Abstract
If diagnosed at early stages, patients with hepatocellular carcinoma (HCC) can receive curative therapies, whereas therapeutic options at later stages are very limited. Here, we addressed the potential of soluble Axl (sAxl) as a biomarker of early HCC by analyzing levels of sAxl in 311 HCC and 237 control serum samples from centers in Europe and China. Serum concentrations of sAxl were significantly increased in HCC (18.575 ng/mL) as compared to healthy (13.388 ng/mL) or cirrhotic (12.169 ng/mL) controls. Receiver operating characteristic curve analysis of sAxl in very early stage HCC patients (BCLC 0) showed an area under the curve (AUC) of 0.848, with a sensitivity of 76.9% and a specificity of 69.2%. α-Fetoprotein (AFP)-negative HCC patients displayed an AUC of 0.803, with sensitivity and specificity of 73% and 70.8%. Combination of sAxl and AFP improved diagnostic accuracy to 0.936 in very early HCC patients and to 0.937 in all HCC. Differential diagnosis of very early HCC versus liver cirrhosis showed a combined performance for sAxl and AFP of 0.901 with a sensitivity of 88.5% and a specificity of 76.7%. Furthermore, sAxl levels failed to be elevated in primary ovarian, colorectal and breast carcinomas as well as in secondary hepatic malignancies derived from colon. In summary, sAxl outperforms AFP in detecting very early HCC as compared to healthy or cirrhotic controls and shows high diagnostic accuracy for AFP-negative patients. sAxl is specific for HCC and suggested as a biomarker for routine clinical use.
Keywords: soluble Axl, hepatocellular carcinoma, biomarker, α-fetoprotein
Hepatocellular carcinoma (HCC) is the most frequently diagnosed liver malignancy and the third most common cause of cancer-related mortality worldwide.1 Even though patients with early HCC achieve a 5-year survival rate of 70% after liver resection or transplantation, the majority of tumors are diagnosed at advanced stages, leading to a median survival of <1 year.2,3
Various screening procedures such as abdominal ultrasonography or measurement of serum α-fetoprotein (AFP) have been implemented for high-risk patients to detect HCC at an early stage. However, ultrasound exhibits only moderate sensitivity of 60%, which is highly dependent on operator experience. With respect to AFP, sensitivity ranges from only 25% to 65% with limited specificity.4,5 Consequently, several new biomarkers have been suggested to increase the accuracy of early HCC detection, such as des-γ-carboxyprothrombin (DCP), lectin-bound AFP (AFP-L3%) and Dickkopf-1 (DKK1). Reports about the performance of these markers are conflicting and a recent study has identified DKK1 to be more sensitive as compared to DCP and AFP-L3% in detecting early HCC.6–8 In addition, combination of AFP, DCP and AFP-L3% only modestly increases sensitivity as compared to AFP alone, while DKK1 together with AFP displayed higher performance. These prototypic examples highlight the need for more reliable biomarkers.9,10
The receptor tyrosine kinase Axl has been implicated in several pathological conditions, including cancer. Axl expression is upregulated in many tumor types, such as breast, lung, brain and liver cancer and correlates with poor prognosis and metastasis in lung and breast cancer as well as in mesothelioma.11–13 Axl is activated by the binding of its ligand growth-arrest specific protein 6 to the extracellular domain (ECD) leading to subsequent phosphorylation of downstream targets. Interestingly, the ECD can be proteolytically processed, possibly by matrix metalloproteinases, resulting in the release of an 80 kDa soluble protein (sAxl) that can be detected in serum.14,15
In this study, we show that the expression of total Axl correlates with the release of sAxl in human hepatoma cell lines. In a retrospective multicenter analysis, we further assessed sAxl levels in sera from 311 HCC, 10 breast cancer, 10 ovarian cancer and 62 colorectal cancer patients as well as 125 healthy and 30 cirrhotic controls from centers in Europe and China. The setting allowed us to evaluate the accuracy of sAxl as a biomarker of early stage HCC and to investigate the relationship of sAxl concentrations with clinicopathological parameters.
Patients and Methods
Study population
Serum samples from HCC patients (n = 311) as well as healthy (n = 125) and cirrhotic (n = 30) controls were collected in the Eastern Hepatobiliary Surgery Hospital (Shanghai, China; HCC, n = 171; healthy controls, n = 66), the Vienna General Hospital (Vienna, Austria; HCC, n = 18; healthy controls, n = 31; cirrhotic controls, n = 30) and the Masaryk Memorial Cancer Institute (Brno, Czech Republic; HCC, n = 22; healthy controls, n = 9) from 2011 to 2013 as well as in the Li Ka Shing Faculty of Medicine (Hong Kong, SAR; HCC, n = 100; healthy controls, n = 20) from 1999 to 2001 (Supporting Information Fig. S1). AFP levels were determined at time of diagnosis via enzyme-linked immunosorbent assay (ELISA). In addition, serum samples from breast (n = 10), ovarian (n = 10) and colorectal (n = 62) cancer (CRC) patients were obtained. All samples were collected prior to therapeutic intervention, with the exception of those from Brno, where 17 patients were included that have undergone treatment but still exhibited stable or progressing disease. For 11 of these patients, multiple samples were collected at different time points ranging from two months to two years post diagnosis. Samples from Vienna were partially collected as plasma into anticoagulant-coated tubes (13 of 18 samples). All samples were centrifuged to eliminate cellular components and stored at −80 °C until testing. The study protocol was approved by the Chinese, Austrian as well as Czech Ethics Committees. Informed consent was obtained both from patients and healthy controls. All patients were diagnosed by ultrasound, computed tomography or magnetic resonance imaging, AFP and liver enzyme serology, and histopathologically confirmed by two individual board certified pathologists after surgical resection. Patients with liver malignancies of different cellular origin, such as cholangiocellular carcinomas were excluded. Age- and sex-matched healthy controls were recruited from routine physical examination. Exclusion criteria were alterations in liver serology, viral or nonviral liver disease, as well as other malignancies. Cirrhotic controls were histopathologically confirmed and screened for tumor formation by ultrasound, computed tomography or magnetic resonance imaging. Clinical information about age, gender, tumor/node/metastasis (TNM) stage, cirrhosis, hepatitis virus infection, tumor size, number of tumors, vascular involvement, lymph node metastasis and AFP level determined at diagnosis was available (Supporting Information Table S1). Follow-up survival data was available for 122 patients with HCC. In the case of CRC, liver metastasis status was known and positive in 52 of 62 patients. We classified patients into very early, early and advanced HCC according to the established Barcelona Clinic Liver Cancer (BCLC) classification. Very early HCC (n = 26) was defined as BCLC stage 0 (single nodule < 2 cm) and early HCC (n = 78) as BCLC stage A (single nodule < 5 cm or 3 nodules < 3 cm). BCLC stage B, C and D (large, multiple nodules, vascular invasion or extrahepatic secondary tumors) were classified as advanced HCC (n = 200).16 Seven HCC cases remained unclassified due to missing data.
Enzyme-linked immunosorbent assay
Sandwich ELISAs for human sAxl in sera were carried out from December 2012 to October 2013 according to the manufacturer’s protocol (R&D Systems, Minneapolis, USA). Details are provided in the Supporting Information.
Cell culture
Details of culturing human hepatoma cell lines and detection of sAxl and total Axl in cell culture are provided in the Supporting Information.
Receiver operating characteristics
Receiver operating characteristic (ROC) curves were generated by plotting sensitivity against the false positive rate for sAxl and AFP using IBM SPSS software v20.0 (IBM Corp., Armonk, USA). In addition, a variable combining both markers was generated by binary logistic regression through an iterative maximum likelihood procedure, according to the equation:
| (1) |
Equations for all comparisons are provided in Supporting Information Table S2. Diagnostic performance was evaluated by ROC curve analysis and quantified using the area under the curve (AUC) with 95% confidence interval (CI). Optimal cutoff values for sAxl were selected at concentrations exhibiting the highest sum of sensitivity and specificity (Youden Index (J)). For AFP, the clinically well-established cutoff value of 20 ng/mL was used.8,10,17,18
Statistical analysis
Datasets were compared using IBM SPSS software v20.0 (IBM Corp., Armonk, USA) and Medcalc version 12.5 (Med-Calc Software, Belgium). For continuous data, nonparametric, two-sided Mann–Whitney U tests were used for single comparisons and nonparametric Kruskal–Wallis tests with Dunn’s multiplicity correction for multiple comparisons. Two-sided Fisher’s exact tests were used for categorical data. Survival curves were compared with the Gehan–Breslow–Wilcoxon test. p Values < 0.05, p < 0.01, p < 0.001 or p < 0.0001 were considered statistically significant.
Results
Axl expressing HCC cell lines produce sAxl
We examined established human HCC cell lines for expression of total Axl in cell extracts and for release of sAxl into cell culture supernatants by ELISA. Well differentiated 3p, HepG2, HuH6 and HuH7 hepatoma cells displayed low to undetectable amounts of Axl (Supporting Information Fig. S2a). In contrast, 7 out of 11 hepatoma cell lines (64%) exhibited significant expression of Axl, which was highest in poorly differentiated SNU423 and SNU449 cells. By comparison of total Axl and sAxl levels, we observed a close correlation of Axl expression and sAxl release in almost all HCC cell lines (Supporting Information Fig. S2a). Ten out of 11 HCC cell lines showed closely corresponding Axl and sAxl values, while only SNU398 cells revealed a slight decrease of sAxl concentration relative to Axl expression (Supporting Information Fig. S2a). As expected, cells that failed to express significant Axl were devoid of sAxl production. Together, these data provide strong evidence that sAxl levels reflect total Axl expression in human hepatoma cells (Supporting Information Fig. S2b).
High sAxl levels in sera of HCC patients
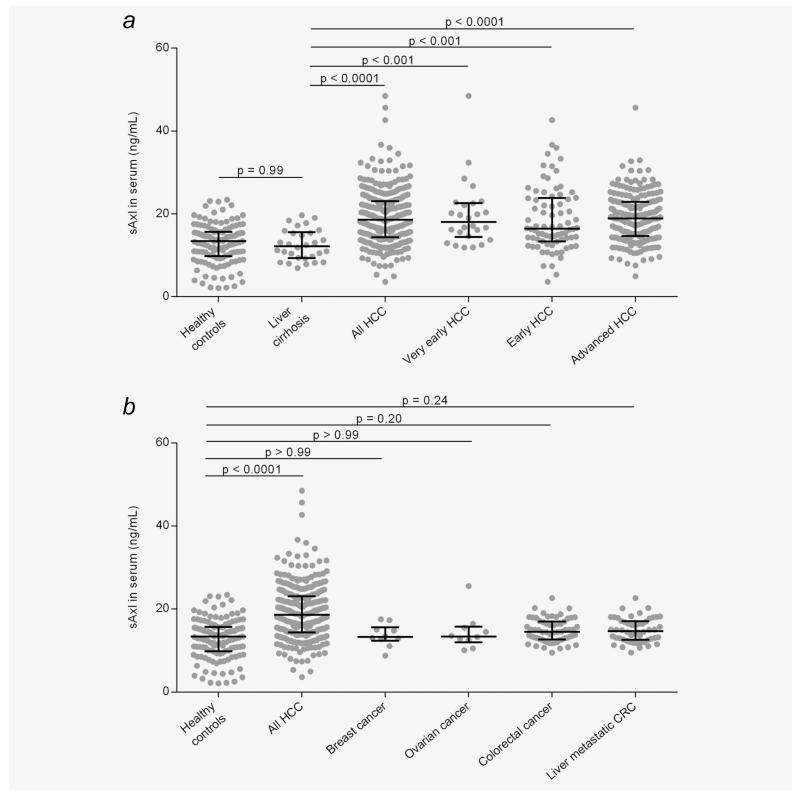
Next, we addressed the question whether enhanced sAxl levels can be detected in HCC patients, since elevated Axl expression has been reported in primary HCC.19 Therefore, sera of 311 HCC patients were analyzed for sAxl levels by ELISA. Anticoagulant-treated blood samples from Vienna (13 out of 18) did not show any alteration in sAxl levels as compared to serum samples, confirming previous findings (Supporting Information Fig. S3).14 Patients were grouped into very early, early and advanced HCC according to established BCLC criteria. HCC patients exhibiting sAxl concentrations above the median value (18.575 ng/mL) were considered “high sAxl” cases, whereas lower concentrations were classified as “low sAxl.” Cirrhotic controls did not display significantly higher sAxl concentrations (12.169 ng/mL) as compared to healthy controls (13.388 ng/mL). Importantly, significantly increased median levels of sAxl were found in all HCC (18.575 ng/mL), very early HCC (18.064 ng/mL) and early HCC (16.430 ng/mL) as compared to healthy or cirrhotic controls (Fig. 1a and Supporting Information Table S3). A further rise in sAxl levels was observed in late HCC (18.880 ng/mL). The increase in HCC patients was significant across all centers included in this study (Supporting Information Fig. S4 and Table S3; Shanghai, 16.82 ng/mL; Hong Kong, 20.03 ng/mL; Brno, 19.95 ng/mL; Vienna 17.08 ng/mL). No changes in sAxl amounts could be determined in HCC with different status of hepatitis B, hepatitis C or cirrhosis (Table 1). In addition, sAxl serum concentrations were assessed in a cohort of breast, ovarian and CRC patients. Notably, sAxl serum levels remained unchanged in patients suffering from these carcinomas as compared to healthy controls. Importantly, no changes in serum sAxl were detected in CRC patients exhibiting liver metastases (Fig. 1b). In addition, significant differences in sAxl concentrations were detected between HCC depending on the presence or absence of vessel invasion or lymph node metastasis (Supporting Information Fig. S5; Table 1). In particular, 54.9% of HCC accompanied by vascular invasion exhibited high levels of sAxl, while 58.6% of noninvasive HCC cases showed low sAxl (Supporting Information Fig. S5a; Table 1). Similarly, 70.6% of HCC patients with lymph node metastasis showed augmented sAxl levels, whereas 54.7% of patients without spreading into lymph nodes exhibited low sAxl (Supporting Information Fig. S5b; Table 1). These data suggest that sAxl levels specifically detect very early, early and late stage HCC in patients’ sera, either alone or associated with invasion into blood vessels or lymph nodes.
Figure 1.
sAxl levels in HCC patients. (a) sAxl serum concentrations in healthy controls (n = 125), cirrhotic controls (n = 30) and patient samples with HCC (n = 311) or very early HCC (BCLC 0; n = 26), early HCC (BCLC A; n = 78) or advanced HCC (BCLC > A; n = 200) as assessed by ELISA. Horizontal bars indicate median levels with interquartile ranges. (b) Correlation of sAxl release with other cancer types. HCC, hepatocellular carcinoma; CRC, colorectal carcinoma.
Table 1.
Correlation of sAxl serum levels with various clinicopathological parameters
| sAxl |
||||||
|---|---|---|---|---|---|---|
| Variable | Number of cases | High | Low | OR | CI 95% | P |
| Age (years) | ||||||
| <55 | 162 | 73 (45.1%) | 89 (54.9%) | 1.497 | 0.939–2.388 | 0.098 |
| ≥55 | 127 | 70 (55.1%) | 57 (44.9%) | |||
| Gender | ||||||
| Male | 256 | 125 (48.8%) | 131 (51.2%) | 1.354 | 0.753–2.434 | 0.373 |
| Female | 55 | 31 (56.4%) | 24 (43.6%) | |||
| HBV status | ||||||
| Negative | 33 | 13 (39.4%) | 20 (60.6%) | 1.578 | 0.750–3.318 | 0.267 |
| Positive | 237 | 120 (50.6%) | 117 (49.4%) | |||
| HCV status | ||||||
| Negative | 275 | 137 (49.8%) | 138 (50.2%) | 0.863 | 0.283–2.635 | 1.000 |
| Positive | 13 | 6 (46.2%) | 7 (53.8%) | |||
| Cirrhosis | ||||||
| No | 53 | 18 (34.0%) | 35 (66.0%) | 1.734 | 0.906–3.318 | 0.110 |
| Yes | 157 | 74 (47.1%) | 83 (52.9%) | |||
| Vascular invasion | ||||||
| No | 145 | 60 (41.4%) | 85 (58.6%) | 1.728 | 1.018–2.932 | 0.045 |
| Yes | 91 | 50 (54.9%) | 41 (45.1%) | |||
| Lymph node metastasis | ||||||
| No | 256 | 116 (45.3%) | 140 (54.7%) | 2.897 | 1.511–5.552 | 0.001 |
| Yes | 51 | 36 (70.6%) | 15 (29.4%) | |||
CI, confidence interval; OR, odds ratio; HBV, hepatitis B virus; HCV, hepatitis C virus.
low sAxl < 18.575 ng/mL < high sAxl.
High diagnostic accuracy of sAxl in very early HCC and AFP-negative HCC patients
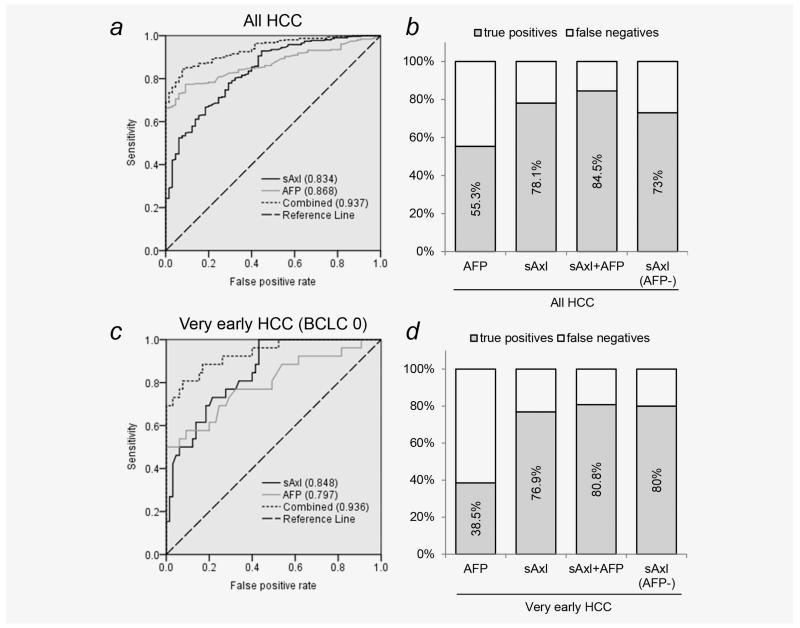
We further assessed the diagnostic value of sAxl in HCC by comparison with the established serum marker AFP. ROC curve analysis revealed a comparable diagnostic performance of sAxl (AUC 0.834) and AFP (AUC 0.868) in all HCC patients, whereas sensitivity was higher for sAxl (78.1%) at the optimal cutoff of 14.053 ng/mL as compared to AFP (55.3%) at the clinically used cutoff of 20 ng/mL (Figs. 2a and 2b; Table 2). Again, diagnostic performance of sAxl was high across all centers included in this study (Shanghai, AUC 0.789; Hong Kong, AUC, 0.901; Brno, AUC 0.866; Vienna AUC 0.854; Supporting Information Figs. S4b–S4e). Remarkably, sAxl outperformed AFP in detecting very early HCC (sAxl, AUC 0.848; AFP, AUC 0.797). Again, sensitivity of sAxl was much higher (76.9%) than that of AFP (38.5%; Figs. 2c and 2d; Table 2). To assess the combination of both markers, the predicted probability was calculated via binary logistic regression. Combined analysis of sAxl and AFP revealed an exceptional accuracy of 0.937 with a sensitivity of 84.5% and a specificity of 92.3% in detecting HCC (Figs. 2a and 2b; Table 2). This was shown to be valid throughout all stages, with AUC 0.936 in very early HCC, AUC 0.921 in early HCC and AUC 0.943 in advanced stage HCC (Fig. 2c and Supporting Information Fig. S6). In AFP-negative HCC, sAxl was also indicated as a valid marker for HCC detection (AUC 0.803; Supporting Information Fig. S6) with a sensitivity of 73% and a specificity of 70.8% (Fig. 2b; Table 2), allowing to overcome the absence of the diagnostic marker AFP. Among very early, AFP-negative patients, sAxl showed even higher sensitivity of 80% and specificity of 69.2% (Fig. 2d; Supporting Information Fig. S6 and Table 2).
Figure 2.
Detection of HCC by sAxl. (a) ROC curve of AFP, sAxl and a combination of both in healthy controls (n = 65) versus HCC patients (n = 311). Numbers in parentheses represent the area under the curve. (b) True positive rate of AFP, sAxl or a combination of both in all HCC and of sAxl in AFP-negative HCC. Diagnostic cutoff for AFP was 20 ng/mL. Diagnostic cutoff for sAxl was 14.053. (c) ROC curves of AFP, sAxl or both in very early HCC patients (n = 26). (d) True positive rate of AFP, sAxl or both in very early HCC and of sAxl in very early, AFP-negative HCC.
Table 2.
Performance of sAxl and AFP in the detection of HCC
| AUC (95% CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden’s index | |
|---|---|---|---|---|---|---|
| All HCC vs. HC | ||||||
| AFP | 0.868 (0.829–0.900) | 55.3 | 100 | 100 | 69.1 | 0.55 |
| sAxl | 0.834 (0.792–0.870) | 78.1 | 70.8 | 72.8 | 76.4 | 0.49 |
| sAxl+AFP | 0.937 (0.907–0.959) | 84.5 | 92.3 | 91.6 | 85.6 | 0.77 |
| Very early HCC vs. HC | ||||||
| AFP | 0.797 (0.699–0.874) | 38.5 | 100 | 100 | 61.9 | 0.39 |
| sAxl | 0.848 (0.757–0.914) | 76.9 | 69.2 | 71.4 | 75.0 | 0.46 |
| sAxl+AFP | 0.936 (0.864–0.976) | 80.8 | 92.3 | 91.3 | 82.8 | 0.73 |
| All AFP negative HCC vs. HC | ||||||
| sAxl | 0.803 (0.741–0.855) | 73 | 70.8 | 71.4 | 72.4 | 0.44 |
| Very early AFP negative HCC vs. HC | ||||||
| sAxl | 0.863 (0.767–0.929) | 80 | 69.2 | 72.2 | 77.6 | 0.49 |
| All HCC vs. LC | ||||||
| AFP | 0.771 (0.710–0.833) | 55.3 | 93.3 | 89.2 | 67.6 | 0.49 |
| sAxl | 0.815 (0.747–0.884) | 78 | 66.7 | 70.1 | 75.2 | 0.45 |
| sAxl+AFP | 0.891 (0.847–0.936) | 85.1 | 80.0 | 81.0 | 84.3 | 0.65 |
| Very early HCC vs. LC | ||||||
| AFP | 0.662 (0.513–0.810) | 42.3 | 93.3 | 86.3 | 61.8 | 0.36 |
| sAxl | 0.838 (0.738–0.939) | 80.8 | 66.7 | 70.8 | 77.6 | 0.48 |
| sAxl+AFP | 0.901 (0.823–0.979) | 88.5 | 76.7 | 79.2 | 87.0 | 0.65 |
| All AFP negative HCC vs. LC | ||||||
| sAxl | 0.780 (0.698–0.861) | 73 | 66.7 | 68.7 | 71.2 | 0.40 |
| Very early AFP negative HCC vs. LC | ||||||
| sAxl | 0.858 (0.746–0.969) | 86.7 | 66.7 | 72.3 | 83.4 | 0.53 |
AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; HCC, hepatocellular carcinoma; AFP, α-fetoprotein; HC, healthy controls; LC, liver cirrhosis.
Diagnostic cutoffs for AFP and sAxl were 20 ng/mL and 14.053 ng/mL, respectively.
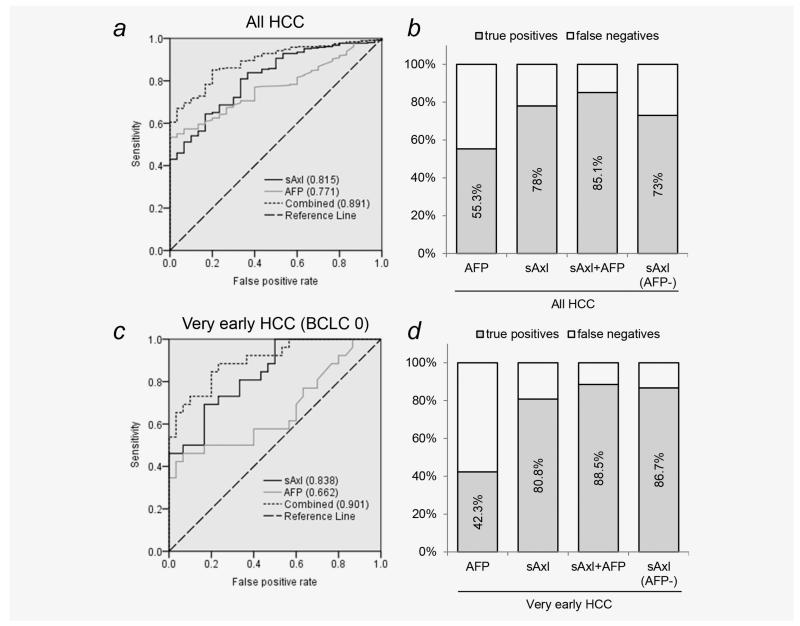
In differential diagnosis of HCC versus liver cirrhosis, sAxl (AUC 0.815) also outperformed AFP (AUC 0.771) and showed increased sensitivity (78%) as compared to AFP (55.3%; Figs. 3a and 3b; Table 2). sAxl also displayed much higher accuracy (AUC 0.838) and sensitivity (80.8%) in discriminating between very early HCC and liver cirrhosis as compared to AFP (AUC 0.662; sensitivity 42.3%; Figs. 3c and 3d; Table 2). Remarkably, combination of both markers enhanced diagnostic accuracy in all HCC (AUC 0.891; sensitivity 85.1%; specificity 80%) and in very early HCC (AUC 0.901; sensitivity 88.5%; specificity 76.7%) vs.cirrhotic controls (Figs. 3a–3d; Table 2). In summary, these data suggest that sAxl is a highly accurate diagnostic marker for very early and AFP-negative HCC, and that sAxl alone or in combination with AFP allows discrimination between very early HCC and liver cirrhosis.
Figure 3.
Discrimination between liver cirrhosis and HCC by sAxl. (a) ROC curve of AFP, sAxl and a combination of both in cirrhotic controls (n = 30) versus HCC patients (n = 311). Numbers in parentheses represent the area under the curve. (b) True positive rate of AFP, sAxl or a combination of both in all HCC and of sAxl in AFP-negative HCC. Diagnostic cutoff for AFP was 20 ng/mL. Diagnostic cutoff for sAxl was 14.053. (c) ROC curves of AFP, sAxl or both in very early HCC patients (n = 26) versus cirrhotic controls. (d) True positive rate of AFP, sAxl or both in very early HCC and of sAxl in very early, AFP-negative HCC.
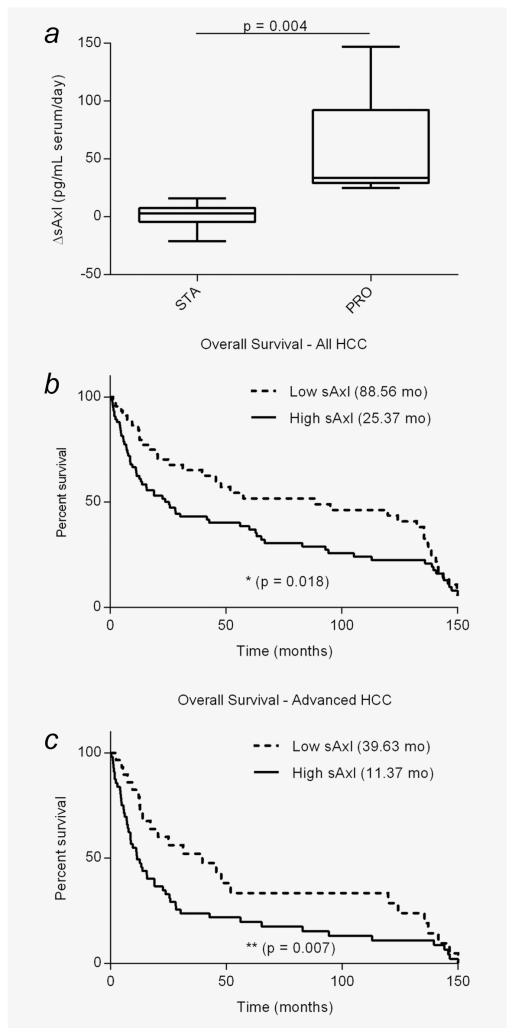
sAxl levels reflect disease progression
We further addressed a prognostic role of sAxl by analyzing samples from different time points after diagnosis of patients that have undergone treatment, ranging from two months to two years. The data revealed a significantly higher rate of change of sAxl levels in patients exhibiting tumor progression (median 33.518 pg/mL/day, n = 5) as compared to those showing stable disease (median 3.06 pg/mL/day, n = 6; Fig. 4a). Furthermore, analysis of patients’ survival was performed by comparison of high sAxl versus low sAxl HCC. Among all HCC stages, patients exhibiting high sAxl show a significantly decreased overall survival (median 25.37 months) as compared to those with low sAxl serum levels (median 88.56 months; Fig. 4b). This decrease was even more pronounced among advanced HCC patients (high sAxl median 11.37 months; low sAxl median 39.63 months; Fig. 4c). These data suggest that sAxl levels reflect disease progression.
Figure 4.
sAxl and survival of HCC patients. (a) Rate of change in sAxl serum levels in patients with stable (n = 6) or progressing disease (n = 5). (b) Kaplan–Meier plot showing the overall survival of all HCC patients with high (> 18.575 ng/mL) and low sAxl serum levels (n = 122). (c) Overall survival among advanced HCC patients exhibiting high/low axl (n = 86). Numbers in brackets represent median survival in months. STA, stable disease; PRO, progressing disease; HCC, hepatocellular carcinoma.
Discussion
Alterations of sAxl levels in human sera have been documented with different outcomes in a number of pathological conditions including cancer.20,21 One study reported a reduction of sAxl in sera of renal cell carcinoma patients as compared to healthy controls, suggesting that the contribution of tumor-released sAxl might be too low to significantly alter total sAxl serum concentrations.20 In contrast, we now show that most cultured HCC cell lines produce sAxl and that sAxl serum levels of HCC patients are significantly higher as compared to healthy and cirrhotic controls, indicating that HCC-derived sAxl is a major contributor to the overall sAxl serum concentration (Fig. 1a). Importantly, cirrhotic controls do not exhibit higher sAxl concentrations as compared to healthy controls and cirrhotic HCC patients show no increase versus noncirrhotic patients arguing against a significant contribution of myofibroblast-derived sAxl (Fig. 1a; Table 1). Similarly, patients suffering from breast, ovarian or colorectal cancer show no changes in sAxl levels either, underlining a specific role of sAxl as a biomarker of HCC (Fig. 1b). Remarkably, liver metastasis of colon cancer does not alter sAxl serum levels, allowing a clear discrimination between HCC and secondary hepatic malignancy (Fig. 1b).22
Axl signaling regulates cellular processes relevant for tumorigenesis such as proliferation, survival and chemoresistance as well as those involved in tumor progression including migration and invasion.23 Therefore, multiple Axl-specific functions might be involved in all stages of HCC. Accordingly, we detected increased sAxl levels already in very early as well as in advanced stages HCC.
Due to the lack of suitable biomarkers, most HCCs remain undetected until they reach advanced stages. This greatly reduces treatment options as compared to very early HCC where liver resection and percutaneous ablation are the therapies of choice, leading to a high 5-year survival of 70%.24 Despite its limited performance, AFP has been extensively used as a biomarker for HCC.5 Many diagnostic thresholds for AFP have been proposed, ranging from 10 to 2000 ng/mL. We applied the most commonly used value of 20 ng/mL to mimic the most probable clinical situation.8,10,17,18 Furthermore, we followed a threshold-independent approach by ROC curve analysis. In this context, sAxl exhibits higher performance as compared to AFP in detecting very early HCC. Importantly, combination of both biomarkers shows exceptional accuracy (Fig. 2c; Table 2). Additionally, almost half (45%) of all patients included in this study exhibited AFP levels below the clinically used cutoff and thus would not have been identified. Among very early HCC, this proportion is even worse (58%) and in these patients, sAxl shows high performance in detecting HCC (Fig. 2d; Table 2). It has to be noted that AFP alone exhibits higher specificity (100% in very early HCC) and PPV as compared to sAxl (69.2%). Similarly, the combination of both markers also results in a decrease of specificity (92.3%) as compared to AFP alone; however, this slight reduction represents only a small trade-off as compared to the vast gain in sensitivity (80.8% for sAxl/AFP versus 38.5% for AFP alone in very early HCC), which is highly desired in diagnostic screening procedures.
For monitoring of high-risk groups, accurate differential diagnosis of HCC versus other risk factors, most notably cirrhosis, is desired.25 sAxl shows higher performance as compared to AFP in discriminating between cirrhotic controls and very early HCC, resulting in higher sensitivity. Combination of both markers again leads to an increase in accuracy with very high sensitivity and specificity (Figs. 3c and 3d; Table 2). Since etiology of HCC strongly differs between China and Europe, we recruited patients from both regions. sAxl was shown to be increased in HCC patients from all centers, further underlining its potential as a biomarker of HCC (Supporting Information Fig. S4). Remarkably, the combined median sAxl levels of HCC patients recruited in Asian centers (18.762 ng/mL) did not differ from those in Europe (18.450 ng/mL). Nevertheless, a bias due to differences in etiology is conceivable, as a higher proportion of Chinese HCC patients were included in this study and cirrhotic controls were exclusively collected in Vienna. Thus, these data need to be verified in a prospective study conducted according to the guidelines of highest quality management and including further controls, such as hepatitis and fibrosis patients as well as cirrhotic patients from Chinese centers and additional HCC patients from Europe. With respect to AFP, we observed higher median levels in Chinese patients as compared to Europe. This might be caused by the inclusion of a higher number of very advanced HCC cases (TNM > 5) from Asia, which were completely absent in Europe, possibly due to regional differences in HCC surveillance and stage at diagnosis.26
sAxl concentrations are slightly higher in advanced HCC cases as compared to early HCCs, although not statistically significant. In addition, high sAxl serum concentrations are associated with vascular invasion and lymph node metastasis (Supporting Information Fig. S5; Table 1), suggesting a prominent role of Axl in HCC progression. Accordingly, patients exhibiting high sAxl show decreased overall survival as compared to those having low levels (Figs. 4b and 4c). Furthermore, patients escaping therapy during tumor progression show an increased rate of change in sAxl levels as compared to patients with stable disease (Fig. 4a). Thus, sAxl may also serve as a candidate prognostic and surveillance marker for HCC.
In summary, we report that sAxl shows high sensitivity in detecting early stages of HCC, as compared to AFP alone. Combination of sAxl and AFP further increases performance and shows high accuracy in differential diagnosis between HCC and hepatic cirrhosis. Additionally, sAxl performs well in AFP-negative HCC patients. Therefore, sAxl represents a valuable candidate biomarker for routine screening of very early HCC. As sAxl levels are elevated in very early as well as in advanced HCC, various Axl-mediated functions might be relevant in the different stages of HCC. These findings justify further prospective studies evaluating the diagnostic and prognostic value of sAxl.
Supplementary Material
What’s new?
If diagnosed at early stages, patients with hepatocellular carcinoma (HCC) can receive curative therapies, whereas therapeutic options at later stages are limited. Detection of early stage hepatocellular carcinoma by measuring serum α-fetoprotein (AFP) however exhibits only moderate sensitivity. This study shows that serum concentrations of soluble Axl (sAxl) are increased in very early, early and advanced HCC as well as in AFP-negative HCC patients, as compared to cirrhotic controls. Assessment of sAxl levels allows accurate differential diagnosis of very early HCC versus cirrhosis and other types of cancer, suggesting that sAxl is a promising diagnostic biomarker for routine clinical use.
Acknowledgements
This work was supported by the Austrian Science Fund, FWF, P25356 (W.M.); European Union, FP7 Health Research, HEALTH-F4-2008-202047 (W.M.); China National Key Projects for Infectious Disease, No. 2012ZX10002-016 (C.F.G.); BBMRI_CZ LM2010004 (D.V.), RECAMO CZ.1.05/2.1.00/03.0101 (D.V.), MHCZ DRO MMCI00209805 (D.V.), BioPersMed (COMET K-project 825329), which is funded by the Austrian Federal Ministry of Transport, Innovation and Technology (BMVIT) and the Austrian Federal Min istry of Economy, Family and Youth (BMWFJ) and the Styrian Busi ness Promotion Agency (SFG) (M.T.); and German Research Foundation, SFB-TRR77 (S.D.).
Grant sponsor: Austrian Science Fund, FWF; Grant number: P25356; Grant sponsor: European Union, FP7 Health Research; Grant number: HEALTH-F4-2008-202047; Grant sponsor: China National Key Projects for Infectious Disease; Grant number: 2012ZX10002-016; Grant sponsor: BBMRI_CZ; Grant number: LM2010004; Grant sponsor: RECAMO; Grant number: CZ.1.05/2.1.00/03.0101; Grant sponsor: MHCZ DRO; Grant number: MMCI00209805; Grant sponsor: Austrian Federal Ministry of Transport, Innovation and Technology (BMVIT) and the Austrian Federal Ministry of Economy, Family and Youth (BMWFJ) and the Styrian Business Promotion Agency (SFG); Grant number: BioPersMed COMET K-project 825329; Grant sponsor: German Research Foundation; Grant number: SFB-TRR77
Abbreviations
- AFP
α-fetoprotein
- AFP-L3%
lectin-bound AFP
- AUC
area under the curve
- BCLC
Barcelona Clinic Liver Cancer staging
- BRN
Brno
- CI
confidence interval
- CRC
colorectal carcinoma
- DCP
des-γ-carboxyprothrombin
- DKK1
Dickkopf-1
- ECD
extracellular domain
- ELISA
enzyme-linked immunosorbent assay
- HBV
hepatitis B virus
- HC
healthy control
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HK
Hong Kong
- IQR
inter-quartile range
- J
Youden index
- LC
liver cirrhosis
- LN
lymph node
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- PRO
progressing disease
- ROC
receiver operating characteristics
- sAxl
soluble Axl
- SHG
Shanghai
- STA
stable disease
- TNM
tumor/node/metastasis staging
- VIE
Vienna
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: K.S. received travel grants from Roche, MSD and Novartis as well as speaker honorarium from Roche and Biotest.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the united states from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 4.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul SB, Gulati MS, Sreenivas V, et al. Evaluating patients with cirrhosis for hepatocellular carcinoma: value of clinical symptomatology, imaging and alpha-fetoprotein. Oncology. 2007;72(Suppl 1):117–23. doi: 10.1159/000111717. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Feng Z, Wang Y, et al. Alpha-feto-protein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–8. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durazo FA, Blatt LM, Corey WG, et al. Des-gamma-carboxyprothrombin, α fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–8. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen Q, Fan J, Yang XR, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–26. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 9.Carr BI, Kanke F, Wise M, et al. Clinical evaluation of lens culinaris agglutinin-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin in histologically proven hepatocellular carcinoma in the united states. Dig Dis Sci. 2007;52:776–82. doi: 10.1007/s10620-006-9541-2. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5–10. doi: 10.1177/1756283X10385964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gjerdrum C, Tiron C, Hoiby T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA. 2010;107:1124–9. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa M, Sonobe M, Nakayama E, et al. Higher expression of receptor tyrosine kinase axl, and differential expression of its ligand, Gas6, predict poor survival in lung adenocarcinoma patients. Ann Surg Oncol. 2013;20(Suppl 3):S467–S476. doi: 10.1245/s10434-012-2795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinato DJ, Mauri FA, Lloyd T, et al. The expression of axl receptor tyrosine kinase influences the tumour phenotype and clinical outcome of patients with malignant pleural mesothelioma. Br J Cancer. 2013;108:621–8. doi: 10.1038/bjc.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekman C, Stenhoff J, Dahlback B. Gas6 is complexed to the soluble tyrosine kinase receptor axl in human blood. J Thromb Haemost. 2010;8:838–44. doi: 10.1111/j.1538-7836.2010.03752.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Bryan JP, Fridell YW, Koski R, et al. The transforming receptor tyrosine kinase, axl, is post-translationally regulated by proteolytic cleavage. J Biol Chem. 1995;270:551–7. doi: 10.1074/jbc.270.2.551. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 17.da Costa AN, Plymoth A, Santos-Silva D, et al. Osteopontin and latent-TGF beta binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. Int J Cancer. 2015;136:172–81. doi: 10.1002/ijc.28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Rui JA, Wang SB, et al. The significance of serum AFP cut-off values, 20 and 400 ng/mL in curatively resected patients with hepatocellular carcinoma and cirrhosis might be of difference. Hepatogastroenterology. 2012;59:840–3. doi: 10.5754/hge10404. [DOI] [PubMed] [Google Scholar]
- 19.Tsou AP, Wu KM, Tsen TY, et al. Parallel hybridization analysis of multiple protein kinase genes: identification of gene expression patterns characteristic of human hepatocellular carcinoma. Genomics. 1998;50:331–40. doi: 10.1006/geno.1998.5338. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson A, Martuszewska D, Johansson M, et al. Differential expression of axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res. 2009;15:4742–9. doi: 10.1158/1078-0432.CCR-08-2514. [DOI] [PubMed] [Google Scholar]
- 21.Ekman C, Site DF, Gottsater A, et al. Plasma concentrations of growth arrest specific protein 6 and the soluble form of its tyrosine kinase receptor axl as markers of large abdominal aortic aneurysms. Clin Biochem. 2010;43:110–14. doi: 10.1016/j.clinbiochem.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Tzeng CW, Aloia TA. Colorectal liver metastases. J Gastrointest Surg. 2013;17:195–201. doi: 10.1007/s11605-012-2022-3. quiz p 2. [DOI] [PubMed] [Google Scholar]
- 23.Korshunov VA. Axl-dependent signalling: a clinical update. Clin Sci (Lond) 2012;122:361–8. doi: 10.1042/CS20110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1:144–58. doi: 10.1159/000343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Fuster J, Bruix J. Barcelona-clinic liver cancer G. The barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.