Abstract
Macrophage recruitment to the central nervous system (CNS) during AIDS pathogenesis is poorly understood. We measured the accumulation of brain perivascular (CD163+) and inflammatory (MAC387+) macrophages in SIV-infected monkeys. Monocyte progenitors were 5-bromo-2′-deoxyuridine (BrdU) labeled in bone marrow, and CNS macrophages were labeled serially with fluorescent dextrans injected into the cisterna magna. MAC387+ macrophages accumulated in the meninges and choroid plexus in early inflammation and in the perivascular space and SIV encephalitis (SIVE) lesions late. CD163+ macrophages accumulated in the perivascular space and SIVE lesions with late inflammation. Most of the BrdU+ cells were MAC387+; however, CD163+BrdU+ macrophages were present in the meninges and choroid plexus with AIDS. Most (81.6% ± 1.8%) of macrophages in SIVE lesions were present in the CNS before SIVE lesion formation. There was a 2.9-fold increase in SIVp28+ macrophages entering the CNS late compared with those entering early (P < 0.05). The rate of CD163+ macrophage recruitment to the CNS inversely correlated with time to death (P < 0.03) and increased with SIVE. In SIVE animals, soluble CD163 correlated with CD163+ macrophage recruitment (P = 0.02). Most perivascular macrophages that comprise SIVE lesions and multinucleated giant cells are present in the CNS early, before SIVE lesions are formed. Most SIV-infected macrophages traffic to the CNS terminally with AIDS.
HIV-associated neurological disorders are associated with central nervous system (CNS) pathologies and include motor, behavioral, and cognitive impairment.1–3 Proposed explanations for the high prevalence of HIV-associated neurological disorders (approximately 50%), despite effective antiretroviral therapy (ART), include incomplete CNS drug penetrance, continued viral replication in the brain, persistent and chronic macrophage activation, CNS toxicity associated with ART, and the normal effects of aging.2–7 In SIV-infected monkeys, HIV-infected humans pre-ART, and some HIV-infected humans after ART, infection of the CNS may be associated with encephalitic lesions composed of a focal accumulation of macrophages and microglia, and productive viral infection. Macrophages and microglia that drive CNS pathology are targets of productive viral infection.3,8–16
The timing of monocyte and macrophage entry and accumulation in the CNS as well as the entry of HIV and SIV quasispecies that are associated with pathogenesis are not well understood because of the technical limitations of such studies in humans. An increased rate of monocyte egress from the bone marrow, increased numbers and percentages of CD16+ monocytes in the blood, and the accumulation of macrophages in the CNS support the idea that a basal rate of monocyte and macrophage recruitment to the CNS is augmented with HIV infection.16–22 In a macaque model of SIV infection, carboxyfluorescein succinimidyl ester–labeled autologous monocytes were shown to traffic to the cerebral perivascular space and choroid plexus during acute SIV infection [12 to 14 days postinfection (dpi)] at an accelerated rate compared with uninfected controls.23 Analyses of macrophage recruitment to and turnover within the CNS from early infection up to the development of AIDS and SIV encephalitis (SIVE) have not been performed.
CNS macrophages are heterogeneous and can be classified on the basis of tissue localization and/or immune phenotype.9,24,25 Parenchymal microglia, the resident macrophages of the CNS, are yolk sac–derived, myeloid lineage cells that engraft the CNS during embryonic development and are then maintained as a stable population.26,27 Perivascular, meningeal, and choroid plexus macrophages are of bone marrow origin and are thought to be replenished from circulating monocytes in rodents, nonhuman primates, and humans, likely at different rates.17,28–32 In the normal CNS, microglia express the pan-macrophage marker CD68 and have low to undetectable hemoglobin-haptoglobin scavenger receptor CD163 (CD68+CD163−).25 Perivascular macrophages express both CD163 and CD68 (CD163+CD68+).25 A third phenotypic population of macrophages is labeled by the antibody MAC387, which recognizes migration inhibitory factor–related protein (MRP) 14 or the MRP8/MRP14 heterodimer, but does not have detectable CD68 or CD163 (MAC387+CD68−CD163−).14 MAC387+ macrophages are not present in the uninfected or noninflamed CNS but are recruited with inflammation.14,33–36 Parenchymal microglia and CD163+ perivascular macrophages are considered primary targets of HIV and SIV infection in the CNS.5,7 MAC387+ macrophages are rarely found to be productively infected.9,13,14,25,37 The timing of monocyte and macrophage entry into the CNS and the role of macrophage subsets mediating the progression or resolution of CNS inflammation due to HIV and SIV infection are not well defined.
Current research suggests that virus enters the CNS via trafficking of infected monocytes and macrophages, although other mechanisms, including infection of or transcytosis through endothelial cells and direct transmission of free virus from the blood to the cerebrospinal fluid, have been suggested.9,20,38 Productive infection of the CNS can be detected within days to weeks of initial infection, primarily within perivascular macrophages, but then resolves until the development of AIDS (pre-ART) or chronic infection with HIVE lesion formation (post-ART).23,32,38–42 Productive infection of the CNS at end-stage disease may represent recrudescence of virus seeded in the CNS with primary/acute infection, reintroduction of virus into the CNS terminally with AIDS, or both. Regardless, productive infection of macrophages in the perivascular space, encephalitis lesions, and meninges and choroid plexus likely results in production of toxic factors, including cytokines and viral proteins, which contribute metabolic encephalopathy with resultant neuronal and glial cell aberrations.8,43
We studied macrophage recruitment to the CNS in a rapid simian model of neuro-AIDS by 5-bromo-2′-deoxyuridine (BrdU) labeling of myeloid progenitors in the bone marrow, which traffic to the CNS, and serial labeling perivascular macrophages, within the CNS, by intracisternal injection of dextran conjugates in the cerebrospinal fluid. We compared macrophage recruitment rates between early/acute and terminal disease, or between animals with SIVE and animals with SIV but no encephalitis (SIVnoE). Early CNS inflammation was characterized by an influx of MAC387+ macrophages in acute infection. Later, recruitment of both MAC387+ and CD163+ macrophages was ongoing and was greater terminally in animals that developed AIDS and SIVE. BrdU+ macrophages present in CNS tissues were primarily MAC387+, but CD163+BrdU+ macrophages were present in the meninges and choroid plexus terminally with AIDS. Overall, few BrdU+ macrophages were present in the perivascular space and SIVE lesions. The ratio of CD163+/MAC387+ macrophages in the CNS was greater in animals with SIVE compared with SIV-infected animals without SIVE. Recruitment of CD163+ macrophages in the CNS correlated with plasma soluble CD163 (sCD163) in animals with SIVE. In all animals, a greater rate of CD163+ macrophage recruitment correlated with shorter time to death. Terminally with AIDS, CD163+ macrophages accumulated in the perivascular space and SIVE lesions, and not in the meninges or choroid plexus. Interestingly, SIVE lesions were composed primarily of CD163+ macrophages that were present in the CNS early in infection, by 20 dpi, before SIVE lesions are typically present. In SIVE lesions, the percentage of productively infected CD163+ macrophages was 2.9 times higher in macrophages that entered the CNS terminally with AIDS compared with macrophages present in the CNS at 20 dpi. These data indicate a role for resident perivascular macrophages that line CNS blood vessels and migrate to form SIVE lesions and suggest that virus is reintroduced to the CNS terminally with AIDS.
Materials and Methods
Ethics Statement
All animals were handled in accordance with good animal practice, as defined by the Tulane National Primate Research Center Institutional Animal Care and Use Committee. All animal work was approved by the Tulane National Primate Research Center and Boston College (Chestnut Hill, MA) Institutional Animal Care and Use Committee.
Animals, Viral Infection, and CD8+ Lymphocyte Depletion
Twelve adult male rhesus macaques were infected i.v. with 1 ng of SIVp27 of SIVmac251, a generous gift from Dr. Ronald Desrosiers (New England Regional Primate Research Center, Boston, MA). CD8+ lymphocytes were depleted to achieve rapid AIDS (3 to 4 months) with >75% incidence of SIVE.14,17,18,44
Although there is an increase in the number of monocytes, macrophages, and virus in the CNS of CD8+ lymphocyte depleted compared with nondepleted animals, the neuropathology with SIVE is the same. A chimeric anti-CD8 antibody, cM-T807 (NIH Non-Human Primate Reagent Resource, Boston, MA), was administered s.c. (10 mg/kg) at 6 dpi and i.v. (5 mg/kg) on 8 and 12 dpi.17,21 Complete blood cell counts and flow cytometry to monitor leukocyte populations and CD8+ lymphocyte depletion were performed before infection and weekly thereafter. Blood and cerebrospinal fluid were sampled weekly, and CNS and other tissues were collected at necropsy. Choroid plexus tissue was available from 7 of 12 animals.
SIV-infected animals were sacrificed with any of the following criteria, indicative of AIDS when present: >15% decrease of body weight in 2 weeks or >30% decrease of body weight in 2 months; documented opportunistic infection; persistent anorexia >3 days without explicable cause; severe, intractable diarrhea; progressive neurological symptoms; or significant cardiac or pulmonary symptoms. Before sacrifice, animals were anesthetized with ketamine–hydrogen chloride, euthanized by an i.v. pentobarbital overdose, and exsanguinated. A postmortem diagnosis of AIDS was confirmed by the presence of AIDS-defining lesions, including the following: Pneumocystis pneumonia, Mycobacterium avium infection, and intestinal adenovirus infection. SIVE was defined by the presence of multinucleated giant cells, accumulation of macrophages in the CNS, and productive viral infection.17,45–47
Necropsy tissues were: i) collected in 10% neutral-buffered formalin and embedded in paraffin, ii) fixed with 2% paraformaldehyde for 4 hours and embedded in OCT compound (Miles Scientific, Naperville, IL), and iii) embedded in OCT without fixation and snap frozen. Formalin-fixed, paraffin-embedded tissues were cut into sections (5 μm thick), and frozen tissues were divided into sections (10 μm thick). CNS tissues from three uninfected control animals that received autologous CD34+ bone marrow stem cells, transduced with an enhanced green fluorescence protein–expressing lentiviral construct, were used to determine basal turnover of CNS macrophages.28
BrdU Administration
A stock solution of 30 mg/mL BrdU (Sigma-Aldrich, St. Louis, MO) was prepared in calcium and magnesium-free phosphate-buffered saline (USP grade), as previously described.18,48 BrdU was administered as an i.v. injection at a dose of 60 mg BrdU/kg body weight. BrdU was administered at either 6 and 20 dpi (termed early) or 49 dpi and 48 hours before necropsy (termed late). The percentage of BrdU+ monocytes was determined by flow cytometry 24 hours after administration.
Dextran Uptake by Uninfected Monocytes ex Vivo
To investigate if monocyte subsets have differential dextran uptake, ex vivo studies using whole blood were performed. EDTA anticoagulated whole blood from normal, uninfected rhesus macaques (n = 6) was incubated with 1 mg/mL fluorescein-conjugated dextran (Molecular Probes, Eugene, OR) for 15 minutes at 37°C. Erythrocytes were lysed using the ImmunoPrep Reagent System (Beckman Coulter, Jersey City, NJ), washed twice with phosphate-buffered saline containing 2% fetal bovine serum, and incubated for 15 minutes at room temperature with fluorochrome-conjugated surface antibodies: anti–HLA-DR-ECD (Immu-357), anti–CD16-PE-Cy7 (3G8), anti–CD3-APC (SP34-2), anti–CD8-APC (RPA-T8), anti–CD20-APC (2H7), and anti–CD14–Pacific blue (M5E2). Data were acquired on a BD FACS Aria (BD Biosciences, Franklin Lakes, NJ) and analyzed with Tree Star Flow Jo software version 8.7.
Intracisternal Injection of Dextran Amines and Detection in CNS Tissues
Animals were tranquilized with ketamine or telazol and anesthetized with sodium pentobarbital. One milliliter of dextran amines (25 mg/mL) dissolved in 0.9% saline was injected into the cerebellomedullary cistern using a stereotaxic apparatus. After intracranial injection, the hydrophilic fluorescent dextran dyes diffuse along the perivascular space and are absorbed by essentially all perivascular macrophages (>98%) via non-specific micropinocytosis.25,30,49 To establish a baseline for subsequent perivascular macrophage turnover, all animals (n = 12) received fluorescein-conjugated dextran (abbreviated Dextran:FITC; Molecular Probes) at 20 dpi (Table 1). Five animals received a second injection of Alexa Fluor 647–conjugated dextran (abbreviated Dextran:AF647; Molecular Probes) 48 hours before necropsy to determine macrophage recruitment from 20 dpi necropsy. Four additional animals received a second injection of Alexa Fluor 647–conjugated dextran at 49 dpi and a third injection of biotinylated dextran (Molecular Probes) 48 hours before necropsy to determine macrophage recruitment from 20, 49, and 49 dpi necropsy, respectively (Table 1).
Table 1.
Experimental Design
| Treatment∗ | SIV, 22 dpi |
AIDS |
|||
|---|---|---|---|---|---|
| Early BrdU |
Early BrdU |
Late BrdU |
|||
| One dye (n = 3) | Two dyes (n = 2) | Three dyes (n = 2) | Two dyes (n = 3) | Three dyes (n = 2) | |
| BrdU | 6 and 20 dpi | 6 and 20 dpi | 6 and 20 dpi | 49 dpi and Nec | 49 dpi and Nec |
| Dextran:FITC (dpi) | 20 | 20 | 20 | 20 | 20 |
| Dextran:AF647 (dpi) | Nec | 49 | Nec | 49 | |
| Dextran:biotin | Nec | Nec | |||
| Sacrificed (dpi) | 22 | AIDS | AIDS | AIDS | AIDS |
BrdU, 5-bromo-2′-deoxyuridine; dpi, days postinfection; FITC, fluorescein isothiocyanate; Nec, administration of BrdU or dextran 48 hours before necropsy.
All animals were infected with SIVmac251 (1 ng SIVp27, i.v.) at day 0. Anti-CD8 antibody was administered at 6, 8, and 12 dpi.
The fluorescent dextran conjugates are directly visualized by fluorescence microscopy in sections of paraformaldehyde-fixed, OCT-embedded frozen tissue. Biotinylated dextran was detected with streptavidin conjugated to Alexa Fluor 568 or Alexa Fluor 647 after blocking with a solution of 10% normal goat serum, 0.2% fish skin gelatin (Sigma-Aldrich), and 0.1% Triton X-100 (Sigma-Aldrich). Background endogenous autofluorescence was reduced by incubating sections with 50 mmol/L CuSO4, as previously described, before mounting in Prolong Gold Antifade reagent (Invitrogen-Life Technologies, Carlsbad, CA).50 Dextran dye–labeled macrophages in CNS tissues were counted using a four-color Zeiss Axio Imager M1 microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) by sampling 3000 to 4000 cells from frontal, temporal, and parietal cortices; 1000 cells from meninges; and two sections of choroid plexus per animal. In addition, 10 or more SIVE lesions were evaluated for animals with AIDS and SIVE (n = 5). One SIVE animal only had three SIVE lesions and was excluded from the analysis of macrophage turnover in SIVE lesions.
Fluorescence Microscopy of Macrophage Phenotype and Determination of SIV Infection
Indirect immunofluorescence was used to determine the immune phenotype of macrophages in the CNS using anti-CD163 (EDHu-1; AbD Serotec, Raleigh, NC) or anti-myeloid histiocyte antigen (MAC387; Dako, Carpinteria, CA) antibodies. Productive SIV infection was determined by anti-SIVp28 (clone 3F7; Fitzgerald Industries International, Acton, MA). Alexa Fluor 568– or Alexa Fluor 350–conjugated secondary antibodies were used to detect primary antibodies according to standard protocols.25
Immunohistochemistry for BrdU and Macrophage Markers
Macrophage accumulation and the phenotype of BrdU-labeled macrophages were assessed by immunohistochemistry for myeloid histiocyte antigen (MAC387; Dako), CD163 (EDHu-1; AbD Serotec), and BrdU (Bu20; Dako). A serum-free protein block (Dako) was applied before immunostaining, followed by visualization with EnVision+ horseradish peroxidase system (Dako) using 3,3′-diaminobenzidine tetrahydrochloride as the chromogen. For detection of two epitopes, the EnVision G2 doublestain system (Dako) was used with 3,3′-diaminobenzidine tetrahydrochloride as the horseradish peroxidase substrate and Vector Blue (Vector Labs, Burlingame, CA) as the alkaline phosphatase substrate. Isotype-matched immunoglobulins (Dako) served as controls. Tissue sections were visualized under a Zeiss Axio Imager M1 microscope using Plan-Apochromat 620/0.8 and 640/0.95 Korr objectives. More than twenty ×20 fields were examined from each of three cortical regions with associated meninges and one section of choroid plexus per animal for each stain. For cell counting, the number of CD163+, MAC387+, and BrdU+ macrophages was determined for each tissue section by dividing the total number of cells counted in the ×20 field by the area of CNS tissue examined to give cells per mm2.
sCD163 Enzyme-Linked Immunosorbent Assay
Plasma levels of sCD163, a marker of monocyte activation, were quantified by enzyme-linked immunosorbent assay, according to the manufacturer's protocol (Trillium Diagnostics, Bangor, ME), as previously described.17,51,52
Results
Early CNS Inflammation with SIV Infection Is Characterized by Recruitment of MAC387+ Macrophages to the Perivascular Space, Meninges, and Choroid Plexus
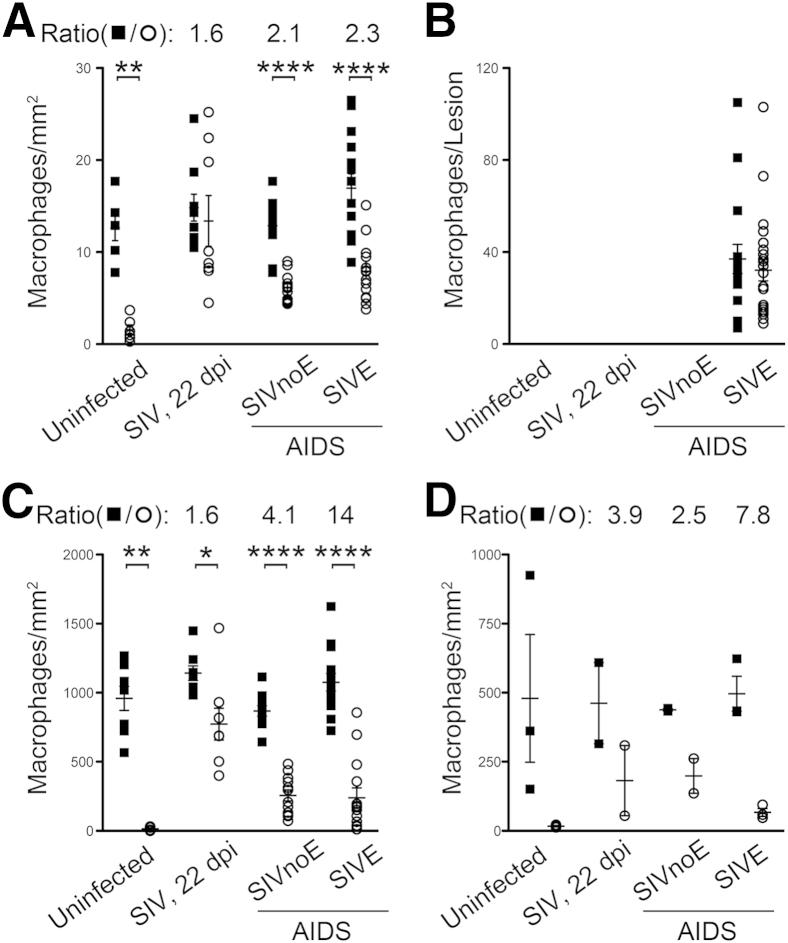
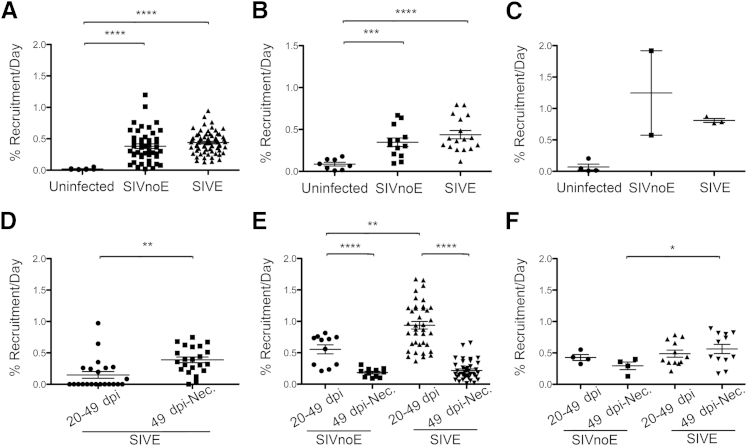
By using single-color immunohistochemistry, we counted the number of CD163+ and MAC387+ macrophages in the CNS of uninfected animals (Uninfected, n = 3), SIV-infected animals sacrificed at 22 dpi (SIV 22 dpi, n = 3), and SIV-infected animals that progressed to AIDS with SIVE (SIVE, n = 5) and without SIVE (SIVnoE, n = 4) (Figure 1). There were no significant differences in the plasma viral load at the same time points after infection between animals sacrificed at 21 dpi, or those sacrificed when they developed AIDS with or without SIVE (Supplemental Figure S1). MAC387+ macrophages were absent from CNS tissues of uninfected animals but were numerous at 22 dpi [perivascular space: 2 ± 1 macrophages/mm2 uninfected and 13 ± 3 macrophages/mm2 SIV 22 dpi (P = 0.0007); meninges: 14 ± 6 macrophages/mm2 uninfected and 770 ± 100 macrophages/mm2 SIV 22 dpi (P = 0.002); choroid plexus: 17 ± 3 macrophages/mm2 uninfected and 180 ± 100 macrophages/mm2 SIV 22 dpi] (Figure 1). The number of CD163+ macrophages did not significantly increase in infected animals sacrificed at 22 dpi compared with uninfected animals (Figure 1). No SIVE lesions were present in the CNS of animals sacrificed at 22 dpi. These data indicate that with SIV infection, early CNS inflammation is characterized primarily by recruitment of MAC387+ macrophages.
Figure 1.
CD163+ and MAC387+ macrophages accumulate in the perivacsular space and SIV encephalitis (SIVE) lesions in SIV-infected animals. Single-label immunohistochemistry for CD163 (closed squares) or MAC387 (open circles) in uninfected animals, SIV-infected animals sacrificed at 22 days postinfection (dpi; SIV, 22 dpi), and SIV-infected animals that progressed to AIDS with encephalitis (SIVE) or without encephalitis (SIVnoE). Each data point represents the cell count from one tissue section or SIVE lesion. MAC387+ macrophages are absent in the normal, uninfected central nervous system (CNS) but are recruited to the CNS with SIV infection. CD163+ macrophages outnumber MAC387+ macrophages in uninfected and SIV-infected animals. A and B: In animals with AIDS, CD163+ and MAC387+ macrophages accumulate primarily in the perivascular space (A) and in SIVE lesions (B). B: SIVE lesions are only observed terminally in animals that progressed to AIDS. C and D: In the meninges (C) and choroid plexus (D), MAC387+ macrophages are more numerous at 22 dpi than terminally with AIDS. A–D: Animals with AIDS and SIVE have higher ratios of CD163+/MAC387+ macrophages than animals with AIDS and no SIVE or animals sacrificed at 22 dpi. The numbers above each graph are the average ratio of CD163+/MAC387+ macrophages for SIV-infected animals. n = 3 (A–D, uninfected animals and SIV, 22 dpi); n = 5 (A–D, SIVE); n = 4 (A–D, SIVnoE). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001. NA, not applicable.
CD163+ Macrophages Accumulate in the Perivascular Space and SIVE Lesions with AIDS and SIVE
There was no significant accumulation of CD163+ macrophages at 22 dpi compared with uninfected animals in the perivascular space (13 ± 1 macrophages/mm2 uninfected and 15 ± 1 macrophages/mm2 SIV 22 dpi), the meninges (960 ± 90 macrophages/mm2 uninfected and 1100 ± 50 macrophages/mm2 SIV 22 dpi), and choroid plexus (480 ± 230 macrophages/mm2 uninfected and 460 ± 150 macrophages/mm2 SIV 22 dpi). In animals with AIDS and no encephalitis (SIVnoE), the number of CD163+ macrophages was similar to uninfected and SIV 22 dpi animals in the perivascular space (13 ± 1 macrophages/mm2 SIVnoE), the meninges (870 ± 40 macrophages/mm2 SIVnoE), and the choroid plexus (440 ± 5 macrophages/mm2 SIVnoE). Last, we found there were more CD163+ macrophages in SIVE animals compared with SIVnoE animals, and CD163+ macrophages accumulated primarily in the perivascular space (17 ± 2 macrophages/mm2 SIVE) and SIVE lesions (37 ± 6 macrophages per lesion SIVE).
There Is a Greater Ratio of CD163+/MAC387+ Macrophages in the Perivascular Space, Meninges, and Choroid Plexus of Animals with AIDS and SIVE
By using single-color immunohistochemistry cell counting data, we determined the ratio of CD163+/MAC387+ macrophages in the perivascular space, meninges, and choroid plexus of SIV-infected animals (ratio = absolute cell count CD163+ macrophages/absolute count MAC387+ macrophages per region). Because SIVE lesions were not present in SIVnoE animals, or animals sacrificed at 22 dpi, intergroup comparisons were not made with regard to SIVE lesions. A higher ratio of CD163+/MAC387+ macrophages (representing increased numbers of CD163+ macrophages and decreased numbers of MAC387+ macrophages) was found in SIVE animals compared with SIVnoE animals or SIV-infected animals sacrificed at 22 dpi (Figure 1, A, C, and D). Extending our prior observation that increased severity of SIVE lesions was reflected in a greater ratio of CD163+/MAC387+ macrophages in SIVE lesions,14 we found that the ratio of CD163+/MAC387+ macrophages was also higher in the perivascular space, meninges, and choroid plexus. This observation suggests that more CD163+ macrophages relative to MAC387+ macrophages are associated with the development of AIDS and SIVE. Overall, both MAC387+ and CD163+ macrophages accumulated primarily in the perivascular space and SIVE lesions and not in the meninges or choroid plexus.
The Phenotype of BrdU+ Macrophages Recruited to the CNS Varies by CNS Compartment
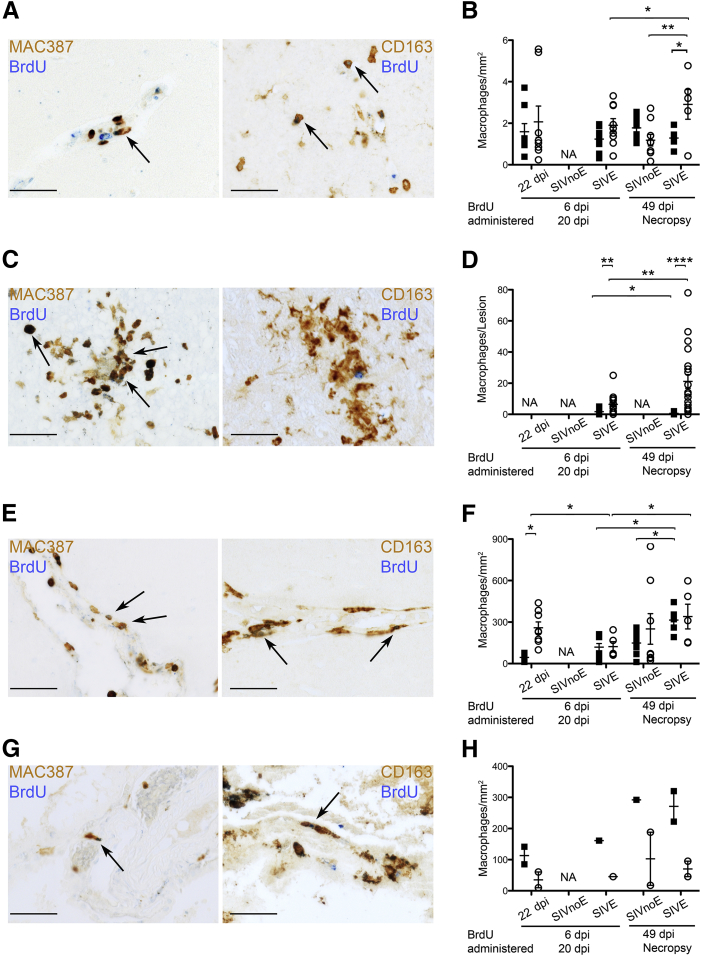
To investigate differences in macrophage traffic from the bone marrow to the CNS in acute infection versus AIDS, monocyte progenitors in the bone marrow were labeled by BrdU administered at 6 and 20 dpi (early, n = 7) or 49 dpi and 48 hours before necropsy (late, n = 5), and BrdU+ cells in the CNS were counted (Table 1). Analysis of CNS tissue was stratified by timing of BrdU administration and CNS pathology, and BrdU+ cells were counted in the perivascular space, SIVE lesions (when present), meninges, and choroid plexus (Table 2 and Figure 2). MAC387+BrdU+ and CD163+BrdU+ macrophages together accounted for essentially all of the BrdU+ cells in the CNS (Figure 2). We did not find CD3+BrdU+ T lymphocytes, as previously reported (data not shown).17
Table 2.
Macrophage Traffic from the Bone Marrow to the CNS Is Highest Terminally with AIDS
| Group∗ | Subset | Perivascular space† | SIVE lesions‡ | Meninges† | Choroid plexus† |
|---|---|---|---|---|---|
| SIV, 22 dpi, BrdU early (n = 3) | CD163+ | 2 ± 0.4 | NP | 45 ± 8 | 110 ± 30§ |
| MAC387+ | 2 ± 0.8 | NP | 260 ± 40 | 35 ± 30§ | |
| SIVE, AIDS, BrdU early (n = 3) | CD163+ | 1 ± 0.2 | 2 ± 0.4 | 120 ± 30 | 160 |
| MAC387+ | 2 ± 0.3 | 6 ± 2 | 120 ± 40 | 45 | |
| SIV, AIDS, BrdU late (n = 3) | CD163+ | 2 ± 0.2 | NP | 150 ± 30 | 290 |
| MAC387+ | 1 ± 0.3 | NP | 250 ± 100 | 100 ± 90§ | |
| SIVE, AIDS, BrdU late (n = 2) | CD163+ | 1 ± 0.3 | 1 ± 0.2 | 310 ± 40 | 270 ± 50§ |
| MAC387+ | 4 ± 0.5 | 21 ± 4 | 340 ± 90 | 70 ± 20§ |
BrdU, 5-bromo-2′-deoxyuridine; CNS, central nervous system; dpi, days postinfection; NP, SIVE lesions not present; SIVE, SIV encephalitis.
Early, BrdU administered at 6 and 20 dpi; late, BrdU administered at 49 dpi and 48 hours before necropsy.
Counts are the means ± SEM of the number of CD163+BrdU+ or MAC387+BrdU+ macrophages per mm2. More than twenty ×20 fields were examined from three different cortical regions, and one section of choroid plexus was examined per animal.
Counts are the means ± SEM of the number of CD163+BrdU+ or MAC387+BrdU+ macrophages per SIVE lesion.
Choroid plexus tissue was available from two animals in this group.
Figure 2.
Recruitment of MAC387+ and CD163+ macrophages from the bone marrow differs in timing and magnitude by central nervous system (CNS) region. Double-label immunohistochemistry for 5-bromo-2′-deoxyuridine (BrdU; blue) and MAC387 or CD163 (brown) was used to quantify traffic of BrdU+ macrophages to the CNS during early [6 and 20 days postinfection (dpi)] and late (49 dpi and 48 hours prenecropsy) SIV infection. Each data point represents cells counted in one tissue section or one SIVE lesion. Data for cell counts are in Table 2. Arrows indicate MAC387+BrdU+ (open circles) or CD163+BrdU+ (closed squares) macrophages. A and B: There are few BrdU+ macrophages in the perivascular space overall, and more MAC387+BrdU+ macrophages in animals with AIDS and SIV encephalitis (SIVE) given BrdU late. C and D: Most BrdU+ macrophages in SIVE lesions are MAC387+ with few CD163+BrdU+ macrophages. More MAC387+BrdU+ macrophages are present in SIVE animals given BrdU late. E and F: BrdU+ macrophages in the meninges are MAC387+ at 22 dpi and both MAC387+ and CD163+ in animals with AIDS. More MAC387+BrdU+ and CD163+BrdU+ macrophages are present in animals with SIVE given BrdU late. G and H: Most BrdU+ macrophages in the choroid plexus are CD163+ with few MAC387+BrdU+ macrophages. More CD163+BrdU+ macrophages are present in animals given BrdU late. n = 3, early BrdU, SIVnoE; early BrdU, SIVE; and late BrdU, SIVnoE. n = 2, late BrdU, SIVE. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001. Scale bar = 50 μm (A, C, E, and G).
Few BrdU+ macrophages were found in the perivascular space (range, 0 to 6 BrdU+ macrophages/mm2), where both MAC387+BrdU+ and CD163+BrdU+ macrophages were present (Table 2 and Figure 2, A and B). In SIVE lesions, most BrdU+ macrophages were MAC387+BrdU+, with rare CD163+BrdU+ macrophages, as previously reported (Table 2 and Figure 2, C and D).14,17 In the meninges, most BrdU+ macrophages were MAC387+BrdU+ in animals sacrificed at 22 dpi, but equal numbers of MAC387+BrdU+ and CD163+BrdU+ macrophages were present in the meninges of animals sacrificed with AIDS, with and without SIVE (Table 2 and Figure 2, E and F). In the choroid plexus, CD163+BrdU+ macrophages outnumbered MAC387+BrdU+ macrophages, regardless of the timing of BrdU administration or CNS pathology (Table 2 and Figure 2, G and H). The observation that CD163+ macrophages present in the meninges at 22 dpi and in SIVE lesions terminally were not BrdU+ suggests these CD163+ macrophages were already present in the CNS before BrdU administration or, alternatively, they may not be recruited from the bone marrow. The differences we find in the relative proportion of MAC387+BrdU+ and CD163+BrdU+ macrophages in SIVE lesions, meninges, and choroid plexus may reflect different mechanisms of recruitment between these CNS compartments or between macrophage subsets.
Recruitment of BrdU+ Macrophages to the CNS Is Greatest Terminally with AIDS and SIVE
More BrdU+ macrophages were present in the CNS of SIVE animals compared with SIVnoE animals, and in animals given BrdU late compared with animals given BrdU early (Table 2 and Figure 2). Recruitment of MAC387+BrdU+ macrophages to the CNS was greatest terminally in animals with AIDS and SIVE in the perivascular space (P < 0.05), SIVE lesions (P < 0.01), and meninges (P < 0.05), but not choroid plexus (Table 2 and Figure 2). The number of CD163+BrdU+ macrophages recruited to the CNS was greater terminally in animals with AIDS and SIVE in the meninges (P < 0.05) and choroid plexus, but not the perivascular space or SIVE lesions (Table 2 and Figure 2). The presence of CD163+BrdU+ macrophages in the meninges terminally suggests that the lack of CD163+BrdU+ macrophages in SIVE lesions is likely not due to differences in labeling affinity between macrophage subsets. Overall, these data indicate that recruitment of CNS macrophages from the bone marrow is greatest during end-stage disease in animals with SIVE, and recruitment of MAC387+BrdU+ macrophages is more widespread within the CNS compared with CD163+BrdU+ macrophages.
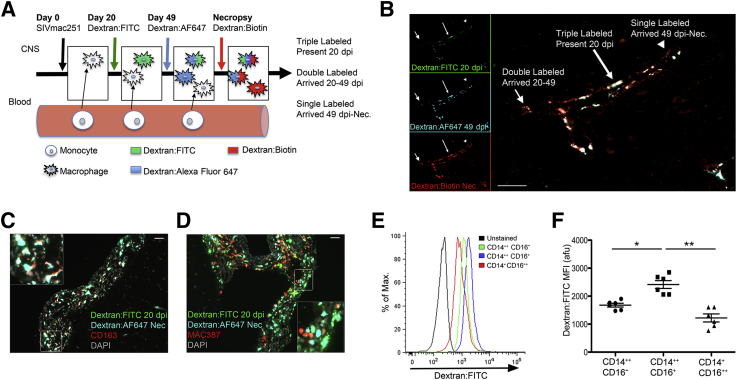
Dextran Dyes Preferentially Label CD163+ Macrophages in Vivo and CD163+ Monocytes in Vitro
Because systemically injected BrdU only labels monocyte/macrophage precursors in bone marrow that traffic to the CNS, we used dextran dyes injected into the cerebrospinal fluid, which label CNS macrophages in the perivascular space, to assess macrophage turnover within the CNS. Macrophages within the perivascular space, meninges, and choroid plexus were labeled by serial injection of fluorescently conjugated dextran dyes into the cerebromedullary cistern of 12 rhesus macaques (Table 1 and Figure 3, A and B). Across all CNS regions, postmortem examination showed that dextran-labeled cells were CD163+ and MAC387+ macrophages, but CD163+ macrophages were the primary population labeled. CD163+ macrophages accounted for 94.2% ± 1.1% of all dextran-labeled cells, and 98.7% ± 0.4% of CD163+ macrophages were dextran labeled. MAC387+ macrophages accounted for 7.0% ± 1.0% of all dextran-labeled cells, and 16.5% ± 2.4% of MAC387+ macrophages were dextran labeled (Figure 3, C and D). No other CNS cell types, including endothelial cells, astrocytes, and parenchymal microglia, were dextran labeled.
Figure 3.
Recruitment of CD163+, but not MAC387+, macrophages to the central nervous system (CNS) with SIV infection is quantitated by serial labeling with soluble dextran dyes. A: Injection of fluorescently conjugated dextran dyes into the cisterna magna labels macrophages in the perivascular space, meninges, and choroid plexus at the time of administration. Serial injection of dextran conjugated to different fluorophores allows for determination of time of entry to the CNS: three dyes [fluorescein, 20 days postinfection (dpi); Alexa Fluor 647, 49 dpi; biotin, 48 hours prenecropsy (Nec)] or two dyes (fluorescein, 20 dpi; Alexa Fluor 647, 48 hours prenecropsy). In animals that were given all three dyes, triple-labeled macrophages (fluorescein + Alexa Fluor 647 + biotin) are present at 20 dpi, double-labeled macrophages (Alexa Fluor 647 + biotin) enter the CNS at 20 to 49 dpi, and single-labeled macrophages (biotin) enter the CNS after 49 dpi. B: Three-color immunofluorescence of a blood vessel and associated perivascular macrophages in the cerebral cortex of an animal sacrificed with AIDS and SIV encephalitis (SIVE). Triple-labeled (long arrow), double-labeled (short arrow), and single-labeled (arrowhead) macrophages are present. Most macrophages are present in the CNS at 20 dpi. Insets: Single-channel images. C: Immunofluorescence staining for CD163 shows that almost all CD163+ macrophages are dextran labeled. D: Immunofluorescence staining for MAC387 shows that few MAC387+ macrophages are dextran labeled. C and D: Images are representative of all CNS regions examined from 12 animals. Insets: Enlargements of the area indicated by a white rectangle. E and F: CD163+CD14+CD16+ monocytes have greater uptake of dextran than CD163+CD14+CD16− and CD163−CD14+CD16+ monocytes in in vitro labeling of whole blood with fluorescein isothiocyanate–conjugated dextran (Dextran:FITC). E: The histogram of Dextran:FITC fluorescence in the three monocyte subsets presented herein is representative of six animals. F: Dextran:FITC is absorbed by all monocyte subsets. Dextran uptake is greatest in CD14++CD16+ intermediate monocytes, putative CD163+ macrophage precursors. n = 4 (A, three dyes); n = 5 (A, two dyes). ∗P < 0.05, ∗∗P < 0.01. Scale bar = 50 μm (B–D). afu, arbitrary fluorescence units; Max., maximum.
Because we found preferential uptake of dextran dyes by CD163+ macrophages compared with MAC387+ macrophages, both of which are monocyte derived, we assessed dextran uptake in ex vivo monocytes from uninfected animals (n = 6) (Figure 3, E and F). After a 15-minute incubation of whole blood with dextran dyes in vitro, all monocyte subsets were dextran labeled. Dextran uptake was greater in CD14++CD16+ intermediate monocytes compared with CD14++CD16− classic monocytes (P = 0.03) or CD14+CD16++ non-classic monocytes (P = 0.002) (Figure 3, E and F). These data could indicate that differences in labeling affinity between monocyte and macrophage subsets reflect differences in lineage, maturation, or activation.
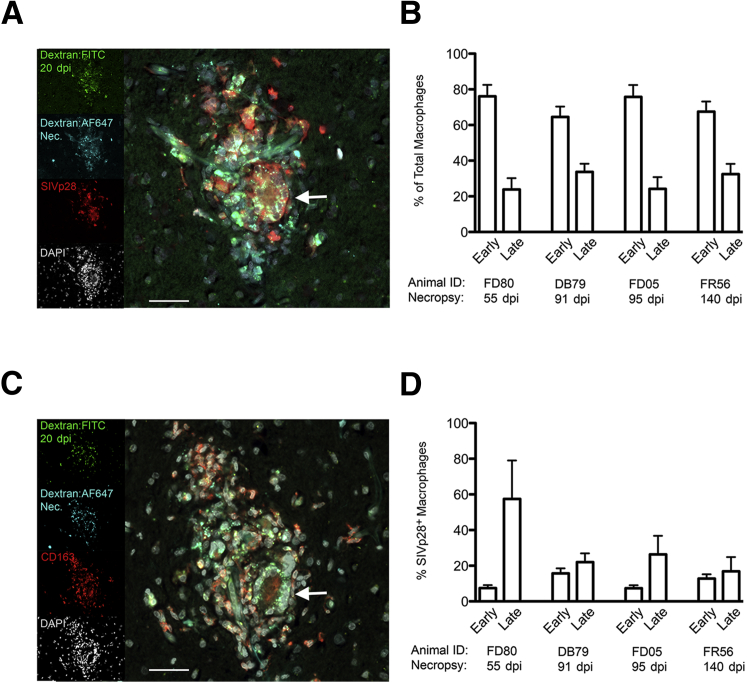
There Is a 2.9-Fold Increase in the Percentage of Productively Infected Macrophages that Enter the CNS Late versus Early in Animals with AIDS and SIVE
SIVE lesions were evaluated to determine at which time macrophages that comprise SIVE lesions entered the CNS and to determine which cells are productively infected (n = 4 animals, 21 SIVE lesions total) (Figure 4). By using the dextran labeling scheme (Table 1 and Figure 3A), macrophages labeled with Dextran:FITC (administered at 20 dpi) were present in the CNS before or on day 20 pi (termed early macrophages), and macrophages that were not Dextran:FITC labeled were recruited to the CNS after 20 dpi (termed late macrophages). Surprisingly, we found 70.7% ± 3% of cells in SIVE lesions (21 lesions examined) were Dextran:FITC labeled, early macrophages, and 29.3% ± 3% were late macrophages (Figure 4, A and B). This was not expected because we do not typically observe SIVE lesions in SIV-infected CD8 lymphocyte-depleted animals sacrificed at 22 dpi. Interestingly, CD163+ multinucleated giant cells are productively infected and were Dextran:FITC labeled, indicating they also are likely derived from macrophages that were present in the CNS early before lesion formation (Figure 4, A and C). These data suggest that CD163+ macrophages present in the CNS early contribute to SIVE lesion formation terminally with AIDS and comprise most macrophages in SIVE lesions.
Figure 4.
The percentage of productively infected macrophages in SIV encephalitis (SIVE) lesions is 2.9-fold higher for macrophages that enter the central nervous system (CNS) late compared with macrophages present in the CNS at 20 days postinfection (dpi). Four-color immunofluorescence was used to determine the timing of CNS entry of productively infected macrophages in SIVE lesions. Data are from >20 lesions from four SIVE animals. A: Immunofluorescence staining for SIVp28 (red) in an SIVE lesion from animal FR56 given Dextran:FITC (green, 20 dpi) and Dextran:AF647 (cyan, 138 dpi), sacrificed at 140 dpi with AIDS and SIVE. DAPI was used to stain nuclei (gray). Productively infected macrophages are early (green + cyan + red = yellow/white) and late (cyan + red = orange/red). The arrow indicates a productively infected multinucleated giant cell (MNGC) that is composed of early macrophages. Insets: Single-channel images. B: Immunofluorescence for CD163 (red) in a serial section of the lesion in A. Dextran-labeled, SIVp28+ cells in SIVE lesion are CD163+ macrophages. The MNGC is CD163+. C: Most macrophages in SIVE lesions are early, and CD163+ macrophages present in the CNS by 20 dpi. D: The percentage of SIVp28+ macrophages is greater for late macrophages compared with early macrophages (means ± SEM, 31.8% ± 9% late versus 10.8% ± 2% early; P = 0.03). Scale bar = 25 μm (A and C).
In SIVE lesions, most productively infected (SIVp28+) cells were dextran-labeled (CD163+) macrophages, which is consistent with previous observations that CD163+ macrophages are a primary target cell for productive infection in the CNS (Figure 4, A and C).14,25 In these cells, CD163+ expression was confirmed in adjacent serial sections (Figure 4C). By using four-color immunofluorescence, we found that both early and late macrophages were productively infected (Figure 4, A and D). When analyzing the data in terms of early and late macrophages for the total on n = 4 animals, we found that 31.8% ± 9% of the late macrophages were SIVp28+ and 10.8% ± 2% of the early macrophages were SIVp28+, representing a 2.9-fold increase of late versus early macrophages that were SIVp28+ (P = 0.03) (Figure 4, A and D). Scattered SIVp28+ cells that were not dextran labeled were also present. These cells most likely are parenchymal microglia or infected macrophages that were not present in the perivascular space at the time of dextran labeling. These data indicate that both early and late macrophages are productively infected and that the frequency of productive infection is 2.9-fold higher in macrophages that entered the CNS late compared with early macrophages.
The Distribution of Dextran-Labeled CD163+ Macrophages in CNS Tissues
By using a combination of dextran dye injections, we characterized the distribution of dextran-labeled CNS macrophages to determine the timing of CD163+ macrophage recruitment to CNS compartments in animals sacrificed with AIDS (Table 3). We determined CD163+ macrophage recruitment from 20 dpi to necropsy (percentage late macrophages) in all animals with AIDS (n = 9). In a subset of animals (n = 4) that received all three dyes, the recruitment period was divided into 20 to 49 dpi and 49 dpi to necropsy, which allowed us to evaluate macrophage recruitment after viremia and terminally with AIDS. For all animals with AIDS, the percentage of late macrophages was greater in the choroid plexus (73.0% ± 6.2%; range, 61.9% to 92.4%) than the meninges (27.3% ± 4.0%; range, 13.0% to 55.9%; P = 0.001) or perivascular space (29.0% ± 3.9%; range, 10.7% to 47.2%; P = 0.001; all data presented as means ± SEM) (Table 3). Interestingly, in SIVE animals, the percentage of macrophages that arrive late was lower in SIVE lesions (18.4% ± 1.8%; range, 14.1% to 21.7%) than in the perivascular space (30.1% ± 4.3%; range, 13.8% to 37.9%) (Table 3). Surprisingly, the percentage of late macrophages was not significantly different between SIVnoE and SIVE animals in the perivascular space (27.6% ± 7.6% SIVnoE and 30.1% ± 4.3% SIVE), meninges (25.8% ± 2.5% SIVnoE and 28.5% ± 7.3% SIVE), and choroid plexus (65.5% ± 1.7% SIVnoE and 77.9% ± 8.8% SIVE) (Table 3). We only found differences between SIVnoE and SIVE animals in the number of late macrophages in SIVE lesions, underlining the importance of these cells in pathogenesis.
Table 3.
Distribution of Dextran-Labeled CD163+ Macrophages in CNS Tissues
| CNS region | CNS pathology | Present (20 dpi)∗ | Entered (20 dpi-Nec)∗ | Entered (20-49 dpi)† | Entered (49 dpi-Nec)† |
|---|---|---|---|---|---|
| Perivascular space | SIVE | 69.9 ± 4.3 | 30.1 ± 4.3 | 27.6 ± 2.8 | 7.3 ± 0.5 |
| SIVnoE | 72.4 ± 7.6 | 27.6 ± 7.6 | 16.1 | 12.7 | |
| SIVE lesion | SIVE | 81.6 ± 1.8 | 18.4 ± 1.8 | 4.3 ± 0.1 | 17.1 ± 0.1 |
| SIVnoE | NP | NP | NP | NP | |
| Meninges | SIVE | 71.5 ± 7.3 | 28.5 ± 7.3 | 14.2 ± 3.6 | 21.3 ± 6.8 |
| SIVnoE | 74.2 ± 2.5 | 25.8 ± 2.5 | 12.4 | 20.3 | |
| Choroid plexus | SIVE | 22.1 ± 8.8‡ | 77.9 ± 8.8‡ | ND | ND |
| SIVnoE | 34.5 ± 1.7‡ | 65.5 ± 1.7‡ | ND | ND |
Values are the means ± SEM percentages of all dextran-labeled macrophages for n animals. Dextran-labeled macrophages in the CNS were counted by sampling 3000 to 4000 cells from frontal, temporal parietal, and occipital cortices, 1000 cells from meninges, and two sections of choroid plexus per animal. When present, 10 or more SIVE lesions were counted per animal.
CNS, central nervous system; dpi, days postinfection; ND, not done; Nec, necropsy; NP, SIVE lesions not present; SIVE, SIV encephalitis; SIVnoE, SIV-infected animals that progressed to AIDS without encephalitis.
SIVE (n = 5) and SIVnoE (n = 4).
For animals that received three dyes, the period 20 dpi to Nec was subdivided into 20 to 49 dpi and 49 dpi to Nec. SIVE (n = 3) and SIV (n = 1).
P < 0.02, choroid plexus vs perivascular space, meninges, or SIVE lesions.
The Rate of CD163+ Macrophage Recruitment to the CNS Increases with SIV Infection and Correlates with Rapid Death
The time to death of all animals in this study sacrificed with AIDS ranged from 55 to 141 dpi and was shorter in animals with SIVE (91 ± 14 days) compared with animals without SIVE (111 ± 19 days). To account for differences in the time to death from 20 dpi, we compared rates of CD163+ macrophage recruitment by normalizing the percentage of dextran-labeled cells that entered the CNS in a given time period by the number of days in that period. For all animals sacrificed with AIDS (n = 9), the CD163+ macrophage recruitment rate was determined for the time period from 20 dpi to necropsy. We found that a shorter time to death correlated with an increased rate of recruitment of CD163+ macrophages in the perivascular space (r = −0.8, P = 0.03), SIVE lesions (r = −1.0, P = 0.08), meninges (r = −0.8, P = 0.02), and choroid plexus (r = −0.9, P = 0.03) for all animals (Figure 5).
Figure 5.
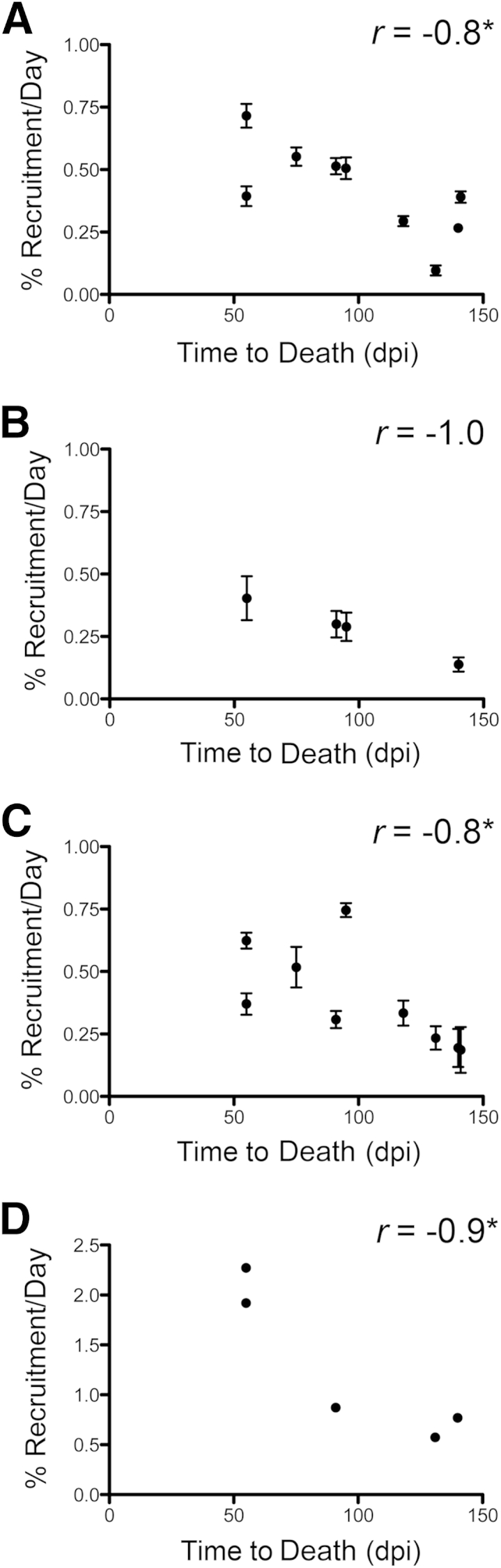
The recruitment rate of CD163+ macrophages to the central nervous system is inversely proportional to time to death. Paired xy values for time to death and CD163+ macrophage recruitment rate are plotted for animals sacrificed with AIDS. Time to death is inversely correlated with the rate of CD163+ macrophage recruitment to the perivascular space (A), SIV encephalitis (SIVE) lesions (B), meninges (C), and choroid plexus (D) in SIVE and SIV-infected animals that progressed to AIDS without encephalitis (SIVnoE) animals. A Spearman rank test is used for correlation statistics. n = 9 (animals sacrificed with AIDS) perivascular space; n = 4 animals with SIVE lesions; n = 9 animals with meninges; n = 5 animals with choroid plexus. ∗P < 0.05. dpi, Days postinfection.
For all animals with AIDS, SIV infection increased the rate of CD163+ macrophage recruitment from 20 dpi to necropsy in the perivascular space (range, 4.9- to 36.3-fold; means ± SEM, 21.0 ± 3; P < 0.0001), the meninges (range, 2.2- to 8.7-fold; means ± SEM, 4.6 ± 0.8; P < 0.001), and the choroid plexus (range, 8.4- to 27.9-fold; means ± SEM, 14.3 ± 3.5; P < 0.05) compared with uninfected controls (Figure 6, A–C).28 The CD163+ macrophage recruitment rate in animals with SIVE was 16% greater in the perivascular space and 26% greater in the meninges compared with animals with AIDS and SIVnoE, although the differences did not reach statistical significance (Figure 6, A and B). These data indicate that, overall, SIV infection greatly increases the rate of recruitment of CD163+ macrophages to the CNS, which is, in turn, associated with rapid death.
Figure 6.
The rate of CD163+ macrophage recruitment to the central nervous system (CNS) is increased with SIV infection and is greater in animals with SIV encephalitis (SIVE). The rate of CD163+ macrophage recruitment was derived by normalizing the percentage of dextran-labeled cells that entered the CNS in a given period (Table 3) by the number of days in that period. Each data point represents one lesion or one tissue section (11 to 14 cortical sections, 3 to 4 sections of meninges, and 1 choroid plexus section per animal). A–C: The recruitment rate was determined 20 days postinfection (dpi) to necropsy (Nec) for all animals that progressed to AIDS. Basal turnover of perivascular, meningeal, and choroid plexus macrophages was determined in uninfected animals reconstituted with enhanced green fluorescence protein (EGFP)–expressing CD34+ stem cells. Compared with uninfected animals, the recruitment rate of CD163+ macrophages 20 dpi to Nec in animals sacrificed with AIDS is increased 21-fold in the perivascular space (A), 4.6-fold in the meninges (B), and 14.3-fold in the choroid plexus (C). A and B: The CD163+ macrophage recruitment rate is higher in animals with SIVE than in SIV-infected animals that progressed to AIDS without encephalitis (SIVnoE), although not statistically significant. D–F: In a subset of animals that progressed to AIDS and received all three dyes, the recruitment rate was determined 20 to 49 dpi and 49 dpi to Nec to compare differences in recruitment during postacute infection and terminally with AIDS, respectively. D: In SIVE lesions, the rate of CD163+ macrophage recruitment is greater terminally, 49 dpi to Nec, compared with 20 to 49 dpi. E: In the perivascular space, the CD163+ macrophage recruitment rate is higher 20 to 49 dpi compared with 49 dpi to Nec in both SIVE and SIVnoE animals. The CD163+ macrophage recruitment rate is 68% higher in animals with SIVE than animals with SIVnoE postacute infection, 20 to 49 dpi, but is not significantly different terminally with AIDS, 49 dpi to Nec. F: In the meninges, the CD163+ macrophage recruitment rate is 91% higher in SIVE animals than in SIVnoE animals terminally with AIDSn = 9 (A–C, animals that progressed to AIDS); n = 3 (A–C, uninfected animals); n = 5 (A and B, animals with SIVE); n = 4 (A and B, animals with SIVnoE). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
For the subset of animals that received all three dyes (n = 4), the rate of recruitment of CD163+ macrophages was compared from 20 to 49 dpi and from 49 dpi to necropsy (Figure 6, D–F). We found that in SIVE lesions, 21.4% ± 0.01% of CD163+ macrophages entered the CNS after 20 dpi (Table 3). For these cells, the rate of recruitment of CD163+ macrophages in SIVE lesions was greater from 49 dpi to necropsy than from 20 to 49 dpi (range, 2.4- to 2.8-fold; means ± SEM, 2.6 ± 0.2; P = 0.0003) (Figure 6D). In contrast, the rate of CD163+ macrophage recruitment in the perivascular space was greater between 20 and 49 dpi than 49 dpi to necropsy in all animals (range, 2.32- to 7.1-fold; means ± SEM, 4.7 ± 1.2; P < 0.001) (Figure 6E). The recruitment rate in the perivascular space was 68% greater in animals with SIVE than in animals with SIVnoE during the postacute period from 20 to 49 dpi (P = 0.002) but was not significantly different with regard to SIVE terminally with AIDS (Figure 6E). In the meninges, the CD163+ macrophage recruitment rate was 91% greater in animals with SIVE compared with animals with SIVnoE terminally with AIDS (P < 0.05) (Figure 6F). These data may suggest that the rate of CD163+ macrophage recruitment in the perivascular space is greatest after acute infection and CD163+ macrophage recruitment in the meninges and SIVE lesions is greatest terminally with AIDS.
Increased Monocyte Activation Is Associated with Greater CD163+ Macrophage Recruitment and SIVE
It has previously been demonstrated that increased monocyte activation and expansion from the bone marrow are associated with the development of HIV-associated neurological disorders and SIVE lesions.17,53–55 We found the average CD14+CD16+ monocyte count from 20 dpi to necropsy was greater in animals with SIVE compared with animals with SIVnoE, as previously demonstrated (SIVnoE, 36 ± 4 cells/μL; SIVE, 104 ± 16 cells/μL; P = 0.02). In addition, higher sCD163 in plasma correlated with a greater percentage of late CD163+ macrophages recruited in the perivascular space and SIVE lesions in animals with AIDS and SIVE (perivascular space: r = 1.0, P = 0.02; SIVE lesions: r = 1.0, P = 0.08), but not in animals without SIVE. There was no correlation between plasma sCD163 and the percentage of late macrophages in the meninges and choroid plexus. This suggests that monocyte activation in the periphery is associated with accumulation of CD163+ macrophages in the perivascular space and SIVE lesions in animals with SIVE.
Discussion
Herein, we characterized recruitment of CD163+ perivascular macrophages and inflammatory MAC387+ macrophages to the CNS with SIV infection. We observed that early CNS inflammation is characterized by the recruitment of MAC387+ macrophages throughout the CNS. The meninges and choroid plexus had greater recruitment of BrdU+ macrophages from the blood, with a high rate of turnover. In contrast, CD163+ and MAC387+ macrophages accumulated in the perivascular space and SIVE lesion with the development of AIDS and SIVE. We observed that throughout the CNS, higher ratios of CD163+/MAC387+ macrophages were associated with AIDS and SIVE. These studies indicate that the basal rate of macrophage recruitment is increased with SIV infection and the higher rates of recruitment are associated with shorter time to death. In addition, increased macrophage activation in the periphery is associated with the development of SIVE in these animals. More important, we find that SIVE lesions are composed primarily of CD163+ macrophages present in the CNS early, at 20 dpi, in addition to productively infected macrophages recruited to the CNS terminally with AIDS.
SIVE Lesions Are Formed by Redistribution of Resident CD163+ Macrophages and Recruitment of Hematogenous Macrophages
A major and surprising observation of this study was that 81.6% of CD163+ macrophages in SIVE lesions were present in the CNS early in infection before the development of SIVE lesions. After 20 dpi, approximately 4.3% of CD163+ macrophages in SIVE lesions entered the CNS from 20 to 49 dpi and 17.1% entered the CNS after 49 dpi. These data fit the hypothesis that SIVE lesions arise more so from the focal redistribution of perivascular macrophages already present in the CNS than from the recruitment of monocytes from the periphery. It has been previously demonstrated that perivascular macrophages and juxtavascular microglia are able to migrate along the CNS vasculature in response to activation.56 In addition, data exist that the choroid plexus, in addition to the meninges, is a site of macrophage precursor cells that can potentially repopulate the CNS parenchyma, which may be a source of CD163-positive macrophages that are not BrdU+ cells labeled with the early dye.57 Low macrophage recruitment that we observed in SIVE lesions from 20 to 49 dpi could also be explained by occlusion of CNS blood vessels, which would prevent extravasation until recanalization of the blood vessel.58,59
Formation of CNS lesions by redistribution of resident macrophages and recruitment of hematogenous macrophages has been previously described in multiple sclerosis (MS).60–62 In MS, aggregation of parenchymal CD68+HLA-DR+ macrophages is thought to represent the earliest stage of lesion formation.60 These preactive lesions progress to early active MS lesions that contain CD68+ microglia and hematogenous CD68+ perivascular macrophages and MRP14+ (MAC387+) macrophages.61,62 These are likely the same three populations of CNS macrophages we describe with HIVE and SIVE.14,17,61,62 In contrast to early active lesions, late active or inactive MS lesions consist primarily of CD68+ macrophages; few MRP14+ macrophages are also present.62 These observations parallel ours in that an increased ratio of CD163+/MAC387+ macrophages is associated with disease progression. In active lesion formation, the transition from early active MS lesions to late/inactive MS lesions is thought to be mediated by macrophage activation and redistribution of CNS macrophages rather than accumulation of recruited hematogenous macrophages.61,62 A redistribution of macrophages to CNS lesions was also reported in a mouse model of spinal cord injury,63 where blood-derived monocytes and macrophages were present diffusely throughout the spinal cord at 1 and 3 days after injury but coalesced into a lesion 7 to 14 days after injury before resolution of the lesion.63 These similarities between HIVE and SIVE, MS, and spinal cord suggest that recruitment of hematogenous macrophages and redistribution of local macrophages are involved in CNS lesion formation.
Timing of SIV Infection of the CNS and Development of AIDS and SIVE
Data from our study demonstrate that there is a 2.9-fold higher percentage of productive infection in macrophages that enter the CNS late compared with cells that enter early. In addition, multinucleated giant cells, many of which are SIVp28+, are also composed of CD163+ macrophages that were present in the CNS at 20 dpi. This is consistent with two hypotheses: SIV is introduced into the CNS early but is controlled by the immune response until it recrudesces with AIDS, and/or acute virus is cleared from the CNS and reintroduction of infectious virus by traffic on infected monocytes/macrophages at the onset of AIDS is necessary to reestablish and maintain CNS infection.23,38–42,64–66 Previous analysis of viral sequences from peripheral and CNS tissue from two of the animals sacrificed at 22 dpi indicates compartmentalization of SIV in the CNS as early as 20 dpi.67 In addition, by using molecular clocks, in phylogenetic analysis of peripheral and CNS viral sequences from four of the animals sacrificed with AIDS, we found multiple instances of late SIV neuroinvasion beginning at approximately 50 dpi in animals sacrificed with AIDS.67 Together, the phylogenetic data discussed above and our observation that late macrophages have a higher frequency of productive infection support a hypothesis of late SIV neuroinvasion by traffic of infected CD163+ macrophages into the perivascular space. Our studies discussed herein used CD8+ lymphocyte depletion, which compresses the window of disease from 1 to 3 years to 2 to 4 months such that early and end-stage events are not as temporally distant as is the case with SIV infection without CD8 lymphocyte depletion.
Resident CD163+ Perivascular Macrophages Are Important in the Development of AIDS and SIVE
Perivascular macrophages are considered important in the development of HIV and SIVE and neuronal dysfunction, because they are the primary cell type productively infected.3,8,9,13 A significant role for perivascular macrophages in the development of CNS pathology is supported by the observation that rate of turnover of perivascular macrophages increased from <0.2% per week in uninfected macaques to approximately 3% per week in SIV-infected animals; in addition, 71% of perivascular macrophages were present in the CNS at 20 dpi, suggesting that there is an accumulation of perivascular macrophages with little or no turnover. Previous studies on the rate of perivascular macrophage turnover in rodents (approximately 8% per week, as determined in mouse bone marrow chimeras, and approximately 2% per week, as determined by serial dextran labeling in rats) indicate that the total population of perivascular macrophages is replaced over several months, although individual macrophages may reside in the CNS for years.30,31,49,68 The slow turnover rate of perivascular macrophages in the CNS, some of which may be infected, likely contributes to the maintenance of a viral reservoir in the CNS with HIV or SIV infection. Previous research indicates that the number of activated macrophages in the CNS better correlates with HIV dementia than viral replication in the CNS.69 We observed an increased rate of recruitment of perivascular macrophages in SIVE animals compared with SIVnoE animals from 20 to 49 dpi, which may represent perivascular cuffing in the CNS; more important, we suggest that the increased recruitment of perivascular macrophages before end-stage disease may predict which animals will develop SIVE terminally with AIDS. The accumulation of macrophages and perivascular macrophages with HIV and SIV infection contributing to metabolic encephalopathy has been implicated in glial and neuronal perturbation and ultimately CNS disease,43 through the simple increase in traffic of monocytes to the CNS10,15,37,44,70 (the release of toxic proteins, cytokines, and chemokines,37,71 as well as possibly viral proteins).
Peripheral Monocyte Activation Predicts CD163+ Macrophage Recruitment in SIVE Animals
Perivascular macrophages are monocyte derived, and activation and expansion of the CD16+ monocyte compartment with AIDS leads to increased trafficking of monocytes to the CNS.17,19,25,72 Peripheral correlates of SIVE underline the important concept that the mechanisms that drive neuropathogenesis are not limited to factors within the CNS compartment only. Notably, the range of values for plasma sCD163 and CD14+CD16+ monocyte counts is greater between SIVE animals than between SIVnoE animals, suggesting that the animals that develop SIVE may have lost the ability to regulate monocyte homeostasis. We observed that peripheral monocytes in SIVE animals show increased activation relative to SIVnoE animals and concomitantly developed a more severe CNS pathology with increased CD163+ macrophage recruitment and presence of SIVE lesions. This relationship between increased monocyte activation, increased macrophage recruitment to the CNS, and SIVE supports the hypothesis that lack of immune regulation in the periphery contributes to the development of SIVE. The increased monocyte/macrophage activation we have demonstrated in the periphery by elevated sCD163 and increased numbers of CD14 and CD16 monocytes correlates with increased neurological deficits in patients undergoing effective ART73 and increased macrophage accumulation in the CNS,17 cardiac tissues of humans undergoing effective ART52 and SIV-infected monkeys,74 and cardiac tissues of elite controllers who are not undergoing ART.75 Thus, chronic immune activation and dysregulation of monocytes/macrophages likely plays a role in increased numbers of CD163 macrophages entering the CNS, and increased percentage of CD163 cells that are HIV+ and SIV+ entering the CNS late, which we observed herein.
Macrophage Turnover via Cell Death or Traffic Out of the CNS
A major assumption of our dextran-labeling model is that once macrophages are present in the CNS, they remain. Thus, we do not account for cell death or macrophages that exit the CNS. Apoptosis of perivascular macrophages and microglia is considered to be low to negligible in the normal CNS, and HIV-1 infection has been shown to reduce the frequency of apoptotic CD68+ macrophages in vivo.76,77 Overall, studies of macrophage death in the normal CNS and in response to activation and infection have not been performed. CNS macrophages are able to actively migrate from the brain to the cervical lymph nodes along olfactory nerves that penetrate the cribriform plate, but the factors that drive this process are not known.78–80 Recirculation of macrophages from the CNS to the blood is thought to be impaired with HIV or SIV infection, which may be a mechanism of macrophage retention and SIVE lesion formation.81–83 Osteopontin, a cytokine that is elevated in the CNS with HIVE and SIVE, inhibits macrophage apoptosis and promotes macrophage retention in the CNS.81 All of these factors contribute to macrophage retention in the CNS and accumulation of macrophages over time.
A Higher Ratio of CD163+/MAC387+ Macrophages Is Associated with the Development of AIDS and SIVE and May Reflect a Switch to an M2-Polarized Microenvironment
We have previously observed that the severity of SIVE was related to an increased ratio of CD68+/MAC387+ macrophages in HIVE and SIVE lesions.14 In this study, we extend this observation and report that the CD163/MAC387 ratio is greater in animals with AIDS compared with SIV-infected animals without AIDS, and in animals with SIVE compared with animals with SIVnoE. We have previously suggested that resident perivascular macrophages are M2 polarized and inflammatory MAC387+ macrophages are M1 polarized.14,37,84 M1-polarized macrophages are considered to be proinflammatory, classically activated macrophages that mediate antiviral type 1 helper T-cell immune responses. Conversely, M2 alternatively activated macrophages mediate type 2 helper T-cell immune response and have been associated with tissue repair and resolution of inflammation. We observed an increased influx of MAC387+ macrophages at 22 dpi throughout the CNS before significant accumulation of CD163+ macrophages. This may suggest that during the early stages of CNS infection, an M1-polarized, antiviral immune response predominates and is a response to CNS infection. In animals sacrificed with AIDS, particularly with SIVE, the number of MAC387+ macrophages had decreased relative to 22 dpi and more CD163+ macrophages were present, which may indicate a switch to an M2 microenvironment. This observation suggests that a shift from M1 to M2 polarization is associated with the progression of neuro-AIDS and the development of SIVE.
MAC387+ Macrophages Do Not Appear to Differentiate into CD163+CD68+ Resident Macrophages in the CNS
We have previously demonstrated that MAC387 is a marker of recently recruited macrophages in the CNS.14,17 It is not known whether MAC387+ macrophages are able to differentiate into CD163+CD68+ macrophages or CD68+CD163− microglia. The BrdU data show that MAC387+ macrophages found at necropsy had entered the CNS before 20 dpi, indicating that with long-term residence in the CNS (35 to 121 days in this study), these cells did not differentiate into a CD163+CD68+ macrophage phenotype.
CD163+BrdU− Macrophages in the CNS May Represent Proliferation of Resident Macrophages or Recruitment from a Site Other than the Bone Marrow
Few CD163+BrdU+ macrophages were present in the perivascular space and SIVE lesions of animals sacrificed with AIDS and SIVE, despite the higher number of total CD163+ macrophages in SIVE animals. The lack of CD163+BrdU+ macrophages in SIVE lesions is consistent with the notion that SIVE lesions are made up of local perivascular macrophages recruited from within the CNS, and may also represent macrophage proliferation in situ. In mice, tissue macrophages have been shown to proliferate in response to IL-4 produced in type 2 helper T-cell immune responses.85 Although historically macrophages are not thought to divide in humans and nonhuman primates, the local microenvironment in SIVE or HIVE may support proliferation of CD163+ macrophages. Another possibility is that CD163+ macrophages are recruited to the perivascular space and SIVE lesions from sites other than the bone marrow. In HIV-infected humans and SIV-infected macaques, virus isolated from the CNS was shown to have sequences similar to virus isolated from the spleen, which could indicate traffic of splenic monocytes to the CNS.86–88 A splenic reservoir of monocytes has been identified in mice, which is mobilized in response to myocardial infarction and lung adenocarcinoma.89,90 Alternatively, emerging studies support the notion of parenchymal macrophage repopulation in tissues and not from bone marrow, which is an interesting field of future study that may, in part, account for a lack of BrdU+ CD163 macrophages in parenchymal lesions in our study.91,92
The Presence of CD163+BrdU+ Macrophages in the Meninges and Choroid Plexus Underscores Differences between CNS Compartments and a Possible Route of Viral Entry into the CNS
Given that we did not observe CD163+BrdU+ macrophages in the perivascular space and SIVE lesions, it is interesting that we observed CD163+BrdU+ macrophages in the meninges and choroid plexus in numbers equal to or greater than MAC387+BrdU+ macrophages. This observation suggests that these two compartments are more similar to each other, and possibly to blood, than to the perivascular space and CNS parenchyma. As the vascular compartment of the CNS, the meninges are a direct link between the blood and the brain. More important, cell-associated HIV has been shown to traffic to the brain through the meninges.93 Thus, it is possible that virus may be continually reintroduced into the brain in end-stage disease because of traffic of infected CD163+ macrophages through the meninges. In addition, the choroid plexus is reported as a source of dendritic and macrophage precursor cells57 that might also migrate into the CNS parenchyma with HIV and SIV infection and also be a source of productively infected CD163+ cells or CD163 macrophages that comprise the HIV and SIV encephalitic lesions.
Overall, these studies identify differences in recruitment of inflammatory and perivascular macrophage subsets across different CNS compartments. Differences in macrophage recruitment and turnover suggest that the meninges and choroid plexus are routes of monocyte traffic, and that macrophages associated with pathogenesis accumulate in the perivascular space and SIVE lesions. These studies identify late infiltrating CD163+ macrophages as a primary target of SIV infection with AIDS and SIVE. More important, our observations indicate that formation of SIVE lesions is a dynamic process involving multiple populations of resident and recruited CNS macrophages that are likely dependent on viral replication in the CNS during end-stage disease.
Acknowledgments
We thank the veterinary staff at the Tulane National Primate Research Center (Covington, LA) for animal care and for assisting with tissue collection, Michael Piatak and Jeffrey Lifson for plasma viral load determination, Ronald Desrosiers (New England Regional Primate Research Center, Boston, MA) for providing the SIV mac251, and Caroline Soulas for critical input and intellectual support.
Footnotes
Supported by NIH/National Institute of Neurological Disorders and Stroke grants NS040237 (K.C.W.), NS063897 (K.C.W.), NS063897 (M.S.), and NS082116 (T.H.B.); and NIH Nonhuman Primate Reagent Resource grants RR016001 and AI040101 (in vivo CD8 lymphocyte depletion antibody).
Disclosures: None declared.
See related Commentary on page 1548
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2015.01.033.
Supplemental Data
Supplemental Figure S1.
There are no differences in the plasma viral loads and average plasma viral loads in animals sacrificed at 21 days postinfection (DPI 21) and terminally with AIDS, with and without simian immunodeficiency virus encephalitis (SIVE). A: Plasma viral loads for individual animals sacrificed at DPI 21 (gray symbols), terminally without SIVE (SIVnoE; black symbols), and terminally with SIVE (SIVE; white symbols). B: The average plasma viral loads of animals sacrificed at DPI 21, animals sacrificed with AIDS without SIVE (SIVnoE), and animals sacrificed with AIDS and SIVE (SIVE).
References
- 1.Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22:315–320. doi: 10.1097/WCO.0b013e328329cf3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 3.Gannon P., Khan M.Z., Kolson D.L. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24:275–283. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 5.Burdo T.H., Lackner A., Williams K.C. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254:102–113. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lackner A.A., Lederman M.M., Rodriguez B. HIV pathogenesis: the host. Cold Spring Harb Perspect Med. 2012;2:a007005. doi: 10.1101/cshperspect.a007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft-Terry S.D., Stothert A.R., Buch S., Gendelman H.E. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persidsky Y., Gendelman H.E. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- 9.Williams K.C., Hickey W.F. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 10.Campbell J.H., Hearps A.C., Martin G.E., Williams K.C., Crowe S.M. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS. 2014;28:2175–2187. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams D.W., Veenstra M., Gaskill P.J., Morgello S., Calderon T.M., Berman J.W. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res. 2014;12:85–96. doi: 10.2174/1570162x12666140526114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorantla S., Makarov E., Finke-Dwyer J., Castanedo A., Holguin A., Gebhart C.L., Gendelman H.E., Poluektova L. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am J Pathol. 2010;177:2938–2949. doi: 10.2353/ajpath.2010.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams K.C., Corey S., Westmoreland S.V., Pauley D., Knight H., deBakker C., Alvarez X., Lackner A.A. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soulas C., Conerly C., Kim W.K., Burdo T.H., Alvarez X., Lackner A.A., Williams K.C. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell J.H., Ratai E.M., Autissier P., Nolan D.J., Tse S., Miller A.D., Gonzalez R.G., Salemi M., Burdo T.H., Williams K.C. Anti-alpha4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014;10:e1004533. doi: 10.1371/journal.ppat.1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams D.W., Byrd D., Rubin L.H., Anastos K., Morgello S., Berman J.W. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm. 2014;1:e36. doi: 10.1212/NXI.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdo T.H., Soulas C., Orzechowski K., Button J., Krishnan A., Sugimoto C., Alvarez X., Kuroda M.J., Williams K.C. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa A., Liu H., Ling B., Borda J.T., Alvarez X., Sugimoto C., Vinet-Oliphant H., Kim W.K., Williams K.C., Ribeiro R.M., Lackner A.A., Veazey R.S., Kuroda M.J. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulliam L., Gascon R., Stubblebine M., McGuire D., McGrath M.S. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 20.Yadav A., Collman R.G. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4:430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams K., Westmoreland S., Greco J., Ratai E., Lentz M., Kim W.K., Fuller R.A., Kim J.P., Autissier P., Sehgal P.K., Schinazi R.F., Bischofberger N., Piatak M., Lifson J.D., Masliah E., Gonzalez R.G. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thieblemont N., Weiss L., Sadeghi H.M., Estcourt C., Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 23.Clay C.C., Rodrigues D.S., Ho Y.S., Fallert B.A., Janatpour K., Reinhart T.A., Esser U. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J Virol. 2007;81:12040–12048. doi: 10.1128/JVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ransohoff R.M., Kivisakk P., Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 25.Kim W.K., Alvarez X., Fisher J., Bronfin B., Westmoreland S., McLaurin J., Williams K. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 27.Barron K.D. The microglial cell: a historical review. J Neurol Sci. 1995;(134 Suppl):57–68. doi: 10.1016/0022-510x(95)00209-k. [DOI] [PubMed] [Google Scholar]
- 28.Soulas C., Donahue R.E., Dunbar C.E., Persons D.A., Alvarez X., Williams K.C. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am J Pathol. 2009;174:1808–1817. doi: 10.2353/ajpath.2009.081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hickey W.F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 30.Bechmann I., Kwidzinski E., Kovac A.D., Simburger E., Horvath T., Gimsa U., Dirnagl U., Priller J., Nitsch R. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- 31.Chinnery H.R., Ruitenberg M.J., McMenamin P.G. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J Neuropathol Exp Neurol. 2010;69:896–909. doi: 10.1097/NEN.0b013e3181edbc1a. [DOI] [PubMed] [Google Scholar]
- 32.Unger E.R., Sung J.H., Manivel J.C., Chenggis M.L., Blazar B.R., Krivit W. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. J Neuropathol Exp Neurol. 1993;52:460–470. doi: 10.1097/00005072-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Bruck W., Porada P., Poser S., Rieckmann P., Hanefeld F., Kretzschmar H.A., Lassmann H. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- 34.Adams C.W., Poston R.N. Macrophage histology in paraffin-embedded multiple sclerosis plaques is demonstrated by the monoclonal pan-macrophage marker HAM-56: correlation with chronicity of the lesion. Acta Neuropathol. 1990;80:208–211. doi: 10.1007/BF00308926. [DOI] [PubMed] [Google Scholar]
- 35.Guignard F., Mauel J., Markert M. The monoclonal antibody Mac 387 recognizes three S100 proteins in human neutrophils. Immunol Cell Biol. 1996;74:105–107. doi: 10.1038/icb.1996.14. [DOI] [PubMed] [Google Scholar]
- 36.Goebeler M., Roth J., Teigelkamp S., Sorg C. The monoclonal antibody MAC387 detects an epitope on the calcium-binding protein MRP14. J Leukoc Biol. 1994;55:259–261. doi: 10.1002/jlb.55.2.259. [DOI] [PubMed] [Google Scholar]
- 37.Fischer-Smith T., Bell C., Croul S., Lewis M., Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis L.E., Hjelle B.L., Miller V.E., Palmer D.L., Llewellyn A.L., Merlin T.L., Young S.A., Mills R.G., Wachsman W., Wiley C.A. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 39.Witwer K.W., Gama L., Li M., Bartizal C.M., Queen S.E., Varrone J.J., Brice A.K., Graham D.R., Tarwater P.M., Mankowski J.L., Zink M.C., Clements J.E. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One. 2009;4:e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakrabarti L., Hurtrel M., Maire M.A., Vazeux R., Dormont D., Montagnier L., Hurtrel B. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 41.Lane J.H., Sasseville V.G., Smith M.O., Vogel P., Pauley D.R., Heyes M.P., Lackner A.A. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- 42.Bissel S.J., Wang G., Bonneh-Barkay D., Starkey A., Trichel A.M., Murphey-Corb M., Wiley C.A. Systemic and brain macrophage infections in relation to the development of simian immunodeficiency virus encephalitis. J Virol. 2008;82:5031–5042. doi: 10.1128/JVI.02069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persidsky Y., Zheng J., Miller D., Gendelman H.E. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J Leukoc Biol. 2000;68:413–422. [PubMed] [Google Scholar]
- 44.Campbell J.H., Burdo T.H., Autissier P., Bombardier J.P., Westmoreland S.V., Soulas C., Gonzalez R.G., Ratai E.M., Williams K.C. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westmoreland S.V., Halpern E., Lackner A.A. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4:260–268. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- 46.Sasseville V.G., Lackner A.A. Neuropathogenesis of simian immunodeficiency virus infection in macaque monkeys. J Neurovirol. 1997;3:1–9. doi: 10.3109/13550289709015787. [DOI] [PubMed] [Google Scholar]
- 47.Bell J.E. The neuropathology of adult HIV infection. Rev Neurol (Paris) 1998;154:816–829. [PubMed] [Google Scholar]
- 48.Wang X., Das A., Lackner A.A., Veazey R.S., Pahar B. Intestinal double-positive CD4+CD8+ T cells of neonatal rhesus macaques are proliferating, activated memory cells and primary targets for SIVMAC251 infection. Blood. 2008;112:4981–4990. doi: 10.1182/blood-2008-05-160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bechmann I., Priller J., Kovac A., Bontert M., Wehner T., Klett F.F., Bohsung J., Stuschke M., Dirnagl U., Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 50.Schnell S.A., Staines W.A., Wessendorf M.W. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- 51.Burdo T.H., Lentz M.R., Autissier P., Krishnan A., Halpern E., Letendre S., Rosenberg E.S., Ellis R.J., Williams K.C. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burdo T.H., Lo J., Abbara S., Wei J., DeLelys M.E., Preffer F., Rosenberg E.S., Williams K.C., Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crowe S., Zhu T., Muller W.A. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 54.Williams K., Alvarez X., Lackner A.A. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- 55.Williams K., Burdo T.H. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol. 2012;7:363–371. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- 56.Grossmann R., Stence N., Carr J., Fuller L., Waite M., Dailey M.E. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 2002;37:229–240. [PubMed] [Google Scholar]
- 57.Nataf S., Strazielle N., Hatterer E., Mouchiroud G., Belin M.F., Ghersi-Egea J.F. Rat choroid plexuses contain myeloid progenitors capable of differentiation toward macrophage or dendritic cell phenotypes. Glia. 2006;54:160–171. doi: 10.1002/glia.20373. [DOI] [PubMed] [Google Scholar]
- 58.Chalifoux L.V., Simon M.A., Pauley D.R., MacKey J.J., Wyand M.S., Ringler D.J. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest. 1992;67:338–349. [PubMed] [Google Scholar]
- 59.Yanai T., Lackner A.A., Sakai H., Masegi T., Simon M.A. Systemic arteriopathy in SIV-infected rhesus macaques (Macaca mulatta) J Med Primatol. 2006;35:106–112. doi: 10.1111/j.1600-0684.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 60.van Horssen J., Singh S., van der Pol S., Kipp M., Lim J.L., Peferoen L., Gerritsen W., Kooi E.J., Witte M.E., Geurts J.J., de Vries H.E., Peferoen-Baert R., van den Elsen P.J., van der Valk P., Amor S. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J Neuroinflammation. 2012;9:156. doi: 10.1186/1742-2094-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trebst C., Sorensen T.L., Kivisakk P., Cathcart M.K., Hesselgesser J., Horuk R., Sellebjerg F., Lassmann H., Ransohoff R.M. CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis. Am J Pathol. 2001;159:1701–1710. doi: 10.1016/s0002-9440(10)63017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henderson A.P., Barnett M.H., Parratt J.D., Prineas J.W. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 63.Mawhinney L.A., Thawer S.G., Lu W.Y., Rooijen N., Weaver L.C., Brown A., Dekaban G.A. Differential detection and distribution of microglial and hematogenous macrophage populations in the injured spinal cord of lys-EGFP-ki transgenic mice. J Neuropathol Exp Neurol. 2012;71:180–197. doi: 10.1097/NEN.0b013e3182479b41. [DOI] [PubMed] [Google Scholar]
- 64.Gray F., Scaravilli F., Everall I., Chretien F., An S., Boche D., Adle-Biassette H., Wingertsmann L., Durigon M., Hurtrel B., Chiodi F., Bell J., Lantos P. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 65.Reinhart T.A., Rogan M.J., Huddleston D., Rausch D.M., Eiden L.E., Haase A.T. Simian immunodeficiency virus burden in tissues and cellular compartments during clinical latency and AIDS. J Infect Dis. 1997;176:1198–1208. doi: 10.1086/514113. [DOI] [PubMed] [Google Scholar]
- 66.Thompson K.A., Varrone J.J., Jankovic-Karasoulos T., Wesselingh S.L., McLean C.A. Cell-specific temporal infection of the brain in a simian immunodeficiency virus model of human immunodeficiency virus encephalitis. J Neurovirol. 2009;15:300–311. doi: 10.1080/13550280903030125. [DOI] [PubMed] [Google Scholar]
- 67.Strickland S.L., Gray R.R., Lamers S.L., Burdo T.H., Huenink E., Nolan D.J., Nowlin B., Alvarez X., Midkiff C.C., Goodenow M.M., Williams K., Salemi M. Efficient transmission and persistence of low-frequency SIVmac251 variants in CD8-depleted rhesus macaques with different neuropathology. J Gen Virol. 2012;93:925–938. doi: 10.1099/vir.0.039586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kida S., Steart P.V., Zhang E.T., Weller R.O. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 1993;85:646–652. doi: 10.1007/BF00334675. [DOI] [PubMed] [Google Scholar]
- 69.Glass J.D., Fedor H., Wesselingh S.L., McArthur J.C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 70.Kusao I., Shiramizu B., Liang C.Y., Grove J., Agsalda M., Troelstrup D., Velasco V.N., Marshall A., Whitenack N., Shikuma C., Valcour V. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams D.W., Eugenin E.A., Calderon T.M., Berman J.W. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91:401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pulliam L., Sun B., Rempel H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J Neuroimmunol. 2004;157:93–98. doi: 10.1016/j.jneuroim.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 73.Burdo T.H., Weiffenbach A., Woods S.P., Letendre S., Ellis R.J., Williams K.C. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–1395. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker J.A., Sulciner M.L., Nowicki K.D., Miller A.D., Burdo T.H., Williams K.C. Elevated numbers of CD163+ macrophages in hearts of simian immunodeficiency virus-infected monkeys correlate with cardiac pathology and fibrosis. AIDS Res Hum Retroviruses. 2014;30:685–694. doi: 10.1089/aid.2013.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pereyra F., Lo J., Triant V.A., Wei J., Buzon M.J., Fitch K.V., Hwang J., Campbell J.H., Burdo T.H., Williams K.C., Abbara S., Grinspoon S.K. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26:2409–2412. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kosel S., Egensperger R., Bise K., Arbogast S., Mehraein P., Graeber M.B. Long-lasting perivascular accumulation of major histocompatibility complex class II-positive lipophages in the spinal cord of stroke patients: possible relevance for the immune privilege of the brain. Acta Neuropathol. 1997;94:532–538. doi: 10.1007/s004010050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cosenza M.A., Zhao M.L., Lee S.C. HIV-1 expression protects macrophages and microglia from apoptotic death. Neuropathol Appl Neurobiol. 2004;30:478–490. doi: 10.1111/j.1365-2990.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 78.Alvarez A, Conerly C, Lackner A, Williams KC: Brain perivasacular cells leave the CNS: implications for AIDS pathogenesis. Presented at the 28th Annual Symposium on Nonhuman Primate Models for AIDS, 2010 Oct 19-22, New Orleans, LA
- 79.Kaminski M., Bechmann I., Pohland M., Kiwit J., Nitsch R., Glumm J. Migration of monocytes after intracerebral injection at entorhinal cortex lesion site. J Leukoc Biol. 2012;92:31–39. doi: 10.1189/jlb.0511241. [DOI] [PubMed] [Google Scholar]
- 80.Johnston M., Zakharov A., Papaiconomou C., Salmasi G., Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burdo T.H., Wood M.R., Fox H.S. Osteopontin prevents monocyte recirculation and apoptosis. J Leukoc Biol. 2007;81:1504–1511. doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hochmeister S., Zeitelhofer M., Bauer J., Nicolussi E.M., Fischer M.T., Heinke B., Selzer E., Lassmann H., Bradl M. After injection into the striatum, in vitro-differentiated microglia- and bone marrow-derived dendritic cells can leave the central nervous system via the blood stream. Am J Pathol. 2008;173:1669–1681. doi: 10.2353/ajpath.2008.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Westhorpe C.L., Zhou J., Webster N.L., Kalionis B., Lewin S.R., Jaworowski A., Muller W.A., Crowe S.M. Effects of HIV-1 infection in vitro on transendothelial migration by monocytes and monocyte-derived macrophages. J Leukoc Biol. 2009;85:1027–1035. doi: 10.1189/jlb.0808501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cassol E., Cassetta L., Alfano M., Poli G. Macrophage polarization and HIV-1 infection. J Leukoc Biol. 2010;87:599–608. doi: 10.1189/jlb.1009673. [DOI] [PubMed] [Google Scholar]
- 85.Jenkins S.J., Ruckerl D., Cook P.C., Jones L.H., Finkelman F.D., van Rooijen N., MacDonald A.S., Allen J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burkala E.J., He J., West J.T., Wood C., Petito C.K. Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. AIDS. 2005;19:675–684. doi: 10.1097/01.aids.0000166090.31693.aa. [DOI] [PubMed] [Google Scholar]
- 87.Ryzhova E.V., Crino P., Shawver L., Westmoreland S.V., Lackner A.A., Gonzalez-Scarano F. Simian immunodeficiency virus encephalitis: analysis of envelope sequences from individual brain multinucleated giant cells and tissue samples. Virology. 2002;297:57–67. doi: 10.1006/viro.2002.1395. [DOI] [PubMed] [Google Scholar]
- 88.Reddy R.T., Achim C.L., Sirko D.A., Tehranchi S., Kraus F.G., Wong-Staal F., Wiley C.A. Sequence analysis of the V3 loop in brain and spleen of patients with HIV encephalitis. AIDS Res Hum Retroviruses. 1996;12:477–482. doi: 10.1089/aid.1996.12.477. [DOI] [PubMed] [Google Scholar]
- 89.Swirski F.K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.L., Kohler R.H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T.R., Libby P., Weissleder R., Pittet M.J. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cortez-Retamozo V., Etzrodt M., Newton A., Rauch P.J., Chudnovskiy A., Berger C., Ryan R.J., Iwamoto Y., Marinelli B., Gorbatov R., Forghani R., Novobrantseva T.I., Koteliansky V., Figueiredo J.L., Chen J.W., Anderson D.G., Nahrendorf M., Swirski F.K., Weissleder R., Pittet M.J. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., Samokhvalov I.M., Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S.W., Forsberg E.C., Tanaka M., van Rooijen N., Garcia-Sastre A., Stanley E.R., Ginhoux F., Frenette P.S., Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lamers S.L., Gray R.R., Salemi M., Huysentruyt L.C., McGrath M.S. HIV-1 phylogenetic analysis shows HIV-1 transits through the meninges to brain and peripheral tissues. Infect Genet Evol. 2011;11:31–37. doi: 10.1016/j.meegid.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]