SUMMARY
Studies in flies, mice, and human models have provided a conceptual framework for how paracrine interactions between damaged cells and the surrounding tissue control tissue repair. These studies have amassed evidence for an evolutionarily conserved secretory program that regulates tissue homeostasis. This program coordinates cell survival and proliferation during tissue regeneration and repair in young animals. By virtue of chronic engagement, however, it also contributes to the age-related decline of tissue homeostasis leading to degeneration, metabolic dysfunction and cancer. Here we review recent studies that shed light on the nature and regulation of this evolutionary conserved secretory program.
Keywords: age-related pathology, cellular senescence, danger signals, inflammation, juxtacrine and paracrine signaling, secreted factors
Introduction
Homeostasis in multicellular organisms depends on a continuous, coordinated response to external and internal insults that challenge cellular and tissue integrity throughout life. Loss of homeostasis is a hallmark of aging, resulting in pathologies often caused by defective or deregulated tissue damage responses.
One characteristic of aged mammalian tissues is an accumulation of senescent cells – cells that have ceased dividing, essentially irreversibly, in response to damage or stress that is potentially oncogenic (Campisi, 2013). Most senescent cells secrete a suite of cytokines, growth factors and proteases, known as the senescence-associated secretory phenotype (SASP) (Coppe et al., 2008). Because the SASP includes many pro-inflammatory cytokines and chemokines, it is thought to be a driving force behind the low level, chronic inflammation that causes or exacerbates many age-related pathologies, including cancer (Coppé et al., 2010). Recent evidence from a transgenic, prematurely aging mouse model showed that senescent cells are indeed causal for at least a subset of age-related degenerative diseases, including cataracts and sarcopenia (Baker et al., 2011).
While the deleterious consequences of the SASP in aging animals suggest a maladaptive role for senescence in adults, its coordinated development and complex composition, including several growth factors, points to an evolved and adaptive origin. Indeed, cellular senescence was recently shown to occur during discrete steps of human and mouse embryonic morphogenesis (Muñoz-Espín et al., 2013; Storer et al., 2013), indicating at least two adaptive roles in young organisms: tumor suppression and the fine-tuning of morphogenesis. Further, the SASP was recently shown to be important for limiting fibrosis and accelerating tissue repair in the liver and skin in mice (Demaria et al., 2014 in press; Jun and Lau, 2010; Krizhanovsky et al., 2008).
Understanding the origin and physiological role of this secretory program will not only provide insights into the development of age-related pathologies, but will also contribute to our understanding of homeostasis in young organisms. Strikingly, the mammalian SASP resembles secretory programs observed in epithelial cells of Drosophila melanogaster (fruit flies). In flies, this secretory program is evident during development and in response to tissue injury, suggesting it is an evolutionarily conserved wounding and tissue damage response. Thus, dissecting the regulation and function of the secretory program in flies may elucidate the mechanisms and consequences of the SASP in mammals. In this review, we aim to bridge the gap between research in flies and mammals with regard to cell non-autonomous mechanisms of growth control and tissue repair. We posit that developing a common perspective of the conserved mechanisms that regulate tissue damage responses will accelerate the development of effective strategies for treating a host of age–related pathologies, ultimately in humans.
Paracrine signaling during organogenesis
Organogenesis and pattern formation in the embryo, and tissue homeostasis in adults, require precise coordination of individual and collective cellular decisions toward cell proliferation, growth and death. Understanding processes that govern this coordination has therefore been a longstanding focus of developmental biology, regenerative biology and cancer research. Studies in flies show that tissue homeostasis in epithelia is governed by ‘collective’ decision mechanisms that determine cell death and proliferation across tissues. These mechanisms include Cell Competition (CC) and Compensatory Proliferation (CP) (Vincent et al., 2013). CC and CP have been identified and studied extensively in flies, and recent studies reveal the existence and importance of similar processes in mammals, where they maintain tissue homeostasis, particularly during tissue repair (Claveria et al., 2013; Martins et al., 2014).
Cell competition in fly imaginal discs
CC was initially described in the 1970’s in developing Drosophila wing imaginal discs as a mechanism of cell interaction in which weaker, yet viable, cells (referred to as ‘loser’ cells) are eliminated by their ‘fitter’ neighbors (referred to as ‘winner’ cells). The first process in which CC was described was the elimination of Minute cells, which harbor defective ribosomal proteins. (Morata and Ripoll, 1975). Decades later, Moreno et al. (2002) proposed that the competitive disadvantage of Minute cells is due to their defective response to the TGF-β/BMP homologue Decapentaplegic (Dpp) (see Table 1), which can provide a pro-survival signal in flies. According to this model, the defective TGF-β/BMP response in Minute cells results in apoptotic cell death through activation of the evolutionarily conserved Jun-N-terminal Kinase (JNK) signaling pathway (Table 1) (Moreno et al., 2002). Later, it was proposed that the cellular interactions at population boundaries during CC require not only the apoptotic death of the Minute cell but also the induction of engulfment genes (drpr, wasp and psr) in the ‘winner’ cells (Li and Baker, 2007). A similar mechanism was described in the context of pro-oncogenic clonal expansion in imaginal discs, in which mutant cells are eliminated by their neighbors. In this case, the engulfment activity is derived from non-apoptotic JNK-dependent activation of PVR signaling (Ohsawa et al., 2011). This mechanism remains controversial, however, as it was recently proposed that, at least in the context of d-myc-induced cell competition, engulfment activity of winner cells is dispensable for loser cell apoptosis and that most of the cellular clearance is performed by hemocytes (Lolo et al., 2012). Moreno and colleagues further proposed that one mechanism that determines relative cell viability is mediated by Flower (Fwe), a membrane receptor that is required for the elimination of loser cells (Rhiner et al., 2010). This mechanism appears to be conserved in mammals, where, as in Drosophila, FWE deficiency is associated with decreased clonal expansion of pre-malignant cells. It has been proposed that this decrease is brought about in FWE-deficient mice by a delay in positive selection for cells with a proliferative advantage (Petrova et al., 2012; Rhiner et al., 2010).
Table 1.
Signaling pathways involved in tissue damage responses in flies and vertebrates.
| SIGNALLING PATHWAY |
LIGANDS | RECEPTORS | INTRACELLULAR PATHWAY | |
|---|---|---|---|---|
|
Decapentalegic
(Dpp) signaling |
D | Decapentalegic (Dpp) Glass bottom boat (Gbb) Screw (Scw) |
Punt (Put) Thickveins (Tkv) Saxophone (Sax) |
Mothers against dpp (Mad) Medea |
|
| ||||
|
Transforming
growth factor β (TGF-β)/Bone morphogenic pathway (BMP) signaling |
V |
BMP2 and BMP4
BMP 5,6,7 ? |
BMP/Activin receptor type II (ACTRII) BMP receptor type I (BMPR1A/B or ALKs) BMP receptor type I (BMPR1A/B or ALKs) |
SMAD 1/5/8/9
SMAD-4 |
|
| ||||
|
Wingless (Wg)
signaling |
D | Wingless (Wg) DWnt-2-6,8,10 |
Frizzeled (Fz) Frizzeled 2-4 (DFz-2-4) |
Armadillo (Arm) Dishevelled (Dsh) Shaggy (Sgg) Axin |
|
| ||||
| Wnt Signaling | V |
WNT-1
WNT-5-8, 10, 14, 15 |
Frizzeled receptors (FZD1-10) |
Beta-catenin (beta-CAT) Segment polarity protein dishevelled homolog (DVL1-3) Glycogen synthase kinase 3 β (GSK3β) Axin 1-2 |
|
| ||||
|
JAK/STAT
signaling |
D | Unpaired proteins (Upd, Upd-2, Upd-3) |
Domeless (Dome) | Hopscotch (Hop) Stat92E |
|
| ||||
| V | Interleukin-6 (IL-6) | Type I cytokine receptors |
Janus kinase (JAKs) Signal Transducer and Activator of Transcription (STATs) |
|
|
| ||||
|
Epidermal
growth factor receptor (EGFR) signaling |
D | Vein (Vn) Gurken (Grk) Spitz (Spi) Keren (Krn) Argos (Aos) |
Epidermal growth factor receptor (Egfr) |
Ras
Pole hole (Phl)/Raf Downstream of raf1 (DSor1) Rolled (Rl) |
|
| ||||
| V | EGF-like ligand similar to neuregulins TGF-α ligands |
Epidermal growth factor receptorr (EGFR) |
Rat sarcoma (RAS) RAF-1 MAPK/ERK kinase (MEK) extracellular-signal-regulated kinases (ERKs) |
|
|
| ||||
|
Stress
signaling pathways |
D | Hemipterous (Hep) DMKK4 Licorne (Lic) Basket (Bsk) D-p38a/b |
||
|
| ||||
| V | JNK kinases (JNKKs) Mitogen-activated protein KK 4 (MKK4) Mitogen-activated protein KK 3 (MKK3) c-Jun N-terminal kinases (JNKs) p38 MAP kinases (p38 MAPKs) |
|||
Drosophila (D) names for the main protein components of pathways involved in paracrine signaling during tissue damage responses and cell competition are shown. The vertebrate (V) rows identify known homologs of the Drosophila proteins and is not an exhaustive list of all the components and pathways known to be part of tissue damage responses in vertebrates. When possible, the nomenclature for human proteins is used. Due to the complexity of the signaling cascades, the ligands and receptors involved in stress signaling pathways have been omitted.
Compensatory proliferation in fly imaginal discs
CC is tightly linked to compensatory proliferation (CP), a process driven by paracrine signaling mechanisms that ensure collective decisions in the epithelium. Apoptotic cells not only collaborate in the competition process by inducing engulfing activity in their neighbors, but also produce mitogenic signals that promote CP in the remaining cells (de la Cova et al., 2004; de la Cova et al., 2014; Moreno and Basler, 2004). Early observations by Alder and Bryant suggested that lethally irradiated imaginal disc tissues can promote the regeneration of neighboring tissues (Adler and Bryant, 1977). In recent years, the molecular signals promoting CP have been elucidated in apoptotic fly cells in which effector caspase activity was artificially blocked by expression of transgenic p35 (Huh et al., 2004; Perez-Garijo et al., 2004; Perez-Garijo et al.; Ryoo et al., 2004). Notably, these so-called “undead cells” strongly resemble the phenotype of senescent mammalian cells. Undead cells are characterized by their ability to influence the behavior of surrounding cells, including stimulating cell proliferation by expressing Dpp and Wingless (Wg), the fly Wnt homologue (Table 1) (Huh et al., 2004; Perez-Garijo et al., 2004; Ryoo et al., 2004). This paracrine activity of undead cells occurs in a JNK-dependent manner (Kondo et al., 2006; Ryoo et al., 2004), and resembles the ability of ‘loser’ cells to stimulate ‘winner’ cell proliferation during CC. It remains unclear, however, whether the same signaling molecules mediate CP and CC-induced winner cell proliferation.
Senescent cells, both mouse and human, also stimulate the growth of neighboring cells by secreting SASP factors, some of which are potent mitogens (Coppe et al., 2010; Coppe et al., 2008). The expression of SASP genes depends on at least two signaling pathways: the DNA damage response (DDR) and p38 mitogen-activated protein kinase-nuclear factor kappa light chain enhancer of activated B cells (p38MAPK-NF-kB) pathways (Freund et al., 2011; Rodier et al., 2009).
Studies of CC in which a growth advantage was conferred by elevated levels of d-myc, the Drosophila homologue of the mammalian proto-oncogene MYC, showed that paracrine communication between ‘weaker’ and ‘fitter’ cells is bi-directional (Figure 1). In this context, cells overexpressing d-myc, a transcriptional regulator, induced apoptosis in their neighbors through expression of the pro-apoptotic gene hid in a JNK independent manner (de la Cova et al., 2004). The competitive advantage of d-myc overexpressing cells is p53 dependent (de la Cova et al., 2014). Studies in cultured Drosophila cells further suggested that this process is regulated by secreted factors (Senoo-Matsuda and Johnston, 2007). Both cell types – weaker and fitter – participate in the competition process through paracrine signaling to induce both apoptotic and pro-proliferative signals, although specific factors were not identified in this study (Senoo-Matsuda and Johnston, 2007).
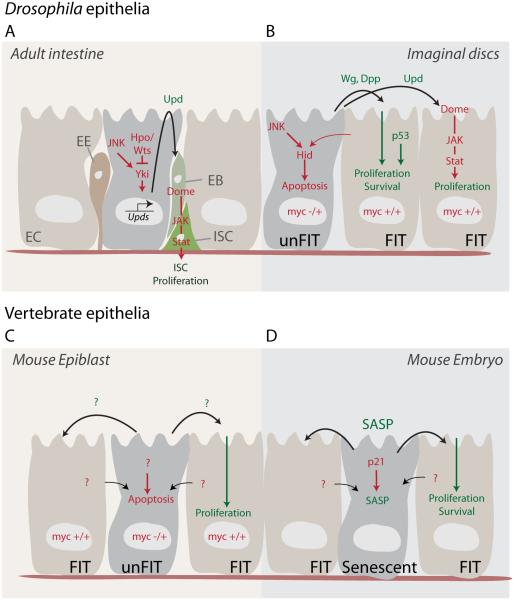
Figure 1. Paracrine signaling in the control of tissue regeneration, homeostasis and remodeling.
(A) In the posterior midgut epithelium of Drosophila, damaged enterocytes (EC) secrete Upd cytokines upon the activation of stress signaling involving JNK and Yki. Upds activate the JAK/STAT signaling pathway of intestinal stem cells (ISCs), inducing their proliferation and generating enteroblasts (EB), which go on to differentiate into ECs or enteroendocrine (EE) cells.
(B) In imaginal discs, cell competition results in the induction of apoptosis in slow growing cells (‘unfit cells’, here exemplified by cells with a lower dose of myc). Faster growing (‘fit’) cells induce apoptosis in unfit cells in a p53 and JNK-dependent manner. Apoptotic cells, in turn, promote compensatory proliferation by inducing Dpp, Wg and Upds.
(C) In the mouse epiblast, fit MYC over-expressing cells induce apoptosis and engulfment of unfit cells, which in turn promote the proliferation of fit cells through paracrine signals.
(D) In the mouse embryo, activation of the cell cycle inhibitor p21 can induce senescence. Senescent cells, through the SASP, fine-tune patterning and growth by supporting the survival and proliferation of neighboring fit cells.
Moreno and Basler suggested that one mechanism of CC is mediated by intrinsic changes in the loser cells, which diminish their ability to respond to a Dpp signal. Accordingly, CC, can be perturbed by enhancing Dpp signaling capacity in the loser cells (Moreno and Basler, 2004). A recent study on cell competition in mammalian cells supports the conservation of both paracrine and cell-intrinsic mechanisms, since defective Bmp signaling capacity (through the deletion of Bmp receptors) is sufficient to induce cell elimination by wild type cells, and this elimination is mediated by secreted factors (Sancho et al., 2013). Further supporting the involvement of paracrine signals in CC is the fact that defects in the endocytic pathway affect the efficiency of CC (Moreno and Basler, 2004) and can also promote non-autonomous overgrowth in imaginal epithelia (Takino et al., 2014). Finally, differential activation of JAK/STAT signaling has been proposed as yet another mechanism that regulates competitive advantage in CC interactions (Rodrigues et al., 2012).
It should be noted that in mosaic tissues ‘winner’ cells do not necessarily proliferate faster, but may gain a competitive advantage by growing faster (as in the case of d-myc-over-expressing cells) and/or killing loser cells. In cell culture, on the other hand, d-myc over-expressing cells proliferate faster, suggesting that CC and CP within the epithelial context is influenced by constraints that are lacking in culture (Senoo-Matsuda and Johnston, 2007).
Senoo-Matsuda and Johnston’s analysis of cell communication during CC uncovered the importance of reciprocal cell signaling in this process. These paracrine interactions between winner and loser cells in flies are reminiscent of the SASP in mammals (Coppe et al., 2008). Recent studies highlight the pleiotropic effects of the SASP on the surrounding tissue environment, which range from autocrine and paracrine reinforcement of senescence to promotion of proliferation and cell recruitment (Tasdemir and Lowe, 2013). It is likely that both the secretory phenotype and the intrinsic properties of cells exposed to the SASP may ultimately determine the tissue outcome. In contrast to the importance of p53 in fly CC (de la Cova et al., 2014), however, development of the SASP in mammalian cells is p53-independent (Coppe et al., 2008), raising the interesting question of whether and why this paracrine mechanism lost its p53 dependence during evolution. On the other hand, senescent mammalian cells also secrete the evolutionarily conserved, pro-inflammatory alarmin HMGB1 in a p53-dependent manner (Davalos et al., 2013). Thus, HMGB1 secretion may be the ancestral paracrine mediator, with the SASP developing later during evolution.
Paracrine signaling during mammalian development
Recently, CC and the SASP were shown to contribute to developmental programs in mammals. In the mouse embryo, CC was described in the epiblast as a mechanism to select for cells with higher MYC levels and anabolic activity, which results in refinement of the initial cell population (Figure 1) (Claveria et al., 2013). Cellular and molecular mechanisms governing CC in the Drosophila wing disc were also observed in this context, including induction of a paracrine apoptotic signal by cells over-expressing MYC and engulfment of apoptotic cells by their neighbors. Although CC in the mammalian epiblast requires cell-cell contact (Claveria et al., 2013), paracrine factors influencing CC were identified in cultured ES cells (Sancho et al., 2013). As in the fly model (Senoo-Matsuda and Johnston, 2007), this finding indicates that cell culture systems fail to replicate constraints on cell growth and proliferation in intact tissues, yet are useful for identifying paracrine mediators of CC and tissue damage responses, an approach that was also used to identify SASP factors (Coppe et al., 2008).
Two recent reports have further identified senescence in the mammalian embryo as a mechanism of fine-tuning patterning and growth control (Figure 1) (Muñoz-Espín et al., 2013; Storer et al., 2013). These embryonic senescent cells shared a secretory profile (SASP) with senescent cells found in adult tissues. In the embryo, as can occur in adult tissues (Iannello et al., 2013; Kang et al., 2011; Xue et al., 2007), the SASP attracted innate immune cells, which eventually cleared the senescent cells from developing embryonic structures. In contrast to adult tissues, however, embryos in which cells failed to undergo senescence did not appear to be tumor prone. In adults, the senescence response depends on two tumor suppressor pathways – the p53/p21 pathway and the p16INK4a/pRB pathway. Senescence in the embryo, however, was independent of p53, yet strictly dependent on p21. Indeed, p21-null, but not p53-null, mice showed defects in embryonic senescence, which was partially compensated by apoptosis. Loss of senescence did not completely impair embryogenesis, but resulted in delayed patterning (Muñoz-Espín et al., 2013; Storer et al.). Thus, the senescence response and SASP appear to fine-tune morphogenesis, a function that is reminiscent of the role of CC during morphogenesis in the fly, where CC and CP are observed primarily in response to genetic or environmental perturbations that confer a growth advantage or disadvantage to individual cells.
These data highlight the existence of specific signaling mechanisms that coordinate the removal of extraneous or abnormal cells and control the growth and proliferation of neighboring cells to ensure proper organ morphogenesis. As discussed below, a strong link between these developmental processes and tumor suppression in mammals has been established, suggesting a common origin for these paracrine signaling mechanisms. Of importance for the maladaptive effects of cellular senescence, senescent cells in adult tissues are resistant to apoptosis; when immune-mediated mechanisms of senescent cell clearance are impaired, senescent cells persist beyond the time of their physiological role and tissue homeostasis is compromised (Adams, 2009; Campisi, 2013). Similarly, artificially preventing apoptosis under conditions in which tissues use CP to compensate for the loss of damaged cells ultimately leads to tissue overgrowth in flies (Huh et al., 2004; Perez-Garijo et al., 2004; Ryoo et al., 2004). It is important to note that the work in Drosophila described above also highlights the complexity of the paracrine processes that control tissue homeostasis, as multiple (and often conflicting) signaling interactions between ‘winner’ and ‘loser’ cells occur. This complexity is likely a reflection of the heterogeneity in tissue microenvironments used to study these interactions, and may provide hints for understanding the pleiotropic effects that senescent cells exert upon their tissue environment (Ohsawa et al., 2014).
Paracrine signaling during tissue regeneration, repair and remodeling
The paracrine signaling interactions described thus far are critical for organogenesis during development, and the amazing regenerative capability of larval imaginal discs has been exploited to reveal many of the fundamental mechanisms that are expected to govern epithelial regeneration in many contexts. Accordingly, similar cell-cell signaling events have been described in recent years that control tissue homeostasis in the adult and influence the long-term maintenance of tissue function. Two general strategies for replacing damaged cells while maintaining tissue size in adults can be distinguished: compensatory proliferation of tissue-resident stem cells, and compensatory hypertrophy of post-mitotic cells. Here, also, the parallels between signaling interactions uncovered in Drosophila and interactions between senescent cells and normal cells that influence aging and cancer in vertebrates are striking.
Paracrine communication between epithelial cells: lessons from the fly
Compensatory Cellular Hypertrophy (CCH) was recently reported in post-mitotic tissues in Drosophila, where tissue resident stem cells are absent and therefore cannot participate in regeneration, and was also observed in mammalian tissues, including the liver, cornea and heart (Tamori and Deng, 2014). In Drosophila follicular epithelia, cell competition between post-mitotic cells results in CCH to repair tissue loss and fill in lost volume. This compensatory growth is achieved by paracrine Insulin/IGF-like signaling (IIS), which accelerates endoreplication in winner cells (Tamori and Deng, 2013). Similarly, a phenomenon of compensatory hypertrophy involving polyploidization and cell fusion, controlled by the YAP homologue Yorkie (Yki)/Hippo (Hpo) signaling pathway, was described as a critical mechanism in epidermal wound closure (Losick et al., 2013). While conceptual similarities exist between CP and CCH, these results also highlight the diversity of strategies to achieve compensation for cell loss in different contexts.
In mitotically active tissues, regenerative processes that rely on tissue resident stem cells can maintain homeostasis. Such processes are particularly important in tissues with high turnover, in which stem cells are either continuously cycling, or can rapidly re-enter the cell cycle to replenish lost cells. The posterior midgut epithelium of Drosophila (Figure 1) has been extensively used to study regenerative processes (Biteau et al., 2011; Buchon et al., 2013), where stem cells with similar properties to those found in mammalian tissues were identified (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). In this tissue, regeneration after tissue damage is regulated by mechanisms that are highly reminiscent of CP in developing imaginal discs: damaged/apoptotic enterocytes (ECs, the major cell type in the Drosophila midgut) induce proliferation and differentiation of neighboring intestinal stem cells (ISCs) through paracrine signals (Jiang et al., 2009). Upon stress, a signaling pathway involving JNK and the Yki induce the expression of Unpaired cytokines (Upd, Upd2 and Upd3), which signal to ISCs to activate JAK/STAT signaling and promote their proliferation and differentiation (Jiang et al., 2009). Based on their receptors, Upds are likely functional homologues of mammalian interleukins, especially interleukin 6 (Table 1) (Agaisse et al., 2003; Arbouzova and Zeidler, 2006; Harrison et al., 1998).
Further characterization of Upd-mediated paracrine signaling in the gut has refined our understanding of regenerative signaling events: Upd1 appears to be the main mediator of stem cell proliferation during homeostatic cell replacement, while Upd2 and 3 act mostly to trigger increased ISC proliferation in response to acute stress, such as bacterial infection (Osman et al., 2012).
While paracrine signaling through Upds promotes control of epithelial regeneration in the gut, ISC proliferation in response to stress is also modulated by other paracrine and cell-autonomous signals. The EGFR ligand Vein, for example, is expressed in the visceral muscle surrounding the intestinal epithelium and activates the EGFR/Mitogen-Activated Protein Kinase (MAPK) pathway in ISCs, synergizing with JAK/STAT signaling to promote ISCs proliferation (see Table 1 for analogies in vertebrates) (Biteau and Jasper, 2011; Buchon et al., 2010; Jiang et al., 2011). The AP1 transcription factor Fos integrates JNK stress signals with EGFR growth factor signals in ISCs to promote proliferation after stress (Biteau and Jasper, 2011). Similarly, inhibition of the Nrf2 transcription factor CncC in ISCs is critical for the induction of regenerative responses to a wide range of damaging stimuli (Hochmuth et al., 2011; Wang et al., 2014).
Several groups reported involvement of the Hpo pathway in initiating paracrine Upd and EGF-like signaling (Karpowicz et al., 2010; Ren et al.; Shaw et al., 2010). Hpo signaling serves to monitor epithelial integrity, and Hpo inactivation in enterocytes induces Upd and EGF, triggering JAK/STAT and EGFR signaling, and subsequent proliferation of ISCs. Interestingly, cell autonomous inactivation of the Hpo pathway in ISCs is also required to trigger the regenerative response (Karpowicz et al., 2010; Shaw et al., 2010). Yki, which is activated in response to Hpo silencing, seems to be the mediator of both paracrine and cell autonomous functions, and is repressed by Hpo in ISCs under non-stress conditions (Karpowicz et al., 2010).
Paracrine communication between epithelial cells: the mammalian story
Paracrine signaling also plays a critical role in tissue homeostasis in adult mammals, and the signaling mechanisms are strikingly similar to those described in flies. IL-6, for example, is one of the most versatile of mammalian cytokines (Rincon, 2012). While IL-6 was originally discovered as an essential factor in the maturation of B cells (Hirano et al., 1986), it was later found to have pleiotropic effects in many contexts. In the liver, it is a critical acute-phase inducer upon injury, and it promotes regeneration in response to chemical damage or partial hepatectomy (Nakamura et al., 2004). In a rat model of spinal cord injury, IL-6 promotes axonal sprouting, synapse formation and functional recovery (Yang et al., 2012). And during acute kidney injury, IL-6 can both promote an injurious inflammatory response and protect cells from excessive injury by limiting oxidative stress (Nechemia-Arbely et al., 2008). In most of these cases, IL-6 signaling mainly activates the transcription factor STAT3, which exerts several pro-survival, proliferative, migration and inflammatory functions (Levy and Lee, 2002).
IL-6 is a prominent component of the human and mouse SASPs (Coppe et al., 2010; Coppe et al., 2008). Its role in regenerative responses and tissue homeostasis highlights a recent appreciation for the role senescent cells play in these processes (Adams, 2009; Campisi, 2011). As noted above, the senescence response comprises a cell autonomous growth arrest, dependent on the p53/p21 and p16/pRb pathways, which protects mammalian organisms from cancer (Prieur and Peeper, 2008). In addition, senescent cells secrete high levels of IL-6 (Coppe et al., 2008), which in part reinforces the senescence growth arrest (Acosta et al., 2008; Kuilman et al., 2008), but also has potent paracrine effects (e.g., inducing a mesenchymal-epithelial transition in neighboring epithelial cells (Coppe et al., 2008)). Further, the SASP comprises a large number of other secreted molecules, including TGF-β, CCL-20, CCL-2 and VEGF (Acosta et al., 2013; Coppe et al., 2008). Through the SASP, senescent cells can propagate the senescence response to a limited number of neighboring cells, thus further deregulating homeostasis (Acosta et al., 2013).
Other examples of paracrine effects of the SASP include IGFBP7 (insulin-like growth factor binding protein 7), which is secreted by cells driven into senescence by oncogenic B-RAF signaling and induces senescence or apoptosis in surrounding cells (Wajapeyee et al., 2008, 2010), and PAI-1 (plasminogen activator inhibitor 1), which is an important mediator of the senescence growth arrest in both wild type and p53 deficient cells (Kortlever et al., 2006).
An important part of the cell non-autonomous effects of senescent cells is the ability of SASP components to attract and activate immune cells, which can remove nearby senescent, damaged and/or potentially tumorigenic cells. For example, re-activation of p53 in a mouse model of liver carcinoma leads to cellular senescence within the tumor and activation of an innate immune response, which mediates tumor regression (Xue et al., 2007). In a model of liver cancer due to fibrosis and cirrhosis, a p53-dependent senescence program in hepatic stellate cells promotes an anti-tumor microenvironment through the differentiation of macrophages toward tumor inhibiting functions (Lujambio et al., 2013). The SASP can also harness the adaptive immune system to induce CD4+T-cell-mediated clearance of pre-malignant heaptocytes in the liver, where senescent cells are targeted by specific Th1 lymphocytes (Kang et al., 2011).
In addition to their anti-cancer function, cellular senescence and the SASP play a complex role in in promoting tissue recovery and homeostasis during regenerative episodes. For example, in response to chemical (carbon tetrachloride) injury in the liver, activated hepatic stellate cells enter a period of highly active proliferation, during which time they produce the extracellular matrix that forms a fibrotic scar. These activated stellate cells eventually become senescent, whereupon they secrete matrix degrading enzymes (prominent SASP components) and promote immunosurveillance (Krizhanovsky et al., 2008). Similarly, in a model of cutaneous wound healing, cells in the granulation tissue are induced to become senescent, whereupon, again, they secrete enzymes that reduced fibrosis during the last stage of remodeling (Jun and Lau, 2010). During wound healing, senescent mesenchymal and endothelial cells also appear to be important for timely wound closure through the secretion of PDGF-AA during the proliferative and contraction stage (Demaria et al., 2014 in press).
When homeostasis goes wrong: cell non-autonomous signaling in aging
The function of IL-6-like factors in regenerative responses and wound healing in both flies and vertebrates highlights the evolutionary conservation of paracrine processes that control tissue repair. At the same time, work in vertebrates has established a critical role for the same paracrine signals in senescent cell-mediated tissue degeneration and even cancer in the aging adult (Adams, 2009; Campisi, 2013; Rodier and Campisi, 2011). These findings suggest that in vertebrates the senescence response might be antagonistically pleiotropic – having evolved to promote fitness in young organisms by suppressing cancer and promoting tissue repair but driving aging phenotypes and pathologies in older organisms. Here, also, studies in flies may significantly advance our mechanistic understanding of the degenerative processes associated with aging. We discuss below the negative consequences of paracrine tissue repair signaling.
During fly development, cell non-autonomous tumor growth has been described in Polycomb Group (PcG) mutants (Classen et al., 2009). Here, PcG genes, which are important regulators of chromatin state, act as tumor suppressors, and their loss results in hyper-proliferation accompanied by loss of tissue integrity in eye imaginal discs. This is mediated by the induction of Upds, which appear to be direct targets of PcG-mediated repression. As in the intestinal epithelium, Upds act in a paracrine manner to activate JAK/STAT signaling and induce the proliferation of surrounding cells (Classen et al., 2009).
Non-autonomous tissue overgrowth of fly imaginal discs has further been described in conditions in which JNK is chronically activated in imaginal disc cells, yet its pro-apoptotic function is masked by pro-survival oncogenic mutations (Figure 2). This phenomenon is observed in Ras/Raf induced tumors, where cells with increased ERK activity are refractory to JNK-induced apoptosis and promote hyperplasia in adjacent wild-type tissue (Brumby and Richardson, 2003; Igaki et al., 2006; Uhlirova et al., 2005). This process thus resembles increased tissue growth induced by ‘undead’ cells. While suppression of PcG protein expression may be one mechanism by which JNK promotes the expression of Upds to drive proliferation of neighboring cells in this context (Lee et al., 2005), JNK activation also weakens cell adhesions, and can promote invasive cell behavior in the oncogenic background (Igaki et al., 2006).
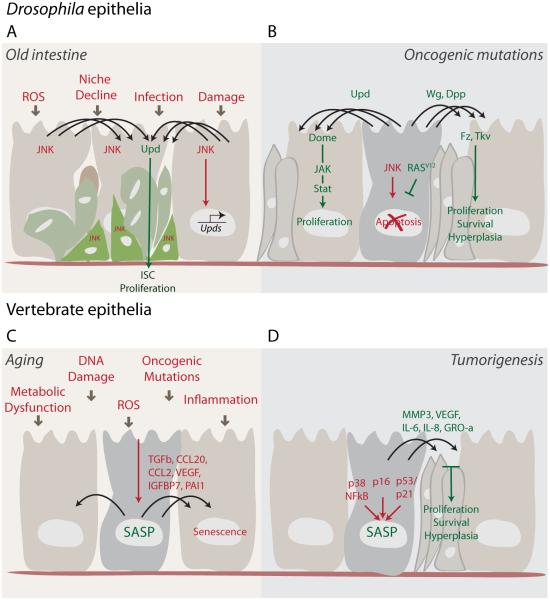
Figure 2. Paracrine signaling promotes tissue degeneration and oncogenic phenotypes with age.
(A) The Drosophila intestinal epithelium deteriorates with age, becoming populated by abnormal and dysplastic cells. These cells accumulate as a consequence of JNK-dependent ISC proliferation and defective differentiation. Different types of damage, including that caused by infection and reactive oxygen species (ROS), create an imbalance of normal signaling events, resulting in the disruption of tissue integrity due to chronic activation of stress signaling and chronic engagement of signaling processes required for tissue repair in the young epithelium (such as Upd secretion).
(B) Oncogenic mutations (such as RasV12) in imaginal disc cell clones can also result in imbalanced paracrine signaling that ultimately causes non-autonomous overgrowth of the tissue. Rasv12 can protect cells from JNK-induced apoptosis. These ‘undead’ cells then promote hyperplasia in the surrounding tissue by continuous secretion of Upd, Wg and Dpp.
(C) In vertebrates, senescent cells induced by different stresses can promote chronic inflammation, which increases during aging due to an accumulation of senescent cells. Some SASP factors (e.g. TGF-β, IGFBP7, PAI-1, CCL2) also induce a senescence response in neighboring cells. Inflammation and the spread of senescence might be the basis for several age-related pathologies, which are characterized by a disruption of normal tissue functions.
(D) Several SASP factors (MMP3, VEGF, IL6, IL8, GRO-α) can also participate in various steps in tumorigenesis, ranging from proliferation to migration and invasion. Thus, senescent cells that accumulate with age and after anti-cancer DNA damaging therapies (chemotherapy, irradiation) can create a milieu for hyperproliferation and tumor growth in the surrounding tissue.
These findings highlight how a beneficial protective mechanism (i.e. JNK-mediated apoptosis of damaged cells) can be converted into a deleterious condition that promotes hyperplasia and tumor invasion through paracrine signals. An interesting aspect to such a hijacking of normal protective mechanisms is the observation that cells expressing oncogenic signaling molecules can evade high-sugar-diet-induced insulin resistance, acquiring an ‘undead’ phenotype that promotes invasiveness and metastatic behavior by promoting the secretion of wingless (Hirabayashi et al., 2013).
Aging itself is a risk factor in the development of such phenotypes. The intestinal epithelium increasingly deteriorates in aging flies and becomes populated by abnormal clusters of cells that carry stem cell markers but are polyploid and reach enterocyte-like size without expressing enterocyte marker genes (Figure 2) (Biteau et al., 2008; Choi et al., 2008). These changes derive from increased ISC proliferation and defective differentiation in aged guts, caused by an imbalance in the normal signaling events that lead to tissue repair (Biteau et al., 2008, 2011). JNK signaling is a central player in this context. It is chronically activated in both ECs and ISCs of aged guts, promoting ISC proliferation and, in cooperation with Notch signaling, misdifferentiation of ISC daughter cells. The resulting dysplasia disrupts tissue integrity and contributes to an age-related increase in mortality (Biteau et al., 2010; Rera et al., 2011 2012). Promoting proliferative homeostasis in the intestinal epithelium by limiting JNK activation and/or insulin signaling can delay these age-associated phenotypes and increase lifespan (Biteau et al., 2010).
The JNK-associated hyperplastic phenotypes resemble those induced by undead cells in imaginal discs, suggesting that JNK activation in the absence of apoptosis may be a general mechanism through which malignant cells propagate (Morata et al., 2011). Recent data support this hypothesis. Moderate caspase activity drives cell invasion without promoting apoptosis, and this phenotype is JNK-dependent (Rudrapatna et al., 2013).
In mammals, chronic inflammation is an important characteristic of aging tissues. It is thought to be a major driver of cellular damage, and to disrupt cellular turnover and tissue homeostasis, eventually causing age-related diseases, including cancer (Chung et al., 2009; Franceschi, 2007). The precise origin of age-related inflammation, or inflammaging, is unknown. One strong candidate, however, is the SASP of senescent cells that persist in aged tissues (Figure 2).
In support of this hypothesis, eliminating senescent cells prevents or delays the onset of some age-related pathologies in a mouse model of accelerated aging (Baker et al., 2011). Further, in cell culture models, SASP components can disrupt normal tissue structures and function and promote malignant phenotypes in neighboring cells. For example, SASP matrix metalloproteinases prevent mammary alveolar morphogenesis and milk protein production (Parrinello et al., 2005). The SASP cytokines IL-6, IL-8 and GRO-α stimulate the proliferation of premalignant or malignant epithelial cells (Coppe et al., 2008; Wang et al., 2006). VEGF, another SASP component, can further promote angiogenesis to provide progressing tumors with an adequate nutrient supply (Coppe et al., 2006). Interestingly, a recent study in flies identified loss of Lamin-B, which also occurs in senescent cells (Freund et al., 2012), in the fly adipose tissue (fatbody) as a potential driver of systemic inflammation (Chen et al., 2014 In Press). Immune modulators secreted from the inflamed fatbody influence innate immune function and regenerative homeostasis also in distant tissues like the gut epithelium, suggesting an endocrine mechanism by which accumulated senescent cells promote loss of proliferative homeostasis systemically (Chen et al., 2014 In Press). Immune senescence and the loss of proliferative homeostasis in the intestinal epithelium of flies are tightly linked and contribute to age-related mortality (Biteau et al., 2010; Guo et al., 2014; Rera et al., 2012).
Studies on CC in flies have also inspired new approaches to understanding and potentially treating proliferative and degenerative diseases in mammals. Thus, CC has been suggested to play a central role in cancer in mammals (Moreno, 2008). The hypothesis put forward by Moreno suggests that targeting the process of CC, which potentiates the propagation of cells with a proliferative advantage, could be an effective anti-cancer therapy. This idea has been supported by the observation that flower (FWE)-deficient mice, in which tumorigenic ‘winner’ cells are expected to lose their competitive advantage, have reduced susceptibility to skin papilloma formation (Petrova et al., 2012). Furthermore, in the thymus, CC was recently shown to act as a tumor suppressor mechanism. Here, introducing improved CC efficiency by progenitor re-population with young-fit bone marrow-derived progenitors prevented malignant transformation (Martins et al., 2014). Finally, artificially induced CC has been proposed as a cell replacement strategy in the mammalian heart, where it can efficiently promote cardiomyocyte elimination and substitution without affecting heart anatomy or function (Villa Del Campo et al., 2014).
A striking example of how the SASP can fuel aging phenotypes in vertebrates is the cellular response to DNA damaging therapies for cancer treatment (Figure 2). These therapies can cause cellular senescence in the cancerous lesion and surrounding normal tissue. A recent analysis of middle-aged adults who were treated with genotoxic therapies for childhood cancer showed a striking acceleration of multiple age-related pathologies, including vascular disease and new cancers unrelated to their childhood tumors (Hudson et al., 2013). Further, therapy-induced senescent cells were shown to reduce the effect of subsequent therapies through the secretion of a WNT protein, which promoted cancer cell survival and disease progression (Sun et al., 2012). Finally, a recent study reported a role for the SASP in promoting obesity-induced cancer (Yoshimoto et al., 2013). Dietary or genetic obesity induced cellular senescence of hepatic stellate cells through DNA damage caused by a bacterial metabolite produced by the obesity-modified gut microbiome. The SASP of these senescent cells contained pro-inflammatory and pro-tumorigenic factors that, in turn, promoted the development and progression of hepatocellular carcinoma.
Conclusions
Cell competition and compensatory proliferation, mediated by paracrine signals, are clearly ancient mechanisms employed by developing metazoans to ensure proper morphogenesis and organogenesis -- and by adult organisms to promote tissue homeostasis. In young adult organisms, paracrine signaling thus ensures balanced tissue maintenance and repair. Later in life, however, these signaling mechanisms can be maladaptive, and drive both degenerative and hyperplastic changes associated with aging. A striking feature of both the positive and negative effects of paracrine signaling is its evolutionary conservation. Thus, organisms as different as fruit flies and mice (and humans) share many features of positive and negative paracrine signaling. The ability to harness the power of genetics in less complex organisms thus holds great promise for obtaining unique insights and developing novel therapies geared toward optimizing the beneficial effects of paracrine signaling in the aging organism.
ACKNOWLEDGEMENTS
Work in Dr. Jasper’s lab is supported by NIH grants AG028127, GM100196 and EY018177. Work in Dr. Campisi’s lab is supported by NIH grants AG009909, AG017242 and AG041122. Dr. Neves is supported by the Glenn Medical Foundation, and Dr. Demaria is supported by the American Italian Cancer Foundation.
REFERENCES
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Furnagalli M, DaCosta M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Adams PD. Healing and hurting: molecular mechanisms, functions and pathologies of cellular senescence. Molec Cell. 2009;36:2–14. doi: 10.1016/j.molcel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Adler PN, Bryant PJ. Participation of lethally irradiated imaginal disc tissue in pattern regulation in Drosophila. Developmental biology. 1977;60:298–304. doi: 10.1016/0012-1606(77)90126-9. [DOI] [PubMed] [Google Scholar]
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Developmental cell. 2003;5:441–450. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van deursen JM. Clearance of p16INK4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell stem cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell stem cell. 2011;9:402–411. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS genetics. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC biology. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nature reviews Microbiology. 2013;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev. 2011;21:107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zheng X, Zheng Y. Age-Associated Loss of Lamin-B Leads to Systemic Inflammation and Gut Hyperplasia. Cell. 2014 doi: 10.1016/j.cell.2014.10.028. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Bunker BD, Harvey KF, Vaccari T, Bilder D. A tumor suppressor activity of Drosophila Polycomb genes mediated by JAK-STAT signaling. Nature genetics. 2009;41:1150–1155. doi: 10.1038/ng.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claveria C, Giovinazzo G, Sierra R, Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500:39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour C, Parrinello S, Hodgson G, Chin K, Desprez PY, Campisi J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz D, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell non-automous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201:613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Senoo-Matsuda N, Ziosi M, Wu DC, Bellosta P, Quinzii CM, Johnston LA. Supercompetitor status of Drosophila Myc cells requires p53 as a fitness sensor to reprogram metabolism and promote viability. Cell metabolism. 2014;19:470–483. doi: 10.1016/j.cmet.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell RJ, Laberge R, Vijg J, Van Steeg H, Dollé MET, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental cell. 2014 doi: 10.1016/j.devcel.2014.11.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:173–176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Molecular biology of the cell. 2012;23:2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Patil PK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Karpac J, Tran SL, Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156:109–122. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes & development. 1998;12:3252–3263. doi: 10.1101/gad.12.20.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi S, Baranski TJ, Cagan RL. Transformed Drosophila cells evade diet-mediated insulin resistance through wingless signaling. Cell. 2013;154:664–675. doi: 10.1016/j.cell.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell stem cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Current biology : CB. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Current biology : CB. 2006;16:1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell stem cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nature Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Molecular and cellular biology. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nature Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, A AL, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Lee N, Maurange C, Ringrose L, Paro R. Suppression of Polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs. Nature. 2005;438:234–237. doi: 10.1038/nature04120. [DOI] [PubMed] [Google Scholar]
- Levy DE, Lee CK. What does Stat3 do? The Journal of clinical investigation. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129:1215–1225. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Lolo FN, Casas-Tinto S, Moreno E. Cell competition time line: winners kill losers, which are extruded and engulfed by hemocytes. Cell reports. 2012;2:526–539. doi: 10.1016/j.celrep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Losick VP, Fox DT, Spradling AC. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Current biology : CB. 2013;23:2224–2232. doi: 10.1016/j.cub.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins VC, Busch K, Juraeva D, Blum C, Ludwig C, Rasche V, Lasitschka F, Mastitsky SE, Brors B, Hielscher T, et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature. 2014;509:465–470. doi: 10.1038/nature13317. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Developmental biology. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Morata G, Shlevkov E, Perez-Garijo A. Mitogenic signaling from apoptotic cells in Drosophila. Development, growth & differentiation. 2011;53:168–176. doi: 10.1111/j.1440-169X.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- Moreno E. Is cell competition relevant to cancer? Nature reviews Cancer. 2008;8:141–147. doi: 10.1038/nrc2252. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635–644. doi: 10.1002/hep.20086. [DOI] [PubMed] [Google Scholar]
- Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH. IL-6/IL-6R axis plays a critical role in acute kidney injury. Journal of the American Society of Nephrology : JASN. 2008;19:1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, Igaki T. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Developmental cell. 2011;20:315–328. doi: 10.1016/j.devcel.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Takemoto D, Igaki T. Dissecting tumour heterogeneity in flies: genetic basis of interclonal oncogenic cooperation. Journal of biochemistry. 2014;156:129–136. doi: 10.1093/jb/mvu045. [DOI] [PubMed] [Google Scholar]
- Osman D, Buchon N, Chakrabarti S, Huang YT, Su WC, Poidevin M, Tsai YC, Lemaitre B. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. Journal of cell science. 2012;125:5944–5949. doi: 10.1242/jcs.113100. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. Journal of cell science. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Struhl G, Morata G. Dpp signaling and the induction of neoplastic tumors by caspase-inhibited apoptotic cells in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17664–17669. doi: 10.1073/pnas.0508966102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova E, Lopez-Gay JM, Rhiner C, Moreno E. Flower-deficient mice have reduced susceptibility to skin papilloma formation. Disease models & mechanisms. 2012;5:553–561. doi: 10.1242/dmm.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur A, Peeper DS. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol. 2008;20:150–155. doi: 10.1016/j.ceb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T, Jr., Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell metabolism. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhiner C, Lopez-Gay JM, Soldini D, Casas-Tinto S, Martin FA, Lombardia L, Moreno E. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Developmental cell. 2010;18:985–998. doi: 10.1016/j.devcel.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends in immunology. 2012;33:571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AB, Zoranovic T, Ayala-Camargo A, Grewal S, Reyes-Robles T, Krasny M, Wu DC, Johnston LA, Bach EA. Activated STAT regulates growth and induces competitive interactions independently of Myc, Yorkie, Wingless and ribosome biogenesis. Development. 2012;139:4051–4061. doi: 10.1242/dev.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrapatna VA, Bangi E, Cagan RL. Caspase signalling in the absence of apoptosis drives Jnk-dependent invasion. EMBO reports. 2013;14:172–177. doi: 10.1038/embor.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Developmental cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Sancho M, Di-Gregorio A, George N, Pozzi S, Sanchez JM, Pernaute B, Rodriguez TA. Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Developmental cell. 2013;26:19–30. doi: 10.1016/j.devcel.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo-Matsuda N, Johnston LA. Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18543–18548. doi: 10.1073/pnas.0709021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takino K, Ohsawa S, Igaki T. Loss of Rab5 drives non-autonomous cell proliferation through TNF and Ras signaling in Drosophila. Developmental biology. 2014;395:19–28. doi: 10.1016/j.ydbio.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Tamori Y, Deng WM. Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Developmental cell. 2013;25:350–363. doi: 10.1016/j.devcel.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y, Deng WM. Compensatory cellular hypertrophy: the other strategy for tissue homeostasis. Trends in cell biology. 2014;24:230–237. doi: 10.1016/j.tcb.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir N, Lowe SW. Senescent cells spread the word: non-cell autonomous propagation of cellular senescence. EMBO J. 2013;32:1975–1976. doi: 10.1038/emboj.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13123–13128. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa Del Campo C, Claveria C, Sierra R, Torres M. Cell competition promotes phenotypically silent cardiomyocyte replacement in the Mammalian heart. Cell reports. 2014;8:1741–1751. doi: 10.1016/j.celrep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Fletcher AG, Baena-Lopez LA. Mechanisms and mechanics of cell competition in epithelia. Nature reviews Molecular cell biology. 2013;14:581–591. doi: 10.1038/nrm3639. [DOI] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Role for IGFBP7 in senescence induction by BRAF. Cell. 2010;141:746–747. doi: 10.1016/j.cell.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- Wang L, Zeng X, Ryoo HD, Jasper H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS genetics In Press. 2014 doi: 10.1371/journal.pgen.1004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wen H, Ou S, Cui J, Fan D. IL-6 promotes regeneration and functional recovery after cortical spinal tract injury by reactivating intrinsic growth program of neurons and enhancing synapse formation. Experimental neurology. 2012;236:19–27. doi: 10.1016/j.expneurol.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]