Significance
The innate immune system provides a first line of defense against invading pathogens. The inflammasome is an innate immune complex that activates inflammatory caspases upon infection, causing cell death and IL-1 cytokine release, which initiate defense against gram-negative bacterial pathogens but also mediate septic shock. Many inflammasome studies have been performed using cells from mice, but mice and humans differ in their complement of inflammatory caspases. Instead of caspase-11, humans encode the putative orthologs caspase-4 and caspase-5. Here, we show that caspase-4 plays a conserved role in inflammasome activation in response to virulent gram-negative pathogens in primary human macrophages. Our findings provide important insight into how inflammasomes are regulated in human cells.
Keywords: inflammasome, caspase-4, innate immunity, primary macrophages, gram-negative bacteria
Abstract
Inflammasomes are critical for host defense against bacterial pathogens. In murine macrophages infected by gram-negative bacteria, the canonical inflammasome activates caspase-1 to mediate pyroptotic cell death and release of IL-1 family cytokines. Additionally, a noncanonical inflammasome controlled by caspase-11 induces cell death and IL-1 release. However, humans do not encode caspase-11. Instead, humans encode two putative orthologs: caspase-4 and caspase-5. Whether either ortholog functions similar to caspase-11 is poorly defined. Therefore, we sought to define the inflammatory caspases in primary human macrophages that regulate inflammasome responses to gram-negative bacteria. We find that human macrophages activate inflammasomes specifically in response to diverse gram-negative bacterial pathogens that introduce bacterial products into the host cytosol using specialized secretion systems. In primary human macrophages, IL-1β secretion requires the caspase-1 inflammasome, whereas IL-1α release and cell death are caspase-1–independent. Instead, caspase-4 mediates IL-1α release and cell death. Our findings implicate human caspase-4 as a critical regulator of noncanonical inflammasome activation that initiates defense against bacterial pathogens in primary human macrophages.
Pattern recognition receptors (PRRs) of the innate immune system are critical for promoting defense against bacterial pathogens (1). Cytosolic PRRs are key for discriminating between pathogenic and nonpathogenic bacteria, because many pathogens access the host cytosol, a compartment where microbial products are typically not found (2). Cytosolic PRRs respond to patterns of pathogenesis that are often associated with virulent bacteria, such as the use of pore-forming toxins or injection of effector molecules through specialized secretion systems (3). A subset of cytosolic PRRs induces the formation of multiprotein complexes known as inflammasomes (4). In mice, the canonical inflammasome activates caspase-1, an inflammatory caspase that mediates cell death and IL-1 family cytokine secretion (5, 6). Additionally, the noncanonical inflammasome activates caspase-11 in response to many gram-negative bacteria (7–14). The canonical and noncanonical inflammasomes differentially regulate release of IL-1α and IL-1β (7). Caspase-11 mediates LPS-induced septic shock in mice (7, 15), and caspase-11 responds to cytoplasmic LPS independent of Toll-like receptor 4 (16, 17).
In addition to its pathologic role in septic shock, the noncanonical inflammasome is critical for host defense in mice (11, 18). However, in humans, it is unclear whether an analogous noncanonical inflammasome exists. Whereas mice encode caspase-11, humans encode two putative functional orthologs: caspase-4 and caspase-5 (19–21). All three inflammatory caspases bind directly to LPS in vitro (22). In murine macrophages, caspase-1 and caspase-11 have both distinct and overlapping roles in the release of IL-1α and IL-1β and the induction of cell death (7). However, the precise role of the human inflammatory caspases in the context of infection by bacterial pathogens remains unclear.
To elucidate how human inflammasome activation is regulated, we investigated the contribution of inflammatory caspases to the response against gram-negative bacterial pathogens in human macrophages. Here, we show that both canonical caspase-1–dependent and noncanonical caspase-1–independent inflammasomes are activated in primary human macrophages and that caspase-4 mediates caspase-1–independent inflammasome responses against several bacterial pathogens, including Legionella pneumophila, Yersinia pseudotuberculosis, and Salmonella enterica serovar Typhimurium (S. Typhimurium). Importantly, noncanonical inflammasome activation in human macrophages is specific for virulent strains of these bacteria that translocate bacterial products into the host cytosol via the virulence-associated type III secretion system (T3SS) or type IV secretion system (T4SS). Thus, caspase-4 is critical for noncanonical inflammasome responses against virulent gram-negative bacteria in human macrophages.
Results
L. pneumophila Induces Both IL-1α and IL-1β Release from Human Macrophages.
In murine macrophages, a canonical inflammasome leads to caspase-1 activation and IL-1β secretion, whereas a noncanonical inflammasome results in caspase-11 activation, cell death, and IL-1α and IL-1β release (7). In human macrophages, it is unclear whether both canonical (caspase-1–dependent) and noncanonical (caspase-1–independent) inflammasomes are activated during bacterial infection (12). To determine if both canonical and noncanonical inflammasomes are activated, we first used L. pneumophila, a pathogen that triggers robust inflammasome activation in murine macrophages and causes a severe form of pneumonia, Legionnaires’ disease, in humans (23). To replicate within macrophages, L. pneumophila uses a T4SS to inject effector proteins into the host cell cytosol (24–26). Because L. pneumophila activates both the canonical and noncanonical inflammasomes in murine macrophages (13, 14), we examined whether the bacterium induces IL-1α and IL-1β release from human macrophages as well. First, we differentiated and infected the THP-1 monocytic cell line. Upon infection, THP-1 cells underwent death and released IL-1α and IL-1β in a manner requiring the presence of the bacterial T4SS (Fig. 1A). THP-1 cells infected with bacterial mutants lacking a functional T4SS [T4SS− L. pneumophila (Lp)] did not activate the inflammasome but still up-regulated pro–IL-1β, suggesting that the cells are capable of sensing T4SS− Lp (Fig. 1B).
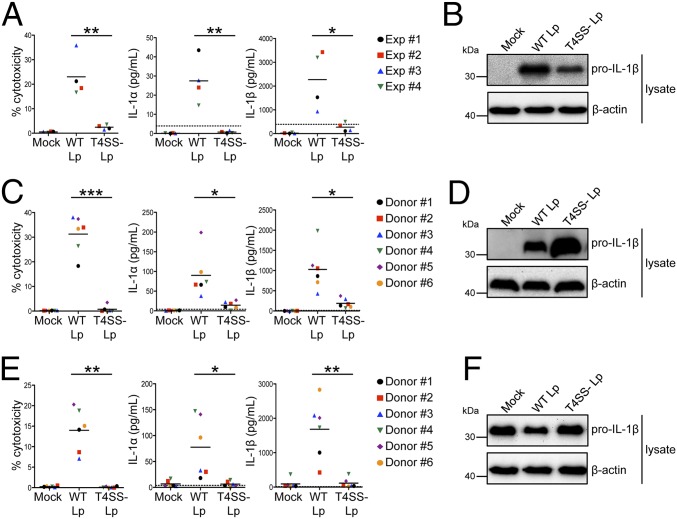
Fig. 1.
L. pneumophila induces both IL-1α and IL-1β release from human macrophages. Phorbol 12-myristate 13-acetate (PMA)-differentiated THP-1 cells (A and B) or primary human MDMs (C and D) were infected with WT Lp, infected with T4SS− Lp, or mock-infected with PBS (Mock) for 20 h. (E and F) Primary human MDMs were primed with LPS and infected with WT Lp or T4SS− Lp or mock-infected for 4 h. Cell death (% cytotoxicity) was measured using a lactate dehydrogenase release assay and normalized to mock-infected cells. IL-1α and IL-1β levels in the supernatants were measured by ELISA. Immunoblot analysis was performed on lysates for full-length IL-1β (pro–IL-1β), and blots were reprobed for β-actin as a loading control. Western blots (B, D, and F) are representative of at least three independent experiments. Shown are the pooled results of four independent experiments in THP-1 cells (A) or the pooled results of six independent infections of cells from different healthy human donors (C and E). Each data point shows the mean of triplicate infected wells. For A, *P < 0.05 and **P < 0.01 by unpaired t test. For C and E, ***P < 0.001, **P < 0.01, and *P < 0.05 by paired t test. The dashed line is the limit of detection.
Inflammasome activation has been extensively analyzed in murine macrophages and in murine and human monocytic and epithelial cell lines. In human epithelial cell lines, noncanonical inflammasome activation leads to IL-18 release and cell death during S. Typhimurium infection (27). Additionally, noncanonical inflammasome activation occurs in a number of transformed cell lines in response to intracellular LPS (22, 27). However, we have limited understanding of inflammasome biology in primary human innate immune cells, particularly with respect to pathways that regulate IL-1α and IL-1β release. We therefore infected primary human monocyte-derived macrophages (MDMs) from healthy human donors with L. pneumophila. Importantly, cell death and IL-1 release in primary human macrophages required the presence of the bacterial T4SS (Fig. 1C). Although T4SS− bacteria did not elicit IL-1β secretion, pro–IL-1β was up-regulated in all infected donor cells (Fig. 1D). Mature IL-1β was detected in the supernatants of cells infected with WT L. pneumophila (WT Lp) but not with T4SS− Lp (Fig. S1). The absence of inflammasome activation in T4SS− Lp-infected cells was not due to a lack of priming, because MDMs that were first directly primed with LPS and then infected with T4SS− Lp also did not activate the inflammasome (Fig. 1 E and F). Thus, L. pneumophila triggers robust T4SS-dependent inflammasome responses in primary human macrophages.
Caspase-1–Dependent and –Independent Inflammasome Pathways Are Activated During Infection of Human Macrophages.
To determine whether both canonical (caspase-1–dependent) and noncanonical (caspase-1–independent) inflammasomes are triggered in human macrophages, we first examined the contribution of caspase-1 to inflammasome responses. We infected primary human MDMs and found that L. pneumophila infection induced caspase-1 cleavage into a 10-kDa (p10) subunit that was released into the supernatant (Fig. 2A). Caspase-1 was processed in response to WT Lp and not T4SS− Lp, suggesting that cytosolic sensing of T4SS activity controls caspase-1 cleavage into its active form. We next asked whether caspase-1 catalytic activity is required for inflammasome activation in human macrophages. We pretreated primary human MDMs with Ac-YVAD-cmk, a chemical inhibitor of caspase-1 activity, and examined inflammasome responses. In primary MDMs, caspase-1 activity played a major role in controlling IL-1β secretion in response to WT Lp (Fig. 2B). Inhibition of caspase-1 catalytic activity had no significant effect on cell death or IL-1α release. These data imply that as in murine cells, caspase-1–independent pathways contribute to IL-1α release in human cells, and these results were consistent with what was observed in THP-1 cells (Fig. 2C). Importantly, the caspase-1–independent cytokine TNF was unaffected by inhibitor treatment. To strengthen the link between caspase-1 and IL-1β secretion in human macrophages, we also knocked down caspase-1 in THP-1 cells (Fig. 2D). Caspase-1 knockdown did not affect expression of caspase-4 or pro–IL-1β during infection with WT Lp but significantly reduced IL-1β secretion (Fig. 2E). Thus, caspase-1 mediates IL-1β release but not IL-1α release, suggesting that another inflammatory caspase may mediate IL-1α release during bacterial infection of human macrophages.
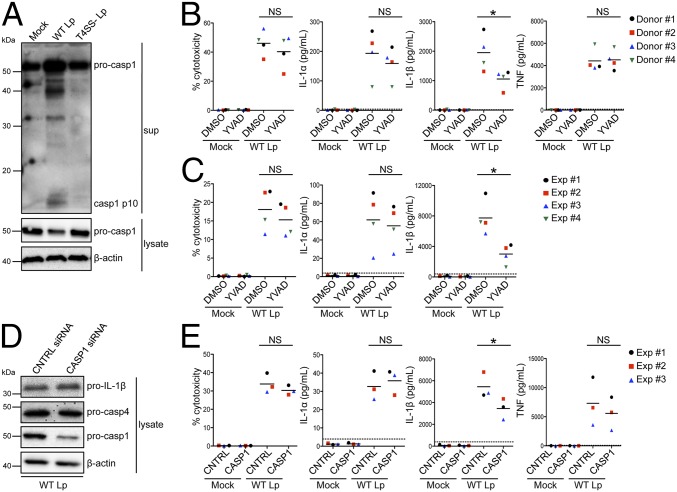
Fig. 2.
Caspase-1–dependent and –independent inflammasomes are activated during infection of human macrophages. (A) Primary human MDMs were infected with WT Lp, infected with T4SS− Lp, or mock-infected for 20 h. Immunoblot analysis was performed on supernatants (sup) for cleaved caspase-1 (casp1 p10) and on lysates for full-length caspase-1 (pro-casp1). Lysates were reprobed for β-actin. Primary human MDMs (B) or PMA-differentiated THP-1 cells (C) were pretreated with 40 μM caspase-1 inhibitor [Ac-YVAD-cmk (YVAD)] or vehicle control (DMSO) and infected with WT Lp or mock-infected for 20 h. (D and E) PMA-differentiated THP-1 cells were transfected with control siRNA (CNTRL) or siRNA against caspase-1 and infected with WT Lp or mock-infected for 20 h. Immunoblot analysis was performed on lysates for pro-casp1, pro-casp4, and pro–IL-1β, and blots were reprobed for β-actin. Cell death was measured using a lactate dehydrogenase release assay and normalized to mock-infected cells. IL-1α, IL-1β, and TNF levels in the supernatants were measured by ELISA. (A and D) Western blots are representative of three independent experiments. Shown are the pooled results of four independent infections of cells from different donors (B) or the pooled results of four (C) or three (E) independent experiments in THP-1 cells. Each data point shows the mean of triplicate infected wells. *P < 0.05 by paired t test (B and E) or unpaired t test (C). NS, not significant. The dashed line is the limit of detection.
Caspase-4 Contributes to Noncanonical Inflammasome Activation in L. pneumophila-Infected Primary Human Macrophages.
Because IL-1α release and cell death were independent of caspase-1, we considered the possibility that other inflammatory caspases mediate this response via noncanonical inflammasome activation. In murine macrophages, caspase-11 is robustly activated and cleaved in response to L. pneumophila (14), but whether caspase-4 or caspase-5 is activated during infection of primary human cells is not known. Notably, we observed that caspase-4 was processed into a 32-kDa (p32) subunit in primary human MDMs infected with WT Lp but not with T4SS− Lp, again implying that primary human macrophages respond to the activity of the virulence-associated T4SS (Fig. 3A). In contrast, we did not observe caspase-5 processing in response to WT Lp (Fig. S2).
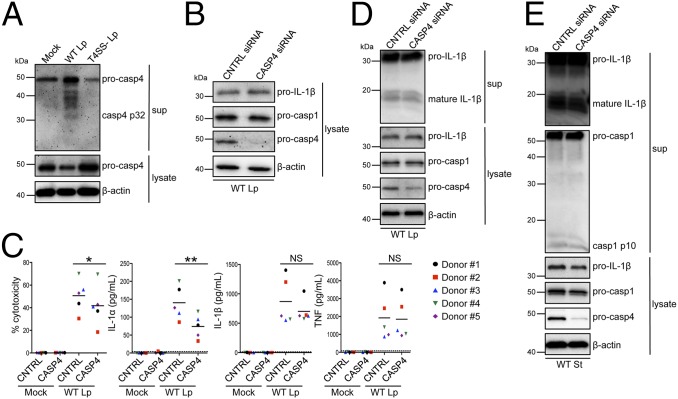
Fig. 3.
Caspase-4 contributes to noncanonical inflammasome activation in L. pneumophila-infected primary human macrophages. (A) Primary human MDMs were infected with WT Lp, infected with T4SS− Lp, or mock-infected for 20 h. Immunoblot analysis was performed on supernatants for cleaved caspase-4 (casp4 p32) and on lysates for pro-casp4. Lysates were reprobed for β-actin. Primary human MDMs (B–D) or THP-1 cells (E) were transfected with control siRNA or siRNA against caspase-4 and infected with WT Lp or mock-infected for 20 h. Immunoblot analysis was performed on supernatants for cleaved IL-1β (mature IL-1β) and casp1 p10 and on lysates for pro–IL-1β, pro-casp1, and pro-casp4, and blots were reprobed for β-actin. Cell death was measured using a lactate dehydrogenase release assay and normalized to mock-infected cells. IL-1α, IL-1β, and TNF levels in the supernatants were measured by ELISA. Western blots (A, B, D, and E) are representative of at least three independent experiments. (C) Shown are the pooled results of five independent infections of cells from different donors. Each data point shows the mean of triplicate infected wells. **P < 0.01 and *P < 0.05 by paired t test. The dashed line is the limit of detection.
In murine macrophages, caspase-11 is up-regulated in response to LPS, type I IFN, and IFN-γ (8, 11). In immortalized epithelial cells, caspase-4 and caspase-5 are both transcriptionally induced by IFN-γ, and caspase-5 is up-regulated by LPS in THP-1 cells (28, 29). However, the effect of type I IFN and LPS on caspase-4 and caspase-5 expression in primary MDMs has not been examined. In MDMs, we observed that although both caspase-4 and caspase-5 were transcriptionally induced by LPS and IFN-β (Fig. S3A), only caspase-4 was translationally up-regulated in response to both stimuli, whereas caspase-5 protein levels increased in response to LPS but not IFN-β (Fig. S3B). Thus, similar to murine caspase-11, caspase-4 is up-regulated by both LPS and type I IFN.
Because caspase-4 is processed specifically in response to WT Lp and is up-regulated by both LPS and type I IFN, we interrogated whether caspase-4 has a role in inflammasome activation. We used siRNA to knock down caspase-4 expression in primary human MDMs and observed robust silencing of caspase-4 and no effect on caspase-1 (Fig. 3B). Caspase-4 played a significant role in mediating cell death in MDMs from four of five donors, and knockdown of caspase-4 significantly reduced IL-1α release for every set of donor cells tested (Fig. 3C). However, in contrast to the role of caspase-11 in murine cells (7), knockdown of caspase-4 did not significantly affect IL-1β secretion or maturation from primary MDMs (Fig. 3D). Release of TNF, an inflammasome-independent cytokine, was unaffected by silencing caspase-4. Similar results were obtained in LPS-primed primary MDMs infected with L. pneumophila (Fig. S4 A and B). Additionally, both IL-1β maturation and caspase-1 processing did not require caspase-4 during infection of THP-1 cells, although IL-1α release was still dependent on caspase-4 (Fig. 3E and Fig. S4C). In contrast, when we infected THP-1 cells that are deficient for the inflammasome adaptor apoptosis-associated speck-like protein containing a carboxy-terminal CARD (ASC) (ASC-def), IL-1β release was greatly diminished (Fig. S5 A and B), thus placing ASC upstream of caspase-1 activation and IL-1β release. These data support a key role for caspase-4 in noncanonical inflammasome activation in primary human macrophages during L. pneumophila infection. Furthermore, these data imply that noncanonical inflammasome activation in human macrophages in response to L. pneumophila infection specifically regulates IL-1α release and cell death separately from IL-1β secretion.
Caspase-4 Mediates Cell Death and IL-1 Release in Primary Human Macrophages in Response to Intracellular LPS.
Intracellular LPS is a trigger for noncanonical, caspase-11–dependent inflammasome activation in murine macrophages (16, 17). Both human caspase-4 and caspase-5 directly bind LPS, and transfection of LPS induces caspase-4–dependent cell death in human monocytic, keratinocyte, and epithelial cell lines and IL-18 release from a human colonic epithelial cell line (22, 27). However, it is unknown whether primary human macrophages respond to cytosolic LPS by activating the noncanonical inflammasome and, if so, whether caspase-4 is responsible. Therefore, we first determined whether transfecting LPS into primary human MDMs induces inflammasome activation. We observed that transfection of LPS into primary human macrophages induced cell death, whereas extracellular LPS treatment did not (Fig. 4A). Silencing of caspase-4 before LPS transfection resulted in a significant reduction in cell death for every donor (Fig. 4 B and C), and both IL-1α release and IL-1β release were also significantly reduced (Fig. 4C). In agreement with prior studies (4), we also found that primary MDMs treated with extracellular LPS for 20 h induced cleavage of caspase-5 but that LPS transfection did not increase this processing (Fig. S6A). These data indicate that caspase-4 is primarily responsible for noncanonical inflammasome responses to intracellular LPS in primary human macrophages; however, unlike the response to bacterial infection, caspase-4 appears to control both IL-1α and IL-1β release during LPS transfection (see Fig. S9).
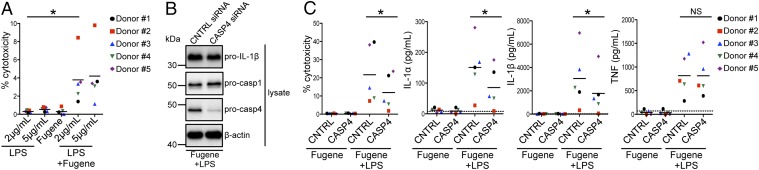
Fig. 4.
Caspase-4 mediates inflammasome activation in primary human macrophages in response to intracellular LPS. (A) Primary human MDMs were primed with Pam3CSK4 (Invivogen) and treated with extracellular LPS at the indicated concentrations, mock-transfected with Fugene HD (Promega) alone, or transfected with Fugene HD and LPS at the indicated concentrations for 20 h. (B and C) Primary human MDMs were transfected with control siRNA or siRNA against caspase-4, primed with Pam3CSK4, and mock-transfected with Fugene HD alone or transfected with Fugene HD and 2 μg/mL LPS for 20 h. Immunoblot analysis was performed on lysates for pro-casp1, pro-casp4, and pro–IL-1β, and blots were reprobed for β-actin. Cell death was measured using a lactate dehydrogenase release assay and normalized to LPS alone (A) or mock-transfected cells (C). IL-1α, IL-1β, and TNF levels in the supernatants were measured by ELISA. (B) Western blots are representative of at least four independent experiments. (A and C) Shown are the pooled results of five independent infections of cells from different donors. Each data point shows the mean of triplicate infected wells. *P < 0.05 by paired t test. The dashed line is the limit of detection.
Caspase-4 Has a Conserved Role in Noncanonical Inflammasome Activation Against Gram-Negative Bacterial Pathogens.
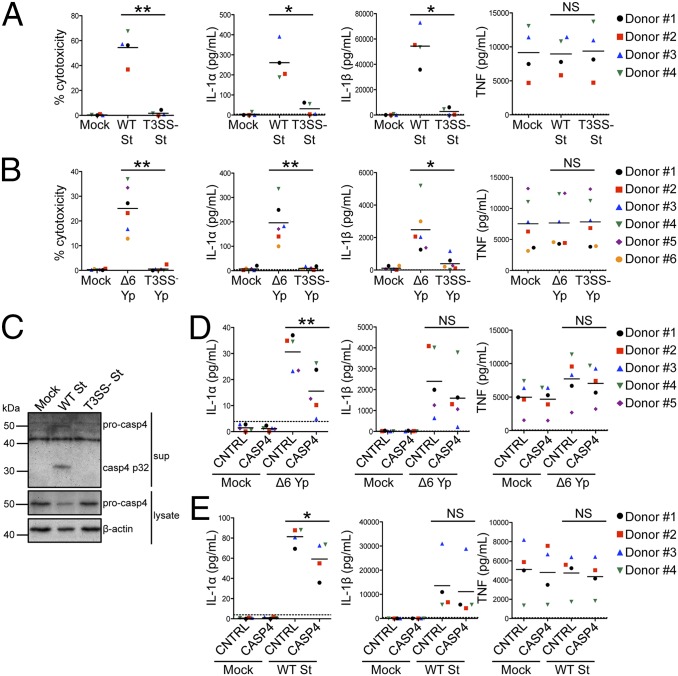
In murine macrophages, caspase-11 controls noncanonical inflammasome responses to a wide variety of gram-negative bacteria, and caspase-11 is activated in response to bacteria that introduce bacterial products into the host cytosol via virulence-associated secretion systems (8–10, 14). We thus hypothesized that caspase-4 has a conserved role in inflammasome activation against other pathogens that use specialized secretion systems to deliver bacterial components into the host cytosol. Similar to the L. pneumophila T4SS, other gram-negative pathogens, including S. Typhimurium and Y. pseudotuberculosis, use a T3SS to inject bacterial effectors that modify host signaling (30). Although the T3SS is evolutionarily quite distinct from the T4SS, both secretion systems perform analogous functions to introduce bacterial products into the host cytosol. Therefore, we infected primary human MDMs with S. Typhimurium and Y. pseudotuberculosis to test whether these bacteria also activate noncanonical inflammasome responses in human cells. S. Typhimurium triggered robust cell death and IL-1 release in a manner requiring the Salmonella pathogenicity island I (SPI-1) T3SS, because bacteria lacking SPI-1 [T3SS− S. Typhimurium (St)] induced little inflammasome activation (Fig. 5A). Because Y. pseudotuberculosis encodes effectors that block inflammasome activation (31, 32), we infected macrophages with a strain of Y. pseudotuberculosis lacking the six known secreted effectors (Δ6 Yp), as Δ6 Yp induces robust caspase-11–dependent inflammasome activation in murine macrophages (14). For Y. pseudotuberculosis, robust inflammasome activation in human macrophages also required the T3SS (Fig. 5B). For both bacteria, the inflammasome-independent cytokine TNF was secreted independent of the presence of a T3SS.
Fig. 5.
Caspase-4 has a conserved role in noncanonical inflammasome activation against gram-negative bacterial pathogens. (A and C) Primary human MDMs were primed with LPS and infected with WT S. Typhimurium (WT St), infected with T3SS− St, or mock-infected for 4 h. Immunoblot analysis was performed on supernatants for casp4 p32 and on lysates for pro-casp4. Blots were reprobed for β-actin. (B) Primary human MDMs were primed with LPS and infected with T3SS-expressing effectorless Y. pseudotuberculosis (Δ6 Yp) or T3SS− Yp or mock-infected for 4 h. (D and E) Primary human MDMs were transfected with control siRNA or siRNA against caspase-4, primed with LPS, and infected with Δ6 Yp (D) or WT St (E) or mock-infected for 4 h. Cell death was measured using a lactate dehydrogenase release assay and normalized to mock-infected cells. IL-1α, IL-1β, and TNF levels in the supernatants were measured by ELISA. (C) Western blots are representative of at least three independent experiments. Shown are the pooled results of four (A and E), five (D), or six (B) independent infections of cells from different donors. Each data point shows the mean of triplicate infected wells. *P < 0.05 and **P < 0.01 by paired t test. The dashed line is the limit of detection.
We next tested whether caspase-4 has a conserved role in inflammasome responses against Y. pseudotuberculosis and S. Typhimurium by using siRNA to knock down caspase-4 in primary human MDMs (Fig. S7 A and B). Indeed, caspase-4 silencing in MDMs significantly reduced IL-1α release (Fig. 5 D and E). Similar to infection with L. pneumophila, IL-1β secretion was caspase-4–independent during infection with Y. pseudotuberculosis, and IL-1β secretion and maturation and caspase-1 processing were also caspase-4–independent during S. Typhimurium infection (Fig. S8). Additionally, infection of ASC-def THP-1 cells showed that ASC is required for IL-1β release during both Y. pseudotuberculosis and S. Typhimurium infection, again placing ASC upstream of caspase-1 (Fig. S5C). Caspase-4 knockdown also significantly reduced cell death upon Y. pseudotuberculosis infection (Fig. S7C), although cell death during S. Typhimurium infection was independent of caspase-4, implying that at least two distinct pathways that mediate cell death are activated in human cells during infection (Fig. S7D). Furthermore, upon infection with WT S. Typhimurium, caspase-4 was processed into the p32 subunit and released into the supernatant (Fig. 5C). Caspase-4 processing requires the presence of the T3SS, because infection with T3SS− St did not induce caspase-4 cleavage. Unlike caspase-4, caspase-5 was not processed during either Y. pseudotuberculosis or S. Typhimurium infection (Fig. S6B). Thus, supporting our findings with L. pneumophila, caspase-4 mediates noncanonical, caspase-1–independent inflammasome activation during infection with Y. pseudotuberculosis and S. Typhimurium as well. Collectively, these data implicate caspase-4 as a critical mediator of inflammasome activation against gram-negative bacterial pathogens that translocate bacterial products into the cytosol of primary human macrophages.
Discussion
Our data demonstrate that caspase-4 regulates noncanonical inflammasome responses against gram-negative bacterial pathogens in primary human macrophages. Recent findings also indicate a role for caspase-4 in nonhematopoietic cells, because caspase-4 mediates secretion of IL-18, another IL-1 family cytokine, in epithelial cell lines infected with S. Typhimurium (27). Interestingly, Shigella flexneri encodes an effector protein that blocks caspase-4 activity (33), and overexpression of caspase-4 in cell lines restricts growth of L. pneumophila (18), supporting a critical role for caspase-4 in defense against bacterial pathogens. Further studies will be important for understanding how caspase-4 responds specifically to virulent bacteria. Because human caspase-4 can bind LPS directly and enhances inflammasome activation in response to LPS when ectopically expressed in mouse macrophages (22, 34), caspase-4 may respond to LPS that is somehow released into the cytosol during infection with virulent bacteria. In murine cells, IFN-inducible guanylate-binding proteins (GBPs) enhance disruption of phagosomes carrying bacterial cargo and allow bacterial products to enter the host cell cytosol, thus promoting caspase-11 activation (35, 36). It would be of interest to determine if GBPs enhance caspase-4 activation in human macrophages as well.
Because L. pneumophila and S. Typhimurium reside within pathogen-containing vacuoles and Y. pseudotuberculosis has an extracellular lifestyle, it is still unclear if caspase-4 is also activated by gram-negative bacterial pathogens that reside within the macrophage cytosol. Presumably, because virulent strains of these pathogens reside and replicate within the cytosol, their LPS could be sensed directly via caspase-4. However, many of these pathogens may have also evolved to evade caspase-4–mediated sensing by blocking caspase-4 activity, as demonstrated by S. flexneri (33), or by encoding LPS that is not readily detectable, as is the case for Francisella novicida in murine macrophages (16).
We observed that although caspase-11 contributes to IL-1β release from murine macrophages in response to both cytosolic LPS and bacterial infection, caspase-4 contributes to IL-1β release in response to cytosolic LPS but does not play a major role in controlling IL-1β release from human macrophages during bacterial infection. We found that human ASC is upstream of caspase-4–independent caspase-1 activation and IL-1β secretion in response to bacterial infection. We speculate that ASC is working in conjunction with NLRP3 to activate caspase-1 during gram-negative bacterial infection, because NLRP3 is recruited to ASC foci during infection of THP-1 cells with S. Typhimurium (37) and both ASC and NLRP3 contribute to IL-1β secretion (38). It is possible that during bacterial infection, another cytosolic pathogen-associated molecular pattern dominantly triggers a canonical NLRP3/ASC/caspase-1 inflammasome and IL-1β release independent of caspase-4. Alternatively, even though we do not detect caspase-5 processing, it is possible that caspase-5 contributes to caspase-1 activation and IL-1β secretion independent of caspase-4 during bacterial infection (Fig. S9).
Intriguingly, in addition to our finding that caspase-4 is activated during bacterial infection, caspase-4 is activated in response to endoplasmic reticulum (ER) stress (39) and UVB irradiation (40). These data imply that both exogenous and endogenous stressors may trigger caspase-4 activation and that a common mechanism may be involved. Both UVB irradiation and ER stress result in elevated cytoplasmic calcium, which has been linked to inflammasome activation (41–43). Because L. pneumophila intercepts ER-derived vesicles to establish its replicative vacuole (44, 45), it is possible that perturbations to ER and calcium homeostasis during bacterial infection provide common signals that induce caspase-4 activation.
Overall, our data implicate caspase-4 as a critical mediator of host defense against virulent gram-negative bacteria in primary human macrophages and reveal unexpected differences in the regulation of noncanonical inflammasome pathways in murine and human cells. Caspase-4 plays an important role as an innate immune effector for discrimination between pathogenic and nonpathogenic bacteria in humans, and further studies will examine the basis for differences in how noncanonical inflammasomes function in different organisms. Like caspase-11 in mice, caspase-4 may play a dual role in humans both to protect the host and to mediate septic shock during bacterial infection. Therefore, studying caspase-4 is critical for our understanding of how the human immune system coordinates an appropriate response during bacterial infection.
Materials and Methods
Primary Human Samples.
All studies on human peripheral blood mononuclear cells (PBMCs) were performed in compliance with the requirements of the US Department of Health and Human Services and the principles expressed in the Declaration of Helsinki. Samples obtained from the University of Pennsylvania Human Immunology Core are considered to be a secondary use of deidentified human specimens and are exempt via Title 55 Part 46, Subpart A of 46.101 (b) of the Code of Federal Regulations.
Bacterial Strains.
All experiments using L. pneumophila were performed with L. pneumophila serogroup 1 strains. The strain Lp02 (thyA), a thymidine auxotroph derived from Lp01 (25), and the ΔdotA (T4SS−) isogenic mutant strain (46) were used as previously described (14). For 48 h before infection, L. pneumophila strains were grown in a stationary patch on charcoal yeast extract agar plates at 37 °C. All experiments using S. Typhimurium were performed with S. Typhimurium SL1344 strains. The strain SL1344 and the ΔsipB (T3SS−) isogenic mutant strain were used. S. Typhimurium strains were grown overnight in LB broth with aeration at 37 °C. Three hours before infection, S. Typhimurium strains were diluted into fresh LB with 300 mM NaCl and then grown for 3 h standing at 37 °C to induce SPI-1 expression. All experiments using Y. pseudotuberculosis were performed with Y. pseudotuberculosis IP2666 strains. The strain IP2666 ΔyopHOJMEK (Δ6 Yp) (47) and the ΔyopB (T3SS−) isogenic mutant (48) strain were used. Y. pseudotuberculosis strains were grown overnight in 2× yeast extract tryptone (YT) broth with aeration at 26 °C. Three hours before infection, Y. pseudotuberculosis strains were diluted into fresh 2× YT with 20 mM sodium oxalate and 20 mM MgCl2, and then grown for 1 h with aeration at 26 °C followed by 2 h with aeration at 37 °C.
Macrophage Infections.
THP-1 cells (TIB-202; American Type Culture Collection) were maintained in RPMI supplemented with 10% (vol/vol) heat-inactivated FBS, 0.05 mM β-mercaptoethanol, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified incubator. One day before infection, THP-1 cells were replated in media without antibiotics at a concentration of 2.0 × 105 cells per well of a 48-well plate and incubated overnight with 200 nM phorbol 12-myristate 13-acetate to induce differentiation into macrophages. The media were replaced with warm media without antibiotics on the day of infection.
Primary human PBMCs from deidentified healthy human donors were obtained from the University of Pennsylvania Human Immunology Core. PBMCs were pelleted at 200 × g for 12 min and washed two times with PBS containing 0.5% BSA and 2 mM EDTA. Monocytes were negatively selected using the Pan Monocyte Isolation Kit, Human (Miltenyi), which enriches for both CD14- and CD16-expressing human monocytes. After selection, monocytes were cultured in RPMI supplemented with 10% (vol/vol) heat-inactivated FBS, 2 mM l-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 50 ng/mL recombinant human M-CSF (Gemini Bio Products). Cells were cultured for 4 d in 10 mL of media in 10-cm dishes at 0.5 × 106 cells per milliliter; fresh media with 50 ng/mL M-CSF was then added, and cells were cultured for an additional 2 d to complete differentiation into macrophages. One day before infection, MDMs were gently detached and replated in media without antibiotics and with 25 ng/mL M-CSF at a concentration of 1.25 × 105 cells per well of a 48-well plate. LPS pretreatment, siRNA knockdown experiments, transfection of intracellular LPS, cytotoxicity assays, ELISA, immunoblot analysis, quantitative RT-PCR analysis, and statistical analysis were performed as described in SI Materials and Methods. Differences were considered statistically significant if the P value was <0.05.
Supplementary Material
Acknowledgments
We thank the Human Immunology Core of the Penn Center for AIDS Research and Abramson Cancer Center for primary human PBMCs. We thank Igor Brodsky for critical reading of the manuscript, the Brodsky laboratory for valuable feedback on experimental design and for use of the Y. pseudotuberculosis and S. Typhimurium strains, and Leigh Knodler for valuable technical advice. This work was supported, in part, by NIH Grants K99/R00AI087963 (to S.S.) and T32GM007229 (to A.M.C.), as well as by American Lung Association Grant RG-268528-N (to S.S.), the University of Pennsylvania University Research Foundation (S.S.), American Heart Association Grant 13BGIA14780070 (to S.S.), and a University of Pennsylvania Institute for Immunology Pilot Grant (to S.S.). This material is based on work supported by the National Science Foundation under Grant DGE-0822 (to C.N.C., graduate research fellowship).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421699112/-/DCSupplemental.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Harton JA, Linhoff MW, Zhang J, Ting JP-Y. Cutting edge: CATERPILLER: A large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J Immunol. 2002;169(8):4088–4093. doi: 10.4049/jimmunol.169.8.4088. [DOI] [PubMed] [Google Scholar]
- 3.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: Discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6(1):10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 5.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80(3):401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 6.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 7.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 8.Rathinam VAK, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150(3):606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurung P, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem. 2012;287(41):34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broz P, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490(7419):288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aachoui Y, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339(6122):975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Case CL, et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci USA. 2013;110(5):1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casson CN, et al. Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog. 2013;9(6):e1003400. doi: 10.1371/journal.ppat.1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92(4):501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 16.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 18.Akhter A, et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity. 2012;37(1):35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamens J, et al. Identification and characterization of ICH-2, a novel member of the interleukin-1 beta-converting enzyme family of cysteine proteases. J Biol Chem. 1995;270(25):15250–15256. doi: 10.1074/jbc.270.25.15250. [DOI] [PubMed] [Google Scholar]
- 20.Munday NA, et al. Molecular cloning and pro-apoptotic activity of ICErelII and ICErelIII, members of the ICE/CED-3 family of cysteine proteases. J Biol Chem. 1995;270(26):15870–15876. doi: 10.1074/jbc.270.26.15870. [DOI] [PubMed] [Google Scholar]
- 21.Kamada S, Funahashi Y, Tsujimoto Y. Caspase-4 and caspase-5, members of the ICE/CED-3 family of cysteine proteases, are CrmA-inhibitable proteases. Cell Death Differ. 1997;4(6):473–478. doi: 10.1038/sj.cdd.4400268. [DOI] [PubMed] [Google Scholar]
- 22.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 23.McDade JE, et al. Legionnaires’ disease: Isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297(22):1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 24.Marra A, Blander SJ, Horwitz MA, Shuman HA. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. 1992;89(20):9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7(1):7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 26.Isberg RR, O’Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: Making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7(1):13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knodler LA, et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe. 2014;16(2):249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ossina NK, et al. Interferon-gamma modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem. 1997;272(26):16351–16357. doi: 10.1074/jbc.272.26.16351. [DOI] [PubMed] [Google Scholar]
- 29.Lin XY, Choi MS, Porter AG. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma. J Biol Chem. 2000;275(51):39920–39926. doi: 10.1074/jbc.M007255200. [DOI] [PubMed] [Google Scholar]
- 30.Galán JE, Lara-Tejero M, Marlovits TC, Wagner S. Bacterial type III secretion systems: Specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol. 2014;68:415–438. doi: 10.1146/annurev-micro-092412-155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodsky IE, et al. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe. 2010;7(5):376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaRock CN, Cookson BT. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe. 2012;12(6):799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi T, et al. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe. 2013;13(5):570–583. doi: 10.1016/j.chom.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Kajiwara Y, et al. A critical role for human caspase-4 in endotoxin sensitivity. J Immunol. 2014;193(1):335–343. doi: 10.4049/jimmunol.1303424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meunier E, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509(7500):366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 36.Pilla DM, et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci USA. 2014;111(16):6046–6051. doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Man SM, et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci USA. 2014;111(20):7403–7408. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Willingham SB, Ting JP-Y, Re F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hitomi J, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165(3):347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sollberger G, Strittmatter GE, Kistowska M, French LE, Beer H-D. Caspase-4 is required for activation of inflammasomes. J Immunol. 2012;188(4):1992–2000. doi: 10.4049/jimmunol.1101620. [DOI] [PubMed] [Google Scholar]
- 41.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17(13):1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 42.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami T, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA. 2012;109(28):11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horwitz MA, Silverstein SC. Legionnaires’ disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63(9):3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger KH, Merriam JJ, Isberg RR. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14(4):809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 47.Lilo S, Zheng Y, Bliska JB. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect Immun. 2008;76(9):3911–3923. doi: 10.1128/IAI.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer LE, Hobbie S, Galán JE, Bliska JB. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27(5):953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.