were created and the cholesterol transfer assay was designed based on previous methods [27]. Briefly, the SUVs were formed from 0.5 mM POPC, 0.4 mM cholesterol and 0.01 mM dicetyl phosphate (DCP) and [14C]cholesterol (1 µCi/1 ml of SUVs) in phosphate-buffered saline (PBS) using an extrusion method. [14C]Cholesterol-containing SUVs (0.05 mM, final lipid concentration) were incubated with L1210 cells (2×107 cells/ml) in the presence and absence of recombinant SCP-2 (final concentration of 50–200 µg/ml in a total volume of 1.5 ml) at 37 °C. Aliquots (250 µl) of the incubation mixture were chilled on ice then centrifuged at 2,000×g for 1 min at room temperature to separate SUVs and L1210 cells; [14C] content of the cell pellet was determined using liquid scintillation counting and was used as an index of the transfer capacity of SCP-2. L1210 cells were maintained in DMEM with 10 % fetal bovine serum and 1 % penicillin streptomycin.
Expression of SCP-2 in HEK 293 Cells
HEK 293 were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10 % fetal bovine serum. HEK 293 cells were plated at 300,000 cells/well on poly-d-lysine coated plates. Cells are transfected with Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Immunofluorescence was detected in 100 % of the cells, indicating highly efficient transfection (data not shown).
Determination of AEA Cellular Accumulation
Twenty-four hours after transfection, [3H]AEA uptake/accumulation in HEK 293 cells was determined. Briefly, cells were incubated in transport buffer (118 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 2.5 mM CaCl2, 10 mM HEPES pH 7.4) for 30 min at desired temperature. Buffer was replaced and [3H]AEA (0.2 nM, final concentration) was added to each well. At desired time intervals, buffer was removed and cells were scraped in water and [3H] contents of both were determined using liquid scintillation counting. Percent uptake was calculated as dpm cells/(dpm cells + dpm buffer). Nonspecific uptake was measured in the presence of 100 µM AEA.
Statistics
One- or two-way analyses of variance (ANOVAs) were carried out using GraphPad Prism. Significant effects in the ANOVA were followed by Bonferroni’s t-tests. For the AEA uptake studies, comparisons planned prior to the study were analyzed in the absence of a significant ANOVA. A p value of less than 0.05 was considered as the threshold for a significant difference.
Results
Arachidonate Analogues Bind Within the Proposed Sterol Binding Pocket of SCP-2
Automated docking of cholesterol, arachidonic acid, AEA, 2-AG, and AM404 to SCP-2 was accomplished using the SCP-2 NMR structure determined by Garcia et al. [21], focusing on the hydrophobic cavity containing Thr85 and Gly86. Of the compounds examined, cholesterol (ΔG=−6.62 kcal/mol) docked with the lowest free energy of binding in this site (Table 1). AEA (ΔG=−3.60 kcal/mol) and 2-AG (ΔG=−2.80 kcal/mol) bound SCP-2 with higher free energy than cholesterol. Arachidonic acid bound with lower energy (ΔG=−4.60 kcal/mol) than AEA. N-Arachidonylaminophenol (AM404), an inhibitor of AEA accumulation by neurons [28], showed the lowest mean free energy of binding (ΔG=−4.80 kcal/mol) of the fatty acid analogues studied.
Table 1.
Calculated mean free energy of binding (ΔG) of lipids docked into SCP-2
| Ligand | ΔG (kcal/mol) |
| Cholesterol | −6.62 |
| AM404 | −4.80 |
| Arachidonic acid | −4.60 |
| AEA | −3.60 |
| 2-AG | −2.80 |
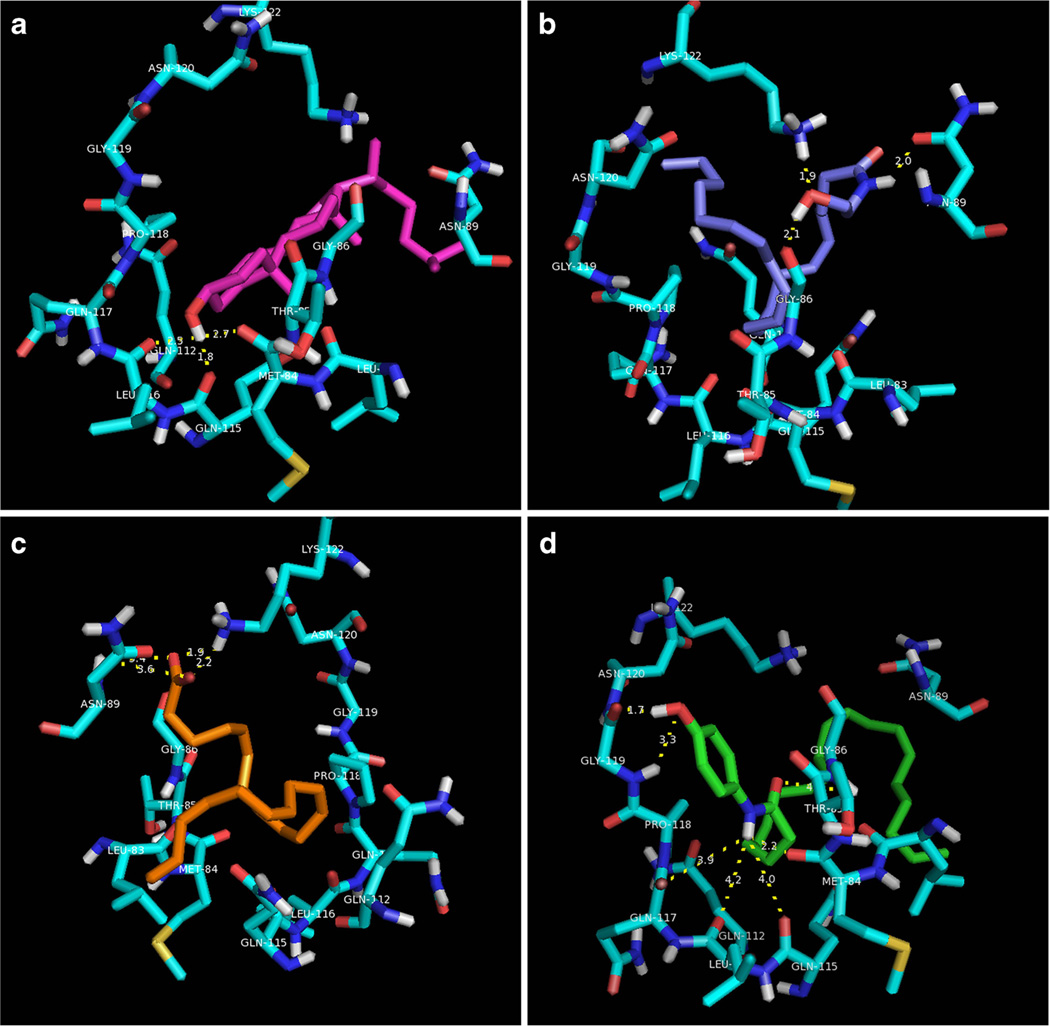
Figure 1 shows orientations of cholesterol, AEA, arachidonic acid, and AM404 within the active site. Cholesterol was found to engage in hydrogen bond-donating interactions with Met84 and Gln115. The lipophilic tail recognizes the backbone region extending from Met109 to Gln112. The amide of AM404 is within similar distance for hydrogen bond-donating activity with Met84 (2.2 Å) as cholesterol. In addition, the phenol is capable of both hydrogen bond-donating (1.7 Å) and accepting (3.3 Å) interactions with Gly119. The polar head group of AEA is oriented toward the entrance of the cavity, optimizing charge–dipole interactions with the side-chain of Lys122 and hydrogen bond-donating interactions with the backbone of Gly86. The lipophilic tail group extends into the pocket lined by Thr85, Met84, and Gln115. The anionic head group of arachidonic acid engages in electrostatic interactions with the cationic side-chain of Lys122. The tail extends into a lipophilic pocket lined by Leu83 and Gln115. 16-Doxyl-stearic acid, which was found to significantly broaden Thr85 and Gly86 NMR signals [21], shared similar electrostatic interactions with Lys122 as arachidonic acid. Interestingly, 16-doxyl-stearic acid was found to preferentially maximize hydrophobic interactions with the backbone extending from Asn120–Pro118, thus binding in an alternate orientation than other arachidonate analogues modeled here. Further discussion of the molecular docking results can be found in Supplemental Information.
Fig. 1.
Automated docking of SCP-2 substrates reveals unique binding interactions among lipids bound within the putative substrate recognition site. a Cholesterol (magenta) donates hydrogen bonds with Met84, Gln115, and Leu116. The lipophilic tail extends into the cleft in contact with Asn89. b AEA (light blue) engages in hydrogen bond-donating interactions with the side-chain of Gln89 and Gly86, and hydrogen bond-accepting interactions with the cationic side-chain of Lys122. The arachidonate tail extends toward the pocket lined by Asn120. c The polar head group of arachidonic acid engages in an energetically favourable electrostatic interaction with Lys122. Dissimilar to AEA, the arachidonate tail extends toward the lipophilic pocket lined by Leu83, Met 84, and Gln115. d The 4-aminophenol head group of AM404 simultaneously engages in similar hydrogen bond-donating interactions as cholesterol (Met84, Gln112, Gln115, Gln117) and unique hydrogen bonding interactions with the backbone of Gly119. The arachidonate tail orients toward Leu83, similar to arachidonic acid
SCP-2-Mediated Transfer of Cholesterol In Vitro is Inhibited by AEA
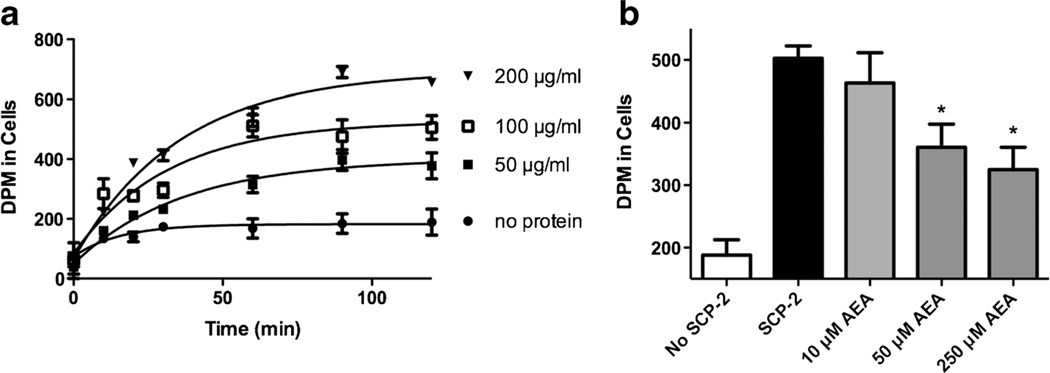
In order to determine whether AEA can bind SCP-2, we performed in vitro experiments using purified SCP-2 protein. In accord with earlier studies, we found that the presence of recombinant SCP-2 enhances the transfer of cholesterol from SUVs to acceptor L1210 cells in a dose and time dependent fashion (Fig. 2a). Following a 1-h incubation, nearly three times more [14C]cholesterol associates with L1210 cells in the presence of SCP-2 as in its absence. The addition of AEA significantly reduces the transfer of [14C]cholesterol (one-way ANOVA F3,15=5.2, p<0.05) (Fig. 2b). Post-hoc tests reveal that both 50 and 250 µM AEA produce significant reductions in the amount of [14C]cholesterol associated with cells.
Fig. 2.
AEA inhibits transfer of cholesterol by SCP-2. a [14C]Cholesterol (0.4 mM) was incorporated into SUVs incubated with recombinant SCP-2 at the concentrations indicated and L1210 cells for 0–120 min. The amount of [14C] was determined in L1210 cells as an index of the cholesterol transfer efficacy of SCP-2. Each point is the mean of three independent experiments. b [14C]Cholesterol-containing SUVs were incubated without (open bar) or with recombinant SCP-2 (200 µg/ml; total volume of 1.5 ml) and increasing concentrations of AEA for 60 min at 37 °C and the amount of [14C] was determined in L1210 cells. Each bar represents four replicates. *p<0.05, **p<0.01
Initial AEA Uptake into HEK 293 Cells is Increased by SCP-2 Expression
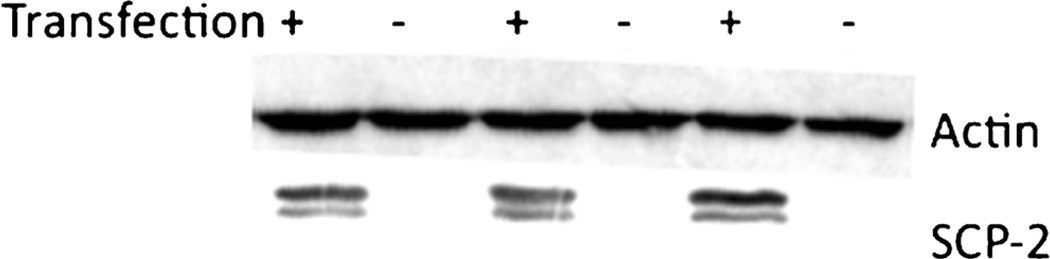
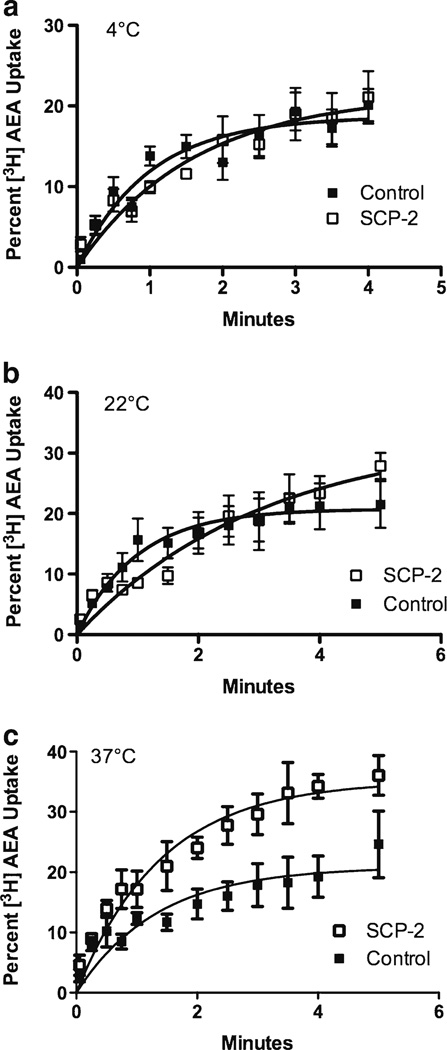
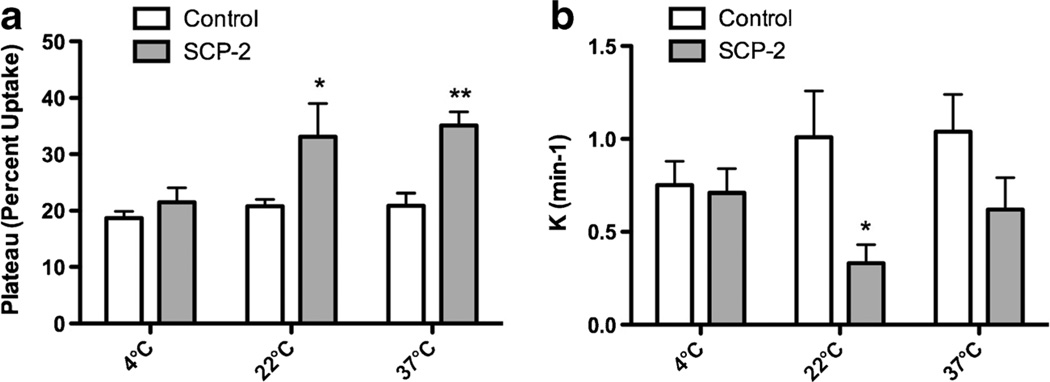
To determine whether SCP-2 facilitates cellular uptake of AEA, SCP-2 was transiently expressed in HEK 293 cells. Untransfected HEK 293 cells do not exhibit detectable amounts of SCP-2 and expression of the SCP-2 plasmid led to a measureable amount of SCP-2 protein (Fig. 3). Untransfected HEK 293 cells rapidly accumulate [3H]AEA (Fig. 4). However, the plateau values determined by fitting the uptake data to the single order association equation are the same regardless of the incubation temperature of the cells (Fig. 5a). The expression of SCP-2 exerts a temperature-sensitive increase in the uptake of AEA in the first 5 min after addition (Fig. 4). Two-way analysis of the plateau values reveals significant main effects of both SCP-2 (F1,27=17.2; p<0.0005) and temperature (F2,27=4.4; p<0.05) without a significant interaction. Planned post hoc comparisons reveal that AEA uptake in SCP-2 expressing cells reaches a significantly higher plateau at incubation temperatures of 22° and 37°, but not at 4 °C (Fig. 5a). Two-way ANOVA was also applied to the rate constant (K) for the uptake process (Fig. 5b). The presence of SCP-2 exerted a significant main effect on the rate constant (F1,27=7.7; p<0.01) but temperature did not (F2,27=2.4, n.s.). Planned post hoc comparisons indicate that K is significantly decreased in cells expressing SCP-2 at 22 °C incubation.
Fig. 3.
Representative Western blot of untransfected HEK 293 cells and those transfected with a plasmid containing full length human SCP-2. Western blots were probed with SCP-2 antisera or antisera against β-actin for a loading control. The image is representative of three different transfections
Fig. 4.
Uptake of AEA by HEK 293 cells is increased in a temperature-dependent manner. For all experiments, HEK 293 cells were treated with empty vector (“Control”) or with SCP-2 containing plasmid for 24 h in lipofectamine 2000 prior to the uptake study. Cells were washed with transport buffer and incubated in that buffer for 30 min at the temperature indicated. [3H]AEA (1 nM) was added and, at the times indicated, buffer and cells were separated and the amount of [3H] was determined in both fractions. The percent [3H] in the cells was determined. Each isotherm shown is the least squares fit of the single site, association equation (GraphPad Prism) to the mean data of five to six replicates, vertical lines represents the SEM. Closed squares are control and open squares SCP-2 expressing HEK 293 cells. Incubation temperatures were: a 4 °C, b 22 °C and c 37 °C
Fig. 5.
Kinetic parameters of the effects of SCP-2 on AEA uptake into HEK 293 cells. These parameters were obtained from the nonlinear fit of the data shown in Fig. 4 to a single site association equation using GraphPad Prism. a The values for the plateau and b the rate constants. Vertical lines represent SEM. Data were analyzed using two-way ANOVA, with SCP-2 transfection status and incubation temperature as main effects. *p<0.05 and **p<0.01 compared to the control transfected data at the same temperature using Bonferroni’s t-tests
SCP-2 Does Not Alter [3H]AEA Accumulation at Equilibrium
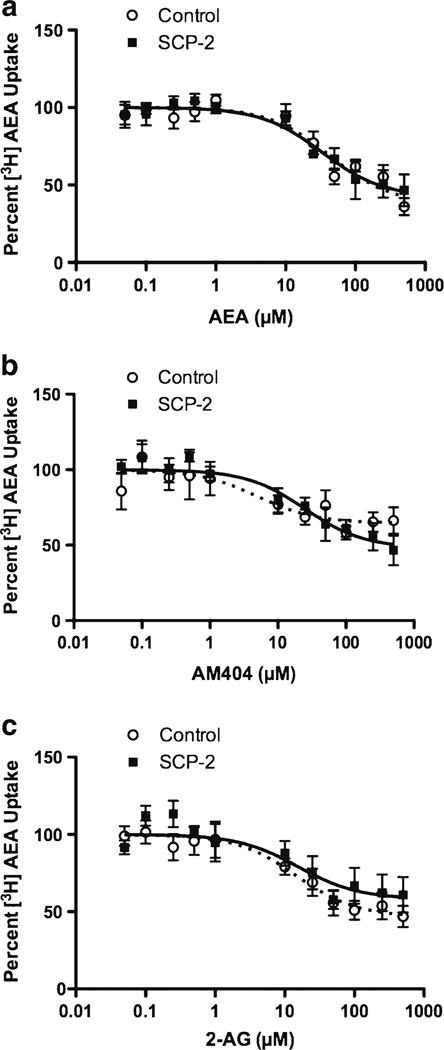
To test the hypothesis that SCP-2 serves as a sequestration site for AEA, we examined the amount of [3H]AEA present in cells after a 10-min incubation with the radioligand, a time at which equilibrium has been reached (data not shown). Surprisingly, there is no significant difference in the percent [3H]AEA that is present within the cells between control and SCP-2 expressing HEK 293 cells (27.1±2.6% for control and 29.3±2.6 % for SCP-2 expressing) at equilibrium. The accumulation of [3H]AEA at equilibrium is reduced by unlabelled AEA and by AM404 and 2-AG (Fig. 6), which is consistent with a saturable sequestration of AEA. However, the addition of SCP-2 to the HEK 293 cells did not alter the competition isotherms for any of the competing ligands. The composite IC50 values and the bottom of the inhibition curves are provided in Table 2. In all cases, the 95% confidence intervals are overlapping, which indicates that there are no significant effects of SCP-2 expression on these parameters.
Fig. 6.
Competition for [3H]AEA accumulation in HEK 293 cells. Control (open circles and dashed lines) and SCP-2-transfected (closed squares and solid lines) HEK 293 cells were incubated with 1 nM a AEA alone or in combination with the competitors at the concentrations indicated for 10 min at 37 °C. The fractional [3H]AEA accumulation was calculated at each concentration of competitor and normalized to the value obtained in the absence of competitor. Each data point is the mean of at least four replicates; nonlinear regression was used to fit the data to a single site competition equation, with the upper limit confined to 100. a Inhibition by unlabeled AEA. b Inhibition byAM404. c Inhibition by 2-AG
Table 2.
IC50 values and bottom value obtained from nonlinear least squares curve fit of competition data shown in Fig. 6
| Control transfected | SCP-2 transfected | |
|---|---|---|
| Competitor | IC50 values (95 % confidence interval) | |
| AEA | 38 µM (20–90) | 42 µM (15–75) |
| AM404 | 5 µM (0.6–42) | 25 µM (9–67) |
| 2-AG | 47 µM (6–39) | 58 µM (4–70) |
| Bottom value (95 % confidence interval) | ||
| AEA | 38 % (25–51) | 42 % (29–55) |
| AM404 | 65 % (54–75) | 48 % (36–60) |
| 2-AG | 47 % (36–57) | 58 % (45–72) |
The bottom value is the percent of [3H]AEA that is uninhibited by the addition of the competitor
Discussion
These studies support the hypothesis that SCP-2 can bind to AEA and other arachidonic acid analogs. In silico docking studies indicate that the arachidonates bind to the same site as cholesterol but with higher relative free energy, which suggests they have lower affinity for the binding site than cholesterol. In support of the predictions from the docking studies, we found that AEA competes with cholesterol for SCP-2 binding using an in vitro cholesterol transfer assay; however, the concentration of AEA required is high. Although heterologous expression of SCP-2 in HEK 293 cells enhances the initial uptake of AEA at 22° and 37 °C, the presence of SCP-2 does not increase the amount of AEA associated with cells at equilibrium. SCP-2 does not alter the IC50 values for competitors to reduce AEA sequestration at equilibrium. We conclude that SCP-2 reduces a barrier to the entry of AEA into cells, but is not functioning as a sequestration or long term binding site for the lipid. It is possible that SCP-2 serves as a transport protein for AEA, moving it to an ultimate sequestration site that has higher affinity for AEA than SCP-2.
SCP-2 is a multi-purpose lipid binding protein that shuttles cholesterol and other lipids from the endoplasmic reticulum, where they are synthesized, to the cell surface [18]. SCP-2 can bind many types of lipid, including fatty acids, fatty acyl CoA derivatives and phospholipids [18]. SCP-2 is expressed in the brain and is enriched in synaptosomal preparations [19, 29], suggesting that it plays a role in synaptic transmission. Schroeder and colleagues have described a mechanism by which SCP-2 shuttles its lipophilic cargo to a desired area in the cell [18]. According to their model, the N terminus of SCP-2 contains an amphipathic alpha helix that allows for association with acidic-phospholipid-rich membranes. This results in the association of SCP-2 with the internal leaflet of the plasma membrane. SCP-2 preferentially associates with negatively charged lipids and the radius of lipid curvature is an important determinant of SCP-2 interactions [30, 31].
Our automated docking studies provide the first detailed (atomic-level) evaluation of a molecular mechanism of action governing SCP-2 recognition and trafficking of endogenous lipids. Previous structural studies of SCP-2 [21] point to a singular lipophilic cavity that binds and transports hydrophobic substrates. Docking and comparison with homologous human proteins have allowed us to identify the likely pocket in SCP-2 that is responsible for lipid/eicosanoid and steroid transport. Focusing our efforts on this cavity gave us detailed information regarding putative binding modes for various proposed ligands and the relative free energies (ΔG) of binding for sterol and eicosanoid structural motifs. It is interesting to note that arachidonate analogues did not adopt consensus orientations within the binding site. Anionic arachidonates (arachidonic acid, 16-doxyl-stearic acid) maximized ionic interactions with Lys122; however, N-arachidonoyl carboxamides (AEA, AM404) differed in the orientations of their respective head groups. The ethanolamide of AEA maximizes hydrogen bonding interactions with Lys122 and Asn89, though the hydrophobic tails of arachidonic acid and AEA orient in opposite directions toward the lipophilic cleft (Fig. 1b, c). AM404 is not predicted to engage with Lys122, instead maximizing hydrogen bond-donating character with Met84, similar to cholesterol (Fig. 1a). Unique to AM404, the 4-phenol functionality simultaneously engages in hydrogen bond-donating and -accepting interactions with Gly119 (Fig. 1d). Relative calculated ΔG values indicate favourable energetics of binding of rank order of: cholesterol > AM404=arachidonic acid > AEA > 2-AG. Thus, the docking studies predict that arachidonates, including those with a modified head group, bind to the putative lipid cargo binding site of SCP-2.
A prediction of the docking studies is that the arachidonates should compete for and, thus, inhibit, the binding and transport of cholesterol by SCP-2. To test this prediction, we used an in vitro assay of SCP-2 enhancement of cholesterol transport. In agreement with the prediction of the docking studies, we found that AEA inhibited the transfer of cholesterol between SUVs and cells at micromolar concentrations. We attempted to establish an AEA transport assay, using radiolabelled AEA in place of cholesterol, but could not measure a reliable signal. We assume this is because the binding of AEA is too low to be detected.
Previous studies have shown that extracellularly administered AEA rapidly enters cells in a manner that is consistent with facilitated diffusion [32]. Since SCP-2 has been shown to traffic lipids, such as cholesterol, across aqueous barriers, we tested the hypothesis that the addition of SCP-2 would enhance cellular uptake of AEA. A second hypothesis that we considered is that SCP-2 serves as an intracellular sequestration site for AEA. To test the first hypothesis, HEK 293 cells were transiently transfected with SCP-2 for 24 h then the accumulation of labeled AEA was determined for 5 min after its addition to the extracellular media. Untransfected HEK 293 cells take up AEA and the plateau reached is the same whether the cells are incubated at 4 °C or 37 °C. These data are consistent with cell entry via passive diffusion. When SCP-2 is expressed, a clear increase in accumulation is seen throughout the time course in cells incubated at 37 °C. Comparison of the plateau and rate constant values predicted by the fit of these data to a single site association equation demonstrate that the SCP-2 expressing cells accumulate AEA to a greater plateau value than control without a significant change in the rate constant. Interestingly, the cells incubated at 22 °C, but not those incubated at 4 °C, also have a significant increase in plateau value. These data are consistent with the hypothesis that the presence of SCP-2 decreases a barrier that retards the initial accumulation of AEA by HEK 293 cells. It is our hypothesis that SCP-2 functions to desorb AEA from the plasma membrane and traffic it to other intracellular organelles, a mechanism that is consistent with the known effect of SCP-2 to traffic lipids, such as cholesterol, between intracellular compartments [33].
Our second hypothesis was that SCP-2 also functions as an intracellular sequestration site for AEA. A prediction of this hypothesis is that the addition of SCP-2 to a cell should increase its intracellular concentration at equilibrium. Our results with competing arachidonates (unlabeled AEA; AM404 and 2-AG) demonstrate that all three compete with the accumulation of [3H]AEA under equilibrium conditions in untransfected HEK 293 cells at 37 °C. The IC50 values are in the range of those previously reported in other cell types [34]. These data indicate that the steady state accumulation of [3H]AEA is saturable, which is consistent with a protein binding site serving as a sequestration mechanism [3]. Surprisingly, the expression of SCP-2 affected neither the total [3H]AEA accumulated in HEK 293 cells at equilibrium nor the IC50 values for competition by AEA, AM404 or 2-AG. Thus, we conclude that, although the presence of SCP-2 enhances the initial uptake of [3H]AEA, it does not function as a sequestration site for AEA in HEK 293 cells. It is possible that the affinity of SCP-2 for AEA is too low to contribute significantly to its cellular accumulation. Treatment of the HEK 293 cells with the fatty acid amide hydrolase (FAAH) inhibitor, URB597, did not affect AEA uptake (data not shown), thus the accumulation of AEA is not driven by FAAH-mediated hydrolysis [35].
These findings suggest that there is another protein or process in HEK 293 cells that serves as an AEA sequestration site. A series of studies by Kaczocha and Deutsch demonstrate that two FABPs, 5 (also called EFABP and KFABP) and 7 (also called BFABP) bind AEA and other N-acylethanolamines at micromolar concentrations and function as intracellular transporters in several cell types [9, 36]. HEK293 cells have been shown to express FABP7 [37], so it is possible that this protein is responsible for the accumulation of AEA. Recent data in sensory neurons support the importance of FABP’s in the cellular uptake of AEA, particularly under pathological conditions [38].
Through multiple approaches, these data support the hypothesis that SCP-2 can bind to the AEA in the micromolar range. Given that the concentrations of AEA in brain tissue are measured in the nanomolar range, it is likely that a small fraction of the available AEA would be bound to SCP-2. However, our data also indicate that the presence of SCP-2 in a model cell increases the initial uptake of AEA. Furthermore, the hypothesis that brain AEA is inactivated through a reuptake process has been proposed and is supported by data from isolated neurons and pharmacological inhibitors [3]. SCP-2 is present in brain, specifically in synaptosomes, which are preparations from brain that are enriched in presynaptic nerve terminals [20]. Interestingly, ethanol also competes with cholesterol for binding to SCP-2 at pharmacologically relevant concentrations [19] and acute exposure of rats to ethanol decreases total [39] and extracellular [40] AEA concentrations in the nucleus accumbens. As the effects of ethanol on tissue AEA concentrations are not due to changes in synthesis or FAAH-mediated hydrolysis of AEA [39], the present study suggests the hypothesis that ethanol and AEA share binding to SCP-2 and could influence each other through that mechanism. Genetic screens have found that SCP-2 gene (scp2) expression is reduced in several important neuropsychiatric disorders. For example, scp2 expression is reduced significantly in the frontal cortex, striatum and midbrain in the wiggle rat, a congenic rat with behavioral characteristics that are similar to attention deficit, hyperactivity disorder in humans [41]. A genome wide association study recently identified scp2 as a candidate gene for narcolepsy [42]. Thus, regardless of the interactions with the ECS, SCP-2 likely has important roles in brain function that deserve more attention.
Supplementary Material
Acknowledgements
These studies were supported by NIH grants DA09155 and DA026996 and by the Advancing a Healthier Wisconsin Endowment of the Medical College of Wisconsin. CWC was supported by a New Investigator Award from the American Association of Colleges of Pharmacy (AACP).
Abbreviations
- 2-AG
2-Arachidonoylglycerol
- AEA
N-Arachidonylethanolamine
- ANOVA
Analysis of variance
- CB1R
Type 1 cannabinoid receptor
- eCBs
Endocannabinoids
- ECS
Endocannabinoid signaling
- SCP-2
Sterol carrier protein 2
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12035-014-8651-7) contains supplementary material, which is available to authorized users.
Conflicts of Interest and Roles in the Study None of the authors have any conflicts of interest to report. ESL carried out the biological studies; CDV, DSS and CWC carried out the docking studies. ESL, DSS, CWC and CJH conceived the studies and wrote the manuscript.
Contributor Information
Elizabeth Sabens Liedhegner, Neuroscience Research Center and Departments of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA.
Caleb D. Vogt, Department of Pharmaceutical Sciences, Concordia University of Wisconsin, School of Pharmacy, Mequon, WI 53097, USA
Daniel S. Sem, Department of Pharmaceutical Sciences, Concordia University of Wisconsin, School of Pharmacy, Mequon, WI 53097, USA
Christopher W. Cunningham, Department of Pharmaceutical Sciences, Concordia University of Wisconsin, School of Pharmacy, Mequon, WI 53097, USA
Cecilia J. Hillard, Email: chillard@mcw.edu, Neuroscience Research Center and Departments of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA.
References
- 1.Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler CJ. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 2013;280(9):1895–1904. doi: 10.1111/febs.12212. [DOI] [PubMed] [Google Scholar]
- 3.Hillard CJ, Jarrahian A. Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol. 2003;140(5):802–808. doi: 10.1038/sj.bjp.0705468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez ER. Chaperoning steroidal physiology: lessons from mouse genetic models of Hsp90 and its cochaperones. Biochim Biophys Acta. 2012;1823(3):722–729. doi: 10.1016/j.bbamcr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muccioli GG. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov Today. 2010;15(11–12):474–483. doi: 10.1016/j.drudis.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Tsou K, Nogueron MI, Muthian S, Sanudo-Pena MC, Hillard CJ, Deutsch DG, Walker JM. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett. 1998;254(3):137–140. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26(18):4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20(2):441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A. 2009;106(15):6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oddi S, Fezza F, Pasquariello N, D’Agostino A, Catanzaro G, De Simone C, Rapino C, Finazzi-Agro A, Maccarrone M. Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem Biol. 2009;16(6):624–632. doi: 10.1016/j.chembiol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285(43):32679–32683. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boneva NB, Mori Y, Kaplamadzhiev DB, Kikuchi H, Zhu H, Kikuchi M, Tonchev AB, Yamashima T. Differential expression of FABP 3, 5, 7 in infantile and adult monkey cerebellum. Neurosci Res. 2010;68(2):94–102. doi: 10.1016/j.neures.2010.07.2028. [DOI] [PubMed] [Google Scholar]
- 13.Kipp M, Gingele S, Pott F, Clarner T, van derValk P, Denecke B, Gan L, Siffrin V, Zipp F, Dreher W, Baumgartner W, Pfeifenbring S, Godbout R, Amor S, Beyer C. BLBP-expression in astrocytes during experimental demyelination and in human multiple sclerosis lesions. Brain Behav Immun. 2011;25(8):1554–1568. doi: 10.1016/j.bbi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70) Mol Med Today. 1999;5(12):525–531. doi: 10.1016/s1357-4310(99)01599-3. [DOI] [PubMed] [Google Scholar]
- 15.Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, Bandiera T, Reggiani A, Mor M, Cavalli A, Piomelli D. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2012;15(1):64–69. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallegos AM, Atshaves BP, Storey SM, McIntosh AL, Petrescu AD, Schroeder F. Sterol carrier protein-2 expression alters plasma membrane lipid distribution and cholesterol dynamics. Biochemistry. 2001;40(21):6493–6506. doi: 10.1021/bi010217l. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder F, Atshaves BP, McIntosh AL, Gallegos AM, Storey SM, Parr RD, Jefferson JR, Ball JM, Kier AB. Sterol carrier protein-2: new roles in regulating lipid rafts and signaling. Biochim Biophys Acta. 2007;1771(6):700–718. doi: 10.1016/j.bbalip.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avdulov NA, Chochina SV, Igbavboa U, Warden CS, Schroeder F, Wood WG. Lipid binding to sterol carrier protein-2 is inhibited by ethanol. Biochim Biophys Acta. 1999;1437(1):37–45. doi: 10.1016/s0005-2760(98)00178-7. [DOI] [PubMed] [Google Scholar]
- 20.Myers-Payne SC, Fontaine RN, Loeffler A, Pu L, Rao AM, Kier AB, Wood WG, Schroeder F. Effects of chronic ethanol consumption on sterol transfer proteins in mouse brain. J Neurochem. 1996;66(1):313–320. doi: 10.1046/j.1471-4159.1996.66010313.x. [DOI] [PubMed] [Google Scholar]
- 21.Garcia FL, Szyperski T, Dyer JH, Choinowski T, Seedorf U, Hauser H, Wuthrich K. NMR structure of the sterol carrier protein-2: implications for the biological role. J Mol Biol. 2000;295(3):595–603. doi: 10.1006/jmbi.1999.3355. [DOI] [PubMed] [Google Scholar]
- 22.Moncecchi D, Murphy EJ, Prows DR, Schroeder F. Sterol carrier protein-2 expression in mouse L-cell fibroblasts alters cholesterol uptake. Biochim Biophys Acta. 1996;1302(2):110–116. doi: 10.1016/0005-2760(96)00044-6. [DOI] [PubMed] [Google Scholar]
- 23.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazeley PS, Prithivi S, Struble CA, Povinelli RJ, Sem DS. Synergistic use of compound properties and docking scores in neural network modeling of CYP2D6 binding: predicting affinity and conformational sampling. J Chem Inf Model. 2006;46(6):2698–2708. doi: 10.1021/ci600267k. [DOI] [PubMed] [Google Scholar]
- 25.O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kernstock RM, Girotti AW. Lipid transfer protein binding of unmodified natural lipids as assessed by surface plasmon resonance methodology. Anal Biochem. 2007;365(1):111–121. doi: 10.1016/j.ab.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila A, Levchenko VV, Korytowski W, Girotti AW. Sterol carrier protein-2-facilitated intermembrane transfer of cholesterol- and phospholipid-derived hydroperoxides. Biochemistry. 2004;43(39):12592–12605. doi: 10.1021/bi0491200. [DOI] [PubMed] [Google Scholar]
- 28.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277(5329):1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 29.Myers-Payne SC, Hubbell T, Pu L, Schnutgen F, Borchers T, Wood WG, Spener F, Schroeder F. Isolation and characterization of two fatty acid binding proteins from mouse brain. J Neurochem. 1996;66(4):1648–1656. doi: 10.1046/j.1471-4159.1996.66041648.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Ball JM, Billheimer JT, Schroeder F. The sterol carrier protein-2 amino terminus: a membrane interaction domain. Biochemistry. 1999;38(40):13231–13243. doi: 10.1021/bi990870x. [DOI] [PubMed] [Google Scholar]
- 31.Butko P, Hapala I, Scallen TJ, Schroeder F. Acidic phospholipids strikingly potentiate sterol carrier protein 2 mediated intermembrane sterol transfer. Biochemistry. 1990;29(17):4070–4077. doi: 10.1021/bi00469a007. [DOI] [PubMed] [Google Scholar]
- 32.Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69(2):631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- 33.Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh AL, Martin GG, Chao H, Kier AB, Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res. 2001;40(6):498–563. doi: 10.1016/s0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 34.Hillard CJ, Jarrahian A. Accumulation of anandamide: evidence for cellular diversity. Neuropharmacology. 2005;48(8):1072–1078. doi: 10.1016/j.neuropharm.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci U S A. 2003;100(7):4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG. Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem. 2012;287(5):3415–3424. doi: 10.1074/jbc.M111.304907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takaoka N, Takayama T, Teratani T, Sugiyama T, Mugiya S, Ozono S. Analysis of the regulation of fatty acid binding protein 7 expression in human renal carcinoma cell lines. BMC Mol Biol. 2011;12:31. doi: 10.1186/1471-2199-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khasabova IA, Holman M, Morse T, Burlakova N, Coicou L, Harding-Rose C, Simone DA, Seybold VS. Increased anandamide uptake by sensory neurons contributes to hyperalgesia in a model of cancer pain. Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrer B, Bermudez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez- Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, Parsons LH, Piomelli D, Rodriguez de Fonseca F. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404(1):97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009;111(1):37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuo Y, Ishido M, Morita M, Sawa H, Nagashima K, Niki E. Behavioural characteristics and gene expression in the hyperactive wiggling (Wig) rat. Eur J Neurosci. 2007;25(12):3659–3666. doi: 10.1111/j.1460-9568.2007.05613.x. [DOI] [PubMed] [Google Scholar]
- 42.Shimada M, Miyagawa T, Kawashima M, Tanaka S, Honda Y, Honda M, Tokunaga K. An approach based on a genome-wide association study reveals candidate loci for narcolepsy. Hum Genet. 2010;128(4):433–441. doi: 10.1007/s00439-010-0862-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.