Significance
Genetically identical individuals display variability in their behaviors even when reared in essentially identical environments. This variation underlies both personality and individuality, but there is little mechanistic understanding of how such differences arise. Here, we investigated individual-to-individual variation in locomotor behaviors of fruit flies. Surprisingly, individual flies exhibit significant bias in their left vs. right locomotor choices during exploratory locomotion, with some flies being strongly left biased or right biased. Using the Drosophila genetic toolkit, we find that the magnitude of locomotor handedness is under the control of neurons within a brain region implicated in motor planning and execution. This observation intriguingly implies that the brain may be able to dynamically regulate behavioral individuality.
Keywords: behavior, individuality, personality, circuit mapping, central complex
Abstract
Genetically identical individuals display variability in their physiology, morphology, and behaviors, even when reared in essentially identical environments, but there is little mechanistic understanding of the basis of such variation. Here, we investigated whether Drosophila melanogaster displays individual-to-individual variation in locomotor behaviors. We developed a new high-throughout platform capable of measuring the exploratory behavior of hundreds of individual flies simultaneously. With this approach, we find that, during exploratory walking, individual flies exhibit significant bias in their left vs. right locomotor choices, with some flies being strongly left biased or right biased. This idiosyncrasy was present in all genotypes examined, including wild-derived populations and inbred isogenic laboratory strains. The biases of individual flies persist for their lifetime and are nonheritable: i.e., mating two left-biased individuals does not yield left-biased progeny. This locomotor handedness is uncorrelated with other asymmetries, such as the handedness of gut twisting, leg-length asymmetry, and wing-folding preference. Using transgenics and mutants, we find that the magnitude of locomotor handedness is under the control of columnar neurons within the central complex, a brain region implicated in motor planning and execution. When these neurons are silenced, exploratory laterality increases, with more extreme leftiness and rightiness. This observation intriguingly implies that the brain may be able to dynamically regulate behavioral individuality.
Hand dominance—better performance using either the left or right hand—is a familiar human trait, moderately heritable (1), and regulated by many genes (2), including those involved in general body symmetry (3). However, behavioral handedness in general, i.e., the preferential performance of a behavior on one side of the body or with a particular chiral twist, is a multifaceted phenomenon. For example, in the absence of visual feedback, people display clockwise or counterclockwise biases in their walking behavior (4). This “locomotor handedness” is uncorrelated to hand dominance or gross morphological asymmetry and instead may be due to asymmetries in the collection and processing of sensory information, resulting in individual locomotor biases with a neurological basis (4, 5).
Handed behavioral tendencies specific to individuals are also prevalent throughout the animal kingdom and have been shown in species as disparate as mice (paw use) (6), octopi (eye use) (7), and tortoises (side rolled on during righting) (8). There is also evidence that, at the population mean level, some species of insects have handed behaviors and asymmetric neurophysiological patterns (9). However, there has been little investigation of the differences in handed behaviors among individuals of the same insect species, and the mechanisms by which asymmetries are instilled in behavior are unknown.
Considering behavioral handedness at the level of individuals offers insight into another major open question in behavioral neuroscience: what is the mechanistic basis of behavioral intragenotypic variability (i.e., the differences in behavior among individuals with the same genotype, reared in identical environments) (10)? There is growing acceptance that individual-to-individual differences in experimental observations of behavior reflect persistent idiosyncrasies (11) rather than just statistical errors, with significant potential impacts on species fitness and ecology (12). Quantifying idiosyncrasy requires large sample sizes, and invertebrate species, with their small size and rapid life cycles, may be particularly valuable (13). Moreover, to probe causal mechanisms underlying idiosyncratic differences in behavior, a paradigm is needed in a molecular model system. We wondered if the fruit fly Drosophila melanogaster would exhibit behavioral handedness during exploratory locomotion. If so, the power of high-throughput imaging systems would allow us to automate the quantification of many individuals, opening up the study of idiosyncrasy and behavioral handedness to the powerful screening approaches available in this species.
In flies and other insects, locomotion is under the control of a prominent midline brain structure known as the central complex (in some clades central body). Work from several species has shown that the integration of sensory information and the execution of locomotor patterns are associated with neural activity in these cells (14–17). The central complex consists of four symmetrical neuropils: the protocerebral bridges, the fan shaped body, the ellipsoid body, and the noduli; mutations that disrupt the development of these structures disrupt normal walking behavior (18). Despite substantial insight into processes that depend on the central complex, understanding of the specific neural computations taking place to produce motor outputs is incomplete.
Here we show that Drosophila melanogaster flies exhibit striking locomotor handedness, which varies significantly among individuals. Very strongly biased “lefty” and “righty” individuals are common in every line assayed. The bias of each individual persists for its lifetime. However, mating two lefty flies does not result in lefty progeny, suggesting that mechanisms other than genetics determine individual biases. We use the Drosophila transgenic toolkit to map a specific set of neurons within the central complex that regulates the magnitude of locomotor handedness within a line. These findings give insights into choice behaviors and behavioral handedness in a simple model organism and demonstrate that individuals from isogenic populations reared under experimentally identical conditions nevertheless display idiosyncratic behaviors.
Results
Flies Have Idiosyncratic Locomotor Handedness.
To investigate whether flies display individual locomotor handedness, we developed a simple high-throughput assay to quantify turning. Flies were placed individually in Y-shaped mazes and allowed to walk freely for 2 h, with their centroids tracked in two dimensions (Fig. 1 A–C and Movies S1 and S2). Each maze was symmetrical and evenly lit, so that choices were by design unbiased rather than stimulus driven. The fraction of times the fly passed through the center of the maze and chose to go right defined a turn bias score (Fig. 1D). Each fly typically performed hundreds of choices per experiment (Fig. S1A). Precise quantification of the distribution of individual behaviors requires large sample sizes, so many mazes were arrayed in parallel (Fig. 1 A and B and Fig. S1 B–D). Thus, our results reflect more than 25,000 individual flies and 16,000,000 turn choices.
Fig. 1.
Individual flies exhibit biases in left-right turning. (A) Schematic of a device for assaying left-right turning in individuals. Flies were placed into an array containing many individual Y-mazes. The mazes were illuminated from below and imaged from above, and the positions of the flies were recorded. (B) Detail of Y-mazes containing individual flies. (C) One hundred example turn paths through the Y-maze recorded from a single fly over 2 h (blue). Other colors highlight individual turns. (D) Left and right turn sequences for example flies of varying turn biases. Magenta ticks indicate left turns; green, right. (E) Observed distribution of turn bias scores (fraction right turns) measured from WT lines (solid lines), and corresponding expected distributions of turn bias scores (dashed lines). Sample sizes given in F. BK, Berlin-K; CA, Cambridge-A; CS, Canton-S. (F) The breadth of the distribution of turn bias scores for seven lines as measured by MAD. Error bars are ±SE estimated by bootstrap resampling. Dashed lines indicate MADs expected under a binomial null model. All lines other than w1118 (transgenic background line) are nominally WT.
We measured the turn biases of hundreds of individual flies from seven different fly lines: Berlin-K (BK), Canton-S (CS), Cambridge-A (CA) (19), two lines of CS that were independently inbred for 10 generations, CA that was inbred for 10 generations (19), and w1118, the background line for many transgenic flies (Fig. 1 E and F and Fig. S1E). The probability of turning right (the turn bias score), averaged across all individuals within each line was statistically indistinguishable from 50%, an observation that held across all experimental groups. However, this consistency belied profound individual-to-individual variability, and an individual fly’s probability of turning right often diverged markedly from the population average. For example, nearly one quarter (23.5%) of CS flies turned right greater than 70% of the time or less than 30% of the time. This distribution would be unlikely indeed if all flies were choosing to turn right with identical probabilities. This null hypothesis can be modeled using the binomial distribution, with each fly performing ni choices (equal to the number it performed in the experiment) and a probability of turning right p (equal to the mean probability observed across all flies of a given strain). Use of the binomial is statistically justified because sequential turns were essentially independent of one another (Fig. S1F). Compared with this null hypothesis, biased righty and lefty individuals are vastly overrepresented (P < 10−16 and 10−4 by χ2 test of variance and bootstrap resampling, respectively). To quantify the extent of variation in turn bias, we calculated the mean absolute deviation from the mean (MAD) of individual turn bias scores (Fig. 1F). Higher MAD scores indicate greater individual-to-individual differences in behavior, i.e., more extreme left and right biases.
We were unable to identify any trivial sources of left-right turning bias. Neither the light boxes, nor the maze arrays, nor the positions of the mazes within the arrays had any significant effect on the observed mean turning bias (Fig. S1 B–D). Anosmic flies (20) displayed the same variability as control flies (Fig. S1G), suggesting that flies were not following odor cues within the mazes. Last, activity level did not explain the strong biases of flies; there is no correlation between turn bias score and number of turns completed in the 2-h experiment (Fig. S1H).
Individual Locomotor Handedness Is Persistent.
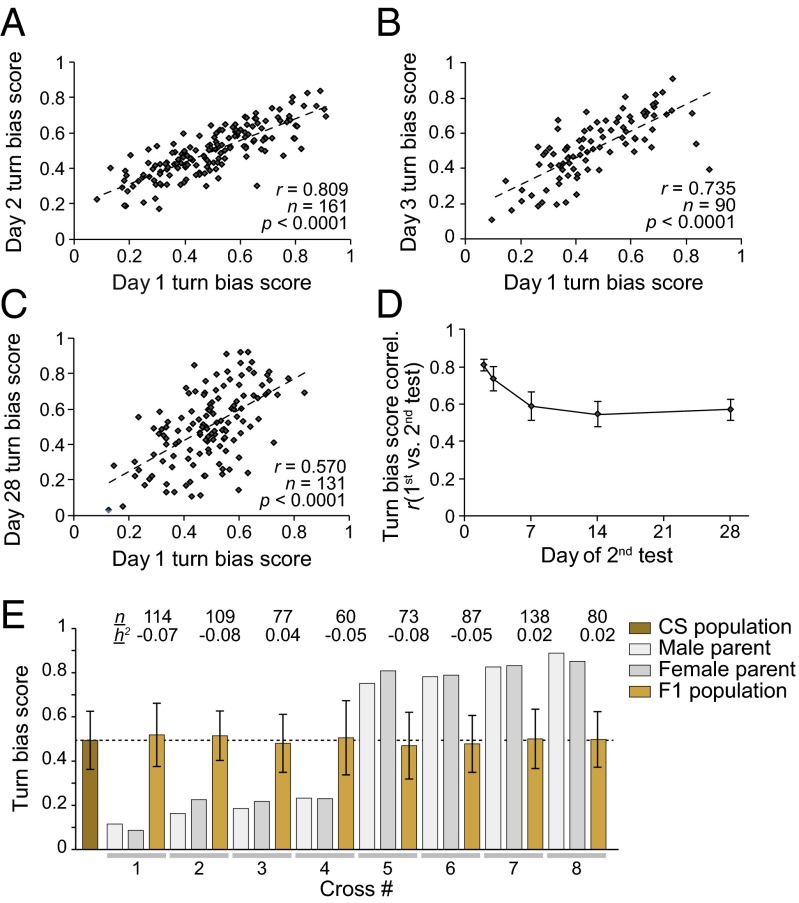
Next, we evaluated the persistence of locomotor handedness. Individual flies were tested in the Y-mazes, recovered, stored individually, and then tested a second time, in a different maze, either 1, 2, 6, 13, or 27 d later. Individual turn bias scores were highly correlated across time, ranging from r = 0.57 for day 1 vs. day 28 to r = 0.81 for day 1 vs. day 2 (all P < 0.0001; Fig. 2 A–D). The persistence of locomotor handedness through time provides further evidence that biases are not introduced by some experimental artifact. If, for example, flies were following a wall or a trail of odors or pheromones, these results would require that they do so in a highly reproducible manner, over long timescales, and in different Y-mazes.
Fig. 2.
An individual's handedness is persistent over time. (A–C) Turn scores from individual flies measured in sequential experiments. Flies were assayed in the Y-mazes, stored individually, and then assayed a second time 1 d (A), 2 d (B), or 4 wk (C) later. (D) Correlation coefficient (r) of turn bias scores across flies tested in the Y-maze, stored individually, and then tested a second time either 1 d, 2 d, 1 wk, 2 wk, or 4 wk later. Error bars indicate ±SE as estimated by bootstrap resampling. n = 85 to n = 184 for all time points. (E) Mean turn bias of parental (brown) and F1 generations (tan) derived from strongly biased CS individuals (gray bars). n indicates number of F1s assayed. h2 indicates estimated heritability. The dashed line indicates 50%. Error bars are ±1 MAD, as a measure of variability rather than error. F1 and parental distributions are statistically indistinguishable.
Locomotor handedness is evidently a persistent property of individual flies—perhaps it reflects a single master regulator of behavioral handedness. We tested this hypothesis by measuring two additional handed behaviors: the direction of spontaneous exploration in circular arenas (Fig. S2) and the folding arrangement of the wings at rest (Fig. S3). We found that individual flies demonstrate a characteristic preference in the direction in which they circle. On average, flies spend equal amounts of time moving clockwise and counterclockwise, but individuals within the population often show strong tendencies to circle in one direction or the other (Fig. S2 D–F). Likewise, individual flies exhibit preferences in which wing is placed on top at rest. Some fold left on top of right, others right on top of left (Fig. S3A). As with turn bias in the Y-mazes, both arena circling bias and wing-folding bias persist across days (Figs. S2F and S3B). We tested individual flies in two assays each, maintained their identities, and found that turn bias scores in the Y-maze positively correlate with a clockwise circling bias in the arena (Fig. S2G). In contrast, circling bias was completely uncorrelated to wing-folding bias (Fig. S3C). From these observations, we conclude that behavioral handedness is multifaceted in Drosophila, like humans, but that the turning biases we see in the Y-maze likely reflect an assay-independent locomotor handedness phenomenon.
Genes Tune the Distribution of Locomotor Handedness.
There are numerous possible causes of individual turning bias. One potential source of variation is the presence of polymorphic lefty and righty alleles in the population. However, the turn bias of individual flies was not heritable (Fig. 2E; mean h2 = −0.03, SE = 0.018, Fisher selection test of heritability) (21), and we found no evidence that inbreeding reduces variability in locomotor bias (Fig. 1F and Fig. S1E). Although an individual's locomotor handedness is not heritable, the total degree of variability at the population level is under genetic control, with some lines being more variable than others (Fig. 1; CS vs. w1118). The extent of this variation was further confirmed in a companion study of wild-derived inbred lines (22). Another potential source of persistent locomotor handedness is morphological asymmetry. We examined whether variability in leg lengths could account for turning biases. We tested 28 metrics of leg length asymmetry and found that just one correlates with turning bias, and the correlation is weak (r2 = 0.11, P = 0.007, P = 0.18 after multiple comparisons correction; Fig. S4). Likewise, gut morphological handedness cannot explain locomotor biases because the twist of the gut is identical among individuals (we confirmed this by noting the meconium position in 50 virgin flies).
Given that neither cryptic genetic variation segregating within lines nor morphological asymmetry is a major source of variation in locomotor handedness, perhaps idiosyncratic locomotor asymmetry has a neurobiological basis. The central complex (CC) is a protocerebral structure with integral roles in processing sensory information and controlling locomotor output across arthropods (15, 16, 18, 23, 24). We examined whether disrupting the CC can alter a population's distribution of turn bias scores. First, we tested seven mutants that perturb central complex development and morphology (19). Of these, no-bridge, central-complex-deranged, and central-body-defect (cbdKS96) showed a significant increase in individual variation in turning compared with heterozygous controls (Fig. 3A). cbdKS96 is a missense mutant of Ten-a (25), which encodes a transmembrane protein involved in axon targeting and synapse formation (26, 27), and causes severe and widespread defects in the fan-shaped body (FB), ellipsoid body (EB), and noduli (No), leading to high individual-to-individual variation in the gross morphology of the CC (19). Furthermore, a genome-wide association study in a panel of inbred lines implicated SNPs within Ten-a as affecting variability in locomotor handedness (22). This association links natural genetic variation in Ten-a, variability in the function of central complex circuits, and variability in turn bias at the population level.
Fig. 3.
The central complex regulates variability in turn bias. (A) The degree of variability in handedness for seven fly lines carrying mutations that disrupt the development of the central complex (purple bars). Three mutations significantly increase the MAD of the distribution of turn bias scores compared with heterozygous controls (gray bars). **P < 0.01, ***P < 0.001, as estimated by comparing bootstrap resampling of MAD values (15), Bonferroni corrected for multiple comparisons. Error bars are ±SE estimated by bootstrap resampling. Numbers indicate sample sizes. Purple boxes indicate neuropils grossly disrupted by each mutation. (B) Turn score variability (MAD) of lines with c465-GAL4 driving expression of temperature-sensitive modulators of neuronal activity (GAL80ts;Kir2,1, Shibirets, dTRPA1, and control lines) at 23 °C (blue bars) and 33 °C (orange bars) temperatures. Error bars and P values as in A. (C–E) Max fluorescence z-projections of c465-driven expression of membrane localized (mCD8) or presynapse localized (nSyb) GFP (cyan), within the central brain (C) and central complex (D and E). Red counterstain is actin. Diagram indicates anterior-posterior extent of z-projection. Cal, mushroom body calyx; PB, protocerebral bridges; No, noduli; EB, ellipsoid body; d/vFB, dorsal/ventral fan-shaped body; PFN, PB-FB-No neurons.
Neural Activity Tunes the Magnitude of Variability in Locomotor Handedness.
We next sought to perturb central complex function more specifically with inducible transgenes (28). We selectively silenced different subsets of CC neurons using a panel of GAL4 lines to express a temperature-sensitive inhibitor of vesicle fusion (Shibirets) (29). By comparing the MADs of the distributions of turn bias scores at the permissive (23 °C) and nonpermissive (33 °C) temperatures, we identified three GAL4 drivers that regulate the amount of turn bias variability in a population (Figs. 3B and 4 and Figs. S5 and S6). Acutely disrupting the function of c465, R16D01, or R73D06 cells by silencing them with Shibirets caused large increases in the variability of turn bias scores. A similar effect resulted from acutely silencing c465 cells with GAL80ts;Kir2.1 (24), or by hyperactivating them with dTRPA1 (30) (Fig. 3B).
Fig. 4.
PFNs regulate variability in turn bias. (A) Maximum fluorescence z-projections of R16D01-GAL4–driven expression of membrane localized mCD8::GFP (cyan), within the central complex. Red counterstain is actin. Diagram indicates anterior-posterior extent of z-projection. (B) As in A for the R73D06-GAL4 driver. (C) Lateral views of CD8::GFP driven by R16D01, R73D06, and c465-GAL4. PB, protocerebral bridges; FB, fan-shaped body; No, noduli; EB, ellipsoid body. d, e, f, layers of the ventral fan-shaped body, 1, 2, 3, 4, domains of the noduli. (D) Turn score variability of lines with various GAL4 lines driving shibire at 23 °C (blue bars) and 33 °C (orange bars). Bars are ±SE estimated by bootstrap resampling. Numbers indicate sample sizes. *P < 0.05, ***P < 0.001. Red boxes indicate PFN subtypes with high GAL4 expression; pink boxes indicate lower expression.
The GAL4 lines c465, R16D01, and R73D06 drive expression in subsets of columnar neurons (PFNs) projecting from the protocerebral bridges (PB) to the FB and contralateral No (Figs. 3 D and E and 4 and Figs. S5, S7, and S8), with dendritic fields in the PB and axonal fields in the FB and No (31, 32) (Fig. 3E). c465 is also expressed in the mushroom bodies, but silencing them had no effect on turn bias variability (Fig. 3B). The only cell type present in all three of these lines are the PFNs (Fig. 4 and Figs. S5 and S7). PFNs can be subclassified into one of three types based on the regions of innervation within the FB and No (32). Our data suggest that PFNs projecting to No domain 3 may specifically be the regulators of turn bias variability (Fig. 4 C and D). Of the six GAL4 lines in our screen that had PFN expression, the three that had no effect all share strong expression in No domain 4 (Fig. S8), hinting that silencing domain 4 PFNs might counteract or gate the effect of silencing domain 3 PFNs, a possibility that has some statistical support in our data (Discussion and SI Discussion).
Discussion
We find that Drosophila exhibit profound handedness in their locomotor behavior. By developing a high-throughput assay for left-right turning, we are able to demonstrate that individual flies show idiosyncratic left-right biases when walking, that strongly left- or right-biased flies are common in the population, and that an individual's locomotor handedness persists throughout its lifetime. This individual-to-individual variation remains in inbred fly populations, is not heritable, and is explained predominantly by factors other than limb morphological asymmetry. Genetic background and mutants affecting the CC, an interconnected group of neuropils involved in the processing of sensory information and the execution of locomotion, can modulate the degree of turn bias variability across individuals. Indeed, the activity level of specific neurons within the central complex alters the breadth of the distribution of turn biases. Our results suggest that genetically and environmentally matched fruit flies exhibit individual differences in the neural processing of sensory information and the execution of locomotor patterns, resulting in profound levels of idiosyncratic locomotor handedness.
Columnar PFNs of the CC may be involved in the integration of bilateral sensory information or the modulation of stimulus signal-to-noise ratios, and asymmetries in their functions may result in asymmetric behavioral outputs. To more rigorously examine this possibility (33), we developed an average firing rate model of a simple left-right decision-making circuit (SI Discussion and Fig. S9). This model consists of two input units whose activity reflects the aggregated inputs in favor of left or right turning, respectively. Each input activates one of two outputs that inhibit each other and activate themselves. The activity of these output units determines the motor output—if the left output unit is active, then a left motor instruction is generated and vice versa. Reciprocal inhibition circuits similar to this one have been implicated in sensory signal processing in vertebrates and invertebrates (34, 35). Thus, our purpose here was not to reinvent known circuit models, but rather to rigorously test how, in this established framework, a symmetrical perturbation (e.g., silencing the PFNs) could enhance both left- and right-biased asymmetries.
Our model assumes that a network of reciprocal inhibitory connections amplify differences in inputs, resulting in a winner-takes-all output. Such an inhibitory network may be present in GABAergic EB neurons. Upstream neuropils of the EB within the CC include the FB and the PB, implicating the c465 neurons as inputs to the decision-making circuit. In our model, we found that circuit perturbations that increase the signal-to-noise-ratio of the inputs evoking choice behavior result in an increase in behavioral variability by increasing the relative importance of any small network asymmetry inherent to an individual. Conversely, when the signal-to-noise ratio is reduced, noise begins to dominate, and every individual behaves more like a fair coin, thus reducing variability. No other perturbation had this effect. Thus, a reasonable hypothesis for the effects we observed is that silencing the PFNs increases the signal-to-noise ratio in the stimuli driving locomotor choice behavior.
How might silencing PFNs increase the signal-to-noise ratio of inputs driving left-right decisions? Activity in the protocerebral bridges may encode information useful for turning decision making in circumstances other than the experimental setting of our Y-mazes. Indeed, visual information flows via at least two routes to the CC: from the polarized light-sensitive dorsal rim ommatidia to the protocerebral bridges (17) and from the higher-order feature detectors of the optic lobe and optic glomeruli to the lateral triangle (and EB) (36). Because there are no polarized light sources in our experiment, it is plausible that activity in c465 neurons constitutes noise with respect to useful visual stimuli. Because presynapses of c465 neurons are found in the ventral FB and No, these neuropils may be the sites at which stimuli relevant and irrelevant for locomotor turn decision making are integrated. This framework may also shed light on our observation that both silencing c465 neurons (with Shibirets) and increasing their activity (with dTRPA1) increased variability. Both of these manipulations have the potential to “peg” neural activity in a regime that cannot encode any information. Although Shibirets increased variability, neither Kir2.1 nor dTRPA1 had an effect when driven by R16D01 and R73D06. These GAL4 lines appear to have lower expression than c465, so perhaps they did not drive these effectors strongly enough to peg neural activity and block information transmission.
Our intersectional analysis suggests that the role of PFNs in regulating locomotor handedness may vary between PFN subtypes. Lines with the strongest expression in PFNs projecting to the third domain of the noduli had the greatest effect on turn bias variability, whereas those with expression in the fourth domain had the least effect. There may be a gating or additive relationship between the PFN subtypes, such that silencing PFNs projecting to domain 3 of the noduli increases turn bias variability, but simultaneously silencing PFNs projecting to domain 4 blocks or counters the domain 3 effect. This scenario has some statistical support (SI Discussion). The development of GAL4 lines specific to these subtypes will allow us to rigorously test such hypotheses.
Locomotor handedness has been observed in humans (4, 5). In these studies, subjects were asked to walk in a straight line in the absence of visual feedback, a task that proved difficult and resulted in subjects veering off course and circling. Testing across multiple trials revealed a spectrum of consistency, with some subjects always veering left, some always veering right, and others showing inconsistent biases. An individual's circling bias correlated to asymmetries in their posture, which in turn is based on the integration of neural inputs and the internal representation of their body's position in space. Individual asymmetries in the collection and processing of information from the vestibular, proprioceptive, and other sensory systems may therefore result in inherent biases in locomotor behaviors. There are obvious parallels between these results and our findings in flies. Fly locomotor handedness manifests itself as either a bias in left-right turning, or a propensity to turn clockwise or counterclockwise in an arena, and we demonstrate a clear role for brain regions implicated in the integration of multiple sensory inputs.
Just as locomotor handedness, hand clasping, arm folding, and hand dominance are all independent in humans (37), we found that fly locomotor handedness and wing folding are uncorrelated. It remains an appealing research direction to determine whether flies exhibit preferential left vs. right limb use in tasks requiring dexterity. One paradigm that could be illuminating is gap crossing (38, 39) in which flies walk along a raised platform and, by first extending their forelegs, are able to cross gaps wider than their body length. Notably, columnar neurons in the PB are required for coordinated gap crossing. Perhaps flies exhibit a consistent preference in which foreleg they lead with. Using an instrument that rapidly tracks the position of each leg, we previously observed that individual flies show persistent idiosyncrasies in their limb positioning during the transitions between behavioral states like postural adjustment and walking (40). Thus, it is plausible that they exhibit an equivalent of hand dominance.
We and others have shown that genetically and environmentally similar individuals can develop idiosyncratic behaviors, morphology, and gene expression profiles. For example, stochastic DNA methylation may contribute to phenotypic variation that is uncorrelated to genetic variation (41). Stochastic gene expression may underlie the phenomenon of partial penetrance of mutations and variability in the escape responses of the nematode Caenorhabditis elegans (42). Morphologically, invertebrates can display a remarkable degree of individual variation in the development of neural connectivity (43, 44). For example, a detailed anatomical study of lobula descending neurons (LDNs) (45), a pair of cells that project from the optic lobes to the ventral nerve cord in several species of insect, revealed that these cells can have highly variable dendritic morphologies. Strikingly, some flies have more than twice as many LDN dendritic spines on one side of the brain compared with the other, and one individual displayed 29 postsynaptic dendritic branches in the left lobula plate but none in the right. These types of stochastic differences in neuronal morphology may be common across the nervous system.
In addition to variable morphology, individuals with identical genotypes and raised in identical environments can display variable behaviors. Genetically identical offspring of the facultatively asexual aphid Acyrthosiphon pisum display surprising variability in their predator avoidance behaviors, a phenomenon that may help the aphid population escape a variety of insectivores (46). Similarly, a quantitative analysis of locomotion in Aphis fabae, another clonal aphid, suggested that individual insects display idiosyncrasy in their food-foraging behaviors (47). In Drosophila, WT siblings display idiosyncratic preferences when given the choice of two odors (48). Work in our laboratory has shown that inbred flies reared identically show broad diversity in their phototactic behavior and the extent of this variation is under the control of serotonin (19). Individual variation in both wiring and behavior may prove to be a very general feature of neural circuits.
Taken together, our results suggest that when a fly must make a left vs. right decision in the absence of an asymmetric stimulus, asymmetries within the brain predispose the animal to go one way rather than the other and that neural activity influences the degree of variation between animals. Perhaps it is a feature of noisy biological systems that allows the animal to avoid detrimental inaction when presented with ambivalent stimuli. In either case, brain asymmetry is implicated in psychiatric disorders (49), suggesting that regulation of individual-to-individual variability may have clinical dimensions. Individual variation in wiring (44, 45, 50), physiology (51), and behavior (19, 40) may prove to be a very general feature of neural circuits, with broad implications both for our basic understanding of developmental neurobiology and the emergence of behavioral phenotypes at the individual level.
Materials and Methods
See SI Materials and Methods for details. All raw data, data acquisition software, and analysis scripts are available at lab.debivort.org/neuronal-control-of-locomotor-handedness/.
Fly Care.
Flies were housed on modified Cal Tech medium according to standard protocols. A full list of the lines used in this study is available in Table S1. Flies used for Shibirets experiments were reared at 25 °C and transferred to 23 or 33 °C 30 min before and during data collection. GAL80ts;Kir2.1 experimental groups were reared at 18 °C, transferred to 30 °C for 48 h before testing, and transferred to 33 °C for data collection; controls were kept at 18 °C until testing at 33 °C.
Behavior.
Four- to 8-d-old flies were placed into individual Y-mazes or arenas and allowed to walk freely for 2 h. Mazes were illuminated from below with white LEDs (5,500 K; LuminousFilm) and imaged with 2-MP digital cameras (Logitech; Point Gray), and the X-Y positions of the flies' centroids were automatically tracked using background subtraction and recorded with software custom written in LabView (National Instruments). Data were then analyzed with custom written scripts in MatLab (The MathWorks). Data from flies making fewer than 50 turns were discarded. For day-to-day experiments, identity was maintained by storing flies individually in labeled culture vials between tests.
Statistics and Modeling.
All statistical calculations were done in MatLab. Expected turn bias distributions (Fig. 1E and Fig. S1E) were calculated by summing binomial distributions with ni equal to the number of choices made by fly i within the corresponding experimental group, and pi equal to the average right turn probability of the entire population. P values determined by bootstrapping are reported as 95% CI upper bounds. P values were Bonferroni corrected for multiple comparisons as appropriate. Model simulations (Fig. S9) were performed in MatLab (The MathWorks) using Euler approximation.
Supplementary Material
Acknowledgments
We thank Mike Burns, Chris Stokes, and other members of The Rowland Institute for fruitful scientific discussions and technical assistance, as well as Julien Ayroles, Kit Longden, Frank Hirth, Tom Maniatis, Charles Zuker, and members of their laboratories for helpful feedback. We thank Shmuel Raz, Roland Strauss, Michael Reiser, Aravi Samuel, Sam Kunes, Chuntao Dan, Douglas Armstrong, and Hiromu Tanimoto for sharing fly lines. We thank the Janelia Farm FlyLight consortium for allowing us to reuse and modify their GAL4 expression images. This research was funded in part by the Junior Fellows Program at The Rowland Institute at Harvard.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.J.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500804112/-/DCSupplemental.
References
- 1.Medland SE, et al. Genetic influences on handedness: Data from 25,732 Australian and Dutch twin families. Neuropsychologia. 2009;47(2):330–337. doi: 10.1016/j.neuropsychologia.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McManus IC, Davison A, Armour JA. Multilocus genetic models of handedness closely resemble single-locus models in explaining family data and are compatible with genome-wide association studies. Ann N Y Acad Sci. 2013;1288:48–58. doi: 10.1111/nyas.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandler WM, et al. Common variants in left/right asymmetry genes and pathways are associated with relative hand skill. PLoS Genet. 2013;9(9):e1003751. doi: 10.1371/journal.pgen.1003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souman JL, Frissen I, Sreenivasa MN, Ernst MO. Walking straight into circles. Curr Biol. 2009;19(18):1538–1542. doi: 10.1016/j.cub.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 5.Bestaven E, Guillaud E, Cazalets J-R. Is “circling” behavior in humans related to postural asymmetry? PLoS ONE. 2012;7(9):e43861. doi: 10.1371/journal.pone.0043861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins RL. On the inheritance of handedness. I. Laterality in inbred mice. J Hered. 1968;59(1):9–12. doi: 10.1093/oxfordjournals.jhered.a107656. [DOI] [PubMed] [Google Scholar]
- 7.Byrne RA, Kuba MJ, Meisel DV. Lateralized eye use in Octopus vulgaris shows antisymmetrical distribution. Anim Behav. 2004;68(5):1107–1114. [Google Scholar]
- 8.Stancher G, Clara E, Regolin L, Vallortigara G. Lateralized righting behavior in the tortoise (Testudo hermanni) Behav Brain Res. 2006;173(2):315–319. doi: 10.1016/j.bbr.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Frasnelli E. Brain and behavioral lateralization in invertebrates. Front Psychol. 2013;4:939. doi: 10.3389/fpsyg.2013.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamps JA, Saltz JB, Krishnan VV. Genotypic differences in behavioural entropy: Unpredictable genotypes are composed of unpredictable individuals. Anim Behav. 2013;86(3):641–649. doi: 10.1016/j.anbehav.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: An intergrative overiew. Q Rev Biol. 2004;79(3):241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- 12.Dall SRX, Houston AI, McNamara JM. The behavioral ecology of personality: Consistent individual differences from an adaptive perspective. Ecol Lett. 2004;7(8):734–739. [Google Scholar]
- 13.Kralj-Fiser S, Schuett W. Studying personality in invertebrates: Why bother? Anim Behav. 2014;91:41–52. [Google Scholar]
- 14.Bender JA, Pollack AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr Biol. 2010;20(10):921–926. doi: 10.1016/j.cub.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439(7076):551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 16.Ritzmann RE, Ridgel AL, Pollack AJ. Multi-unit recording of antennal mechano-sensitive units in the central complex of the cockroach, Blaberus discoidalis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194(4):341–360. doi: 10.1007/s00359-007-0310-2. [DOI] [PubMed] [Google Scholar]
- 17.Vitzthum H, Muller M, Homberg U. Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J Neurosci. 2002;22(3):1114–1125. doi: 10.1523/JNEUROSCI.22-03-01114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13(5):1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kain JS, Stokes C, de Bivort BL. Phototactic personality in fruit flies and its suppression by serotonin and white. Proc Natl Acad Sci USA. 2012;109(48):19834–19839. doi: 10.1073/pnas.1211988109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Fisher RA. The correlation between relatives on the supposition of Mendelian inheritance. Trans R Soc Edinb. 1918;52:399–433. [Google Scholar]
- 22.Ayroles JF, et al. Behavioral idiosyncracy reveals genetic control of phenotypic variability. Proc Natl Acad Sci USA. 2015;112:6706–6711. doi: 10.1073/pnas.1503830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12(6):633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- 24.Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011;474(7350):204–207. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng X, et al. Ten-a affects the fusion of central complex primordia in Drosophila. PLoS ONE. 2013;8(2):e57129. doi: 10.1371/journal.pone.0057129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong W, Mosca TJ, Luo L. Teneurins instruct synaptic partner matching in an olfactory map. Nature. 2012;484(7393):201–207. doi: 10.1038/nature10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosca TJ, Hong W, Dani VS, Favaloro V, Luo L. Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature. 2012;484(7393):237–241. doi: 10.1038/nature10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 29.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47(2):81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 30.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454(7201):217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young JM, Armstrong JD. Structure of the adult central complex in Drosophila: Organization of distinct neuronal subsets. J Comp Neurol. 2010;518(9):1500–1524. doi: 10.1002/cne.22284. [DOI] [PubMed] [Google Scholar]
- 32.Lin CY, et al. A comprehensive wiring diagram of the protocerebral bridge for visual information processing in the Drosophila brain. Cell Reports. 2013;3(5):1739–1753. doi: 10.1016/j.celrep.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Servedio MR, et al. Not just a theory—the utility of mathematical models in evolutionary biology. PLoS Biol. 2014;12(12):e1002017. doi: 10.1371/journal.pbio.1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452(7190):956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA. 1995;92(8):3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mota T, Yamagata N, Giurfa M, Gronenberg W, Sandoz JC. Neural organization and visual processing in the anterior optic tubercle of the honeybee brain. J Neurosci. 2011;31(32):11443–11456. doi: 10.1523/JNEUROSCI.0995-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McManus IC, Mascie-Taylor CGN. Hand-clasping and arm-folding: A review and a genetic model. Ann Hum Biol. 1979;6(6):527–558. doi: 10.1080/03014467900003931. [DOI] [PubMed] [Google Scholar]
- 38.Pick S, Strauss R. Goal-driven behavioral adaptations in gap-climbing Drosophila. Curr Biol. 2005;15(16):1473–1478. doi: 10.1016/j.cub.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Triphan T, Poeck B, Neuser K, Strauss R. Visual targeting of motor actions in climbing Drosophila. Curr Biol. 2010;20(7):663–668. doi: 10.1016/j.cub.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 40.Kain J, et al. Leg-tracking and automated behavioural classification in Drosophila. Nat Commun. 2013;4:1910. doi: 10.1038/ncomms2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukens LN, Zhan S. The plant genome’s methylation status and response to stress: Implications for plant improvement. Curr Opin Plant Biol. 2007;10(3):317–322. doi: 10.1016/j.pbi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Topalidou I, Chalfie M. Shared gene expression in distinct neurons expressing common selector genes. Proc Natl Acad Sci USA. 2011;108(48):19258–19263. doi: 10.1073/pnas.1111684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bucher D, Prinz AA, Marder E. Animal-to-animal variability in motor pattern production in adults and during growth. J Neurosci. 2005;25(7):1611–1619. doi: 10.1523/JNEUROSCI.3679-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou YH, et al. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13(4):439–449. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nässel DR, Strausfeld NJ. A pair of descending neurons with dendrites in the optic lobes projecting directly to thoracic ganglia of dipterous insects. Cell Tissue Res. 1982;226(2):355–362. doi: 10.1007/BF00218365. [DOI] [PubMed] [Google Scholar]
- 46.Schuett W, et al. Personality variation in a clonal insect: The pea aphid, Acyrthosiphon pisum. Dev Psychobiol. 2011;53(6):631–640. doi: 10.1002/dev.20538. [DOI] [PubMed] [Google Scholar]
- 47.Petrovskii S, Mashanova A, Jansen VA. Variation in individual walking behavior creates the impression of a Levy flight. Proc Natl Acad Sci USA. 2011;108(21):8704–8707. doi: 10.1073/pnas.1015208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139(2):405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klar AJS. An epigenetic hypothesis for human brain laterality, handedness, and psychosis development. Cold Spring Harb Symp Quant Biol. 2004;69:499–506. doi: 10.1101/sqb.2004.69.499. [DOI] [PubMed] [Google Scholar]
- 50.Caron SJ, Ruta V, Abbott LF, Axel R. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature. 2013;497(7447):113–117. doi: 10.1038/nature12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakurai A, Tamvacakis AN, Katz PS. Hidden synaptic differences in a neural circuit underlie differential behavioral susceptibility to a neural injury. eLife. 2014;3:e02598. doi: 10.7554/eLife.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.