Significance
Ammonites went extinct at the time of the end-Cretaceous asteroid impact, as did more than 90% of species of calcium carbonate-shelled plankton (coccolithophores and foraminifera). Comparable groups not possessing calcium carbonate shells were less severely affected, raising the possibility that ocean acidification, as a side effect of the collision, might have been responsible for the apparent selectivity of the extinctions. We investigated whether ocean acidification could have caused the disappearance of the calcifying organisms. In a first detailed modelling study we simulated several possible mechanisms from impact to seawater acidification. Our results suggest that acidification was most probably not the cause of the extinctions.
Keywords: ocean acidification, asteroid impact, K/Pg boundary, mass extinction
Abstract
Most paleo-episodes of ocean acidification (OA) were either too slow or too small to be instructive in predicting near-future impacts. The end-Cretaceous event (66 Mya) is intriguing in this regard, both because of its rapid onset and also because many pelagic calcifying species (including 100% of ammonites and more than 90% of calcareous nannoplankton and foraminifera) went extinct at this time. Here we evaluate whether extinction-level OA could feasibly have been produced by the asteroid impact. Carbon cycle box models were used to estimate OA consequences of (i) vaporization of up to 60 × 1015 mol of sulfur from gypsum rocks at the point of impact; (ii) generation of up to 5 × 1015 mol of NOx by the impact pressure wave and other sources; (iii) release of up to 6,500 Pg C as CO2 from vaporization of carbonate rocks, wildfires, and soil carbon decay; and (iv) ocean overturn bringing high-CO2 water to the surface. We find that the acidification produced by most processes is too weak to explain calcifier extinctions. Sulfuric acid additions could have made the surface ocean extremely undersaturated (Ωcalcite <0.5), but only if they reached the ocean very rapidly (over a few days) and if the quantity added was at the top end of literature estimates. We therefore conclude that severe ocean acidification might have been, but most likely was not, responsible for the great extinctions of planktonic calcifiers and ammonites at the end of the Cretaceous.
From preindustrial times up to 2008, ca. 530 Pg of carbon were added to the atmosphere through burning of fossil fuels and deforestation (1). This has led to an increase in atmospheric CO2 of 40% (from 280 in 1750 to 400 ppm today). Simultaneously, about 160 Pg C has been taken up by the ocean, causing ocean acidification (OA) (2).
OA is of particular concern for calcifying organisms, because it leads to lower CO32− concentrations and hence lower seawater saturation states with respect to CaCO3 (Ω). In theory, lower Ω should make it energetically more costly for organisms to synthesize CaCO3 shells and skeletons and, subsequently, if Ω falls below 1.0, to maintain them against dissolution. A large variety of short-term experiments have been carried out to test for such consequences (2). It is widely recognized, however, that one aspect that these experiments generally do not address is the degree to which organisms can evolve in response to the changing carbonate chemistry and thereby become more tolerant of the new conditions. As a result, there is a need for approaches that reveal the long-term response to OA with evolutionary adaptation factored in.
Motivation
Using Earth History to Understand Ocean Acidification Impacts.
Events in the past could potentially shed more light on the evolutionary response to OA. However, a recent review (3) highlighted a major difficulty: During most suspected OA events, CO2 levels rose so slowly that the carbonate compensation process, that is, the automatic stabilizing mechanism opposing changes in Ω (4), must have interposed to alter the nature of the impacts, making them less useful for understanding the future.
In contrast, at the end of the Cretaceous the asteroid impact induced very sudden changes. Here we investigate the possibility that there was a sharp and sudden acidification event concentrated in surface waters [deep waters experience delayed and less severe acidification in response to an atmospheric source of acidity (5)]. Because there are no paleo records with which to constrain seawater chemistry changes during the critical few years following the impact (the slow speed at which most ocean sediments accumulate limits the resolution of sediment records to thousands of years), we use models to calculate how dramatic the surface OA may have been at the end of the Cretaceous.
Extinctions of Calcifiers at the End of the Cretaceous.
Another reason for being particularly interested in the Cretaceous/Paleogene (K/Pg) boundary in the context of OA is that many surface-dwelling calcifiers went extinct at this time (6). Ammonites had existed on Earth for some 300 million years and had survived previous extinction events, including the one at the end of the Permian when more than 95% of all marine species were lost, but they succumbed at the K/Pg (7). Within other groups of marine organisms there seems also to have been a strong extinction bias toward calcifiers. Among autotrophs, for example, more than 90% of all calcareous nannoplankton (coccolithophore) species went extinct at this time (8, 9). By contrast, there were much lower extinction rates for comparable noncalcareous groups, such as siliceous diatoms, of which at most 50% of species went extinct (10), organic-walled dinoflagellates, which experienced no significant extinction (11), and noncalcifying haptophyte phytoplankton, of which many clades survived the K/Pg (12). Similarly, among heterotrophs, more than 95% of carbonate-shelled planktic foraminifera were lost (10), whereas only a few planktic silica-shelled radiolaria went extinct (10). The particular severity of extinctions for calcifiers has led to suggestions (e.g., refs. 13 and 14) that they were caused by OA.
We used biogeochemical box models of the global carbon cycle (simulating particle fluxes, mixing, and air–sea CO2 exchange, explained in greater detail in Methods and Supporting Information) to assess whether severe OA might have occurred. Because of the absence of accompanying paleodata at this timescale, our aim is not to pin down the exact pattern of carbon chemistry changes that took place at the K/Pg. Instead, we focus our attention on delineating the upper bound of OA severity. Our aim is to calculate the maximum degree of OA that might plausibly have occurred, not the most likely.
Previous Work on Carbon Chemistry at the K/Pg
A few other studies (14–18) have previously addressed carbon cycle and OA changes at the K/Pg. Beerling et al. (16) used data (the stomatal index of land plant leaves) and a box model; they suggested that atmospheric pCO2 increased from 350 to 500 to 2,300 ppmv across the K/Pg boundary [although such a rise is not seen in other proxy data (17)], from which they inferred an instantaneous transfer of ca. 4,600 Pg C from rocks to the atmosphere.
D’Hondt et al. (18) used calculations rather than models to investigate the severity of surface OA at the K/Pg boundary. They calculated the consequences of acid creation (0.1–1.3 × 1017 mol H2SO4) from vaporization of gypsum rocks at the site of impact and, secondarily, from nitric acid. The lower amounts do not significantly affect surface ocean pH, according to their calculations, but the highest amounts would be large enough (>1.2 × 1017) to destroy entirely the carbonate buffer capacity of the upper 100 m of the modern global ocean and drive pH transiently to values as low as 3. However, such extreme pH changes are by no means necessary to make seawater strongly corrosive to CaCO3 (Ω ≪1), which can be achieved by a pH drop of less than one unit from its modern value of just over 8.
We revisit the OA estimates of D’Hondt et al. (18), carrying out the first evaluation to our knowledge using an established dynamic ocean carbon cycle model. Whereas D’Hondt et al. (18) focused on how much acid is needed to overwhelm the entire buffering capacity of seawater, we focus instead on the consequences for Ωcalcite (the saturation state of seawater with respect to the calcite form of CaCO3). We explicitly calculate and compare the potential of different hypothesized sources of acidity to lower surface (0–100 m) ocean pH and Ωcalcite at the end of the Cretaceous.
Results
Sulfate Aerosols Due to Impact on Gypsum-Rich Rocks.
An asteroid estimated at ∼10 km in diameter (19) hit the Earth on the Yucatan peninsula in Mexico, producing the Chicxulub crater (diameter ∼200 km). The target rocks (carbonate- and gypsum/anhydrite-rich sediments underlain by granite crust) were partly ejected and partly volatilized by the impact. In addition to sulfur from the impact rocks, 1–5 × 1015 mol S could have come from the asteroid itself (20). Thermal decomposition of gypsum or anhydrite (we refer only to gypsum henceforth) is presumed (on the basis of laboratory volatilization experiments, e.g., ref. 21) to have led to the near-instantaneous release of SO3 (14) to the atmosphere according to the reaction
According to this equation there was also a simultaneous equimolar production of base (in the form of lime, CaO), some of which could have counteracted the effects of the acid (22). However, consistent with our goal of estimating maximum possible impacts, our simulations did not include either lime effects on pH or the possible loss of SO3 through recombining with CaO within the plume to reform solid CaSO4 (22, 23).
After being injected into the atmosphere, SO3 would have been transformed to sulfuric acid (H2SO4), which would have rained out to the ocean. There is debate about the rapidity of this transport. Sulfur dynamics following the eruption of Mount Pinatubo in 1991 suggest (24, 25) a timescale of a few years, with injection of S into the dry stratosphere delaying its return to Earth’s surface. In line with this, earlier K/Pg modeling studies (23, 26) used atmospheric residence times of S of several months to a few years. However, an alternative and very different scenario has recently been proposed for the K/Pg. It is suggested (14) that, immediately after the impact, most of the sulfuric acid aerosols could have been scavenged by large silicate particles falling rapidly back to Earth, delivering the H2SO4 to the ocean within only one or a few days.
D’Hondt et al. (18) estimated total sulfuric acid production to have been in the range 10–130 × 1015 mol H2SO4 (320–4,160 Pg S), based on several earlier studies (27–29). Later studies suggested ∼10-fold smaller total production of only 0.9–9 × 1015 mol H2SO4 (30–300 Pg S, ref. 23) and 2.4–11 × 1015 mol H2SO4 (78–364 Pg S, ref. 30).
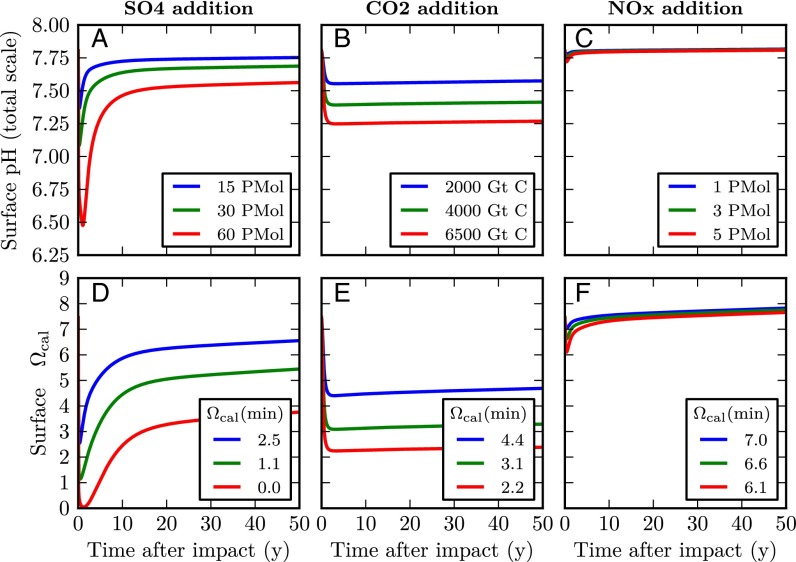
We implemented this hypothesis in the model through a family of runs of different total sulfur addition (15, 30, and 60 × 1015 mol, corresponding to 480, 960, and 1,920 Pg). A main set of runs used longer e-folding times of H2SO4 addition (0.5, 1, 5, and 10 y). Additional runs used a shorter e-folding time of 10 h, following ref. 14. H2SO4 addition reduces surface water total alkalinity (TA) in the molar ratio H2SO4:TA = 1:−2 (31). Results of the main sulfuric acid addition runs are shown in Fig. 1 A and D (with sensitivity to rate of addition shown in Fig. S1). The results of the additional runs with e-folding timescale (τ) = 10 h are shown in Fig. 2. There are very large impacts on pH and Ωcalcite for large sulfur inputs, which are exacerbated by rapid addition. The greatest effects are in the immediate aftermath and are of short duration (a few years).
Fig. 1.
Impacts of different scenarios of environmental change on surface ocean chemistry: (A–C) pH and (D–F) saturation state for calcite (Ωcalcite). Each column shows the acidification impacts for a different type of forcing (same vertical axis scale for each) when the forcing is applied with an e-folding time of 6 mo. Minimum Ωcalcite values for each model run are shown in the boxes. The color of each line indicates the magnitude of the forcing for that run.
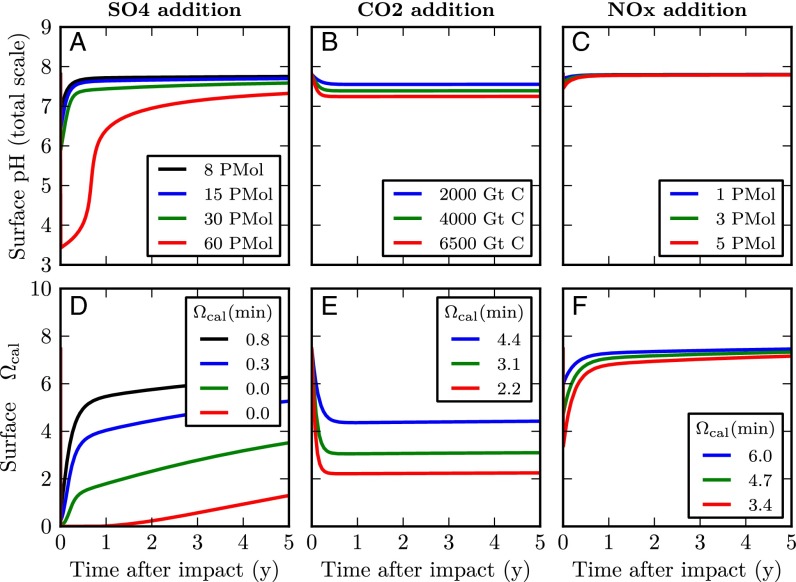
Fig. 2.
Impacts of very rapid additions (e-folding time of 10 h, ref. 14) of H2SO4 (A and D), CO2 (B and E), and HNO3 (C and F) on pH (A–C) and saturation state for calcite (D–F). Minimum Ωcalcite values for each model run are shown in the boxes. The color of each line indicates the magnitude of the forcing for that run. The axis scale for pH differs from that in Fig. 1.
Carbon Dioxide from Carbonate Rocks and Organic Carbon.
We consider all CO2 sources together. In the same way that vaporization of gypsum rocks yields sulfur compounds, vaporization of carbonate rocks yields CO2. It has been estimated that an asteroid of diameter ∼10 km hitting the 3- or 4-km-thick layer of sedimentary carbonates of the Yucatan peninsula (19) would have released between about 5,000 and 9,000 Pg CO2 (32), or in other words 1,300–2,500 Pg carbon. This may, however, be an overestimate, because the total amount released was most likely greatly reduced by rapid back-reactions (33) in which 40–80% of volatilized CaO and CO2 immediately recombined within the plume to reform CaCO3 (22). As for sulfur, we do not include this or lime effects on pH in the model calculations.
A global heat shock (34) following the impact may have ignited woody biomass, leading to wildfires (35), and thence CO2 release to the atmosphere. In the Late Cretaceous the total biomass of living vegetation was possibly larger than it is today (∼600 Pg C), because forests extended closer to the poles at that time. Although there are much larger estimates of the amount of vegetation burnt [up to 2,700 Pg C (36), based on the amount of soot produced and assuming it all came from wildfires], it seems unlikely to us that burning of terrestrial vegetation carbon could have contributed much more than 1,500 Pg C, both because of midcontinent aridity in warmer climates and because of finite habitat space.
An additional potential carbon source comes from soils. The throwing up of large amounts of soot, aerosols, and dust to the atmosphere probably led to an extended period of darkness on Earth, during which photosynthesis was strongly inhibited by low light levels (37). During this period, decay of soil organic carbon [turnover time today of about 50 y (38)] would not have been balanced by replenishment from production of leaf litter and other carbon-rich matter by living plants. We assumed a maximum total for the Late Cretaceous of 2,500 Pg C (compared with 1,600 Pg C today), taking into account that soil carbon stocks on Earth today are much higher toward the poles, particularly in permafrost regions. Although the planet was warmer on the whole in the Late Cretaceous, the lack of ice sheets on Antarctica could have allowed large soil carbon stocks to accumulate there (39).
We modeled the effect on ocean carbonate chemistry from all of these sources combined, through a family of model runs with carbon additions of 2,000, 4,000, and 6,500 Pg C. The sources most likely released carbon both rapidly (volatilization of carbonate rocks at the point of impact and wildfires) and slowly (decay of soil carbon). We therefore carried out runs in which CO2 was added both more slowly (e‐folding times of between 0.5 and 10 y) and more rapidly (e‐folding time of 10 h). Results are shown in Figs. 1 and 2 and Fig. S1. Large impacts are produced, although not as severe as from the largest sulfuric acid additions. In contrast to SO4, slower and faster additions of CO2 to the atmosphere elicit similar responses in the ocean, because slow air–sea gas exchange of CO2 delays the onset of OA.
Nitrogen Oxides Due to Atmospheric Shock Wave.
As the asteroid (and subsequent ejecta) traveled at high speed through the atmosphere, the associated intense pressure wave would have led to conversion of N2 and O2 in the atmosphere to NOX. Upon conversion to nitric acid (HNO3) and incorporation into rain, this would have induced acidification of the ocean as it rained out over the following months or years (or possibly days if also scavenged by large silicate particles). Although the total amount of HNO3 produced directly by the initial pressure wave [∼1 × 1015 mol (40)] is relatively small, it could have been doubled by HNO3 from ejecta pressure waves (41), and supplemented by ∼3 × 1015 mol HNO3 from wildfires (36), giving rise to maximum total additions of up to 5 × 1015 mol HNO3. Nitric acid addition reduces surface water total alkalinity (TA) in the molar ratio HNO3:TA = 1:−1 (31). We implemented this hypothesis with a set of runs of different NOx additions (1, 3, and 5 × 1015 mol) over a range of e-folding times (0.5, 1, 5, and 10 y). The results are shown in Figs. 1 and 2 and Fig. S1. The impact is similar to that from sulfuric acid, but considerably smaller (5 × 1015 mol of HNO3 has a smaller impact than the minimum sulfur run).
Breakdown of Ocean Stratification.
Large tsunamis occurred within 1,000 km of Chicxulub (19) and other effects (the shock wave, and secondary tsunamis following impact-induced earthquakes and the return of ejecta) would have led to more widespread consequences for ocean stratification. If the overall disturbance was sufficient to bring about a global mixing between surface and intermediate-depth (lower pH) waters, then surface ocean pH would have dropped. This scenario was implemented in model runs by increasing the amount of mixing [initially by a factor (α) of 2, 5, 10, or 100-fold above normal], with the mixing rate (K) over time (t) subsequently decaying back to the baseline value (K′) over e-folding timescales (τ) of between 6 mo and 10 y, according to the equation . The resulting impacts are small, as shown in Table 1 and Fig. S1.
Table 1.
Severity of impacts in different models
| Scenario | Minimum surface Ωcalcite* | |||||
| Alkalinity removal from surface ocean, Pmol | Carbon addition to atm, Pg | JModel (Cretaceous) | LOSCAR† (Paleocene) | JModel (preindustrial) | Duration of peak impact‡ | |
| Gypsum vaporization (SO4) | 120 | — | 0.0 | 0.0 | 0.0 | <5 y |
| NOx generation | 5 | — | 6.1 | 3.6 | 3.8 | <5 y |
| Carbon dioxide (CO2) | — | 6,500 | 2.2 | 1.4 | 0.7 | Thousands of years |
| Stirring | — | — | 5.4 | — | 3.8 | <5 y |
| All | 125 | 6,500 | 0.0 | 0.0 | 0.0 | <5 y |
| All except SO4 | 5 | 6,500 | 2.1 | 1.0 | 0.6 | <5 y |
| Rapid addition of SO4 | 120 | — | 0.0 | 0.0 | — | <1 y |
| Rapid addition of NOx | 5 | — | 3.4 | — | — | <1 y |
| Rapid addition of CO2 | — | 6,500 | 2.2 | — | — | — |
For each scenario the lowest minimum Ωcalcite is taken from the run in which the largest amount of substance (for instance 60 × 1015 mol, for SO4) is added over an e-folding time of 6 mo, except for rapid additions where the e-folding time was 10 h. Initial (preaddition) values of surface Ωcalcite were 7.5 (JModel Cretaceous), 4.9 (LOSCAR Paleocene, ref. 52), and 4.9 (JModel preindustrial).
Average across all ocean basins. A run with increased stirring was not implemented for this model.
Time for which Ωcalcite was at least 80% of the maximum distance from the initial value.
Combined Scenarios.
Additional runs (τ = 6 mo or τ = 10 h) were carried out in which all acidifying factors were set to their maximum amounts, except for the input of SO4 from sulfate aerosols, which was the only factor varied between runs. The input of nitric acid was thus set to 5 × 1015 mol and the input of CO2 to 6,500 Gt C. Changes to ocean stratification were not simultaneously modeled because they weaken the combined impact. The results from these runs are shown in Table 1 and Fig. S2.
Discussion
Sulfate Aerosols Were Probably the Dominant Acidifying Factor.
It is clear that some processes have much greater potential than others to drive extinction-level OA. Considering first the inputs of CO2 (from wildfires, decay, and release from rocks), it seems that the potential for mass extinction-scale OA impacts in this case is limited. Even for the largest total amount (6,500 Pg C) over the shortest timescale (10 h), the model produces a minimum Ωcalcite of 2.2. Such an impact would not have been sufficiently severe to produce complete global extinction of most calcifying species via shell dissolution. This maximum amount of 6,500 Pg C at the K/Pg compares to ∼600 Pg C of anthropogenic carbon released to date (2015) in the Anthropocene (1) and estimated total available fossil fuel reserves of about 4,000 Pg C. It can be seen (Table 1), however, that the addition of the same amount of carbon to the modern system would reduce Ωcalcite to a much lower average value (0.7).
Breakdown of ocean stratification is seen to have only a relatively small potential to lower seawater pH and Ωcalcite. This is not surprising, because the pH of intermediate waters in most oceans is about 7.7 and this sets a limit to the decrease in pH that can be achieved by a sudden stirring of the oceans. Although very deep waters are undersaturated with respect to calcium carbonate, this is primarily due to the effect of pressure. For instance, if water from 3.5 km deep in the North Atlantic [dissolved inorganic carbon (DIC) = 2,180 µmol⋅kg−1, TA = 2,340 µmol⋅kg−1] is raised to the surface and the pressure effect removed, its Ωcalcite is ∼2.8. Likewise for the North Pacific, the same procedure yields Ωcalcite of ∼1.8. In neither case is Ωcalcite <1. Although long-term sustained changes in mixing can have a great impact over hundreds of thousands of years on the depth of the calcite compensation depth (CCD) and the δ13C of CaCO3 (42), short-term effects on surface ocean Ωcalcite are modest and this mechanism can also be ruled out as a cause of severe OA at the K/Pg.
Of the two processes in which acid is directly added to the surface ocean (sulfuric and nitric acid additions), the former far outweighs the latter in terms of maximum possible OA impacts. The nitric acid additions do not cause severe OA (Table 1) even for the highest amount added (5 × 1015 mol N). Therefore, this too can be discounted as a sole cause of severe OA.
The only process capable by itself of producing severe undersaturation (here defined as Ωcalcite <0.5; the choice of this threshold is discussed below) is the deposition of sulfuric acid. In fact, even when all other acidifying factors are set to maximum values and combined, in the absence of sulfate aerosols then the total effect is insufficient to induce undersaturation (Table 1, All except SO4). In the rest of this paper we thus focus on impacts from sulfuric acid additions.
How Much Sulfur Was Released?
A key uncertainty in the assessment of OA at the K/Pg is therefore the magnitude of sulfur release, which depends on several factors. An important uncertainty is the size of the impactor, with early calculations made for bodies of diameter 10, 15, or 20 km but later calculations for 10 km, in line with downward revision of the size of the impactor (19). There has been disagreement about the pressure required to vaporize gypsum and release sulfur oxides. Earlier papers such as that by Sigurdsson et al. (27) assumed that 20–40 GPa are required, leading to higher estimates of the amounts vaporized. Later studies (e.g., ref. 23) used 100 GPa. There is also uncertainty about the angle at which the impactor hit the Earth. It may have been a vertical, full-on impact [angle of incidence = 90° (ref. 43)] or a shallower angle impact [20–30° (ref. 19)]. Additional uncertainty comes from lack of complete knowledge about the nature of the impact site geology and more specifically the amount of gypsum in the impacted rocks. Another source of uncertainty is that most estimates assume that 100% of the volatilized sulfur ended up as sulfuric acid added to the ocean, whereas others take into consideration that about 50% (22, 23) may have been reincorporated almost immediately back into solid CaSO4.
These uncertainties lead to a greater than 10-fold range in the predictions of sulfuric acid release. The highest estimate [used by D’Hondt et al. (18) in their calculations] is that of Sigurdsson et al. (27), of up to 60 × 1015 mol. This number is 5- to 20-fold higher than the maximum estimate from the other two studies cited by D’Hondt et al. (18) and is nearly an order of magnitude higher than the top end of the range (9 × 1015 mol) from the most recent analysis (23). The difference has been ascribed (41) primarily to the assumption by Sigurdsson et al. (27) that gypsum vaporization can occur at lower pressures.
Could OA at the K/Pg Have Been Severe Enough to Cause Calcifier Extinctions?
To answer this question, it is necessary to estimate a lower limit value of Ω below which calcifiers could not have survived. Despite large amounts of ongoing research into the impacts of OA on the marine biota, a precise value for such a Ω threshold is not yet available, and in any case seems to differ between calcifier clades (44). We use a critical threshold value of Ωcalcite of 0.5 in the surface ocean. This criterion of Ωcalcite <0.5 corresponds to those waters becoming strongly undersaturated for calcite, the less soluble form of CaCO3 produced by coccolithophores (calcareous nannoplankton) and foraminifera to form shells. Because high latitudes and low latitudes are not distinguished in this simple model, there is only one surface box and hence only one value of Ωcalcite. In nature, however, there is a latitudinal gradient in Ω in surface waters, with highest values (at low latitudes) on average about 20% higher than the global average. A global average value of Ωcalcite of 0.5 therefore corresponds to surface oceans being quite strongly undersaturated for both calcite and aragonite at all latitudes. However, some calcifying species continue to calcify quite well (in the laboratory at least) even at Ωcalcite of 0.5 (e.g., ref. 44). This threshold should therefore be considered as the minimum degree of seawater corrosivity to CaCO3 that is required to account for widespread calcifer extinctions, and it is possible that even lower average values would actually be required.
To assess whether this threshold of Ωcalcite of 0.5 could have been exceeded, additional model runs (Figs. S2 and S3 and Table S1) were carried out to calculate the critical amount of SO4 needed to reduce Ωcalcite to 0.5. This critical amount is found to be between 8 and 43 × 1015 mol, depending on the rapidity of addition and on the intensity of other acidifying processes. Between 30 and 43 × 1015 mol is required if SO4 is added relatively slowly (τ = 6 mo), whereas between 8 and 10 × 1015 mol is required if SO4 is added very rapidly (τ = 10 h). Our model runs therefore suggest that Ωcalcite could have fallen below the threshold of 0.5 for the high value of SO4 emissions (27) used by D’Hondt et al. (18), but only for the upper end of the range proposed by the most recent study (23).
Overall, our model results do not point to extremely severe OA at the K/Pg, although they do not completely rule it out. We conclude that it is possible but not likely that the numerous calcifier extinctions were due to OA. Some reasons for this conclusion are as follows. (i) Out of several factors considered in the simulated scenarios, only one (sulfuric acid) made the surface ocean strongly corrosive to calcite (Ωcalcite <0.5). (ii) Even for sulfuric acid, the amount required to produce severe OA (Table S1) is above the upper ends of most (including the most recent) estimates of ranges of possible emissions (Table S2). (iii) The amounts of H2SO4 reaching the surface ocean were probably at least twofold less than the early estimates of the amounts of S released, because they ignored rapid back-reactions consuming sulfur in the plume (22). (iv) The release of S to the atmosphere may have been accompanied by a production of lime (CaO) (22) and/or other basic compounds (30), which if subsequently falling into the surface ocean may have dissolved there, raising Ωcalcite and pH. Other explanations for selective extinctions should therefore continue to be explored, such as the suggestion (45) that groups with resting stages (e.g., dinoflagellates cysts or diatom spores) were able to survive better than those without (including calcareous nannoplankton). Further progress on this question would be assisted by a better understanding of both the magnitude and rapidity of sulfuric acid additions.
Although a preliminary inspection of the K/Pg paleontological record strongly supports OA-driven calcifier extinction, upon closer inspection the evidence seems less compelling. For instance, in contrast to calcareous nannoplankton, another group of calcifying plankton, the calcareous dinoflagellates, experienced no major extinction at the K/Pg (11). All calcareous rudist bivalve species were lost, but ∼40% of other bivalve genera survived (46). In contrast to planktics, benthic foraminifera with calcareous shells survived the event relatively intact, whether inhabiting shallow or deep waters (47). Inoceramid clams underwent 100% extinction but up to 13% of bryozoans and only rather few marine gastropods went extinct (10).
Zooxanthellate scleractinian corals (those that host photosynthetic algal symbionts, restricting them to living in shallow waters where peak OA impacts would have been greatest) suffered much greater species extinction rates than azooxanthellate scleractinian corals inhabiting a much larger depth range (48). In fact, deep-water corals, far from being preferentially killed off, instead preferentially survived the end-Cretaceous mass extinction (48). This is compatible with our model results that show much more severe OA in surface than in deeper waters. However, among scleractinian corals as a whole (zooxanthellate and azooxanthellate combined) only ∼50% of all species were lost, which would seem surprising if OA was the cause for other calcifier extinctions, given the experimental and field evidence showing coral sensitivity to OA. According to Kiessling and Simpson (49), “During the major mass extinctions at the end of the Ordovician, Permian and Cretaceous periods [the calcifying groups] corals and coralline sponges have indistinguishable extinction rates from other taxa.”
Comparison with Previous Calculations.
Some of these acidifying processes were considered in earlier work by D’Hondt et al. (18). Although D’Hondt et al. calculated the effects of adding acids to the ocean without the aid of a dynamical model such as that used here, their earlier predictions are broadly consistent with our model results. We have considered a wider range of possible acidifying processes but concur that the process of gypsum vaporization is likely to be quantitatively the most important, at least if the larger estimates are correct. Our estimate of the amount of sulfur required to explain extinctions, in the absence of other processes, is however much less than theirs: 43 × 1015 mol SO4 (or 10 × 1015 mol SO4 if added very rapidly, but see Table S1 for sensitivity of these numbers to assumptions) compared with their estimate of 61 × 1015 mol SO4. In agreement with Ohno et al. (14), our model results show lower Ωcalcite values when SO4 is added more rapidly than when it is added more slowly. However, contrary to their calculations (14), in our model we find that 1 × 1014 kg H2SO4 (∼1 × 1015 mol SO4) is not nearly enough to produce strong undersaturation (Fig. S3B). Our model results support an earlier assessment (41) that nitric acid could only have caused minor acidification and that sulfuric acid was unlikely to have led to significant acidification.
Conclusions
Results have been presented from our carbon cycle modeling study of OA following the asteroid impact at the end of the Cretaceous. The effects of several acidifying mechanisms were simulated, including wildfires emitting CO2 to the atmosphere and vaporization of gypsum rocks leading to deposition of sulfuric acid on the ocean surface. Our assessment of the potential for OA from these mechanisms finds that most produce too small an impact on the CaCO3 saturation of the surface ocean to be able to explain the simultaneous extinctions of calcifiers. Only sulfuric acid deposition is capable of making the surface oceans strongly corrosive to calcite. However, to produce severe CaCO3 undersaturation (Ωcalcite <0.5), very large quantities (greater than between 8 and 43 × 1015 mol, depending on other assumptions) of sulfur must have been volatilized from gypsum and anhydrite in sedimentary rocks and deposited on the surface of the ocean as sulfuric acid; 8 × 1015 mol is right at the top of recent estimates (0.9–9 × 1015 mol). Hence, we think it rather unlikely, although not completely impossible, that biologically catastrophic OA occurred at the K/Pg boundary. The great extinctions of calcifiers at this time (100% of ammonites and rudist bivalves and more than 90% of calcareous nannoplankton and planktic foraminifera species) were most likely due to some other cause.
Methods
The main biogeochemical box model (JModel) used here is a variant of one used previously to study various carbon cycle and OA problems (e.g., refs. 42 and 50). The model (for more details see Supporting Information) represents the coupled global ocean and atmosphere. It includes phytoplankton, phosphate, DIC, total alkalinity, and atmospheric CO2 as state variables. The model fully resolves the carbonate system; ocean carbon chemistry is linked, through air–sea gas exchange, with atmospheric CO2.
Simulations runs were started in steady state with geochemical conditions appropriate for the Late Cretaceous (66 Mya). The atmospheric CO2 concentration was 1,000 ppmv, the calcium ion concentration 20 mmol⋅kg−1, and the magnesium ion concentration 30 mmol⋅kg−1 (e.g., ref. 51) (preindustrial values are 280 ppmv, 10.3 mmol⋅kg−1, and 53 mmol⋅kg−1, respectively). The effects of altered [Ca2+] and [Mg2+] on K1, K2 (the carbonate system equilibrium constants), and Ksp (the CaCO3 solubility product) were calculated following ref. 51. The starting state of the model was obtained by holding the atmospheric CO2 fixed at its target value (1,000 ppmv) and then running the model out to equilibrium. For each hypothesis, the rate of addition (R) over time (t) of a total amount (A) of an acidifying substance was calculated using the e-folding timescale (τ) according to .
To assess the robustness of the results obtained, we carried out sensitivity analyses (to different surface layer depths, Ksp values, and initial atmospheric CO2 values; Table S1) and repeated the tests in different model setups: (i) the same model (JModel), but in its preindustrial configuration, that is, without the atm CO2, [Ca2+], and [Mg2+] alterations just described (results shown in Table 1 and Figs. S4 and S5) and (ii) the independent LOSCAR model [a box model with more boxes than the JModel (Fig. S6) and with explicit sediments (52)] configured to resemble the late Paleocene ocean (results shown in Table 1).
Supplementary Material
Acknowledgments
We thank Andy Ridgwell, Ken Caldeira, Eric Achterberg, Jay Melosh, and Dani Schmidt for insightful discussions, and Richard Zeebe for assistance with use of his LOSCAR model. This work was a contribution to the European Project on Ocean Acidification, which received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant 211384. T.T. received funding from the Natural Environment Research Council (NERC); Department for Environment, Food, and Rural Affairs (Defra); and Department of Energy and Climate Change (DECC) to the UK Ocean Acidification Programme (Grant NE/H017348/1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418604112/-/DCSupplemental.
References
- 1.Raupach MR, Canadell JG. Carbon and the Anthropocene. Curr Opin Sust. 2010;2(4):210–218. [Google Scholar]
- 2.Gattuso J-P, Hansson L. Ocean Acidification. Oxford Univ Press; Oxford: 2011. [Google Scholar]
- 3.Hönisch B, et al. The geological record of ocean acidification. Science. 2012;335(6072):1058–1063. doi: 10.1126/science.1208277. [DOI] [PubMed] [Google Scholar]
- 4.Zeebe RE, Westbroek P. A simple model for the CaCO3 saturation state of the ocean: The “Strangelove,” the “Neritan,” and the “Cretan” Ocean. Geochem Geophys Geosyst. 2003;4(12) doi: 10.1029/2003GC000538. [DOI] [Google Scholar]
- 5.Caldeira K, Wickett ME. Oceanography: Anthropogenic carbon and ocean pH. Nature. 2003;425(6956):365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- 6.Renne PR, et al. Time scales of critical events around the Cretaceous-Paleogene boundary. Science. 2013;339(6120):684–687. doi: 10.1126/science.1230492. [DOI] [PubMed] [Google Scholar]
- 7.Marshall CR, Ward PD. Sudden and gradual molluscan extinctions in the latest Cretaceous of western European Tethys. Science. 1996;274(5291):1360–1363. doi: 10.1126/science.274.5291.1360. [DOI] [PubMed] [Google Scholar]
- 8.Bown P. Selective calcareous nannoplankton survivorship at the Cretaceous-Tertiary boundary. Geology. 2005;33(8):653–656. [Google Scholar]
- 9.Jiang SJ, Bralower TJ, Patzkowsky ME, Kump LR, Schueth JD. Geographic controls on nannoplankton extinction across the Cretaceous/Palaeogene boundary. Nat Geosci. 2010;3(4):280–285. [Google Scholar]
- 10.Macleod N, et al. The Cretaceous-Tertiary biotic transition. J Geol Soc London. 1997;154:265–292. [Google Scholar]
- 11.Wendler J, Willems H. 2002. Distribution pattern of calcareous dinoflagellate cysts across the Cretaceous-Tertiary boundary (Fish Clay, Stevns Klint, Denmark): Implications for our understanding of species-selective extinction. Catastrophic Events and Mass Extinctions: Impacts and Beyond, Geological Society of America Special Papers, eds Koeberel C, MacLeod KG (Geological Society of America, Boulder, CO), Vol 356, pp 265–275.
- 12.Medlin LK, Saez AG, Young JR. A molecular clock for coccolithophores and implications for selectivity of phytoplankton extinctions across the K/T boundary. Mar Micropaleontol. 2008;67(1–2):69–86. [Google Scholar]
- 13.Alegret L, Thomas E, Lohmann KC. End-Cretaceous marine mass extinction not caused by productivity collapse. Proc Natl Acad Sci USA. 2012;109(3):728–732. doi: 10.1073/pnas.1110601109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno S, et al. Production of sulphate-rich vapour during the Chicxulub impact and implications for ocean acidification. Nat Geosci. 2014;7(4):279–282. [Google Scholar]
- 15.Caldeira K, Rampino MR. Aftermath of the end-Cretaceous mass extinction — Possible biogeochemical stabilization of the carbon-cycle and climate. Paleoceanography. 1993;8(4):515–525. [Google Scholar]
- 16.Beerling DJ, Lomax BH, Royer DL, Upchurch GR, Jr, Kump LR. An atmospheric pCO2 reconstruction across the Cretaceous-Tertiary boundary from leaf megafossils. Proc Natl Acad Sci USA. 2002;99(12):7836–7840. doi: 10.1073/pnas.122573099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Retallack GJ, Wang C, Huang Q. Paleoatmospheric pCO2 fluctuations across the Cretaceous–Tertiary boundary recorded from paleosol carbonates in NE China. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;385:95–105. [Google Scholar]
- 18.D’Hondt S, Pilson MEQ, Sigurdsson H, Hanson AK, Carey S. Surface-water acidification and extinction at the Cretaceous-Tertiary boundary. Geology. 1994;22(11):983–986. [Google Scholar]
- 19.Schulte P, et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science. 2010;327(5970):1214–1218. doi: 10.1126/science.1177265. [DOI] [PubMed] [Google Scholar]
- 20.Kring DA. The Chicxulub impact event and its environmental consequences at the Cretaceous-Tertiary boundary. Palaeogeogr Palaeocl. 2007;255(1–2):4–21. [Google Scholar]
- 21.Gerasimov MV, Dikov YP, Yakovlev OI, Wlotzka F. Lunar and Planetary Institute Science Conference Abstracts. Vol 25. Lunar and Planetary Institute; Houston, TX: 1994. High-temperature vaporization of gypsum and anhydrites: Experimental results; p. 413. [Google Scholar]
- 22.Agrinier P, Deutsch A, Schärer U, Martinez I. Fast back-reactions of shock-released CO2 from carbonates: An experimental approach. Geochim Cosmochim Acta. 2001;65(15):2615–2632. [Google Scholar]
- 23.Pierazzo E, Hahmann AN, Sloan LC. Chicxulub and climate: Radiative perturbations of impact-produced S-bearing gases. Astrobiology. 2003;3(1):99–118. doi: 10.1089/153110703321632453. [DOI] [PubMed] [Google Scholar]
- 24.Thornton DC, Bandy AR, Blomquist BW, Driedger AR, Wade TP. Sulfur dioxide distribution over the Pacific Ocean 1991–1996. J Geophys Res Atmos. 1999;104(D5):5845–5854. [Google Scholar]
- 25.Barnes JE, Hofmann DJ. Lidar measurements of stratospheric aerosol over Mauna Loa Observatory. Geophys Res Lett. 1997;24(15):1923–1926. [Google Scholar]
- 26.Pope KO, Baines KH, Ocampo AC, Ivanov BA. Impact winter and the Cretaceous/Tertiary extinctions: Results of a Chicxulub asteroid impact model. Earth Planet Sci Lett. 1994;128(3):719–725. doi: 10.1016/0012-821x(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 27.Sigurdsson H, Dhondt S, Carey S. The impact of the Cretaceous Tertiary bolide on evaporite terrane and generation of major sulfuric-acid aerosol. Earth Planet Sci Lett. 1992;109(3–4):543–559. [Google Scholar]
- 28.Brett R. The Cretaceous-Tertiary extinction — A lethal mechanism involving anhydrite target rocks. Geochim Cosmochim Acta. 1992;56(9):3603–3606. [Google Scholar]
- 29.Pope KO, Ocampo AC, Baines KH, Ivanov BA. Lunar and Planetary Institute Science Conference Abstracts. Vol 24. Lunar and Planetary Institute; Houston, TX: 1993. Global blackout following the K/T Chicxulub impact: Results of impact and atmospheric modeling; p. 1165. [Google Scholar]
- 30.Maruoka T, Koeberl C. Acid-neutralizing scenario after the Cretaceous-Tertiary impact event. Geology. 2003;31(6):489–492. [Google Scholar]
- 31.Doney SC, et al. Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proc Natl Acad Sci USA. 2007;104(37):14580–14585. doi: 10.1073/pnas.0702218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Keefe JD, Ahrens TJ. Impact production of CO2 by the Cretaceous Tertiary extinction bolide and the resultant heating of the Earth. Nature. 1989;338(6212):247–249. [Google Scholar]
- 33.Yancey TE, Guillemette RN. Carbonate accretionary lapilli in distal deposits of the Chicxulub impact event. Geol Soc Am Bull. 2008;120(9–10):1105–1118. [Google Scholar]
- 34.Goldin TJ, Melosh HJ. Self-shielding of thermal radiation by Chicxulub impact ejecta: Firestorm or fizzle? Geology. 2009;37(12):1135–1138. [Google Scholar]
- 35.Morgan J, Artemieva N, Goldin T. Revisiting wildfires at the K-Pg boundary. J Geophys Res-Biogeo. 2013;118(4):1508–1520. [Google Scholar]
- 36.Crutzen PJ. Mass extinctions — Acid-rain at the K/T Boundary. Nature. 1987;330(6144):108–109. [Google Scholar]
- 37.Vellekoop J, et al. Rapid short-term cooling following the Chicxulub impact at the Cretaceous-Paleogene boundary. Proc Natl Acad Sci USA. 2014;111(21):7537–7541. doi: 10.1073/pnas.1319253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giardina CP, Ryan MG. Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature. 2000;404(6780):858–861. doi: 10.1038/35009076. [DOI] [PubMed] [Google Scholar]
- 39.DeConto RM, et al. Past extreme warming events linked to massive carbon release from thawing permafrost. Nature. 2012;484(7392):87–91. doi: 10.1038/nature10929. [DOI] [PubMed] [Google Scholar]
- 40.Zahnle KJ. 1990. Atmospheric chemistry by large impacts. Global Catastrophes in Earth History; An Interdisciplinary Conference on Impacts, Volcanism, and Mass Mortality, Geological Society of America Special Papers, eds Sharpton VL, Ward PD (Geological Society of America, Boulder, CO), Vol 247, pp 271–288.
- 41.Toon OB, Zahnle K, Morrison D, Turco RP, Covey C. Environmental perturbations caused by the impacts of asteroids and comets. Rev Geophys. 1997;35(1):41–78. [Google Scholar]
- 42.Merico A, Tyrrell T, Wilson PA. Eocene/Oligocene ocean de-acidification linked to Antarctic glaciation by sea-level fall. Nature. 2008;452(7190):979–982. doi: 10.1038/nature06853. [DOI] [PubMed] [Google Scholar]
- 43.Kring DA, Durda DD. Trajectories and distribution of material ejected from the Chicxulub impact crater: Implications for postimpact wildfires. J Geophys Res Planets. 2002;107(E8) 6-1–6-22. [Google Scholar]
- 44.Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009;37(12):1131–1134. [Google Scholar]
- 45.Ribeiro S, et al. Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nat Commun. 2011;2:311. doi: 10.1038/ncomms1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jablonski D. Extinction and the spatial dynamics of biodiversity. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11528–11535. doi: 10.1073/pnas.0801919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Culver SJ. Benthic foraminifera across the Cretaceous–Tertiary (K–T) boundary: A review. Mar Micropaleontol. 2003;47:177–226. [Google Scholar]
- 48.Kiessling W, Baron-Szabo RC. Extinction and recovery patterns of scleractinian corals at the Cretaceous-Tertiary boundary. Palaeogeogr Palaeocl. 2004;214(3):195–223. [Google Scholar]
- 49.Kiessling W, Simpson C. On the potential for ocean acidification to be a general cause of ancient reef crises. Glob Change Biol. 2011;17(1):56–67. [Google Scholar]
- 50.Bernie D, Lowe J, Tyrrell T, Legge O. Influence of mitigation policy on ocean acidification. Geophys Res Lett. 2010;37(15) doi: 10.1029/2010GL043181. [DOI] [Google Scholar]
- 51.Tyrrell T, Zeebe RE. History of carbonate ion concentration over the last 100 million years. Geochim Cosmochim Acta. 2004;68:3521–3530. [Google Scholar]
- 52.Zeebe RE. LOSCAR: Long-term Ocean-atmosphere-Sediment CArbon cycle Reservoir Model v2.0.4. Geosci Model Dev. 2012;5(1):149–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.