Summary
Resistant hypertension is a clinically distinct subgroup of hypertension defined by the failure to achieve blood pressure control on optimal dosing of at least 3 antihypertensive medications of different classes, including a diuretic. The pathophysiology of hypertension can be attributed to aldosterone excess in more than 20% of patients with resistant hypertension. Existing dogma attributes the increase in blood pressure seen with increases in aldosterone to its antinatriuretic effects in the distal nephron. However, emerging research, which has identified and has begun to define the function of amiloride-sensitive sodium channels and mineralocorticoid receptors in the systemic vasculature, challenges impaired natriuresis as the sole cause of aldosterone-mediated resistant hypertension. This review integrates these findings to better define the role of the vasculature and aldosterone in the pathophysiology of resistant hypertension. In addition, a brief guide to the treatment of resistant hypertension is presented.
Keywords: Sodium channel, aldosterone, resistant hypertension, pathophysiology, amiloride
Maintaining an appropriate arterial pressure is essential for human life. Over hundreds of millions of years, the processes responsible for regulating blood pressure (BP) have evolved to respond to challenges such as change in position, extremes in diet, changes in tissue demands, and acute blood loss. As a result, a complex communication and feedback network has developed to control BP. Since the discovery of renin by Goldblatt et al1 in an animal model of hypertension, 2 primary regulators of BP, the renin-angiotensin-aldosterone system and the autonomic nervous system, have been identified.2 Although the interaction of these systems in physiologic models of hypertension is still being debated, research continues to define each system’s effects.3,4 The vasculature, seen as a responder to both systems (ie, vasoconstriction in response to angiotensin II or norepinephrine), may have a direct role in the development of hypertension.5–10 This article reviews the pathophysiology of hypertension that initially is resistant to medical therapy with a particular focus on aldosterone: its direct effect on the vasculature and the role of aldosterone blockade in the treatment of resistant hypertension.
DEFINING RESISTANT AND PSEUDORESISTANT HYPERTENSION
Resistant hypertension is defined based on BP response to standard therapy, and identifies a group of high-risk patients who may benefit from specialized care, including evaluation and treatment of secondary causes of hypertension. The definition was established in an American Heart Association scientific statement as “BP that remains above goal despite optimal doses of 3 antihypertensive agents of different classes, one ideally being a diuretic.”11
Resistant hypertension does not represent a single pathologic entity. Some individuals initially classified as resistant instead may have pseudoresistant hypertension, a distinction arising from limitations in BP measurement and management. Resistant individuals who have increased office BPs as a result of white-coat hypertension, improperly measured BPs, or medication nonadherence are reclassified as having pseudoresistant hypertension.11,12 This difference is useful not only in identifying pathology, but also in predicting outcomes. Patients with true resistant BP have an increased risk of cardiovascular events including stroke, myocardial infarction, and end-stage renal disease.13–16
PATHOPHYSIOLOGY OF RESISTANT HYPERTENSION
Our understanding of the pathology and physiology of hypertension stems from animal models of hypertension, genetic disorders of hypertension in human beings, kidney transplantation, computer models of BP physiology, and responses to pharmacologic therapy.17 With few exceptions, all of these areas converge on the kidney as an active participant in the development of hypertension.
Based on computer models, Guyton and Coleman18 concluded that the kidney’s regulation of sodium excretion made up the critical pathway that determines the chronic level of intra-arterial pressure. The high gain (ie, the capacity to return any aberrant pressure back into normal control) of the renal function curve (pressure-natriuresis relationship) are posited in the long run to override any extrarenal mechanisms of BP control.19 Under this theory, a rightward shifted renal function curve would be observed in all forms of hypertension; rightward shifts have been confirmed both in animal models of hypertension (spontaneously hypertensive rat, Goldblatt hypertension, aldosterone infusion, and angiotensin II infusion) and human hypertension (renovascular hypertension and primary aldosteronism)18–20.
Perhaps the strongest support for the Guytonian theory of the pathophysiology of hypertension is its survival through more than 40 years of experimentation and discovery in the field of hypertension. Kidney transplantation in human beings along with studies of cross-transplantation in animal models provides compelling evidence in support of the underlying theory. In the study by Curtis et al,21 6 individuals with hypertension resulting in nephrosclerosis and kidney failure underwent bilateral nephrectomy and kidney transplantation from normotensive unrelated donors. After 4.5 years of follow-up evaluation, all 6 participants were normotensive and had evidence of reversal of hypertensive damage to the heart and retinal vessels.21 Although the effects of bilateral renal denervation cannot be isolated from the return of a normal renal function curve, this study definitely identifies the kidney as a central mediator of human hypertension, and is consistent with kidney transplantation in animal models, in which BP nearly always follows the kidney.22–24
The mouse angiotensin type 1A (AT1A)-receptor knockout model is worth discussing in detail because it relates to our understanding of the pathophysiology of hypertension. In a cross-renal transplant model of AT1A-receptor–deficient mice and their wild-type litter-mates, identical levels of BP reduction were seen in the mice with whole-body AT1A-receptor deficiency plus intact kidney AT1A receptors and mice with intact extrarenal AT1A receptors plus deficient kidney AT1A receptors.21,22 The reduction in BP seen with a lack of whole-body AT1A receptors was shown to be independent of aldosterone or sympathetic nervous system effects, suggesting that AT1-receptor actions in systemic tissues such as the vascular and/or the central nervous systems make additional contributions to BP regulation.21,22 Regulation of BP by the renin-angiotensin system is mediated both within and outside the kidney.
These same cross-renal transplant models were investigated in the setting of hypertension. Throughout 4 weeks of continuous infusion of angiotensin II, hypertension was sustained only in mice with intact kidney AT1A receptors.24 Therefore, angiotensin II causes hypertension primarily through AT1 receptors in the kidney, which is consistent with Guyton’s hypothesis.18,19 The persistently low BP seen in mice without extrarenal AT1A receptors can be explained by a leftward shift of the renal function curve, providing evidence for an extrarenal mechanism of adjusting the pressure natriuresis set point.
The pathophysiology of resistant hypertension also involves a rightward shift of the renal function curve. However, it offers specific phenotypes for which a cause of hypertension can be identified (Table 1). Resistance to standard pharmacologic therapies (ie, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, thiazide diuretics, β-blockers, α-blockers, central acting agents, and peripheral vasodilators) characterizes secondary forms of hypertension.11 The majority of secondary causes of hypertension can be grouped by plasma renin activity level, with low-renin causes involving the distal nephron’s handling of sodium either through dysfunction of the mineralocorticoid receptor (MR) or direct tubular pathology (ie, epithelial sodium channel [ENaC] or sodium chloride co-transporter) (Table 1). Rare (eg, glucocorticoid-remediable aldosteronism) and very rare (eg, Liddle’s syndrome and familial hyperkalemic hypertension) secondary causes of hypertension have been well described.17,25 Primary aldosteronism is a common secondary cause of hypertension with a prevalence among individuals with resistant hypertension ranging from 20% to 23%.26,27
Table 1.
Pathobiology and Screening Method for Secondary Causes of Hypertension
| Secondary Cause | Pathobiology | Screening Method |
|---|---|---|
| Mineralocorticoid-receptor related |
||
| Primary aldosteronism | Functional macro-adrenal or micro-adrenal adenoma |
ARR, 24-h urine aldosterone |
| Syndrome of AME | Inactivation of 11 p-HSD2 | 24-h urine free Cortisol and free cortisone |
| Cushing’s syndrome | Grouping of many different diseases resulting in excess Cortisol |
24-h urine total Cortisol (in absence of exogenous glucocorticoids), dexamethasone suppression test |
| Glucocorticoid-remediable aldosteronism |
Chimeric gene results in ACTH-mediated aldosterone synthase activity |
ARR, 24-h urine aldosterone, genetic testing |
| Congenital adrenal hyperplasia |
11p–hydroxylase deficiency results in increased deoxycorticosterone |
In adolescents and adults: measurement of ARR, ACTH, Cortisol, 11-deoxycortisol, and 11-deoxycorticosterone |
| HTN exacerbated by pregnancy |
Mutation in ligand binding portion of the MR allows progesterone to activate |
ARR, urine protein assessment, genetic testing |
| Liddle’s syndrome | Gain-of-function mutation in β or γ subunit of ENaC |
ARR, genetic testing |
| Familial hyperkalemic hypertension |
WNK kinase 1 or 4 mutation leading to NCC overactivity |
Serum potassium, ARR, genetic testing |
| Chronic kidney disease | Multiple, including renal parenchyma loss | Kidney imaging, urine protein assessment (eg, ACR, UPR), serum creatinine-based eGFR |
| Atherosclerotic RAS | Renal artery narrowing from atherosclerotic plaque |
Renal duplex ultrasonography |
| RAS resulting from fibromuscular dysplasia |
Renal artery narrowing from tortuosity and abnormal wall growth |
CTA, MRA, renal angiogram |
| Pheochromocytoma | Catecholamine-secreting tumor | Plasma metanephrines, 24-h urine for catecholamines |
| Aortic coarctation | Narrowing of aorta results in upper-extremity HTN and lower-extremity hypotension |
Physical examination, 2-dimensional echocardiogram |
Abbreviations: 11β-HSD2, 11β-hydroxysteroid dehydrogenase 2; ACR, albumin-to-creatinine ratio; ACTH, adrenocorticotropic hormone; AME, apparent mineralocorticoid excess; ARR, plasma aldosterone-to-renin ratio; CTA, computed tomography angiography; eGFR, estimated glomerular filtration rate; HTN, hypertension; MRA, magnetic resonance angiography; NCC, sodium chloride cotransporter; RAS, renal artery stenosis; UPR, urinary protein-to-creatinine ratio; WNK, with no lysine = K.
ALDOSTERONE AND THE MINERALOCORTICOID RECEPTOR
Aldosterone is a mineralocorticoid produced in the zona glomerulosa of the adrenal cortex in response to angiotensin II, increased serum potassium, and corticotropin. Classically, it regulates total body sodium and potassium balance through genomic effects that follow binding and activating MR in the distal collecting duct of the kidney.28–30 More recently, extrarenal effects of aldosterone have been described in vascular endothelial and smooth muscle cells.5–10 The effects of aldosterone on vascular cells include inflammation, fibrosis, hypertrophic remodeling, endothelial stiffening, and oxidative stress, which are exacerbated in experimental animals on a high-salt diet.10,31
Similar to the AT1 receptor, the extrarenal effects of aldosterone also may contribute to the control of systemic BP. Aldosterone excess is undoubtedly a cause of hypertension, resulting in a rightward shift of the renal function curve.18–20 However, increases in BP also are seen with increases in plasma aldosterone levels within physiologic ranges. Normotensive participants of the Framingham Offspring Study showed an incremental increase in BP and development of hypertension at the 4-year follow-up evaluation with each increase in quartile of plasma aldosterone level.32 Furthermore, MR antagonists such as eplerenone or spironolactone reduced BP in essential hypertension33 and normotensive subjects,34 in addition to the highly responsive resistant hypertension group.35 In resistant hypertension, BP reduction occurs at low doses (25 mg spironolactone) and is not related directly to plasma aldosterone levels.36,37 Together, these data suggest that the BP-lowering effect of aldosterone antagonists may include renal as well as extrarenal components.
The study by McCurley et al6 supports the hypothesis that aldosterone can make extrarenal contributions to BP control. Mice lacking MR specifically in vascular smooth muscle cells had reduced BP with aging without alteration in renal sodium handling; the reduced BP was not affected by dietary sodium intake but could be overcome with aldosterone plus sodium chloride infusion. Furthermore, aged mice lacking vascular smooth muscle MR showed no significant vasoconstrictive or BP responses to angiotensin II infusion.6 Thus, the MR in vascular smooth muscle cells contributes to angiotensin II–induced vasoconstriction and BP increase. This finding is consistent with the well-known crosstalk between angiotensin II and aldosterone in the vascular smooth muscle cells.38–40 Cross-talk between the AT1 and MR receptors has been described in several systems, and provides a parsimonious explanation for the so-called nongenomic effects of aldosterone.41–44
SODIUM AND POTASSIUM INTAKE, ALDOSTERONE, AND HYPERTENSION
Diets low in sodium and high in potassium reduce BP despite increases in aldosterone levels.45–47 In adults with hypertension, plasma levels of renin activity, angiotensin II, and aldosterone all increase after 5 days of a very low sodium diet (10 mEq/d) with unaltered potassium intake.48 In this study, activation of the renin-angiotensin-aldosterone system (RAAS) correlated significantly with BP reduction; however, salt-sensitive black participants showed the greatest BP reduction with a less responsive RAAS 48 Less RAAS activation is required to maintain sodium homeostasis in salt-sensitive individuals who have a flatter renal function curve.
Individuals chronically consuming a low-sodium diet, such as the Yanomama Indians in southern Venezuela and northern Brazil whose average sodium intake is less than 10 mEq/d,49 require a chronically activated RAAS to maintain sodium homeostasis. When measured, aldosterone levels in Yanomama Indians (average 24-h urine aldosterone level, 70 µg) were higher than those seen in patients with primary aldosteronism.50 However, cardiovascular disease and hypertension rarely occur among Yanomama Indians. Low BP is expected in the setting of very low sodium intake when sodium balance is maintained on the low portion of the renal function curve. In this case, high aldosterone levels would not be expected to over-compensate and lead to hypertension. The beneficial effects of a low BP and an active lifestyle may offset any cardiovascular risk from high aldosterone levels.
Yanomama Indians also consume large amounts of potassium,50 which contributes to both a lower BP and cardiovascular risk.51 After adjustment for covariates in the International Study of Salt and BP, the statistical association with BP was stronger for the urinary sodium-to-potassium ratio than individual levels of urinary sodium and potassium.46 These findings and others suggest that diets resulting in a physiologic activation of the RAAS reduce cardiovascular risk.52 Therefore, high levels of aldosterone alone are not sufficient to increase BP or cardiovascular risk. The causal relationship between aldosterone and hypertension exists when aldosterone levels remain unsuppressed from high-sodium and low-potassium intake, the typical American diet. Proposed mechanisms of this diet-dependent effect include central nervous system activation and increased vascular resistance.53
AMILORIDE-SENSITIVE SODIUM CHANNELS IN THE VASCULAR SYSTEM
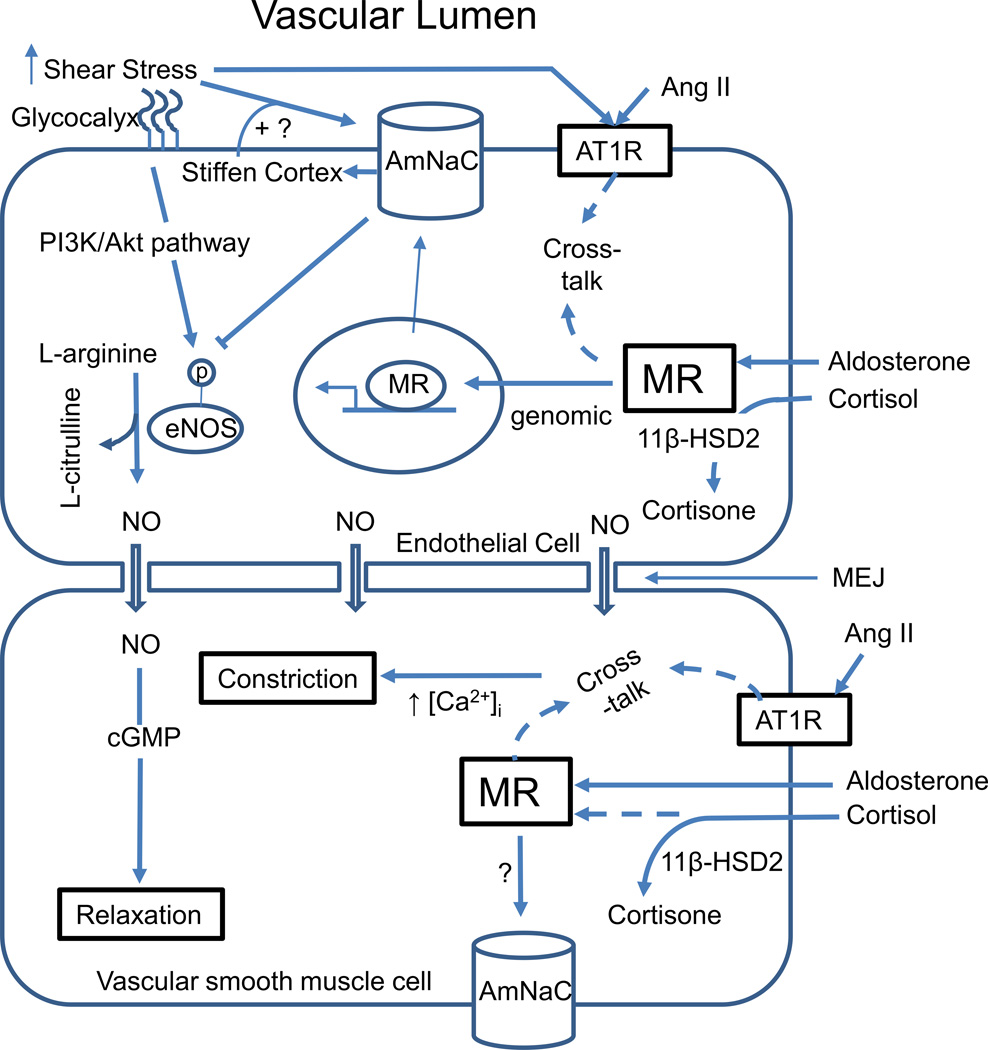
Discovery of amiloride-sensitive sodium channels in both endothelial and vascular smooth muscle cells offers potential mechanisms through which MR activation and/or flow sensors can affect vascular function and BP (Fig. 1).10 ENaC function and response to aldosterone in the distal nephron has been well described.28–30 In the vasculature, in vitro experiments indicate that amiloride-sensitive sodium channels regulate the mechanical properties of the endothelial cell (ie, the cell’s stiffness).9,54,55 Aldosterone leads to acute endothelial cell swelling, likely from sodium and water uptake associated with increased amiloride-sensitive sodium channel abundance in the luminal membrane, re-organization of the subcortical cytoskeleton, and increased mechanical stiffness of the endothelial cell surface membrane.54–56 In addition, local nitric oxide production is reduced, leading to vasoconstriction of the neighboring vascular smooth muscle cells.57,58 Importantly, the vascular response to aldosterone can occur within minutes with plasma membrane insertion of sodium channels (Fig. 1).55 In addition, treatment with amiloride can prevent acute endothelial cell swelling.56 Taken together these findings suggest that endothelial amiloride-sensitive sodium channels contribute to vascular function, and aldosterone is an important regulator of these functions.
Figure 1.
Interaction between the mineralocorticoid receptor and amiloride-sensitive sodium channel in the vasculature. Barauna et al87 showed that shear stress can activate AT1R on the surface of the endothelial cell; an effect that is blocked by the presence of an angiotensin-receptor blocker. 11β-HSD2, 11β-hydroxysteroid dehydrogenase 2; AmNaC, amiloride-sensitive sodium channel; Ang II, angiotensin II; cGMP, cyclic guanosine monophosphate; MEJ, myoendothelial junction; PI3K/Akt, phosphoinositide-3-kinase/(protein kinase B).
Perez et al59 investigated the role of endothelial amiloride-sensitive sodium channels in catecholamine-mediated vasoconstriction. In rat mesenteric arteries, phenylephrine-induced vasoconstriction was blunted markedly by amiloride, an effect lost in endothelium-denuded arteries and greatly diminished by an endothelial nitric oxide synthase (eNOS) inhibitor, or under conditions of reduced flow. From these experiments, the investigators concluded that the endothelial sodium channel is a negative modulator of eNOS and vasodilation in response to shear stress (Fig. 1).
Shear stress, the tangential force derived by the friction of the flowing blood on the endothelial surface, is known to initiate vasodilation through increased eNOS activity as well as directly affecting amiloride-sensitive sodium channel activity.59,60 Kusche-Vihrog et al55 hypothesized that the endothelial glycocalyx, which acts as a sodium buffer, may be disrupted in the setting of increased shear stress, thereby interfering with endothelial function and responses to changes in blood flow. Conditions such as atherosclerotic disease with altered plaque-related changes in blood flow and shear stress may have different vascular responses to aldosterone61.
The vascular effects of aldosterone differ by study population with the greatest benefit of MR antagonists seen among individuals with cardiovascular disease and clinically significant heart failure.62,63 Improvement in endothelial function has been put forward as an explanation for the reduced mortality seen with MR antagonists in clinical outcome trials of heart failure.64–66 The dynamic relationship between shear stress, the endothelial glycocalyx, eNOS activity, and vascular amiloride-sensitive sodium channels may be involved in the increased cardiovascular risk associated with hypertension.
TREATMENT OF RESISTANT HYPERTENSION
In the absence of well-defined understanding of the underlying vascular physiology, current BP management has evolved largely from trial and error. The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial showed the importance of overall efficacy regardless of choice in initial BP agent (lisinopril, amlodipine, or chlorthalidone). 67 However, if more than one antihypertensive agent is required, which is the case for approximately 30% of all hypertensive individuals in the United States,68 then amlodipine plus benazepril appears superior to benazepril plus hydrochlorothiazide in reducing cardiovascular events.69 The most recent guidelines70–72 support the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, dihydropyridine calcium channel blockers, and thiazide diuretics as initial therapies.
Treatment is more complicated for individuals who do not achieve BP control with optimal doses of those 3 classes of medications. Once pseudoresistance has been excluded and conditions interfering with BP control have been addressed (Table 2), screening for a secondary cause of hypertension should be performed to identify an etiology for targeted therapy (Table 1). More than 20% of patients with resistant hypertension will have aldosterone excess and may require additional work-up for primary aldosteronism.26–27 Suppressed plasma renin activity levels with an inappropriately low aldosterone level for the serum concentration of potassium is consistent, with very rare secondary causes such as Liddle’s syndrome or familial hyperkalemic hypertension.73,74 Detailed recommendations for the evaluation of secondary causes is summarized in the American Heart Association statement on resistant hypertension.11
Table 2.
Comorbidities and Drugs Interfering With Blood Pressure Control
| Comorbidities | Interfering Drugs |
|---|---|
| Smoking | NSAIDs |
| Smokeless tobacco | Sympathomimetics |
| Obesity/insulin resistance | Nasal decongestants |
| Obstructive sleep apnea | Appetite suppressants |
| Excess alcohol intake | Cocaine |
| Anxiety | Stimulants |
| Hyperventilation | Methylphenidate |
| Panic attacks | Amphetamine |
| Chronic pain syndromes | Caffeine |
| Oral contraceptives (estrogen) | |
| Adrenal steroids | |
| Natural licorice | |
| Tacrolimus, cyclosporine | |
| Erythropoietin | |
| Herbals (ephedra, ma huang) |
NSAIDs, nonsteroidal anti-inflammatory drugs.
Individuals with resistant hypertension are frequently salt-sensitive, particularly those with chronic kidney disease and black race. This phenotype, which is characterized by low plasma renin activity, may represent a dysregulation of aldosterone or ENaC; however, efforts to identify a broad genetic cause have not been unsuccessful.75 When clinically feasible, instituting a low-sodium diet in individuals with resistant hypertension can have dramatic effects on BP reduction. In a controlled cross-over trial, mean 24-hour BP decreased by an average of 20/10 mm Hg by reducing dietary sodium intake from 250 to 50 mmol/d in individuals with resistant hypertension.76 In the United States, where the typical diet consists of 153 mmol/d (3.5 g/d) of sodium,77 effectively reducing dietary sodium can be challenging, and requires ongoing dietary counseling and feedback based on measured 24-hour urinary sodium excretion.
Diuretics effectively decrease BP in the majority of patients with resistant hypertension. Chlorthalidone, a thiazide-like diuretic, rapidly concentrates in erythrocytes, and with long-term dosing the erythrocyte pool acts as a depot slowly releasing stored chlorthalidone into the plasma.78,79 The steady depot release creates a long duration of effect (48–72 hours with long-term dosing).80 This is in contrast to the 16- to 24-hour duration of effect with long-term dosing of hydrochlorothiazide. This difference in effective half-life between hydrochlorothiazide and chlorthalidone may have importance beyond concerns of medication adherence. Chlorthalidone is more effective at decreasing BP, particularly in patients who are salt-sensitive,81 and is recommended as the preferred diuretic in the treatment of resistant hypertension.11
In addition to salt sensitivity, hypokalemic metabolic alkalosis is also common among individuals with resistant hypertension. Even with normal aldosterone levels, chlorthalidone-mediated kaliuresis can be ameliorated by the use of a potassium-sparing agent. However, before considering the addition of a potassium-sparing diuretic to thiazides, serious attention should be paid to restriction of dietary salt intake.23 The combination of a MR antagonist and a thiazide-like diuretic make physiologic sense, especially in resistant hypertension, in which baseline aldosterone levels are high. In experimental studies with rats, aldosterone infusion or oral fludrocortisone increases sodium chloride co-transporter expression, the thiazide-sensitive sodium chloride cotransporter.82 Furthermore, chlorthalidone-induced increases in sympathetic nervous system activity, as measured by peroneal microneurography, are returned back to baseline levels with the addition of spironolactone.83
The benefit of MR antagonism in resistant hypertension goes beyond counteracting the adverse effects of thiazide-like diuretics. MR antagonists are extremely effective at reducing BP in patients with resistant hypertension. In a follow-up analysis of the Anglo-Scandinavian Cardiac Outcomes Trial, 1,411 participants received spironolactone at a median dose of 25 mg in addition to a mean of 3 other antihypertensive medications. With a median treatment duration of 1.3 years, BP decreased from 157/85 mm Hg by 22/10 mm Hg with the addition of spironolactone.35 These effects on BP as well as vascular remodeling can be seen with doses of spironolactone that are below those required to completely block the renal effects of aldosterone.36,37,84–86 With the recent appreciation of the effects of aldosterone on the vasculature, it is reasonable to consider that the BP reductions with spironolactone in resistant hypertension may reflect vascular effects in addition to the classic renal effects, including natriuresis.10 In this case a vascular-related leftward shift in the renal function curve would be needed to sustain BP reduction, which is an interesting extension of the classic Guytonian theory of BP regulation.
CONCLUSIONS
A systematic investigation into the pathophysiology of hypertension has been initiated with the classification of resistant hypertension. In the majority of cases this search will show an abnormality involving aldosterone. Although aldosterone-mediated impaired natriuresis is the principle source of the hypertension, the vascular effects of aldosterone also may contribute to hypertension. A complex and dynamic interplay between endothelial function, vascular amiloride-sensitive sodium channel activity, shear stress, and vascular MR modulate the vascular responses to aldosterone. As future research continues to define the role of aldosterone in the vasculature, new therapeutic targets may arise for the treatment of hypertension as well as other diseases.86
Acknowledgments
Financial support: Supported by the National Institutes of Health (T32 HL007457), the University of Alabama at Birmingham’s Department of Medicine Walter B. Frommeyer Jr Award (E.J.), and by the University of Alabama at Birmingham (UAB)-University of California at San Diego (UCSD) O’Brien Core Center for Acute Kidney Injury Research (DK079337). D.G.W. is the Hilda B. Anderson Endowed Professor of Nephrology at the University of Alabama at Birmingham.
Footnotes
Conflict of interest statement: David Warnock is a consultant for Gilead Sciences and Parion Sciences, and has provided research support for Bayer. David Calhoun is a consultant for Bayer, and has provided research support for Novartis and Medtronic.
REFERENCES
- 1.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso N, Terragno NA. History about the discovery of the reninangiotensin system. Hypertension. 2001;38:1246–1249. doi: 10.1161/hy1201.101214. [DOI] [PubMed] [Google Scholar]
- 3.Montani JP, Van Vliet BN. Understanding the contribution of Guyton’s large circulatory model to long-term control of arterial pressure. Exp Physiol. 2009;94:382–388. doi: 10.1113/expphysiol.2008.043299. [DOI] [PubMed] [Google Scholar]
- 4.Osborn JW, Averina VA, Fink GD. Current computational models do not reveal the importance of the nervous system in long-term control of arterial pressure. Exp Physiol. 2009;94:389–396. doi: 10.1113/expphysiol.2008.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, et al. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–2463. doi: 10.1096/fj.09-147926. [DOI] [PubMed] [Google Scholar]
- 6.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao ML, Metzger D, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol. 2012;350:256–265. doi: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffrin EL. Vascular mineralocorticoid receptors regulate blood pressure effects on myogenic tone and role in aging. Circ Res. 2013;112:415–417. doi: 10.1161/CIRCRESAHA.113.300883. [DOI] [PubMed] [Google Scholar]
- 9.Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, et al. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension. 2013;61:1053–1059. doi: 10.1161/HYPERTENSIONAHA.111.199455. [DOI] [PubMed] [Google Scholar]
- 10.Warnock DG, Kusche-Vihrog K, Tarjus A, Sheng S, Oberleithner H, Kleyman TR, et al. Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nat Rev Nephrol. 2014;10:146–157. doi: 10.1038/nrneph.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis evaluation, treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 12.Sarafidis PA, Georgianos P, Bakris GL. Resistant hypertension-its identification and epidemiology. Nat Rev Nephrol. 2013;9:492. doi: 10.1038/nrneph.2012.260. [DOI] [PubMed] [Google Scholar]
- 13.Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422–1428. doi: 10.1016/j.amjhyper.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168:2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 15.Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutierrez OM, Irvin MR, et al. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8:1583–1590. doi: 10.2215/CJN.00550113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutierrez OM, Irvin MR, et al. Incident ESRD and treatment-resistant hypertension: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Kidney Dis. 2014;63:781–788. doi: 10.1053/j.ajkd.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 18.Guyton AC, Coleman TG. Long-term regulation of the circulation: interrelationships with body fluid volumes. In: Reeve EB, Guyton AC, editors. Physical bases of circulatory transport regulation and exchange. Philadelphia: Saunders; 1967. pp. 179–201. [Google Scholar]
- 19.Guyton AC. Renal function curve-a key to understanding the pathogenesis of hypertension. Hypertension. 1987;10:1–6. doi: 10.1161/01.hyp.10.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Kimura G, Saito F, Kojima S, Yoshimi H, Abe H, Kawano Y, et al. Renal function curve in patients with secondary forms of hypertension. Hypertension. 1987;10:11–15. doi: 10.1161/01.hyp.10.1.11. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JJ, Luke RG, Dustan HP, Kashgarian M, Whelchel JD, Jones P, et al. Remission of essential hypertension after renal transplantation. N Engl J Med. 1983;309:1009–1015. doi: 10.1056/NEJM198310273091702. [DOI] [PubMed] [Google Scholar]
- 22.Rettig R, Grisk O. The kidney as a determinant of genetic hypertension: evidence from renal transplantation studies. Hypertension. 2005;46:463–428. doi: 10.1161/01.HYP.0000178189.68229.8a. [DOI] [PubMed] [Google Scholar]
- 23.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffman TM, Crowley SD. Kidney in hypertension: Guyton redux. Hypertension. 2008;51:811–816. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 25.Jain G, Ong S, Warnock DG. Genetic disorders of potassium homeostasis. Semin Nephrol. 2013;33:300–309. doi: 10.1016/j.semnephrol.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 27.Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–2226. doi: 10.1097/00004872-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Rossier BC. 1996 Homer Smith Award Lecture. Cum grano salis: the epithelial sodium channel and the control of blood pressure. J Am Soc Nephrol. 1997;8:980–992. doi: 10.1681/ASN.V86980. [DOI] [PubMed] [Google Scholar]
- 29.Studer RA, Person E, Robinson-Rechavi M, Rossier BC. Evolution of the epithelial sodium channel and the sodium pump as limiting factors of aldosterone action on sodium transport. Physiol Genomics. 2011;43:844–854. doi: 10.1152/physiolgenomics.00002.2011. [DOI] [PubMed] [Google Scholar]
- 30.Rossier BC, Staub O, Hummler E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: importance in the control of blood pressure and hypertension. FEBS Lett. 2013;587:1929–1941. doi: 10.1016/j.febslet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Park JB, Schiffrin EL. ET(A) receptor antagonist prevents blood pressure elevation and vascular remodeling in aldosterone-infused rats. Hypertension. 2001;37:1444–1449. doi: 10.1161/01.hyp.37.6.1444. [DOI] [PubMed] [Google Scholar]
- 32.Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 33.Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension. 2008;51:432–439. doi: 10.1161/HYPERTENSIONAHA.107.103267. [DOI] [PubMed] [Google Scholar]
- 34.Pratt JH, Eckert GJ, Newman S, Ambrosius WT. Blood pressure responses to small doses of amiloride and spironolactone in normotensive subjects. Hypertension. 2001;38:1124–1129. doi: 10.1161/hy1101.095010. [DOI] [PubMed] [Google Scholar]
- 35.Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–345. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 36.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. doi: 10.1016/s0895-7061(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 37.Oxlund CS, Henriksen JE, Tarnow L, Schousboe K, Gram J, Jacobsen IA. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens. 2013;31:2094–2102. doi: 10.1097/HJH.0b013e3283638b1a. [DOI] [PubMed] [Google Scholar]
- 38.Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, et al. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res. 2007;76:506–516. doi: 10.1016/j.cardiores.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Yamada M, Kushibiki M, Osanai T, Tomita H, Okumura K. Vasoconstrictor effect of aldosterone via angiotensin II type 1 (ATI) receptor: possible role of ATI receptor dimerization. Cardiovasc Res. 2008;79:169–178. doi: 10.1093/cvr/cvn064. [DOI] [PubMed] [Google Scholar]
- 40.Batenburg WW, Jansen PM, van den Bogaerdt AJ, Danser AH. Angiotensin II-aldosterone interaction in human coronary microarteries involves GPR30, EGFR, and endothelial NO synthase. Cardiovasc Res. 2012;94:136–143. doi: 10.1093/cvr/cvs016. [DOI] [PubMed] [Google Scholar]
- 41.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocorticoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 42.Di Zhang A, Nguyen Dinh Cat A, Soukaseum C, Escoubet B, Cherfa A, Messaoudi S, et al. Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension. 2008;52:1060–1067. doi: 10.1161/HYPERTENSIONAHA.108.117531. [DOI] [PubMed] [Google Scholar]
- 43.Jain G, Campbell RC, Warnock DG. Mineralocorticoid receptor blockers and chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1685–1691. doi: 10.2215/CJN.01340209. [DOI] [PubMed] [Google Scholar]
- 44.Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76:834–839. doi: 10.1016/j.steroids.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Anonymous. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;267:1213–1220. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]
- 46.Anonymous. Intersalt: an international study of electrolyte excretion, blood pressure. Results for 24 hour urinary sodium, potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium, the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 48.He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension. 1998;32:820–824. doi: 10.1161/01.hyp.32.5.820. [DOI] [PubMed] [Google Scholar]
- 49.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52:146–151. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- 50.Nowaczynski W, Oliver WJ, Neel JV. Serum aldosterone and protein-binding variables in Yanomama Indians: a no-salt culture as compared to partially acculturated Guaymi Indians. Clin Physiol Biochem. 1985;3:289–306. [PubMed] [Google Scholar]
- 51.Whelton PK. Potassium and blood pressure. In: Sica DA, Black HR, editors. Hypertension primer. 4th ed. Dallas, TX: American Heart Association: Council on High Blood Pressure Research; 2008. pp. 304–306. [Google Scholar]
- 52.Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169:32–40. doi: 10.1001/archinternmed.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adrogue HJ, Madias NE. Sodium surfeit and potassium deficit: keys to the pathogenesis of hypertension. J Am Soc Hypertens. 2014;8:203–213. doi: 10.1016/j.jash.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Oberleithner H. Aldosterone makes human endothelium stiff and vulnerable. Kidney Int. 2005;67:1680–1682. doi: 10.1111/j.1523-1755.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 55.Kusche-Vihrog K, Jeggle P, Oberleithner H. The role of ENaC in vascular endothelium. Pflugers Arch. 2014;466:851–859. doi: 10.1007/s00424-013-1356-3. [DOI] [PubMed] [Google Scholar]
- 56.Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, et al. Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch. 2008;455:849–857. doi: 10.1007/s00424-007-0341-0. [DOI] [PubMed] [Google Scholar]
- 57.Kusche-Vihrog K, Callies C, Fels J, Oberleithner H. The epithelial sodium channel (ENaC): mediator of the aldosterone response in the vascular endothelium? Steroids. 2010;75:544–549. doi: 10.1016/j.steroids.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Druppel V, Kusche-Vihrog K, Grossmann C, Gekle M, Kasprzak B, Brand E, et al. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013;27:3652–3659. doi: 10.1096/fj.13-228312. [DOI] [PubMed] [Google Scholar]
- 59.Perez FR, Venegas F, Gonzalez M, Andres S, Vallejos C, Riquelme G, et al. Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3-kinase/Akt in small-diameter mesenteric arteries. Hypertension. 2009;53:1000–1007. doi: 10.1161/HYPERTENSIONAHA.108.128520. [DOI] [PubMed] [Google Scholar]
- 60.Wang S, Meng F, Mohan S, Champaneri B, Gu Y. Functional ENaC channels expressed in endothelial cells: a new candidate for mediating shear force. Microcirculation. 2009;16:276–287. doi: 10.1080/10739680802653150. [DOI] [PubMed] [Google Scholar]
- 61.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 62.Collier TJ, Pocock SJ, McMurray JJ, Zannad F, Krum H, van Veldhuisen DJ, et al. The impact of eplerenone at different levels of risk in patients with systolic heart failure and mild symptoms: insight from a novel risk score for prognosis derived from the EMPHASIS-HF trial. Eur Heart J. 2013;34:2823–2829. doi: 10.1093/eurheartj/eht247. [DOI] [PubMed] [Google Scholar]
- 63.Krum H, Shi H, Pitt B, McMurray J, Swedberg K, van Veldhuisen DJ, et al. Clinical benefit of eplerenone in patients with mild symptoms of systolic heart failure already receiving optimal best practice background drug therapy: analysis of the EMPHASIS-HF study. Circ Heart Fail. 2013;6:711–718. doi: 10.1161/CIRCHEARTFAILURE.112.000173. [DOI] [PubMed] [Google Scholar]
- 64.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity, mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 65.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 66.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 67.Anonymous. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 68.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 70.Krause T, Lovibond K, Caulfield M, McCormack T, Williams B. Management of hypertension: summary of NICE guidance. Br Med J. 2011;343:d4891. doi: 10.1136/bmj.d4891. [DOI] [PubMed] [Google Scholar]
- 71.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 72.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;11:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 73.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, et al. Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 74.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 75.Warnock DG. Aldosterone-related genetic effects in hypertension. Curr Hypertens Rep. 2000;2:295–301. doi: 10.1007/s11906-000-0013-3. [DOI] [PubMed] [Google Scholar]
- 76.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957–2003: a systematic review. Am J Clin Nutr. 2010;92:1172–1180. doi: 10.3945/ajcn.2010.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collste P, Garle M, Rawlins MD, Sjoqvist F. Interindividual differences in chlorthalidone concentration in plasma and red cells of man after single and multiple doses. Eur J Clin Pharmacol. 1976;9:319–325. doi: 10.1007/BF00561667. [DOI] [PubMed] [Google Scholar]
- 79.Riess W, Dubach UC, Burckhardt D, Theobald W, Vuillard P, Zimmerli M. Pharmacokinetic studies with chlorthalidone (Hygroton) in man. Eur J Clin Pharmacol. 1977;12:375–382. doi: 10.1007/BF00562454. [DOI] [PubMed] [Google Scholar]
- 80.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4–9. doi: 10.1161/01.HYP.0000103632.19915.0E. [DOI] [PubMed] [Google Scholar]
- 81.Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 82.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raheja P, Price A, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, et al. Spironolactone prevents chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients. Hypertension. 2012;60:319–325. doi: 10.1161/HYPERTENSIONAHA.112.194787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ori Y, Chagnac A, Korzets A, Zingerman B, Herman-Edelstein M, Bergman M, et al. Regression of left ventricular hypertrophy in patients with primary aldosteronism/low-renin hypertension on low-dose spironolactone. Nephrol Dial Transplant. 2013;28:1787–1793. doi: 10.1093/ndt/gfs587. [DOI] [PubMed] [Google Scholar]
- 85.Funder JW. Primary aldosteronism and low-renin hypertension: a continuum? Nephrol Dial Transplant. 2013;28:1625–1627. doi: 10.1093/ndt/gft052. [DOI] [PubMed] [Google Scholar]
- 86.Warnock DG. The amiloride-sensitive endothelial sodium channel and vascular tone. Hypertension. 2013;61:952–954. doi: 10.1161/HYPERTENSIONAHA.113.00768. [DOI] [PubMed] [Google Scholar]
- 87.Barauna VG, Mantuan PR, Magalhaes FC, Campos LC, Krieger JE. ATI receptor blocker potentiates shear-stress induced nitric oxide production via modulation of eNOS phosphorylation of residues Thr(495) and Ser(1177.) Biochem Biophys Res Commun. 2013;441:713–719. doi: 10.1016/j.bbrc.2013.10.108. [DOI] [PubMed] [Google Scholar]