Significance
Mating interactions in Drosophila melanogaster depend on a number of sensory cues targeting different modalities like hearing, taste, and olfaction. From an olfactory perspective, only negative fly-derived signals had been identified, whereas a positive signal mediating mating was missing. Here we demonstrate the presence of such a signal (methyl laurate) and dissect the neural mechanism underlying its detection. We also show that the same odorant together with two additional fly-derived odorants (methyl myristate and methyl palmitate) mediate attraction via a pathway separated from that involved in courtship. Interestingly, the odorants identified are attractive to several closely related species. Thus, we describe two highly important neural circuits involved in mating and attraction that seem to be conserved in Drosophila.
Keywords: Drosophila, pheromone, mating, olfaction, olfactory circuit
Abstract
Intraspecific olfactory signals known as pheromones play important roles in insect mating systems. In the model Drosophila melanogaster, a key part of the pheromone-detecting system has remained enigmatic through many years of research in terms of both its behavioral significance and its activating ligands. Here we show that Or47b-and Or88a-expressing olfactory sensory neurons (OSNs) detect the fly-produced odorants methyl laurate (ML), methyl myristate, and methyl palmitate. Fruitless (fruM)-positive Or47b-expressing OSNs detect ML exclusively, and Or47b- and Or47b-expressing OSNs are required for optimal male copulation behavior. In addition, activation of Or47b-expressing OSNs in the male is sufficient to provide a competitive mating advantage. We further find that the vigorous male courtship displayed toward oenocyte-less flies is attributed to an oenocyte-independent sustained production of the Or47b ligand, ML. In addition, we reveal that Or88a-expressing OSNs respond to all three compounds, and that these neurons are necessary and sufficient for attraction behavior in both males and females. Beyond the OSN level, information regarding the three fly odorants is transferred from the antennal lobe to higher brain centers in two dedicated neural lines. Finally, we find that both Or47b- and Or88a-based systems and their ligands are remarkably conserved over a number of drosophilid species. Taken together, our results close a significant gap in the understanding of the olfactory background to Drosophila mating and attraction behavior; while reproductive isolation barriers between species are created mainly by species-specific signals, the mating enhancing signal in several Drosophila species is conserved.
In the vinegar fly Drosophila melanogaster, cuticular hydrocarbons (CHCs) act as pheremones and play important roles in courtship and aggregation behaviors. These pheremones include the female-specific aphrodisiacs (Z,Z)-7,11-heptacosadiene (7,11-HD) and (Z,Z)-7,11-nonacosadiene (7,11-ND) and the male specific antiaphrodisiacs (Z)-7-tricosene (7-T) and 11-cis-vaccenyl acetate (cVA) (1). However, several lines of evidence suggest that other unidentified pheromones likely contribute to courtship and aggregation behaviors. Previous studies have demonstrated that an unidentified volatile sex pheromone produced by female flies stimulates male courtship (2–6). Flies anosmic to cVA exhibit residual attraction to live male flies, suggesting that other attractive cues are produced by flies that are independent of cVA and its neural circuit (7). Furthermore, no specific ligands other than cVA have been identified for the potential pheromone receptors expressed in OSNs of antennal trichoid sensilla (8). Moreover, OSNs expressing olfactory receptors Or47a and Or88a housed in trichoid sensilla respond to unidentified odors in male and female body wash extracts (9).
Although the CHC profile of D. melanogaster has been characterized by several analytical techniques (10–14), it is not yet complete (3). In the present study, we used thermal desorption-gas chromatography-mass spectrometry (TD-GC-MS) to determine whether flies harbor so far unidentified CHCs. TD-GC-MS provides a highly sensitive and labor-saving alternative to solvent extraction, and allows analysis of a wider volatility range of components than all previously mentioned techniques. In addition, this method has been applied to confirm the composition of sex pheromones in other insect species (15, 16).
Here we demonstrate the presence of a truly positive fly-produced signal mediating mating and dissect the neural mechanism underlying its detection. With our findings, the understanding of male olfactory-based sexual arousal is becoming more complete, with all fru-positive OSNs now with known ligands. We also report the presence of the first fly odorants that exclusively mediate attraction in both sexes via a pathway separated from that involved in sexual and social behaviors. Interestingly, both systems and their ligands are remarkably conserved over a number of drosophilid species.
Results and Discussion
Flies Produce Previously Unidentified Candidate Pheromones.
To determine whether D. melanogaster harbors so far unidentified CHCs, we used TD-GC-MS to measure CHC profiles of individual flies. Intact flies of different ages were placed in thermal desorption tubes, which were subsequently heated. The cuticular compounds released were trapped by cooling and then transferred to the GC-MS device by rapid heating. Eighty-five cuticular compounds, including alkanes, methyl-alkanes, monoenes, dienes, aldehydes, ketones, esters, and amides, were identified (Fig. S1 and Table S1). Sixty-four were found in both males and females, whereas 11 were female-specific and 10 were male-specific.
OR47b- and OR88a-Expressing OSNs Detect Methyl Laurate, Methyl Myristate, and Methyl Palmitate.
To test for olfactory detection of the fly-produced compounds identified in the analytical study, we obtained single-sensillum recording (SSR) measurements from all OSN types housed in trichoid sensilla (at1 and at4) using 42 synthetic compounds as stimuli. These compounds were chosen to represent all chemical classes identified. In addition to cVA, three other fly-produced odorants activated two OSN types, both present in the antennal trichoid sensillum type 4 (at4) (Fig. 1 A and B). The at4 sensillum in total houses three OSNs (A–C), which have been shown to respond to previously unidentified odors secreted by both male and female flies (9). The at4A OSN responded exclusively to methyl laurate (ML), whereas the at4C OSN responded to ML, methyl myristate (MM), and methyl palmitate (MP) (Fig. 1 A and B).
Fig. 1.
OR47b- and OR88a-expressing OSNs detect ML, MM, and MP. (A) Average SSR responses from all OSNs housed in trichoid sensilla after stimulation with 42 cuticular compounds (10−1 dilution) (n = 5). (B) Representative SSR traces from measurements of WT at4 OSNs stimulated with ML, MM, and MP (10−1 dilution). (C) Representative GC-SSR measurements from at4 OSNs stimulated with GC-fractionated fly body wash extracts (n = 4). (D) Heat map of the average SSR responses from all OSN classes stimulated with ML, MM, and MP (10−1 dilution) (n = 3). Asterisks denote the total activity of an OSN when spike sorting failed. (E) Dose–response curves from at4A and at4C OSNs to ML, MM, and MP (n = 5). (F) Average SSR responses from ∆ab3A: Or47b, ∆ab3A: Or65a, and ∆ab3A: Or88a to ML, MM, and MP (10−1 dilution) (n = 5). (G) Representative SSR traces from Δab3A: Or47b and Δab3A: Or88a stimulated with ML and MM (10−1 dilution). (H) Average SSR responses from at4A and at4C OSNs of Or47b[3] mutant flies stimulated with ML, MM, and MP (10−1 dilution) (n = 5). (I) Representative SSR traces from at4 OSNs of Or47b[3] mutant flies stimulated with ML, MM, and MP (10−1 dilution). (J) Average SSR responses from at4A and at4C OSNs of Or88a−/− mutant flies stimulated with ML, MM, and MP (10−1 dilution) (n = 5). (K) Representative SSR traces from at4 OSNs of Or88a−/− mutant flies stimulated with ML, MM, and MP (10−1 dilution).
Because not all fly odors were tested in our initial screening, we proceeded to obtain linked GC-SSR measurements from at4 OSNs using fly body wash extracts to further test whether the three fly odors were the exclusive ligands for at4A and at4C OSNs. In these experiments, only three flame ionization detector (FID) peaks corresponded to responses from the at4 OSNs (Fig. 1C). Using GC-MS and synthetic standards, we found that these three FID peaks are ML, MM, and MP. Thus, we conclude that ML is the sole fly-produced ligand for at4A OSNs, whereas ML, MM, and MP are the ligands for at4C OSNs.
To establish whether these three active compounds activate other OSNs types as well, we proceeded to test them in SSR experiments including all OSN types located on the third antennal segment and maxillary palp. None of the compounds elicited a reliable response from any OSN type beyond at4A and at4C (Fig. 1D); thus, we conclude that these three active fly odorants activate exclusively at4A and at4C OSNs.
We next examined dose–response relationships of at4A and at4C OSNs for ML, MM, and MP. In contrast to the strong sexual dimorphism in antennal responses to pheromones observed in moths (17, 18), responses of at4A and at4C OSNs to the three fly odorants were quantitatively indistinguishable between the sexes (Fig. 1E). However, the at4A OSNs were two orders of magnitude more sensitive to ML compared with the at4C OSNs, whereas at4C OSNs were activated by MP at lower doses than by MM or ML (Fig. 1E).
The three neurons of the at4 sensillum express Or47b, Or88a, and the closely related genes Or65a, Or65b, and Or65c (19). To identify the Or expressed in at4A and at4C OSNs, we misexpressed Or47b, Or88a, and Or65a in ∆ab3A OSNs using the Drosophila empty neuron system (20). OSNs misexpressing Or47b responded exclusively to ML, whereas OSNs misexpressing Or88a responded to ML and MM, but not to MP (Fig. 1 F and G). The latter finding is enigmatic, but the detection of MP may require other crucial factors in the native trichoid environment, such as odorant-binding proteins (7, 21). This relationship remains to be investigated, however. None of the three fly odorants activated OSNs misexpressing Or65a (Fig. 1F). Furthermore, in an Or47b mutant (22), which has two identical independent knockout alleles, Or47b[2] and Or47b[3] (in all experiments, we used only Or47b[3] after backcrossing it to the Canton-S background to minimize genetic background effects), the responses of at4A OSNs to ML were completely abolished, whereas at4C OSNs still responded to the three fly odorants (Fig. 1 H and I). In contrast, in an Or88a mutant, which was generated by imprecise excision (as a gift from L. B. Vosshall) and validated by RT-PCR experiments (Fig. S2), the responses of at4C OSNs to the three fly odorants were abolished, whereas the responses of at4A OSNs to ML remained unaffected (Fig. 1 J and K). These results suggest that the responsiveness of at4A OSNs to ML is due to the expression of Or47b, whereas the responsiveness of at4C OSNs to ML, MM, and MP is due to the expression of Or88a.
ML, MM, and MP Peripheral Signals Are Transferred from the Antennal Lobe to Higher Brain Centers in Dedicated Lines.
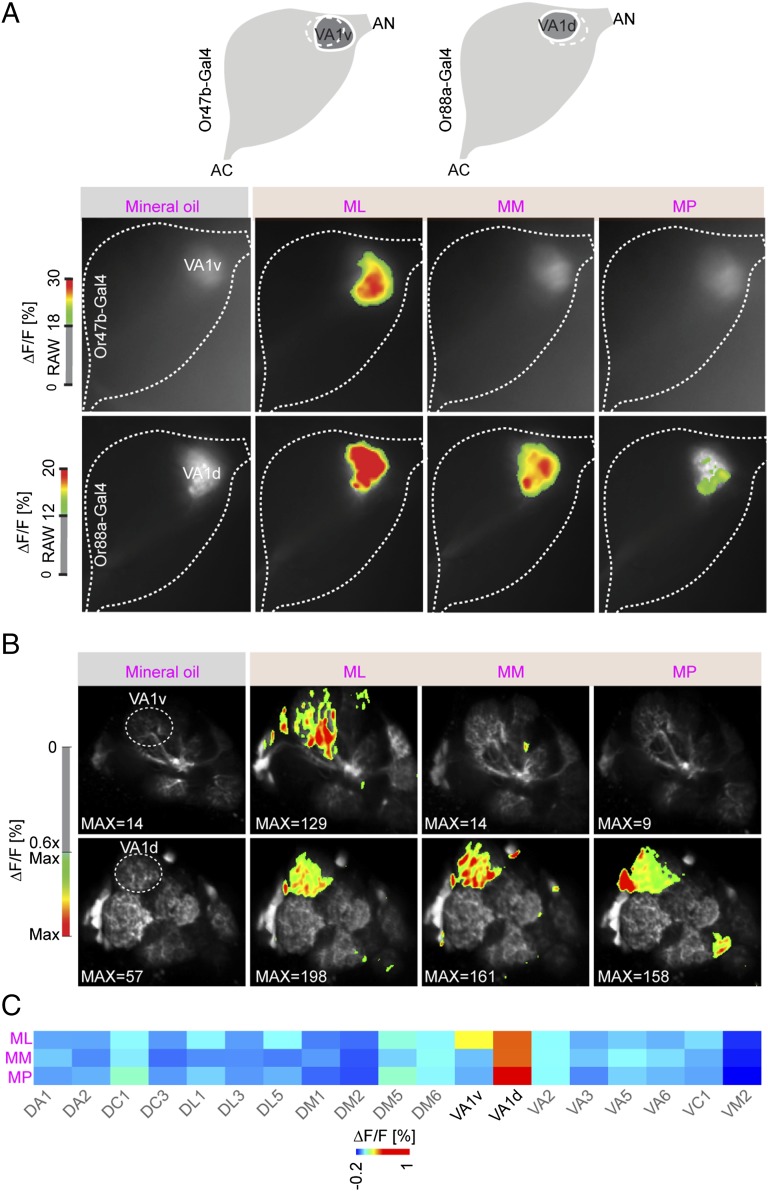
We verified that Or47b- and Or88a-expressing OSNs are the peripheral channels for the three fly odorants by expressing the calcium-sensitive protein GCaMP (23) under control of the two corresponding Or lines (19) (Fig. 2A). To further investigate how the input signals were transferred via projection neurons (PNs) to higher processing centers, we expressed GCaMP (24) under control of the GH146 (25) driver line and performed two-photon calcium imaging at the level of PN dendrites in the antennal lobe (AL). As expected, the VA1v glomerulus, which receives input from Or47b (19), was exclusively activated by ML but not by MM or MP, whereas the VA1d glomerulus, which receives input from Or88a (19), was activated by all three fly odorants (Fig. 2 B and C). Thus, we conclude from the SSR and imaging data that ML, MM, and MP are detected exclusively by Or47b- and Or88a-expressing OSNs, and that this information enters and leaves the AL through these two channels only.
Fig. 2.
ML, MM, and MP peripheral signals are transferred via dedicated neural lines from the antennal lobe to higher brain centers. (A) False color-coded images showing mineral oil-, ML-, MM-, and MP-induced calcium dependent fluorescence changes in the AL of a representative fly expressing the activity reporter GCaMP3.0 from Or47b and Or88a promotors (10−1 dilution) (n = 5). (B) False color-coded images of a representative fly showing mineral oil-, ML-, MM-, and MP-induced calcium signals in PNs of the AL via GCaMP6s expression under control of the GH146-GAL4 driver (10−1 dilution). (C) Heat map of the average ML-, MM-, and MP-evoked calcium signals in PNs in the AL as shown in B (n = 3).
ML Acts as a Stimulatory Pheromone to Promote Male Copulation.
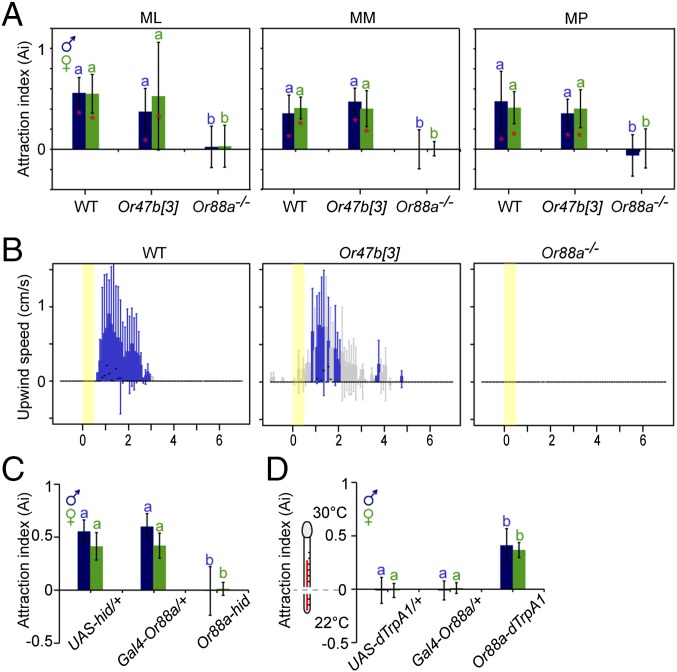
Male courtship behavior is controlled by neural circuitry expressing male-specific isoforms of the transcription factor Fruitless (fruM) (26, 27). Blocking of synaptic transmission of all fru-expressing neurons significantly reduces male courtship (27). The Or47b OSN population is one of only three expressing fruM (26, 27). In addition, the VA1v glomerulus, the target of Or47b neurons in the AL, is larger in males than in females (27). These facts suggest a role for ML, the sole ligand of Or47b-expressing neurons, in mediating male courtship behavior. We investigated this hypothesis in single pair courtship assays. Coating WT females with 100 pg of ML (the equivalent quantity of an individual fly; Fig. S3) significantly increased the number of copulation attempts and copulation success in WT males (Fig. 3 A and B). The other sequences of the male courtship ritual remained unaffected (Fig. S4). WT females coated with 100 pg of MM, MP, or acetone elicited no significant change in the courtship behavior of WT males (Fig. 4 A and B and Fig. S4). Thus, we conclude that only ML, and not MM or MP, acts as a stimulatory pheromone to promote male copulation behavior.
Fig. 3.
Or47b promotes male copulation. (A) Average number of copulation attempts of WT males courting WT females painted with acetone (Ac), ML, MM, or MP (n = 20). Error bars represent SD. Significant differences are denoted by letters (P < 0.05, ANOVA followed by Tukey’s test). (B) Percentage of copulation success of WT males courting WT females painted with acetone, ML, MM, or MP (n = 20) (Fisher’s exact test). (C) Percentage of copulation success of WT, Or47b[3], Or88a−/−, and Or47b rescue males courting WT females (Fisher’s exact test). Sample sizes are given in brackets above bars. (D) Representative SSR traces from at4 OSNs of Or47b[3] and Or47b rescue flies stimulated with ML (10−1 dilution). (E) WT males competing with either Or47b[3] (gray) or Or47b rescue (green) males for mating with WT females in competition assays (n = 25) (χ2 test). (F) Percentage of copulation success of males expressing UAS-hid/+, Gal4-Or47b/+, and UAS-hid from Or47b promoter courting WT females (n = 20) (Fisher’s exact test). (G) Males expressing dTrpA1 from Or47b promoter competing with WT males for mating with WT females in competition assays (n = 25) (χ2 test). (H) Copulation latency of WT and Or47b[3] males courting either WT or oe− females. Error bars represent SD. Significant differences are denoted by letters (P < 0.05, ANOVA followed by Tukey’s test). Sample sizes are given in brackets above bars. (I) Percentage of copulation success of WT and Or47b[3] males courting either WT or oe− females (Fisher’s exact test). Sample sizes are given in brackets above bars. (J) Average quantity of ML, MM, and MP in WT and oe− flies (P > 0.05, independent-samples t test; n = 6).
Fig. 4.
Or88a is required for the attraction behavior toward ML, MM, and MP. (A) Attraction indices of WT, Or47b[3], and Or88a−/− in a binary choice assay between ML, MM, or MP against solvent control. Error bars represent SD. Deviation of the response indices against zero was tested with the Student t test; significant differences are denoted by asterisks. For comparison between groups, ANOVA followed by Tukey’s test was used, and significant differences are denoted by letters (P < 0.05). (B) Boxplot representation of odor-induced changes in the FlyWalk assay in upwind speed. Black lines indicate median values; box, interquartile range; whiskers, 90th and 10th percentiles; blue boxplots, significantly increased upwind speed compared with the upwind speed during the solvent control situation within the corresponding 100-ms time frame (P < 0.05, Wilcoxon signed-rank test; n = 30 flies); gray boxplots, no significant difference in upwind speed. (C) Attraction indices of flies expressing UAS-hid/+, Gal4-Or88a/+, and UAS-hid from Or88a promoter in a binary choice assay between ML and solvent control. Error bars represent SD. ANOVA followed by Tukey’s test was used for comparisons between groups. Significant differences are denoted by letters (P < 0.05). (D) Attraction indices of flies expressing UAS-dTrpA1/+, Gal4-Or88a/+, and UAS-dTrpA1 from Or88a promoter in a binary choice assay between 22 °C and 30 °C. Error bars represent SD. ANOVA followed by Tukey’s test was used for comparisons between groups. Significant differences are denoted by letters (P < 0.05).
Or47b- and Or47b-Expressing OSNs Are Required for Optimal Male Copulation Behavior.
Because ML activates both Or47b- and Or88a-expressing OSNs, we asked whether normal levels of male copulation behavior require only one or both of these receptors. Pairs of either Or47b[3] or Or88a−/− males with virgin WT females were placed in courtship chambers and the percentage of copulation success was observed after 30 min. When courting WT females, Or47b[3] males, but not Or88a mutant males, displayed a significant reduction in copulation success compared with control males (Fig. 3C). This result is consistent with a previous finding that a reduced size of the VA1v glomerulus, the target of Or47b-expressing neurons, causes courtship deficits (28). To verify that the observed phenotype was due to the loss of Or47b function, we rescued this function by introducing UAS-Or47b under control of Or47b-Gal4 into Or47b[3]. Restoration of Or47b function was accompanied by restoration of normal levels of spontaneous activity and responses to ML in at4A OSNs (Fig. 3D). As expected, Or47b rescue males, in contrast to Or47b[3] males, copulated as much as control males when courting WT females (Fig. 3C).
To avoid any variation dependent on female receptivity, we further examined the importance of Or47b for male copulation success in competitive mating assays. In these assays, one WT male with intact Or47b and one male with mutation in Or47b were allowed to compete for copulation with the same WT female for 30 min. The genotypes of the competing males were verified by eye color. Indeed, males with mutation in Or47b had significantly lower copulation success than WT males when competing for copulation with WT females (Fig. 3E). This defect was fully restored to the levels of WT males by rescuing Or47b function (Fig. 3E). Thus, we conclude that Or47b is required for optimal male copulation behavior.
We proceeded to examine whether Or47b-expressing OSNs are also required for promoting male copulation behavior. We expressed the programmed cell death gene, head involution defective (UAS-hid) (29), coupled with UAS-Stinger II from the Or47b promoter to generate flies lacking Or47b neurons. The combination of StingerGFP with hid allowed us to visualize the absence of GFP-labeled Or47b neurons from males lacking Or47b neurons in the fluorescence microscope. Indeed, in single pair courtship assays, males lacking Or47b neurons had significantly less copulation with WT females compared with control males (Fig. 3F). The percentage of copulation success with WT females was similar in males lacking Or47b neurons and males with disrupted Or47b. Thus, we conclude that the activity of Or47b neurons is required for optimal male copulation behavior.
Activation of Or47b-Expressing OSNs Provides a Competitive Mating Advantage.
We next tested whether activation of Or47b OSNs is sufficient to provide a competitive mating advantage. We therefore generated males expressing the heat-activatable cation channel, dTrpA1 (UAS-dTrpA1), from the Or47b promoter to artificially activate Or47b neurons by shifting the temperature to 30 °C. Indeed, males carrying UAS-dTrpA1 (30) from the Or47b promoter exhibited significantly greater copulation success than WT males when competing for copulation with WT females at 30 °C (Fig. 3G). This effect was not observed in males of the same genotype at the permissive temperature (20 °C), or in the parental lines at the restrictive temperature (30 °C) (Fig. 3G). Thus, activation of Or47b neurons is important for providing a competitive mating advantage.
Vigorous Courtship Toward Oenocyte-Less Flies Is Due to Sustained Production of the Or47b Ligand, ML.
In D. melanogaster, CHCs are synthesized in specialized cells called oenocytes (31). Genetic manipulation of oenocyte cells (oe−) eliminates CHCs (32), but does not affect the level of cVA, which is synthesized in the ejaculatory bulb (33). A previous study reported that WT males exhibit decreased copulation latency toward oe− females compared with WT females (32). We investigated whether this decreased copulation latency requires Or47b. For this purpose, we paired either WT or Or47b mutant males with oe− females in single pair assays and observed copulation latency and copulation success. Compared with WT males, Or47b mutant males exhibited increased copulation latency (Fig. 3H) and reduced copulation success when courting oe− females (Fig. 3I). This result, together with the previously reported idea that mutation in Or47b suppresses increased levels of courtship toward oe− males (22), strongly suggest that oe− flies still synthesize the ligand for Or47b. We investigated this hypothesis by analyzing CHC profiles of oe− flies. Indeed, we found no significant difference in the average quantity of ML found on oe− and WT flies, even though all other known nonvolatile pheromones except cVA were completely eliminated from oe− flies (Fig. 3J). These findings provide further support that Or47b and its ligand ML mediate the vigorous courtship observed toward oe− flies, and that ML is the key stimulatory pheromone necessary for optimal male copulation behavior in D. melanogaster.
ML, MM, and MP Elicit Attraction in Males and Females.
Aggregation can facilitate mate finding. Drosophilid flies use aggregation pheromones to assemble on breeding substrates, where they feed, mate, and oviposit communally (34, 35). The well-known aggregation pheromone in D. melanogaster is cVA, which in addition to its role in social and sexual behaviors elicits aggregation in both males and females (36). Flies anosmic to cVA display residual attraction to live male flies, indicating that other attractive cues are produced by flies that are independent of cVA and its neural circuit (7). Therefore, we investigated whether the three so far unidentified fly odorants mediate a behavior similar to the aggregation function of cVA. None of these three fly odorants elicited any significant upwind long-range flight attraction in wind tunnel assays; however, in the trap assay (37), the three fly odorants elicited short-range attraction in both males and females (Fig. 4A). Furthermore, pulses of ML presented in the FlyWalk assay (38, 39) were attractive to both males and females (Fig. 4B). Thus, we conclude that ML, MM, and MP mediate short-range attraction in both males and females.
Or88a- and Or88a-Expressing OSNs Are Required for the Attraction Behavior Toward ML, MM, and MP.
We next asked whether both receptors, Or47b and Or88a, are necessary for the observed attraction behavior. Although Or88a mutant flies were not attracted to any of the three fly odorants in the trap assay, Or47b mutant flies were still attracted to all three (Fig. 4A). Correspondingly, the ML attraction in the FlyWalk assay disappeared in Or88a mutant flies, but not in Or47b mutant flies (Fig. 5B and Fig. S5). In addition, we verified that the observed phenotype of Or88a mutant flies does not reflect a general deficit in attraction behavior by exposing Or88a mutant flies in the FlyWalk assay to pulses of ethyl acetate (EtA), a well-known attractant to flies. Indeed, both Or88a mutant males and females were attracted to EtA, similar to WT flies (Fig. S5). Consequently, we conclude that ML, MM, and MP activate Or88a to mediate short-range attraction in both sexes.
Fig. 5.
Both Or47b- and Or88a-based systems and their ligands are remarkably conserved over a number of drosophilid species. (A) Average SSR responses of ML, MM, and MP from at4A and at4C OSNs of D. simulans (Dsim), D. mauritiana (Dmau), D. yakuba (Dyak), and D. erecta (Dere) (10−1 dilution) (n = 5). (B) Attraction indices from a binary choice assay between ML, MM, or MP and solvent control. Error bars represent SD. Deviation of the response indices against zero was tested with the Student t test, and all were found to be significant (P < 0.05) (n = 10). (C) Average quantity of ML, MM, and MP (n = 3).
We further investigated whether Or88a-expressing OSNs are required for the observed attraction behavior. We generated flies expressing UAS-head involution defective (UAS-hid) and UAS-Stinger II in Or88a neurons to ablate Or88a neurons. Attraction toward ML was abolished in flies lacking Or88a neurons, but not in the corresponding parental lines (Fig. 4C). These experiments suggest that Or88a neurons are necessary for fly attraction behavior induced by Or88a ligands.
We next determined the sufficiency of Or88a OSN activity to induce attraction behavior. For this purpose, we drove the expression of dTrpA1 in Or88a neurons, to conditionally activate this specific OSN population at 30 °C. Consistent with the attraction behavior induced by Or88a ligands, flies carrying Gal4-Or88a and UAS-dTrpA1, but not the corresponding parental lines, preferred traps heated to 30 °C over traps held at 20 °C (Fig. 4D). In short, we conclude that Or88a neurons are necessary and sufficient for the observed attraction toward ML, MM, and MP.
Both Or47b- and Or88a-Based Systems and Their Ligands Are Remarkably Conserved over a Number of Drosophilid Species.
In addition to ML, we also found MM and MP present in oe− flies (Fig. 3J). Interestingly, oe− females are courted by males of four D. melanogaster sibling species (4, 32). Based on these results, we hypothesized that male copulation and aggregation behaviors are driven by the novel pheromones also in these other species. Notably, we found that the other four species detect all three compounds with the same set of OSNs (Fig. 5A) and also show attraction toward these fly odors in trap assays (Fig. 5B). Finally, we found ML and MM (but not MP, which seems to be D. melanogaster-specific) in the CHC profiles of all four sibling species (Fig. 5C). These data suggest that closely related drosophilid species rely on these pheromones to promote male copulation and aggregation behaviors, although the last common ancestor with D. melanogaster lies 2–10 million years back through evolutionary time (40).
Conclusions
The mating of D. melanogaster is clearly governed by a number of sensory cues targeting different detector systems. Already the complexity of the olfactory signals involved in the interplay between positive and negative cues determining the ultimate outcome of an encounter between the sexes is quite astounding. One factor lacking among the so-far unidentified chemical signals has been a truly positive fly-derived olfactory signal mediating mating. We have demonstrated the presence of such a signal (ML) and dissected the neural mechanism (Or47b) underlying its detection. With our findings, the understanding of male olfactory-based sexual arousal is becoming more complete, with all fru-positive OSNs now having known ligands. We also demonstrate the presence of the first fly odorants, MM and MP, which, together with ML, exclusively mediate attraction in both sexes via a pathway (Or88a) separated from that involved in sexual behavior. Interestingly, the compounds identified are attractive to several closely related species. We conclude that in several Drosophila species, the mating enhancing signal is conserved, whereas reproductive isolation barriers between species are created mainly by species-specific signals.
Materials and Methods
TD-GC-MS.
Individual flies were placed in standard microvials in thermal desorption tubes and transferred using a GERSTEL MPS 2 XL multipurpose sampler into a GERSTEL thermal desorption unit (www.gerstel.de). After desorption at 200 °C for 5 min with solvent venting, the analytes were trapped in the liner of a GERSTEL CIS 4 Cooled Injection System at −50 °C, using liquid nitrogen for cooling. The components were transferred to the GC column by heating the programmable temperature vaporizer injector at 12 °C/s up to 210 °C and then held for 5 min. The GC-MS device (Agilent GC 7890A fitted with an MS 5975C inert XL MSD unit; www.agilent.com) was equipped with an HP5-MS UI column (19091S-433UI; Agilent Technologies) and operated as follows. The temperature of the gas chromatograph oven was held at 40 °C for 3 min and then increased by 5 grd/min to 200 °C and then by 20 grd/min to 260 °C, with the final temperature held for 15 min. For MS, the transfer line was held at 260 °C, the source was held at 230 °C, and the quad was held at 150 °C. Mass spectra were taken in EI mode (at 70 eV) in the range from 33 m/z to 500 m/z. The structures of most of the cuticular compounds were confirmed by comparison with reference compounds measured at the same conditions.
Details on Drosophila stocks, compound quantification, genetic elimination of female CHCs, perfuming of female flies with cuticular compounds, single sensillum recordings, imaging, and the different behavioral assays are provided in SI Materials and Methods.
Or88a Mutant Generation and Genotyping Information.
The Or88a mutant was generated by Leslie Vosshall in collaboration with Tim Tully in 2001–2003 by imprecise excision of a P-element from the E4365 strain. This line was generated at Cold Spring Harbor Laboratory as part of a large-scale learning and memory mutant screen in the Tully Lab, supported by the John A. Hartford Foundation. The original strain contains a P-element with the white eye color marker inserted 728 bp upstream of the Or88a ATG translation initiation codon. The P-element insertion site E4365 is indicated by <X> in the following sequence:
TAAGTGTTTGCGTAAACTTACCCCCGTTTTGAGCAGTGCACGCCTCGGAC<X>ATATTACGAAATGCACGAGGGGCATCCACTACGCACAAATAATAGCTCAATTTCAT
Standard P-element mobilization was carried out, and white−/− strains were isolated and genotyped by PCR to detect deletions 3′ of the P-element insertion site. A single imprecise excision line, E4365#181, was isolated and contains a 1,229-bp deletion that stretches from the P-element insertion site downstream to the middle of the first protein-coding exon. In addition to this deletion, there is a 25-bp insertion in the breakpoint region. The breakpoint of the E4365-181 deletion is indicated by <Δ>, and the 25-bp insertion is indicated in lowercase bold type below:
TAAGTGTTTGCGTAAACTTACCCCCGTTTTGAGCAGTGCACGCCTCGGAC<Δ>catgatgaaataacaataatagata<Δ>ATACTCCTGTTGCCCAGCACGAGCAGCTCCTTGGAGGATGGCTGCCATGCGGTG
This deletion removes the first 168 amino acids of Or88a and is predicted to be a null mutation. The strain is homozygous viable, and the deletion does not affect any other known protein-coding genes in this part of the genome. However, in the time since the mutant was generated and characterized, the Drosophila genome consortium has annotated a noncoding RNA (CR44237) located on the other strand and contiguous with the Or88a gene. This theoretical gene has no known function and has not been characterized.
Supplementary Material
Acknowledgments
We thank J. R. Carlson for critical comments on the manuscript and for providing empty neuron fly lines, J.-C. Billeter for providing the oe− fly lines, L. B. Vosshall for providing the Or88a mutant allele, K. Weniger and R. Stieber for technical support, and G. Walther for conducting the blind analyses of the courtship assay. This work was supported by the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504527112/-/DCSupplemental.
References
- 1.Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu Rev Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 2.Averhoff WW, Richardson RH. Pheromonal control of mating patterns in Drosophila melanogaster. Behav Genet. 1974;4(3):207–225. doi: 10.1007/BF01074155. [DOI] [PubMed] [Google Scholar]
- 3.Ferveur JF, Sureau G. Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc Biol Sci. 1996;263(1373):967–973. doi: 10.1098/rspb.1996.0143. [DOI] [PubMed] [Google Scholar]
- 4.Savarit F, Sureau G, Cobb M, Ferveur JF. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc Natl Acad Sci USA. 1999;96(16):9015–9020. doi: 10.1073/pnas.96.16.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shorey HH, Bartell RJ. Role of a volatile female sex pheromone in stimulating male courtship behaviour in Drosophila melanogaster. Anim Behav. 1970;18(1):159–164. doi: 10.1016/0003-3472(70)90085-0. [DOI] [PubMed] [Google Scholar]
- 6.Tompkins L. Genetic analysis of sex appeal in Drosophila. Behav Genet. 1984;14(5):411–440. doi: 10.1007/BF01065443. [DOI] [PubMed] [Google Scholar]
- 7.Xu P, Atkinson R, Jones DN, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45(2):193–200. doi: 10.1016/j.neuron.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446(7135):542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 9.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17(7):606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antony C, Jallon JM. The chemical basis for sex recognition in Drosophila melanogaster. J Insect Physiol. 1982;28(10):873–880. [Google Scholar]
- 11.Everaerts C, Farine JP, Cobb M, Ferveur JF. Drosophila cuticular hydrocarbons revisited: Mating status alters cuticular profiles. PLoS ONE. 2010;5(3):e9607. doi: 10.1371/journal.pone.0009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pechine JM, Antony C, Jallon JM. Precise characterization of cuticular compounds in young Drosophila by mass spectrometry. J Chem Ecol. 1988;14(4):1071–1085. doi: 10.1007/BF01019336. [DOI] [PubMed] [Google Scholar]
- 13.Yew JY, Cody RB, Kravitz EA. Cuticular hydrocarbon analysis of an awake behaving fly using direct analysis in real-time time-of-flight mass spectrometry. Proc Natl Acad Sci USA. 2008;105(20):7135–7140. doi: 10.1073/pnas.0802692105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yew JY, et al. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr Biol. 2009;19(15):1245–1254. doi: 10.1016/j.cub.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griepink FC, Drijfhout FP, Van Beek TA, Visser JH, De Groot A. Analysis of sex pheromone gland content of individual Symmetrischema tangolias by means of direct gland introduction into a two-dimensional gas chromatograph. J Chem Ecol. 2000;26(4):1013–1023. [Google Scholar]
- 16.Drijfhout FP, Van Beek TA, Visser JH, De Groot A. On-line thermal desorption-gas chromatography of intact insects for pheromone analysis. J Chem Ecol. 2000;26(6):1383–1392. [Google Scholar]
- 17.Kasang G, Kaissling KE, Vostrowsky O, Bestmann HJ. Bombykal, a second pheromone component of the silkworm moth Bombyx mori. Angew Chem Int Ed Engl. 1978;17:60. [Google Scholar]
- 18.Kaissling KE, Hildebrand JG, Tumlinson JH. Pheromone receptor cells in the male moth Manduca sexta. Arch Insect Biochem Physiol. 1989;10(4):273–279. [Google Scholar]
- 19.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15(17):1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37(5):827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 21.Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450(7167):289–293. doi: 10.1038/nature06328. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, et al. Hierarchical chemosensory regulation of male–male social interactions in Drosophila. Nat Neurosci. 2011;14(6):757–762. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6(12):875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414(6860):204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- 26.Manoli DS, et al. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436(7049):395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 27.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121(5):795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Kayser MS, Yue Z, Sehgal A. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science. 2014;344(6181):269–274. doi: 10.1126/science.1250553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igaki T, et al. Drob-1, a Drosophila member of the Bcl-2/CED-9 family that promotes cell death. Proc Natl Acad Sci USA. 2000;97(2):662–667. doi: 10.1073/pnas.97.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454(7201):217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferveur JF. Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35(3):279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 32.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461(7266):987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- 33.Butterworth FM. Lipids of Drosophila: A newly detected lipid in the male. Science. 1969;163(3873):1356–1357. doi: 10.1126/science.163.3873.1356. [DOI] [PubMed] [Google Scholar]
- 34.Spieth HT. Courtship behavior in Drosophila. Annu Rev Entomol. 1974;19:385–405. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- 35.Wertheim B, van Baalen EJ, Dicke M, Vet LE. Pheromone-mediated aggregation in nonsocial arthropods: An evolutionary ecological perspective. Annu Rev Entomol. 2005;50:321–346. doi: 10.1146/annurev.ento.49.061802.123329. [DOI] [PubMed] [Google Scholar]
- 36.Bartelt RJ, Schaner AM, Jackson LL. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol. 1985;11(12):1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- 37.Knaden M, Strutz A, Ahsan J, Sachse S, Hansson BS. Spatial representation of odorant valence in an insect brain. Cell Reports. 2012;1(4):392–399. doi: 10.1016/j.celrep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Steck K, et al. A high-throughput behavioral paradigm for Drosophila olfaction: The Flywalk. Sci Rep. 2012;2:361. doi: 10.1038/srep00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thoma M, Hansson BS, Knaden M. Compound valence is conserved in binary odor mixtures in Drosophila melanogaster. J Exp Biol. 2014;217(Pt 20):3645–3655. doi: 10.1242/jeb.106591. [DOI] [PubMed] [Google Scholar]
- 40.Russo CA, Takezaki N, Nei M. Molecular phylogeny and divergence times of drosophilid species. Mol Biol Evol. 1995;12(3):391–404. doi: 10.1093/oxfordjournals.molbev.a040214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.