Abstract
The skin is our largest sensory organ, transmitting pain, temperature, itch, and touch information to the central nervous system. Touch sensations are conveyed by distinct combinations of mechanosensory end organs and the low-threshold mechanoreceptors (LTMRs) that innervate them. Here we explore the various structures underlying the diverse functions of cutaneous LTMR end organs. Beyond anchoring of LTMRs to the surrounding dermis and epidermis, recent evidence suggests that the non-neuronal components of end organs play an active role in signaling to LTMRs and may physically gate force-sensitive channels in these receptors. Combined with LTMR intrinsic properties, the balance of these factors comprises the response properties of mechanosensory neurons and, thus, the neural encoding of touch.
The skin, our largest organ, encompasses the entire body and mediates our sense of touch. Neurophysiologically complex, the skin is innervated by a wide variety of sensory neuron subtypes, including nociceptors, which sense painful stimuli; pruriceptors, which convey itch; thermoreceptors, which register temperature information; and low-threshold mechanoreceptors (LTMRs), which encode non-painful mechanical stimuli, or touch. We use our sense of touch to recognize and manipulate objects, to communicate and socially interact with one another, to appreciate the textures of the foods we eat, for procreation and sexual pleasure, and in maternal nursing. The cutaneous end organs and the mechanosensory neurons that innervate them have evolved to underlie a range of sensory functions, as evidenced by the multitude of skin type specializations that are each innervated by a distinct array of sensory neuron subtypes, reflecting the diversity of functions of touch neurons.
Mammalian skin comprises both hairy and nonhairy, or glabrous, skin. Glabrous skin is predominantly found on the hands and feet of most mammals. In this context, glabrous skin is specialized for discriminative touch, determining texture and shape to accurately recognize objects and providing feedback to the central nervous system to mediate proper grip control, reaching, and locomotion. Hairy skin covers more than 90% of the body surface. It also serves a discriminative touch role, albeit with considerably lower spatial acuity as compared with nonhairy skin. Hairy skin is strongly associated with affective touch—that is, touch that evokes an emotional response, such as during nurturing. Other types of skin are highly specialized for the functional roles they play. The genitalia, specifically the glans penis and glans clitoris, are specialized forms of glabrous skin, fine-tuned for sexual pleasure sensation and reproductive reflexes. The skin of the lips, tongue, and inner cheeks are specialized to aid in food localization and movement and to define textural components of taste. In mammalian females, milk secretion is triggered by suckling stimulation of the nipples. Moreover, some species have evolved skin that is highly specialized for particular functions. Mystacial pads of nocturnal rodents have long whiskers and are specialized for navigation and spatial orientation. The snouts of star-nosed moles and the bills of tactile-foraging birds are specialized for locating prey. These particular skin regions are associated with different combinations of LTMRs, making each region neurophysiologically and functionally distinct.
Key to our understanding of the neurobiological basis of touch is determining how LTMR end organs encode complex forms of tactile stimulation and how this encoding is then integrated and processed within the central nervous system. LTMR subtype central projections terminate within somatotopic columns in the dorsal horn of the spinal cord, with a subset also sending collaterals to the dorsal column nuclei of the brainstem (1). Dorsal horn columns contain interneurons that are thought to process touch information, as well as projection neurons that carry this processed information to the brainstem and higher cortical areas [for a review of central processing, see (2)]. Here we explore the mechanosensory end organs of the skin, focusing on the physiological, morphological, and ultrastructural properties of LTMRs and their associated non-neuronal cells, and we hypothesize how different end organs give rise to the distinct response properties and functions that define mammalian touch neurons.
LTMRs of hairy and glabrous skin
The LTMRs are a heterogeneous group of sensory neurons. Just as the gustatory system has distinct sensory receptors optimally tuned to detect sweet, sour, salty, umami, or bitter tastants, LTMRs are divided into subtypes distinguished by their distinct sensitivities, conduction velocities, and adaptation to sustained mechanical stimulation. For example, slowly-adapting (SA) touch receptors are indentation detectors, firing continuously during a sustained stimulus, whereas rapidly-adapting (RA) touch receptors are velocity detectors that respond only to the onset and offset of indentation. In glabrous skin, four types of LTMRs with fast conduction velocity (Aβ LTMRs) have been defined (Fig. 1), each with a distinct terminal morphology and tuning property (3–5): (i) Aβ SA1-LTMRs innervate Merkel cells in the basal epidermis and report the static nature of touch stimuli, (ii) Aβ SA2-LTMRs are hypothesized to terminate in Ruffini corpuscles in the dermis and are particularly sensitive to skin stretch, (iii) Aβ RA1-LTMRs innervate Meissner’s corpuscles in dermal papillae and are sensitive to movement across the skin, and (iv) Aβ RA2-LTMRs terminate in Pacinian corpuscles deep in the dermis and are tuned to high-frequency vibration (6–8).
Fig. 1. LTMR innervation of glabrous skin.
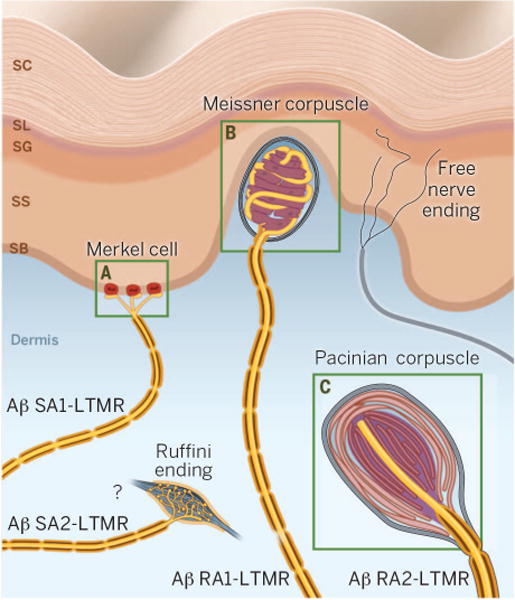
Glabrous skin is innervated by Aβ LTMRs, including Aβ SA1-LTMRs terminating in Merkel cells, Aβ SA2-LTMRs hypothesized to end in Ruffini endings, Aβ RA1-LTMRs innervating Meissner corpuscles, and Aβ RA2-LTMRs ending in Pacinian corpuscles. Green boxed regions are shown in greater detail in Fig. 3 (here, the letters “A,” “B,” and “C” correspond to panels with the same names in Fig. 3). SC, stratum corneum; SL, stratum lucidum; SG, stratum granulosum; SS, stratum spinosum; SB, stratum basale.
In hairy skin, several LTMRs form specialized terminals associated with hair follicles, allowing the sense of touch to extend beyond the skin surface (Fig. 2). Aβ SA1-LTMRs and Merkel cells form complexes called touch domes to detect skin indentation (9, 10). The hair follicle shaft is supplied by collars of mechanoreceptor terminals, including at least three LTMR subtypes that exhibit longitudinal lanceolate endings and one type that has circumferential endings. These subtypes differ in their sensitivities, adaptation properties, and conduction velocities (1, 11). The neck of the hair follicles and the adjacent epidermis are penetrated by unmyelinated LTMRs with free nerve endings (12). At least one type of unmyelinated, slowly-conducting LTMR has endings localized solely to hairy skin and is implicated in pleasurable touch sensation in humans (13). Thus, morphological and neurophysiological differences in glabrous- and hairy-skin LTMRs define the distinct sensory functions of glabrous and hairy skin.
Fig. 2. LTMR innervation of hairy skin.
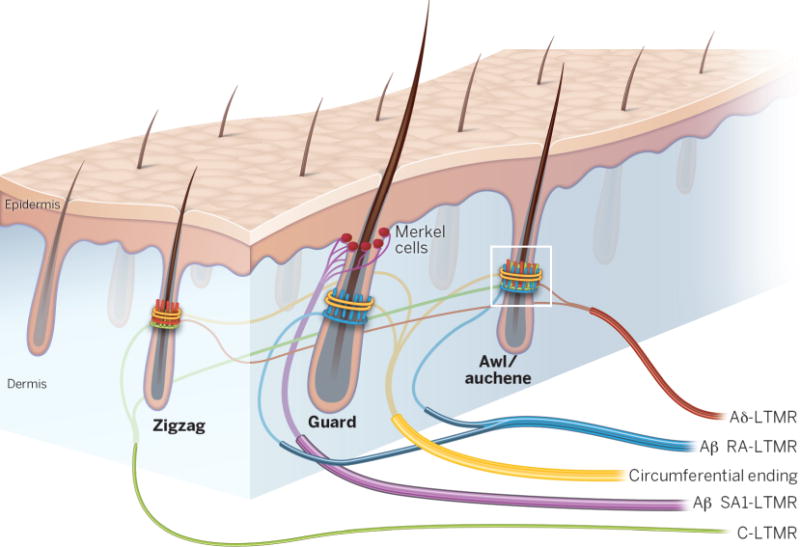
Hairy skin in rodents is innervated by distinct combinations of LTMRs. Touch domes of Merkel cells and associated Aβ SA1-LTMRs are found above the level of the sebaceous glands of guard hair follicles. Guard hairs are also innervated by Aβ RA-LTMR lanceolate endings. Awl/auchene hairs are innervated by all three types of lanceolate-ending LTMRs: Aβ RA-LTMRs, Aδ-LTMRs, and C-LTMRs. Zigzag hairs, the most numerous, are innervated by Aδ-LTMRs and C-LTMRs. Circumferential endings encircle the longitudinal lanceolate endings of all three types of hair follicles. The white boxed region is shown in greater detail in Fig. 4.
The form that underlies function of cutaneous touch receptors
Merkel cell/Aβ SA1-LTMR mechanotransduction
How do ultrastructural features of mechanosensory end organs in hairy and glabrous skin underlie LTMR response properties and functions? It is instructive to first consider the end organ that is shared by hairy and glabrous skin, the Merkel cell/Aβ SA1-LTMR complex. Originally described as “touch corpuscles” by Friedrich Merkel in 1875, they are a group of specialized oval cells in the epidermis of glabrous skin that are innervated by sensory fibers and were thus reasoned to mediate mechanosensation. Later termed “Merkel cells,” clusters of these cells are observed in the basal layer of the epidermis of many specialized skin types, including a dome-shaped bulge close to guard hairs of rodent hairy skin, mammalian glabrous skin, the noses of moles, the wings of bats, whisker pads, the mucosa of the mouth and lips, and elsewhere (14). These complexes are infrequent or absent in skin regions in which spatial acuity is not paramount, such as the genitalia (15, 16). Aβ SA1-LTMR responses to static indentation include a high-frequency dynamic phase during initial skin indentation and lower-frequency, tonic firing during prolonged indentation. Functionally, studies mainly performed in humans and other primates indicate that the glabrous-skin Merkel cell/Aβ SA1-LTMR complex conveys information about texture, curvature, and object shape with high spatial acuity (7, 17).
The Merkel cell is anchored within the epidermis by thin cytoplasmic protrusions projecting to keratinocytes and by desmosomes (9). These physical connections link movement and compression of the skin to mechanical stress on Merkel cells. Aβ SA1-LTMR endings exhibit stereotyped discoid enlargement and connect to the dermal side of each Merkel cell. Synapse-like structures at the junction between Aβ SA1-LTMR endings and Merkel cells have been described in multiple species and include a postsynaptic-like thickening of Aβ SA1-LTMR axon terminal membranes and the presence of presynaptic protein and neurotransmitter in the Merkel cells (18–20). However, small, clear synaptic vesicles typically associated with fast neurotransmission are absent in Merkel cells, and instead dense-core vesicles are seen clustered near the presumptive postsynaptic region of axonal endings (Fig. 3A). These dense-core vesicles are hypothesized to release neuropeptides rather than classical neurotransmitters, thus modulating Aβ SA1-LTMR responses.
Fig. 3. LTMR end organs of glabrous skin.
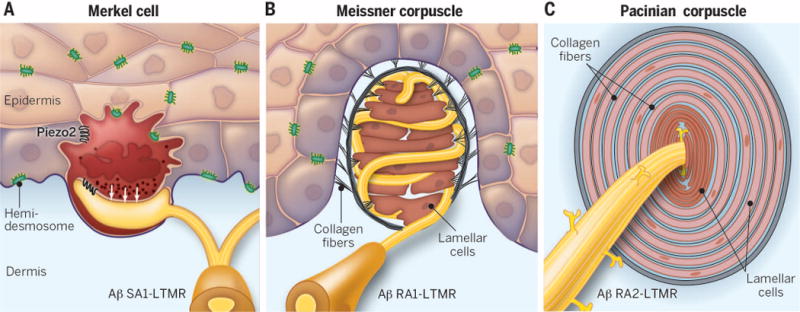
(A) Merkel cells are located within the basal layer of the epidermis, innervated by a single Aβ SA1-LTMR. Cytoplasmic protrusions of the Merkel cell and hemidesmosomes physically link Merkel cells to surrounding epithelial cells. Dense-core vesicles are located inside the Merkel cell in close proximity to the enlarged axon terminal and are thought to be involved in signaling between the Merkel cell and the neurite. Recent evidence revealed Merkel cells to be mechanically sensitive and to play an active role in mechanotransduction (white arrows). (B) Meissner corpuscles are located within dermal papillae and are innervated by one or more Aβ RA1-LTMRs. The external capsule is linked to both the lamellar cells and the epidermis via collagen fibers. (C) Pacinian corpuscles are located in the deep dermis, contain layered lamellar cells, and are innervated by a single Aβ RA2-LTMR. Axonal protrusions project from the neurite into the cleft between inner-core lamellar cells and are thought to be the sites of generator potentials. Longitudinal and circumferential collagen fibers anchor the inner core and outer zone, respectively.
Taste receptor cells in the gustatory system and hair cells of the auditory system are prominent examples of non-neuronal cells participating in stimulus transduction (21, 22), yet it was only recently shown that an analogous situation occurs in touch sensation. Merkel cells, both in culture and ex vivo, exhibit mechanically activated currents, which are absent after loss of the mechanically activated cation channel Piezo2 (23, 24). Moreover, optogenetic activation of Merkel cells is alone sufficient to evoke a SA discharge in Aβ SA1-LTMRs, whereas ablation or functional inactivation of Merkel cells leads to a reduction in both the dynamic and static phases of Aβ SA1-LTMR firing in response to skin indentation (24, 25). Thus, both Merkel cells and Aβ SA1-LTMRs directly respond to mechanical force applied to the skin, and Merkel cells signal to Aβ SA1-LTMRs to achieve optimal activation of the LTMR. A new principle, that non-neuronal components of cutaneous touch complexes detect stimuli and potentiate LTMR responses, has thus begun to emerge.
Glabrous corpuscle LTMR transduction mechanisms
Do non-neuronal cells of the skin mediate mechanotransduction in other cutaneous mechanosensory end organs? We explore two different corpuscles, both of which are rapidly adapting end organs discovered more than 150 years ago. Nestled within dermal papillae of glabrous skin, Meissner corpuscles are composed of flattened lamellar cells that form an ellipsoid structure perpendicular to the skin surface, with one or more tortuous Aβ RA1-LTMR axons meandering throughout (26, 27). In contrast, the larger Pacinian corpuscles are found deep in the dermis of glabrous skin and, in some species, in hairy skin and non-cutaneous tissues including the mesentery and periosteum. Pacinian corpuscles are oval-shaped, contain layered lamellae, and reach up to 3 to 4 mm in length in adult human hands (28, 29). Both Meissner and Pacinian corpuscles are innervated by RA LTMRs tuned to vibration and motion across the skin, in contrast to the static mechanical indentation encoded by Merkel cells/Aβ SA1-LTMRs. Psychophysical studies in humans have described two coding channels of vibration, with low-frequency sinusoids perceived as flutter and high-frequency stimulation detected as vibration (8). Aβ RA1-LTMRs, which are tuned to low-frequency vibrations under 40 Hz, can detect the slip of an object in the hand (5, 8, 30) and may be essential for reflex grip control. On the other hand, Pacinian corpuscle afferents, or Aβ RA2-LTMRs, are tuned to high-frequency stimulation, with optimal activation around 200 Hz, and thus are involved in detecting vibration of held objects (3, 31, 32).
The non-neuronal components of Meissner and Pacinian corpuscles are quite distinct, and their arrangement within the corpuscle offers clues about how vibration and dynamic movement across the skin are encoded. Each disc-like unit of the Meissner corpuscle consists of an unmyelinated axon terminal swelling surrounded by flattened Schwann cell–derived lamellar cells (26, 33). These discoid units are serrated on their external surfaces and smooth on their inner surfaces and are connected to collagen fibers that traverse the surrounding fibroblast capsule (Fig. 3B) (27, 34). Substantial convergence occurs, a s a single Aβ RA1-LTMR innervates multiple Meissner corpuscles (6). During indentation, force is transduced via collagen fibers connected to the serrated edges of the lamellar cells, which leads to bending of Aβ RA1-LTMR axon terminals until the smooth lamellar cell middle compresses. This compression generates action potentials during stimulus onset and produces a RA response (33, 34). How this mechanism results in Meissner corpuscle sensitivity at the low end of the frequency stimulation range is unknown.
“We use our sense of touch to recognize and manipulate objects, to communicate and socially interact with one another, to appreciate the textures of the foods we eat, for procreation and sexual pleasure, and in maternal nursing.”
In contrast to the layered lamellae organization of Meissner corpuscles, the non-neuronal components of Pacinian corpuscles are arranged in concentric lamellae, consisting of an inner core, an intermediate layer or growth zone, and an outer zone. The inner core is composed of tightly packed, bilaterally symmetric hemilamellar cells distributed along both sides of the Aβ RA2-LTMR axon terminal, with small-diameter collagen fibers coursing longitudinally in the clefts between them (6, 35). The outer zone, about three times thicker than the inner core, is composed of concentrically arranged, flattened, and overlapping lamellar cells interspersed with circularly oriented type II collagen fibers in the fluid-filled extracellular space (35, 36). A single heavily myelinated axon penetrates each corpuscle. Its unmyelinated axonal branches, or filopodia, project radially into the clefts between the inner-core hemilamellar cells (Fig. 3C) (29, 37). The lamellar composition is believed to be responsible for the Aβ RA2-LTMR’s encoding of high-frequency vibration, postulated in a model proposed more than 50 years ago: Pacinian corpuscle outer-core lamellar cells and surrounding fluid act as a high-pass filter that dampens low-frequency mechanical stimuli while allowing the slightest high-frequency vibration to reach axonal filopodia via interconnected collagen fibers. The depolarizing generator potentials of the filopodia converge and summate on the primary axon (38). Therefore, the cylindrically layered ultrastructure of Pacinian corpuscles facilitates the high-frequency sensitivity of Aβ RA2-LTMRs.
Do corpuscle lamellar cells actively govern Aβ RA1-LTMR and Aβ RA2-LTMR responses, analogous to what is observed in Merkel cell/Aβ SA1-LTMRs, or do they simply serve a structural role, positioning the respective LTMR endings to detect vibration and movement within the dermis? For the Pacinian corpuscle, studies in cats have revealed that inner-core lamellar cells have glutamate and γ-aminobutyric acid (GABA) receptors, vesicular glutamate transporters, synaptic proteins, synapse-like thickenings, and clear-core vesicles on both sides of the area adjacent to axonal filopodia and lamellae intersections. Glutamate and GABA receptor antagonists modulate electrophysiological responses of Aβ RA2-LTMRs (39, 40). These findings suggest that Pacinian corpuscle lamellar cells can modulate Aβ RA2-LTMR responses and potentially even transduce force directly. Future studies are needed to determine whether an active role of non-neuronal cells of cutaneous LTMR end organs is the rule rather than the exception during mechanotransduction.
Hair follicle LTMR mechanotransduction mechanisms
Hair follicles are neurophysiologically complex mechanosensory organs. Apart from the SA1/Merkel cell complex, hair follicles are innervated by collars of LTMR terminals located just below the level of the sebaceous gland in both rodents and primates. The outer region of this sensory collar contains circumferential endings, whose physiological properties and functions remain unknown (11, 41). The inner region is composed of three types of longitudinal lanceolate terminals, comb-like protrusions aligned parallel to the hair follicle. These longitudinal lanceolate endings—belonging to Aβ RA-LTMRs, Aδ-LTMRs, and C-LTMRs—are all sensitive to hair deflection and light touch of the skin, yet they have distinct conduction velocities (with Aβ > Aδ > C) (1, 42, 43). Similar to Aβ RA1-LTMRs associated with Meissner corpuscles in glabrous skin, lanceolate Aβ RA-LTMRs and Aδ-LTMRs are rapidly adapting and sensitive to movement and low-frequency vibration, despite slight differences in tuning properties (43–45). In rodent trunk skin, lanceolate C-LTMRs are intermediately adapting. Their electrophysiological properties resemble those of C-LTMRs in humans, which are optimally tuned to stroking of the skin at rates that are deemed pleasurable, thus implicating C-LTMRs in “emotional touch” (46, 47).
Despite their differences in sensitivity and encoding, the three types of lanceolate-ending LTMRs have virtually identical terminal structures (Fig. 4) (1, 48). All lanceolate terminals are shaped like flattened cylinders and are sandwiched between two or three terminal Schwann cell processes. The inner face of the axon directly abuts the basal lamina of hair follicle outer root sheath cells, with no intervening Schwann cell process, enabling a close apposition of LTMR axon terminal membranes and hair follicle keratinocytes. Longitudinally oriented collagen fibers fill the extracellular space between and around each lanceolate ending-terminal Schwann cell unit, which may provide structural support for the lanceolate complex (48, 49).
Fig. 4. LTMRs associated with mouse hair follicles.
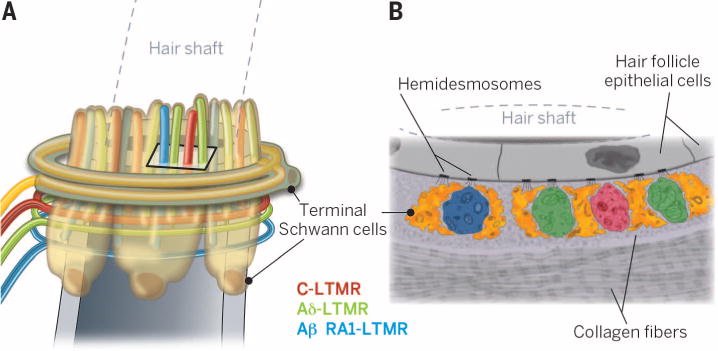
(A) Innervation pattern of Awl/auchene hair follicles. Terminal Schwann cells surround all three types of interdigitated lanceolate endings, as well as the circumferential ending(s). (B) Cross-sectional view of (A), based on electron microscopic analysis. Terminal Schwann cells surround lanceolate endings on either side, with a gap in coverage facing the hair follicle hair cells. Putative protein tethers may connect hair follicle epithelial cells with LTMRs and terminal Schwann cells, while both longitudinal and circumferential collagen fibers provide a supporting role.
How does hair deflection result in excitation of LTMR lanceolate endings and, subsequently, LTMR firing? Electron microscopy has been used to demonstrate that large numbers of hemidesmosomes are distributed along hair follicle epithelial cell membranes and that fine filaments appear to project from these hemidesmosomes to directly contact both LTMR lanceolate endings and terminal Schwann cell processes (Fig. 4B) (48). These filaments may serve to either simply anchor axon terminals to hair follicles or perhaps function as a kind of protein tether necessary for mechanotransduction of lanceolate-ending LTMRs. The protein tether hypothesis posits a mechanism analogous to that seen in the auditory system, in which tip links that connect stereocilia of cochlear hair cells transduce stereocilia movements to the opening of force-gated ion channels (22). In support of such a model for hair follicle lanceolate endings, protein tethers extend between cultured somatosensory neuron axons and fibroblasts, and chemical ablation of these tethers leads to a loss of mechanically activated currents (50). Thus, a physical connection between hair follicle epithelial cells and LTMR lanceolate endings may underlie LTMR excitation during hair deflection. Future challenges include understanding the contributions of hair follicle epithelial cells, terminal Schwann cells, and putative mechanical tethers in the transduction of hair deflection to LTMR excitation.
Cutaneous LTMRs and the neural encoding of touch
Like individual instruments in an orchestra, each LTMR subtype conveys a specific feature of the forces acting on the skin, collectively culminating in a musical symphony of neural impulses that the brain translates as a touch. Each LTMR end organ has similar basic components: sensory axon terminals, associated non-neuronal components (e.g., lamellar cells, Merkel cells, epithelial cells, and/or terminal Schwann cells), and contacts between them and the surrounding cells of the skin. Deciphering the underlying principles that govern transduction at LTMR axon terminals in the skin will reveal the mechanisms by which LTMR subtypes are tuned to deliver distinct components of touch sensation.
As neuroscientists, our instinct is to focus on how intrinsic physiological properties of neurons define their diverse tuning properties. As in the case of distinguishing lanceolate-ending LTMRs from one another, intrinsic determinants appear to be a predominant factor underlying physiological differences of LTMR subtypes [for a detailed discussion, see (45)]. Yet the ultrastructure of mechanosensory end organs plays an equal, if not more important, role in defining the responses of LTMRs. Be it structural filtering, physical tethering, or active propagation or modulation of force sensation, non-neuronal end organ components contribute heavily to the neural dialogue between the skin and the brain. The challenge for future research is to explore how the balance of intrinsic LTMR physiological properties and structural features of LTMR end organs underlies the neural encoding of each LTMR subtype. Ultimately, it is the combination of distinctively constructed touch end organs, highly specialized for each skin type, that produces myriad ensembles of LTMR activity patterns that are represented and processed in the spinal cord and brain and enables the richness of touch perceptions.
Acknowledgments
We thank members of the Ginty laboratory for helpful discussions and comments on this manuscript Our research addressing the organization and function of LTMRs and their circuits is supported by NIH grants R01 NS34814 and R01 DE022750 (to D.D.G.). D.D.G. is an investigator of the Howard Hughes Medical Institute.
REFERENCES AND NOTES
- 1.Li L, et al. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraira VE, Ginty DD. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iggo A, Ogawa H. J Physiol. 1977;266:275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jänig W. Brain Res. 1971;28:203–216. doi: 10.1016/0006-8993(71)90655-x. [DOI] [PubMed] [Google Scholar]
- 5.Johansson RS. J Physiol. 1978;281:101–125. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paré M, Smith AM, Rice FL. J Comp Neurol. 2002;445:347–359. doi: 10.1002/cne.10196. [DOI] [PubMed] [Google Scholar]
- 7.Blake DT, Hsiao SS, Johnson KO. J Neurosci. 1997;17:7480–7489. doi: 10.1523/JNEUROSCI.17-19-07480.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. J Neurophysiol. 1968;31:301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- 9.Iggo A, Muir AR. J Physiol. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodbury CJ, Koerber HR. J Comp Neurol. 2007;505:547–561. doi: 10.1002/cne.21517. [DOI] [PubMed] [Google Scholar]
- 11.Biemesderfer D, Munger BL, Binck J, Dubner R. Brain Res. 1978;142:197–222. doi: 10.1016/0006-8993(78)90631-5. [DOI] [PubMed] [Google Scholar]
- 12.Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ. Nature. 2013;493:669–673. doi: 10.1038/nature11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olausson H, et al. Nat Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- 14.Halata Z, Grim M, Bauman KI. Anat Rec A Discov Mol Cell Evol Biol. 2003;271A:225–239. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- 15.Halata Z, Munger BL. Brain Res. 1986;371:205–230. doi: 10.1016/0006-8993(86)90357-4. [DOI] [PubMed] [Google Scholar]
- 16.Shih C, Cold CJ, Yang CC. J Sex Med. 2013;10:1783–1789. doi: 10.1111/jsm.12191. [DOI] [PubMed] [Google Scholar]
- 17.Maricich SM, et al. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartschuh W, Weihe E. J Invest Dermatol. 1980;75:159–165. doi: 10.1111/1523-1747.ep12522555. [DOI] [PubMed] [Google Scholar]
- 19.Mihara M, Hashimoto K, Ueda K, Kumakiri M. J Invest Dermatol. 1979;73:325–334. doi: 10.1111/1523-1747.ep12550322. [DOI] [PubMed] [Google Scholar]
- 20.Fagan BM, Cahusac PMB. Neuroreport. 2001;12:341–347. doi: 10.1097/00001756-200102120-00032. [DOI] [PubMed] [Google Scholar]
- 21.Finger TE, et al. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 22.LeMasurier M, Gillespie PG. Neuron. 2005;48:403–415. doi: 10.1016/j.neuron.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda R, et al. Cell. 2014;157:664–675. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo SH, et al. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maksimovic S, et al. Nature. 2014;509:617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauna N. Am J Anat. 1956;99:315–350. doi: 10.1002/aja.1000990206. [DOI] [PubMed] [Google Scholar]
- 27.Idé C. Am J Anat. 1976;147:329–355. doi: 10.1002/aja.1001470307. [DOI] [PubMed] [Google Scholar]
- 28.Bentivoglio M, Pacini P. Brain Res Bull. 1995;38:161–165. doi: 10.1016/0361-9230(95)00083-q. [DOI] [PubMed] [Google Scholar]
- 29.Cauna N, Mannan G. J Anat. 1958;92:1–20. [PMC free article] [PubMed] [Google Scholar]
- 30.Knibestöl M. J Physiol. 1973;232:427–452. doi: 10.1113/jphysiol.1973.sp010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brisben AJ, Hsiao SS, Johnson KO. J Neurophysiol. 1999;81:1548–1558. doi: 10.1152/jn.1999.81.4.1548. [DOI] [PubMed] [Google Scholar]
- 32.Sato M. J Physiol. 1961;159:391–409. doi: 10.1113/jphysiol.1961.sp006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cauna N, Ross LL. J Biophys Biochem Cytol. 1960;8:467–482. doi: 10.1083/jcb.8.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi-Iwanaga H, Shimoda H. J Neurocytol. 2003;32:363–371. doi: 10.1023/B:NEUR.0000011330.57530.2f. [DOI] [PubMed] [Google Scholar]
- 35.Pease DC, Quilliam TA. J Biophys Biochem Cytol. 1957;3:331–342. doi: 10.1083/jcb.3.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawson L, Slepecky NB, Bolanowski SJ. Somatosens Mot Res. 2000;17:159–170. doi: 10.1080/08990220050020571. [DOI] [PubMed] [Google Scholar]
- 37.Spencer PS, Schaumburg HH. J Neurocytol. 1973;2:217–235. doi: 10.1007/BF01474721. [DOI] [PubMed] [Google Scholar]
- 38.Loewenstein WR, Skalak R. J Physiol. 1966;182:346–378. doi: 10.1113/jphysiol.1966.sp007827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawson L, Pack AK, Bolanowski SJ. Somatosens Mot Res. 2007;24:85–95. doi: 10.1080/08990220701388364. [DOI] [PubMed] [Google Scholar]
- 40.Pawson L, et al. J Neurosci. 2009;29:2695–2705. doi: 10.1523/JNEUROSCI.5974-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice FL, Munger BL. J Comp Neurol. 1986;252:186–205. doi: 10.1002/cne.902520205. [DOI] [PubMed] [Google Scholar]
- 42.Iggo A. J Physiol. 1960;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown AG, Iggo A. J Physiol. 1967;193:707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koltzenburg M, Stucky CL, Lewin GR. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 45.Lechner SG, Lewin GR. Physiology. 2013;28:142–150. doi: 10.1152/physiol.00059.2012. [DOI] [PubMed] [Google Scholar]
- 46.Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- 47.Vallbo A, Olausson H, Wessberg J, Norrsell U. Brain Res. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Ginty DD. eLife. 2014;3:e01901. doi: 10.7554/eLife.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto T. J Electron Microsc. 1966;15:158–166. [PubMed] [Google Scholar]
- 50.Hu J, Chiang LY, Koch M, Lewin GR. EMBO J. 2010;29:855–867. doi: 10.1038/emboj.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]


