To keep the mechanical integrity of an organism it seems obvious that cells, as the building blocks, must be solid. Although it is clear that switching to a fluid would be catastrophic for organization of the body, it turns out that living cells do change their mechanical properties to a more fluid-like behavior when it comes to migration and force generation. Being fluid-like allows cells to adapt to any arbitrary shape posed by the environment, which is crucial for movement through complex tissue. The mechanical integrity of healthy cells is therefore closely regulated to ensure that cells are solid enough to maintain tissue shape while also being fluid enough to allow dynamic remodeling. Physics provides powerful tools in the framework of viscoelasticity to characterize this fundamental solid and fluid-like behavior (1), and it is evident that cells need to dynamically regulate their viscoelastic properties to support physiological pressures and forces generated during lung expansion, muscle contraction, blood filtration, etc., while still allowing growth, remodeling, and repair over the lifetime of the organism. However, when this precise mechanical regulation is disturbed, cells often transition to diseased states (2). In PNAS, Ehrlicher et al. (3) study a genetic defect in the actin cross-linker alpha-actinin 4 that is known to lead to the severe kidney disease focal segmental glomerulosclerosis. Their study shows that the mutation affects cell movement, force generation, and cytoplasmic mobility, thus providing a connection between physical properties at the molecular scale and human disease.
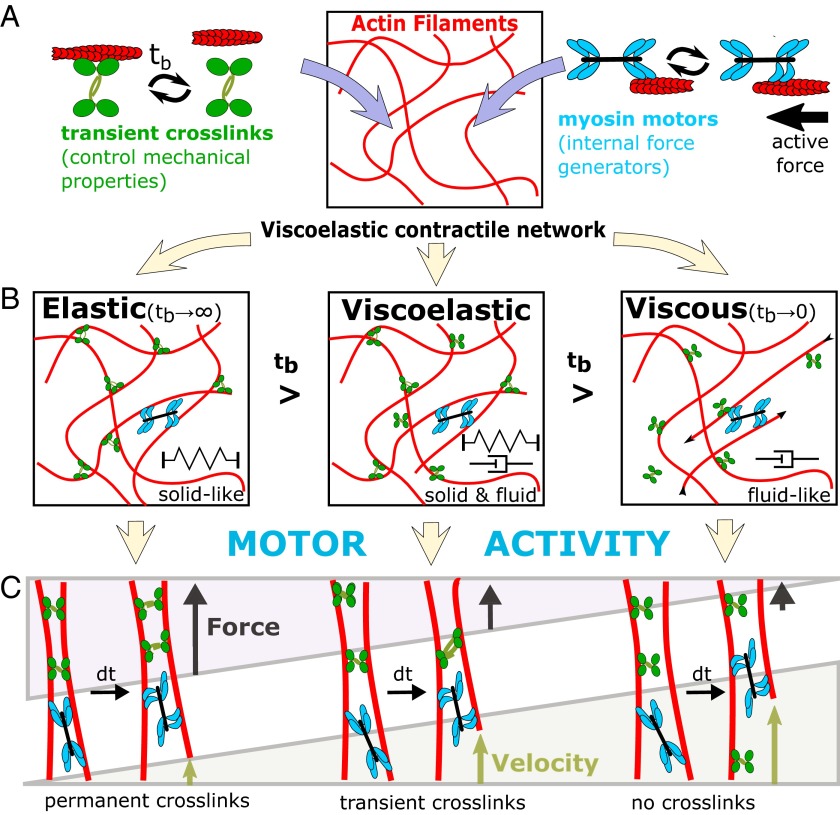
Thanks to a number of fundamental physical studies in simplified in vitro model systems (4–7), the mechanical properties of actin networks have been well characterized, thus setting the stage to understand cellular viscoelasticity. Two important ingredients control the mechanical properties of actin networks in cells: cross-linkers and molecular motors (Fig. 1A). Permanent cross-linking of actin networks (via scruin) is known to cause a dominantly elastic behavior (4). In contrast, transient cross-linking (via heavy meromyosin) allowed stress relaxation in the network, hence resulting in a dominantly viscous behavior at long timescales (5). These previous studies hint that cross-link kinetics provides a mechanism to tune the viscoelastic properties of the actin network. Groundbreaking in vitro studies of myosin-II in actin networks further emphasized the importance of cross-linking dynamics (6, 7). At low cross-linking density, the activity of myosin-II motors allowed faster stress relaxation to occur leading to an even more fluid-like network (6). However, when permanent cross-links were introduced into a similar network, the activity of myosin-II motors resulted in elastic stiffening by two orders of magnitude (7). Thus, the combination of dynamic cross-links and myosin motor activity provides a sensitive mechanism to tune actin network viscoelasticity between a dominantly elastic solid to a viscous fluid (Fig. 1 B and C).
Fig. 1.
Schematic diagram of the properties of actin networks with cross-linkers and myosin motors. (A) Actin filaments form the bulk polymeric material of the network. Transient cross-linkers bind actin filaments together and myosin motors allow the generation of internal forces. (B) The binding time of cross-linkers (tb) determines the transition from solid to fluid-like behavior. In the limit where the cross-links are bound infinitely (tb →∞), the network becomes elastic, whereas for vanishing binding times it is fluid. (C) In a solid-like network (permanent cross-links), motor forces lead to build up of elastic energy, which is stored in the deformation of actin filaments and cross-linkers. In a fluid-like network (no cross-links), motor forces are able to slide uncross-linked filaments through the network leading to force relaxation through viscous dissipation. Tuning the cross-linking dynamics of the actin cytoskeleton allows cells to generate and resist forces while also allowing remodeling.
Recent in vivo studies are beginning to reveal the role of cross-linkers and molecular motors on the mechanical properties of living cells (8–10). For instance, in mouse oocytes myosin-V acts as a motor and cross-linker of the actin meshwork that drives fluidization of the cytoskeleton to allow positioning of the nucleus during prophase I, which is necessary for proper division (8). In a similar fashion, myosin-II motors provide a random shaking force in the cytoskeleton to stir the elastic cytoplasm, creating mobility of subcellular organelles and molecules resembling a fluid (9, 10). Because molecular motors serve as both cross-linkers and force generators, they provide an added level of complexity that can be difficult to decouple.
Ehrlicher et al. (3) circumvent this difficulty by elegantly tuning the dynamic cross-linking kinetics directly in living cells. Alpha-actinin is a cross-linker that forms loose bundles of actin filaments, but a mutated version known as K255E, leads to kidney dysfunction. Using fluorescence recovery after photobleaching, Ehrlicher et al. (3) show that the mutated alpha-actinin (K255E) binds actin filaments together for nearly three times as long as the normal alpha-actinin in WT cells. This suggests that stress relaxation in the cytoskeleton of K255E cells will occur more slowly, allowing these cells to resist larger forces than normal. Indeed, tracking endocytosed particles shows that mutated cells exhibit three times less intracellular motion due to the increased resistance of the actin network. In other words, the increased binding time of alpha-actinin shifts the cytoskeleton to a more solid-like regime.
Because the cytoskeleton does not only provide mechanical resistance but also transmits forces to the surrounding environment, the cross-linker mutation was tested for changes in cell force application. By using traction force microscopy, Ehrlicher et al. (3) show that K255E cells generate higher traction forces resulting in increased work done on the environment. These solid-like
Overall, the study by Ehrlicher et al. shows that, by only changing the dynamic of actin cross-linkers, it is possible to tune the solid and fluid-like behavior of living cells.
characteristics of mutant K255E cells come with the added cost of decreased migration speed, most likely due to longer waiting times required to reorganize the cytoskeleton. Additionally, Ehrlicher et al. calculate the strain energy, a measure of the work done by the cell on the substrate, and show that K255E cells exert five times the amount of work, which is attributed to less work being dissipated by actin filament sliding. Thus, the cross-linking kinetics is able to tune how myosin-II motor forces are translated from inside to outside of the cell. In future studies, it would be exciting to investigate independently the contribution of cross-linkers and motor activity on how mechanics and forces are partitioned in the cytoplasm, which could be done using recently developed techniques such as force spectrum microscopy (10).
Overall, the study by Ehrlicher et al. (3) shows that, by only changing the dynamics of actin cross-linkers, it is possible to tune the solid and fluid-like behavior of living cells. On one hand, the implication is that a molecular-scale defect can dramatically change the ability of a cell to function under the pressure and force typically experienced in physiological tissues (e.g., kidney, heart, lungs, etc.). On the other hand, the tuning of dynamic cross-links provides cells a way to tune their properties from being like an Olympic runner (intermediate strength with high agility) to more like a professional bodybuilder (strong force generation but little movement). Additionally, it is not far-fetched to say that athletic ability at the organismal scale is related to cross-linked actin-myosin networks at the molecular scale (11).
In broader scope, these results are a clear example of the relevance of basic biophysical studies to cell biology and human disease. A fundamental understanding of actin network physics was developed using in vitro systems (4–7), setting the stage for recent discoveries of active diffusion driven processes in vivo (8–10). The current study by Ehrlicher et al. (3) relates fundamental actin network behavior to a known human kidney disease by manipulating dynamic cross-linking to tie it all together.
Acknowledgments
W.W.A. was supported by La Fondation Pierre-Gilles de Gennes and Marie Curie Actions. T.B. was supported by French Agence Nationale de la Recherche Grants ANR-11-JSV5-0002.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6619.
References
- 1.Janmey PA, Georges PC, Hvidt S. Basic rheology for biologists. Methods Cell Biol. 2007;83:3–27. doi: 10.1016/S0091-679X(07)83001-9. [DOI] [PubMed] [Google Scholar]
- 2.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrlicher AJ, et al. Alpha-actinin binding kinetics modulate cellular dynamics and force generation. Proc Natl Acad Sci USA. 2015;112:6619–6624. doi: 10.1073/pnas.1505652112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin JH, Gardel ML, Mahadevan L, Matsudaira P, Weitz DA. Relating microstructure to rheology of a bundled and cross-linked F-actin network in vitro. Proc Natl Acad Sci USA. 2004;101(26):9636–9641. doi: 10.1073/pnas.0308733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieleg O, Claessens MM, Luan Y, Bausch AR. Transient binding and dissipation in cross-linked actin networks. Phys Rev Lett. 2008;101(10):108101. doi: 10.1103/PhysRevLett.101.108101. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey D, Duggan C, Saha D, Smith D, Käs J. Active fluidization of polymer networks through molecular motors. Nature. 2002;416(6879):413–416. doi: 10.1038/416413a. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno D, Tardin C, Schmidt CF, Mackintosh FC. Nonequilibrium mechanics of active cytoskeletal networks. Science. 2007;315(5810):370–373. doi: 10.1126/science.1134404. [DOI] [PubMed] [Google Scholar]
- 8.Almonacid M, et al. Active diffusion positions the nucleus in mouse oocytes. Nat Cell Biol. 2015;17(4):470–479. doi: 10.1038/ncb3131. [DOI] [PubMed] [Google Scholar]
- 9.Fakhri N, et al. High-resolution mapping of intracellular fluctuations using carbon nanotubes. Science. 2014;344(6187):1031–1035. doi: 10.1126/science.1250170. [DOI] [PubMed] [Google Scholar]
- 10.Guo M, et al. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell. 2014;158(4):822–832. doi: 10.1016/j.cell.2014.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Étienne J, et al. Cells as liquid motors: Mechanosensitivity emerges from collective dynamics of actomyosin cortex. Proc Natl Acad Sci USA. 2015;112(9):2740–2745. doi: 10.1073/pnas.1417113112. [DOI] [PMC free article] [PubMed] [Google Scholar]