Abstract
Drug-induced gingival overgrowth is caused by the antiseizure medication phenytoin, calcium channel blockers, and ciclosporin. Characteristics of these drug-induced gingival overgrowth lesions differ. We evaluate the ability of a mouse model to mimic human phenytoin-induced gingival overgrowth and assess the ability of a drug to prevent its development. Lovastatin was chosen based on previous analyses of tissue-specific regulation of CCN2 production in human gingival fibroblasts and the known roles of CCN2 in promoting fibrosis and epithelial to mesenchymal transition. Data indicate that anterior gingival tissue overgrowth occurred in phenytoin-treated mice based on gross tissue observations and histomorphometry of tissue sections. Molecular markers of epithelial plasticity and fibrosis were regulated by phenytoin in gingival epithelial tissues and in connective tissues similar to that seen in humans. Lovastatin attenuated epithelial gingival tissue growth in phenytoin-treated mice and altered the expressions of markers for epithelial to mesenchymal transition. Data indicate that phenytoin-induced gingival overgrowth in mice mimics molecular aspects of human gingival overgrowth and that lovastatin normalizes the tissue morphology and the expression of the molecular markers studied. Data are consistent with characterization of phenytoin-induced human gingival overgrowth in vivo and in vitro characteristics of cultured human gingival epithelial and connective tissue cells. Findings suggest that statins may serve to prevent or attenuate phenytoin-induced human gingival overgrowth, although specific human studies are required.
Gingival overgrowth is principally a tissue-specific adverse effect of the antiseizure drug phenytoin, antihypertensive calcium channel blockers, and the immunosuppressant ciclosporin (formerly cyclosporine A). Oral complications of gingival overgrowth include difficulty in maintaining adequate oral hygiene that can have systemic consequences related to excess inflammation. More than 3 million Americans have seizure disorders, 20% of whom continued to receive phenytoin principally because of grand mal epilepsy. Alternative medications can sometimes be prescribed, but this is not possible for patients with grand mal epileptic seizures, which are optimally treated with phenytoin. Some patients require the calcium channel blocker nifedipine to treat hypertension and cannot tolerate other antihypertensive drugs.1 Similarly, although tacrolimus is now often substituted for cyclosporin, data indicate that gingival overgrowth still occurs, albeit with delayed onset and perhaps reduced severity.1,2 The current surgical therapy for drug-induced gingival overgrowth is gingivectomy. Gingivectomy is unsatisfactory because in many cases there is a need for repeated operations due to recurrence of the disease. In addition, the surgical approach leads to substantial patient discomfort and risk of infection. There remains, therefore, a need to investigate tissue-specific mechanisms and develop therapeutic approaches to treat this disfiguring and detrimental craniofacial disease.
Analyses of tissue-specific characteristics of human gingival overgrowth clinical samples and in vitro pathway analyses using primary human gingival fibroblasts and epithelial cells have resulted in identification of specific pathological processes and pharmacological approaches to potentially prevent or alleviate gingival overgrowth.3–5 These studies have focused on pathways that regulate CCN2, also known as connective tissue growth factor, which is highly elevated in phenytoin-induced gingival overgrowth and in idiopathic human gingival fibromatosis, which are both fibrotic, unlike some other forms of gingival overgrowth, which are not fibrotic.6 CDC42 and RAC1 are small G-proteins that require lipid modification for activity and are critical mediators of transforming growth factor (TGF)-β1–induced CCN2 expression in a tissue-specific manner.4 Lipid modifications of CDC42 and RAC1 are required for activation and are derived from the cholesterol biosynthetic pathway.7 Inhibition of the cholesterol biosynthetic pathway by the HMG-CoA reductase inhibitors lovastatin and simvastatin inhibits TGF-β1–induced CCN2 expression in primary human gingival fibroblasts.4 Lovastatin is an approved drug in widespread use for the treatment of hypercholesteremia. Therefore, we wished to determine whether in vivo administration of lovastatin could prevent the development of gingival overgrowth in a mouse model. We report both the development of a mouse model of phenytoin-induced gingival overgrowth in mice and its use to determine the effectiveness of lovastatin to prevent the development of gingival overgrowth and to normalize the expression of Ccn2 and other proteins associated with phenytoin-induced gingival overgrowth. Data are consistent with earlier in vitro studies that identified molecular pathways that drive phenytoin-induced gingival overgrowth in humans in a tissue-specific manner.3,4
Materials and Methods
Phenytoin was purchased from Henry Schein Corporation (NDC 0641-0493; Roswell, GA). Lovastatin was purchased from EMD Millipore (Calbiochem, 438186; Billerica, MA). Physiological sterile saline was purchased from the Laboratory Animal Science Center at Boston University. Propylene glycol was purchased from MP Biomedical, (151957; Santa Ana, CA). Harris modified hematoxylin (SH-30), eosin B (E514), and eosin Y (E511) were purchased from Fisher Scientific (Hampton, NH). Rabbit polyclonal anti-Tgfβ1 IgG and blocking peptide were purchased from Santa Cruz Biotechnology (sc-146; Dallas, TX). Rabbit polyclonal anti-CTGF/CCN2 (ab6992) antibody was purchased from Abcam (Cambridge, MA). Rabbit polyclonal anti LOXL2 IgG (105085) was purchased from GeneTex (Irvine, CA) and mouse LOXL2 shRNA plasmids were purchased from Sigma (St Louis, MO). Mouse monoclonal anti E-cadherin IgG was purchased from BD Bioscience (610181; San Jose, CA). Immunoperoxidase detection kits and reagents were purchased from Vector Laboratories (Burlingame, CA).
Human Gingival Samples
Human gingival samples were collected from patients with severe gingival overgrowth who were treated with phenytoin for epilepsy and subjected to gingivectomy and from systemically healthy individuals undergoing crown-lengthening surgery. Samples were collected at the Department of Periodontology and Oral Biology and the Clinical Research Center of Boston University at the Goldman School of Dental Medicine and the Franciscan Children's Hospital and Rehabilitation Center. Informed consent forms were signed by all donors. The consent forms and study protocols were approved by the Institutional Review Board of Boston University Medical Center. All excised tissues were fixed in 4% paraformaldehyde in phosphate-buffered saline at 4°C for 4 hours and then incubated in cold 30% sucrose overnight. Tissues were then stored in 2-methylbutane at −80°C. At least twenty 5-μm serial sections per sample were made on a cryostat and were stored at −80°C.
Mouse Model
Eight-week-old BALB/cByJ male mice were purchased from Jackson Laboratory (Cat# 001026; Bar Harbor, ME). Mice were housed for 1 week before any procedure for acclimation. Mice were then divided into groups.
Control groups consisted of a negative control group receiving physiological saline (NaCl 0.9%, n = 8) and a control group receiving vehicle (50% water, 41% propylene glycol, and 9% ethanol) (n = 3). The treatment groups consisted of a group receiving phenytoin (NDC 0641-0493, 30 mg/kg; Henry Schein Corporation) (n = 10) and a group receiving phenytoin plus lovastatin (0.65 mg/kg) (n = 7). Phenytoin was supplied from the vendor as pharmaceutical-grade phenytoin sodium in liquid form with a concentration of 50 mg/mL in 50% water, 41% propylene glycol, and 9% ethanol. Lovastatin was supplied as the lovastatin sodium salt and was dissolved directly in either 4.2 mL of phenytoin sodium solution or 4.2 mL of the vehicle (50% water, 41% propylene glycol, and 9% ethanol) to give a concentration of 1.13 mg/mL of lovastatin. Phenytoin, control vehicle, or physiologic saline were delivered with miniosmotic pumps (ALZET Osmotic Pumps, 2002, Cupertino, CA) at a 0.5-μL/h delivery rate for a period of 2 weeks. Pumps were replaced every 2 weeks as needed for the duration of the experiment. Lovastatin, when present, was dissolved in the same vehicle as phenytoin and when administered together with phenytoin was dissolved in the same solution and applied simultaneously in a single osmotic minipump. Although a detailed time course for gingival overgrowth development was not performed, a pilot study indicated that only minimal levels of gingival overgrowth could be observed at an experimental period of 6 weeks. Therefore, the duration of the experimental period reported here was 12 weeks. The dose of lovastatin of 0.65 mg/kg is equivalent to an intermediate human dose, which ranges from 0.3 to 1.2 mg/kg.8
Mouse Operations
Mice were preinjected with buprenorphine for analgesia 30 minutes before surgery. Then, mice were placed in an induction chamber to induce anesthesia with isoflurane. Fur was shaved from the mouse posterior to the shoulder, and skin was wiped with povidone-iodine and 70% ethanol. Using a scalpel blade 11, a small incision was made, and then blunt dissection was performed with a hemostat. A pump that contained physiological saline, vehicle, lovastatin, phenytoin, or phenytoin plus lovastatin was placed subcutaneously. Then, the wound was closed with autoclips (427631; Clay Adams, New York, NY). All pumps were primed in sterile physiological saline overnight at 37°C to ensure immediate release of the drugs on implantation. Pumps were replaced every 2 weeks throughout the 12-week experimental period. Mice were then euthanized and heads were immediately placed in freshly made 4% paraformaldehyde for 24 hours for fixation.
Mouse Tissue Processing
After fixation, mouse heads were washed under running tap water to remove excess fixative for 3 minutes. Then, each head was immersed in 50 mL 0.5 mol/L EDTA at pH 8.0 for decalcification. The EDTA was changed every other day, and the heads were incubated with EDTA at 4°C on a shaker for a 6- to 8-week period. Serial X-rays assessed the degree of decalcification. Anterior maxillae and mandibles were separated from posterior dentition. Samples were trimmed so that each sample included either maxillary central incisors or maxillary molars or mandibular incisors or mandibular molars, with their respective surrounding gingival tissues.
Gross Morphology
Gross morphology images were taken with a stereomicroscope (Carl Zeiss AG, Jena, Germany). To compare the gross morphological differences among control and treatment groups, papillary height and papillary area were analyzed on labial and buccal gingival tissue of anterior and posterior upper and lower jaws. All measurements were performed using the Olympus Microsuite Five software version 5 (Richmond Hill, ON, Canada). Means of these measurements were calculated and subjected to one-way analysis of variance statistical analysis with the Bonferroni post hoc test.
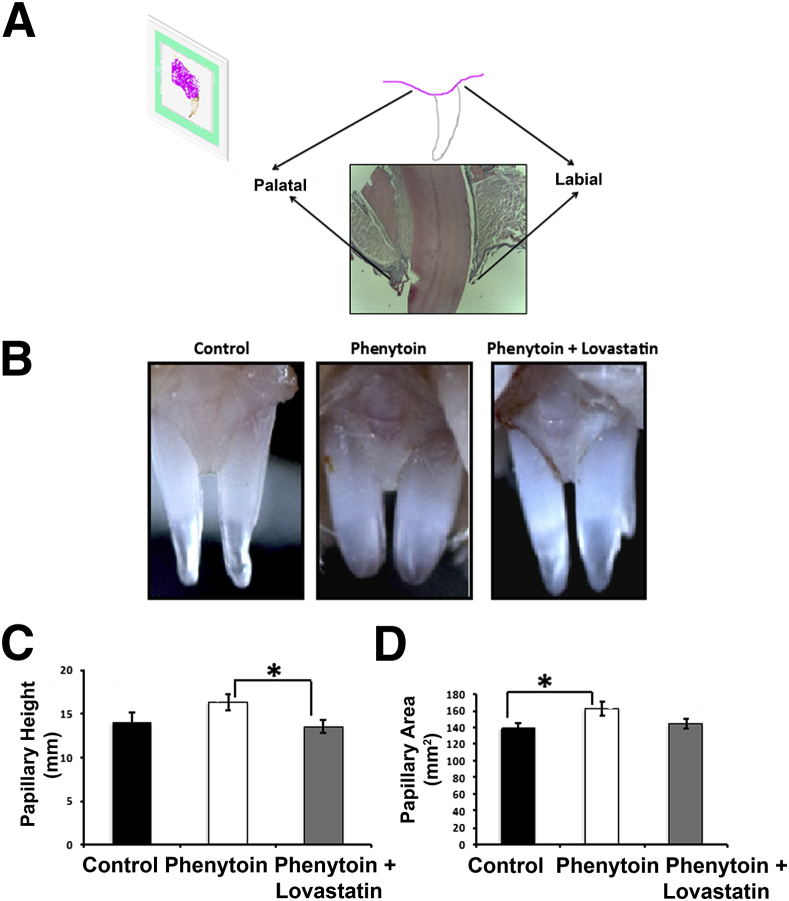
For embedding, samples were dehydrated in serial ethanol concentrations: 50%, 70%, 95%, and 100% for half a day, overnight, half a day, and overnight, respectively. Samples were then placed in 100% acetone followed by 100% chloroform at 60°C for 45 minutes each. Samples were placed in paraffin for 3 successive hourly changes. Samples were then embedded in paraffin using metal molds, cooled down at room temperature, and kept at 4°C overnight. The orientation of the samples during embedding was set up for sagittal sectioning (Figure 1A), whereas posterior tissues were for transverse sectioning. Sectioning was performed using a microtome with stainless steel blades (Fisher Scientific) producing 5-μm- thick sections. Slides were baked at 60°C for 2 hours to adhere sections to slides. For each sample, each fifth slide (three sections per slide) was stained with hematoxylin-eosin to determine the range of the fine sections that were used for histomorphometric and immunohistochemistry (IHC) analyses.
Figure 1.
A: Representation of orientation for embedding and sectioning procedures of maxillary anterior tissues. B: Representative images of gross morphology. C and D: Quantitation of papillary height and papillary area of all samples obtained. These results were drawn from a sample size of seven control, seven phenytoin-treated, and seven phenytoin plus lovastatin–treated mice. Data are expressed as means ± SEM. ∗P < 0.05, Bonferroni post hoc tests.
Histomorphometry
Slides were selected that contained buccal and lingual gingival tissue and the decalcified tooth structure that provided a landmark for orientation. Two different parameters were measured in three representative central sections from mandibles (lower jaw) and maxillae (upper jaw) from both the anterior and posterior teeth to perform a comparative histomorphometric analysis. The variables were the width of the epithelium and the area of the epithelium.
For measuring the width of the epithelium, at least 10 arbitrary distances were measured from the outer layer of the epithelium to the basement membrane in buccal and lingual gingival tissue, separately. Then, the means of these measurements were calculated, and one-way analysis of variance with the Bonferroni post hoc test statistical analysis was performed. For measuring the area of the epithelium, two area measurements for each of the buccal and lingual sides from each representative section were obtained, and the means were calculated followed by one-way analysis of variance with the Bonferroni post hoc statistical analyses. All measurements were performed using the Olympus Microsuite software. Significance was declared at P < 0.05.
Immunohistochemistry
The expression levels of different epithelial to mesenchymal transition (EMT) and fibrosis markers were assessed by IHC. Four sections per sample per marker were assayed. The IHC technique used heat-based antigen retrieval followed by immunoperoxidase staining. Sections were deparafinized in xylene for 10 minutes, followed by rehydration in serial concentrations of ethanol down to 75% and distilled water. Then, sections were subjected to high-temperature antigen retrieval in 10 mmol/L citric acid at pH 6.0. Endogenous peroxidase was inactivated with 3% hydrogen peroxide in methanol for 30 minutes. Endogenous immunoglobulin blocking was performed using 10% normal serum in phosphate-buffered saline from the same species in which the secondary antibody was raised for 30 minutes. Endogenous avidin and biotin were blocked for 20 minutes each to eliminate nonspecific binding and background. Rabbit polyclonal primary antibodies were used for detection of TGF-β1, CCN2, and LOXL2. A mouse monoclonal primary antibody was used for detection of E-cadherin. Sections stained with nonimmune rabbit and mouse IgG primary antibody were used as the negative control. Sections were incubated with primary antibody diluted in 5% normal serum in phosphate-buffered saline overnight at 4°C. Bound primary antibody was detected using Vectastain Elite kits (Vector Laboratories) with biotinylated secondary antibody for 30 minutes and immunoperoxidase staining as described by the manufacturer. For IHC analysis of LOXL2 expression in human tissues, frozen sections were fixed in 100% acetone for 10 minutes at 37°C and stained as previously described.9
Antibody Specificity
Antibody specificity for CCN2 was established by Western blotting of nontreated and TGF-β1–treated gingival fibroblasts; for TGF-β1, this was established by probing mouse gingival tissue sections with anti–TGF-β1 alone or with anti–TGF-β preabsorbed with blocking peptide; for E-cadherin, by Western blotting of cultured gingival epithelial cells; and for LOXL2, by shRNA knockdown of LOXL2 and Western blotting of extracts of control and Ccn2 knockdown gingival fibroblasts (Supplemental Figure S1).
Analysis of IHC
Threshold analysis was used to quantify the percentage of stained area in two representative sections from each sample using ImageJ version 1.46 (NIH, Bethesda, MD). In this technique, the threshold of a digital image is composed of three different variables: hue, saturation, and brightness. By setting the hue and saturation to maximum and adjusting the brightness, the total area of tissue was determined. Next, by adjusting the hue and brightness again, the area of stained tissue in the section was determined.10 Then, the percentage of the stained area normalized to the total tissue area in each section was calculated. One-way analysis of variance statistical analyses with Bonferroni post hoc tests were finally performed.
Results
Gross Morphology
Gross gingival morphology differences between groups were investigated, and gingival overgrowth was apparent in the maxillary anterior gingival tissue of phenytoin-treated mice compared with control and phenytoin plus lovastatin–treated mice (Figure 1B). Quantitative analyses (Figure 1, C and D) revealed a trend toward an increase of papillary height in the phenytoin group compared with the control group (P = 0.094) and a significant reduction of the papillary height in the phenytoin plus lovastatin group when compared with phenytoin (P = 0.046). Area measurements revealed a significant increase in the phenytoin group when compared with the control group (P = 0.028). A comparison between the area measurements of the phenytoin group and the phenytoin plus lovastatin group revealed a trend toward reduction in the papillary area of the phenytoin plus lovastatin group (P = 0.094). No gingival overgrowth could be identified around the anterior mandibles or in the posterior mandible or maxillary molar areas (data not shown). Thus, data suggest that phenytoin successfully induced gingival overgrowth in anterior regions under these experimental conditions. Lovastatin appears to prevent gingival overgrowth development and is further analyzed below.
Histomorphometry of Tissue Sections
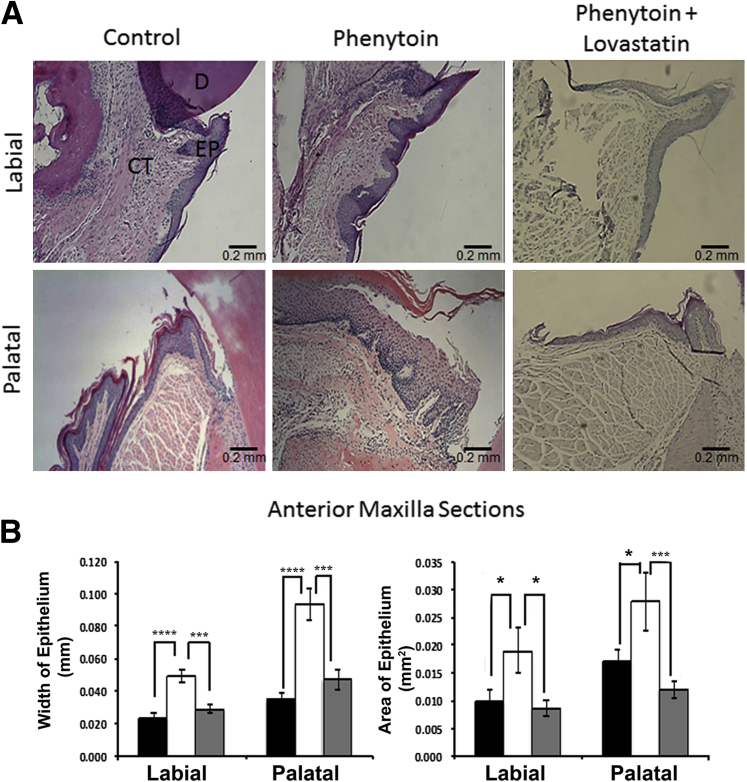
Quantitative histomorphometric analysis of tissue sections from all mouse groups revealed increased epithelial thickness and area in the maxillary anterior zone of phenytoin-treated mice. In addition, lovastatin appeared to reduce the hyperplastic effect of phenytoin on gingival tissue as was observed in the phenytoin plus lovastatin–treated mouse group (Figure 2). The phenytoin-treated group had a 2.1-fold increase in labial epithelial width and a 2.7-fold increase in epithelial palatal width, implying an increased epithelial volume. In addition, the phenytoin group had higher values of epithelial area with a 1.9-fold labial increase and a 1.6-fold increase in palatal area compared with the control group. Interestingly, administration of both lovastatin and phenytoin together prevented the hyperplastic effect of phenytoin and resulted in the width and area of the epithelium essentially equivalent to control levels (Figure 2A). By contrast, maxillary and mandibular posterior gingival epithelia had either no differences or inconsistent results (data not shown). Mice treated with lovastatin alone had no alteration in histomorphometric parameters compared with saline or vehicle controls (Supplemental Figure S2).
Figure 2.
Histomorphometric data of upper anterior area. A: Representative sections of comparisons between sagittal sections of mouse maxillary anterior areas obtained from three different mouse groups (control, phenytoin, and phenytoin plus lovastatin groups) stained with hematoxylin and eosin (three sections per area per mouse). B: Quantification of maxillary anterior histomorphometric data. These results were drawn from a sample size of 9 control (black bars), 10 phenytoin-treated (white bars), and 6 phenytoin plus lovastatin–treated (gray bars) mice. One-way analysis of variance analysis was significant for labial epithelial width (P < 0.00005), palatal epithelial width (P < 0.00005), labial epithelial area (P < 0.05), and palatal epithelial area (P < 0.02). Data are expressed as means ± SEM. ∗P < 0.05, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001, Bonferroni post hoc tests. Scale bar = 0.2 mm. CT, connective tissue; D, dentin; EP, epithelium.
In summary, histomorphometric analysis revealed that phenytoin increases the gingival epithelial volume in the maxillary anterior zone and that lovastatin prevents development of the hyperplastic effect of phenytoin. These observations were greater on the palatal (lingual) side than on the labial side of the gingival tissue.
Immunostaining for Markers of Fibrosis
Ccn2
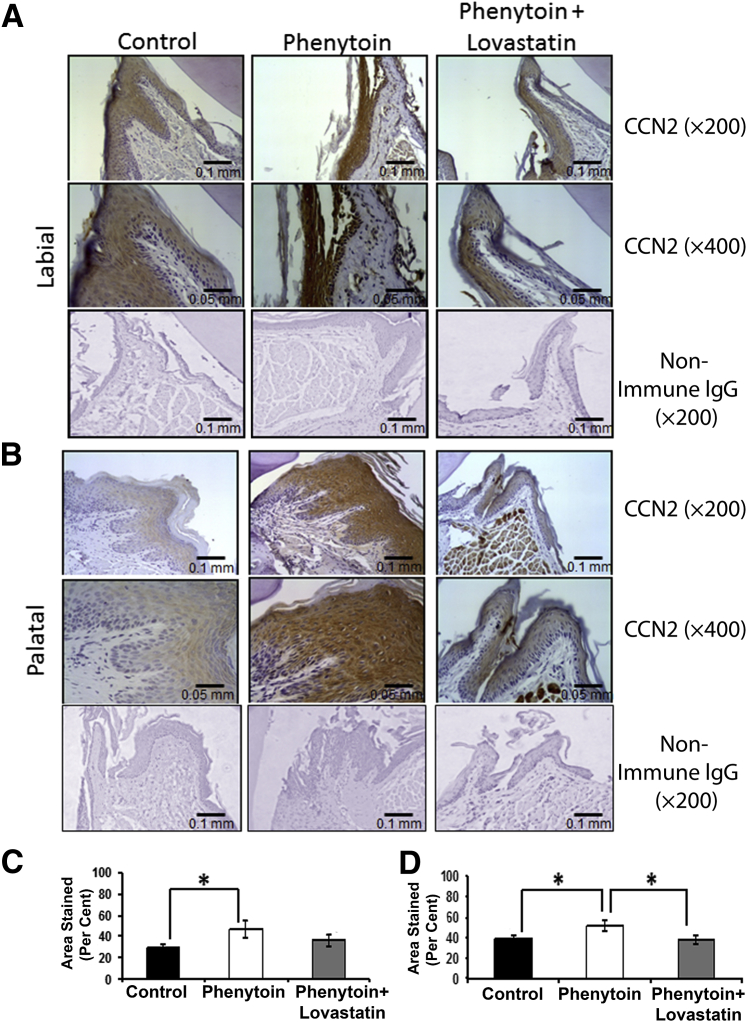
Ccn2 was expressed mostly in the epithelium in all three groups, with some connective tissue expression in the phenytoin group, which was seen only in three phenytoin samples. Qualitatively, the phenytoin group had increased intensity of staining, suggesting overexpression of Ccn2 in all areas of the epithelium when compared with the control and the phenytoin plus lovastatin groups (Figure 3, A and B). Quantitative analysis of Ccn2 staining was consistent with these features except that the effect of lovastatin was significant for the palatal tissues and not for the labial tissues (Figure 3, C and D). Although Ccn2 was detected in the connective tissue stroma, no obvious regulation by phenytoin or lovastatin could be discerned under these conditions. We conclude that phenytoin causes overexpression of Ccn2 primarily in the epithelium of the phenytoin-treated mice and that lovastatin largely prevented the phenytoin-induced overexpression of Ccn2, especially in palatal anterior gingiva.
Figure 3.
A and B: Ccn2 regulation by phenytoin and phenytoin plus lovastatin in mouse gingiva (labial view, A, and palatal view, B). C and D: Quantification of Ccn2 epithelial expression in the mouse maxillary anterior area (labial tissue, C, and palatal tissue, D). These results were drawn from a sample size of eight control, eight phenytoin-treated mice, and seven phenytoin plus lovastatin–treated mice. One-way analysis of variance analyses was not significant for palatal tissues. Data are expressed as means ± SEM. ∗P < 0.05, Bonferroni post hoc tests. Scale bars: 0.1 mm (A); 0.05 mm (B). Original magnification: ×200 (A); ×400 (B).
TGF-β
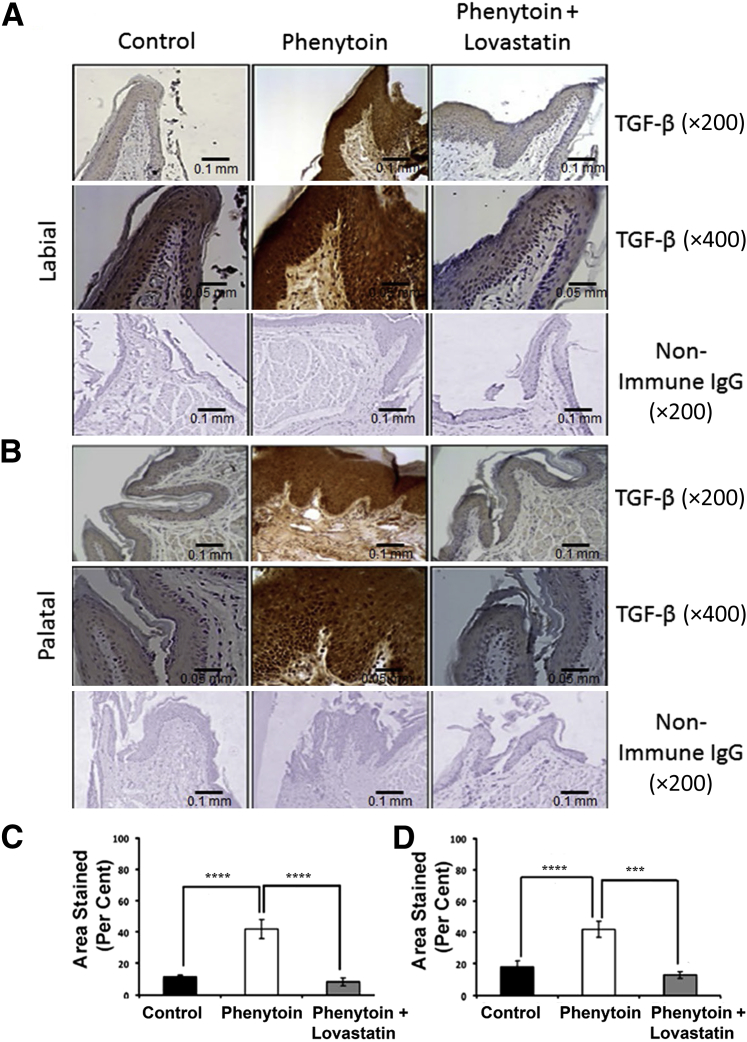
TGF-β is a major inducer of CCN2. We therefore investigated expression of TGF-β1 by IHC in these mouse tissues. Data from mouse tissue sections assayed with anti–TGF-β1 antibody revealed that phenytoin alone increased TGF-β staining in both the gingival epithelium and connective tissue in anterior gingival tissues when compared with the control group (Figure 4, A and B). Interestingly, lovastatin in the phenytoin plus lovastatin group prevented or diminished phenytoin-induced TGF-β1 expression in gingival epithelium and connective tissues. The connective tissue expression of TGF-β1 observed was both extracellular and intracellular. The intensity of the staining was found to be higher on the palatal side than the labial side of the gingival tissue (Figure 4, A and B). Interestingly, connective tissue staining for Tgf-β was similarly regulated in these tissues.
Figure 4.
A and B: Transforming growth factor (TGF)-β regulation by phenytoin and phenytoin plus lovastatin in mouse gingiva (labial view, A, and palatal view, B). C and D: Quantification of TGF-β expression in the mouse maxillary anterior area (labial tissue, C, and palatal tissue, D). Results were drawn from two sections per area per mouse with a sample size of six control mice, seven phenytoin-treated mice, and six phenytoin plus lovastatin–treated mice. One-way analysis of variance analyses were significant for labial (P < 0.00001) and palatal (P < 0.0005) epithelia. Data are expressed as means ± SEM. ∗∗∗P < 0.001 and ∗∗∗∗P < 0.0001, Bonferroni post hoc tests. Scale bars: 0.1 mm (A); 0.05 mm (B). Original magnification: ×200 (A); ×400 (B).
Quantitative analysis revealed that the phenytoin-treated mice exhibited 3.7-fold higher TGF-β1 levels in the labial gingiva and 2.3-fold higher levels in the palatal gingiva compared with the control group (Figure 4, C and D). Mice treated with phenytoin plus lovastatin had significantly reduced levels of TGF-β1 staining, even slightly lower than levels seen in the control group, confirming our qualitative findings. Data indicate that TGF-β1 was overexpressed in the phenytoin-treated mice in both the epithelium and connective tissue. This overexpression was significantly reduced in the phenytoin plus lovastatin group.
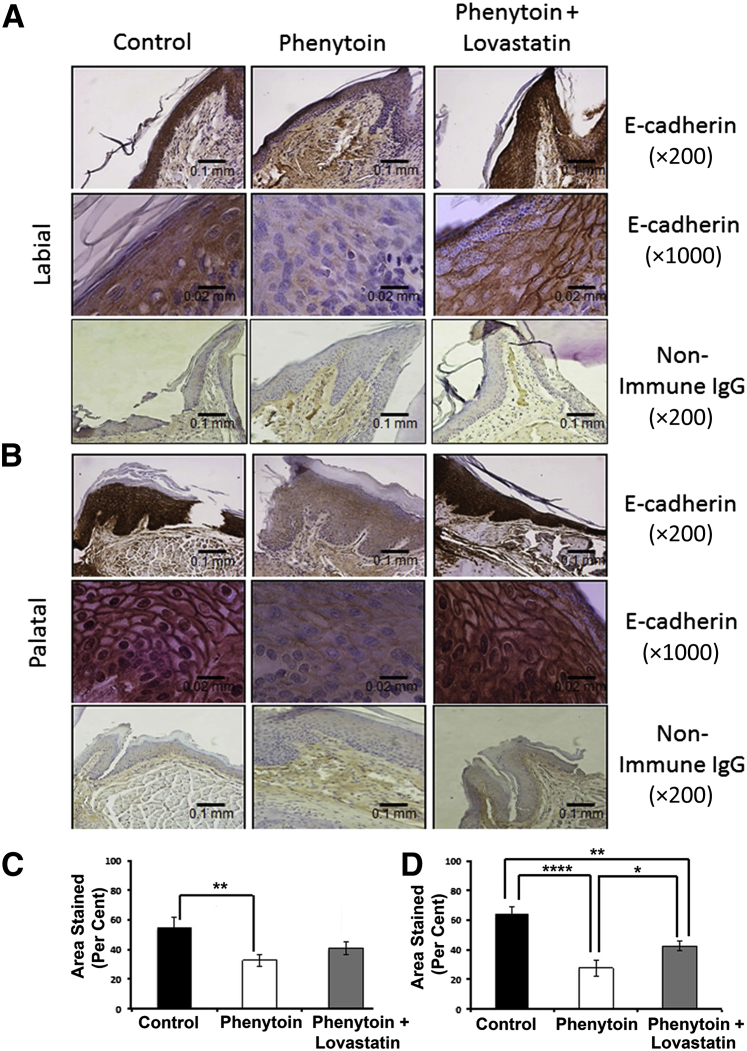
E-Cadherin
Ccn2 and TGF-β are drivers of EMT in epithelial tissues. Therefore, we next stained tissues for the classic epithelial cell marker E-cadherin, which is down-regulated as cells undergo EMT. E-cadherin staining revealed a lower intensity in phenytoin-treated mice compared with both control and lovastatin-treated mice (Figure 5, A and B). Quantitative analyses of areas stained suggest a partial restoration by lovastatin of approximately 25% of E-cadherin staining on the palatal side compared with phenytoin alone, whereas there was no apparent effect of lovastatin on the labial side (Figure 5, C and D). Besides intense epithelial staining, there was some nonspecific immunoreactivity observed in the connective tissue and periodontal ligament area in all groups, including the nonimmune control. However, the nonimmune control exhibited no epithelial staining, which supports that epithelial E-cadherin staining is specific. Similar data were obtained with a polyclonal E-cadherin antibody (data not shown). It can be concluded that phenytoin strongly inhibits E-cadherin staining in gingival epithelia, whereas lovastatin has a limited ability to restore this expression on palatal tissues.
Figure 5.
A and B: E-cadherin regulation by phenytoin and phenytoin plus lovastatin in mouse gingiva (labial view, A, and palatal view, B). C and D: Quantification of E-cadherin expression in the mouse maxillary anterior area (labial tissue, C, and palatal tissue, D). Results were drawn from two sections per area per mouse with a sample size of five control, six phenytoin-treated, and four phenytoin plus lovastatin–treated mice. One-way analysis of variance analyses were significant for labial epithelia (P < 0.05) and palatal epithelia (P < 0.0005). Data are expressed as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001, Bonferroni post hoc tests. Scale bars: 0.1 mm (A); 0.02 mm (B). Original magnification: ×200 (A); ×1000 (B).
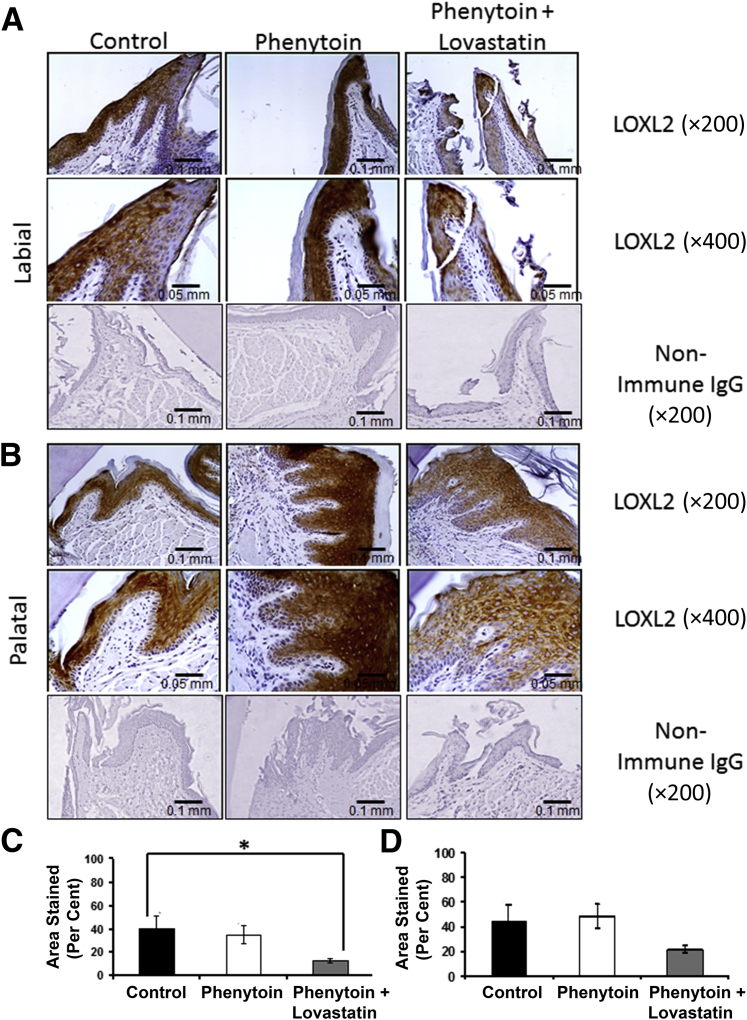
LOXL2
Evidence has been presented that LOXL2 is a driver of EMT in the context of cancer.11,12 Additional ongoing studies suggest that LOXL2 expression may contribute to human gingival overgrowth (D. Saxena and P.C. Trackman, unpublished data). We, therefore, wanted to determine whether Loxl2 was expressed in the epithelium of phenytoin-treated mice and whether lovastatin would modulate this expression. Staining of mouse tissue sections with anti-LOXL2 antibody revealed epithelial expression in all groups, and connective tissue expression was seen only in one phenytoin sample on the palatal side. The intensity of LOXL2 staining appeared to be slightly higher in phenytoin-treated mice compared with the control, whereas lovastatin appeared to lower the intensity of LOXL2 expression below that of the control (Figure 6, A and B). Quantitative analyses of stained areas indicate that the presence of lovastatin significantly lowered LOXL2 expression in labial tissues, whereas a trend toward lowered expression was observed in palatal tissues. Phenytoin alone had no effect on the LOXL2 area stained. We conclude that lovastatin appears to down-regulate LOXL2 expression in gingival epithelia under these experimental conditions. It is currently unclear whether phenytoin alone could have a stimulatory effect on the intensity of LOXL2 epithelial expression, which was not reflected in changes in the percentage of tissue area stained in this semiquantitative model.
Figure 6.
A and B: LOXL2 regulation by phenytoin and phenytoin plus lovastatin in mouse gingiva (labial view, A, and palatal view, B). C and D: Quantification of LOXL2 expression by percentage of area stained in the mouse maxillary anterior area (labial tissue, C, and palatal tissue, D). Data are expressed as means ± SEM. These results were drawn from a sample size of six control, eight phenytoin-treated mice, and six phenytoin plus lovastatin–treated mice. One-way analysis of variance analyses were nonsignificant in labial and palatal tissues. Bonferroni post hoc tests were nonsignificant for differences between control and phenytoin groups, and differences between the phenytoin and phenytoin plus lovastatin groups were also nonsignificant. ∗P < 0.05, Bonferroni post hoc tests. Scale bars: 0.1 mm (A); 0.05 mm (B). Original magnification: ×200 (A); ×400 (B).
LOXL2 in Human Gingival Overgrowth
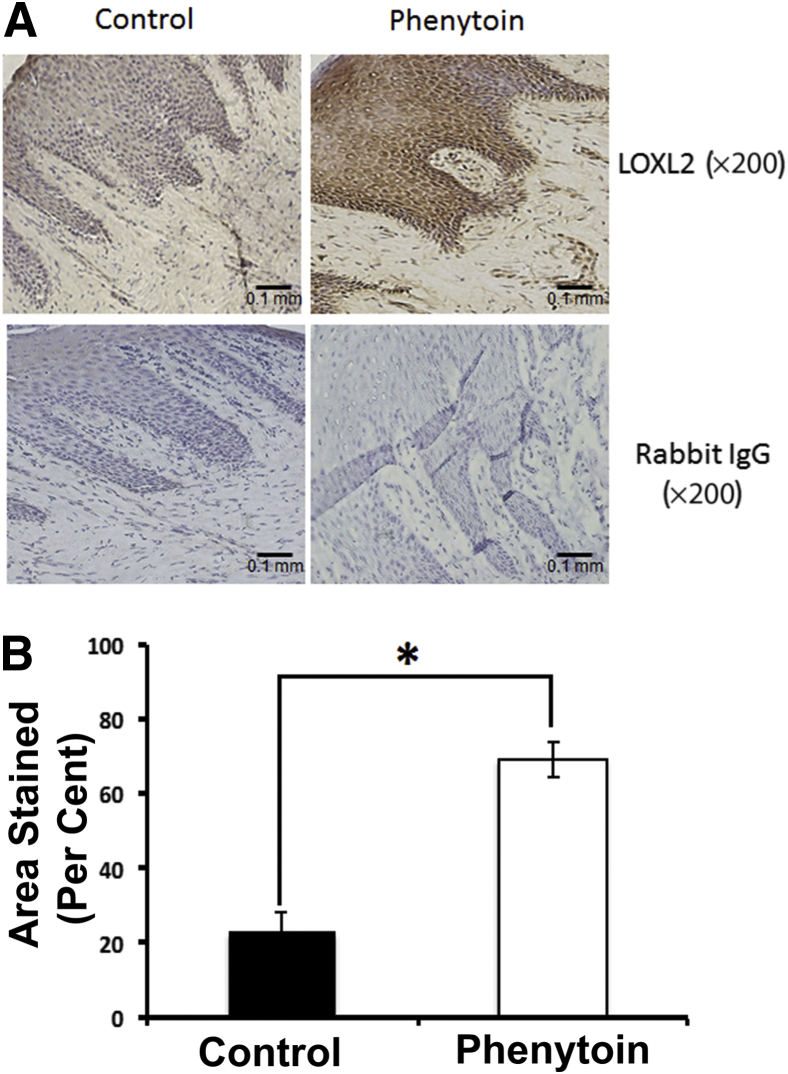
LOXL2 levels in human phenytoin-induced gingival overgrowth tissues have not been previously measured, whereas all other markers studied here have been previously measured in the human gingival overgrowth tissues.5 We, therefore, investigated the expression of LOXL2 in frozen gingival tissue sections from human subjects taking phenytoin (n = 4) and control (n = 4). Results indicate that LOXL2 is expressed in the epithelium and connective tissue of both groups. Interestingly, the phenytoin group had higher expression of LOXL2 in both the epithelium and the connective tissue compared with control tissues (Figure 7A). Quantitative analyses indicate that the phenytoin group had the highest value of epithelial area staining with a 3.05-fold increase when compared with the controls (P = 0.0007) (Figure 7B).
Figure 7.
LOXL2 is up-regulated in human gingival overgrowth tissues. A: Representative human gingival tissue sections stained with anti LOXL2 antibody. B: Quantification of LOXL2 epithelial expression in human samples. These results were drawn from a sample size of four control and four phenytoin-treated human subjects. Student's t-test result was statistically significant for the difference in LOXL2 expression levels between the control and phenytoin groups. Data are expressed as means ± SEM. n = 4. ∗P < 0.05. Scale bar = 0.1 mm (A).
Discussion
Three types of drugs are known to induce gingival overgrowth with variable degrees of fibrosis and inflammation.13 These drugs are the anticonvulsant phenytoin the immunosuppressant ciclosporin, and the calcium channel blocker nifedipine.1–3 All types of drug-induced gingival overgrowth had enlarged gingival epithelial and connective tissues with variable degrees of fibrosis and inflammation in expanded connective tissue.14 Phenytoin-induced gingival overgrowth is clearly the most fibrotic form of drug-induced gingival overgrowth with high expression of CCN2 and the lowest level of inflammatory cells. Ciclosporin-induced gingival overgrowth is the least fibrotic form and contains more inflammatory cells and the lowest CCN2 expression. Nifedipine-induced gingival overgrowth has an intermediate degree of fibrosis, CCN2 expression, and inflammatory cell concentration when compared with phenytoin- and cyclosporin-induced overgrowth.6 Oral hygiene and genetic susceptibility and dose of drugs can partially modulate the degree of gingival overgrowth.14–16
Several studies have been conducted to investigate drug-induced gingival overgrowth pathogenesis. Inconsistent with the clinical findings, some of these studies suggested that the drugs causing gingival overgrowth directly inhibit fibroblast proliferation and/or extracellular matrix production by these cells.17,18 More consistent with the in vivo findings, inflammatory cytokine imbalance and up-regulation of proliferative macrophages that secrete platelet-derived growth factor-β were found to mediate drug-induced gingival overgrowth. Evidence for contributions of EMT in the development of gingival overgrowth in vitro and in vivo has also been reported.5,19
It is important to discover new treatment modalities that will permanently resolve gingival overgrowth lesions or prevent their formation. We called attention to targeting fibrotic signaling pathways based on the mechanistic understanding that lovastatin inhibits TGF-β–induced CCN2 expression through indirect inhibition of CDC42 and RAC1 GTPases, which mediate TGF-β–induced CCN2 expression in human gingival fibroblasts.4 The further evaluation of the mechanisms that lead to drug-induced gingival overgrowth and of consequent therapeutic approaches requires the use of in vivo animal models. Establishment of such models will provide tools to monitor the temporal cellular and molecular associations of gingival overgrowth in vivo. Moreover, these models will permit evaluation of novel therapeutic approaches. Previous studies have reported development of animal models for drug-induced gingival overgrowth, but few have used them to investigate therapeutic approaches. Models have been established in dogs,20,21 monkeys,22 cats,23 rats,24–27 and mice.28–30 The routes of drug administration in these studies varied among daily intraperitoneal injections,29,30 gavage,24,26 or oral administration in diets.27,29 Although these studies were successful in developing drug-induced gingival overgrowth, the drug administration systems were suboptimal regarding the efficacy and consistency of drug administration.
The goals of our study included the induction of gingival overgrowth in a mouse model by continuous and accurate delivery of phenytoin and to test the effects of lovastatin in the same model, also continuously and accurately administered. We chose the BALB/cByJ mouse strain to reduce genetic variability compared with outbred strains. Moreover, a study by Meller et al30 presented BALB/c mouse as a good model for cyclosporin-induced gingival overgrowth.
Gross morphology data revealed significant gingival overgrowth in the phenytoin group when compared with the control and the phenytoin plus lovastatin groups. These effects were observed only in the maxillary anterior gingival area, whereas other areas had less consistent results. Clinical studies of phenytoin-induced gingival overgrowth in humans identified anterior maxillary and mandibular areas as the most affected sites by phenytoin.31,32 The histomorphometric evaluation of mouse tissue sections confirmed gross morphology results. The phenytoin group exhibited overall increases in the volume of the gingival epithelial tissue when compared with the control group. Interestingly, lovastatin was capable of inhibiting the hyperplastic effect of phenytoin. These findings were limited to the maxillary anterior area, which is most severely affected in humans as well. Additional studies of time and dose dependency coupled with both RNA and protein expression studies will provide additional information and potentially novel insights into the molecular events that drive gingival overgrowth development.
EMT has been implicated in phenytoin-induced gingival overgrowth development,5,19 and high epithelial expression of mesenchymal proteins in phenytoin-induced overgrowth observed here is of considerable interest. Several markers of EMT were studied, including TGF-β1, E-cadherin, Ccn2, and Loxl2. Limited by the semiquantitative measures of protein expression and a single drug dose and a single experimental time point used in this study, all markers studied appeared to change either as a function of both phenytoin and lovastatin treatments, with the possible exception of LOXL2, which appears to have been modulated only as a function of lovastatin. The fact that these expressions were most obvious in the epithelium of mouse gingival tissues supports the notion that EMT is occurring. These data may further suggest that gingival overgrowth is dependent on early changes in the epithelium in response to systemic drug treatments. This finding seems to be unexpected in light of the fact that distribution of systemic drugs depends on the systemic vasculature with initial drug exposure occurring in the connective tissue rather than in the avascular epithelium.
TGF-β1 is a strong inducer of EMT.5,33,34 Studies have found high expression of TGF-β1 in phenytoin-induced human samples.6,9 Our results in mice revealed substantially higher expression of TGF-β1 in the phenytoin-treated mouse group when compared with the control group in both the epithelium and connective tissue, which were attenuated in the presence of lovastatin. Consistent with our results, HMG-CoA reductase inhibitors (statins) were found to inhibit TGF-β1 expression in kidney and tooth extraction sockets.35,36
Down-regulation of E-cadherin occurs in EMT and fibrosis in nonoral tissues.37,38 Our results indicate that phenytoin-treated mice have lower expression of E-cadherin and that lovastatin treatment resulted in partial attenuation of phenytoin down-regulation of E-cadherin. The restoration of E-cadherin expression by a RAS farnesylation inhibitor (FTI-277) in human cancer cells39 and the finding that ρ GTPases promote loss of E-cadherin and cancer metastasis40 are consistent with our findings because lovastatin inhibits the formation of the substrates needed for lipidation and activation of these GTPases. The partial inhibition of the phenytoin-induced E-cadherin down-regulation observed here could be due to the presence of other potentially phenytoin-regulated signaling pathways that repress E-cadherin, or alternatively a higher dose of lovastatin may provide a better outcome. The dose of lovastatin used was 0.65 mg/kg and is based on an intermediate, but not maximum, human dose. Human therapeutic doses of lovastatin range from 0.3 to 1.2 mg/kg.8,41
CCN2 levels have been positively correlated with fibrosis and EMT in phenytoin-induced gingival overgrowth and other diseases5,6,42–44 and in the present study. Interestingly, lovastatin in the phenytoin plus lovastatin group reduced the phenytoin effect in inducing Ccn2 expression consistent with in vitro studies.4 LOXL2 participates in EMT and fibrosis.45–47 For example, LOXL2 down-regulates E-cadherin levels resulting in β-catenin activation.47 Our analysis of LOXL2 expression in human phenytoin-induced gingival overgrowth samples revealed substantial overexpression of LOXL2 in both the epithelium and the connective tissue. Increased intensity of LOXL2 expression predominantly in the epithelium was observed in the phenytoin-induced gingival overgrowth mouse tissues, although measurements of the percentage of the area stained did not reveal increased staining. We suspect that the level of LOXL2 expression in the epithelium was higher in phenytoin-treated mice, but because of limitations to accurately quantitate the intensity of staining, we can only suggest that LOXL2 expression was increased based on qualitative observations (Figure 6). Data suggest that the phenytoin plus lovastatin mouse group contains lower levels of LOXL2 expression compared with those observed in the other two groups. Because Loxl2 is reported to regulate differentiation in multiple cell lineages, such as epithelium47 and chondrocytes,48 our findings suggest that lovastatin might modulate gingival epithelial cell differentiation through down-regulation of LOXL2 expression. Any potential functional role of LOXL2 in mediating phenytoin-induced gingival overgrowth clearly requires additional study.
In summary, we report the development of a novel mouse model for phenytoin-induced gingival overgrowth with continuous and accurate drug delivery that replicates many of the morphological and molecular characteristics of phenytoin-induced gingival overgrowth in humans. Moreover, we report the use of this model to evaluate the effectiveness of a statin drug, which, based on earlier in vitro studies, predicted that lovastatin would be effective at attenuating phenytoin-induced gingival overgrowth.3,4 Molecular profiles obtained are consistent with EMT playing a role in the development of gingival overgrowth, and expression of these markers and tissue overgrowth appeared by the methods used to be either attenuated or normalized by lovastatin. Evaluation of the phenytoin-induced gingival overgrowth mouse model at both the RNA and protein levels at earlier and later time points and with different doses of drugs may allow us to better understand the temporal and spatial associations of the molecular markers of EMT and fibrosis. As noted, data so far point to the possibility that early signals in gingival overgrowth development may emanate from the epithelium. Using the same drug delivery system, mouse models for ciclosporin or nifedipine-induced gingival overgrowth may, in addition, be highly informative.
Acknowledgments
We thank Dr. Arthur F. Stucchi (Boston Medical Center) and Dr. Roozbeh Khosravi for expert technical advice and assistance with the ALZET minipump implantations and Debashree Saxena and Manish Bais for assistance with determining LOXL2 antibody specificity.
Footnotes
Supported by NIH/National Institute of Dental and Craniofacial Research grant R01DE011004 (P.C.T.) and by a scholarship from the Faculty of Dentistry, King Abdulaziz University, Ministry of Higher Education, Saudi Arabia (M.A.A.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2015.02.004.
Supplemental Data
Description of antibody specificities. A: Sections from the gingiva of phenytoin-treated mice probed with anti–transforming growth factor (TGF)-β1 alone or preabsorbed with blocking peptide. B: Western blot from human gingival epithelial cell lysates probed with anti–E-cadherin antibody. C: Human gingival fibroblasts were treated with or without 5 ng/mL TGF-β1 for 6 hours, extracted into SDS-PAGE buffer, and then probed with CCN2 antibody, revealing the expected glycosylated and nonglycosylated forms of CCN2 only in lysates from the TGF-β1–treated cells, as expected. D: Western blots from human gingival cell lysates transduced with empty shRNA, nontarget shRNA, or LOXL2 shRNA and probed with the same LOXL2 antibody used in Figures 6 and 7.
Histomorphometric data of the upper anterior area. A: Representative sections reveal comparisons between sagittal sections of mouse maxillary anterior areas obtained from three different mouse control groups (saline, lovastatin, and vehicle groups, respectively) stained with hematoxylin-eosin (three sections per area per mouse). B: Quantification of maxillary anterior histomorphometric data. These results were drawn from a sample size of n = 6 saline (black), n = 4 lovastatin (white), and n = 3 vehicle (gray) mice. CT, connective tissue; D, dentin; EP, epithelium.
References
- 1.Ramon Y., Behar S., Kishon Y., Engelberg I.S. Gingival hyperplasia caused by nifedipine–a preliminary report. Int J Cardiol. 1984;5:195–206. doi: 10.1016/0167-5273(84)90145-1. [DOI] [PubMed] [Google Scholar]
- 2.Rateitschak-Pluss E.M., Hefti A., Lortscher R., Thiel G. Initial observation that cyclosporin-A induces gingival enlargement in man. J Clin Periodontol. 1983;10:237–246. doi: 10.1111/j.1600-051x.1983.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 3.Black S.A., Jr., Palamakumbura A.H., Stan M., Trackman P.C. Tissue-specific mechanisms for CCN2/CTGF persistence in fibrotic gingiva: interactions between cAMP and MAPK signaling pathways, and prostaglandin E2-EP3 receptor mediated activation of the c-JUN N-terminal kinase. J Biol Chem. 2007;282:15416–15429. doi: 10.1074/jbc.M610432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black S.A., Jr., Trackman P.C. Transforming growth factor-beta1 (TGFbeta1) stimulates connective tissue growth factor (CCN2/CTGF) expression in human gingival fibroblasts through a RhoA-independent, Rac1/Cdc42-dependent mechanism: statins with forskolin block TGFbeta1-induced CCN2/CTGF expression. J Biol Chem. 2008;283:10835–10847. doi: 10.1074/jbc.M710363200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sume S.S., Kantarci A., Lee A., Hasturk H., Trackman P.C. Epithelial to mesenchymal transition in gingival overgrowth. Am J Pathol. 2010;177:208–218. doi: 10.2353/ajpath.2010.090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uzel M.I., Kantarci A., Hong H.H., Uygur C., Sheff M.C., Firatli E., Trackman P.C. Connective tissue growth factor in drug-induced gingival overgrowth. J Periodontol. 2001;72:921–931. doi: 10.1902/jop.2001.72.7.921. [DOI] [PubMed] [Google Scholar]
- 7.Alegret M., Silvestre J.S. Pleiotropic effects of statins and related pharmacological experimental approaches. Methods Find Exp Clin Pharmacol. 2006;28:627–656. doi: 10.1358/mf.2006.28.9.1003573. [DOI] [PubMed] [Google Scholar]
- 8.Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582. [PubMed] [Google Scholar]
- 9.Hong H.H., Uzel M.I., Duan C., Sheff M.C., Trackman P.C. Regulation of lysyl oxidase, collagen, and connective tissue growth factor by TGF-beta1 and detection in human gingiva. Lab Invest. 1999;79:1655–1667. [PubMed] [Google Scholar]
- 10.Van Bruaene N.N., Derycke L.L., Perez-Novo C.A.C., Gevaert P.P., Holtappels G.G., De Ruyck N.N., Cuvelier C.C., Van Cauwenberge P.P., Bachert C.C. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–259. doi: 10.1016/j.jaci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Millanes-Romero A., Herranz N., Perrera V., Iturbide A., Loubat-Casanovas J., Gil J., Jenuwein T., Garcia de Herreros A., Peiro S. Regulation of heterochromatin transcription by Snail1/LOXL2 during epithelial-to-mesenchymal transition. Mol Cell. 2013;52:746–757. doi: 10.1016/j.molcel.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Bueno G., Salvador F., Martín A., Floristán A., Cuevas E.P., Santos V., Montes A., Morales S., Castilla M.A., Rojo-Sebastián A., Martínez A., Hardisson D., Csiszar K., Portillo F., Peinado H., Palacios J., Cano A. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011;3:528–544. doi: 10.1002/emmm.201100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassell T.M., Hefti A.F. Drug-induced gingival overgrowth: old problem, new problem. Crit Rev Oral Biol Med. 1991;2:103–137. doi: 10.1177/10454411910020010201. [DOI] [PubMed] [Google Scholar]
- 14.Trackman P.C., Kantarci A. Connective tissue metabolism and gingival overgrowth. Crit Rev Oral Biol Med. 2004;15:165–175. doi: 10.1177/154411130401500305. [DOI] [PubMed] [Google Scholar]
- 15.Kapur R.N., Girgis S., Little T.M., Masotti R.E. Diphenylhydantoin-induced gingival hyperplasia: its relationship to dose and serum level. Dev Med Child Neurol. 2008;15:483–487. doi: 10.1111/j.1469-8749.1973.tb05070.x. [DOI] [PubMed] [Google Scholar]
- 16.Seymour R.A., Ellis J.S., Thomason J.M. Risk factors for drug-induced gingival overgrowth. J Clin Periodontol. 2000;27:217–223. doi: 10.1034/j.1600-051x.2000.027004217.x. [DOI] [PubMed] [Google Scholar]
- 17.Modéer T., Dahllöf G., Otteskog P. The effect of the phenytoin metabolite p-HPPH on proliferation of gingival fibroblasts in vitro. Acta Odontol Scand. 1982;40:353–357. doi: 10.3109/00016358209024080. [DOI] [PubMed] [Google Scholar]
- 18.Salo T., Oikarinen K.S., Oikarinen A.I. Effect of phenytoin and nifedipine on collagen gene expression in human gingival fibroblasts. J Oral Pathol Med. 1990;19:404–407. doi: 10.1111/j.1600-0714.1990.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 19.Kantarci A., Nseir Z., Kim Y.-S., Sume S.S., Trackman P.C. Loss of basement membrane integrity in human gingival overgrowth. J Dent Res. 2011;90:887–893. doi: 10.1177/0022034511404703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heijl L., Sundin Y. Nitrendipine-induced gingival overgrowth in dogs. J Periodontol. 1989;60:104–112. doi: 10.1902/jop.1989.60.2.104. [DOI] [PubMed] [Google Scholar]
- 21.Seibel W., Yahia N.A., McCleary L.B., Lesko L.J., Hassell T.M. Cyclosporine-induced gingival overgrowth in beagle dogs. J Oral Pathol Med. 1989;18:240–245. doi: 10.1111/j.1600-0714.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanno C.M., Oliveira J.A., Garcia J.F., Castro A.L., Crivelini M.M. Effects of cyclosporin, phenytoin, and nifedipine on the synthesis and degradation of gingival collagen in tufted capuchin monkeys (Cebus apella): histochemical and MMP-1 and -2 and collagen I gene expression analyses. J Periodontol. 2008;79:114–122. doi: 10.1902/jop.2008.070267. [DOI] [PubMed] [Google Scholar]
- 23.Hassell T.M., Roebuck S., Page R.C., Wray S.H. Quantitative histopathologic assessment of developing phenytoin-induced gingival overgrowth in the cat. J Clin Periodontol. 1982;9:365–372. doi: 10.1111/j.1600-051x.1982.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 24.Fu E., Nieh S., Chang H.L., Wang S.L. Dose-dependent gingival overgrowth induced by cyclosporin in rats. J Periodontol. 1995;66:594–598. doi: 10.1902/jop.1995.66.7.594. [DOI] [PubMed] [Google Scholar]
- 25.Ishida H., Kondoh T., Kataoka M., Nishikawa S., Nakagawa T., Morisaki I., Kido J.-I., Oka T., Nagata T. Factors influencing nifedipine-induced gingival overgrowth in rats. J Periodontol. 1995;66:345–350. doi: 10.1902/jop.1995.66.5.345. [DOI] [PubMed] [Google Scholar]
- 26.Nieh S., Fu E., Chang H.L., Wang S.L., Wikesjö U.M. Histopathologic alterations of periodontium in cyclosporin-treated rats. Is the periodontium a target tissue for the drug? J Clin Periodontol. 1996;23:730–736. doi: 10.1111/j.1600-051x.1996.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa S., Nagata T., Morisaki I., Oka T., Ishida H. Pathogenesis of drug-induced gingival overgrowth: a review of studies in the rat model. J Periodontol. 1996;67:463–471. doi: 10.1902/jop.1996.67.5.463. [DOI] [PubMed] [Google Scholar]
- 28.Asahara Y., Nishimura F., Yamada H., Naruishi K., Kataoka M., Kido J-i, Nagata T., Murayama Y. Mast cells are not involved in the development of cyclosporin A-induced gingival hyperplasia: a study with mast cell-deficient mice. J Periodontol. 2000;71:1117–1120. doi: 10.1902/jop.2000.71.7.1117. [DOI] [PubMed] [Google Scholar]
- 29.Ashrafi S.H., Atassi B., Erickson R., Sabet T. Migration of epithelium during phenytoin-dependent gingival overgrowth in mice. Scanning Microsc. 1993;7:1247–1253. [PubMed] [Google Scholar]
- 30.Meller A.T., Rumjanek V.M., Sansone C., Allodi S. Oral mucosa alterations induced by cyclosporin in mice: morphological features. J Periodontal Res. 2002;37:412–415. doi: 10.1034/j.1600-0765.2002.01002.x. [DOI] [PubMed] [Google Scholar]
- 31.Hallmon W.W., Rossmann J.A. The role of drugs in the pathogenesis of gingival overgrowth: a collective review of current concepts. Periodontol 2000. 1999;21:176–196. doi: 10.1111/j.1600-0757.1999.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 32.Marshall R.I., Bartold P.M. A clinical review of drug-induced gingival overgrowths. Aust Dent J. 1999;44:219–232. doi: 10.1111/j.1834-7819.1999.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 33.Bhowmick N.A., Zent R., Ghiassi M., McDonnell M., Moses H.L. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 34.Zeisberg M., Hanai J.-I., Sugimoto H., Mammoto T., Charytan D., Strutz F., Kalluri R. BMP-7 counteracts TGF-β1–induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 35.Liu C., Wu Z., Sun H.-C. The effect of simvastatin on mRNA expression of transforming growth factor-beta1, bone morphogenetic protein-2 and vascular endothelial growth factor in tooth extraction socket. Int J Oral Sci. 2009;1:90–98. doi: 10.4248/ijos.08011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song C.Y., Kim B.C., Lee H.S. Lovastatin inhibits oxidized low-density lipoprotein-induced plasminogen activator inhibitor and transforming growth factor-β1 expression via a decrease in Ras/extracellular signal-regulated kinase activity in mesangial cells. Transl Res. 2008;151:27–35. doi: 10.1016/j.trsl.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 37.He M., Kubo H., Ishizawa K., Hegab A.E., Yamamoto Y., Yamamoto H., Yamaya M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1427–L1436. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 38.Moll R., Mitze M., Frixen U.H., Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143:1731–1742. [PMC free article] [PubMed] [Google Scholar]
- 39.Nam J.-S., Ino Y., Sakamoto M., Hirohashi S. Ras farnesylation inhibitor FTI-277 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Jpn J Cancer Res. 2002;93:1020–1028. doi: 10.1111/j.1349-7006.2002.tb02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lozano E., Betson M., Braga V.M.M. Tumor progression: small GTPases and loss of cell-cell adhesion. Bioessays. 2003;25:452–463. doi: 10.1002/bies.10262. [DOI] [PubMed] [Google Scholar]
- 41.Golper T.A., Illingworth D.R., Morris C.D., Bennett W.M. Lovastatin in the treatment of multifactorial hyperlipidemia associated with proteinuria. Am J Kidney Dis. 1989;13:312–320. doi: 10.1016/s0272-6386(89)80038-1. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi A., Nashiro K., Kikuchi K., Sato S., Ihn H., Fujimoto M., Grotendorst G.R., Takehara K. Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. J Invest Dermatol. 1996;106:729–733. doi: 10.1111/1523-1747.ep12345771. [DOI] [PubMed] [Google Scholar]
- 43.Ito Y., Aten J., Bende R.J., Oemar B.S., Rabelink T.J., Weening J.J., Goldschmeding R. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 44.Sonnylal S., Shi-Wen X., Leoni P., Naff K., Van Pelt C.S., Nakamura H., Leask A., Abraham D., Bou-Gharios G., de Crombrugghe B. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akiri G., Sabo E., Dafni H., Vadasz Z., Kartvelishvily Y., Gan N., Kessler O., Cohen T., Resnick M., Neeman M., Neufeld G. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- 46.Barry-Hamilton V., Spangler R., Marshall D., McCauley S., Rodriguez H.M., Oyasu M., Mikels A., Vaysberg M., Ghermazien H., Wai C., Garcia C.A., Velayo A.C., Jorgensen B., Biermann D., Tsai D., Green J., Zaffryar-Eilot S., Holzer A., Ogg S., Thai D., Neufeld G., Van Vlasselaer P., Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 47.Peinado H., del Carmen Iglesias-de la Cruz M., Olmeda D., Csiszar K., Fong K.S.K., Vega S., Nieto M.A., Cano A., Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in Snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iftikhar M., Hurtado P., Bais M.V., Wigner N., Stephens D.N., Gerstenfeld L.C., Trackman P.C. Lysyl oxidase-like-2 (LOXL2) is a major isoform in chondrocytes and is critically required for differentiation. J Biol Chem. 2011;286:909–918. doi: 10.1074/jbc.M110.155622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of antibody specificities. A: Sections from the gingiva of phenytoin-treated mice probed with anti–transforming growth factor (TGF)-β1 alone or preabsorbed with blocking peptide. B: Western blot from human gingival epithelial cell lysates probed with anti–E-cadherin antibody. C: Human gingival fibroblasts were treated with or without 5 ng/mL TGF-β1 for 6 hours, extracted into SDS-PAGE buffer, and then probed with CCN2 antibody, revealing the expected glycosylated and nonglycosylated forms of CCN2 only in lysates from the TGF-β1–treated cells, as expected. D: Western blots from human gingival cell lysates transduced with empty shRNA, nontarget shRNA, or LOXL2 shRNA and probed with the same LOXL2 antibody used in Figures 6 and 7.
Histomorphometric data of the upper anterior area. A: Representative sections reveal comparisons between sagittal sections of mouse maxillary anterior areas obtained from three different mouse control groups (saline, lovastatin, and vehicle groups, respectively) stained with hematoxylin-eosin (three sections per area per mouse). B: Quantification of maxillary anterior histomorphometric data. These results were drawn from a sample size of n = 6 saline (black), n = 4 lovastatin (white), and n = 3 vehicle (gray) mice. CT, connective tissue; D, dentin; EP, epithelium.